Biofilm and Outer Membrane Vesicle Formation in ESKAPE Gram-Negative Bacteria: A Comprehensive Review
Abstract
1. Introduction
2. Biofilms in Gram-Negative ESKAPE Bacteria
2.1. The Main Aspects of Biofilm Formation
2.2. Biofilm Formation in A. baumannii
2.3. Biofilm Formation in P. aeruginosa
2.4. Biofilm Formation in K. pneumoniae
2.5. Biofilm Formation in Enterobacter spp.
3. OMVs in Gram-Negative ESKAPE Bacteria
3.1. Key Aspects of OMV Formation and the Functions of OMVs
Biomedical and Clinical Applications of OMVs
3.2. OMV Production in A. baumannii
3.3. OMV Production in P. aeruginosa
3.4. OMV Production in K. pneumoniae
3.5. OMV Production in Enterobacter spp.
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AbaI | autoinducer synthase |
| AbaR | receptor protein activator AbaR |
| AHLs | N-acyl-homoserine lactones |
| AMG | aminoglycosides |
| AmpC | Ambler class C β-lactamases |
| AMR | antimicrobial resistance |
| ARGs | antimicrobial resistance genes |
| Bap | biofilm-associated proteins |
| c-di-GMP | c-di-guanosine monophosphate |
| CFTR | cystic fibrosis transmembrane conductance regulator |
| COVID-19 | coronavirus disease |
| CPA | common polysaccharide antigen |
| CTX-M-15 | cefotaxime Munich-15 extended spectrum β-lactamase |
| DNA | deoxyribonucleic acid |
| DRP1 | GTPase dynamin-related protein 1 |
| ECP | Escherichia coli common pilus |
| eDNA | extracellular DNA |
| EDTA | ethylenediaminetetraacetic acid |
| EPS | extracellular polymeric substances |
| ESBL | extended-spectrum β-lactamase |
| ESKAPE | Enterococcus faecium (E. faecium), Staphylococcus aureus (S. aureus), Klebsiella pneumoniae (K. pneumoniae), Acetinobacter baumannii (A. baumannii), Pseudomona aeruginosa (P. aeruginosa), Enterobacter spp. |
| FQs | fluoroquinolones |
| Fur | ferric uptake regulator |
| GDP | gross domestic product |
| ICU | intensive care unit |
| IQR | interquartile range |
| KPC-2 | K. pneumoniae carbapenemase |
| KpHCD1 | K. pneumoniae strain HCD1 |
| LOS | lipooligosaccharide |
| Lpps | lipoproteins |
| LPSs | lipopolysaccharides |
| MDR | multi-drug resistance |
| MFS | major facilitator superfamily |
| miRNA | microRNA |
| mRNA | messenger ribonucleic acid |
| MRSA | meticillin-resistant Staphylococcus aureus |
| NDM-1 | New Delhi metallo-β-lactamase-1 |
| OIMVs | outer-inner membrane vesicles |
| OM | outer membrane |
| OMPs | outer membrane proteins |
| OMVs | outer membrane vesicles |
| OXA | oxacillinase |
| PAMPs | pathogen-associated molecular patterns |
| PBP | penicillin-binding protein |
| Pel | pellicle polysaccharide |
| PER-1 | P. aeruginosa extended resistance β-lactamase |
| PG | peptidoglycan |
| PHA | polyhydroxyalkanoate |
| PLs | phospholipids |
| PNAG | poly-β-(1-6)-N-acetylglucosamine |
| PQS | Pseudomonas Quinolone Signal |
| PRRs | pattern recognition receptors |
| Psl | polysaccharide synthesis locus |
| PTK | putative protein tyrosine kinase |
| QS | quorum sensing |
| RND | resistance-nodulation-division |
| ROS | reactive oxygen species |
| SHV | sulfhydryl variable lactamase |
| SMR | small multi-antibiotic resistance |
| T4P | type IV pili |
| TCSs | two-component systems |
| TEM1 | Temoneira-1 extended spectrum β-lactamase |
| VAP | ventilator-associated pneumonia |
| VIM | Verona Integron-Borne Metallo-β-Lactamase |
| WHO | World Health Organization |
References
- National Action Plans and Monitoring and Evaluation (NPM). Implementing the Global Action Plan on Antimicrobial Resistance: First Quadripartite Biennial Report; World Health Organization: Geneva, Switzerland; Food and Agriculture Organization of the United Nations: Rome, Italy; United Nations Environment Programme and World Organisation for Animal Health: Nairobi, Kenya, 2023. [Google Scholar]
- The Global Health Observatory. Proportion of Bloodstream Infection due to Methicillin-Resistant Staphylococcus aureus (MRSA) (%): World Health Statistics. Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/sdg-3.d.2-amr-infect-mrsa (accessed on 26 November 2023).
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655, Erratum in Lancet 2022, 400, 1102. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Chung, P.; Adam, H.; Zelenitsky, S.; Denisuik, A.; Schweizer, F.; Lagacé-Wiens, P.R.; Rubinstein, E.; Gin, A.S.; Walkty, A.; et al. Ceftolozane/tazobactam: A novel cephalosporin/β-lactamase inhibitor combination with activity against multidrug-resistant gram-negative bacilli. Drugs 2014, 74, 31–51. [Google Scholar] [CrossRef]
- Berthe, F.C.J.; Irwin, A.; Jonas, O.B.; Le Gall, F.G.; Marquez, P.V. Drug-Resistant Infections: A Threat to Our Economic Future; World Bank: Washington, DC, USA, 2017. [Google Scholar]
- FAO. Government of Uganda and Stakeholders Commit to Tackling Tick Vector Related Challenges in Uganda; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018; Available online: https://www.fao.org/uganda/news/detail-events/es/c/1151610 (accessed on 9 January 2023).
- Aloke, C.; Achilonu, I. Coping with the ESKAPE pathogens: Evolving strategies, challenges and future prospects. Microb. Pathog. 2023, 175, 105963. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.B. Progress and challenges in implementing the research on ESKAPE pathogens. Infect. Control Hosp. Epidemiol. 2010, 31 (Suppl. S1), S7–S10. [Google Scholar] [CrossRef] [PubMed]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef]
- World Health Organization. Naming the Coronavirus Disease (COVID-19) and the Virus That Causes It. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it (accessed on 31 May 2024).
- Sakalauskienė, G.V.; Malcienė, L.; Stankevičius, E.; Radzevičienė, A. Unseen Enemy: Mechanisms of Multidrug Antimicrobial Resistance in Gram-Negative ESKAPE Pathogens. Antibiotics 2025, 14, 63. [Google Scholar] [CrossRef]
- Sulayyim, H.J.A.; Ismail, R.; Hamid, A.A.; Ghafar, N.A. Antibiotic Resistance during COVID-19: A Systematic Review. Int. J. Environ. Res. Public. Health 2022, 19, 11931. [Google Scholar] [CrossRef]
- One Health Trust. ResistanceMap: Antibiotic Resistance. Available online: https://resistancemap.onehealthtrust.org/CountryPage.php?country=India (accessed on 31 May 2024).
- Makena, A.; Düzgün, A.; Brem, J.; McDonough, M.A.; Rydzik, A.M.; Abboud, M.I.; Saral, A.; Çiçek, A.; Sandalli, C.; Schofield, C.J. Comparison of Verona Integron-Borne Metallo-β-Lactamase (VIM) Variants Reveals Differences in Stability and Inhibition Profiles. Antimicrob. Agents Chemother. 2015, 60, 1377–1384. [Google Scholar] [CrossRef]
- Sawa, T.; Kooguchi, K.; Moriyama, K. Molecular diversity of extended-spectrum β-lactamases and carbapenemases, and antimicrobial resistance. J. Intensive Care 2020, 8, 13. [Google Scholar] [CrossRef]
- Mullié, C.; Lemonnier, D.; Adjidé, C.C.; Maizel, J.; Mismacque, G.; Cappe, A.; Carles, T.; Pierson-Marchandise, M.; Zerbib, Y. Nosocomial outbreak of monoclonal VIM carbapenemase-producing Enterobacter cloacae complex in an intensive care unit during the COVID-19 pandemic: An integrated approach. J. Hosp. Infect. 2022, 120, 48–56. [Google Scholar] [CrossRef]
- Miltgen, G.; Garrigos, T.; Cholley, P.; Deleume, M.; Allou, N.; Allyn, J.; Wilkinson, D.A.; Lugagne, N.; Belmonte, O.; Bertrand, X.; et al. Nosocomial cluster of carbapenemase-producing Enterobacter cloacae in an intensive care unit dedicated COVID-19. Antimicrob. Resist. Infect. Control 2021, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Sun, Z.; Yang, Y.; Zhu, D.; Chen, Z.; Hu, F.; Xie, Y.; Kang, M.; Zhang, F.; Ji, P.; et al. Changing antimicrobial resistance profile of Enterobacter spp. isolates in hospitals across China: A seven-year analysis from the CHINET antimicrobial resistance surveillance program (2015–2021). One Health Adv. 2024, 2, 11. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, T.; Liu, Z. Molecular epidemiology and risk factors for carbapenem-resistant Enterobacteriaceae infections during 2020–2021 in Northwest China. Microb. Pathog. 2024, 106728. [Google Scholar] [CrossRef] [PubMed]
- Hafiz, T.A.; Albloshi, A.; Alhumaidan, O.S.; Mubaraki, M.A.; Alyami, A.S.; Alrashoudi, R.; Alrabiah, M.A.; Alotaibi, F. The Epidemiological Pattern, Resistance Characteristics and Clinical Outcome of Enterobacter cloacae: Recent Updates and Impact of COVID-19 Pandemic. Healthcare 2023, 11, 312. [Google Scholar] [CrossRef]
- Kariyawasam, R.M.; Julien, D.A.; Jelinski, D.C.; Larose, S.L.; Rennert-May, E.; Conly, J.M.; Dingle, T.C.; Chen, J.Z.; Tyrrell, G.J.; Ronksley, P.E.; et al. Antimicrobial resistance (AMR) in COVID-19 patients: A systematic review and meta-analysis (November 2019–June 2021). Antimicrob. Resist. Infect. Control 2022, 11, 45. [Google Scholar] [CrossRef]
- Abdelaziz Abdelmoneim, S.; Mohamed Ghazy, R.; Anwar Sultan, E.; Hassaan, M.A.; Anwar Mahgoub, M. Antimicrobial resistance burden pre and post-COVID-19 pandemic with mapping the multidrug resistance in Egypt: A comparative cross-sectional study. Sci. Rep. 2024, 14, 7176. [Google Scholar] [CrossRef]
- Cureño-Díaz, M.A.; Plascencia-Nieto, E.S.; Loyola-Cruz, M.Á.; Cruz-Cruz, C.; Nolasco-Rojas, A.E.; Durán-Manuel, E.M.; Ibáñez-Cervantes, G.; Gómez-Zamora, E.; Tamayo-Ordóñez, M.C.; Tamayo-Ordóñez, Y.d.J.; et al. Gram-Negative ESKAPE Bacteria Surveillance in COVID-19 Pandemic Exposes High-Risk Sequence Types of Acinetobacter baumannii MDR in a Tertiary Care Hospital. Pathogens 2024, 13, 50. [Google Scholar] [CrossRef]
- López-Jácome, L.E.; Fernández-Rodríguez, D.; Franco-Cendejas, R.; Camacho-Ortiz, A.; Morfin-Otero, M.D.R.; Rodríguez-Noriega, E.; Ponce-de-León, A.; Ortiz-Brizuela, E.; Rojas-Larios, F.; Velázquez-Acosta, M.D.C.; et al. Increment Antimicrobial Resistance During the COVID-19 Pandemic: Results from the Invifar Network. Microb. Drug Resist. 2022, 28, 338–345. [Google Scholar]
- Langford, B.J.; Soucy, J.R.; Leung, V.; So, M.; Kwan, A.T.H.; Portnoff, J.S.; Bertagnolio, S.; Raybardhan, S.; MacFadden, D.R.; Daneman, N. Antibiotic resistance associated with the COVID-19 pandemic: A systematic review and meta-analysis. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2023, 29, 302–309. [Google Scholar] [CrossRef]
- Ibáñez-Cervantes, G.; Cruz-Cruz, C.; Durán-Manuel, E.M.; Loyola-Cruz, M.Á.; Cureño-Díaz, M.A.; Castro-Escarpulli, G.; Lugo-Zamudio, G.E.; Rojo-Gutiérrez, M.I.; Razo-Blanco Hernández, D.M.; López-Ornelas, A.; et al. Disinfection efficacy of ozone on ESKAPE bacteria biofilms: Potential use in difficult-to-access medical devices. Am. J. Infect. Control 2023, 51, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Durán-Manuel, E.M.; Loyola-Cruz, M.; Cruz-Cruz, C.; Ibáñez-Cervantes, G.; Gaytán-Cervantes, J.; González-Torres, C.; Quiroga-Vargas, E.; Calzada-Mendoza, C.C.; Cureño-Díaz, M.A.; Fernández-Sánchez, V.; et al. Massive sequencing of the V3-V4 hypervariable region of bronchoalveolar lavage from patients with COVID-19 and VAP reveals the collapse of the pulmonary microbiota. J. Med. Microbiol. 2022, 71, 001634. [Google Scholar] [CrossRef] [PubMed]
- Loyola-Cruz, M.; Durán-Manuel, E.M.; Cruz-Cruz, C.; Márquez-Valdelamar, L.M.; Bravata-Alcántara, J.C.; Cortés-Ortíz, I.A.; Cureño-Díaz, M.A.; Ibáñez-Cervantes, G.; Fernández-Sánchez, V.; Castro-Escarpulli, G.; et al. ESKAPE bacteria characterization reveals the presence of Acinetobacter baumannii and Pseudomonas aeruginosa outbreaks in COVID-19/VAP patients. Am. J. Infect. Control 2023, 51, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Hernández, O.; Matías-Téllez, B.; Estrada-Hernández, A.; Cureño-Díaz, M.A.; Bello-López, J.M. Incidence and costs of ventilator-associated pneumonia in the adult intensive care unit of a tertiary referral hospital in Mexico. Am. J. Infect. Control 2019, 47, e21–e25. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Olivadese, V.; Trecarichi, E.M.; Torti, C. Bacterial Ventilator-Associated Pneumonia in COVID-19 Patients: Data from the Second and Third Waves of the Pandemic. J. Clin. Med. 2022, 11, 2279. [Google Scholar] [CrossRef]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. BioMed Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef]
- Kim, J.Y.; Suh, J.W.; Kang, J.S.; Kim, S.B.; Yoon, Y.K.; Sohn, J.W. Gram-Negative Bacteria’s Outer Membrane Vesicles. Infect. Chemother. 2023, 55, 1–9. [Google Scholar] [CrossRef]
- Sharma, G.; Rao, S.; Bansal, A.; Dang, S.; Gupta, S.; Gabrani, R. Pseudomonas aeruginosa biofilm: Potential therapeutic targets. Biol. J. Int. Assoc. Biol. Stand. 2014, 42, 1–7. [Google Scholar] [CrossRef]
- Guerra, M.E.S.; Destro, G.; Vieira, B.; Lima, A.S.; Ferraz, L.F.C.; Hakansson, A.P.; Darrieux, M.; Converso, T.R. Klebsiella pneumoniae Biofilms and Their Role in Disease Pathogenesis. Front. Cell. Infect. Microbiol. 2022, 12, 877995, Erratum in Front. Cell. Infect. Microbiol. 2022, 15, 1564010. [Google Scholar] [CrossRef]
- Sartorio, M.G.; Pardue, E.J.; Feldman, M.F.; Haurat, M.F. Bacterial Outer Membrane Vesicles: From Discovery to Applications. Annu. Rev. Microbiol. 2021, 75, 609–630. [Google Scholar] [CrossRef]
- Sakalauskienė, G.V.; Radzevičienė, A. Antimicrobial Resistance: What Lies Beneath This Complex Phenomenon? Diagnostics 2024, 14, 2319. [Google Scholar] [CrossRef]
- Zhao, A.; Sun, J.; Liu, Y. Understanding bacterial biofilms: From definition to treatment strategies. Front. Cell. Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef] [PubMed]
- Markowska, K.; Szymanek-Majchrzak, K.; Pituch, H.; Majewska, A. Understanding Quorum-Sensing and Biofilm Forming in Anaerobic Bacterial Communities. Int. J. Mol. Sci. 2024, 25, 12808. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, U.K.B.; Ballard, J.D. Autoinducing peptide-based quorum signaling systems in Clostridioides difficile. Curr. Opin. Microbiol. 2022, 65, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Muras, A.; Mallo, N.; Otero-Casal, P.; Pose-Rodríguez, J.M.; Otero, A. Quorum sensing systems as a new target to prevent biofilm-related oral diseases. Oral Dis. 2022, 28, 307–313. [Google Scholar] [CrossRef]
- Abisado, R.G.; Benomar, S.; Klaus, J.R.; Dandekar, A.A.; Chandler, J.R. Bacterial Quorum Sensing and Microbial Community Interactions. mBio 2018, 9, e02331-17, Erratum in mBio 2018, 9, e01749-18. [Google Scholar] [CrossRef]
- Gunaratnam, S.; Millette, M.; McFarland, L.V.; DuPont, H.L.; Lacroix, M. Potential role of probiotics in reducing Clostridioides difficile virulence: Interference with quorum sensing systems. Microb. Pathog. 2021, 153, 104798. [Google Scholar] [CrossRef]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef]
- Fteita, D.; Könönen, E.; Gürsoy, M.; Ma, X.; Sintim, H.O.; Gürsoy, U.K. Quorum sensing molecules regulate epithelial cytokine response and biofilm-related virulence of three Prevotella species. Anaerobe 2018, 54, 128–135. [Google Scholar] [CrossRef]
- Paluch, E.; Rewak-Soroczyńska, J.; Jędrusik, I.; Mazurkiewicz, E.; Jermakow, K. Prevention of biofilm formation by quorum quenching. Appl. Microbiol. Biotechnol. 2020, 104, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.P.; Ramachandra, S.S. Quorum Sensing and Quorum Quenching with a Focus on Cariogenic and Periodontopathic Oral Biofilms. Microorganisms 2022, 10, 1783. [Google Scholar] [CrossRef] [PubMed]
- Scheres, N.; Lamont, R.J.; Crielaard, W.; Krom, B.P. LuxS signaling in Porphyromonas gingivalis-host interactions. Anaerobe 2015, 35 Pt A, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Schooling, S.R.; Beveridge, T.J. Membrane vesicles: An overlooked component of the matrices of biofilms. J. Bacteriol. 2006, 188, 5945–5957. [Google Scholar] [CrossRef]
- Grande, R.; Di Marcantonio, M.C.; Robuffo, I.; Pompilio, A.; Celia, C.; Di Marzio, L.; Paolino, D.; Codagnone, M.; Muraro, R.; Stoodley, P.; et al. Helicobacter pylori ATCC 43629/NCTC 11639 Outer Membrane Vesicles (OMVs) from Biofilm and Planktonic Phase Associated with Extracellular DNA (eDNA). Front. Microbiol. 2015, 6, 1369. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, L.; Miao, J.; Zhang, Z.; Ruan, J.; Xu, L.; Guo, H.; Zhang, M.; Qiao, W. Regulation of the formation and structure of biofilms by quorum sensing signal molecules packaged in outer membrane vesicles. Sci. Total Environ. 2022, 806 Pt 4, 151403. [Google Scholar] [CrossRef]
- Kanno, M.; Shiota, T.; Ueno, S.; Takahara, M.; Haneda, K.; Tahara, Y.O.; Shintani, M.; Nakao, R.; Miyata, M.; Kimbara, K.; et al. Identification of genes involved in enhanced membrane vesicle formation in Pseudomonas aeruginosa biofilms: Surface sensing facilitates vesiculation. Front. Microbiol. 2023, 14, 1252155. [Google Scholar] [CrossRef]
- Cooke, A.C.; Florez, C.; Dunshee, E.B.; Lieber, A.D.; Terry, M.L.; Light, C.J.; Schertzer, J.W. Pseudomonas Quinolone Signal-Induced Outer Membrane Vesicles Enhance Biofilm Dispersion in Pseudomonas aeruginosa. mSphere 2020, 5, e01109-20. [Google Scholar] [CrossRef]
- Chen, N.; Li, Y.; Liang, X.; Qin, K.; Zhang, Y.; Wang, J.; Wu, Q.; Gupta, T.B.; Ding, Y. Bacterial extracellular vesicle: A non-negligible component in biofilm life cycle and challenges in biofilm treatments. Biofilm 2024, 8, 100216. [Google Scholar] [CrossRef]
- Esoda, C.N.; Kuehn, M.J. Pseudomonas aeruginosa Leucine Aminopeptidase Influences Early Biofilm Composition and Structure via Vesicle-Associated Antibiofilm Activity. mBio 2019, 10, e02548-19. [Google Scholar] [CrossRef]
- Chiș, A.A.; Rus, L.L.; Morgovan, C.; Arseniu, A.M.; Frum, A.; Vonica-Țincu, A.L.; Gligor, F.G.; Mureșan, M.L.; Dobrea, C.M. Microbial Resistance to Antibiotics and Effective Antibiotherapy. Biomedicines 2022, 10, 1121. [Google Scholar] [CrossRef]
- Abebe, G.M. The Role of Bacterial Biofilm in Antibiotic Resistance and Food Contamination. Int. J. Microbiol. 2020, 2020, 1705814. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, Y.; Ichikawa, S.; Nakajima-Kambe, T.; Uchiyama, H.; Nomura, N. Pseudomonas quinolone signal affects membrane vesicle production in not only gram-negative but also gram-positive bacteria. Microbes Environ. 2010, 25, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Baumgarten, T.; Sperling, S.; Seifert, J.; von Bergen, M.; Steiniger, F.; Wick, L.Y.; Heipieper, H.J. Membrane vesicle formation as a multiple-stress response mechanism enhances Pseudomonas putida DOT-T1E cell surface hydrophobicity and biofilm formation. Appl. Environ. Microbiol. 2012, 78, 6217–6224. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, H.; Osaki, T.; Kurata, S.; Fukuda, M.; Kawakami, H.; Ochiai, K.; Hanawa, T.; Kamiya, S. Outer membrane vesicles of Helicobacter pylori TK1402 are involved in biofilm formation. BMC Microbiol. 2009, 9, 197. [Google Scholar] [CrossRef]
- Baeza, N.; Mercade, E. Relationship Between Membrane Vesicles, Extracellular ATP and Biofilm Formation in Antarctic Gram-Negative Bacteria. Microb. Ecol. 2021, 81, 645–656. [Google Scholar] [CrossRef]
- Gedefie, A.; Demsis, W.; Ashagrie, M.; Kassa, Y.; Tesfaye, M.; Tilahun, M.; Bisetegn, H.; Sahle, Z. Acinetobacter baumannii Biofilm Formation and Its Role in Disease Pathogenesis: A Review. Infect. Drug Resist. 2021, 14, 3711–3719. [Google Scholar] [CrossRef]
- McConnell, M.J.; Actis, L.; Pachón, J. Acinetobacter baumannii: Human infections, factors contributing to pathogenesis and animal models. FEMS Microbiol. Rev. 2013, 37, 130–155. [Google Scholar] [CrossRef]
- Richards, A.M.; Abu Kwaik, Y.; Lamont, R.J. Code blue: Acinetobacter baumannii, a nosocomial pathogen with a role in the oral cavity. Mol. Oral Microbiol. 2015, 30, 2–15. [Google Scholar] [CrossRef]
- Cerqueira, G.M.; Peleg, A.Y. Insights into Acinetobacter baumannii pathogenicity. IUBMB Life 2011, 63, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Su, P.W.; Moi, S.H.; Chuang, L.Y. Biofilm Formation in Acinetobacter baumannii: Genotype-Phenotype Correlation. Molecules 2019, 24, 1849. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, I.; Vasileiou, E.; Pana, Z.D.; Tragiannidis, A. Acinetobacter baumannii Antibiotic Resistance Mechanisms. Pathogens 2021, 10, 373. [Google Scholar] [CrossRef] [PubMed]
- Antunes, L.C.; Visca, P.; Towner, K.J. Acinetobacter baumannii: Evolution of a global pathogen. Pathog. Dis. 2014, 71, 292–301. [Google Scholar] [CrossRef]
- Lin, M.F.; Lan, C.Y. Antimicrobial resistance in Acinetobacter baumannii: From bench to bedside. World J. Clin. Cases 2014, 2, 787–814. [Google Scholar] [CrossRef]
- Mea, H.J.; Yong, P.V.C.; Wong, E.H. An overview of Acinetobacter baumannii pathogenesis: Motility, adherence and biofilm formation. Microbiol. Res. 2021, 247, 126722. [Google Scholar] [CrossRef]
- Mendes, S.G.; Combo, S.I.; Allain, T.; Domingues, S.; Buret, A.G.; Da Silva, G.J. Co-regulation of biofilm formation and antimicrobial resistance in Acinetobacter baumannii: From mechanisms to therapeutic strategies. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2023, 42, 1405–1423. [Google Scholar] [CrossRef]
- Elkheloui, R.; Laktib, A.; Mimouni, R.; Aitalla, A.; Hassi, M.; Elboulani, A.; Hamadi, F. Acinetobacter baumannii Biofilm: Intervening Factors, Persistence, Drug Resistance, and Strategies of Treatment. Mediterr. J. Infect. Microb. Antimicrob. 2020, 9, 7. [Google Scholar] [CrossRef]
- Amala Reena, A.A.; Subramaniyan, A.; Kanungo, R. Biofilm formation as a virulence factor of Acinetobacter baumannii: An emerging pathogen in critical care units. J. Curr. Res. Sci. Med. 2017, 3, 74–78. [Google Scholar] [CrossRef]
- Choudhary, P.; Singh, S.; Agarwal, V. Microbial Biofilms. In Bacterial Biofilms; Dincer, S., Sumengen Ozdenefe, M., Arkut, A., Eds.; IntechOpen: Rijeka, Yugoslavia, 2020. [Google Scholar]
- Eze, E.C.; Chenia, H.Y.; El Zowalaty, M.E. Acinetobacter baumannii biofilms: Effects of physicochemical factors, virulence, antibiotic resistance determinants, gene regulation, and future antimicrobial treatments. Infect. Drug Resist. 2018, 11, 2277–2299. [Google Scholar] [CrossRef]
- Toyofuku, M.; Inaba, T.; Kiyokawa, T.; Obana, N.; Yawata, Y.; Nomura, N. Environmental factors that shape biofilm formation. Biosci. Biotechnol. Biochem. 2016, 80, 7–12. [Google Scholar] [CrossRef]
- Vo, N.; Sidner, B.S.; Yu, Y.; Piepenbrink, K.H. Type IV Pilus-Mediated Inhibition of Acinetobacter baumannii Biofilm Formation by Phenothiazine Compounds. Microbiol. Spectr. 2023, 11, e0102323. [Google Scholar] [CrossRef]
- Piepenbrink, K.H. DNA Uptake by Type IV Filaments. Front. Mol. Biosci. 2019, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Pelicic, V. Type IV pili: E pluribus unum? Mol. Microbiol. 2008, 68, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Ronish, L.A.; Lillehoj, E.; Fields, J.K.; Sundberg, E.J.; Piepenbrink, K.H. The structure of PilA from Acinetobacter baumannii AB5075 suggests a mechanism for functional specialization in Acinetobacter type IV pili. J. Biol. Chem. 2019, 294, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Ramezanalizadeh, F.; Owlia, P.; Rasooli, I. Type I pili, CsuA/B and FimA induce a protective immune response against Acinetobacter baumannii. Vaccine 2020, 38, 5436–5446. [Google Scholar] [CrossRef]
- Miller, D.P.; Wang, Q.; Weinberg, A.; Lamont, R.J. Transcriptome analysis of Porphyromonas gingivalis and Acinetobacter baumannii in polymicrobial communities. Mol. Oral Microbiol. 2018, 33, 364–377. [Google Scholar] [CrossRef]
- Yihunie, W.; Nibret, G.; Aschale, Y. Recent Advances in Messenger Ribonucleic Acid (mRNA) Vaccines and Their Delivery Systems: A Review. Clin. Pharmacol. Adv. Appl. 2023, 15, 77–98. [Google Scholar] [CrossRef]
- Moriel, D.G.; Beatson, S.A.; Wurpel, D.J.; Lipman, J.; Nimmo, G.R.; Paterson, D.L.; Schembri, M.A. Identification of novel vaccine candidates against multidrug-resistant Acinetobacter baumannii. PLoS ONE 2013, 8, e77631. [Google Scholar] [CrossRef]
- Tomaras, A.P.; Dorsey, C.W.; Edelmann, R.E.; Actis, L.A. Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: Involvement of a novel chaperone-usher pili assembly system. Microbiology 2003, 149 Pt 12, 3473–3484. [Google Scholar] [CrossRef]
- Pakharukova, N.; Tuittila, M.; Paavilainen, S.; Malmi, H.; Parilova, O.; Teneberg, S.; Knight, S.D.; Zavialov, A.V. Structural basis for Acinetobacter baumannii biofilm formation. Proc. Natl. Acad. Sci. USA 2018, 115, 5558–5563. [Google Scholar] [CrossRef]
- Hospenthal, M.K.; Costa, T.R.D.; Waksman, G. A comprehensive guide to pilus biogenesis in Gram-negative bacteria. Nat. Rev. Microbiol. 2017, 15, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Gaddy, J.A.; Arivett, B.A.; McConnell, M.J.; López-Rojas, R.; Pachón, J.; Actis, L.A. Role of acinetobactin-mediated iron acquisition functions in the interaction of Acinetobacter baumannii strain ATCC 19606T with human lung epithelial cells, Galleria mellonella caterpillars, and mice. Infect. Immun. 2012, 80, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- de Breij, A.; Dijkshoorn, L.; Lagendijk, E.; van der Meer, J.; Koster, A.; Bloemberg, G.; Wolterbeek, R.; van den Broek, P.; Nibbering, P. Do biofilm formation and interactions with human cells explain the clinical success of Acinetobacter baumannii? PLoS ONE 2010, 5, e10732. [Google Scholar] [CrossRef] [PubMed]
- Montaña, S.; Vilacoba, E.; Traglia, G.M.; Almuzara, M.; Pennini, M.; Fernández, A.; Sucari, A.; Centrón, D.; Ramírez, M.S. Genetic Variability of AdeRS Two-Component System Associated with Tigecycline Resistance in XDR-Acinetobacter baumannii Isolates. Curr. Microbiol. 2015, 71, 76–82. [Google Scholar] [CrossRef]
- Tomaras, A.P.; Flagler, M.J.; Dorsey, C.W.; Gaddy, J.A.; Actis, L.A. Characterization of a two-component regulatory system from Acinetobacter baumannii that controls biofilm formation and cellular morphology. Microbiology 2008, 154 Pt 11, 3398–3409. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, M.H.; Kim, S.I.; Son, J.H.; Kim, S.; Lee, Y.C.; Shin, M.; Oh, M.H.; Lee, J.C. The sensor kinase BfmS controls production of outer membrane vesicles in Acinetobacter baumannii. BMC Microbiol. 2019, 19, 301. [Google Scholar] [CrossRef]
- Liou, M.L.; Soo, P.C.; Ling, S.R.; Kuo, H.Y.; Tang, C.Y.; Chang, K.C. The sensor kinase BfmS mediates virulence in Acinetobacter baumannii. J. Microbiol. Immunol. Infect. 2014, 47, 275–281. [Google Scholar] [CrossRef]
- Chen, R.; Lv, R.; Xiao, L.; Wang, M.; Du, Z.; Tan, Y.; Cui, Y.; Yan, Y.; Luo, Y.; Yang, R.; et al. A1S_2811, a CheA/Y-like hybrid two-component regulator from Acinetobacter baumannii ATCC17978, is involved in surface motility and biofilm formation in this bacterium. MicrobiologyOpen 2017, 6, e00510. [Google Scholar] [CrossRef]
- He, X.; Lu, F.; Yuan, F.; Jiang, D.; Zhao, P.; Zhu, J.; Cheng, H.; Cao, J.; Lu, G. Biofilm Formation Caused by Clinical Acinetobacter baumannii Isolates Is Associated with Overexpression of the AdeFGH Efflux Pump. Antimicrob. Agents Chemother. 2015, 59, 4817–4825. [Google Scholar] [CrossRef]
- Sato, Y.; Unno, Y.; Ubagai, T.; Ono, Y. Sub-minimum inhibitory concentrations of colistin and polymyxin B promote Acinetobacter baumannii biofilm formation. PLoS ONE 2018, 13, e0194556. [Google Scholar] [CrossRef]
- Zhong, S.; He, S. Quorum Sensing Inhibition or Quenching in Acinetobacter baumannii: The Novel Therapeutic Strategies for New Drug Development. Front. Microbiol. 2021, 12, 558003. [Google Scholar] [CrossRef] [PubMed]
- Naeimi Mazraeh, F.; Hasani, A.; Sadeghi, J.; Samadi Kafil, H.; Soroush Barhaghi, M.H.; Yeganeh Sefidan, F.; Rishi Sharabiani, H.; Hematyar, Y.; Ahangarzadeh Rezaee, M. High frequency of blaPER-1 gene in clinical strains of Acinetobacter baumannii and its association with quorum sensing and virulence factors. Gene Rep. 2021, 24, 101232. [Google Scholar] [CrossRef]
- Sun, X.; Ni, Z.; Tang, J.; Ding, Y.; Wang, X.; Li, F. The abaI/abaR Quorum Sensing System Effects on Pathogenicity in Acinetobacter baumannii. Front. Microbiol. 2021, 12, 679241. [Google Scholar] [CrossRef] [PubMed]
- Khidhr Rahman, J.; Ahmed Ahmed, A.; Ganjo, A.R.; Salih Mohamad, T. Assessment of toxicity, anti-quorum sensing and anti-biofilm production effects of Hypericum triquetrifolium Turra extract on multi-drug resistant Acinetobacter baumannii. J. King Saud. Univ. -Sci. 2023, 35, 102714. [Google Scholar] [CrossRef]
- Tang, J.; Chen, Y.; Wang, X.; Ding, Y.; Sun, X.; Ni, Z. Contribution of the AbaI/AbaR Quorum Sensing System to Resistance and Virulence of Acinetobacter baumannii Clinical Strains. Infect. Drug Resist. 2020, 13, 4273–4281. [Google Scholar] [CrossRef]
- Loehfelm, T.W.; Luke, N.R.; Campagnari, A.A. Identification and characterization of an Acinetobacter baumannii biofilm-associated protein. J. Bacteriol. 2008, 190, 1036–1044. [Google Scholar] [CrossRef]
- Brossard, K.A.; Campagnari, A.A. The Acinetobacter baumannii biofilm-associated protein plays a role in adherence to human epithelial cells. Infect. Immun. 2012, 80, 228–233. [Google Scholar] [CrossRef]
- Goh, H.M.; Beatson, S.A.; Totsika, M.; Moriel, D.G.; Phan, M.D.; Szubert, J.; Runnegar, N.; Sidjabat, H.E.; Paterson, D.L.; Nimmo, G.R.; et al. Molecular analysis of the Acinetobacter baumannii biofilm-associated protein. Appl. Environ. Microbiol. 2013, 79, 6535–6543. [Google Scholar] [CrossRef]
- Fallah, A.; Rezaee, M.A.; Hasani, A.; Barhaghi, M.H.S.; Kafil, H.S. Frequency of bap and cpaA virulence genes in drug resistant clinical isolates of Acinetobacter baumannii and their role in biofilm formation. Iran. J. Basic Med. Sci. 2017, 20, 849–855. [Google Scholar] [CrossRef]
- Thummeepak, R.; Kongthai, P.; Leungtongkam, U.; Sitthisak, S. Distribution of virulence genes involved in biofilm formation in multi-drug resistant Acinetobacter baumannii clinical isolates. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2016, 19, 121–129. [Google Scholar] [CrossRef]
- Hua, X.; Zhou, Z.; Yang, Q.; Shi, Q.; Xu, Q.; Wang, J.; Shi, K.; Zhao, F.; Sun, L.; Ruan, Z.; et al. Evolution of Acinetobacter baumannii In Vivo: International Clone II, More Resistance to Ceftazidime, Mutation in ptk. Front. Microbiol. 2017, 8, 1256. [Google Scholar] [CrossRef]
- Su, P.-W.; Yang, E.C.; Moi, S.-H.; Yang, C.-H.; Chuang, L.-Y. Prevalence of Carbapenem Resistance Genes among Acinetobacter baumannii Isolated from a Teaching Hospital in Taiwan. Antibiotics 2023, 12, 1357. [Google Scholar] [CrossRef]
- Choi, C.H.; Lee, J.S.; Lee, Y.C.; Park, T.I.; Lee, J.C. Acinetobacter baumannii invades epithelial cells and outer membrane protein A mediates interactions with epithelial cells. BMC Microbiol. 2008, 8, 216. [Google Scholar] [CrossRef]
- Gaddy, J.A.; Tomaras, A.P.; Actis, L.A. The Acinetobacter baumannii 19606 OmpA protein plays a role in biofilm formation on abiotic surfaces and in the interaction of this pathogen with eukaryotic cells. Infect. Immun. 2009, 77, 3150–3160. [Google Scholar] [CrossRef]
- Choi, C.H.; Lee, E.Y.; Lee, Y.C.; Park, T.I.; Kim, H.J.; Hyun, S.H.; Kim, S.A.; Lee, S.K.; Lee, J.C. Outer membrane protein 38 of Acinetobacter baumannii localizes to the mitochondria and induces apoptosis of epithelial cells. Cell. Microbiol. 2005, 7, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Choi, C.H.; Kim, J.W.; Lee, J.C. Acinetobacter baumannii outer membrane protein A induces dendritic cell death through mitochondrial targeting. J. Microbiol. 2010, 48, 387–392. [Google Scholar] [CrossRef]
- Sherif, M.M.; Elkhatib, W.F.; Khalaf, W.S.; Elleboudy, N.S.; Abdelaziz, N.A. Multidrug Resistant Acinetobacter baumannii Biofilms: Evaluation of Phenotypic-Genotypic Association and Susceptibility to Cinnamic and Gallic Acids. Front. Microbiol. 2021, 12, 716627. [Google Scholar] [CrossRef] [PubMed]
- Petrova, O.E.; Sauer, K. Sticky situations: Key components that control bacterial surface attachment. J. Bacteriol. 2012, 194, 2413–2425. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.K.; Iyer, P.S.; Oak, A.M.; Pardesi, K.R.; Chopade, B.A. Characterization of eDNA from the clinical strain Acinetobacter baumannii AIIMS 7 and its role in biofilm formation. Sci. World J. 2012, 2012, 973436. [Google Scholar] [CrossRef]
- Whitchurch, C.B.; Tolker-Nielsen, T.; Ragas, P.C.; Mattick, J.S. Extracellular DNA required for bacterial biofilm formation. Science 2002, 295, 1487. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Barken, K.B.; Skindersoe, M.E.; Christensen, A.B.; Givskov, M.; Tolker-Nielsen, T. Effects of iron on DNA release and biofilm development by Pseudomonas aeruginosa. Microbiology 2007, 153 Pt 5, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Nait Chabane, Y.; Marti, S.; Rihouey, C.; Alexandre, S.; Hardouin, J.; Lesouhaitier, O.; Vila, J.; Kaplan, J.B.; Jouenne, T.; Dé, E. Characterisation of pellicles formed by Acinetobacter baumannii at the air-liquid interface. PLoS ONE 2014, 9, e111660. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.H.; Slamti, L.; Avci, F.Y.; Pier, G.B.; Maira-Litrán, T. The pgaABCD locus of Acinetobacter baumannii encodes the production of poly-beta-1-6-N-acetylglucosamine, which is critical for biofilm formation. J. Bacteriol. 2009, 191, 5953–5963. [Google Scholar] [CrossRef]
- Ahmad, I.; Nygren, E.; Khalid, F.; Myint, S.L.; Uhlin, B.E. A Cyclic-di-GMP signalling network regulates biofilm formation and surface associated motility of Acinetobacter baumannii 17978. Sci. Rep. 2020, 10, 1991. [Google Scholar] [CrossRef]
- Rao, R.S.; Karthika, R.U.; Singh, S.P.; Shashikala, P.; Kanungo, R.; Jayachandran, S.; Prashanth, K. Correlation between biofilm production and multiple drug resistance in imipenem resistant clinical isolates of Acinetobacter baumannii. Indian. J. Med. Microbiol. 2008, 26, 333–337. [Google Scholar] [CrossRef]
- Badave, G.K.; Kulkarni, D. Biofilm Producing Multidrug Resistant Acinetobacter baumannii: An Emerging Challenge. J. Clin. Diagn. Res. 2015, 9, DC08–DC10. [Google Scholar] [CrossRef]
- Krzyściak, P.; Chmielarczyk, A.; Pobiega, M.; Romaniszyn, D.; Wójkowska-Mach, J. Acinetobacter baumannii isolated from hospital-acquired infection: Biofilm production and drug susceptibility. APMIS Acta Pathol Microbiol. Immunol. Scand. 2017, 125, 1017–1026. [Google Scholar] [CrossRef]
- Rodríguez-Baño, J.; Martí, S.; Soto, S.; Fernández-Cuenca, F.; Cisneros, J.M.; Pachón, J.; Pascual, A.; Martínez-Martínez, L.; McQueary, C.; Actis, L.A.; et al. Biofilm formation in Acinetobacter baumannii: Associated features and clinical implications. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2008, 14, 276–278. [Google Scholar] [CrossRef]
- Perez, L.R. Acinetobacter baumannii displays inverse relationship between meropenem resistance and biofilm production. J. Chemother. 2015, 27, 13–16. [Google Scholar] [CrossRef]
- Chang, C.Y. Surface Sensing for Biofilm Formation in Pseudomonas aeruginosa. Front. Microbiol. 2017, 8, 2671. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, H.; Sivaneson, M.; Filloux, A. Key two-component regulatory systems that control biofilm formation in Pseudomonas aeruginosa. Environ. Microbiol. 2011, 13, 1666–1681. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, M.; Yu, C.; Li, J.; Zhou, X. Biofilm formation: Mechanistic insights and therapeutic targets. Mol. Biomed. 2023, 4, 49. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- O’Toole, G.A.; Kolter, R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 1998, 30, 295–304. [Google Scholar] [CrossRef]
- Jyot, J.; Sonawane, A.; Wu, W.; Ramphal, R. Genetic mechanisms involved in the repression of flagellar assembly by Pseudomonas aeruginosa in human mucus. Mol. Microbiol. 2007, 63, 1026–1038. [Google Scholar] [CrossRef]
- Anyan, M.E.; Amiri, A.; Harvey, C.W.; Tierra, G.; Morales-Soto, N.; Driscoll, C.M.; Alber, M.S.; Shrout, J.D. Type IV pili interactions promote intercellular association and moderate swarming of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2014, 111, 18013–18018. [Google Scholar] [CrossRef]
- Banerjee, P.; Sahoo, P.K.; Sheenu; Adhikary, A.; Ruhal, R.; Jain, D. Molecular and structural facets of c-di-GMP signalling associated with biofilm formation in Pseudomonas aeruginosa. Mol. Asp. Med. 2021, 81, 101001. [Google Scholar] [CrossRef]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H.A. Pseudomonas aeruginosa Biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef]
- Das, T.; Sehar, S.; Manefield, M. The roles of extracellular DNA in the structural integrity of extracellular polymeric substance and bacterial biofilm development. Environ. Microbiol. Rep. 2013, 5, 778–786. [Google Scholar] [CrossRef]
- Strempel, N.; Neidig, A.; Nusser, M.; Geffers, R.; Vieillard, J.; Lesouhaitier, O.; Brenner-Weiss, G.; Overhage, J. Human host defense peptide LL-37 stimulates virulence factor production and adaptive resistance in Pseudomonas aeruginosa. PLoS ONE 2013, 8, e82240. [Google Scholar] [CrossRef]
- Ghafoor, A.; Hay, I.D.; Rehm, B.H. Role of exopolysaccharides in Pseudomonas aeruginosa biofilm formation and architecture. Appl. Environ. Microbiol. 2011, 77, 5238–5246. [Google Scholar] [CrossRef] [PubMed]
- Billings, N.; Millan, M.; Caldara, M.; Rusconi, R.; Tarasova, Y.; Stocker, R.; Ribbeck, K. The extracellular matrix Component Psl provides fast-acting antibiotic defense in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2013, 9, e1003526. [Google Scholar] [CrossRef] [PubMed]
- Gheorghita, A.A.; Wozniak, D.J.; Parsek, M.R.; Howell, P.L. Pseudomonas aeruginosa biofilm exopolysaccharides: Assembly, function, and degradation. FEMS Microbiol. Rev. 2023, 47, fuad060. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Conover, M.; Lu, H.; Parsek, M.R.; Bayles, K.; Wozniak, D.J. Assembly and development of the Pseudomonas aeruginosa biofilm matrix. PLoS Pathog. 2009, 5, e1000354. [Google Scholar] [CrossRef]
- Jones, C.J.; Wozniak, D.J. Psl Produced by Mucoid Pseudomonas aeruginosa Contributes to the Establishment of Biofilms and Immune Evasion. mBio 2017, 8, e00864-17. [Google Scholar] [CrossRef]
- Jiang, Z.; Nero, T.; Mukherjee, S.; Olson, R.; Yan, J. Searching for the Secret of Stickiness: How Biofilms Adhere to Surfaces. Front. Microbiol. 2021, 12, 686793. [Google Scholar] [CrossRef]
- Cooley, B.J.; Thatcher, T.W.; Hashmi, S.M.; L’Her, G.; Le, H.H.; Hurwitz, D.A.; Provenzano, D.; Touhami, A.; Gordon, V.D. The extracellular polysaccharide Pel makes the attachment of P. aeruginosa to surfaces symmetric and short-ranged. Soft Matter 2013, 9, 3871–3876. [Google Scholar] [CrossRef]
- Irie, Y.; Roberts, A.E.L.; Kragh, K.N.; Gordon, V.D.; Hutchison, J.; Allen, R.J.; Melaugh, G.; Bjarnsholt, T.; West, S.A.; Diggle, S.P. The Pseudomonas aeruginosa PSL Polysaccharide Is a Social but Noncheatable Trait in Biofilms. mBio 2017, 8, e00374-17. [Google Scholar] [CrossRef]
- Mishra, M.; Byrd, M.S.; Sergeant, S.; Azad, A.K.; Parsek, M.R.; McPhail, L.; Schlesinger, L.S.; Wozniak, D.J. Pseudomonas aeruginosa Psl polysaccharide reduces neutrophil phagocytosis and the oxidative response by limiting complement-mediated opsonization. Cell. Microbiol. 2012, 14, 95–106. [Google Scholar] [CrossRef]
- Colvin, K.M.; Alnabelseya, N.; Baker, P.; Whitney, J.C.; Howell, P.L.; Parsek, M.R. PelA deacetylase activity is required for Pel polysaccharide synthesis in Pseudomonas aeruginosa. J. Bacteriol. 2013, 195, 2329–2339. [Google Scholar] [CrossRef]
- Jennings, L.K.; Storek, K.M.; Ledvina, H.E.; Coulon, C.; Marmont, L.S.; Sadovskaya, I.; Secor, P.R.; Tseng, B.S.; Scian, M.; Filloux, A.; et al. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. USA 2015, 112, 11353–11358. [Google Scholar] [CrossRef]
- Jackson, K.D.; Starkey, M.; Kremer, S.; Parsek, M.R.; Wozniak, D.J. Identification of psl, a locus encoding a potential exopolysaccharide that is essential for Pseudomonas aeruginosa PAO1 biofilm formation. J. Bacteriol. 2004, 186, 4466–4475. [Google Scholar] [CrossRef]
- Folkesson, A.; Jelsbak, L.; Yang, L.; Johansen, H.K.; Ciofu, O.; Høiby, N.; Molin, S. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: An evolutionary perspective. Nat. Rev. Microbiol. 2012, 10, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Ryall, B.; Carrara, M.; Zlosnik, J.E.; Behrends, V.; Lee, X.; Wong, Z.; Lougheed, K.E.; Williams, H.D. The mucoid switch in Pseudomonas aeruginosa represses quorum sensing systems and leads to complex changes to stationary phase virulence factor regulation. PLoS ONE 2014, 9, e96166. [Google Scholar] [CrossRef]
- Wu, H.; Wang, D.; Tang, M.; Ma, L.Z. The advance of assembly of exopolysaccharide Psl biosynthesis machinery in Pseudomonas aeruginosa. MicrobiologyOpen 2019, 8, e857. [Google Scholar] [CrossRef] [PubMed]
- Limoli, D.H.; Whitfield, G.B.; Kitao, T.; Ivey, M.L.; Davis, M.R., Jr.; Grahl, N.; Hogan, D.A.; Rahme, L.G.; Howell, P.L.; O’Toole, G.A.; et al. Pseudomonas aeruginosa Alginate Overproduction Promotes Coexistence with Staphylococcus aureus in a Model of Cystic Fibrosis Respiratory Infection. mBio 2017, 8, e00186-17. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.; Whitfield, G.B.; Hill, P.J.; Little, D.J.; Pestrak, M.J.; Robinson, H.; Wozniak, D.J.; Howell, P.L. Characterization of the Pseudomonas aeruginosa Glycoside Hydrolase PslG Reveals That Its Levels Are Critical for Psl Polysaccharide Biosynthesis and Biofilm Formation. J. Biol. Chem. 2015, 290, 28374–28387. [Google Scholar] [CrossRef]
- Turnbull, L.; Toyofuku, M.; Hynen, A.L.; Kurosawa, M.; Pessi, G.; Petty, N.K.; Osvath, S.R.; Cárcamo-Oyarce, G.; Gloag, E.S.; Shimoni, R.; et al. Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat. Commun. 2016, 7, 11220. [Google Scholar] [CrossRef]
- Secchi, E.; Savorana, G.; Vitale, A.; Eberl, L.; Stocker, R.; Rusconi, R. The structural role of bacterial eDNA in the formation of biofilm streamers. Proc. Natl. Acad. Sci. USA 2022, 119, e2113723119. [Google Scholar] [CrossRef]
- Chiang, W.C.; Nilsson, M.; Jensen, P.; Høiby, N.; Nielsen, T.E.; Givskov, M.; Tolker-Nielsen, T. Extracellular DNA shields against aminoglycosides in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2013, 57, 2352–2361. [Google Scholar] [CrossRef]
- Allesen-Holm, M.; Barken, K.B.; Yang, L.; Klausen, M.; Webb, J.S.; Kjelleberg, S.; Molin, S.; Givskov, M.; Tolker-Nielsen, T. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol. Microbiol. 2006, 59, 1114–1128. [Google Scholar] [CrossRef] [PubMed]
- Wilton, M.; Charron-Mazenod, L.; Moore, R.; Lewenza, S. Extracellular DNA Acidifies Biofilms and Induces Aminoglycoside Resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2016, 60, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Wilton, M.; Wong, M.J.Q.; Tang, L.; Liang, X.; Moore, R.; Parkins, M.D.; Lewenza, S.; Dong, T.G. Chelation of Membrane-Bound Cations by Extracellular DNA Activates the Type VI Secretion System in Pseudomonas aeruginosa. Infect. Immun. 2016, 84, 2355–2361. [Google Scholar] [CrossRef] [PubMed]
- Gloag, E.S.; Turnbull, L.; Huang, A.; Vallotton, P.; Wang, H.; Nolan, L.M.; Mililli, L.; Hunt, C.; Lu, J.; Osvath, S.R.; et al. Self-organization of bacterial biofilms is facilitated by extracellular DNA. Proc. Natl. Acad. Sci. USA 2013, 110, 11541–11546. [Google Scholar] [CrossRef]
- Fuxman Bass, J.I.; Russo, D.M.; Gabelloni, M.L.; Geffner, J.R.; Giordano, M.; Catalano, M.; Zorreguieta, A.; Trevani, A.S. Extracellular DNA: A major proinflammatory component of Pseudomonas aeruginosa biofilms. J. Immunol. 2010, 184, 6386–6395. [Google Scholar] [CrossRef]
- Reichhardt, C.; Wong, C.; Passos da Silva, D.; Wozniak, D.J.; Parsek, M.R. CdrA Interactions within the Pseudomonas aeruginosa Biofilm Matrix Safeguard It from Proteolysis and Promote Cellular Packing. mBio 2018, 9, e01376-18. [Google Scholar] [CrossRef]
- Cherny, K.E.; Sauer, K. Untethering and Degradation of the Polysaccharide Matrix Are Essential Steps in the Dispersion Response of Pseudomonas aeruginosa Biofilms. J. Bacteriol. 2020, 202, e00575-19. [Google Scholar] [CrossRef]
- Pham, T.H.; Webb, J.S.; Rehm, B.H. The role of polyhydroxyalkanoate biosynthesis by Pseudomonas aeruginosa in rhamnolipid and alginate production as well as stress tolerance and biofilm formation. Microbiology 2004, 150 Pt 10, 3405–3413. [Google Scholar] [CrossRef]
- Moradali, M.F.; Ghods, S.; Rehm, B.H. Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Front. Cell. Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef]
- Moradali, M.F.; Rehm, B.H.A. Bacterial biopolymers: From pathogenesis to advanced materials. Nat. Rev. Microbiol. 2020, 18, 195–210. [Google Scholar] [CrossRef]
- Kang, D.; Turner, K.E.; Kirienko, N.V. PqsA Promotes Pyoverdine Production via Biofilm Formation. Pathogens 2017, 7, 3. [Google Scholar] [CrossRef]
- Storz, M.P.; Maurer, C.K.; Zimmer, C.; Wagner, N.; Brengel, C.; de Jong, J.C.; Lucas, S.; Müsken, M.; Häussler, S.; Steinbach, A.; et al. Validation of PqsD as an anti-biofilm target in Pseudomonas aeruginosa by development of small-molecule inhibitors. J. Am. Chem. Soc. 2012, 134, 16143–16146. [Google Scholar] [CrossRef] [PubMed]
- Elfadadny, A.; Ragab, R.F.; AlHarbi, M.; Badshah, F.; Ibáñez-Arancibia, E.; Farag, A.; Hendawy, A.O.; De Los Ríos-Escalante, P.R.; Aboubakr, M.; Zakai, S.A.; et al. Antimicrobial resistance of Pseudomonas aeruginosa: Navigating clinical impacts, current resistance trends, and innovations in breaking therapies. Front. Microbiol. 2024, 15, 1374466. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, P.N.; Koch, G.; Thompson, J.A.; Xavier, K.B.; Cool, R.H.; Quax, W.J. The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol. Mol. Biol. Rev. 2012, 76, 46–65. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Almpani, M.; Wheeler, K.M.; Rahme, L.G. Interconnections of Pseudomonas aeruginosa Quorum-Sensing Systems in Intestinal Permeability and Inflammation. mBio 2023, 14, e0352422. [Google Scholar] [CrossRef]
- Song, H.; Li, Y.; Wang, Y. Two-component system GacS/GacA, a global response regulator of bacterial physiological behaviors. Eng. Microbiol. 2023, 3, 100051. [Google Scholar] [CrossRef]
- Latour, X. The Evanescent GacS Signal. Microorganisms 2020, 8, 1746. [Google Scholar] [CrossRef]
- Ortet, P.; Fochesato, S.; Bitbol, A.F.; Whitworth, D.E.; Lalaouna, D.; Santaella, C.; Heulin, T.; Achouak, W.; Barakat, M. Evolutionary history expands the range of signaling interactions in hybrid multikinase networks. Sci. Rep. 2021, 11, 11763. [Google Scholar] [CrossRef]
- Wei, X.; Huang, X.; Tang, L.; Wu, D.; Xu, Y. Global control of GacA in secondary metabolism, primary metabolism, secretion systems, and motility in the rhizobacterium Pseudomonas aeruginosa M18. J. Bacteriol. 2013, 195, 3387–3400. [Google Scholar] [CrossRef]
- Parkins, M.D.; Ceri, H.; Storey, D.G. Pseudomonas aeruginosa GacA, a factor in multihost virulence, is also essential for biofilm formation. Mol. Microbiol. 2001, 40, 1215–1226. [Google Scholar] [CrossRef]
- Rasamiravaka, T.; Labtani, Q.; Duez, P.; El Jaziri, M. The formation of biofilms by Pseudomonas aeruginosa: A review of the natural and synthetic compounds interfering with control mechanisms. BioMed Res. Int. 2015, 2015, 759348. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Sarkar, S.; Das, B.; Bhattacharjee, S.; Tribedi, P. Biofilm, pathogenesis and prevention--a journey to break the wall: A review. Arch. Microbiol. 2016, 198, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Chen, L.; Zhao, J.; Shen, T.; Surette, M.G.; Shen, L.; Duan, K. Hybrid sensor kinase PA1611 in Pseudomonas aeruginosa regulates transitions between acute and chronic infection through direct interaction with RetS. Mol. Microbiol. 2013, 88, 784–797. [Google Scholar] [CrossRef] [PubMed]
- Records, A.R.; Gross, D.C. Sensor kinases RetS and LadS regulate Pseudomonas syringae type VI secretion and virulence factors. J. Bacteriol. 2010, 192, 3584–3596. [Google Scholar] [CrossRef]
- Friedman, L.; Kolter, R. Two genetic loci produce distinct carbohydrate-rich structural components of the Pseudomonas aeruginosa biofilm matrix. J. Bacteriol. 2004, 186, 4457–4465. [Google Scholar] [CrossRef]
- Ventre, I.; Goodman, A.L.; Vallet-Gely, I.; Vasseur, P.; Soscia, C.; Molin, S.; Bleves, S.; Lazdunski, A.; Lory, S.; Filloux, A. Multiple sensors control reciprocal expression of Pseudomonas aeruginosa regulatory RNA and virulence genes. Proc. Natl. Acad. Sci. USA 2006, 103, 171–176. [Google Scholar] [CrossRef]
- Zheng, J.; Leung, K.Y. Dissection of a type VI secretion system in Edwardsiella tarda. Mol. Microbiol. 2007, 66, 1192–1206. [Google Scholar] [CrossRef]
- Sadovskaya, I.; Vinogradov, E.; Li, J.; Hachani, A.; Kowalska, K.; Filloux, A. High-level antibiotic resistance in Pseudomonas aeruginosa biofilm: The ndvB gene is involved in the production of highly glycerol-phosphorylated beta-(1->3)-glucans, which bind aminoglycosides. Glycobiology 2010, 20, 895–904. [Google Scholar] [CrossRef]
- Mah, T.F.; Pitts, B.; Pellock, B.; Walker, G.C.; Stewart, P.S.; O’Toole, G.A. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 2003, 426, 306–310. [Google Scholar] [CrossRef]
- Zhang, L.; Hinz, A.J.; Nadeau, J.P.; Mah, T.F. Pseudomonas aeruginosa tssC1 links type VI secretion and biofilm-specific antibiotic resistance. J. Bacteriol. 2011, 193, 5510–5513. [Google Scholar] [CrossRef]
- Zhang, L.; Mah, T.F. Involvement of a novel efflux system in biofilm-specific resistance to antibiotics. J. Bacteriol. 2008, 190, 4447–4452. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ni, M. Regulation of biofilm formation in Klebsiella pneumoniae. Front. Microbiol. 2023, 14, 1238482. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Huang, Y.J.; Fung, C.P.; Peng, H.L. Regulation of the Klebsiella pneumoniae Kpc fimbriae by the site-specific recombinase KpcI. Microbiology 2010, 156 Pt 7, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Alcántar-Curiel, M.D.; Blackburn, D.; Saldaña, Z.; Gayosso-Vázquez, C.; Iovine, N.M.; De la Cruz, M.A.; Girón, J.A. Multi-functional analysis of Klebsiella pneumoniae fimbrial types in adherence and biofilm formation. Virulence 2013, 4, 129–138. [Google Scholar] [CrossRef]
- Khater, F.; Balestrino, D.; Charbonnel, N.; Dufayard, J.F.; Brisse, S.; Forestier, C. In silico analysis of usher encoding genes in Klebsiella pneumoniae and characterization of their role in adhesion and colonization. PLoS ONE 2015, 10, e0116215. [Google Scholar] [CrossRef]
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef]
- Gomes AÉ, I.; Pacheco, T.; Dos Santos, C.D.S.; Pereira, J.A.; Ribeiro, M.L.; Darrieux, M.; Ferraz, L.F.C. Functional Insights From KpfR, a New Transcriptional Regulator of Fimbrial Expression That Is Crucial for Klebsiella pneumoniae Pathogenicity. Front. Microbiol. 2020, 11, 601921. [Google Scholar] [CrossRef]
- Murphy, C.N.; Clegg, S. Klebsiella pneumoniae and type 3 fimbriae: Nosocomial infection, regulation and biofilm formation. Future Microbiol. 2012, 7, 991–1002. [Google Scholar] [CrossRef]
- Fang, R.; Liu, H.; Zhang, X.; Dong, G.; Li, J.; Tian, X.; Wu, Z.; Zhou, J.; Cao, J.; Zhou, T. Difference in biofilm formation between carbapenem-resistant and carbapenem-sensitive Klebsiella pneumoniae based on analysis of mrkH distribution. Microb. Pathog. 2021, 152, 104743. [Google Scholar] [CrossRef]
- Ashwath, P.; Deekshit, V.K.; Rohit, A.; Dhinakaran, I.; Karunasagar, I.; Karunasagar, I.; Akhila, D.S. Biofilm Formation and Associated Gene Expression in Multidrug-Resistant Klebsiella pneumoniae Isolated from Clinical Specimens. Curr. Microbiol. 2022, 79, 73. [Google Scholar] [CrossRef]
- Wilksch, J.J.; Yang, J.; Clements, A.; Gabbe, J.L.; Short, K.R.; Cao, H.; Cavaliere, R.; James, C.E.; Whitchurch, C.B.; Schembri, M.A.; et al. MrkH, a novel c-di-GMP-dependent transcriptional activator, controls Klebsiella pneumoniae biofilm formation by regulating type 3 fimbriae expression. PLoS Pathog. 2011, 7, e1002204. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.W.; Wilksch, J.J.; Hocking, D.M.; Wang, N.; Srikhanta, Y.N.; Tauschek, M.; Lithgow, T.; Robins-Browne, R.M.; Yang, J.; Strugnell, R.A. Positive autoregulation of mrkHI by the cyclic di-GMP-dependent MrkH protein in the biofilm regulatory circuit of Klebsiella pneumoniae. J. Bacteriol. 2015, 197, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.G.; Murphy, C.N.; Sippy, J.; Johnson, T.J.; Clegg, S. Type 3 fimbriae and biofilm formation are regulated by the transcriptional regulators MrkHI in Klebsiella pneumoniae. J. Bacteriol. 2011, 193, 3453–3460. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Lin, C.T.; Cheng, W.Y.; Huang, C.J.; Wang, Z.C.; Peng, H.L. Fur-dependent MrkHI regulation of type 3 fimbriae in Klebsiella pneumoniae CG43. Microbiology 2012, 158 Pt 4, 1045–1056. [Google Scholar] [CrossRef]
- Huang, C.J.; Wang, Z.C.; Huang, H.Y.; Huang, H.D.; Peng, H.L. YjcC, a c-di-GMP phosphodiesterase protein, regulates the oxidative stress response and virulence of Klebsiella pneumoniae CG43. PLoS ONE 2013, 8, e66740. [Google Scholar] [CrossRef]
- Lin, T.H.; Tseng, C.Y.; Lai, Y.C.; Wu, C.C.; Huang, C.F.; Lin, C.T. IscR Regulation of Type 3 Fimbriae Expression in Klebsiella pneumoniae CG43. Front. Microbiol. 2017, 8, 1984. [Google Scholar] [CrossRef]
- Li, L.; Gao, X.; Li, M.; Liu, Y.; Ma, J.; Wang, X.; Yu, Z.; Cheng, W.; Zhang, W.; Sun, H.; et al. Relationship between biofilm formation and antibiotic resistance of Klebsiella pneumoniae and updates on antibiofilm therapeutic strategies. Front. Cell. Infect. Microbiol. 2024, 14, 1324895. [Google Scholar] [CrossRef]
- Boddicker, J.D.; Anderson, R.A.; Jagnow, J.; Clegg, S. Signature-tagged mutagenesis of Klebsiella pneumoniae to identify genes that influence biofilm formation on extracellular matrix material. Infect. Immun. 2006, 74, 4590–4597. [Google Scholar] [CrossRef]
- Wu, M.C.; Lin, T.L.; Hsieh, P.F.; Yang, H.C.; Wang, J.T. Isolation of genes involved in biofilm formation of a Klebsiella pneumoniae strain causing pyogenic liver abscess. PLoS ONE 2011, 6, e23500. [Google Scholar] [CrossRef]
- Vuotto, C.; Longo, F.; Pascolini, C.; Donelli, G.; Balice, M.P.; Libori, M.F.; Tiracchia, V.; Salvia, A.; Varaldo, P.E. Biofilm formation and antibiotic resistance in Klebsiella pneumoniae urinary strains. J. Appl. Microbiol. 2017, 123, 1003–1018. [Google Scholar] [CrossRef]
- Zheng, J.X.; Lin, Z.W.; Chen, C.; Chen, Z.; Lin, F.J.; Wu, Y.; Yang, S.Y.; Sun, X.; Yao, W.M.; Li, D.Y.; et al. Biofilm Formation in Klebsiella pneumoniae Bacteremia Strains Was Found to be Associated with CC23 and the Presence of wcaG. Front. Cell. Infect. Microbiol. 2018, 8, 21. [Google Scholar] [CrossRef]
- Singh, S.; Wilksch, J.J.; Dunstan, R.A.; Mularski, A.; Wang, N.; Hocking, D.; Jebeli, L.; Cao, H.; Clements, A.; Jenney, A.W.J.; et al. LPS O Antigen Plays a Key Role in Klebsiella pneumoniae Capsule Retention. Microbiol. Spectr. 2022, 10, e0151721. [Google Scholar] [CrossRef]
- Gu, Y.; Tian, J.; Zhang, Y.; Wu, R.; Li, L.; Zhang, B.; He, Y. Dissecting signal molecule AI-2 mediated biofilm formation and environmental tolerance in Lactobacillus plantarum. J. Biosci. Bioeng. 2021, 131, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, D.; Haagensen, J.A.; Rich, C.; Forestier, C. Characterization of type 2 quorum sensing in Klebsiella pneumoniae and relationship with biofilm formation. J. Bacteriol. 2005, 187, 2870–2880. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wilksch, J.J.; Liu, H.; Zhang, X.; Torres, V.V.L.; Bi, W.; Mandela, E.; Cao, J.; Li, J.; Lithgow, T.; et al. Investigation of LuxS-mediated quorum sensing in Klebsiella pneumoniae. J. Med. Microbiol. 2020, 69, 402–413. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, C.; Balestrino, D.; Roth, L.; Charbonnel, N.; Forestier, C. Quorum sensing affects biofilm formation through lipopolysaccharide synthesis in Klebsiella pneumoniae. Res. Microbiol. 2010, 161, 595–603. [Google Scholar] [CrossRef]
- Nikibakhsh, M.; Firoozeh, F.; Badmasti, F.; Kabir, K.; Zibaei, M. Molecular study of metallo-β-lactamases and integrons in Acinetobacter baumannii isolates from burn patients. BMC Infect. Dis. 2021, 21, 782. [Google Scholar] [CrossRef]
- Alav, I.; Sutton, J.M.; Rahman, K.M. Role of bacterial efflux pumps in biofilm formation. J. Antimicrob. Chemother. 2018, 73, 2003–2020. [Google Scholar] [CrossRef]
- Tang, M.; Wei, X.; Wan, X.; Ding, Z.; Ding, Y.; Liu, J. The role and relationship with efflux pump of biofilm formation in Klebsiella pneumoniae. Microb. Pathog. 2020, 147, 104244. [Google Scholar] [CrossRef]
- Kvist, M.; Hancock, V.; Klemm, P. Inactivation of efflux pumps abolishes bacterial biofilm formation. Appl. Environ. Microbiol. 2008, 74, 7376–7382. [Google Scholar] [CrossRef] [PubMed]
- Guilhen, C.; Miquel, S.; Charbonnel, N.; Joseph, L.; Carrier, G.; Forestier, C.; Balestrino, D. Colonization and immune modulation properties of Klebsiella pneumoniae biofilm-dispersed cells. NPJ Biofilms Microbiomes 2019, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Escolar, L.; Pérez-Martín, J.; de Lorenzo, V. Opening the iron box: Transcriptional metalloregulation by the Fur protein. J. Bacteriol. 1999, 181, 6223–6229. [Google Scholar] [CrossRef] [PubMed]
- Troxell, B.; Hassan, H.M. Transcriptional regulation by Ferric Uptake Regulator (Fur) in pathogenic bacteria. Front. Cell. Infect. Microbiol. 2013, 3, 59. [Google Scholar] [CrossRef]
- Sanders, W.E., Jr.; Sanders, C.C. Enterobacter spp.: Pathogens poised to flourish at the turn of the century. Clin. Microbiol. Rev. 1997, 10, 220–241. [Google Scholar] [CrossRef]
- Lazar, V.; Holban, A.M.; Curutiu, C.; Chifiriuc, M.C. Modulation of Quorum Sensing and Biofilms in Less Investigated Gram-Negative ESKAPE Pathogens. Front. Microbiol. 2021, 12, 676510. [Google Scholar] [CrossRef]
- Yin, W.F.; Purmal, K.; Chin, S.; Chan, X.Y.; Chan, K.G. Long chain N-acyl homoserine lactone production by Enterobacter sp. isolated from human tongue surfaces. Sensors 2012, 12, 14307–14314. [Google Scholar] [CrossRef]
- Lau, Y.Y.; Sulaiman, J.; Chen, J.W.; Yin, W.F.; Chan, K.G. Quorum sensing activity of Enterobacter asburiae isolated from lettuce leaves. Sensors 2013, 13, 14189–14199. [Google Scholar] [CrossRef]
- Mezzatesta, M.L.; Gona, F.; Stefani, S. Enterobacter cloacae complex: Clinical impact and emerging antibiotic resistance. Future Microbiol. 2012, 7, 887–902. [Google Scholar] [CrossRef]
- Soria-Bustos, J.; Ares, M.A.; Gómez-Aldapa, C.A.; González, Y.M.J.A.; Girón, J.A.; De la Cruz, M.A. Two Type VI Secretion Systems of Enterobacter cloacae Are Required for Bacterial Competition, Cell Adherence, and Intestinal Colonization. Front. Microbiol. 2020, 11, 560488. [Google Scholar] [CrossRef]
- Soares, G.G.; Costa, J.F.; Melo, F.B.S.; Mola, R.; Balbino, T.C.L. Biofilm production and resistance profile of Enterobacter sp. strains isolated from pressure ulcers in Petrolina, Pernambuco, Brazil. J. Bras. Patol. Med. Lab. 2016, 52, 293–298. [Google Scholar] [CrossRef]
- Santajit, S.; Sookrung, N.; Indrawattana, N. Quorum Sensing in ESKAPE Bugs: A Target for Combating Antimicrobial Resistance and Bacterial Virulence. Biology 2022, 11, 1466. [Google Scholar] [CrossRef]
- Shankar, M.; Ponraj, P.; Illakkiam, D.; Rajendhran, J.; Gunasekaran, P. Inactivation of the transcriptional regulator-encoding gene sdiA enhances rice root colonization and biofilm formation in Enterobacter cloacae GS1. J. Bacteriol. 2013, 195, 39–45. [Google Scholar] [CrossRef]
- Rezzonico, F.; Smits, T.H.; Duffy, B. Detection of AI-2 receptors in genomes of Enterobacteriaceae suggests a role of type-2 quorum sensing in closed ecosystems. Sensors 2012, 12, 6645–6665. [Google Scholar] [CrossRef]
- Tay, S.B.; Yew, W.S. Development of quorum-based anti-virulence therapeutics targeting Gram-negative bacterial pathogens. Int. J. Mol. Sci. 2013, 14, 16570–16599. [Google Scholar] [CrossRef]
- Reading, N.C.; Sperandio, V. Quorum sensing: The many languages of bacteria. FEMS Microbiol. Lett. 2006, 254, 1–11. [Google Scholar] [CrossRef]
- Lau, Y.Y.; How, K.Y.; Yin, W.F.; Chan, K.G. Functional characterization of quorum sensing LuxR-type transcriptional regulator, EasR in Enterobacter asburiae strain L1. PeerJ 2020, 8, e10068. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, L.; Wang, L.; Zhou, B.; Ye, D.; Zheng, X.; Lin, Y.; Zeng, W.; Zhou, T.; Ye, J. Cluster Differences in Antibiotic Resistance, Biofilm Formation, Mobility, and Virulence of Clinical Enterobacter cloacae Complex. Front. Microbiol. 2022, 13, 814831. [Google Scholar] [CrossRef] [PubMed]
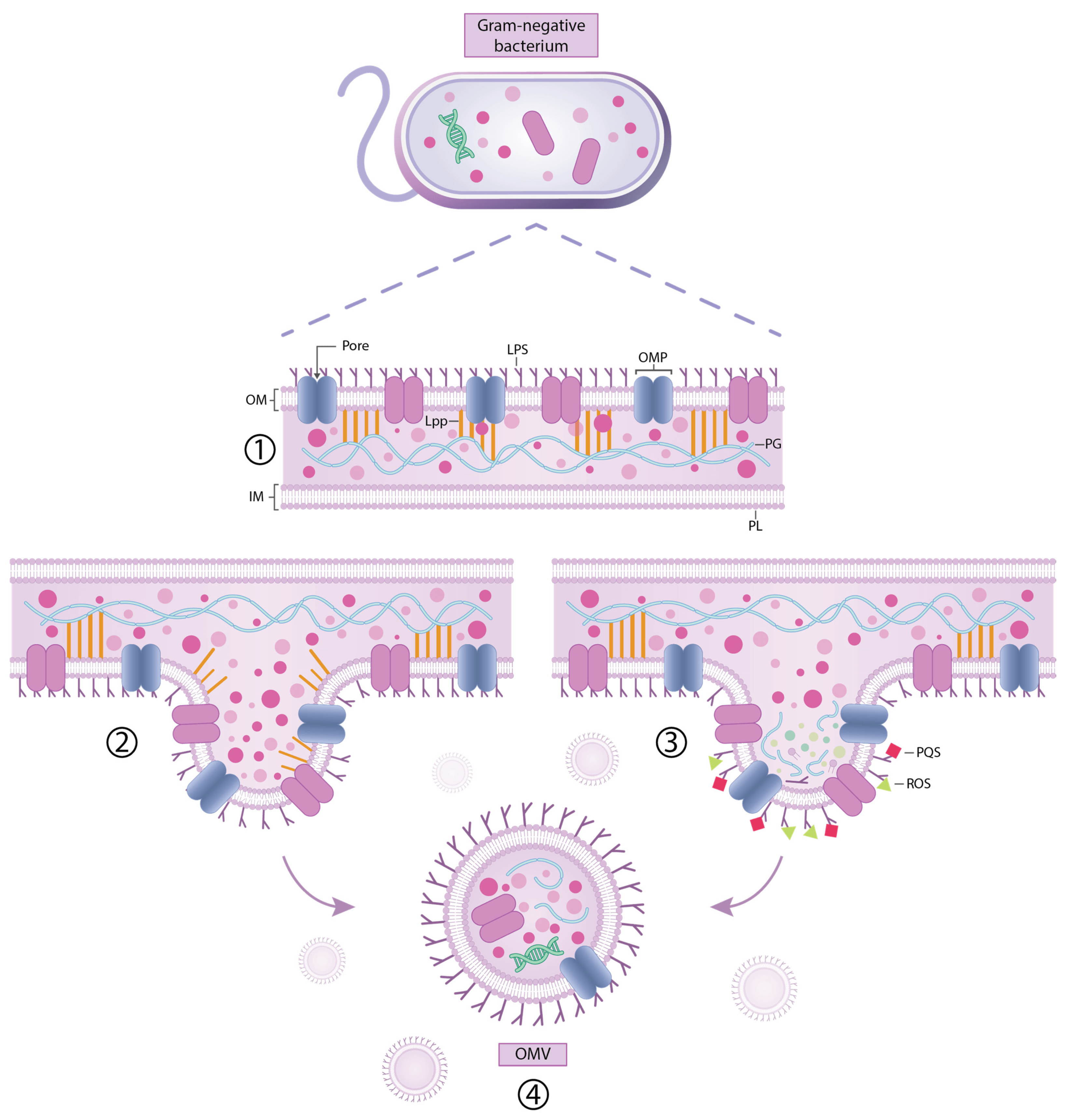
- Juodeikis, R.; Carding, S.R. Outer Membrane Vesicles: Biogenesis, Functions, and Issues. Microbiol. Mol. Biol. Rev. 2022, 86, e0003222. [Google Scholar] [CrossRef] [PubMed]
- Magaña, G.; Harvey, C.; Taggart, C.C.; Rodgers, A.M. Bacterial Outer Membrane Vesicles: Role in Pathogenesis and Host-Cell Interactions. Antibiotics 2023, 13, 32. [Google Scholar] [CrossRef]
- Winkle, M.; Hernández-Rocamora, V.M.; Pullela, K.; Goodall, E.C.A.; Martorana, A.M.; Gray, J.; Henderson, I.R.; Polissi, A.; Vollmer, W. DpaA Detaches Braun’s Lipoprotein from Peptidoglycan. mBio 2021, 12, e00836-21. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Vascon, F.; De Felice, S.; Gasparotto, M.; Huber, S.T.; Catalano, C.; Chinellato, M.; Mezzetti, R.; Grinzato, A.; Filippini, F.; Maso, L.; et al. Snapshots of Pseudomonas aeruginosa SOS response reveal structural requisites for LexA autoproteolysis. iScience 2025, 28, 111726. [Google Scholar] [CrossRef] [PubMed]
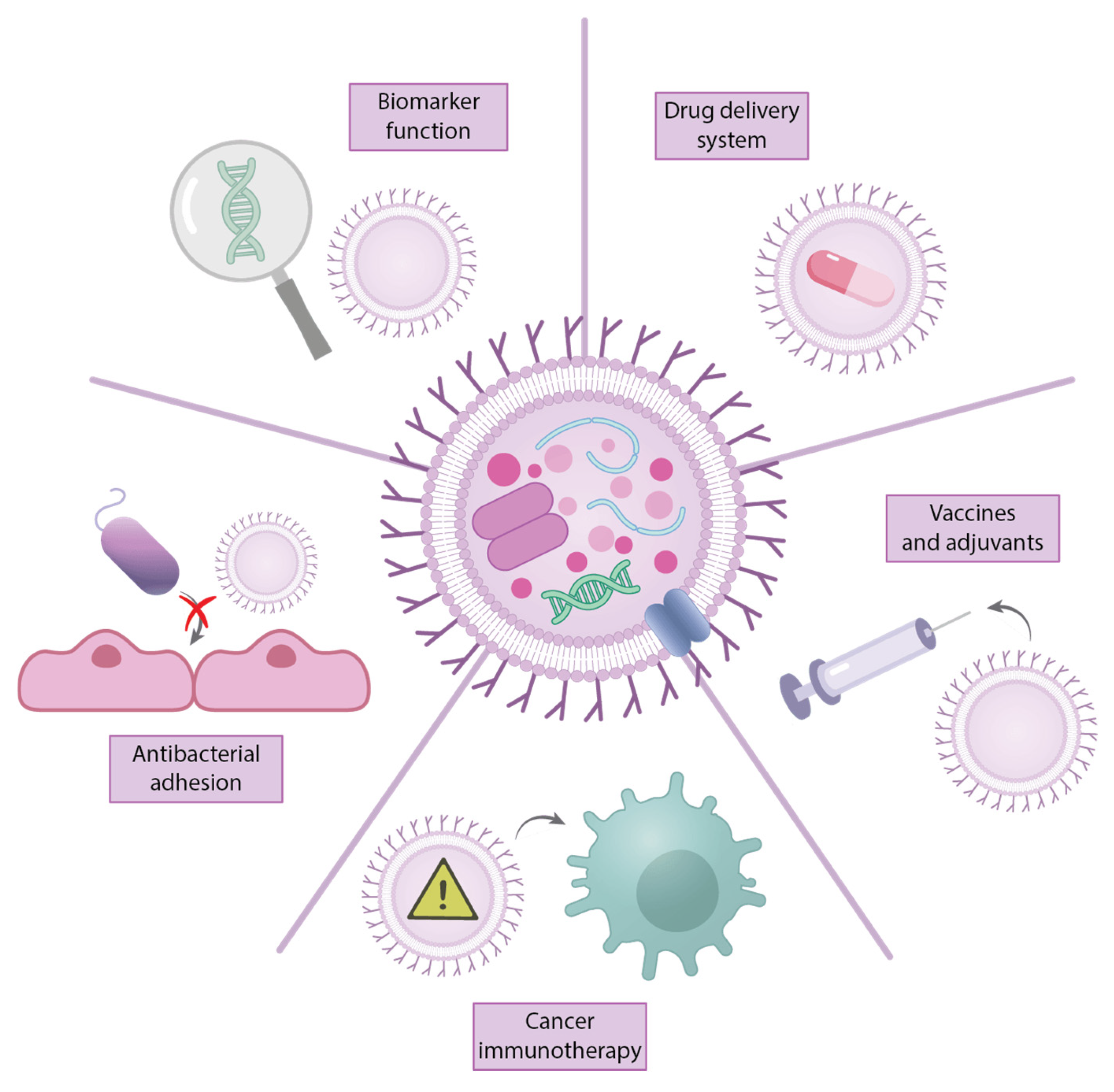
- Li, D.; Zhu, L.; Wang, Y.; Zhou, X.; Li, Y. Bacterial outer membrane vesicles in cancer: Biogenesis, pathogenesis, and clinical application. Biomed. Pharmacother. Biomed. Pharmacother. 2023, 165, 115120. [Google Scholar] [CrossRef]
- Lieberman, L.A. Outer membrane vesicles: A bacterial-derived vaccination system. Front. Microbiol. 2022, 13, 1029146. [Google Scholar] [CrossRef]
- Xiang, S.; Khan, A.; Yao, Q.; Wang, D. Recent advances in bacterial outer membrane vesicles: Effects on the immune system, mechanisms and their usage for tumor treatment. J. Pharm. Anal. 2024, 14, 101049. [Google Scholar] [CrossRef]
- Gonçalves, M.O.; Pincela Lins, P.M.; Kassab, G.; Bagnato, V.; Zucolotto, V. Outer membrane vesicles (OMVs) and their therapeutic potential as anti-infectious agents. Nano Trends 2025, 11, 100129. [Google Scholar] [CrossRef]
- Ajam-Hosseini, M.; Akhoondi, F.; Parvini, F.; Fahimi, H. Gram-negative bacterial sRNAs encapsulated in OMVs: An emerging class of therapeutic targets in diseases. Front. Cell. Infect. Microbiol. 2023, 13, 1305510. [Google Scholar] [CrossRef]
- Li, G.; Pu, S.; You, L.; Gao, Y.; Zhong, Y.; Zhao, H.; Fan, D.; Lu, X. Innovative Strategies in Oncology: Bacterial Membrane Vesicle-Based Drug Delivery Systems for Cancer Diagnosis and Therapy. Pharmaceutics 2025, 17, 58. [Google Scholar] [CrossRef]
- Liu, J.; Wang, T.; Zhou, Y.; Wang, X.; Ma, B.; Su, C.; Duan, X. Bacterial outer membrane vesicles in tumor prevention and treatment: Advancements in research and application. J. Mater. Chem. B 2025, 13, 3786–3805. [Google Scholar] [CrossRef]
- Jalalifar, S.; Morovati Khamsi, H.; Hosseini-Fard, S.R.; Karampoor, S.; Bajelan, B.; Irajian, G.; Mirzaei, R. Emerging role of microbiota derived outer membrane vesicles to preventive, therapeutic and diagnostic proposes. Infect. Agents Cancer 2023, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, Q.; Li, W.; Chen, Y.; Shu, C.; Li, Q.; Zhou, J.; Ye, C.; Bai, H.; Sun, W.; et al. Anti-outer Membrane Vesicle Antibodies Increase Antibiotic Sensitivity of Pan-Drug-Resistant Acinetobacter baumannii. Front. Microbiol. 2019, 10, 1379. [Google Scholar] [CrossRef] [PubMed]
- Pulido, M.R.; García-Quintanilla, M.; Pachón, J.; McConnell, M.J. A lipopolysaccharide-free outer membrane vesicle vaccine protects against Acinetobacter baumannii infection. Vaccine 2020, 38, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Yang, N.; Shi, S.; Xu, Z.; Chen, Z.; Liang, C.; Zhang, X.; Du, X. Outer Membrane Vesicles from Acinetobacter baumannii: Biogenesis, Functions, and Vaccine Application. Vaccines 2023, 12, 49. [Google Scholar] [CrossRef]
- Tiku, V.; Kofoed, E.M.; Yan, D.; Kang, J.; Xu, M.; Reichelt, M.; Dikic, I.; Tan, M.W. Outer membrane vesicles containing OmpA induce mitochondrial fragmentation to promote pathogenesis of Acinetobacter baumannii. Sci. Rep. 2021, 11, 618. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Philippon, A.; Arlet, G.; Labia, R.; Iorga, B.I. Class C β-Lactamases: Molecular Characteristics. Clin. Microbiol. Rev. 2022, 35, e0015021. [Google Scholar] [CrossRef]
- Henriquez, T.; Falciani, C. Extracellular Vesicles of Pseudomonas: Friends and Foes. Antibiotics 2023, 12, 703. [Google Scholar] [CrossRef]
- Augustyniak, D.; Olszak, T.; Drulis-Kawa, Z. Outer Membrane Vesicles (OMVs) of Pseudomonas aeruginosa Provide Passive Resistance but Not Sensitization to LPS-Specific Phages. Viruses 2022, 14, 121. [Google Scholar] [CrossRef]
- Chevalier, S.; Bouffartigues, E.; Bodilis, J.; Maillot, O.; Lesouhaitier, O.; Feuilloley, M.G.J.; Orange, N.; Dufour, A.; Cornelis, P. Structure, function and regulation of Pseudomonas aeruginosa porins. FEMS Microbiol. Rev. 2017, 41, 698–722. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, X.; Huang, Y.; Chen, S.; Liu, M.; Zhang, F.; Li, M.; Wang, X.; Gu, Y.; Yang, Y.; et al. Pseudomonas aeruginosa outer membrane vesicle-packed sRNAs can enter host cells and regulate innate immune responses. Microb. Pathog. 2024, 188, 106562. [Google Scholar] [CrossRef]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Johnston, E.L.; Zavan, L.; Bitto, N.J.; Petrovski, S.; Hill, A.F.; Kaparakis-Liaskos, M. Planktonic and Biofilm-Derived Pseudomonas aeruginosa Outer Membrane Vesicles Facilitate Horizontal Gene Transfer of Plasmid DNA. Microbiol. Spectr. 2023, 11, e0517922. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.N.; Leiman, S.A.; Kuehn, M.J. Naturally produced outer membrane vesicles from Pseudomonas aeruginosa elicit a potent innate immune response via combined sensing of both lipopolysaccharide and protein components. Infect. Immun. 2010, 78, 3822–3831. [Google Scholar] [CrossRef] [PubMed]
- Cooke, A.C.; Nello, A.V.; Ernst, R.K.; Schertzer, J.W. Analysis of Pseudomonas aeruginosa biofilm membrane vesicles supports multiple mechanisms of biogenesis. PLoS ONE 2019, 14, e0212275. [Google Scholar] [CrossRef]
- Kadurugamuwa, J.L.; Beveridge, T.J. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: A novel mechanism of enzyme secretion. J. Bacteriol. 1995, 177, 3998–4008. [Google Scholar] [CrossRef]
- Martora, F.; Pinto, F.; Folliero, V.; Cammarota, M.; Dell’Annunziata, F.; Squillaci, G.; Galdiero, M.; Morana, A.; Schiraldi, C.; Giovane, A.; et al. Isolation, characterization and analysis of pro-inflammatory potential of Klebsiella pneumoniae outer membrane vesicles. Microb. Pathog. 2019, 136, 103719. [Google Scholar] [CrossRef]
- Lee, J.C.; Lee, E.J.; Lee, J.H.; Jun, S.H.; Choi, C.W.; Kim, S.I.; Kang, S.S.; Hyun, S. Klebsiella pneumoniae secretes outer membrane vesicles that induce the innate immune response. FEMS Microbiol. Lett. 2012, 331, 17–24. [Google Scholar] [CrossRef]
- Li, M.; Yu, M.; Yuan, Y.; Li, D.; Ye, D.; Zhao, M.; Lin, Z.; Shi, L. Designing a conjugate vaccine targeting Klebsiella pneumoniae ST258 and ST11. Heliyon 2024, 10, e27417. [Google Scholar] [CrossRef]
- Hussein, M.; Jasim, R.; Gocol, H.; Baker, M.; Thombare, V.J.; Ziogas, J.; Purohit, A.; Rao, G.G.; Li, J.; Velkov, T. Comparative Proteomics of Outer Membrane Vesicles from Polymyxin-Susceptible and Extremely Drug-Resistant Klebsiella pneumoniae. mSphere 2023, 8, e0053722. [Google Scholar] [CrossRef]
- Llobet, E.; March, C.; Giménez, P.; Bengoechea, J.A. Klebsiella pneumoniae OmpA confers resistance to antimicrobial peptides. Antimicrob. Agents Chemother. 2009, 53, 298–302. [Google Scholar] [CrossRef]
- Cai, R.; Deng, H.; Song, J.; Zhang, L.; Zhao, R.; Guo, Z.; Zhang, X.; Zhang, H.; Tian, T.; Ji, Y.; et al. Phage resistance mutation triggered by OmpC deficiency in Klebsiella pneumoniae induced limited fitness costs. Microb. Pathog. 2022, 167, 105556. [Google Scholar] [CrossRef]
- Lucena, A.C.R.; Ferrarini, M.G.; de Oliveira, W.K.; Marcon, B.H.; Morello, L.G.; Alves, L.R.; Faoro, H. Modulation of Klebsiella pneumoniae Outer Membrane Vesicle Protein Cargo under Antibiotic Treatment. Biomedicines 2023, 11, 1515. [Google Scholar] [CrossRef]
- Nafaee, Z.H.; Hunyadi-Gulyás, É.; Gyurcsik, B. Temoneira-1 β-lactamase is not a metalloenzyme, but its native metal ion binding sites allow for purification by immobilized metal ion affinity chromatography. Protein Expr. Purif. 2023, 201, 106169. [Google Scholar] [CrossRef]
- Readman, J.B.; Dickson, G.; Coldham, N.G. Translational Inhibition of CTX-M Extended Spectrum β-Lactamase in Clinical Strains of Escherichia coli by Synthetic Antisense Oligonucleotides Partially Restores Sensitivity to Cefotaxime. Front. Microbiol. 2016, 7, 373. [Google Scholar] [CrossRef] [PubMed]
- Marinacci, B.; Krzyżek, P.; Pellegrini, B.; Turacchio, G.; Grande, R. Latest Update on Outer Membrane Vesicles and Their Role in Horizontal Gene Transfer: A Mini-Review. Membranes 2023, 13, 860. [Google Scholar] [CrossRef] [PubMed]
- Dell’Annunziata, F.; Dell’Aversana, C.; Doti, N.; Donadio, G.; Dal Piaz, F.; Izzo, V.; De Filippis, A.; Galdiero, M.; Altucci, L.; Boccia, G.; et al. Outer Membrane Vesicles Derived from Klebsiella pneumoniae Are a Driving Force for Horizontal Gene Transfer. Int. J. Mol. Sci. 2021, 22, 8732. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Yang, A.; Liu, P.; Wang, Z.; Jian, Z.; Chen, X.; Yan, Q.; Liang, X.; Liu, W. Outer Membrane Vesicles Transmitting bla(NDM-1) Mediate the Emergence of Carbapenem-Resistant Hypervirulent Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2023, 67, e0144422. [Google Scholar] [CrossRef]
- Bhar, S.; Edelmann, M.J.; Jones, M.K. Characterization and proteomic analysis of outer membrane vesicles from a commensal microbe, Enterobacter cloacae. J. Proteom. 2021, 231, 103994. [Google Scholar] [CrossRef]
| Antibiotic | A. baumannii Median % (IQR) | K. pneumonia Median % (IQR) | P. aeruginosa Median % (IQR) | E. cloacae (N = 638) Resistant % | |
|---|---|---|---|---|---|
| Beta–lactam antibiotics | Amoxicillin/ clavulanate | – | 81 (79.3–83.75) | – | 99.84 |
| Ampicillin | – | 100 (90.5–100) | – | 100 | |
| Aztreonam | – | 84.7 (67.27–88.87) | – | – | |
| Cefazolin | – | 93 (78–95.5) | – | – | |
| Cefuroxime | – | 88.9 (79.6–91.42) | – | 79.31 | |
| Cefepime | 94.4 (93–100) | 81.15 (71.7–87.25) | 14.3 (12.5–47.8) | 13 | |
| Ceftazidime | 91.2 (50–100) | 93.5 (83.7–97.9) | 40 (23–41.7) | 23.2 | |
| Cefoperazone/ sulbactam | – | 76.2 (73.8–77.9) | – | – | |
| Ceftriaxone | 76.2 (54.75–95.55) | 84 (77.55–93.4) | 75 (43.75–87.5) | 34 | |
| Meropenem | 92.1 (64.02–95.65) | 71.25 (55.37–77.37) | 38 (18.37–42.17) | 9.56 | |
| Imipenem | 92.1 (80.65–95.72) | 65.7 (19.25–72.87) | 42.9 (19.75–52.9) | 9.87 | |
| Ertapenem | – | 71.4 (55.55–75.05) | – | 9.25 | |
| Piperacillin/ tazobactam | 93.7 (66.9–100) | 77.7 (57.1–79.27) | 11.25 (9.25–13.85) | 13.64 | |
| AMG | Amikacin | 84.6 (56.3–92.95) | 69.85 (58.7–80.12) | 25 (12–28) | – |
| Gentamicin | 95.7 (74.2–97.1) | 57.1 (33.45–86.6) | 25 (19.75–58.75) | 10.7 | |
| FQs | Levofloxacin | 97.05 (91.92–100) | 80.8 (78.55–90.85) | 43.5 (28.6–80) | 7.4 |
| Ciprofloxacin | 91.2 (65–100) | 87.8 (55.1–92.95) | 50 (32.3–62.5) | 10.5 | |
| Other antibiotics | Trimethoprim/ sulfamethoxazole | 50 (46.8–84.2) | 73.5 (32–74) | – | 22.8 |
| Tigecycline | 9.5 (8.8–33.3) | 31.4 (1.7–44) | – | – | |
| Nitrofurantoin | – | 51.8 (38.5–60.6) | – | – | |
| Colistin | 2.5 (0–19.62) | 21.1 (12.42–69.82) | 4 (0–12.25) | – |
| Bacterium | Biofilm Forming Factors | Main Function |
|---|---|---|
| A. baumannii | Fimbriae [64,72,73] | Bacterial mobility [72,79,82,83,84] |
| Pili (type I and type IV) [72] | ||
| Surface-adhesion proteins Baps [64,72,73] | Biofilm initiation and maturation [73,104,105] | |
| Csu pilus (CsuA/BABCDE-mediated pilus) [64,67,72,86,87] | Recognition of adhesion site and biofilm formation on the abiotic surfaces [64,72,91] | |
| TCS BfmRS [72,92] | Regulation of Csu pili operon activity, with downstream impact on cellular structure, surface-associated biofilm architecture, adhesion to inert and biological substrates, and antimicrobial tolerance [64,72,93,94] | |
| Autoinducer synthase AbaI [73,95,97,98,99,100] | Production of signaling molecules involved in QS, supporting normal biofilm development and influencing the expression of antimicrobial resistance determinants, efflux transport components, and motility-associated functions [73,95,97,98,101] | |
| QS, including autoinducer synthase AbaI, receptor protein activator AbaR, along with N-acyl-homoserine lactones (AHLs) [73,99,100] | Cell-to-cell communication [101] | |
| blaPER–1 gene [73] | Contribution to A. baumannii adhesion to respiratory tract linings and development of surface-associated communities on host-derived and inert materials, promoting bacterial persistence and pathogenic potential [73,100] | |
| epsA locus, associated with outer membrane-bound EpsA protein implicated in exopolysaccharide matrix formation; ptk locus, coding for a predicted tyrosine kinase involved in signal transduction and exopolysaccharide regulation [73,100,108,109] | Enhancing the bacterium’s ability to form robust biofilms [73] | |
| Outer membrane porin OmpA [73] | Adhesion to epithelial surfaces, intracellular access mechanisms targeting mitochondrial integrity, and promotion of host cell demise [73,111,112,113,114] Contribution to biofilm formation on abiotic and biotic surfaces [73] | |
| bfmS gene, encoding the cytoplasmic membrane sensor kinase BfmS as part of the TCS BfmRS [73] | Mutations in this gene cause disruption of disrupt AbOmpA regulatory pathways [73] | |
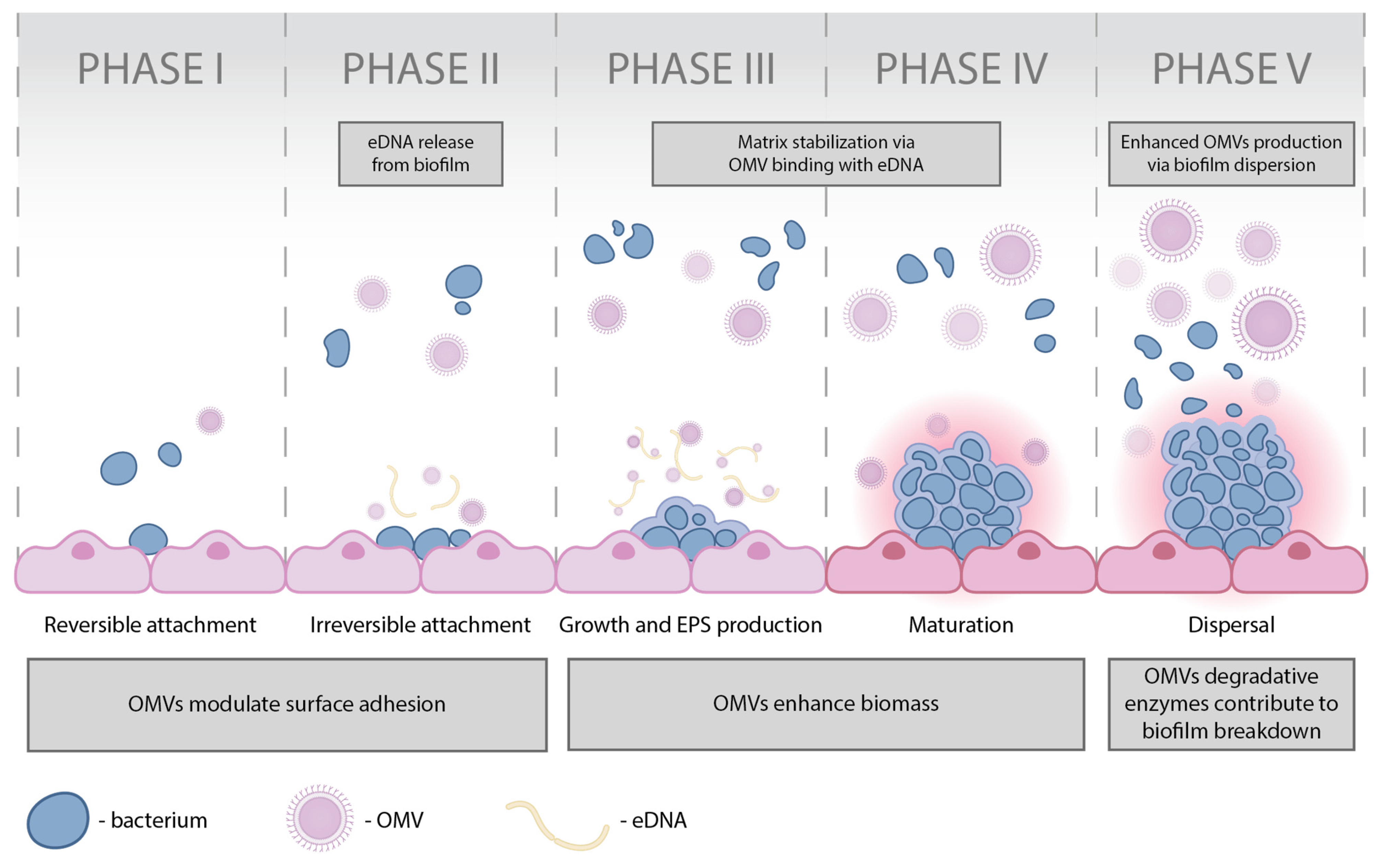
| eDNA [72,116] | Contribution to EPS layer assembly [72,117,118,119] | |
| PNAG, encoded by the pgaABCD gene cluster [72,120,121] | Facilitation of biofilm structuring, bacterial attachment, surface-to-cell adhesion, cell-to-cell interactions, pellicle formation, and contributes to bacterial protection against innate host defenses [72,120,121] | |
| c-di-GMP [72,122] | Regulation of biofilm formation and surface-associated motility) [72,122] | |
| P. aeruginosa | Flagellum [131,132] | Initiation of biofilm formation [131,132] |
| Type IV pili [130,134] | Mediation of irreversible cell-to-surface colonization [130,134] | |
| c-di-GMP, regulated by the Wsp chemosensory-like signal transduction pathway, and promoted by the proteins WspR are FleQ and PelD [130,135] | Production of matrix components, including exopolysaccharides [130,131] | |
| Exopolysaccharides such as alginate, Pel, and Psl [130,131,136,139,140,141] | Contribution to surface attachment and the stability of biofilm architecture [130,131,136,139,140,141] | |
| eDNA [130,136] | Nutrition source for the bacteria within biofilm, cellular organization support, cation chelation, establishment of an optimal environment for bacteria residing in biofilms [131,136,160,161,162,163] | |
| Extracellular adhesin CdrA, secreted with the CdrA- CdrB TCS [130,164] | Bacterial adhesion and preservation of biofilm structural integrity [130,165] | |
| PHA [136,166] | Implication in stress tolerance and attachment to abiotic surfaces like glass, possible contribution to energy-generating metabolic processes [136,166,167,168] | |
| QS circuitry involving Las, Rhl, and PQS-MvfR signaling modules with their respective regulatory elements [131,169,170,171] | Development of mature and differentiated biofilms, also regulation of various processes that contribute to severe systemic infections [131,172,173] | |
| TCSs GacS/GacA and RetS/LadS [173,174] | Contribution of TCS GacS/GacA to biofilm formation, bacterial fitness, motility, stress tolerance, and virulence [173,175,176,177] Contribution of RetS to the repression of biofilm formation, and mediation of biofilm development by LadS [179,180,181,182,183,184,185] | |
| Cyclic glycerophosphorylated β-(1,3)-glucans [131,186] | Contribution to AMR [131,187,188,189] | |
| K. pneumoniae | Type III fimbriae (more dominant) [190] | Biofilm formation: contribution to bacterial adhesion on non-biological surfaces, and modulation of the c-di-GMP-governed shift in growth phenotype from motile to sessile state [190,202,203] Regulation of type III fimbrial expression supporting community establishment, associated with mrkA, mrkD, and the mrkHIJ genetic locus [190,197,198,199,200,203] |
| Type I fimbriae [190] | Biofilm formation, mainly regulated by fimK, fimH, kpfR genes [36,190,195] | |
| E. coli common pilus (ECP) fimbriae [36,190] | Participation in cell adhesion, biofilm formation, and colonization of various environments [190] | |
| Polysaccharide capsule [190,205,206] | Contribution to multiple stages of biofilm formation, including initial adhesion and maturation [190,205,206] The main genes responsible for polysaccharide synthesis include wza homologous, ORF14, treC, and sugE [36,205,207,208] | |
| LPSs [36,208] | Contribution to the initial attachment of K. pneumoniae to abiotic surfaces [36,208]; The main genes responsible for this process are wzm and wbbM [36,208] | |
| Virulence gene wcaG [190,209] | Involvement in capsule biosynthesis and biofilm formation [190,209] | |
| Type II QS pathway based on AI-2 signaling molecules synthesized via LuxS-mediated catalytic activity [36,190,205,212,213] | Regulation of fimbriae, exopolysaccharide, adhesin, and other substance synthesis via signaling molecules, promoting biofilm formation and maturation in bacteria [190,210,211]; Facilitation of interspecific communication, enabling bacteria to respond to both autoinducers: AI-2 produced by other species and their own AI-2 [36,190,205,212,213]; The primary gene responsible for type II QS activity is luxS [36,212] | |
| Efflux pumps AcrA, OqxA, QacEΔ1, and EmrB [205,216] | Contribution to biofilm formation: enhancing antibiotic resistance, regulating exopolysaccharide production, and promoting bacterial survival within the biofilm matrix [205,216]; The primary genes responsible for efflux pump function are acrA, emrB, oqxA, and qacEΔ1 [205,218] | |
| Fur [36,195] | Iron balance regulation within biofilm-associated populations through modulation of genetic pathways linked to iron acquisition and metabolic processing, with downstream effects on community structure, stability, and bacterial virulence [36,195,202,221] | |
| Enterobacter spp. | Curli fimbriae [223,226] | Attachment of bacterial cells to non-living materials and host-associated surfaces [223,226] |
| Secondary type VI secretion apparatus (T6SS-2) [223,227] | Participation in surface association with inert materials and host-derived substrates [223,227] | |
| csgA and cdgD genes [223,228] | Regulation of biofilm formation and control through curli biogenesis [223] | |
| Autoinducers AI-1, AI-2, and AI-3 [223,229] | Decreased bacterial adhesion and biofilm downregulation due to AI-1 [229,230]; Intercellular communication in Enterobacter spp. via Lsr-type receptors induced by AI-2 [223,231,232]; Facilitation of interspecies QS and regulation of biofilm formation through Lsr-type receptors [229,233] |
| Bacterium | OMV Components | Functions |
|---|---|---|
| A. baumannii | Phospholipids and lipopolysaccharides [250] | Maintenance morphology and stability of outer membrane vesicles [250] |
| Outer membrane protein OmpA [251,252] | Determination of vesicle formation sites in the outer membrane, promotion of OMV-mediated pathogenesis, and induction of host cell death, acting as a major virulence factor in humans [251] | |
| Outer membrane protein Omp33-36 [251] | Adhesion, invasion, regulation of autophagy, metabolic adaptability [251] | |
| Outer membrane protein OmpW [251] | Colistin binding, regulation of bacterial iron homeostasis [251] | |
| Outer membrane structure OccAB1 (Carboxylate channel) [251] | Uptaking glycine and ornithine [251] | |
| Outer membrane protein OprD [251] | Facilitating the diffusion of necessary amino acids into the cell [251] | |
| Outer membrane protein CarO [251] | Carbapenem resistance [251] | |
| Phospholipase C, superoxide dismutase, and catalase [251] | Promotion of surface binding and invasive potential [251] | |
| Nucleic acids (DNA, mRNA, miRNA, non-coding RNA) and signaling molecules [253] | Cellular communication, host immune responses, and bacterial nutrition [253] | |
| Enzymatic resistance factors: Ambler class C β-lactamases (AmpC), oxacillinase (OXA), and New Delhi metallo-β-lactamase-1 (NDM-1) [251] | Antimicrobial resistance [251] | |
| blaOXA-24 and blaNDM genes [251] | Facilitating the dissemination of antimicrobial resistance through OMVs [251] | |
| P. aeruginosa | Lipopolysaccharides [255] | Immune system activation, contribution to virulence, participation in the OMV biogenesis and formation [255] |
| Outer membrane protein OprF [255,257] | Possible contribution to OMV interaction with host cells and recognition of external signals from host cell [255,257] | |
| Outer membrane protein OprD [255,257] | Bacterial resistance to carbapenems [255,257] | |
| Outer membrane protein OprE [255,257] | Contribution to bacterial survival in alkaline environment [255,257] | |
| Outer membrane protein OprH [255,257] | Adhesion to the host cell and recognition of external signals from the host cell [255,257] | |
| Small RNA [255] | Entering host cells and influencing the innate immune response [258] | |
| DNA [260] | Promotion of horizontal gene transfer, facilitating the dissemination of antibiotic resistance genes among P. aeruginosa strains [260] | |
| Lipase, nuclease, and protease [256] | Contribution to the extracellular degradation of matrix components during biofilm dispersion [256] | |
| Pseudomonas quinolone signal (PQS) [255] | Contribution to the modulation of virulence traits, biofilm development, iron acquisition, cytotoxicity, and OMV production [255] | |
| K. pneumoniae | OmpA protein embedded in the outer membrane [265] | Immune system activation, contributing to virulence by protecting the bacterium from innate immune responses [267,268] |
| OmpC (OmpK36), a porin embedded in the bacterial outer membrane [265,266] | Enhancement of resistance to β-lactam antibiotics by reducing outer membrane permeability, contribution to carbapenem resistance, particularly in ESBL-positive strains, alteration of the immune response by affecting macrophage inflammatory responses to OMVs, resulting in reduced cytokine secretion [264,269,270] | |
| Outer membrane protein OmpK37 [270] | Contribution to virulence and antimicrobial resistance [270] | |
| Type 1 and type 3 fimbriae genes [271] | Contribution to virulence, bacterial adhesion and biofilm formation [271] | |
| Enterobactin [247,271] | Contribution to virulence [271] | |
| TEM1, CTX-M-15 and KPC-2 [17,271,272] | Contribution to virulence [270] | |
| Efflux pumps AcrAB and OqxAB [270] | Mediation of antimicrobial resistance and contribution to virulence [270] | |
| LptD, MsRA, LptE, ArnT (proteins from lipopolysaccharide transport machinery) [270] | Contribution to maintaining outer membrane integrity and resistance to antimicrobial agents, including polymyxins [270] | |
| Ribosomal proteins (21 from 30S, 15 from 50S), RNA polymerase sigma factor RpoD, RNA polymerase alpha and beta subunits, NlpD, YbiS, MltA, LpoA, TolB proteins [270] | Contribution to resistance to polymyxin B through transcription and translation disruption, participation in vesiculation, which is enhanced by meropenem exposure [270] | |
| QS factors [17] | Involvement in pathogenic potential [17] | |
| Enterobacter spp. | Outer membrane protein OmpX [276] | Contribution to virulence and biofilm formation [276] |
| OmpA family lipoprotein, predicted metalloprotease YggG, BOF-like domain protein, and immunoglobulin-like domain protein [276] | Involvement in bacterial–host engagement [276] | |
| Hydrolases [276] | Facilitation of cell interaction, enhancement of immune tolerance, and mediation of microbial compound transfer across the intestinal epithelial barrier [276] | |
| Endolytic peptidoglycan transglucosylase RlpA, PBP activator LpoA, Lpp-repeat motif-containing lipoprotein, predicted outer membrane lipoprotein, M23B family peptidase [276] | Involvement in peptidoglycan synthesis and metabolism [276] | |
| Copper lipoprotein, uncharacterized protein, periplasmic protein, periplasmic trehalase, and penicillin-binding protein-1b (PBP-1b) [276] | Involvement in stress response [276] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakalauskienė, G.V.; Radzevičienė, A. Biofilm and Outer Membrane Vesicle Formation in ESKAPE Gram-Negative Bacteria: A Comprehensive Review. Int. J. Mol. Sci. 2025, 26, 9857. https://doi.org/10.3390/ijms26209857
Sakalauskienė GV, Radzevičienė A. Biofilm and Outer Membrane Vesicle Formation in ESKAPE Gram-Negative Bacteria: A Comprehensive Review. International Journal of Molecular Sciences. 2025; 26(20):9857. https://doi.org/10.3390/ijms26209857
Chicago/Turabian StyleSakalauskienė, Giedrė Valdonė, and Aurelija Radzevičienė. 2025. "Biofilm and Outer Membrane Vesicle Formation in ESKAPE Gram-Negative Bacteria: A Comprehensive Review" International Journal of Molecular Sciences 26, no. 20: 9857. https://doi.org/10.3390/ijms26209857
APA StyleSakalauskienė, G. V., & Radzevičienė, A. (2025). Biofilm and Outer Membrane Vesicle Formation in ESKAPE Gram-Negative Bacteria: A Comprehensive Review. International Journal of Molecular Sciences, 26(20), 9857. https://doi.org/10.3390/ijms26209857








