Identifying Plasma Biomarkers That Predict Patient-Reported Outcomes Following Treatment for Trapeziometacarpal Osteoarthritis Using Machine Learning
Abstract
1. Introduction
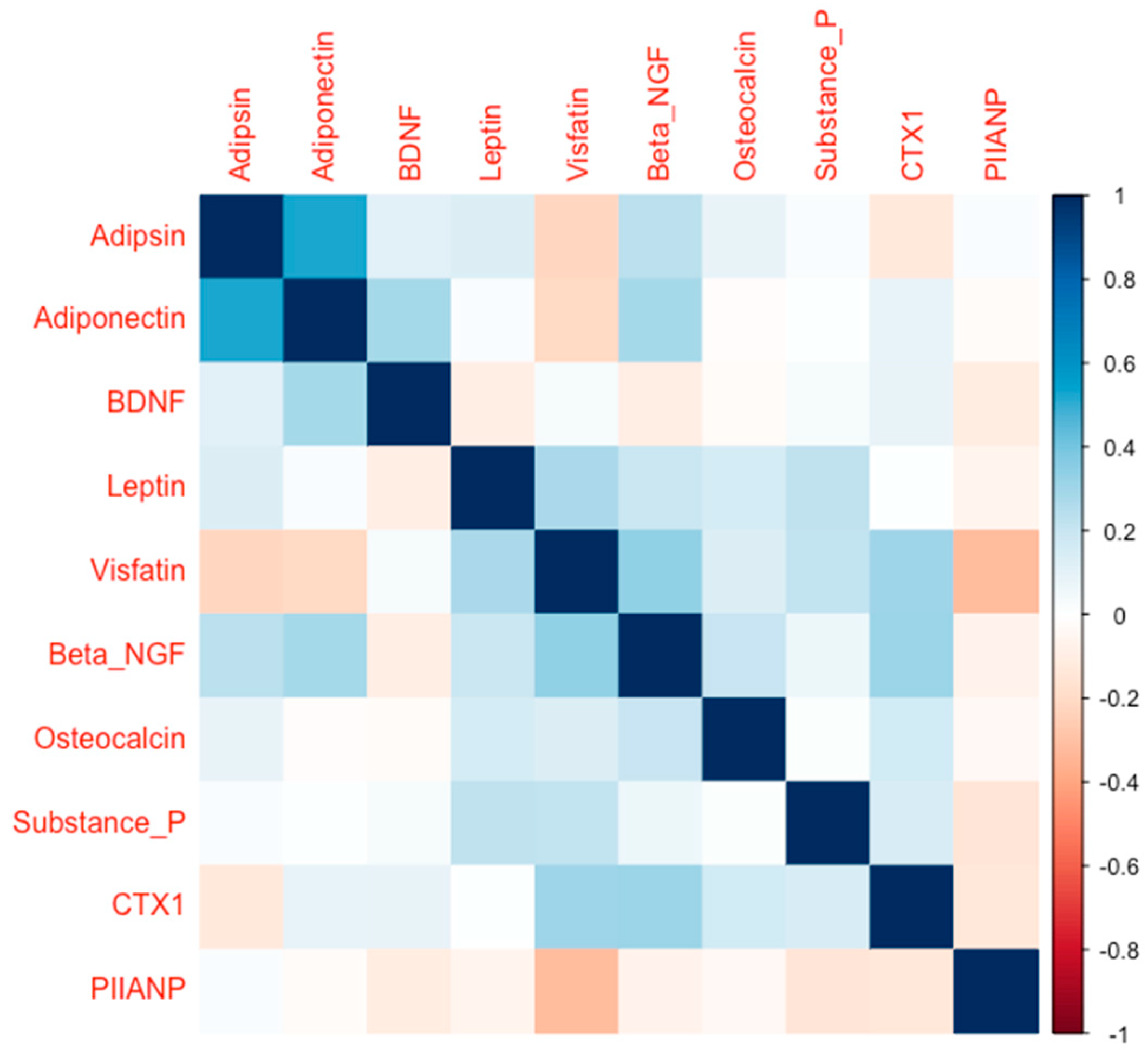
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Sample
4.2. Quantification of Protein Markers in Plasma
4.3. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BDNF | Brain-derived neurotrophic factor |
| Beta-NGF | Beta-nerve growth factor |
| CTX-1 | Type I collagen cross-linked C-telopeptide |
| OA | Osteoarthritis |
| PIIANP | N-propeptide of collagen IIA |
| PROM | Patient-reported outcome measure |
| QuickDASH | Quick Disabilities of the Arm, Shoulder, and Hand. |
| TASD | Trapeziometacarpal Arthrosis Symptoms and Disability |
| TM | Trapeziometacarpal |
| VAS | Visual analog scale |
Appendix A
| Protein Markers | Singleplex/Multiplex | Vendor | Catalog # | |
|---|---|---|---|---|
| 1. | PIIANP | Singleplex | MyBioSource, San Diego, CA, USA | MBS8807733 |
| 2. | Osteocalcin | Singleplex | abcam, Waltham, MA, USA | ab270202 |
| 3. | CTX-1 | Singleplex | Bio-techne, Toronto, ON, Canada | NBP2-69073 |
| 4. | Substance P | Singleplex | abcam, Waltham, MA, USA | ab288318 |
| 5. | Adiponectin | 2-plex | Bio-Rad, Mississauga, ON, Canada | 171A7002M |
| 6. | Adipsin | Bio-Rad, Mississauga, ON, Canada | 171A7002M | |
| 7. | Leptin | 4-plex | Bio-techne, Toronto, ON, Canada | LXSAHM-04 |
| 8. | Visfatin | Bio-techne, Toronto, ON, Canada | LXSAHM-04 | |
| 9. | Beta-NGF | Bio-techne, Toronto, ON, Canada | LXSAHM-04 | |
| 10. | BDNF | Bio-techne, Toronto, ON, Canada | LXSAHM-04 |
References
- Maniglio, M.; Loisay, L.; de Haro, D.; Antoniadis, A.; Hügle, T.; Geurts, J. Subchondral bone marrow adipose tissue lipolysis regulates bone formation in hand osteoarthritis. Osteoarthr. Cartil. 2024, 33, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Ladd, A.L.; Crisco, J.J.; Hagert, E.; Rose, J.; Weiss, A.-P.C. The 2014 ABJS Nicolas Andry award: The puzzle of the thumb: Mobility, stability, and demands in opposition. Clin. Orthop. Relat. Res. 2014, 472, 3605–3622. [Google Scholar] [CrossRef]
- Moriatis Wolf, J.; Turkiewicz, A.; Atroshi, I.; Englund, M. Prevalence of doctor-diagnosed thumb carpometacarpal joint osteoarthritis: An analysis of swedish health care. Arthritis Care Res. 2014, 66, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F. Aging and osteoarthritis: The role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthr. Cartil. 2009, 17, 971–979. [Google Scholar] [CrossRef]
- Maniglio, L.; Maniglio, M.; Aregger, F.; Schweizer, A. Is increased trapezial slope a cause of early trapeziometacarpal osteoarthritis? Hand Surg. Rehabil. 2023, 42, 464–469. [Google Scholar] [CrossRef]
- Ratneswaran, A.; Rockel, J.S.; Antflek, D.; Matelski, J.J.; Shestopaloff, K.; Kapoor, M.; Baltzer, H. Investigating molecular signatures underlying trapeziometacarpal osteoarthritis through the evaluation of systemic cytokine expression. Front. Immunol. 2021, 12, 794792. [Google Scholar] [CrossRef]
- Griffin, T.M.; Guilak, F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc. Sport. Sci. Rev. 2005, 33, 195–200. [Google Scholar] [CrossRef]
- Wolff, J. The Law of Bone Remodelling; Springer: Berlin/Heidelberg, Germany, 1986. [Google Scholar]
- Marshall, M.; van der Windt, D.; Nicholls, E.; Myers, H.; Dziedzic, K. Radiographic thumb osteoarthritis: Frequency, patterns and associations with pain and clinical assessment findings in a community-dwelling population. Rheumatology 2011, 50, 735–739. [Google Scholar] [CrossRef]
- Hoffler, C.E., 2nd; Matzon, J.L.; Lutsky, K.F.; Kim, N.; Beredjiklian, P.K. Radiographic stage does not correlate with symptom severity in thumb basilar joint osteoarthritis. J. Am. Acad. Orthop. Surg. 2015, 23, 778–782. [Google Scholar] [CrossRef]
- Dahaghin, S.; Bierma-Zeinstra, S.M.; Ginai, A.Z.; Pols, H.A.; Hazes, J.M.; Koes, B.W. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the rotterdam study). Ann. Rheum. Dis. 2005, 64, 682–687, Erratum in Ann. Rheum. Dis. 2005, 64, 1248. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, T.; Lalonde, L.; Harris, P.; Bureau, N.J.; Gaudreault, N.; Ziegler, D.; Choinière, M. Efficacy of treatments and pain management for trapeziometacarpal (thumb base) osteoarthritis: Protocol for a systematic review. BMJ Open 2015, 5, e008904. [Google Scholar] [CrossRef]
- Lotz, M.; Martel-Pelletier, J.; Christiansen, C.; Brandi, M.L.; Bruyère, O.; Chapurlat, R.; Collette, J.; Cooper, C.; Giacovelli, G.; Kanis, J.A.; et al. Value of biomarkers in osteoarthritis: Current status and perspectives. Ann. Rheum. Dis. 2013, 72, 1756–1763, Erratum in Ann. Rheum. Dis. 2017, 76, e35. [Google Scholar] [CrossRef]
- Hunter, D.J.; Nevitt, M.; Losina, E.; Kraus, V. Biomarkers for osteoarthritis: Current position and steps towards further validation. Best Pract. Res. Clin. Rheumatol. 2014, 28, 61–71. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Thudium, C.S.; Bay-Jensen, A.-C.; Maleitzke, T.; Geissler, S.; Duda, G.N.; Winkler, T. Biomarkers for osteoarthritis: Current status and future prospects. Best Pract. Res. Clin. Rheumatol. 2023, 37, 101852. [Google Scholar] [CrossRef] [PubMed]
- Aborode, A.T.; Emmanuel, O.A.; Onifade, I.A.; Olotu, E.; Otorkpa, O.J.; Mehmood, Q.; Abdulai, S.I.; Jamiu, A.; Osinuga, A.; Oko, C.I.; et al. The role of machine learning in discovering biomarkers and predicting treatment strategies for neurodegenerative diseases: A narrative review. NeuroMarkers 2025, 2, 100034. [Google Scholar] [CrossRef]
- Sandhu, A.; Rockel, J.S.; Lively, S.; Kapoor, M. Emerging molecular biomarkers in osteoarthritis pathology. Ther. Adv. Musculoskelet. Dis. 2023, 15, 1759720X231177116. [Google Scholar] [CrossRef]
- Bauer, D.C.; Hunter, D.J.; Abramson, S.B.; Attur, M.; Corr, M.; Felson, D.; Heinegård, D.; Jordan, J.M.; Kepler, T.B.; Lane, N.E.; et al. Classification of osteoarthritis biomarkers: A proposed approach. Osteoarthr. Cartil. 2006, 14, 723–727. [Google Scholar] [CrossRef]
- Bay-Jensen, A.C.; Andersen, T.L.; Charni-Ben Tabassi, N.; Kristensen, P.W.; Kjaersgaard-Andersen, P.; Sandell, L.; Garnero, P.; Delaissé, J.M. Biochemical markers of type II collagen breakdown and synthesis are positioned at specific sites in human osteoarthritic knee cartilage. Osteoarthr. Cartil. 2008, 16, 615–623. [Google Scholar] [CrossRef]
- Liao, L.; Chen, Y.; Wang, W. The current progress in understanding the molecular functions and mechanisms of visfatin in osteoarthritis. J. Bone Miner. Metab. 2016, 34, 485–490. [Google Scholar] [CrossRef]
- Santangelo, K.S.; Nuovo, G.J.; Bertone, A.L. In vivo reduction or blockade of interleukin-1β in primary osteoarthritis influences expression of mediators implicated in pathogenesis. Osteoarthr. Cartil. 2012, 20, 1610–1618. [Google Scholar] [CrossRef]
- Saggaf, M.M.; Roy, M.; Antflek, D.; Borkhoff, C.M.; Baltzer, H. Assessing responsiveness of the trapeziometacarpal arthrosis symptoms and disability questionnaire. Hand 2024, 19, 96–103. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. Ser. B (Methodol.) 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Stone, M. Cross-validatory choice and assessment of statistical predictions. J. R. Stat. Soc. Ser. B (Methodol.) 1974, 36, 111–147. [Google Scholar] [CrossRef]
- Zeger, S.L.; Liang, K.Y.; Albert, P.S. Models for longitudinal data: A generalized estimating equation approach. Biometrics 1988, 44, 1049–1060, Erratum in Biometrics 1989, 45, 347. [Google Scholar] [CrossRef]
| Sex | N = 143 |
| Female | 99 (69%) |
| Male | 44 (31%) |
| Age | N = 143 |
| Mean (range) | 61 (42–87) |
| BMI | N = 142 |
| Mean (SD) | 26.8 (5.6) |
| Eaton–Littler Grade | N = 138 |
| 1/2 | 44 (32%) |
| 3 | 58 (42%) |
| 4 | 36 (26%) |
| Scores at baseline | Mean (SD) |
| VAS Pain (N = 140) | 61 (24) |
| QuickDASH Score (N = 142) | 45 (19) |
| TASD Score (N = 143) | 52 (19) |
| TASD Symptom Subscale (N = 143) | 52 (19) |
| TASD Disability Subscale (N = 143) | 53 (23) |
| Marker * | QuickDASH | VAS | TASD | TASD Symptom | TASD Disability |
|---|---|---|---|---|---|
| Adipsin Baseline One-year | |||||
| Adiponectin Baseline One-year | −1.49 | −1.78 | −3.63 | −3.63 | −2.71 |
| BDNF Baseline One-year | |||||
| Leptin Baseline One-year | +0.01 | +1.88 | +0.58 | +0.90 | |
| Visfatin Baseline One-year | +0.77 −1.93 | +3.32 −2.19 | +1.26 −1.35 | +0.81 −1.08 | +1.26 −1.26 |
| Beta-NGF Baseline One-year | |||||
| Osteocalcin Baseline One-year | |||||
| Substance P Baseline One-year | |||||
| CTX-1 Baseline One-year | −0.91 | ||||
| PIIANP Baseline One-year | −2.56 +1.21 | +1.47 | −0.37 +0.38 | −0.23 +0.47 | +1.16 |
| Outcome | Biomarker | Estimate * (95% CI) | p-Value |
|---|---|---|---|
| QuickDASH | Visfatin PIIANP Adiponectin Leptin | 1.01 (−0.97 to 3.13) −3.99 (−5.98 to −1.99) −1.30 (−3.51 to 0.90) −0.02 (−2.31 to 2.26) | 0.30 <0.0001 0.25 0.98 |
| VAS | Visfatin PIIANP Adiponectin Leptin CTX-1 | 3.04 (0.14 to 5.93) −3.09 (−6.07 to −0.11) −2.76 (−5.92 to 0.39) 1.77 (−1.53 to 5.07) −1.23 (−4.13 to 1.67) | 0.04 0.04 0.09 0.29 0.41 |
| TASD | Visfatin PIIANP Adiponectin Leptin | 1.04 (−0.95 to 3.02) −2.42 (−4.51 to −0.33) −1.60 (−3.85 to 0.66) 0.64 (−1.64 to 2.93) | 0.30 0.02 0.17 0.58 |
| TASD Symptom Subscale | Visfatin PIIANP Adiponectin Leptin | 0.69 (−1.16 to 2.54) −1.91 (−3.90 to 0.08) −1.59 (−3.79 to 0.63) 1.17 (−0.99 to 3.32) | 0.47 0.06 0.16 0.29 |
| TASD Disability Subscale | Visfatin PIIANP Adiponectin | 1.52 (−0.92 to 3.96) −3.13 (−5.70 to −0.56) −1.62 (−4.26 to 1.01) | 0.22 0.02 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maniglio, M.; Saggaf, M.; Purohit, N.; Antflek, D.; Rockel, J.S.; Kapoor, M.; Baltzer, H.L. Identifying Plasma Biomarkers That Predict Patient-Reported Outcomes Following Treatment for Trapeziometacarpal Osteoarthritis Using Machine Learning. Int. J. Mol. Sci. 2025, 26, 9856. https://doi.org/10.3390/ijms26209856
Maniglio M, Saggaf M, Purohit N, Antflek D, Rockel JS, Kapoor M, Baltzer HL. Identifying Plasma Biomarkers That Predict Patient-Reported Outcomes Following Treatment for Trapeziometacarpal Osteoarthritis Using Machine Learning. International Journal of Molecular Sciences. 2025; 26(20):9856. https://doi.org/10.3390/ijms26209856
Chicago/Turabian StyleManiglio, Mauro, Moaath Saggaf, Nupur Purohit, Daniel Antflek, Jason S. Rockel, Mohit Kapoor, and Heather L. Baltzer. 2025. "Identifying Plasma Biomarkers That Predict Patient-Reported Outcomes Following Treatment for Trapeziometacarpal Osteoarthritis Using Machine Learning" International Journal of Molecular Sciences 26, no. 20: 9856. https://doi.org/10.3390/ijms26209856
APA StyleManiglio, M., Saggaf, M., Purohit, N., Antflek, D., Rockel, J. S., Kapoor, M., & Baltzer, H. L. (2025). Identifying Plasma Biomarkers That Predict Patient-Reported Outcomes Following Treatment for Trapeziometacarpal Osteoarthritis Using Machine Learning. International Journal of Molecular Sciences, 26(20), 9856. https://doi.org/10.3390/ijms26209856





