Cellular Immunity in Obesity: Pathophysiological Insights and the Impact of Bariatric Surgery
Abstract
1. Introduction
2. Immune Response in Obesity
2.1. Cellular Immunity
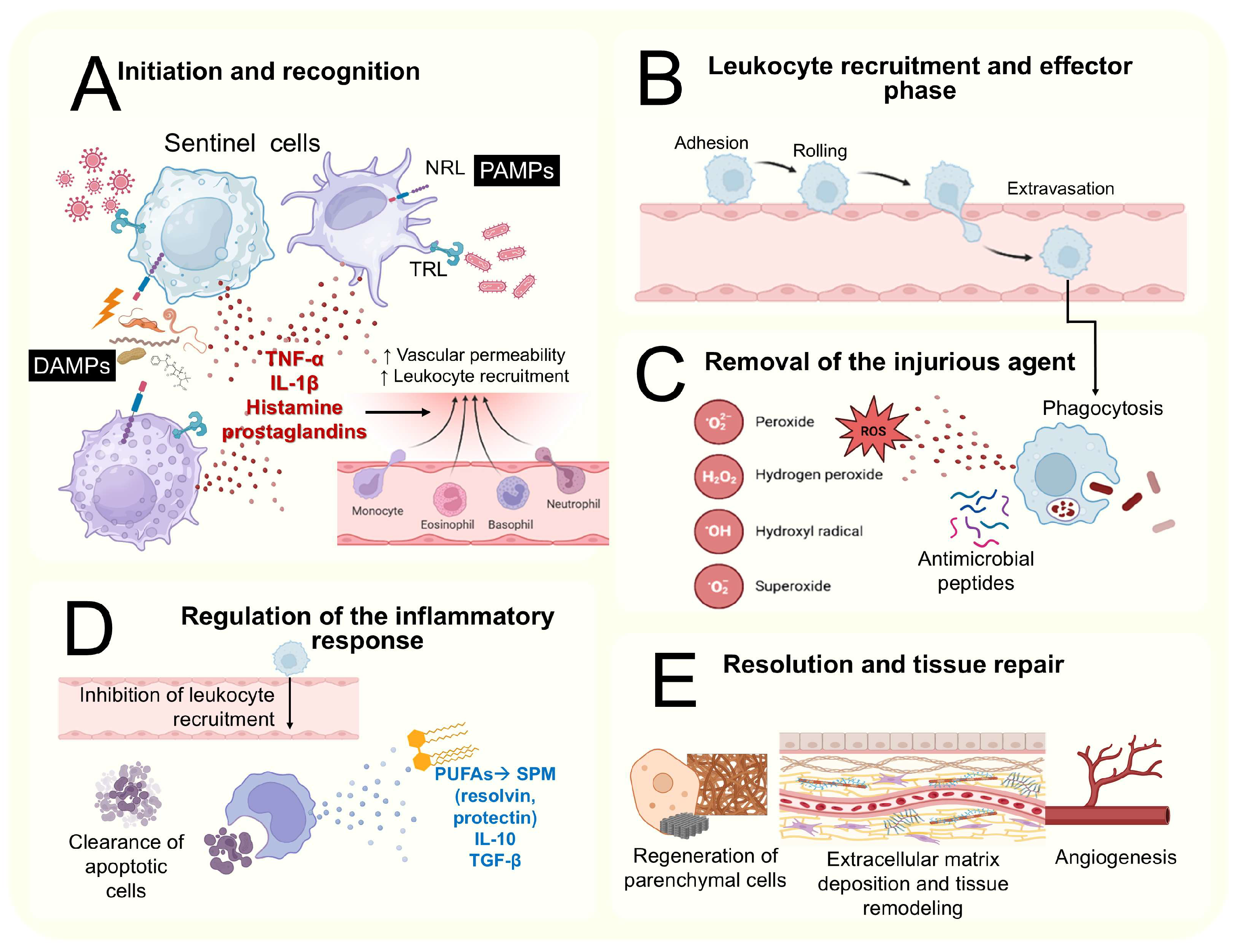
2.2. The Basis of the Inflammatory Process
2.3. Mechanism of Low-Grade Chronic Inflammation Associated with Obesity
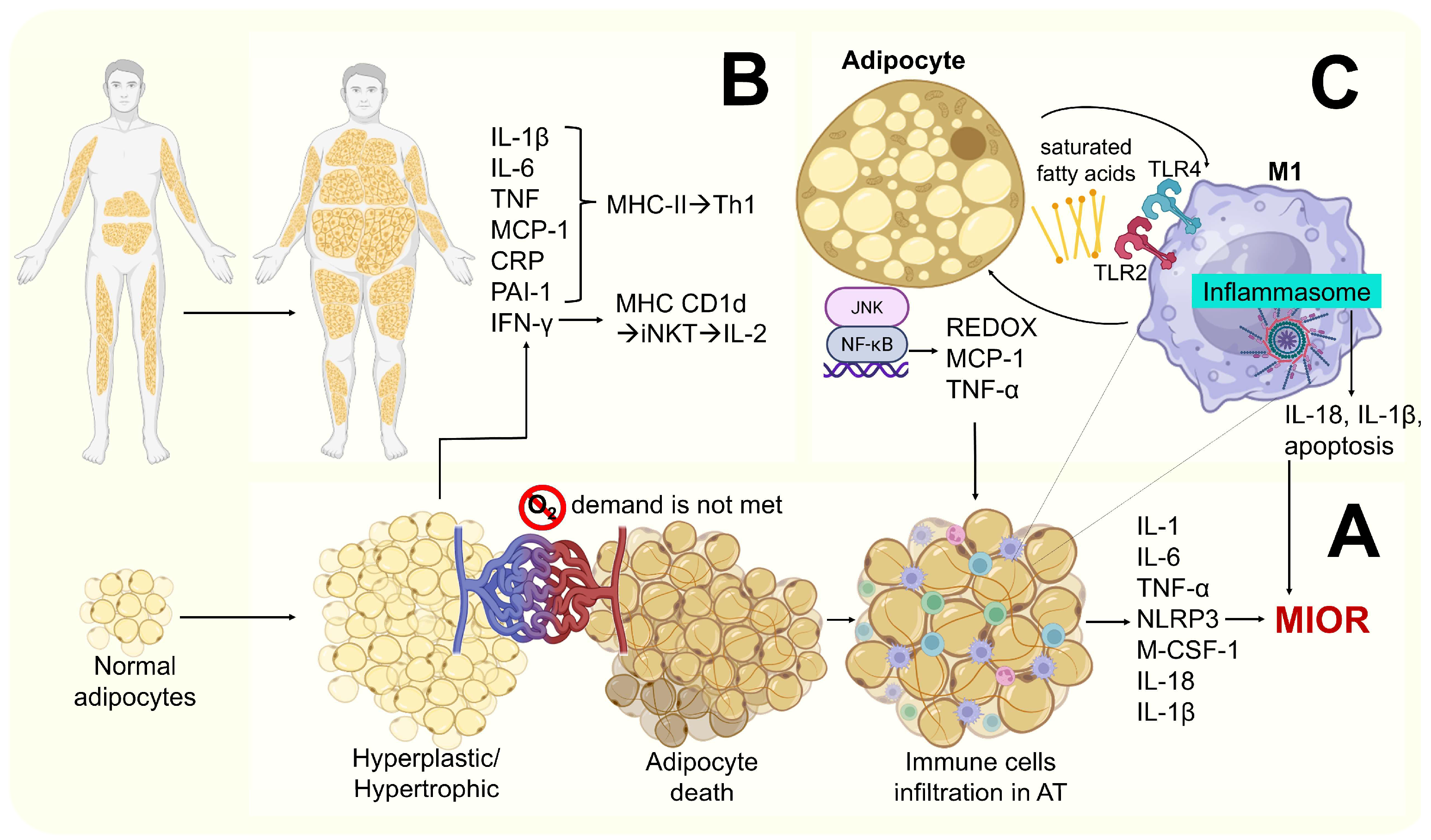
- –
- Saturated fatty acids released by apoptotic adipocytes (recognized as DAMPS) promote M1 activation via indirect binding to toll-like receptor 4 (TLR4) and toll-like receptor 2 (TLR2). This leads to NF-κβ and JNK activation, resulting in the secretion of pro-inflammatory cytokines such as IL-1β, MCP-1, and TNF-α [34,48].
- –
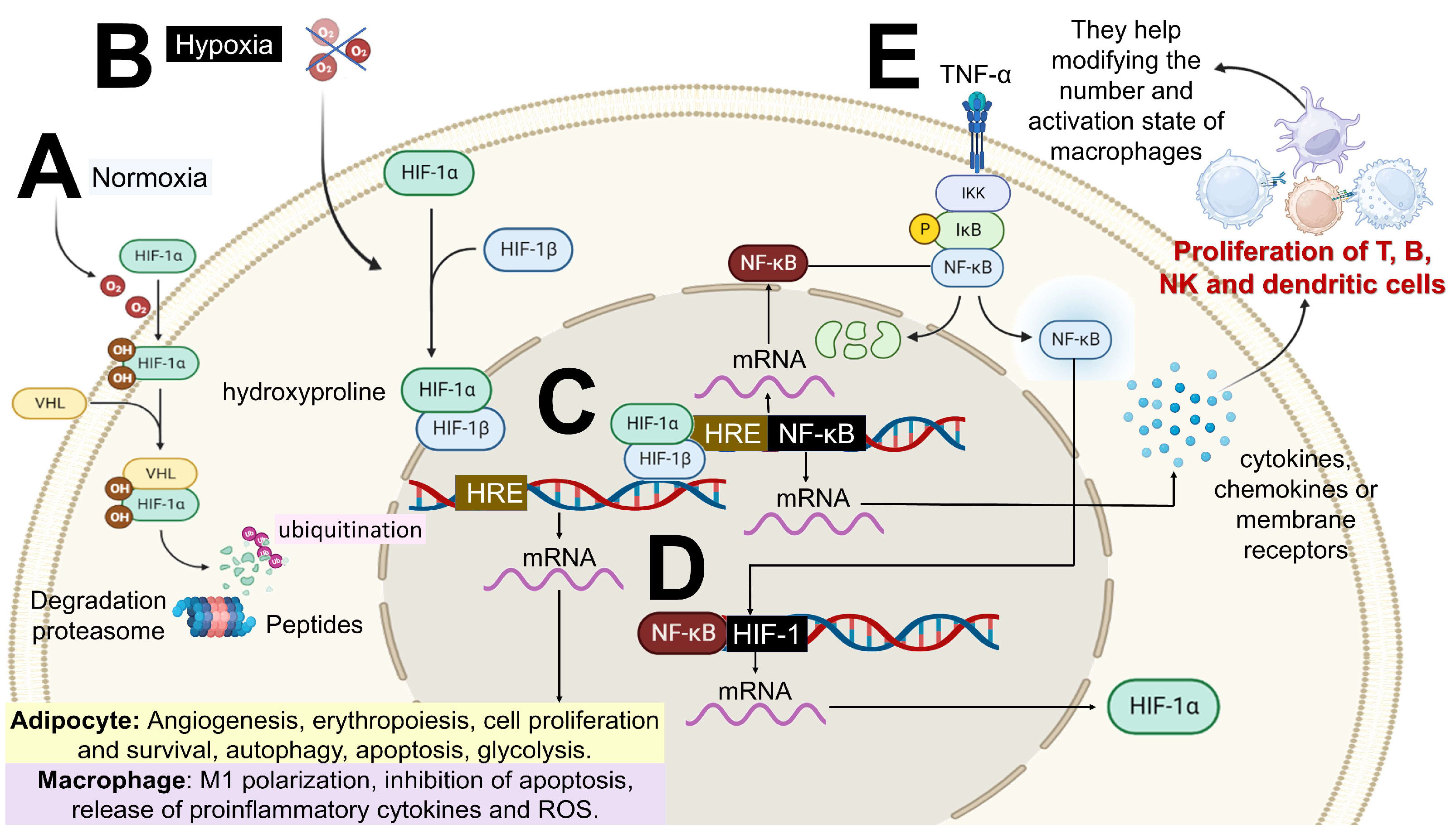
- Hypoxia and hypertrophy adipocytes may contribute to mitochondrial dysfunction, leading to the accumulation of intracellular fatty acids and their metabolites (e.g., fatty acyl-Coenzyme A and diacylglycerols), resulting in lipid peroxidation and oxidative stress. This is characterized by increased ROS production (particularly superoxide ions and nitric oxide) and the further recruitment of immune cells to AT. Elevated levels of TNF-α and leptin, along with reduced levels of IL-10 and adiponectin (anti-inflammatory molecules), have also been reported [45,47] (Figure 4).
2.4. Cancer and Low-Grade Chronic Inflammation Associated with Obesity
2.4.1. Low-Grade Chronic Inflammation Associated with Obesity
2.4.2. Consequences and Complications of Chronic Inflammation Associated with Obesity
3. Cellular Immunity and Its Participation in Obesity
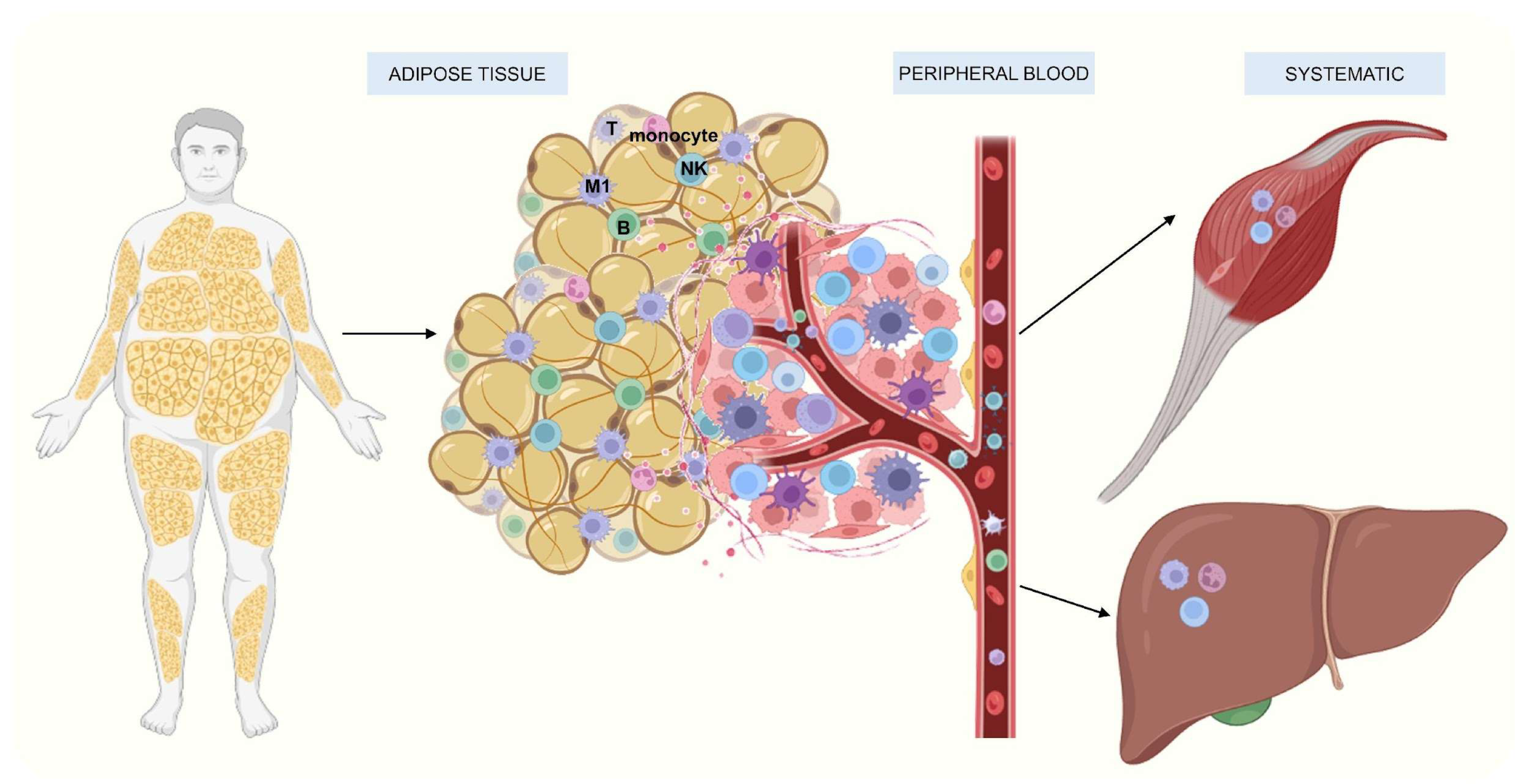
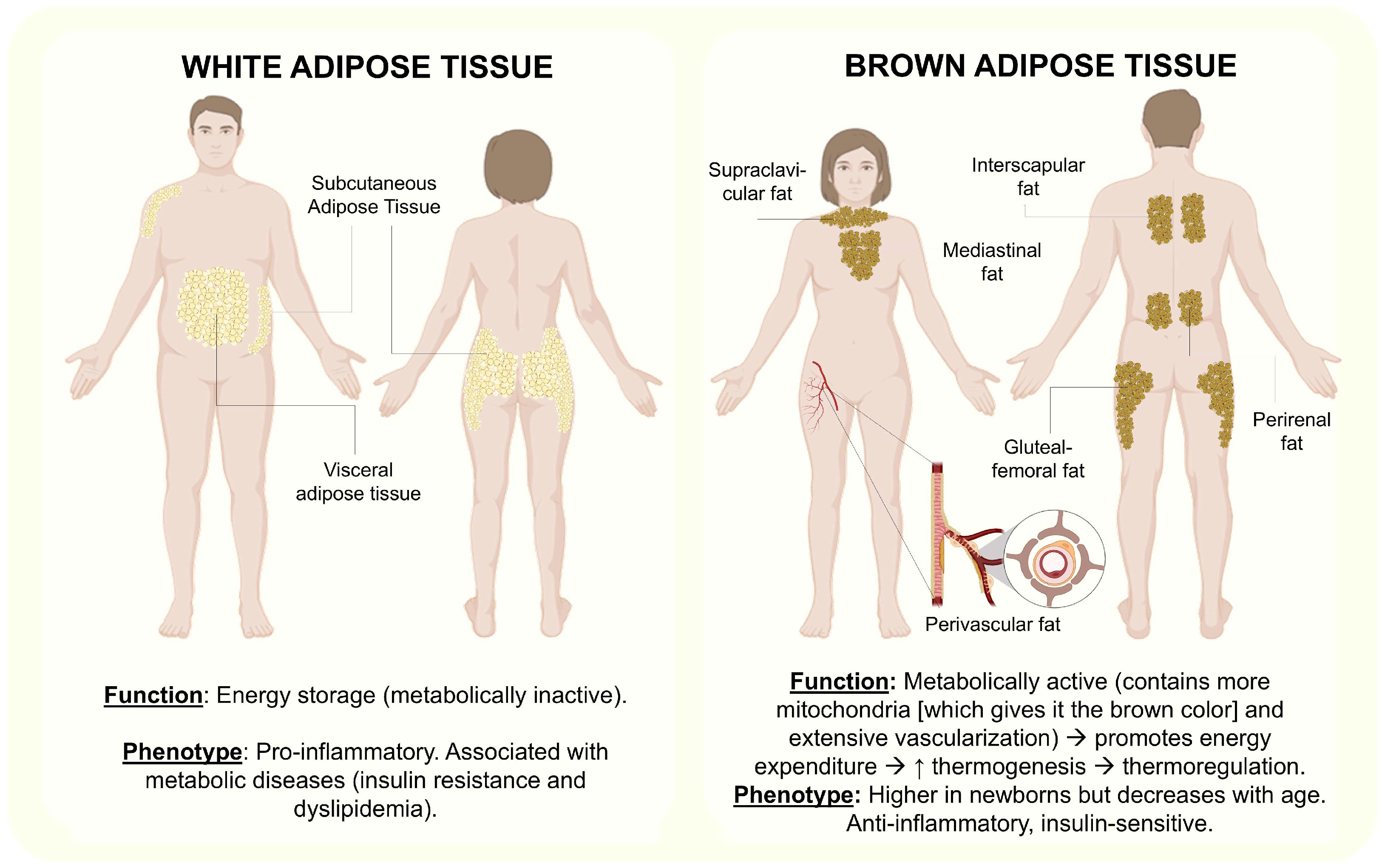
3.1. Adipose Tissue’s Immune Cells
3.2. Endothelial Dysfunction in Obesity
3.3. Cellular Immunity as a Key Factor in Inflammation Amplification
3.4. Evidence of Changes in Lymphocytes in People with Obesity
4. Changes in Cellular Immunity in Obese People Undergoing Bariatric Surgery
4.1. Restrictive Bariatric Surgery Techniques
4.2. Mixed Bariatric Surgery Techniques
4.3. Differences in Peripheral Immune Cell Profiles Between the Two Most Used Bariatric Surgical Techniques (Gastric Bypass and Sleeve Gastrectomy)
5. New Perspectives
5.1. The Modulation of the Inflammatory Response Through Diet and Nutraceutical Interventions
5.2. Metabolic Reprogramming of Immune and Senescent Cells
- Reverse the dysfunctional phenotype of T cells, characterized by metabolic exhaustion, reduced cytotoxicity, and impaired tumor recognition [48].
- Achieve the downregulation of Janus kinase 3 (Jak3), a tyrosine kinase associated with cytokine receptors in the intestinal epithelium [148], NF-κβ signaling mediated by TLRs, and the phosphoinositide-3-kinase-protein kinase B (PI3K-Akt) axis (a pathway controlling insulin receptor signaling) [149], suggesting potential therapeutic strategies targeting metabolic signaling in these cells.
5.3. The Modulation of the Gut Microbiota
- The alteration of the production, metabolism, signaling, and epigenetic modulation of short chain fatty acids (SCFAs). On one hand, the impaired epigenetic modulation of SCFAs changes the expression of inflammatory genes (via histone deacetylase inhibition and acetylation of NF-κβ target genes), thereby suppressing immune cell activation in AT [152]. On the other hand, the deficient binding/activation of SCFAs to G-protein-coupled receptors (GPCRs) and free fatty acid receptors (FFARs) leads to the inappropriate regulation of GPCR43 (expressed in WAT) and FFAR2/FFAR3 coupled to GPCRs. This results in an increased appetite (due to insufficient intracellular Ca2+ elevation to stimulate glucagon like peptide-1 [GLP-1] secretion), decreased energy expenditure, and elevated glucose and insulin levels [143,149].
- A reduction in butyrate levels decreases claudin proteins between enterocytes, increasing intestinal permeability and allowing the translocation of dietary antigens, metabolic endotoxins, and bacterial lipopolysaccharides (LPSs) from the gut into the bloodstream. LPS binds to TLR4 on immune cells, triggering an inflammatory cascade mediated by IL-6 and TNF-α secretion, which further increases the intestinal permeability [144]. In enteroendocrine cells, deficient LPS binding to TLR4 reduces GLP-1 and cholecystokinin secretion [145], highlighting the dual role of TLR4 in metabolic homeostasis [127].
- The alteration of the molecular composition of extracellular vesicles (EV). EV are lipid-bound nanostructures released by donor microbial cells and internalized by target cells, enabling the transfer of bioactive molecules such as nucleic acids, proteins, lipids, and metabolites. An altered EV composition has been linked to metabolic dysfunction, impaired immune cell recruitment, dysregulated adipocyte signaling and thermogenesis, and macrophage and T cell dysfunction in AT [151]. Recent studies describe how microbial and adipocyte-derived EV transport bioactive proteins, metabolites, cytokines, and lipid mediators, thereby regulating Th1/Th17 cell differentiation and suppressing Treg induction in VAT, a paradigm expanding the concept of the gut–adipose immunometabolic communication [153,154].
- –
- Weight loss via BS to restore microbial balance and thereby normalize SCFAs and EV profiles, as well as immune cell function [155].
- –
- Capsaicin supplementation to reduce inflammation via the downregulation of NF-κβ, NLRP3, and LPS activation [147].
- –
- Application of novel multi-omics frameworks (metagenomics, metabolomics, and proteomics) and mass spectrometry platforms integrated with artificial intelligence to measure the production and function of bioactive proteins, inflammatory mediators, enzymes, SCFAs, and EV from fecal samples and/or AT [149,150]. These could serve as novel molecular targets and/or diagnostic biomarkers, enabling personalized intervention strategies targeting the gut–immune interface [153], thereby opening the door to an unprecedented analytical resolution in obesity research [154] (Figure 6).
5.4. Regenerative Approaches with AT and Adipose-Derived Stem Cell Transplantation for Metabolic and Immune Modulation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADSC | Adipose-derived stem cells |
| AKT/PKβ | Serine/threonine kinase or protein kinase β |
| AMPK | Adenosine monophosphate-activated protein kinase |
| AT | Adipose tissue |
| ATP | Adenosine triphosphate |
| BAT | Brown adipose tissue |
| Breg | Regulatory B cell |
| BMI | Body mass index |
| BS | Bariatric surgery |
| CD | Cluster of differentiation |
| CD3+ | Total T lymphocyte |
| CD4+ | T helper lymphocyte |
| CD4+CD45RA+ | Naive T helper lymphocyte |
| CD4+CD45RO+ | Memory T helper lymphocyte |
| CD4+CD62− | Effector help T lymphocyte |
| CD8+/CTLs | Cytotoxic T lymphocytes |
| CD8+CD28− | Effector cytotoxic T lymphocyte |
| CD8+CD45RA+ | Naive cytotoxic T lymphocyte |
| CD8+CD45RO+ | Memory cytotoxic T lymphocyte |
| COX-2 | Ciclooxygenase-2 |
| CXCL | Chemokine (C-X-C motif) ligand |
| Cells/µL | Counting or absolute number |
| CRP | C-reactive protein |
| DAMP | Danger-associated molecular pattern |
| DNA | Deoxyribonucleic acid |
| EV | Extracellular vesicles |
| ERK | Extracellular signal-regulated kinases |
| FGF21 | Circulating protein belonging to the human FGF superfamily |
| FFM | Fat-free mass |
| FFAR | Free fatty acid receptor |
| GM | Gut microbiota |
| GPCRs | G protein-coupled receptors |
| GLP-1 | Glucagon-like peptide-1 |
| HDL-c | High-density lipoprotein cholesterol |
| HbA1c | Glycated hemoglobin |
| HIF-1 | Hypoxia-inducible factor 1 |
| HIF-1α | Hypoxia-inducible factor 1 alpha |
| HIF-1β | Hypoxia-inducible factor 1 beta |
| HMGB1 | High-mobility group box 1 |
| HOMA-IR | Homeostatic model assessment of insulin resistance |
| HRE | Hypoxia response element |
| ICAM-1 | Intercellular adhesion molecule-1 |
| IFN-γ | Interferon gamma |
| Ig | Immunoglobulin |
| IgA | Immunoglobulin A |
| IgG | Immunoglobulin G |
| IgM | Immunoglobulin M |
| IGFBP-2 | Insulin-like growth factor-binding protein 2 |
| IGFBP7 | Insulin-like growth factor binding protein-7 |
| IKK | Ikappaβ kinase |
| IkβK | Inhibition of IκB kinase |
| IL | Interleukin |
| IL-1β | interleukin-1 beta |
| IL-7R | Interleukin 7 receptor |
| ILCs | Innate lymphoid cells |
| IR | Insulin resistance |
| IRS-1 | Insulin receptor substrate 1 |
| IRS-2 | Insulin receptor substrate 2 |
| Iκβ | Ikappaβ |
| Jak3 | Janus kinase 3 |
| JNK | C-Jun N-terminal kinase |
| LBD | Laparoscopic biliopancreatic diversion |
| LDL-c | Low-density lipoprotein cholesterol |
| LPS | Lipopolysaccharide |
| LRYGB | Laparoscopic Roux-en-Y gastric bypass |
| LSG | Laparoscopic sleeve gastrectomy |
| MAPK | p38 mitogen-activated protein kinase |
| MASLD | Metabolic dysfunction-associated steatosis liver disease |
| M1 | Pro-inflammatory macrophage |
| M2 | Anti-inflammatory macrophage |
| MCP-1/CCL2 | Monocyte chemoattractant protein-1 |
| MHC | Major histocompatibility complex |
| MIOR | Metainflammation-related obesity |
| MS | Metabolic syndrome |
| mTOR | Mammalian target of rapamycin |
| NAD+ | Nicotinamide adenine dinucleotide |
| NADP | Nicotinamide adenine dinucleotide phosphate |
| NF-κβ | Nuclear factor kappa-light-chain-enhancer of activated β cell |
| NK | Natural killers |
| NKG2D | Natural killer group 2 member D |
| NLRs | NOD-like receptors |
| NLRP3 | NLR family pyrin domain containing 3 or inflammasome |
| NOTCH1 | Neurogenic locus notch homolog protein 1 |
| OGM | Obesogenic gut |
| OXPHOS | Oxidative phosphorylation |
| PAI-1 | Plasminogen activator inhibitor type-1 |
| PAMPs | Pathogen-associated molecular patterns |
| PI3K-Akt | Phosphoinositide-3-kinase-protein kinase B |
| PUFAs | Polyunsaturated fatty acids |
| PVAT | Perivascular adipose tissue |
| ROS | Reactive oxygen species |
| SASP | Senescence-associated secretory phenotype |
| SIRT1 | Sirtuin-1 deacetylase |
| SCFAs | Short-chain fatty acids |
| SPMs | Specialized pro-resolving mediators |
| REBPs | Sterol regulatory element-binding proteins |
| TBF | Total body fat |
| T2D | Type 2 diabetes |
| TCR | T cell receptor |
| TGF-β | Transforming growth factor beta |
| Th0 | Naive T cells |
| TLRs | Toll-like receptors |
| TLR2 | Toll-like receptor 2 |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor alpha |
| Tregs | Regulatory T cells |
| VAT | Visceral adipose tissue |
| VHL | Hippel–Lindau |
| VF | Visceral fat |
| WAT | White adipose tissue |
References
- Busebee, B.; Ghusn, W.; Cifuentes, L.; Acosta, A. Obesity: A Review of Pathophysiology and Classification. Mayo Clin. Proc. 2023, 98, 1842–1857. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.C.; Kim, M.S.; Pae, M.; Yamamoto, Y.; Eberlé, D.; Shimada, T.; Kamei, N.; Park, H.S.; Sasorith, S.; Woo, J.R.; et al. Adipose Natural Killer Cells Regulate Adipose Tissue Macrophages to Promote Insulin Resistance in Obesity. Cell Metab. 2016, 23, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Nikolajczyk, B.S. Tissue Immune Cells Fuel Obesity-Associated Inflammation in Adipose Tissue and Beyond. Front. Immunol. 2019, 10, 1587. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, J.; Dai, H.; Duan, Y.; An, Y.; Shi, L.; Lv, Y.; Li, H.; Wang, C.; Ma, Q.; et al. Brown and beige adipose tissue: A novel therapeutic strategy for obesity and type 2 diabetes mellitus. Adipocyte 2021, 10, 48–65. [Google Scholar] [CrossRef]
- Weinstock, A.; Moura Silva, H.; Moore, K.J.; Schmidt, A.M.; Fisher, E.A. Leukocyte Heterogeneity in Adipose Tissue, Including in Obesity. Circ. Res. 2020, 126, 1590–1612. [Google Scholar] [CrossRef]
- Honecker, J.; Ruschke, S.; Seeliger, C.; Laber, S.; Strobel, S.; Pröll, P.; Nellaker, C.; Lindgren, C.M.; Kulozik, U.; Ecker, J.; et al. Transcriptome and fatty-acid signatures of adipocyte hypertrophy and its non-invasive MR-based characterization in human adipose tissue. EBioMedicine 2022, 79, 104020. [Google Scholar] [CrossRef]
- Su, J.; Song, Y.; Zhu, Z.; Huang, X.; Fan, J.; Qiao, J.; Mao, F. Cell-cell communication: New insights and clinical implications. Signal Transduct. Target. Ther. 2024, 9, 196. [Google Scholar] [CrossRef]
- Filella, X.; Molina, R.; Ballesta, A.M. Estructura y función de las citocinas. Med. Integral 2002, 39, 63–71. [Google Scholar]
- de Heredia, F.P.; Gómez-Martínez, S.; Marcos, A. Obesity, inflammation and the immune system. Proc. Nutr. Soc. 2012, 71, 332–338. [Google Scholar] [CrossRef]
- Kwon, Y. Immuno-Resolving Ability of Resolvins, Protectins, and Maresins Derived from Omega-3 Fatty Acids in Metabolic Syndrome. Mol. Nutr. Food Res. 2020, 64, e1900824. [Google Scholar] [CrossRef]
- Bhusal, R.P.; Foster, S.R.; Stone, M.J. Structural basis of chemokine and receptor interactions: Key regulators of leukocyte recruitment in inflammatory responses. Protein Sci. 2020, 29, 420–432. [Google Scholar] [CrossRef]
- Sanjabi, S.; Zenewicz, L.A.; Kamanaka, M.; Flavell, R.A. Anti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity. Curr. Opin. Pharmacol. 2009, 9, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tang, Y.; Luo, Y.; Gao, Y.; He, L. Role and mechanism of specialized pro-resolving mediators in obesity-associated insulin resistance. Lipids Health Dis. 2024, 23, 234. [Google Scholar] [CrossRef] [PubMed]
- van de Vyver, M. Immunology of chronic low-grade inflammation: Relationship with metabolic function. J. Endocrinol. 2023, 257, e220271. [Google Scholar] [CrossRef]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2019, 10, 1607. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Püschel, G.P.; Klauder, J.; Henkel, J. Macrophages, Low-Grade Inflammation, Insulin Resistance and Hyperinsulinemia: A Mutual Ambiguous Relationship in the Development of Metabolic Diseases. J. Clin. Med. 2022, 11, 4358. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hu, J.; Wang, Y.; Lei, H.; Xu, D. The role and research progress of the balance and interaction between regulatory T cells and other immune cells in obesity with insulin resistance. Adipocyte 2021, 10, 66–79. [Google Scholar] [CrossRef]
- Wensveen, F.M.; Valentić, S.; Šestan, M.; Turk Wensveen, T.; Polić, B. The “Big Bang” in obese fat: Events initiating obesity-induced adipose tissue inflammation. Eur. J. Immunol. 2015, 45, 2446–2456. [Google Scholar] [CrossRef]
- Gálvez, I.; Dolores Hinchado, M.; Martín-Cordero, L.; Javier Morán-Plata, F.; Graham, G.; Francisco-Morcillo, J.; Ortega, E. The anti-inflammatory and bioregulatory effects of habitual exercise in high-fat diet-induced obesity involve crown-like structures and MCP-1 in white adipose tissue. Exerc. Immunol. Rev. 2023, 29, 111–120. [Google Scholar]
- Yan, L.; Rust, B.M.; Sundaram, S.; Nielsen, F.H. Metabolomic Alteration in Adipose Monocyte Chemotactic Protein-1 Deficient Mice Fed a High-Fat Diet. Nutr. Metab. Insights 2024, 17, 11786388241280859. [Google Scholar] [CrossRef] [PubMed]
- Stone, W.L.; Basit, H.; Zubair, M.; Burns, B. Pathology, Inflammation. In StatPearls; StatPearls Publishing Copyright © 2025; StatPearls Publishing LLC: Treasure Island, FL, USA, 2025. [Google Scholar]
- Barton, G.M. A calculated response: Control of inflammation by the innate immune system. J. Clin. Investig. 2008, 118, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Panigrahy, D.; Gilligan, M.M.; Serhan, C.N.; Kashfi, K. Resolution of inflammation: An organizing principle in biology and medicine. Pharmacol. Ther. 2021, 227, 107879. [Google Scholar] [CrossRef]
- Kourtzelis, I.; Mitroulis, I.; von Renesse, J.; Hajishengallis, G.; Chavakis, T. From leukocyte recruitment to resolution of inflammation: The cardinal role of integrins. J. Leukoc. Biol. 2017, 102, 677–683. [Google Scholar] [CrossRef]
- Ley, K. Molecular mechanisms of leukocyte recruitment in the inflammatory process. Cardiovasc. Res. 1996, 32, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Cross, K.; Vetter, S.W.; Alam, Y.; Hasan, M.Z.; Nath, A.D.; Leclerc, E. Role of the Receptor for Advanced Glycation End Products (RAGE) and Its Ligands in Inflammatory Responses. Biomolecules 2024, 14, 1550. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.; Batterham, R.L.; Koch, M.; Mingrone, G.; le Roux, C.W.; Farooqi, I.S.; Farpour-Lambert, N.; Gregg, E.W.; Cummings, D.E. Lancet Diabetes & Endocrinology Commission on the definition and diagnosis of clinical obesity. Lancet Diabetes Endocrinol. 2023, 11, 226–228. [Google Scholar] [CrossRef]
- Carobbio, S.; Pellegrinelli, V.; Vidal-Puig, A. Adipose Tissue Function and Expandability as Determinants of Lipotoxicity and the Metabolic Syndrome. Adv. Exp. Med. Biol. 2017, 960, 161–196. [Google Scholar] [CrossRef]
- D’Ignazio, L.; Bandarra, D.; Rocha, S. NF-κB and HIF crosstalk in immune responses. FEBS J. 2016, 283, 413–424. [Google Scholar] [CrossRef] [PubMed]
- D’Ignazio, L.; Rocha, S. Hypoxia Induced NF-κB. Cells 2016, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Rivera Carranza, T. Inmunidad Celular y su Asociación con Características Metabólicas y Clínicas en Pacientes con Obesidad y Cirugía Bariátrica. Ph.D. Thesis, Universidad Autónoma Metropolitana Unidad Xochimilco, Ciudad de México, Mexico, 2024. Available online: https://repositorio.xoc.uam.mx/jspui/handle/123456789/43822 (accessed on 8 May 2025).
- Tinahones, F.J.; Coín Aragüez, L.; Murri, M.; Oliva Olivera, W.; Mayas Torres, M.D.; Barbarroja, N.; Gomez Huelgas, R.; Malagón, M.M.; El Bekay, R. Caspase induction and BCL2 inhibition in human adipose tissue: A potential relationship with insulin signaling alteration. Diabetes Care 2013, 36, 513–521. [Google Scholar] [CrossRef]
- Cancello, R.; Henegar, C.; Viguerie, N.; Taleb, S.; Poitou, C.; Rouault, C.; Coupaye, M.; Pelloux, V.; Hugol, D.; Bouillot, J.L.; et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 2005, 54, 2277–2286. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, R.W.; White, A.E.; Metcalf, M.D.; Olivas, A.S.; Mitra, P.; Larison, W.G.; Cheang, E.C.; Varlamov, O.; Corless, C.L.; Roberts, C.T., Jr.; et al. Hypoxia-induced inflammatory cytokine secretion in human adipose tissue stromovascular cells. Diabetologia 2011, 54, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.K.; Gutierrez, D.A.; Kennedy, A.; Hasty, A.H. Weight cycling increases T-cell accumulation in adipose tissue and impairs systemic glucose tolerance. Diabetes 2013, 62, 3180–3188. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Campitiello, R.; Gotelli, E.; Soldano, S. The Role of M1/M2 Macrophage Polarization in Rheumatoid Arthritis Synovitis. Front. Immunol. 2022, 13, 867260. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, Y.; Yi, J.; Zhao, Z.; Ye, R. Hyperglycemia modulates M1/M2 macrophage polarization via reactive oxygen species overproduction in ligature-induced periodontitis. J. Periodontal Res. 2021, 56, 991–1005. [Google Scholar] [CrossRef]
- Scheithauer, T.P.M.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; van Raalte, D.H.; Herrema, H. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front. Immunol. 2020, 11, 571731. [Google Scholar] [CrossRef]
- Lee, J.-W.; Bae, S.-H.; Jeong, J.-W.; Kim, S.-H.; Kim, K.-W. Hypoxia-inducible factor (HIF-1)α: Its protein stability and biological functions. Exp. Mol. Med. 2004, 36, 1–12. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.Z.; Xu, Z.Q.; Han, B.Z.; Su, D.F.; Liu, C. NLRP3 inflammasome and its inhibitors: A review. Front. Pharmacol. 2015, 6, 262. [Google Scholar] [CrossRef] [PubMed]
- León-Pedroza, J.I.; González-Tapia, L.A.; del Olmo-Gil, E.; Castellanos-Rodríguez, D.; Escobedo, G.; González-Chávez, A. Low-grade systemic inflammation and the development of metabolic diseases: From the molecular evidence to the clinical practice. Cir. Cir. 2015, 83, 543–551. [Google Scholar] [CrossRef] [PubMed]
- López-Reyes, A.; Martinez-Armenta, C.; Espinosa-Velázquez, R.; Vázquez-Cárdenas, P.; Cruz-Ramos, M.; Palacios-Gonzalez, B.; Gomez-Quiroz, L.E.; Martínez-Nava, G.A. NLRP3 Inflammasome: The Stormy Link Between Obesity and COVID-19. Front. Immunol. 2020, 11, 570251. [Google Scholar] [CrossRef]
- Fariñas Guerrero, F.; López Gigosos, R.M. Obesity, immunity and vaccination. Vacunas. 2021, 22, 174–182. [Google Scholar] [CrossRef]
- Piening, A.; Ebert, E.; Gottlieb, C.; Khojandi, N.; Kuehm, L.M.; Hoft, S.G.; Pyles, K.D.; McCommis, K.S.; DiPaolo, R.J.; Ferris, S.T.; et al. Obesity-related T cell dysfunction impairs immunosurveillance and increases cancer risk. Nat. Commun. 2024, 15, 2835. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, N.M.; Gucalp, A.; Dannenberg, A.J.; Hudis, C.A. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J. Clin. Oncol. 2016, 34, 4270–4276. [Google Scholar] [CrossRef]
- Sanhueza, S.; Vidal, M.A.; Hernandez, M.A.; Henriquez-Beltran, M.E.; Cabrera, C.; Quiroga, R.; Antilef, B.E.; Aguilar, K.P.; Castillo, D.A.; Llerena, F.J.; et al. Clinical and pulmonary function analysis in long-COVID revealed that long-term pulmonary dysfunction is associated with vascular inflammation pathways and metabolic syndrome. Front. Med. 2023, 10, 1271863. [Google Scholar] [CrossRef]
- Iyengar, N.M.; Hudis, C.A.; Dannenberg, A.J. Obesity and cancer: Local and systemic mechanisms. Annu. Rev. Med. 2015, 66, 297–309. [Google Scholar] [CrossRef]
- Michelet, X.; Dyck, L.; Hogan, A.; Loftus, R.M.; Duquette, D.; Wei, K.; Beyaz, S.; Tavakkoli, A.; Foley, C.; Donnelly, R.; et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat. Immunol. 2018, 19, 1330–1340. [Google Scholar] [CrossRef]
- Wang, Z.; Aguilar, E.G.; Luna, J.I.; Dunai, C.; Khuat, L.T.; Le, C.T.; Mirsoian, A.; Minnar, C.M.; Stoffel, K.M.; Sturgill, I.R.; et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 2019, 25, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Pingili, A.K.; Chaib, M.; Sipe, L.M.; Miller, E.J.; Teng, B.; Sharma, R.; Yarbro, J.R.; Asemota, S.; Al Abdallah, Q.; Mims, T.S.; et al. Immune checkpoint blockade reprograms systemic immune landscape and tumor microenvironment in obesity-associated breast cancer. Cell Rep. 2021, 35, 109285. [Google Scholar] [CrossRef] [PubMed]
- Woodall, M.J.; Neumann, S.; Campbell, K.; Pattison, S.T.; Young, S.L. The Effects of Obesity on Anti-Cancer Immunity and Cancer Immunotherapy. Cancers 2020, 12, 1230. [Google Scholar] [CrossRef] [PubMed]
- Dyck, L.; Prendeville, H.; Raverdeau, M.; Wilk, M.M.; Loftus, R.M.; Douglas, A.; McCormack, J.; Moran, B.; Wilkinson, M.; Mills, E.L.; et al. Suppressive effects of the obese tumor microenvironment on CD8 T cell infiltration and effector function. J. Exp. Med. 2022, 219, e20210042, Erratum in J. Exp. Med. 2022, 219, e2021004202072022c. https://doi.org/10.1084/jem.2021004202072022c. [Google Scholar] [CrossRef]
- Rutkowski, M.R.; Stephen, T.L.; Svoronos, N.; Allegrezza, M.J.; Tesone, A.J.; Perales-Puchalt, A.; Brencicova, E.; Escovar-Fadul, X.; Nguyen, J.M.; Cadungog, M.G. Microbially driven TLR5-dependent signaling governs distal malignant progression through tumor-promoting inflammation. Cancer Cell 2015, 27, 27–40. [Google Scholar] [CrossRef]
- Desharnais, L.; Walsh, L.A.; Quail, D.F. Exploiting the obesity-associated immune microenvironment for cancer therapeutics. Pharmacol. Ther. 2022, 229, 107923. [Google Scholar] [CrossRef]
- Lennon, H.; Sperrin, M.; Badrick, E.; Renehan, A.G. The obesity paradox in cancer: A review. Curr. Oncol. Rep. 2016, 18, 56. [Google Scholar] [CrossRef]
- Liu, R.; Pugh, G.H.; Tevonian, E.; Thompson, K.; Lauffenburger, D.A.; Kern, P.A.; Nikolajczyk, B.S. Regulatory T cells control effector T cell inflammation in human prediabetes. Diabetes 2022, 71, 264–274. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Deiuliis, J.; Shah, Z.; Shah, N.; Needleman, B.; Mikami, D.; Narula, V.; Perry, K.; Hazey, J.; Kampfrath, T.; Kollengode, M.; et al. Visceral adipose inflammation in obesity is associated with critical alterations in tregulatory cell numbers. PLoS ONE 2011, 6, e16376. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Eto, K.; Yamashita, H.; Ohsugi, M.; Otsu, M.; Hara, K.; Ueki, K.; Sugiura, S.; et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 2009, 15, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Cepero-Donates, Y.; Lacraz, G.; Ghobadi, F.; Rakotoarivelo, V.; Orkhis, S.; Mayhue, M.; Chen, Y.G.; Rola-Pleszczynski, M.; Menendez, A.; Ilangumaran, S.; et al. Interleukin-15-mediated inflammation promotes non-alcoholic fatty liver disease. Cytokine 2016, 82, 102–111. [Google Scholar] [CrossRef] [PubMed]
- van der Weerd, K.; Dik, W.A.; Schrijver, B.; Schweitzer, D.H.; Langerak, A.W.; Drexhage, H.A.; Kiewiet, R.M.; van Aken, M.O.; van Huisstede, A.; van Dongen, J.J.; et al. Morbidly obese human subjects have increased peripheral blood CD4+ T cells with skewing toward a Treg- and Th2-dominated phenotype. Diabetes 2012, 61, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, S.; Zernecke, A. CD8(+) T Cells in Atherosclerosis. Cells 2020, 10, 37. [Google Scholar] [CrossRef]
- Rivera-Carranza, T.; Nájera-Medina, O.; Bojalil-Parra, R.; Rodríguez-López, C.P.; Zúñiga-León, E.; Girón, A.L.-T.; Azaola-Espinosa, A. The link between lymphocyte subpopulations in peripheral blood and metabolic variables in patients with severe obesity. PeerJ 2023, 11, e15465. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, C.P.; González, M.C.; Aguilar-Salinas, C.A.; Nájera-Medina, O. Peripheral Lymphocytes, Obesity, and Metabolic Syndrome in Young Adults: An Immunometabolism Study. Metab. Syndr. Relat. Disord. 2018, 16, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.; Sinha, S. Obesity and aging: Molecular mechanisms and therapeutic approaches. Ageing Res. Rev. 2021, 67, 101268. [Google Scholar] [CrossRef]
- Kalinkovich, A.; Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017, 35, 200–221. [Google Scholar] [CrossRef]
- Harmon, D.B.; Srikakulapu, P.; Kaplan, J.L.; Oldham, S.N.; McSkimming, C.; Garmey, J.C.; Perry, H.M.; Kirby, J.L.; Prohaska, T.A.; Gonen, A. Protective role for B-1b B cells and IgM in obesity-associated inflammation, glucose intolerance, and insulin resistance. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 682–691. [Google Scholar] [CrossRef]
- Srikakulapu, P.; McNamara, C.A. B lymphocytes and adipose tissue inflammation. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Diaz, A.; Romero, M.; Thaller, S.; Blomberg, B.B. Secretion of autoimmune antibodies in the human subcutaneous adipose tissue. PLoS ONE 2018, 13, e0197472. [Google Scholar] [CrossRef]
- Frasca, D.; Ferracci, F.; Diaz, A.; Romero, M.; Lechner, S.; Blomberg, B.B. Obesity decreases B cell responses in young and elderly individuals. Obesity 2016, 24, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, M.; Kinoshita, K.; Fagarasan, S.; Yamada, S.; Shinkai, Y.; Honjo, T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 2000, 102, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Sayegh, C.E.; Quong, M.W.; Agata, Y.; Murre, C. E-proteins directly regulate expression of activation-induced deaminase in mature B cells. Nat. Immunol. 2003, 4, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Landin, A.M.; Lechner, S.C.; Ryan, J.G.; Schwartz, R.; Riley, R.L.; Blomberg, B.B. Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells. J. Immunol. 2008, 180, 5283–5290. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Qian, G.; Wang, Y.; Chen, X.; Lu, J.; Zhang, Y.; Huang, Q.; Wang, Q. Elevated B cell activation is associated with type 2 diabetes development in obese subjects. Cell. Physiol. Biochem. 2016, 38, 1257–1266. [Google Scholar] [CrossRef]
- Engin, A. Endothelial Dysfunction in Obesity and Therapeutic Targets. Adv. Exp. Med. Biol. 2024, 1460, 489–538. [Google Scholar] [CrossRef]
- Wang, X.; He, B. Endothelial dysfunction: Molecular mechanisms and clinical implications. MedComm. 2024, 5, e651. [Google Scholar] [CrossRef]
- Velusamy, P.; Buckley, D.J.; Greaney, J.L.; Case, A.J.; Fadel, P.J.; Trott, D.W. IL-6 induces mitochondrial ROS production and blunts NO bioavailability in human aortic endothelial cells. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2025, 328, R509–R514. [Google Scholar] [CrossRef]
- Gkrania-Klotsas, E.; Ye, Z.; Cooper, A.J.; Sharp, S.J.; Luben, R.; Biggs, M.L.; Chen, L.-K.; Gokulakrishnan, K.; Hanefeld, M.; Ingelsson, E. Differential white blood cell count and type 2 diabetes: Systematic review and meta-analysis of cross-sectional and prospective studies. PLoS ONE 2010, 5, e13405. [Google Scholar] [CrossRef]
- Ryder, E.; Diez-Ewald, M.; Mosquera, J.; Fernández, E.; Pedreañez, A.; Vargas, R.; Peña, C.; Fernández, N. Association of obesity with leukocyte count in obese individuals without metabolic syndrome. Diabetes Metab. Syndr. 2014, 8, 197–204. [Google Scholar] [CrossRef]
- Patsouris, D.; Cao, J.J.; Vial, G.; Bravard, A.; Lefai, E.; Durand, A.; Durand, C.; Chauvin, M.A.; Laugerette, F.; Debard, C.; et al. Insulin resistance is associated with MCP1-mediated macrophage accumulation in skeletal muscle in mice and humans. PLoS ONE 2014, 9, e110653. [Google Scholar] [CrossRef] [PubMed]
- Siervo, M.; Rubele, S.; Shannon, O.M.; Prado, C.M.; Donini, L.M.; Zamboni, M.; Homayounfar, R.; Farjam, M.; Faghih, S.; Mazidi, M. Prevalence of sarcopenic obesity and association with metabolic syndrome in an adult Iranian cohort: The Fasa PERSIAN cohort study. Clin. Obes. 2021, 11, e12459. [Google Scholar] [CrossRef] [PubMed]
- Tencerova, M.; Aouadi, M.; Vangala, P.; Nicoloro, S.M.; Yawe, J.C.; Cohen, J.L.; Shen, Y.; Garcia-Menendez, L.; Pedersen, D.J.; Gallagher-Dorval, K.; et al. Activated Kupffer cells inhibit insulin sensitivity in obese mice. FASEB J. 2015, 29, 2959–2969. [Google Scholar] [CrossRef] [PubMed]
- López-Vicario, C.; Titos, E.; Walker, M.E.; Alcaraz-Quiles, J.; Casulleras, M.; Durán-Güell, M.; Flores-Costa, R.; Pérez-Romero, N.; Forné, M.; Dalli, J.; et al. Leukocytes from obese individuals exhibit an impaired SPM signature. FASEB J. 2019, 33, 7072–7083. [Google Scholar] [CrossRef]
- Abad-Jiménez, Z.; López-Domènech, S.; García-Gargallo, C.; Vezza, T.; Gómez-Abril, S.Á.; Morillas, C.; Díaz-Pozo, P.; Falcón, R.; Bañuls, C.; Víctor, V.M.; et al. Roux-en-Y Gastric Bypass Modulates AMPK, Autophagy and Inflammatory Response in Leukocytes of Obese Patients. Biomedicines 2022, 10, 430. [Google Scholar] [CrossRef]
- Ballesteros-Pomar, M.D.; Calleja, S.; Diez-Rodriguez, R.; Calleja-Fernandez, A.; Vidal-Casariego, A.; Nunez-Alonso, A.; Cano-Rodriguez, I.; Olcoz-Goni, J.L. Inflammatory status is different in relationship to insulin resistance in severely obese people and changes after bariatric surgery or diet-induced weight loss. Exp. Clin. Endocrinol. Diabetes 2014, 122, 592–596. [Google Scholar] [CrossRef]
- Mauro, C.; Smith, J.; Cucchi, D.; Coe, D.; Fu, H.; Bonacina, F.; Baragetti, A.; Cermenati, G.; Caruso, D.; Mitro, N. Obesity-induced metabolic stress leads to biased effector memory CD4+ T cell differentiation via PI3K p110δ-Akt-mediated signals. Cell Metab. 2017, 25, 593–609. [Google Scholar] [CrossRef]
- Jongbloed, F.; Meijers, R.W.J.; Ijzermans, J.N.M.; Klaassen, R.A.; Dollé, M.E.T.; Van Den Berg, S.; Betjes, M.G.H.; de Bruin, R.W.F.; van der Harst, E.; Litjens, N.H.R. Effects of bariatric surgery on telomere length and T-cell aging. Int. J. Obes. 2019, 43, 2189–2199. [Google Scholar] [CrossRef]
- Monte, S.V.; Caruana, J.A.; Ghanim, H.; Sia, C.L.; Korzeniewski, K.; Schentag, J.J.; Dandona, P. Reduction in endotoxemia, oxidative and inflammatory stress, and insulin resistance after Roux-en-Y gastric bypass surgery in patients with morbid obesity and type 2 diabetes mellitus. Surgery 2012, 151, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Simar, D.; Versteyhe, S.; Donkin, I.; Liu, J.; Hesson, L.; Nylander, V.; Fossum, A.; Barrès, R. DNA methylation is altered in B and NK lymphocytes in obese and type 2 diabetic human. Metabolism 2014, 63, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, T.; Liu, L.-F.; Lamendola, C.; Shen, L.; Morton, J.; Rivas, H.; Winer, D.; Tolentino, L.; Choi, O.; Zhang, H. T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2637–2643. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Diaz, A.; Romero, M.; Thaller, S.; Blomberg, B.B. Metabolic requirements of human pro-inflammatory B cells in aging and obesity. PLoS ONE 2019, 14, e0219545. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Romero, M.; Diaz, A.; Garcia, D.; Thaller, S.; Blomberg, B.B. B cells with a senescent-associated secretory phenotype accumulate in the adipose tissue of individuals with obesity. Int. J. Mol. Sci. 2021, 22, 1839. [Google Scholar] [CrossRef]
- Frasca, D.; Diaz, A.; Romero, M.; Blomberg, B.B. Phenotypic and functional characterization of double negative B cells in the blood of individuals with obesity. Front. Immunol. 2021, 12, 616650. [Google Scholar] [CrossRef]
- Li, W.; Chen, W. Weight cycling based on altered immune microenvironment as a result of metaflammation. Nutr. Metab. 2023, 20, 13. [Google Scholar] [CrossRef]
- Garcia, J.N.; Wanjalla, C.N.; Mashayekhi, M.; Hasty, A.H. Immune Cell Activation in Obesity and Cardiovascular Disease. Curr. Hypertens. Rep. 2022, 24, 627–637. [Google Scholar] [CrossRef]
- Rivera-Carranza, T.; Azaola-Espinosa, A.; Bojalil-Parra, R.; Zúñiga-León, E.; León-Téllez-Girón, A.; Rojano-Rodríguez, M.E.; Nájera-Medina, O. Immunometabolic Changes Following Gastric Bypass and Sleeve Gastrectomy: A Comparative Study. Obes. Surg. 2025, 35, 481–495. [Google Scholar] [CrossRef]
- Ruze, R.; Liu, T.; Zou, X.; Song, J.; Chen, Y.; Xu, R.; Yin, X.; Xu, Q. Obesity and type 2 diabetes mellitus: Connections in epidemiology, pathogenesis, and treatments. Front. Endocrinol. 2023, 14, 1161521. [Google Scholar] [CrossRef]
- Cuellar-Tamez, R.X.; Villarreal-Calderon, J.R.; Rubio-Infante, N.; Castillo, E.C.; García-Garza, M.; Elizondo-Montemayor, L.; García-Rivas, G. Bariatric surgery-induced weight loss reduces B cell activating cytokines and IgG immunoglobulins related to autoimmunity. Surg. Endosc. 2021, 35, 5147–5158. [Google Scholar] [CrossRef]
- Lindegaard, K.K.; Jorgensen, N.B.; Just, R.; Heegaard, P.M.H.; Madsbad, S. Effects of Roux-en-Y gastric bypass on fasting and postprandial inflammation-related parameters in obese subjects with normal glucose tolerance and in obese subjects with type 2 diabetes. Diabetol. Metab. Syndr. 2015, 7, 12. [Google Scholar] [CrossRef]
- Netto, B.D.; Bettini, S.C.; Clemente, A.P.; Ferreira, J.P.; Boritza, K.; Souza Sde, F.; Von der Heyde, M.E.; Earthman, C.P.; Dâmaso, A.R. Roux-en-Y gastric bypass decreases pro-inflammatory and thrombotic biomarkers in individuals with extreme obesity. Obes. Surg. 2015, 25, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Cunha, F.M.; Saavedra, A.; Barbosa, J.; Freitas, P.; Carvalho, D.; Varela, A. Effect of different bariatric surgery type on the leukocyte formula. Surg. Obes. Relat. Dis. 2016, 12, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Rizk, N.M.; Fadel, A.; AlShammari, W.; Younes, N.; Bashah, M. The immunophenotyping changes of peripheral CD4+ T lymphocytes and inflammatory markers of class III obesity subjects after laparoscopic gastric sleeve surgery–a follow-up study. J. Inflamm. Res. 2021, 1743–1757. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xia, B.; Wang, J.; Lyu, X.; Wang, Y.; Huang, W.; Tan, B.; Deng, H.; Yin, Y. Effect of roux-En-Y gastric bypass on the short-term immune function in patients with type 2 diabetes Mellitus. Zhonghua Wei Chang. Wai Ke Za Zhi=Chin. J. Gastrointest. Surg. 2016, 19, 312–316. [Google Scholar]
- Lylloff, L.; Bathum, L.; Madsbad, S.; Grundtvig, J.L.G.; Nordgaard-Lassen, I.; Fenger, M. S100A8/A9 (calprotectin), interleukin-6, and C-reactive protein in obesity and diabetes before and after Roux-en-Y gastric bypass surgery. Obes. Facts 2017, 10, 386–395. [Google Scholar] [CrossRef]
- Schmatz, R.; Bitencourt, M.R.; Patias, L.D.; Beck, M.; Alvarez, G.d.C.; Zanini, D.; Gutierres, J.M.; Diehl, L.N.; Pereira, L.B.; Leal, C.A. Evaluation of the biochemical, inflammatory and oxidative profile of obese patients given clinical treatment and bariatric surgery. Clin. Chim. Acta 2017, 465, 72–79. [Google Scholar] [CrossRef]
- Askarpour, M.; Khani, D.; Sheikhi, A.; Ghaedi, E.; Alizadeh, S. Effect of bariatric surgery on serum inflammatory factors of obese patients: A systematic review and meta-analysis. Obes. Surg. 2019, 29, 2631–2647. [Google Scholar] [CrossRef]
- Aasbrenn, M.; Farup, P.G.; Videm, V. Changes in C-reactive protein, neopterin and lactoferrin differ after conservative and surgical weight loss in individuals with morbid obesity. Sci. Rep. 2019, 9, 17695. [Google Scholar] [CrossRef]
- Rega-Kaun, G.; Kaun, C.; Ebenbauer, B.; Jaegersberger, G.; Prager, M.; Wojta, J.; Hohensinner, P.J. Bariatric surgery in morbidly obese individuals affects plasma levels of protein C and thrombomodulin. J. Thromb. Thrombolysis 2019, 47, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, J.; Ázaro, E.; Weiner, R.; Higa, K.D.; Neto, M.G.; Teixeira, A.F.; Jawad, M. Gastric Bypass: Bariatric and metabolic Surgery Perspectives; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Zhang, J.; Chen, X.; Liu, W.; Zhang, C.; Xiang, Y.; Liu, S.; Zhou, Z. Metabolic surgery improves the unbalanced proportion of peripheral blood myeloid dendritic cells and T lymphocytes in obese patients. Eur. J. Endocrinol. 2021, 185, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Montecucco, F.; Lenglet, S.; Gayet-Ageron, A.; Bertolotto, M.; Pelli, G.; Palombo, D.; Pane, B.; Spinella, G.; Steffens, S.; Raffaghello, L.; et al. Systemic and intraplaque mediators of inflammation are increased in patients symptomatic for ischemic stroke. Stroke 2010, 41, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Irani, M.; Merhi, Z. Role of vitamin D in ovarian physiology and its implication in reproduction: A systematic review. Fertil. Steril. 2014, 102, 460–468.e463. [Google Scholar] [CrossRef] [PubMed]
- Fathy, S.M.; Morshed, G. Peripheral blood lymphocyte subsets (CD4+, CD8+ T cells), leptin level and weight loss after laparoscopic greater curvature plication in morbidly obese patients. Arch. Med. Sci. 2014, 10, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Moulin, C.M.; Marguti, I.; Peron, J.P.; Halpern, A.; Rizzo, L.V. Bariatric surgery reverses natural killer (NK) cell activity and NK-related cytokine synthesis impairment induced by morbid obesity. Obes. Surg. 2011, 21, 112–118. [Google Scholar] [CrossRef]
- Dixon, J.B.; O’Brien, P.E. Obesity and the White Blood Cell Count: Changes with Sustained Weight Loss. Obes. Surg. 2006, 16, 251–257. [Google Scholar] [CrossRef]
- Nuttall, F.Q. Body mass index: Obesity, BMI, and health: A critical review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef]
- Touch, S.; Clément, K.; André, S. T Cell Populations and Functions Are Altered in Human Obesity and Type 2 Diabetes. Curr. Diab Rep. 2017, 17, 81. [Google Scholar] [CrossRef]
- Tobón, G.J.; Ospina, F.E.; Suso, J.P.; Posso-Osorio, I.; Echeverri, A.F.; Muñoz-Buitrón, E.; Martínez, J.D.; Castaño, G.L.; Agualimpia, A.; Bonilla-Abadía, F.; et al. Autoantibodies production and immunological abnormalities after bariatric surgery. J. Transl. Autoimmun. 2019, 2, 100024. [Google Scholar] [CrossRef]
- Lee, M.; Song, S.J.; Choi, M.S.; Yu, R.; Park, T. IL-7 receptor deletion ameliorates diet-induced obesity and insulin resistance in mice. Diabetologia 2015, 58, 2361–2370. [Google Scholar] [CrossRef]
- Lucas, S.; Taront, S.; Magnan, C.; Fauconnier, L.; Delacre, M.; Macia, L.; Delanoye, A.; Verwaerde, C.; Spriet, C.; Saule, P.; et al. Interleukin-7 regulates adipose tissue mass and insulin sensitivity in high-fat diet-fed mice through lymphocyte-dependent and independent mechanisms. PLoS ONE 2012, 7, e40351. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wijngaarden, L.H.; Taselaar, A.E.; Nuijten, F.; van der Harst, E.; Klaassen, R.A.; Kuijper, T.M.; Jongbloed, F.; Ambagtsheer, G.; Klepper, M.; IJzermans , J.N.M.; et al. T and B Cell Composition and Cytokine Producing Capacity Before and After Bariatric Surgery. Front. Immunol. 2022, 13, 888278. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.Y.; Deng, Y.; Wen, Y.L.; Cheng, Y.Q.; Li, K.X.; Chen, H.P. Chronic low-grade inflammation is involved in TLR4 knockout-induced spontaneous obesity in aged mice. Biomed. Pharmacother. 2022, 147, 112637. [Google Scholar] [CrossRef]
- Dai, X.; Zhao, W.; Zhan, J.; Zeng, S.; Ran, D.; Zhang, H.; Song, Z.; Song, K.H.; Wu, L. B cells present skewed profile and lose the function of supporting T cell inflammation after Roux-en-Y gastric bypass. Int. Immunopharmacol. 2017, 43, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Cottam, M.A.; Caslin, H.L.; Winn, N.C.; Hasty, A.H. Multiomics reveals persistence of obesity-associated immune cell phenotypes in adipose tissue during weight loss and weight regain in mice. Nat. Commun. 2022, 13, 2950. [Google Scholar] [CrossRef] [PubMed]
- Kavyani, Z.; Najafi, K.; Naghsh, N.; Karvane, H.B.; Musazadeh, V. The effects of curcumin supplementation on biomarkers of inflammation, oxidative stress, and endothelial function: A meta-analysis of meta-analyses. Prostaglandins Other Lipid Mediat. 2024, 174, 106867. [Google Scholar] [CrossRef]
- Dehzad, M.J.; Ghalandari, H.; Nouri, M.; Askarpour, M. Effects of curcumin/turmeric supplementation on obesity indices and adipokines in adults: A grade-assessed systematic review and dose-response meta-analysis of randomized controlled trials. Phytother. Res. 2023, 37, 1703–1728. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, P.; Venkatesan, K.; Abdulla Khan, N.; Raghavendra, N.M.; Venugopal, V.; Bharathi, D.R.; Fuloria, N.K. Mechanistic insights into the beneficial effects of curcumin on insulin resistance: Opportunities and challenges. Drug Discov. Today 2023, 28, 103627. [Google Scholar] [CrossRef]
- Islam, T.; Scoggin, S.; Gong, X.; Zabet-Moghaddam, M.; Kalupahana, N.S.; Moustaid-Moussa, N. Anti-Inflammatory Mechanisms of Curcumin and Its Metabolites in White Adipose Tissue and Cultured Adipocytes. Nutrients 2023, 16, 70. [Google Scholar] [CrossRef]
- Zhu, J.; He, L. The Modulatory Effects of Curcumin on the Gut Microbiota: A Potential Strategy for Disease Treatment and Health Promotion. Microorganisms 2024, 12, 642. [Google Scholar] [CrossRef]
- Bahramzadeh, A.; Bolandnazar, K.; Meshkani, R. Resveratrol as a potential protective compound against skeletal muscle insulin resistance. Heliyon 2023, 9, e21305. [Google Scholar] [CrossRef]
- Gong, W.; Sun, P.; Li, X.; Wang, X.; Zhang, X.; Cui, H.; Yang, J. Investigating the Molecular Mechanisms of Resveratrol in Treating Cardiometabolic Multimorbidity: A Network Pharmacology and Bioinformatics Approach with Molecular Docking Validation. Nutrients 2024, 16, 2488. [Google Scholar] [CrossRef]
- Ren, Z.Q.; Zheng, S.Y.; Sun, Z.; Luo, Y.; Wang, Y.T.; Yi, P.; Li, Y.S.; Huang, C.; Xiao, W.F. Resveratrol: Molecular Mechanisms, Health Benefits, and Potential Adverse Effects. MedComm 2025, 6, e70252. [Google Scholar] [CrossRef] [PubMed]
- Kamińska-Omasta, K.; Omasta, B.; Romańczuk, K.; Krupa, O.; Pietrukaniec, P.; Wójcik, Z.; Furtak, K.; Stolarczyk, S.; Czerska, M.; Rybak, D. Berberine in obesity therapy: From molecular mechanisms to clinical applications. Qual. Sport 2025, 38, 58195. [Google Scholar] [CrossRef]
- Och, A.; Och, M.; Nowak, R.; Podgórska, D.; Podgórski, R. Berberine, a Herbal Metabolite in the Metabolic Syndrome: The Risk Factors, Course, and Consequences of the Disease. Molecules 2022, 27, 1351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting cellular senescence with senotherapeutics: Senolytics and senomorphics. FEBS J. 2023, 290, 1362–1383. [Google Scholar] [CrossRef] [PubMed]
- Nga, H.T.; Nguyen, T.L.; Yi, H.S. T-Cell Senescence in Human Metabolic Diseases. Diabetes Metab. J. 2024, 48, 864–881. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huo, T.; Lu, M.; Zhao, Y.; Zhang, J.; He, W.; Chen, H. Recent Advances in Aging and Immunosenescence: Mechanisms and Therapeutic Strategies. Cells 2025, 14, 499. [Google Scholar] [CrossRef]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome–brain–β-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef]
- Vetrani, C.; Di Nisio, A.; Paschou, S.A.; Barrea, L.; Muscogiuri, G.; Graziadio, C.; Savastano, S.; Colao, A.; On Behalf of The Obesity Programs of Nutrition Education Research and Assessment Opera Group. From Gut Microbiota through Low-Grade Inflammation to Obesity: Key Players and Potential Targets. Nutrients 2022, 14, 2103. [Google Scholar] [CrossRef]
- Sun, L.J.; Li, J.N.; Nie, Y.Z. Gut hormones in microbiota-gut-brain cross-talk. Chin. Med. J. 2020, 133, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Shastri, N. The Role of T Cells in Obesity-Associated Inflammation and Metabolic Disease. Immune Netw. 2022, 22, e13. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, W.; Wang, Y. Capsaicin Reduces Obesity by Reducing Chronic Low-Grade Inflammation. Int. J. Mol. Sci. 2024, 25, 8979. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Verma, R.K.; Alpini, G.; Meng, F.; Kumar, N. Role of Janus Kinase 3 in Predisposition to Obesity-associated Metabolic Syndrome. J. Biol. Chem. 2015, 290, 29301–29312. [Google Scholar] [CrossRef]
- Yang, C.; Camargo Tavares, L.; Lee, H.-C.; Steele, J.R.; Ribeiro, R.V.; Beale, A.L.; Yiallourou, S.; Carrington, M.J.; Kaye, D.M.; Head, G.A. Faecal metaproteomics analysis reveals a high cardiovascular risk profile across healthy individuals and heart failure patients. Gut Microbes 2025, 17, 2441356. [Google Scholar] [CrossRef]
- Biemann, R.; Buß, E.; Benndorf, D.; Lehmann, T.; Schallert, K.; Püttker, S.; Reichl, U.; Isermann, B.; Schneider, J.G.; Saake, G.; et al. Fecal Metaproteomics Reveals Reduced Gut Inflammation and Changed Microbial Metabolism Following Lifestyle-Induced Weight Loss. Biomolecules 2021, 11, 726. [Google Scholar] [CrossRef]
- Kang, Y.; Cai, Y. Gut microbiota and obesity: Implications for fecal microbiota transplantation therapy. Hormones 2017, 16, 223–234. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.; Li, Y.; Bai, G.; Pang, J.; Wu, M.; Li, J.; Zhao, X.; Xia, Y. Implications of gut microbiota-mediated epigenetic modifications in intestinal diseases. Gut Microbes 2025, 17, 2508426. [Google Scholar] [CrossRef]
- Shaikh, S.R.; Beck, M.A.; Alwarawrah, Y.; MacIver, N.J. Emerging mechanisms of obesity-associated immune dysfunction. Nat. Rev. Endocrinol. 2024, 20, 136–148. [Google Scholar] [CrossRef]
- Gkrinia, E.M.M.; Belančić, A. The Mechanisms of Chronic Inflammation in Obesity and Potential Therapeutic Strategies: A Narrative Review. Curr. Issues Mol. Biol. 2025, 47, 357. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.; Lehne, C.; Weiland, A.; Archid, R.; Ritze, Y.; Bauer, K.; Zipfel, S.; Penders, J.; Enck, P.; Mack, I. Gut Microbiota, Probiotics and Psychological States and Behaviors after Bariatric Surgery-A Systematic Review of Their Interrelation. Nutrients 2020, 12, 2396. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, B.A. Adipose Tissue-Derived Mesenchymal Stem Cells. Cells 2021, 10, 3433. [Google Scholar] [CrossRef] [PubMed]
- Dewal, R.S.; Yang, F.T.; Baer, L.A.; Vidal, P.; Hernandez-Saavedra, D.; Seculov, N.P.; Ghosh, A.; Noé, F.; Togliatti, O.; Hughes, L.; et al. Transplantation of committed pre-adipocytes from brown adipose tissue improves whole-body glucose homeostasis. iScience 2024, 27, 108927. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Song, Y.; Zhang, B.; Cao, G.; Zhou, H.; Li, H.; Sun, H.; Deng, M.; Qiu, Y.; Yi, W.; et al. Progress and application of adipose-derived stem cells in the treatment of diabetes and its complications. Stem Cell Res. Ther. 2024, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Gunawardana, S. Brown Adipose Tissue Transplantation. Methods Mol. Biol. 2023, 2662, 193–202. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Cauli, O.; Cabrera-Pastor, A. Obesity and Adipose-Derived Extracellular Vesicles: Implications for Metabolic Regulation and Disease. Biomolecules 2025, 15, 231. [Google Scholar] [CrossRef]
- Al-Sammarraie, S.H.A.; Ayaz-Güner, Ş.; Acar, M.B.; Şimşek, A.; Sınıksaran, B.S.; Bozalan, H.D.; Özkan, M.; Saraymen, R.; Dündar, M.; Özcan, S. Mesenchymal stem cells from adipose tissue prone to lose their stemness associated markers in obesity related stress conditions. Sci. Rep. 2024, 14, 19702. [Google Scholar] [CrossRef]
- Zhou, B.; Chen, Q.; Zhang, Q.; Tian, W.; Chen, T.; Liu, Z. Therapeutic potential of adipose-derived stem cell extracellular vesicles: From inflammation regulation to tissue repair. Stem Cell Res. Ther. 2024, 15, 249. [Google Scholar] [CrossRef]
- Rodriguez-Muñoz, A.; Motahari-Rad, H.; Martin-Chaves, L.; Benitez-Porres, J.; Rodriguez-Capitan, J.; Gonzalez-Jimenez, A.; Insenser, M.; Tinahones, F.J.; Murri, M. A Systematic Review of Proteomics in Obesity: Unpacking the Molecular Puzzle. Curr. Obes. Rep. 2024, 13, 403–438. [Google Scholar] [CrossRef]
- Mauney, E.E.; Wibowo, M.C.; Tseng, Y.-H.; Kostic, A.D. Adipose tissue–gut microbiome crosstalk in inflammation and thermogenesis. Trends Endocrinol. Metab. 2025, 36, 721–732. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Carranza, T.; León-Téllez-Girón, A.; González-Vázquez, R.; Vázquez-Cárdenas, P.; Esquivel-Campos, A.L.; Mendoza-Pérez, F.; Rojano-Rodríguez, M.E.; Mimiaga-Hernández, C.; Cifuentes-Goches, J.C.; Peralta-Valle, O.E.; et al. Cellular Immunity in Obesity: Pathophysiological Insights and the Impact of Bariatric Surgery. Int. J. Mol. Sci. 2025, 26, 9867. https://doi.org/10.3390/ijms26209867
Rivera-Carranza T, León-Téllez-Girón A, González-Vázquez R, Vázquez-Cárdenas P, Esquivel-Campos AL, Mendoza-Pérez F, Rojano-Rodríguez ME, Mimiaga-Hernández C, Cifuentes-Goches JC, Peralta-Valle OE, et al. Cellular Immunity in Obesity: Pathophysiological Insights and the Impact of Bariatric Surgery. International Journal of Molecular Sciences. 2025; 26(20):9867. https://doi.org/10.3390/ijms26209867
Chicago/Turabian StyleRivera-Carranza, Tania, Angélica León-Téllez-Girón, Raquel González-Vázquez, Paola Vázquez-Cárdenas, Ana Laura Esquivel-Campos, Felipe Mendoza-Pérez, Martín E. Rojano-Rodríguez, Claudia Mimiaga-Hernández, Juan Carlos Cifuentes-Goches, Omar Edgar Peralta-Valle, and et al. 2025. "Cellular Immunity in Obesity: Pathophysiological Insights and the Impact of Bariatric Surgery" International Journal of Molecular Sciences 26, no. 20: 9867. https://doi.org/10.3390/ijms26209867
APA StyleRivera-Carranza, T., León-Téllez-Girón, A., González-Vázquez, R., Vázquez-Cárdenas, P., Esquivel-Campos, A. L., Mendoza-Pérez, F., Rojano-Rodríguez, M. E., Mimiaga-Hernández, C., Cifuentes-Goches, J. C., Peralta-Valle, O. E., Zúñiga-León, E., & Bojalil-Parra, R. (2025). Cellular Immunity in Obesity: Pathophysiological Insights and the Impact of Bariatric Surgery. International Journal of Molecular Sciences, 26(20), 9867. https://doi.org/10.3390/ijms26209867