Bio-Based Mulching Films and Soil Conditioners for Non-Irrigated Tomato Cultivation: Toward Plastic-Free and Water-Efficient Crop Production
Abstract
1. Introduction
2. Results and Discussion
2.1. Evaluation of Yield Characteristic
2.2. Physico-Chemical Analysis of Tomato Fruits
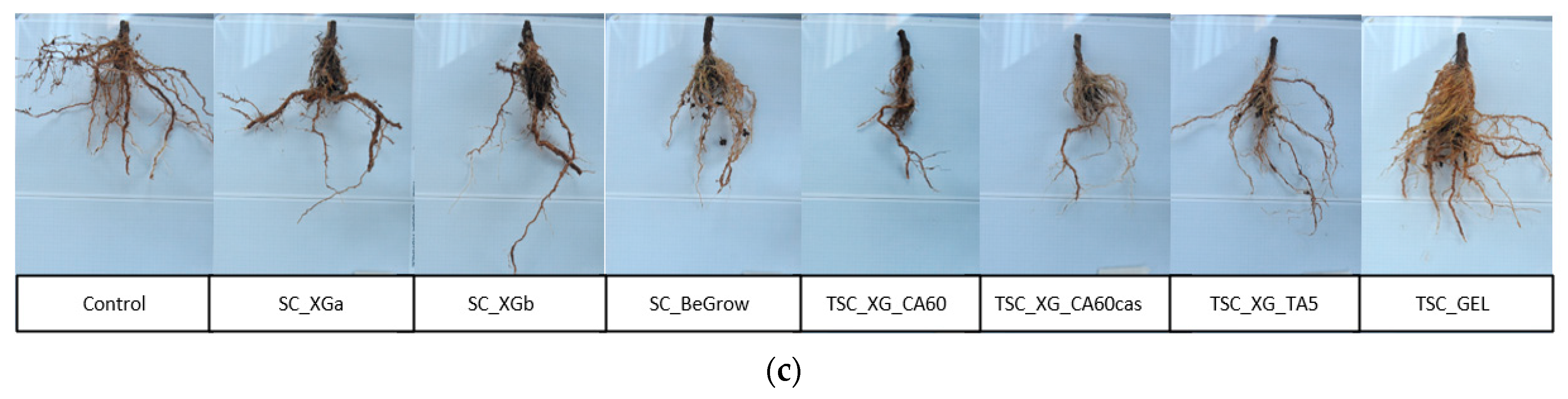
2.3. Evaluation of Plant Root System
2.4. Evaluation of Mulching Residues
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation
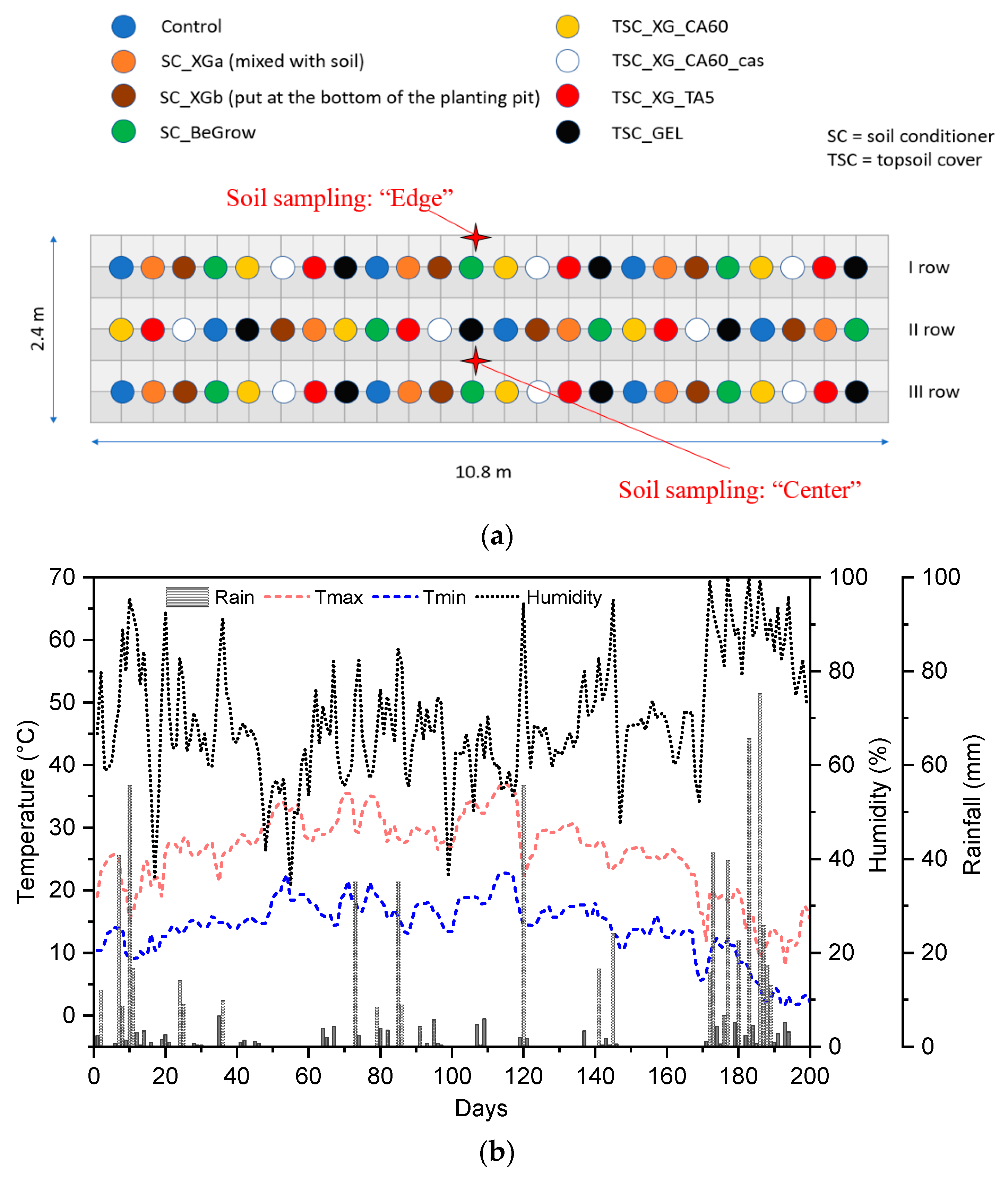
3.3. Planting Experiment
| Determination | Edge | Centre |
|---|---|---|
| Sand (2.0–0.05 mm) | 412 g/kg | 405 g/kg |
| Silt (0.05–0.002 mm) | 458 g/kg | 465 g/kg |
| Clay (<0.002 mm) | 130 g/kg | 130 g/kg |
| pH (in water ratio 1:2.5) | 8.1 | 8.4 |
| Total limestone | 349 g/kg CaCO3 | 345 g/kg CaCO3 |
| Active limestone | 15 g/kg CaCO3 | 20 g/kg CaCO3 |
| Organic substance | 33 g/kg | 12 g/kg |
| Assimilable phosphorus | 27 mg/kg P2O5 | 15 mg/kg P2O5 |
| Potassium | 166 mg/kg K2O | 114 mg/kg K2O |
| Magnesium | 317 mg/kg MgO | 284 mg/kg MgO |
3.4. Evaluation of Yield Characteristics
3.5. Physico-Chemical Analysis of Tomato Fruits
3.5.1. Evaluation of the Dry Matter
3.5.2. Evaluation of the Brix Degree
3.5.3. Evaluation of Lutein, Lycopene and β-Carotene Content
3.5.4. Evaluation of Potassium Content
3.6. Evaluation of Mulching Residues
3.7. Evaluation of Plant Root System
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TSC | Topsoil cover |
| SC | Soil conditioner |
| XG | Xanthan gum |
| CA | Citric acid |
| TA | Tannic acid |
| GEL | Gelatine |
| WF | Wood Fibres |
| NFT | Number fruit grown per plant |
References
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef]
- Oliver, M.A.; Gregory, P. Soil, food security and human health: A review. Eur. J. Soil Sci. 2015, 66, 257–276. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Sauer, T.J.; Cruse, R.M. Soil: The forgotten piece of the water, food, energy nexus. Adv. Agron. 2017, 143, 1–46. [Google Scholar]
- Siebert, S.; Ewert, F.; Rezaei, E.E.; Kage, H.; Graß, R. Impact of heat stress on crop yield—On the importance of considering canopy temperature. Environ. Res. Lett. 2014, 9, 044012. [Google Scholar] [CrossRef]
- Lobell, D.B.; Hammer, G.L.; Chenu, K.; Zheng, B.; McLean, G.; Chapman, S.C. The shifting influence of drought and heat stress for crops in northeast Australia. Glob. Change Biol. 2015, 21, 4115–4127. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Holm, P.; Liu, F. Alternate partial root-zone drying irrigation improves fruit quality in tomatoes. Hortic Sci. 2014, 41, 185–191. [Google Scholar] [CrossRef]
- Perveen, R.; Suleria, H.A.R.; Anjum, F.M.; Butt, M.S.; Pasha, I.; Ahmad, S. Tomato (Solanum lycopersicum) carotenoids and lycopenes chemistry; metabolism, absorption, nutrition, and allied health claims—A comprehensive review. Crit. Rev. Food Sci. Nutr. 2015, 55, 919–929. [Google Scholar] [CrossRef]
- Amuji, C.F.; Beaumont, L.J.; Atwell, B.J. The effect of co-occurring heat and water stress on reproductive traits and yield of tomato (Solanum lycopersicum). Hort. J. 2020, 89, 530–536. [Google Scholar] [CrossRef]
- Osei-Bonsu, I.; Osei, M.; Agyare, R.; Adjebeng-Danquah, J.; Asare Bediako, K.; Gyau, J.; Adomako, J.; Ofori, P.; Prempeh, R.; Cho, M.-C. Assessing the heat stress tolerance potential of tomato lines under poly-house and open field conditions. Cogent Food Agric. 2022, 8, 2115665. [Google Scholar] [CrossRef]
- Mukherjee, S.; Dash, P.K.; Das, D.; Das, S. Growth, Yield and Water Productivity of Tomato as Influenced by Deficit Irrigation Water Management. Environ. Process. 2023, 10, 10. [Google Scholar] [CrossRef]
- Barbagallo, R.N.; Di Silvestro, I.; Patane, C. Yield, physicochemical traits, antioxidant pattern, polyphenol oxidase activity and total visual quality of field-grown processing tomato cv. Brigade as affected by water stress in Mediterranean climate. J. Sci. Food Agric. 2013, 93, 1449–1457. [Google Scholar] [CrossRef]
- Fernandes, Â.; Chaski, C.; Pereira, C.; Kostić, M.; Rouphael, Y.; Soković, M.; Barros, L.; Petropoulos, S.A. Water Stress Alleviation Effects of Biostimulants on Greenhouse-Grown Tomato Fruit. Horticulturae 2022, 8, 645. [Google Scholar] [CrossRef]
- Kumar, P.S.; Singh, Y.; Nangare, D.D.; Bhagat, K.; Kumar, M.; Taware, P.B.; Kumari, A.; Minhas, P.S. Influence of growth stage specific water stress on the yield, physico-chemical quality and functional characteristics of tomato grown in shallow basaltic soils. Sci. Hortic. 2015, 197, 261–271. [Google Scholar] [CrossRef]
- Vurro, F.; Manfredi, R.; Bettelli, M.; Bocci, G.; Cologni, A.L.; Cornali, S.; Reggiani, R.; Marchetti, E.; Coppedè, N.; Caselli, S.; et al. In vivo sensing to monitor tomato plants in field conditions and optimize crop water management. Precis. Agric. 2023, 24, 2479–2499. [Google Scholar] [CrossRef]
- Qin, W.; Hu, C.; Oenema, O. Soil mulching significantly enhances yields and water and nitrogen use efficiencies of maize and wheat: A meta-analysis. Sci. Rep. 2015, 5, 16210. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Muñoz, K.; Frör, O.; Schaumann, G.E. Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total Environ. 2016, 550, 690–705. [Google Scholar] [CrossRef]
- Kader, M.; Senge, M.; Mojid, M.; Ito, K. Recent advances in mulching materials and methods for modifying soil environment. Soil Tillage Res. 2017, 168, 155–166. [Google Scholar] [CrossRef]
- Zhang, X.; You, S.; Tian, Y.; Li, J. Comparison of plastic film, biodegradable paper and bio-based film mulching for summer tomato production: Soil properties, plant growth, fruit yield and fruit quality. Sci. Hortic. 2019, 249, 38–48. [Google Scholar] [CrossRef]
- Cuello, J.P.; Hwang, H.Y.; Gutierrez, J.; Kim, S.Y.; Kim, P.J. Impact of plastic film mulching on increasing greenhouse gas emissions in temperate upland soil during maize cultivation. Appl. Soil Ecol. 2015, 91, 48–57. [Google Scholar] [CrossRef]
- Chen, B.; Liu, E.; Mei, X.; Yan, C.; Garré, S. Modelling soil water dynamic in rain-fed spring maize field with plastic mulching. Agric. Water Manag. 2018, 198, 19–27. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Wang, F.-X.; Shock, C.C.; Yang, K.-J.; Kang, S.-Z.; Qin, J.-T.; Li, S.-E. Influence of different plastic film mulches and wetted soil percentages on potato grown under drip irrigation. Agric. Water Manag. 2017, 180, 160–171. [Google Scholar] [CrossRef]
- Mendonça, S.R.; Ávila, M.C.R.; Vital, R.G.; Evangelista, Z.R.; de Carvalho Pontes, N.; dos Reis Nascimento, A. The effect of different mulching on tomato development and yield. Sci. Hortic. 2021, 275, 109657. [Google Scholar] [CrossRef]
- Liu, E.; He, W.; Yan, C. ‘White revolution’to ‘white pollution’—Agricultural plastic film mulch in China. Environ. Res. Lett. 2014, 9, 091001. [Google Scholar] [CrossRef]
- Briassoulis, D.; Giannoulis, A. Evaluation of the functionality of bio-based food packaging films. Polym. Test. 2018, 69, 39–51. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, X.; Pelaez, A.M.; Huerta Lwanga, E.; Beriot, N.; Gertsen, H.; Garbeva, P.; Geissen, V. Macro- and micro- plastics in soil-plant system: Effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci. Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef]
- Wang, J.; Lv, S.; Zhang, M.; Chen, G.; Zhu, T.; Zhang, S.; Teng, Y.; Christie, P.; Luo, Y. Effects of plastic film residues on occurrence of phthalates and microbial activity in soils. Chemosphere 2016, 151, 171–177. [Google Scholar] [CrossRef]
- Russo, R.; Malinconico, M.; Santagata, G. Effect of cross-linking with calcium ions on the physical properties of alginate films. Biomacromolecules 2007, 8, 3193–3197. [Google Scholar] [CrossRef]
- Menossi, M.; Cisneros, M.; Alvarez, V.A.; Casalongué, C. Current and emerging biodegradable mulch films based on polysaccharide bio-composites. A review. Agron. Sustain. Dev. 2021, 41, 53. [Google Scholar] [CrossRef]
- Kayserilioğlu, B.Ş.; Bakir, U.; Yilmaz, L.; Akkaş, N.J.B.t. Use of xylan, an agricultural by-product, in wheat gluten based biodegradable films: Mechanical, solubility and water vapor transfer rate properties. Bioresour. Technol. 2003, 87, 239–246. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, J.; Xu, J.; Gao, X.; Fu, X. Effects of crosslinking modes on the film forming properties of kelp mulching films. Algal Res. 2017, 26, 74–83. [Google Scholar] [CrossRef]
- Merino, D.; Gutiérrez, T.J.; Mansilla, A.Y.; Casalongué, C.A.; Alvarez, V.A. Critical Evaluation of starch-based antibacterial nanocomposites as agricultural mulch films: Study on their interactions with water and light. ACS Sustain. Chem. Eng. 2018, 6, 15662–15672. [Google Scholar] [CrossRef]
- Kim, M.S.; Chang, H.; Zheng, L.; Yan, Q.; Pfleger, B.F.; Klier, J.; Nelson, K.; Majumder, E.L.-W.; Huber, G.W. A review of biodegradable plastics: Chemistry, applications, properties, and future research needs. Chem. Rev. 2023, 123, 9915–9939. [Google Scholar] [CrossRef]
- Hoffmann, R.; Morais, D.D.S.; Braz, C.; Haag, K.; Wellen, R.M.; Canedo, E.; De Carvalho, L.; Koschek, K. Impact of the natural filler babassu on the processing and properties of PBAT/PHB films. Compos. Part A Appl. Sci. Manuf. 2019, 124, 105472. [Google Scholar] [CrossRef]
- Merino, D.; Alvarez, V.A. Green microcomposites from renewable resources: Effect of seaweed (Undaria pinnatifida) as filler on corn starch–chitosan film properties. J. Polym. Environ. 2020, 28, 500–516. [Google Scholar] [CrossRef]
- Sorze, A.; Valentini, F.; Burin Mucignat, M.; Pegoretti, A.; Dorigato, A. Multifunctional xanthan gum/wood fibers based hydrogels as novel topsoil covers for forestry and agricultural applications. Carbohydr. Polym. Technol. Appl. 2024, 7, 100520. [Google Scholar] [CrossRef]
- Merino, D.; Gutiérrez, T.J.; Alvarez, V.A. Potential agricultural mulch films based on native and phosphorylated corn starch with and without surface functionalization with chitosan. J. Polym. Environ. 2019, 27, 97–105. [Google Scholar] [CrossRef]
- Zou, X.; Niu, W.; Liu, J.; Li, Y.; Liang, B.; Guo, L.; Guan, Y. Effects of residual mulch film on the growth and fruit quality of tomato (Lycopersicon esculentum Mill.). Water Air Soil Pollut. 2017, 228, 71. [Google Scholar] [CrossRef]
- Coello, J.; Ameztegui, A.; Rovira, P.; Fuentes, C.; Piqué, M. Innovative soil conditioners and mulches for forest restoration in semiarid conditions in northeast Spain. Ecol. Eng. 2018, 118, 52–65. [Google Scholar] [CrossRef]
- Sojka, R.; Bjorneberg, D.; Entry, J.; Lentz, R.; Orts, W. Polyacrylamide in agriculture and environmental land management. Adv. Agron. 2007, 92, 75–162. [Google Scholar]
- Ritonga, H.; Nurfadillah, A.; Rembon, F.S.; Ramadhan, L.; Nurdin, M. Preparation of Chitosan-EDTA hydrogel as soil conditioner for soybean plant (Glycine max). Groundw. Sustain. Dev. 2019, 9, 100277, Erratum in Groundw. Sustain. Dev. 2021, 13, 100594. [Google Scholar] [CrossRef]
- Parvathy, P.C.; Jyothi, A.N. Water sorption kinetics of superabsorbent hydrogels of saponified cassava starch-graft-poly(acrylamide). Starke 2012, 64, 803–812. [Google Scholar] [CrossRef]
- Hemvichian, K.; Chanthawong, A.; Suwanmala, P. Synthesis and characterization of superabsorbent polymer prepared by radiation-induced graft copolymerization of acrylamide onto carboxymethyl cellulose for controlled release of agrochemicals. Radiat. Phys. Chem. 2014, 103, 167–171. [Google Scholar] [CrossRef]
- Bouranis, D.L.; Theodoropoulos, A.G.; Drossopoulos, J.B. Designing synthetic polymers as soil conditioners. Commun. Soil Sci. Plant Anal. 2008, 26, 1455–1480. [Google Scholar] [CrossRef]
- Chang, I.; Prasidhi, A.K.; Im, J.; Shin, H.-D.; Cho, G.-C. Soil treatment using microbial biopolymers for anti-desertification purposes. Geoderma 2015, 253–254, 39–47. [Google Scholar] [CrossRef]
- Thombare, N.; Mishra, S.; Siddiqui, M.Z.; Jha, U.; Singh, D.; Mahajan, G.R. Design and development of guar gum based novel, superabsorbent and moisture retaining hydrogels for agricultural applications. Carbohydr. Polym. 2018, 185, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Dearfield, K.L.; Abernathy, C.O.; Ottley, M.S.; Brantner, J.H.; Hayes, P.F. Acrylamide: Its metabolism, developmental and reproductive effects, genotoxicity, and carcinogenicity. Mutat. Res.-Rev. Genet. Toxicol. 1988, 195, 45–77. [Google Scholar] [CrossRef]
- Christensen, L.H.; Breiting, V.B.; Aasted, A.; Jørgensen, A.; Kebuladze, I. Long-Term Effects of Polyacrylamide Hydrogel on Human Breast Tissue. Plast. Reconstr. Surg. 2003, 111, 1883–1890. [Google Scholar] [CrossRef]
- Sheng, Q.; Zou, H.-C.; Lü, Z.-R.; Zou, F.; Park, Y.-D.; Yan, Y.-B.; Yao, S.-J. Effects of Acrylamide on the Activity and Structure of Human Brain Creatine Kinase. Int. J. Mol. Sci. 2009, 10, 4210–4222. [Google Scholar] [CrossRef]
- Becker, A.; Katzen, F.; Pühler, A.; Ielpi, L. Xanthan gum biosynthesis and application: A biochemical/genetic perspective. Appl. Microbiol. Biotechnol. 1998, 50, 145–152. [Google Scholar]
- Garcıa-Ochoa, F.; Santos, V.; Casas, J.; Gómez, E. Xanthan gum: Production, recovery, and properties. Biotechnol. Adv. 2000, 18, 549–579. [Google Scholar] [CrossRef]
- Patel, J.; Maji, B.; Moorthy, N.; Maiti, S. Xanthan gum derivatives: Review of synthesis, properties and diverse applications. RSC Adv. 2020, 10, 27103–27136. [Google Scholar] [CrossRef]
- Ramshaw, J.A.; Glattauer, V. Biophysical and Chemical Properties of Collagen: Biomedical Applications; IOP Publishing: Bristol, UK, 2019. [Google Scholar]
- Schrieber, R.; Gareis, H. Gelatine Handbook: Theory and Industrial Practice; John Wiley & Sons: New York, NY, USA, 2007. [Google Scholar]
- Sorze, A.; Valentini, F.; Dorigato, A.; Pegoretti, A. Development of a xanthan gum based superabsorbent and water retaining composites for agricultural and forestry applications. Molecules 2023, 28, 1952. [Google Scholar] [CrossRef]
- Sorze, A.; Valentini, F.; Smolar, J.; Logar, J.; Pegoretti, A.; Dorigato, A. Effect of different cellulose fillers on the properties of xanthan-based composites for soil conditioning applications. Materials 2023, 16, 7285. [Google Scholar] [CrossRef]
- Fortuna, B.; Logar, J.; Sorze, A.; Valentini, F.; Smolar, J. Influence of Xanthan Gum-Based Soil Conditioners on the Geotechnical Properties of Soils. Appl. Sci. 2024, 14, 4044. [Google Scholar] [CrossRef]
- Valentini, F.; Sorze, A.; Coello, J.; Ros, L.; Chowdhury, A.A.; Piergiacomo, F.; Casapiccola, G.; Brusetti, L.; Bösing, J.; Hirschmüller, S.; et al. Xanthan-and gelatine-based composites used as nursery groundcovers: Assessment of soil microbiology and seedling performance. Sustainability 2025, 17, 1265. [Google Scholar] [CrossRef]
- Auriemma, S.; Chowdhury, A.A.; Sorze, A.; Valentini, F.; Piergiacomo, F.; Dorigato, A.; Brusetti, L. Wood-derived topsoil cover positively influences the diversity and activity of tomato plant rhizobacteria. Resour. Environ. Sustain. 2025, 21, 100241. [Google Scholar] [CrossRef]
- Sorze, A.; Bösing, J.; Hirschmüller, S.; Dorigato, A. Investigation of Flame and Thermal Degradation Behavior of Xanthan-and Gelatin-Based Composites Used as Topsoil Covers in Forestry. Molecules 2025, 30, 3324. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Tao, C.; Deng, X.; Liu, H.; Shen, Z.; Liu, Y.; Li, R.; Shen, Q.; Geisen, S. Organic fertilization enhances the resistance and resilience of soil microbial communities under extreme drought. J. Adv. Res. 2023, 47, 1–12. [Google Scholar] [CrossRef]
- Sullivan, P. Drought Resistant Soils; ATTRA: Fayetteville, NC, USA, 2000. [Google Scholar]
- Adamczewska-Sowińska, K.; Bykowy, J.; Jaworska, J. Effect of Biodegradable Mulch and Different Synthetic Mulches on Growth and Yield of Field-Grown Small-Fruited Tomato (Lycopersicon esculentum Mill.). Agriculture 2025, 15, 212. [Google Scholar] [CrossRef]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.S.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef]
- Helmisaari, H.-S.; Lehto, T.; Makkonen, K. Fine roots and soil properties. In Forest Condition in a Changing Environment: The Finnish Case; Springer: Dordrecht, The Netherlands, 2000; pp. 203–217. [Google Scholar]
- Picchio, M.L.; Linck, Y.G.; Monti, G.A.; Gugliotta, L.M.; Minari, R.J.; Alvarez Igarzabal, C.I. Casein films crosslinked by tannic acid for food packaging applications. Food Hydrocoll. 2018, 84, 424–434. [Google Scholar] [CrossRef]
- Bertoldi, D.; Santato, A.; Paolini, M.; Barbero, A.; Camin, F.; Nicolini, G.; Larcher, R. Botanical traceability of commercial tannins using the mineral profile and stable isotopes. J. Mass Spectrom. 2014, 49, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Larcher, R.; Nicolini, G. Survey of 22 mineral elements in wines from Trentino (Italy) using ICP-OES. Ital. J. Food Sci. 2001, 13, 233–242. [Google Scholar]
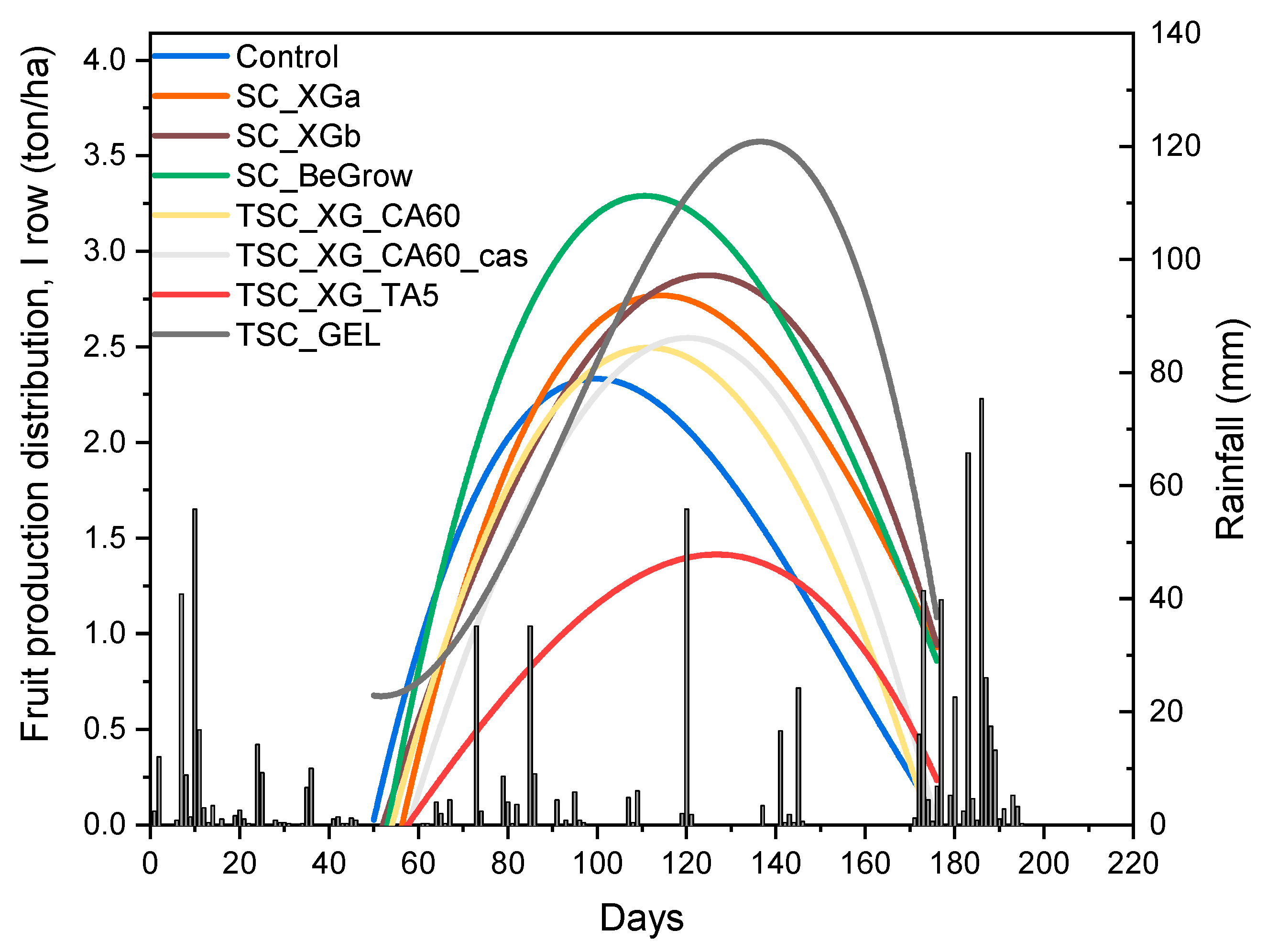


| Sample | Total Yield (Ton/ha) | NFT (Number/Plant) | Relative Yield I Row (%) | Relative Yield II Row (%) | Relative Yield III Row (%) |
|---|---|---|---|---|---|
| Control | 19.1 ± 9.0 | 94 ± 32 | - | - | - |
| SC_XGa | 16.7 ± 13.9 | 87 ± 67 | +17.8 | −61.8 | −20.1 |
| SC_XGb | 20.0 ± 15.1 | 98 ± 63 | +33.9 | −47.2 | +6.5 |
| SC_BeGrow | 22.8 ± 18.2 | 108 ± 77 | +56.3 | −23.2 | −22.6 |
| TSC_XG_CA60 | 15.0 ± 12.7 | 79 ± 65 | +6.3 | −60.2 | −42.1 |
| TSC_XG_CA60_cas | 15.7 ± 11.9 | 72 ± 47 | +5.2 | −60.5 | −12.7 |
| TSC_XG_TA5 | 10.6 ± 5.9 | 57 ± 22 | −39.8 | −49.3 | −51.8 |
| TSC_GEL | 28.1 ± 14.1 | 130 ± 54 | +55.4 | −4.9 | +121.8 |
| Sample | Dry Matter Content (%) | Brix Degree (°) | Lycopene Content (mg/kg) | β-Carotene Content (mg/kg) | Lutein Content (mg/kg) | Potassium Content (mg/kg) |
|---|---|---|---|---|---|---|
| Control | 8.3 ± 0.5 | 7.32 ± 0.31 | 193.7 ± 72.3 | 97.3 ± 37.4 | 8.3 ± 2.1 | 3092.7 ± 45.4 |
| SC_XGa | 7.7 ± 1.8 | 6.93 ± 0.20 | 165.0 ± 22.5 | 138.7 ± 11.8 | 11.5 ± 1.8 | 3158.3 ± 93.7 |
| SC_XGb | 8.1 ± 0.8 | 6.93 ± 0.06 | 152.7 ± 16.9 | 137.0 ± 40.6 | 10.8 ± 2.5 | 3069.7 ± 296.9 |
| SC_BeGrow | 8.1 ± 1.0 | 6.98 ± 0.60 | 143.0 ± 18.9 | 134.0 ± 26.9 | 13.4 ± 1.1 | 2884.0 ± 204.7 |
| TSC_XG_CA60 | 7.9 ± 1.4 | 6.53 ± 0.89 | 145.0 ± 21.3 | 121.0 ± 13.1 | 12.3 ± 1.1 | 2828.3 ± 433.3 |
| TSC_XG_CA60_cas | 8.1 ± 0.9 | 6.95 ± 0.28 | 179.3 ± 33.1 | 122.3 ± 42.8 | 12.3 ± 2.1 | 2964.0 ± 224.2 |
| TSC_XG_TA5 | 8.3 ± 0.1 | 6.97 ± 0.05 | 207.7 ± 82.7 | 105.0 ± 9.9 | 10.6 ± 0.8 | 3028.7 ± 168.3 |
| TSC_GEL | 8.2 ± 2.6 | 7.12 ± 0.16 | 178.0 ± 63.6 | 111.7 ± 28.8 | 8.0 ± 0.7 | 3244.3 ± 173.5 |
| Sample | Root Length (cm) | Stem Diameter (cm) | Root: Shoot Ratio | Root Water Content (%) |
|---|---|---|---|---|
| Control | 32.6 ± 4.2 | 1.0 ± 0.3 | 0.27 ± 0.03 | 80.9 ± 0.8 |
| SC_XGa | 34.8 ± 5.9 | 1.0 ± 0.2 | 0.31 ± 0.06 | 77.8 ± 2.4 |
| SC_XGb | 35.2 ± 3.7 | 1.0 ± 0.3 | 0.41 ± 0.11 | 79.7 ± 1.8 |
| SC_BeGrow | 34.1 ± 5.6 | 1.0 ± 0.1 | 0.36 ± 0.17 | 77.6 ± 2.0 |
| TSC_XG_CA60 | 35.5 ± 6.7 | 0.9 ± 0.2 | 0.41 ± 0.16 | 80.5 ± 1.2 |
| TSC_XG_CA60_cas | 32.9 ± 3.9 | 0.8 ± 0.2 | 0.36 ± 0.10 | 78.8 ± 1.9 |
| TSC_XG_TA5 | 34.7 ± 2.0 | 0.8 ± 0.1 | 0.35 ± 0.08 | 79.1 ± 2.1 |
| TSC_GEL | 33.9 ± 1.5 | 1.0 ± 0.1 | 0.36 ± 0.10 | 76.8 ± 1.8 |
| Sample | Mresidual (%) |
|---|---|
| TSC_XG_CA60 | 36.4 ± 6.7 a |
| TSC_XG_CA60_cas | 29.1 ± 12.1 a |
| TSC_XG_TA5 | 57.6 ± 8.9 b |
| TSC_GEL | 56.3 ± 8.1 b |
| Code | Composition | Characteristics |
|---|---|---|
| SC_XG | Soil conditioner based on xanthan gum and Arbocel® cellulose fibre | Powder of 1–2 mm granulometry |
| SC_BeGrow | Commercial soil conditioner based on potassium polyacrylate | Powder of 100 µm granulometry |
| TSC_XG_CA60 | Mulching film based on xanthan gum and STEICO wood fibres cross-linked with citric acid | Disc of 160 mm diameter and 3 mm thickness (areal density 900 g/m2) |
| TSC_XG_CA60_cas | Mulching film based on xanthan gum and STEICO wood fibres cross-linked with citric acid and coated with casein | Disc of 160 mm diameter and 3 mm thickness (areal density 900 g/m2) |
| TSC_XG_TA5 | Mulching film based on xanthan gum and STEICO wood fibres cross-linked with tannic acid | Disc of 160 mm diameter and 3 mm thickness (areal density 900 g/m2) |
| TSC_GEL | Mulching film based on gelatine and STEICO wood fibres cross-linked with tannic acid | Disc of 160 mm diameter and 8 mm thickness (areal density 2400 g/m2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorze, A.; Valentini, F.; Nardin, T.; Larcher, R.; Bösing, J.; Hirschmüller, S.; Dorigato, A.; Pegoretti, A. Bio-Based Mulching Films and Soil Conditioners for Non-Irrigated Tomato Cultivation: Toward Plastic-Free and Water-Efficient Crop Production. Int. J. Mol. Sci. 2025, 26, 9894. https://doi.org/10.3390/ijms26209894
Sorze A, Valentini F, Nardin T, Larcher R, Bösing J, Hirschmüller S, Dorigato A, Pegoretti A. Bio-Based Mulching Films and Soil Conditioners for Non-Irrigated Tomato Cultivation: Toward Plastic-Free and Water-Efficient Crop Production. International Journal of Molecular Sciences. 2025; 26(20):9894. https://doi.org/10.3390/ijms26209894
Chicago/Turabian StyleSorze, Alessandro, Francesco Valentini, Tiziana Nardin, Roberto Larcher, Janine Bösing, Sebastian Hirschmüller, Andrea Dorigato, and Alessandro Pegoretti. 2025. "Bio-Based Mulching Films and Soil Conditioners for Non-Irrigated Tomato Cultivation: Toward Plastic-Free and Water-Efficient Crop Production" International Journal of Molecular Sciences 26, no. 20: 9894. https://doi.org/10.3390/ijms26209894
APA StyleSorze, A., Valentini, F., Nardin, T., Larcher, R., Bösing, J., Hirschmüller, S., Dorigato, A., & Pegoretti, A. (2025). Bio-Based Mulching Films and Soil Conditioners for Non-Irrigated Tomato Cultivation: Toward Plastic-Free and Water-Efficient Crop Production. International Journal of Molecular Sciences, 26(20), 9894. https://doi.org/10.3390/ijms26209894








