Cold Exposure Induces Swine Brown Adipocytes to Display an Island-like Distribution with Atypical Characteristics
Abstract
1. Introduction
2. Results
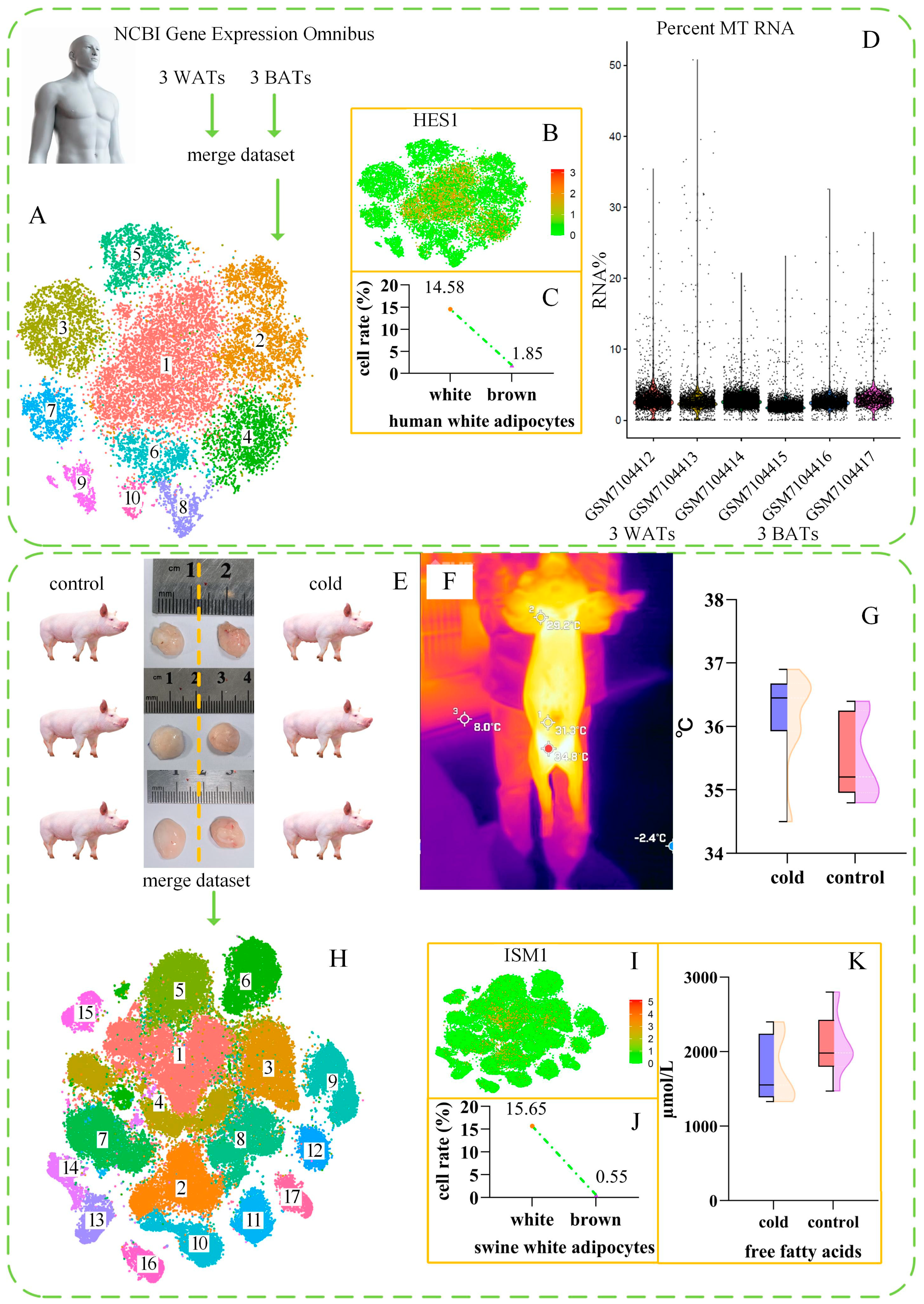
2.1. Human Brown Adipocyte scRNA-Seq Data Analysis
2.2. Swine Brown Adipocyte scRNA-Seq Data Analysis
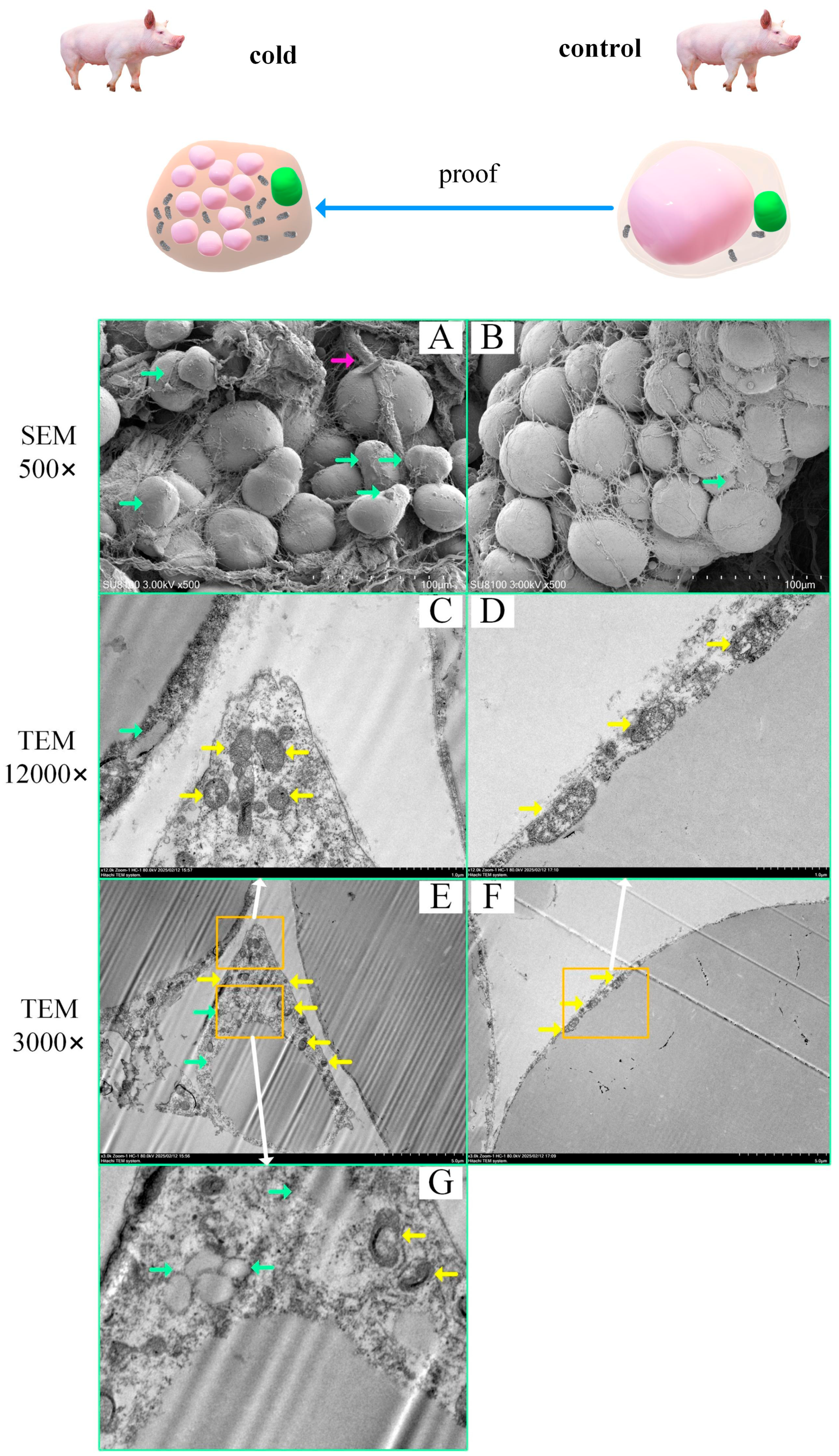
2.3. Histological Evidence of Swine Brown Adipocytes
2.4. Subcellular Structural Evidence of Swine Brown Adipocytes
2.5. Changes in the Serum Components of Pigs After Cold Exposure
2.6. Whole-Transcriptome Sequencing of Groin Adipose Tissue
2.7. ceRNA Network
2.8. DNA Methylation Epigenetic Memory
2.9. Chromatin Accessibility of Epigenetic Memory
2.10. UCP2 and UCP3 Molecular Docking
3. Discussion
3.1. The Possibility of the Presence of Brown Adipocytes in White Adipose Tissue
3.2. Histological Characteristics of Brown Adipocytes
3.3. Gene Expression Characteristics of Brown Adipocytes
3.4. Changes in DNA Methylation After Cold Exposure and Subsequent Return to Warm Temperatures
3.5. The Distinctions and Connections Between Beige and Brown Adipocytes
3.6. Limitations
4. Materials and Methods
4.1. Ethics Statement
4.2. Human Database Search Strategy and scRNA-Seq Data Analysis
4.3. Animals
4.4. scRNA-Seq Data Processing of Swine Inguinal Fat
4.5. Adipocyte Size and Count Analysis
4.6. Transmission and Scanning Electron Microscopy
4.7. qRT–PCR Analysis of UCP2/3 Expression in Adipose Tissue
4.8. Enzyme-Linked Immunosorbent Assay (ELISA)
4.9. Whole-Transcriptome Sequencing to Construct a ceRNA Network
4.10. DNA Methylation
4.11. ATAC–Seq
4.12. Molecular Docking of UCP2/3
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AEBP1 | Adipocyte enhancer-binding protein 1 |
| ap2 | Adipose P2 |
| ATAC–Seq | Assay for transposase-accessible chromatin high-throughput sequencing |
| circrna | Circular RNAs |
| cerna network | Competitive endogenous RNA network |
| dmrs | Differentially methylated regions |
| ffas | Free fatty acids |
| GO | Gene Ontology |
| IH | H+ leakage |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| lncrna | Long non-coding RNAs |
| qrt–PCR | Real-time quantitative PCR |
| SOD | Superoxide dismutase |
| T4 | Thyroxine |
| TSS | Transcription start site |
| T3 | Triiodothyronine |
| UCP1 | Uncoupling protein 1 |
| UCP2/3 | Uncoupling protein 2/3 |
| WGBS | Whole-genome bisulfite sequencing |
References
- Bai, X.; Zhu, Q.; Combs, M.; Wabitsch, M.; Mack, C.P.; Taylor, J.M. GRAF1 deficiency leads to defective brown adipose tissue differentiation and thermogenic response. Sci Rep. 2024, 14, 28692. [Google Scholar] [CrossRef]
- Zhu, Y.; Yao, L.; Gallo-Ferraz, A.L.; Bombassaro, B.; Simoes, M.R.; Abe, I.; Chen, J.; Sarker, G.; Ciccarelli, A.; Zhou, L.; et al. Sympathetic neuropeptide Y protects from obesity by sustaining thermogenic fat. Nature 2024, 634, 243–250. [Google Scholar] [CrossRef]
- Palani, N.P.; Horvath, C.; Timshel, P.N.; Folkertsma, P.; Gronning, A.G.B.; Henriksen, T.I.; Peijs, L.; Jensen, V.H.; Sun, W.; Jespersen, N.Z.; et al. Adipogenic and SWAT cells separate from a common progenitor in human brown and white adipose depots. Nat. Metab. 2023, 5, 996–1013. [Google Scholar] [CrossRef] [PubMed]
- Hinte, L.C.; Castellano-Castillo, D.; Ghosh, A.; Melrose, K.; Gasser, E.; Noe, F.; Massier, L.; Dong, H.; Sun, W.; Hoffmann, A.; et al. Adipose tissue retains an epigenetic memory of obesity after weight loss. Nature 2024, 636, 457–465, Erratum in Nature 2025, 643, E24. [Google Scholar] [CrossRef] [PubMed]
- Loft, A.; Emont, M.P.; Weinstock, A.; Divoux, A.; Ghosh, A.; Wagner, A.; Hertzel, A.V.; Maniyadath, B.; Deplancke, B.; Liu, B.; et al. Towards a consensus atlas of human and mouse adipose tissue at single-cell resolution. Nat. Metab. 2025, 7, 875–894. [Google Scholar] [CrossRef]
- Seki, T.; Yang, Y.; Sun, X.; Lim, S.; Xie, S.; Guo, Z.; Xiong, W.; Kuroda, M.; Sakaue, H.; Hosaka, K.; et al. Brown-fat-mediated tumour suppression by cold-altered global metabolism. Nature 2022, 608, 421–428. [Google Scholar] [CrossRef]
- Sun, W.; Dong, H.; Balaz, M.; Slyper, M.; Drokhlyansky, E.; Colleluori, G.; Giordano, A.; Kovanicova, Z.; Stefanicka, P.; Balazova, L.; et al. snRNA-seq reveals a subpopulation of adipocytes that regulates thermogenesis. Nature 2020, 587, 98–102. [Google Scholar] [CrossRef]
- Grabek, K.R.; Sprenger, R.J. The evolution of thermogenesis in mammals. Science 2024, 384, 1065–1066. [Google Scholar] [CrossRef]
- Keipert, S.; Gaudry, M.J.; Kutschke, M.; Keuper, M.; Dela Rosa, M.A.S.; Cheng, Y.; Monroy Kuhn, J.M.; Laterveer, R.; Cotrim, C.A.; Giere, P.; et al. Two-stage evolution of mammalian adipose tissue thermogenesis. Science 2024, 384, 1111–1117. [Google Scholar] [CrossRef]
- Wolfrum, C.; Gerhart-Hines, Z. Fueling the fire of adipose thermogenesis. Science 2022, 375, 1229–1231. [Google Scholar] [CrossRef] [PubMed]
- De Bock, K.; Wolfrum, C. A ‘replace me’ signal from dying brown fat fires up weight loss. Nature 2022, 609, 252–253. [Google Scholar] [CrossRef]
- Suchacki, K.J.; Stimson, R.H. Nutritional Regulation of Human Brown Adipose Tissue. Nutrients 2021, 13, 1748. [Google Scholar] [CrossRef]
- Dong, H.; Sun, W.; Shen, Y.; Balaz, M.; Balazova, L.; Ding, L.; Loffler, M.; Hamilton, B.; Kloting, N.; Bluher, M.; et al. Identification of a regulatory pathway inhibiting adipogenesis via RSPO2. Nat. Metab. 2022, 4, 90–105. [Google Scholar] [CrossRef]
- Attane, C.; Muller, C. From Fat Providers to Cancer Therapy: Adipocytes as Unexpected Allies. Cancer Res. 2025, 85, 1750–1752. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, A.E.; Dinas, P.C.; Krase, A.; Nintou, E.; Georgakopoulos, A.; Metaxas, M.; Ryan, E.J.; Vliora, M.; Georgoulias, P.; Chatziioannou, S.; et al. Dietary Intake Is Similar Among Adult Men with Different Levels of Cold-Induced Brown Adipose Tissue Activation. Nutrients 2024, 16, 3697. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhong, Y.; Huang, J.; Miao, Y.; Du, M.; Huang, K. TRIM56 Promotes White Adipose Tissue Browning to Attenuate Obesity by Degrading TLE3. Adv. Sci. 2025, 12, e2414073. [Google Scholar] [CrossRef]
- Yildiz, R.; Ganbold, K.; Sparman, N.Z.R.; Rajbhandari, P. Immune Regulatory Crosstalk in Adipose Tissue Thermogenesis. Compr. Physiol. 2025, 15, e70001. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Lu, Y.; Wu, S.; Guo, W.; Ni, J.; Song, J.; Liu, Z.; Chang, X.; Wang, K.; Sun, P.; et al. Blocking Adipocyte YY1 Decouples Thermogenesis From Beneficial Metabolism by Promoting Spermidine Production. Diabetes 2025, 74, 295–307. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, X.; Hong, L.; Zhao, J.; Qian, W.; Pham, L.K.; Willard, B.; Li, X.; Bulek, K.; et al. Adipocyte-specific Steap4 deficiency reduced thermogenesis and energy expenditure in mice. iScience 2025, 28, 111903. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, Y.; Liu, T.; Chen, C.; Chui, L.; Cui, A.; Zhang, X.; Wang, X.; Wang, Y.; Yang, C.; et al. Single-nucleus RNA sequencing defines adipose tissue subpopulations that contribute to Tibetan pig cold adaptation. BMC Biol. 2025, 23, 107. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, Y.; Chen, C.; Zhang, L.; Wang, J.; Yang, C.; Wu, T.; Yang, S.; Tao, C.; Wang, Y. Bone Morphogenetic Protein 2 Enhances Porcine Beige Adipogenesis via AKT/mTOR and MAPK Signaling Pathways. Int. J. Mol. Sci. 2024, 25, 3915. [Google Scholar] [CrossRef]
- Kim, S.; Yazawa, T.; Koide, A.; Yoneda, E.; Aoki, R.; Okazaki, T.; Tomita, K.; Watanabe, H.; Muroi, Y.; Testuka, M.; et al. Potential Role of Pig UCP3 in Modulating Adipocyte Browning via the Beta-Adrenergic Receptor Signaling Pathway. Biology 2024, 13, 284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, S.; Cao, C.; Chen, C.; Liu, J.; Wang, Y.; Liu, J.; Zhao, J.; Tao, C.; Wang, Y. Functional and Genetic Characterization of Porcine Beige Adipocytes. Cells 2022, 11, 751. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Q.; Yang, X.; Tang, Q.; Han, Y.; Meng, J.; Zhang, J.; Lu, X.; Wang, D.; Liu, J.; et al. Mitochondrial GCN5L1 coordinates with YME1L and MICOS to remodel mitochondrial cristae in white adipocytes and modulate obesity. Cell Rep. 2025, 44, 115682. [Google Scholar] [CrossRef]
- Gaudry, M.J.; Jastroch, M. Hotly awaited structures obtained for the human protein UCP1. Nature 2023, 620, 42–43. [Google Scholar] [CrossRef]
- Yang, S.; Ma, H.; Wang, L.; Wang, F.; Xia, J.; Liu, D.; Mu, L.; Yang, X.; Liu, D. The Role of beta3-Adrenergic Receptors in Cold-Induced Beige Adipocyte Production in Pigs. Cells 2024, 13, 709. [Google Scholar] [CrossRef]
- Guo, Z.; Lv, L.; Liu, D.; Ma, H.; Wang, L.; Fu, B.; Wang, F. Network Meta-Analysis: Effect of Cold Stress on the Gene Expression of Swine Adipocytes ATGL, CIDEA, UCP2, and UCP3. Curr. Issues Mol. Biol. 2024, 46, 3866–3876. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Z.; Wang, L.; Ling, D.; Nong, Q.; Xie, J.; Zhu, X.; Shan, T. Cold Exposure Induces Depot-Specific Alterations in Fatty Acid Composition and Transcriptional Profile in Adipose Tissues of Pigs. Front. Endocrinol. 2022, 13, 827523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, L.; Zhu, R.; Zhang, S.; Liu, S.; Wang, Y.; Wu, Y.; Xing, S.; Liao, X.; Mi, J. Porcine gut microbiota in mediating host metabolic adaptation to cold stress. NPJ Biofilms Microbiomes 2022, 8, 18. [Google Scholar] [CrossRef]
- Lin, J.; Cao, C.; Tao, C.; Ye, R.; Dong, M.; Zheng, Q.; Wang, C.; Jiang, X.; Qin, G.; Yan, C.; et al. Cold adaptation in pigs depends on UCP3 in beige adipocytes. J. Mol. Cell Biol. 2017, 9, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Oguri, Y.; Shinoda, K.; Kim, H.; Alba, D.L.; Bolus, W.R.; Wang, Q.; Brown, Z.; Pradhan, R.N.; Tajima, K.; Yoneshiro, T.; et al. CD81 Controls Beige Fat Progenitor Cell Growth and Energy Balance via FAK Signaling. Cell 2020, 182, 563–577.e520. [Google Scholar] [CrossRef]
- Han, J.; Wang, Y.; Qiu, Y.; Sun, D.; Liu, Y.; Li, Z.; Zhou, B.; Zhang, H.; Xiao, Y.; Wu, G.; et al. Single-cell sequencing unveils key contributions of immune cell populations in cancer-associated adipose wasting. Cell Discov. 2022, 8, 122. [Google Scholar] [CrossRef]
- Kyung, D.S.; Lee, E.; Chae, S.; Son, Y.; Moon, Y.J.; Hwang, D.; Kim, J.K.; Lee, Y.H.; Seong, J.K. Single-cell transcriptomic analysis reveals dynamic activation of cellular signaling pathways regulating beige adipogenesis. Exp. Mol. Med. 2024, 56, 2309–2322. [Google Scholar] [CrossRef]
- Abdul Majeed, S.; Dunzendorfer, H.; Weiner, J.; Heiker, J.T.; Kiess, W.; Korner, A.; Landgraf, K. COBL, MKX and MYOC Are Potential Regulators of Brown Adipose Tissue Development Associated with Obesity-Related Metabolic Dysfunction in Children. Int. J. Mol. Sci. 2023, 24, 3085. [Google Scholar] [CrossRef]
- Wang, T.; Sharma, A.K.; Wu, C.; Maushart, C.I.; Ghosh, A.; Yang, W.; Stefanicka, P.; Kovanicova, Z.; Ukropec, J.; Zhang, J.; et al. Single-nucleus transcriptomics identifies separate classes of UCP1 and futile cycle adipocytes. Cell Metab. 2024, 36, 2130–2145.e2137. [Google Scholar] [CrossRef]
- Gao, Y.; Qimuge, N.R.; Qin, J.; Cai, R.; Li, X.; Chu, G.Y.; Pang, W.J.; Yang, G.S. Acute and chronic cold exposure differentially affects the browning of porcine white adipose tissue. Animal 2018, 12, 1435–1441. [Google Scholar] [CrossRef]
- Palomaki, V.A.; Koivukangas, V.; Merilainen, S.; Lehenkari, P.; Karttunen, T.J. A Straightforward Method for Adipocyte Size and Count Analysis Using Open-source Software QuPath. Adipocyte 2022, 11, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Hall, M.L.; Lee, E.; Kim, S.C.; Ramesh, N.; Lee, S.H.; Jang, J.Y.; Bold, R.J.; Ku, J.L.; Hwang, C.I. Whole-genome bisulfite sequencing identifies stage- and subtype-specific DNA methylation signatures in pancreatic cancer. iScience 2024, 27, 109414. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Zhou, B.; Yang, J.; Lu, Y.; Mao, F.; Song, Y. Whole-genome DNA methylation and gene expression profiling in the livers of mice with nonalcoholic steatohepatitis. Life Sci. 2023, 329, 121951. [Google Scholar] [CrossRef] [PubMed]
- Lubojemska, A.; Stefana, M.I.; Sorge, S.; Bailey, A.P.; Lampe, L.; Yoshimura, A.; Burrell, A.; Collinson, L.; Gould, A.P. Adipose triglyceride lipase protects renal cell endocytosis in a Drosophila dietary model of chronic kidney disease. PLoS Biol. 2021, 19, e3001230. [Google Scholar] [CrossRef]
- Yan, S.; Tu, Z.; Liu, Z.; Fan, N.; Yang, H.; Yang, S.; Yang, W.; Zhao, Y.; Ouyang, Z.; Lai, C.; et al. A Huntingtin Knockin Pig Model Recapitulates Features of Selective Neurodegeneration in Huntington’s Disease. Cell 2018, 173, 989–1002.e1013. [Google Scholar] [CrossRef]
- Moretti, A.; Fonteyne, L.; Giesert, F.; Hoppmann, P.; Meier, A.B.; Bozoglu, T.; Baehr, A.; Schneider, C.M.; Sinnecker, D.; Klett, K.; et al. Somatic gene editing ameliorates skeletal and cardiac muscle failure in pig and human models of Duchenne muscular dystrophy. Nat. Med. 2020, 26, 207–214. [Google Scholar] [CrossRef]
- Lin, Y.; Li, C.; Chen, Y.; Gao, J.; Li, J.; Huang, C.; Liu, Z.; Wang, W.; Zheng, X.; Song, X.; et al. RNA-Targeting CRISPR/CasRx system relieves disease symptoms in Huntington’s disease models. Mol. Neurodegener. 2025, 20, 4. [Google Scholar] [CrossRef]
- Graham, F. Daily briefing: Genetically modified pig-organ transplant trial gets green light. Nature 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Mallapaty, S.; Kozlov, M. The science behind the first pig-organ transplant trial in humans. Nature 2025, 638, 303–304. [Google Scholar] [CrossRef] [PubMed]
- Sykes, M. Developing pig-to-human organ transplants. Science 2022, 378, 135–136. [Google Scholar] [CrossRef]
- McMillan, D.B.; Harris, R.J. Chapter D—Connective Tissues. In An Atlas of Comparative Vertebrate Histology; McMillan, D.B., Harris, R.J., Eds.; Academic Press: San Diego, CA, USA, 2018; pp. 75–117. [Google Scholar]
- Treuting, P.M.; Dintzis, S.M.; Montine, K.S. Comparative Anatomy and Histology: A Mouse, Rat, and Human Atlas; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Benammar, A.; Derisoud, E.; Vialard, F.; Palmer, E.; Ayoubi, J.M.; Poulain, M.; Chavatte-Palmer, P. The Mare: A Pertinent Model for Human Assisted Reproductive Technologies? Animals 2021, 11, 2304. [Google Scholar] [CrossRef] [PubMed]
- Imakawa, K.; Kusama, K.; Kaneko-Ishino, T.; Nakagawa, S.; Kitao, K.; Miyazawa, T.; Ishino, F. Endogenous Retroviruses and Placental Evolution, Development, and Diversity. Cells 2022, 11, 2304. [Google Scholar] [CrossRef]
- Mesa, A.M.; Medrano, T.I.; Sirohi, V.K.; Walker, W.H.; Johnson, R.D.; Tevosian, S.G.; Adkin, A.M.; Cooke, P.S. Identification and characterization of novel abdominal and pelvic brown adipose depots in mice. Adipocyte 2022, 11, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Hao, G.; Shao, M.; Nham, K.; An, Y.; Wang, Q.; Zhu, Y.; Kusminski, C.M.; Hassan, G.; Gupta, R.K.; et al. An Adipose Tissue Atlas: An Image-Guided Identification of Human-like BAT and Beige Depots in Rodents. Cell Metab. 2018, 27, 252–262.e253. [Google Scholar] [CrossRef]
- Scheele, C.; Wolfrum, C. Brown Adipose Crosstalk in Tissue Plasticity and Human Metabolism. Endocr. Rev. 2020, 41, 53–65. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, H.; Wang, Q.; Han, X.; Zeng, W. Three-dimensional volume fluorescence-imaging of vascular plasticity in adipose tissues. Mol. Metab. 2018, 14, 71–81. [Google Scholar] [CrossRef]
- Mo, Y.Y.; Han, Y.X.; Xu, S.N.; Jiang, H.L.; Wu, H.X.; Cai, J.M.; Li, L.; Bu, Y.H.; Xiao, F.; Liang, H.D.; et al. Adipose Tissue Plasticity: A Comprehensive Definition and Multidimensional Insight. Biomolecules 2024, 14, 1223. [Google Scholar] [CrossRef]
- Yudasaka, M.; Okamatsu-Ogura, Y.; Tanaka, T.; Saeki, K.; Kataura, H. Cold-induced Conversion of Connective Tissue Skeleton in Brown Adipose Tissues. Acta Histochem. Cytochem. 2021, 54, 131–141. [Google Scholar] [CrossRef]
- Wang, W.; Seale, P. Control of brown and beige fat development. Nat. Rev. Mol. Cell Biol. 2016, 17, 691–702. [Google Scholar] [CrossRef]
- Wang, B.; Fu, X.; Liang, X.; Deavila, J.M.; Wang, Z.; Zhao, L.; Tian, Q.; Zhao, J.; Gomez, N.A.; Trombetta, S.C.; et al. Retinoic acid induces white adipose tissue browning by increasing adipose vascularity and inducing beige adipogenesis of PDGFRalpha(+) adipose progenitors. Cell Discov. 2017, 3, 17036. [Google Scholar] [CrossRef]
- Van Schaik, L.; Kettle, C.; Green, R.; Wundersitz, D.; Gordon, B.; Irving, H.R.; Rathner, J.A. Both caffeine and Capsicum annuum fruit powder lower blood glucose levels and increase brown adipose tissue temperature in healthy adult males. Front. Physiol. 2022, 13, 870154. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ustione, A.; Wilkerson, E.M.; Balakrishnan, R.; Thurmond, D.C.; Goldfarb, D.; Piston, D.W. Regulation of Type 1 Diabetes via Brown Adipocyte-Secreted Proteins and the Novel Glucagon Regulator Nidogen-2. Diabetes 2025, 74, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Narayanan, N.; Cano-Vega, M.A.; Jia, Z.; Ajuwon, K.M.; Kuang, S.; Deng, M. Nanoparticle-Mediated Inhibition of Notch Signaling Promotes Mitochondrial Biogenesis and Reduces Subcutaneous Adipose Tissue Expansion in Pigs. iScience 2020, 23, 101167. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Shamsi, F.; Altemose, N.; Dorlhiac, G.F.; Cypess, A.M.; White, A.P.; Yosef, N.; Patti, M.E.; Tseng, Y.H.; Streets, A. Characterization of transcript enrichment and detection bias in single-nucleus RNA-seq for mapping of distinct human adipocyte lineages. Genome Res. 2022, 32, 242–257. [Google Scholar] [CrossRef]
- Sorek, G.; Haim, Y.; Chalifa-Caspi, V.; Lazarescu, O.; Ziv-Agam, M.; Hagemann, T.; Nono Nankam, P.A.; Bluher, M.; Liberty, I.F.; Dukhno, O.; et al. sNucConv: A bulk RNA-seq deconvolution method trained on single-nucleus RNA-seq data to estimate cell-type composition of human adipose tissues. iScience 2024, 27, 110368. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, L.; Li, M.; Liu, Y.; Shi, Y.; Zhang, J. Plasticity of Adipose Tissues: Interconversion among White, Brown, and Beige Fat and Its Role in Energy Homeostasis. Biomolecules 2024, 14, 483. [Google Scholar] [CrossRef]
- Abbasi, M.; Fan, Z.; Dawson, J.A.; Wang, S. Transdermal Delivery of Metformin Using Dissolving Microneedles and Iontophoresis Patches for Browning Subcutaneous Adipose Tissue. Pharmaceutics 2022, 14, 879. [Google Scholar] [CrossRef]
- Taylor, B.C.; Steinthal, L.H.; Dias, M.; Yalamanchili, H.K.; Ochsner, S.A.; Zapata, G.E.; Mehta, N.R.; McKenna, N.J.; Young, N.L.; Nuotio-Antar, A.M. Histone proteoform analysis reveals epigenetic changes in adult mouse brown adipose tissue in response to cold stress. Epigenetics Chromatin 2024, 17, 12. [Google Scholar] [CrossRef]
- So, J.; Taleb, S.; Wann, J.; Strobel, O.; Kim, K.; Roh, H.C. Chronic cAMP activation induces adipocyte browning through discordant biphasic remodeling of transcriptome and chromatin accessibility. Mol. Metab. 2022, 66, 101619. [Google Scholar] [CrossRef]
- Du, K.; Shi, Y.; Bai, X.; Chen, L.; Sun, W.; Chen, S.; Wang, J.; Jia, X.; Lai, S. Integrated Analysis of Transcriptome, microRNAs, and Chromatin Accessibility Revealed Potential Early B-Cell Factor1-Regulated Transcriptional Networks during the Early Development of Fetal Brown Adipose Tissues in Rabbits. Cells 2022, 11, 2675. [Google Scholar] [CrossRef]
- Inagaki, T. Histone demethylases regulate adipocyte thermogenesis. Diabetol. Int. 2018, 9, 215–223. [Google Scholar] [CrossRef]
- Shuai, L.; Zhang, L.N.; Li, B.H.; Tang, C.L.; Wu, L.Y.; Li, J.; Li, J.Y. SIRT5 Regulates Brown Adipocyte Differentiation and Browning of Subcutaneous White Adipose Tissue. Diabetes 2019, 68, 1449–1461. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cao, Q.; Cui, X.; Jing, J.; Li, F.; Shi, H.; Xue, B.; Shi, H. Dnmt3b Deficiency in Myf5(+)-Brown Fat Precursor Cells Promotes Obesity in Female Mice. Biomolecules 2021, 11, 1087. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.; Lee, C.H.; Jeong, H.; Kim, H.; Kwon, H.M.; Park, S.; Myung, K.; An, J.; Ko, M. Loss of adipose TET proteins enhances beta-adrenergic responses and protects against obesity by epigenetic regulation of beta3-AR expression. Proc. Natl. Acad. Sci. USA 2022, 119, e2205626119. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Holloway, N.; Tran, A.; Lin, F.; Dinh, J.; Yang, C.; Wang, Y.; Yi, D.; Sul, H.S. A distal enhancer with ETV4 binding is critical for UCP1 expression and thermogenesis in brown fat. Genes. Dev. 2025, 39, 808–825. [Google Scholar] [CrossRef]
- Yang, W.; Cui, H.; Chai, Z.; Zou, P.; Shi, F.; Yang, B.; Zhang, G.; Yang, H.; Chen, Q.; Liu, J.; et al. Benzo [a] pyrene inhibits testosterone biosynthesis via NDUFA10-mediated mitochondrial compromise in mouse Leydig cells: Integrating experimental and in silico toxicological approaches. Ecotoxicol. Environ. Saf. 2022, 244, 114075. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Ren, Z.; Han, Q.; Chen, X.; Han, J.; Qiu, G.; Sun, C. NDUFA9 and its crotonylation modification promote browning of white adipocytes by activating mitochondrial function in mice. Int. J. Biochem. Cell Biol. 2024, 171, 106583. [Google Scholar] [CrossRef]
- Dong, L.; Luo, L.; Wang, Z.; Lian, S.; Wang, M.; Wu, X.; Fan, J.; Zeng, Y.; Li, S.; Lv, S.; et al. Targeted degradation of NDUFS1 by agrimol B promotes mitochondrial ROS accumulation and cytotoxic autophagy arrest in hepatocellular carcinoma. Free Radic. Biol. Med. 2024, 220, 111–124. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, L.; Fan, S.; Bai, S.; Xu, B. Single-cell proteomics analysis of human oocytes during GV-to-MI transition. Hum. Reprod. 2025, 40, 1332–1343. [Google Scholar] [CrossRef]
- Kesavanarayanan, K.S.; Sathiya, S.; Ranju, V.; Sunil, A.G.; Ilavarasan, R.; Saravana Babu, C.; Kavimani, S.; Prathiba, D. In vitro cytotoxic, antioxidative and alpha-glucosidase inhibitory potential of a herbal mixture comprised of Allium sativum and Lagerstroemia speciosa. Eur. Rev. Med. Pharmacol. Sci. 2012, 16 (Suppl. 3), 58–68. [Google Scholar]
- Li, X.T.; Zhang, Y.K.; Kuang, H.X.; Jin, F.X.; Liu, D.W.; Gao, M.B.; Liu, Z.; Xin, X.J. Mitochondrial protection and anti-aging activity of Astragalus polysaccharides and their potential mechanism. Int. J. Mol. Sci. 2012, 13, 1747–1761. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wei, D.; Zhang, Z.; Guo, H.; Li, S.; Zhang, J.; Zhang, P.; Zhang, L.; Zhao, Y. FABP5 controls macrophage alternative activation and allergic asthma by selectively programming long-chain unsaturated fatty acid metabolism. Cell Rep. 2022, 41, 111668. [Google Scholar] [CrossRef] [PubMed]
- Kochumon, S.; Malik, M.Z.; Sindhu, S.; Arefanian, H.; Jacob, T.; Bahman, F.; Nizam, R.; Hasan, A.; Thomas, R.; Al-Rashed, F.; et al. Gut Dysbiosis Shaped by Cocoa Butter-Based Sucrose-Free HFD Leads to Steatohepatitis, and Insulin Resistance in Mice. Nutrients 2024, 16, 1929. [Google Scholar] [CrossRef]
- Younes, N.B.; Mohamed, O.A.; Rizk, N.M. Docosahexaenoic Acid Counteracts the Hypoxic-Induced Inflammatory and Metabolic Alterations in 3T3-L1 Adipocytes. Nutrients 2022, 14, 4600. [Google Scholar] [CrossRef]
- Li, X.; Huang, X.; Song, K.; Liu, J.; Jin, Y.; Li, T.; Zhang, L.; Zhang, H. Qingre Sanjie Formula alleviates atherosclerosis by promoting LXR-alpha/ABCG5/G8-mediated reverse cholesterol transport and bile acid synthesis. Phytomedicine 2025, 142, 156691. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wang, J.; Shi, H.; Wei, X.; Zhang, H.; Ji, Y.; Lu, S.; Yan, Y.; Yu, X.; Luo, X.; et al. CCE and EODF as two distinct non-shivering thermogenesis models inducing weight loss. Pflugers Arch. 2023, 475, 961–974. [Google Scholar] [CrossRef]
- Nicholls, D.G.; Brand, M.D. A critical assessment of the role of creatine in brown adipose tissue thermogenesis. Nat. Metab. 2023, 5, 21–28. [Google Scholar] [CrossRef]
- Bunk, J.; Hussain, M.F.; Delgado-Martin, M.; Samborska, B.; Ersin, M.; Shaw, A.; Rahbani, J.F.; Kazak, L. The Futile Creatine Cycle powers UCP1-independent thermogenesis in classical BAT. Nat. Commun. 2025, 16, 3221. [Google Scholar] [CrossRef]
- Rahbani, J.F.; Bunk, J.; Lagarde, D.; Samborska, B.; Roesler, A.; Xiao, H.; Shaw, A.; Kaiser, Z.; Braun, J.L.; Geromella, M.S.; et al. Parallel control of cold-triggered adipocyte thermogenesis by UCP1 and CKB. Cell Metab. 2024, 36, 526–540.e527. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.C.; Tsai, L.T.Y.; Shao, M.; Tenen, D.; Shen, Y.; Kumari, M.; Lyubetskaya, A.; Jacobs, C.; Dawes, B.; Gupta, R.K.; et al. Warming Induces Significant Reprogramming of Beige, but Not Brown, Adipocyte Cellular Identity. Cell Metab. 2018, 27, 1121–1137.e1125. [Google Scholar] [CrossRef] [PubMed]
- Morigny, P.; Boucher, J.; Arner, P.; Langin, D. Lipid and glucose metabolism in white adipocytes: Pathways, dysfunction and therapeutics. Nat. Rev. Endocrinol. 2021, 17, 276–295. [Google Scholar] [CrossRef]
- Majdalawieh, A.F.; Massri, M.; Ro, H.S. AEBP1 is a Novel Oncogene: Mechanisms of Action and Signaling Pathways. J. Oncol. 2020, 2020, 8097872. [Google Scholar] [CrossRef]
- Muise, A.M.; Ro, H.S. Enzymic characterization of a novel member of the regulatory B-like carboxypeptidase with transcriptional repression function: Stimulation of enzymic activity by its target DNA. Biochem. J. 1999, 343 Pt 2, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Ro, H.S.; Zhang, L.; Majdalawieh, A.; Kim, S.W.; Wu, X.; Lyons, P.J.; Webber, C.; Ma, H.; Reidy, S.P.; Boudreau, A.; et al. Adipocyte enhancer-binding protein 1 modulates adiposity and energy homeostasis. Obesity 2007, 15, 288–302. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, Y.; Li, X.; Qin, H.; Yan, S.; Rao, C.; Fan, D.; Liu, D.; Deng, F.; Miao, Y.; et al. PRMT4 Facilitates White Adipose Tissue Browning and Thermogenesis by Methylating PPARgamma. Diabetes 2023, 72, 1095–1111. [Google Scholar] [CrossRef]
- Jeon, Y.G.; Nahmgoong, H.; Oh, J.; Lee, D.; Kim, D.W.; Kim, J.E.; Kim, Y.Y.; Ji, Y.; Han, J.S.; Kim, S.M.; et al. Ubiquitin ligase RNF20 coordinates sequential adipose thermogenesis with brown and beige fat-specific substrates. Nat. Commun. 2024, 15, 940. [Google Scholar] [CrossRef] [PubMed]
- Rosenwald, M.; Perdikari, A.; Rulicke, T.; Wolfrum, C. Bi-directional interconversion of brite and white adipocytes. Nat. Cell Biol. 2013, 15, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Yang, G.; Yoneshiro, T.; Abe, Y.; Ito, R.; Yang, C.; Nakazono, J.; Okamoto-Katsuyama, M.; Uchida, A.; Arai, M.; et al. MYPT1-PP1beta phosphatase negatively regulates both chromatin landscape and co-activator recruitment for beige adipogenesis. Nat. Commun. 2022, 13, 5715. [Google Scholar] [CrossRef]
- Yu, J.; Gu, X.; Guo, Y.; Gao, M.; Cheng, S.; Meng, M.; Cui, X.; Zhang, Z.; Guo, W.; Yan, D.; et al. E3 ligase FBXW7 suppresses brown fat expansion and browning of white fat. EMBO Rep. 2025, 26, 748–767. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Lv, L.; Ma, H.; Wang, L.; Fu, B.; Wang, F.; Yang, S.; Liu, D.; Zhang, D. Cold Exposure Induces Swine Brown Adipocytes to Display an Island-like Distribution with Atypical Characteristics. Int. J. Mol. Sci. 2025, 26, 9871. https://doi.org/10.3390/ijms26209871
Guo Z, Lv L, Ma H, Wang L, Fu B, Wang F, Yang S, Liu D, Zhang D. Cold Exposure Induces Swine Brown Adipocytes to Display an Island-like Distribution with Atypical Characteristics. International Journal of Molecular Sciences. 2025; 26(20):9871. https://doi.org/10.3390/ijms26209871
Chicago/Turabian StyleGuo, Zhenhua, Lei Lv, Hong Ma, Liang Wang, Bo Fu, Fang Wang, Shuo Yang, Di Liu, and Dongjie Zhang. 2025. "Cold Exposure Induces Swine Brown Adipocytes to Display an Island-like Distribution with Atypical Characteristics" International Journal of Molecular Sciences 26, no. 20: 9871. https://doi.org/10.3390/ijms26209871
APA StyleGuo, Z., Lv, L., Ma, H., Wang, L., Fu, B., Wang, F., Yang, S., Liu, D., & Zhang, D. (2025). Cold Exposure Induces Swine Brown Adipocytes to Display an Island-like Distribution with Atypical Characteristics. International Journal of Molecular Sciences, 26(20), 9871. https://doi.org/10.3390/ijms26209871





