Cardioprotective Mechanisms of Beta-Blockers in Myocardial Ischemia and Reperfusion: From Molecular Targets to Clinical Implications
Abstract
1. Introduction
2. Current Classification and Main Actions
2.1. First-Generation Beta-Blockers
2.2. Second-Generation Beta-Blockers
2.3. Third-Generation Beta-Blockers
3. Ischemia-Induced Myocardial Remodeling
3.1. Initial Ischemic Changes at the Cellular Level
3.2. Adverse Myocardial Remodeling
3.3. Biochemical Mechanisms of Adverse Remodeling
3.3.1. Cellular Changes
3.3.2. Extracellular Matrix Changes
3.3.3. Inflammation
3.3.4. Endothelin
3.3.5. Neurohormonal Regulation
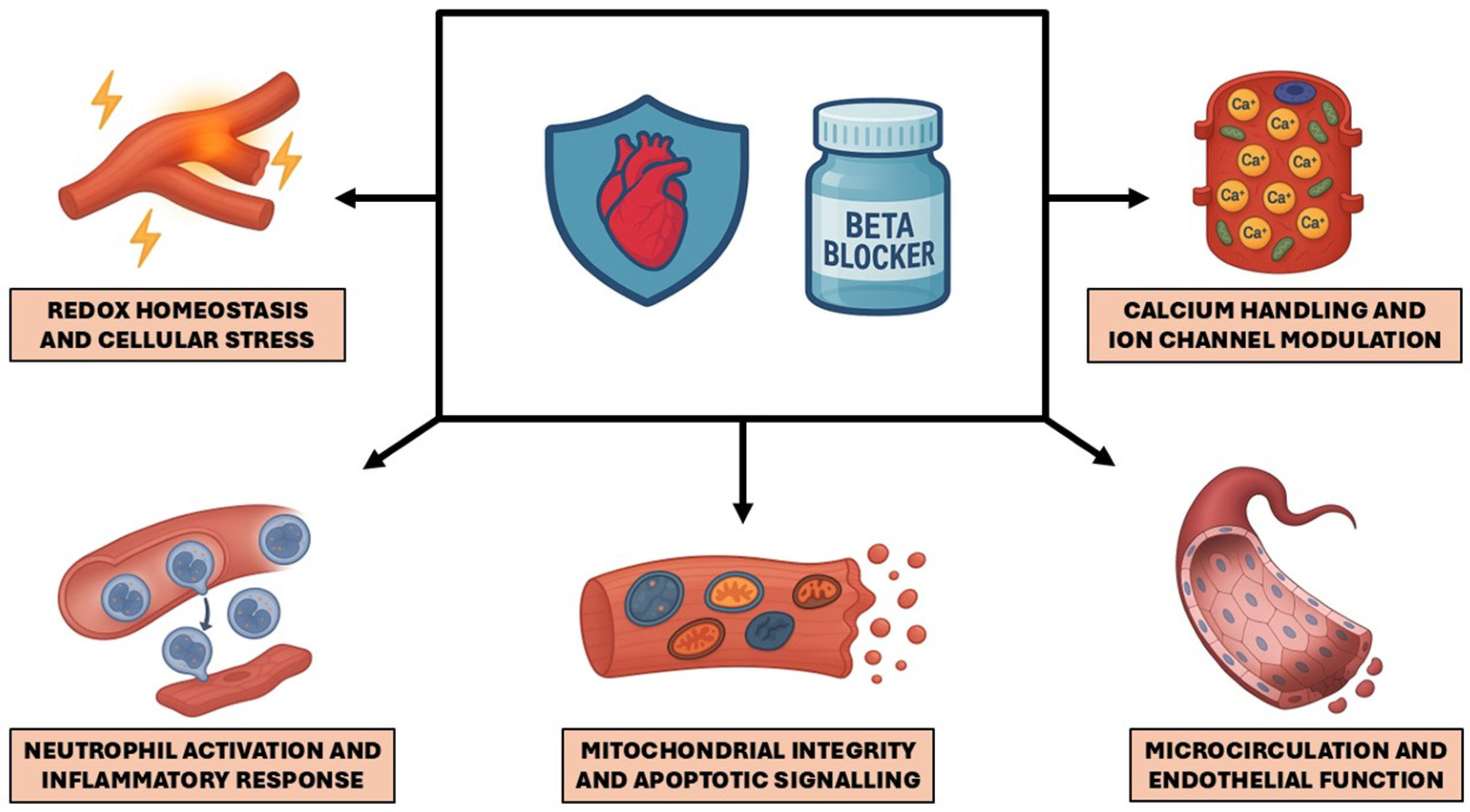
4. Molecular and Cellular Mechanisms of Expanded Beta-Blocker Cardioprotection
4.1. Redox Homeostasis and Cellular Stress
4.2. Neutrophil Activation and Inflammatory Response
4.3. Mitochondrial Integrity and Apoptotic Signaling
4.4. Microcirculation and Endothelial Function
4.5. Calcium Handling and Ion Channel Modulation
5. Current Beta-Blocker Positioning in Guidelines and Data from Clinical Studies
5.1. Current Position in Guidelines
5.2. Recent Clinical Data
5.3. Imaging and Functional Data
5.3.1. Imaging Outcomes in Systolic Dysfunction
5.3.2. Imaging Outcomes in Preserved Systolic Function
5.4. Markers of Myocardial Injury
5.5. Other Results
6. Limitations and Future Research Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4-HNE | 4-hydroxynonenal | IHD | ischemic heart disease |
| AEBP1 | adipocyte enhancer -binding protein 1 | IL | interleukin |
| ACS | acute coronary syndrome | iPSC | induced pluripotent stem cell |
| AMI | acute myocardial infarction | IRI | ischemia/reperfusion injury |
| AP | angina pectoris | JNK | c-Jun N-terminal kinase |
| AT1 | angiotensin II type 1 receptor | LTCC | L-type calcium channel |
| AT2 | angiotensin II type 2 receptor | LV | left ventricle |
| ATP | adenosine triphosphate | LVEF | left ventricular ejection fraction |
| BB | beta-blocker | MACE | major adverse cardiovascular events |
| BH4 | tetrahydrobiopterin | MAPK | mitogen-activated protein kinase |
| BP | blood pressure | MCP-1 | monocyte chemoattractant protein-1 |
| CAD | coronary artery disease | MEK | MAPK kinase |
| CaMKII | Calcium/calmodulin- dependent protein kinase II | miR | microRNA |
| cAMP | cyclic adenosine monophosphate | miRNA | microRNA |
| cGMP | cyclic guanosine monophosphate | MnSOD | manganese superoxide dismutase |
| CILP1 | cartilage intermediate layer protein 1 | mPTP | mitochondrial permeability transition pore |
| CNS | central nervous system | MVO | microvascular obstruction |
| COX-2 | cyclooxygenase-2 | NADPH | nicotinamide adenine dinucleotide phosphate |
| CXCL1 | C-X-C motif chemokine ligand 1 | NCX | sodium/calcium exchanger |
| DNA | deoxyribonucleic acid | NET | neutrophil extracellular trap |
| ECC | excitation–contraction coupling | NF-κB | nuclear factor kappa-light-chain- enhancer of activated B cells |
| ECM | extracellular matrix | NO | nitric oxide |
| EF | ejection fraction | NOX | NADPH oxidase |
| eNOS | endothelial nitric oxide synthase | PAR1 | protease-activated receptor 1 |
| ET-1 | endothelin-1 | PDGF-A | platelet-derived growth factor A |
| fMLP | formyl-Methionyl-Leucyl- Phenylalanine | PI3K | phosphoinositide 3-kinase |
| Gi | inhibitory G protein | PKA | protein kinase A |
| GPCR | G protein-coupled receptor | PKB | protein kinase B |
| GPx | glutathione peroxidase | PKC | protein kinase C |
| GRK | G protein-coupled receptor kinase | PLB | phospholamban |
| GRO | growth-related oncogene | RAAS | renin–angiotensin–aldosterone system |
| Gs | stimulatory G protein | ROS | reactive oxygen species |
| GSH | reduced glutathione | RyR2 | ryanodine receptor 2 |
| GSSG | oxidized glutathione | SAPK | stress-activated protein kinase |
| H2O2 | hydrogen peroxide | SERCA2a | sarcoplasmic/endoplasmic reticulum Ca2+-ATPase 2a |
| HF | heart failure | SOD | superoxide dismutase |
| HFmrEF | heart failure with mildly reduced ejection fraction | SNS | sympathetic nervous system |
| HFpEF | heart failure with preserved ejection fraction | SR | sarcoplasmic reticulum |
| HFrEF | heart failure with reduced ejection fraction | TGFBI | transforming growth factor β-induced |
| HNX | Na+/H+ exchanger | TLR | toll-like receptor |
| HR | heart rate | TNF-α | tumor necrosis factor alpha |
| HSP60 | heat shock protein 60 | VCAM-1 | vascular cell adhesion molecule-1 |
| I/R | ischemia/reperfusion |
References
- Stark, B.; Johnson, C.; Roth, G.A. Global Prevalence of Coronary Artery Disease: An Update from the Global Burden of Disease Study. JACC 2024, 83, 2320. [Google Scholar] [CrossRef]
- Samaras, A.; Papazoglou, A.S.; Moysidis, D.V.; Bekiaridou, A.; Tsoumakas, G.; Tzikas, A.; Chalikias, G.K.; Farmakis, D.; Tsigkas, G.; Lazaros, G.; et al. Clinical Profiles and Temporal Trends of 37,741 Cardiovascular Hospitalizations in Greece over 12 Years: Initial Insights from the CardioMining Database. Hell. J. Cardiol. 2025. online ahead of print. [Google Scholar] [CrossRef]
- Soares, R.O.S.; Losada, D.M.; Jordani, M.C.; Évora, P.; Castro-e-Silva, O. Ischemia/Reperfusion Injury Revisited: An Overview of the Latest Pharmacological Strategies. Int. J. Mol. Sci. 2019, 20, 5034. [Google Scholar] [CrossRef]
- Frishman, W.H. Beta-Adrenergic Blockers: A 50-Year Historical Perspective. Am. J. Ther. 2008, 15, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.; Swedberg, K.; Leong, D.P.; Yusuf, S. The Evolution of β-Blockers in Coronary Artery Disease and Heart Failure (Part 1/5). J. Am. Coll. Cardiol. 2019, 74, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Bristow, M.R. Beta-Adrenergic Receptor Blockade in Chronic Heart Failure. Circulation 2000, 101, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Anderson, P.J.; Rajagopal, S.; Lefkowitz, R.J.; Rockman, H.A. G Protein-Coupled Receptors: A Century of Research and Discovery. Circ. Res. 2024, 135, 174–197. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.V. Beta-Blockers: The Constantly Swinging Pendulum. J. Am. Coll. Cardiol. 2023, 81, 2312–2314. [Google Scholar] [CrossRef]
- Poirier, L.; Tobe, S.W. Contemporary Use of β-Blockers: Clinical Relevance of Subclassification. Can. J. Cardiol. 2014, 30, S9–S15. [Google Scholar] [CrossRef]
- Messerli, F.H.; Bangalore, S.; Yao, S.S.; Steinberg, J.S. Cardioprotection with Beta-Blockers: Myths, Facts and Pascal’s Wager. J. Intern. Med. 2009, 266, 232–241. [Google Scholar] [CrossRef]
- Reiter, M.J. Cardiovascular Drug Class Specificity: Beta-Blockers. Prog. Cardiovasc. Dis. 2004, 47, 11–33. [Google Scholar] [CrossRef] [PubMed]
- Kamp, T.J.; Hell, J.W. Regulation of Cardiac L-Type Calcium Channels by Protein Kinase A and Protein Kinase C. Circ. Res. 2000, 87, 1095–1102. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Kim, S.; Fu, Q.; Parikh, D.; Sridhar, B.; Shi, Q.; Zhang, X.; Guan, Y.; Chen, X.; et al. Phosphodiesterases Coordinate cAMP Propagation Induced by Two Stimulatory G Protein-Coupled Receptors in Hearts. Proc. Natl. Acad. Sci. USA 2012, 109, 6578–6583. [Google Scholar] [CrossRef]
- Lager, I.; Blohmé, G.; Smith, U. Effect of Cardioselective and Non-Selective Beta-Blockade on the Hypoglycaemic Response in Insulin-Dependent Diabetics. Lancet 1979, 1, 458–462. [Google Scholar] [CrossRef]
- Boskabady, M.H.; Snashall, P.D. Bronchial Responsiveness to Beta-Adrenergic Stimulation and Enhanced Beta-Blockade in Asthma. Respirology 2000, 5, 111–118. [Google Scholar] [CrossRef]
- Marsden, C.D.; Foley, T.H.; Owen, D.A.; McAllister, R.G. Peripheral Beta-Adrenergic Receptors Concerned with Tremor. Clin. Sci. 1967, 33, 53–65. [Google Scholar]
- Do Vale, G.T.; Ceron, C.S.; Gonzaga, N.A.; Simplicio, J.A.; Padovan, J.C. Three Generations of β-Blockers: History, Class Differences and Clinical Applicability. Curr. Hypertens. Rev. 2019, 15, 22–31. [Google Scholar] [CrossRef]
- Barrett, A.M.; Carter, J.; Fitzgerald, J.D.; Hull, R.; Le Count, D. A New Type of Cardioselective Adrenoceptive Blocking Drug. Br. J. Pharmacol. 1973, 48, 340P. [Google Scholar] [PubMed]
- Dunlop, D.; Shanks, R.G. Selective Blockade of Adrenoceptive Beta Receptors in the Heart. Br. J. Pharmacol. Chemother. 1968, 32, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Ablad, B.; Carlsson, E.; Ek, L. Pharmacological Studies of Two New Cardioselective Adrenergic Beta-Receptor Antagonists. Life Sci. 1973, 12, 107–119. [Google Scholar] [CrossRef]
- Zhou, X.; Ma, L.; Habibi, J.; Whaley-Connell, A.; Hayden, M.R.; Tilmon, R.D.; Brown, A.N.; Kim, J.-A.; Demarco, V.G.; Sowers, J.R. Nebivolol Improves Diastolic Dysfunction and Myocardial Remodeling through Reductions in Oxidative Stress in the Zucker Obese Rat. Hypertension 2010, 55, 880–888. [Google Scholar] [CrossRef]
- Farmer, J.B.; Kennedy, I.; Levy, G.P.; Marshall, R.J. Pharmacology of AH 5158; a Drug Which Blocks Both- and -Adrenoceptors. Br. J. Pharmacol. 1972, 45, 660–675. [Google Scholar] [CrossRef]
- Kennedy, I.; Levy, G.P. Combined Alpha- and Beta-Adrenoceptor Blocking Drug AH 5158: Further Studies on Alpha-Adrenoceptor Blockade in Anaesthetized Animals. Br. J. Pharmacol. 1975, 53, 585–592. [Google Scholar] [CrossRef]
- Zepeda, R.J.; Castillo, R.; Rodrigo, R.; Prieto, J.C.; Aramburu, I.; Brugere, S.; Galdames, K.; Noriega, V.; Miranda, H.F. Effect of Carvedilol and Nebivolol on Oxidative Stress-Related Parameters and Endothelial Function in Patients with Essential Hypertension. Basic Clin. Pharmacol. Toxicol. 2012, 111, 309–316. [Google Scholar] [CrossRef]
- Fongemie, J.; Felix-Getzik, E. A Review of Nebivolol Pharmacology and Clinical Evidence. Drugs 2015, 75, 1349–1371. [Google Scholar] [CrossRef]
- Jiang, M.; Xie, X.; Cao, F.; Wang, Y. Mitochondrial Metabolism in Myocardial Remodeling and Mechanical Unloading: Implications for Ischemic Heart Disease. Front. Cardiovasc. Med. 2021, 8, 789267. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.-Z.; Zhuang, D.-L.; Vong, C.T.; He, X.; Ouyang, Q.; Liang, J.-H.; Guo, Y.-P.; Wang, Y.-H.; Zhao, S.; Yuan, H.; et al. Role of Autophagy in Myocardial Remodeling After Myocardial Infarction. J. Cardiovasc. Pharmacol. 2025, 85, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.G.; Sharpe, N. Left Ventricular Remodeling after Myocardial Infarction: Pathophysiology and Therapy. Circulation 2000, 101, 2981–2988. [Google Scholar] [CrossRef]
- Leancă, S.A.; Crișu, D.; Petriș, A.O.; Afrăsânie, I.; Genes, A.; Costache, A.D.; Tesloianu, D.N.; Costache, I.I. Left Ventricular Remodeling after Myocardial Infarction: From Physiopathology to Treatment. Life 2022, 12, 1111. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, T.F. The Law of Laplace. Its Limitations as a Relation for Diastolic Pressure, Volume, or Wall Stress of the Left Ventricle. Circ. Res. 1980, 46, 321–331. [Google Scholar] [CrossRef]
- Frantz, S.; Hundertmark, M.J.; Schulz-Menger, J.; Bengel, F.M.; Bauersachs, J. Left Ventricular Remodelling Post-Myocardial Infarction: Pathophysiology, Imaging, and Novel Therapies. Eur. Heart J. 2022, 43, 2549–2561. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.; Fini, M.; Caminiti, G.; Barbaro, G. Cardiac Metabolism in Myocardial Ischemia. Curr. Pharm. Des. 2008, 14, 2551–2562. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, J.; Rong, J. Pharmacological Modulation of Cardiac Remodeling after Myocardial Infarction. Oxidative Med. Cell. Longev. 2020, 2020, 8815349. [Google Scholar] [CrossRef]
- Heusch, G. Myocardial Ischemia/Reperfusion: Translational Pathophysiology of Ischemic Heart Disease. Med 2024, 5, 10–31. [Google Scholar] [CrossRef]
- Mouton, A.J.; Rivera, O.J.; Lindsey, M.L. Myocardial Infarction Remodeling That Progresses to Heart Failure: A Signaling Misunderstanding. Am. J. Physiol. -Heart Circ. Physiol. 2018, 315, H71–H79. [Google Scholar] [CrossRef]
- Mitsis, A.; Avraamides, P.; Lakoumentas, J.; Kyriakou, M.; Sokratous, S.; Karmioti, G.; Drakomathioulakis, M.; Theodoropoulos, K.C.; Nasoufidou, A.; Evangeliou, A.; et al. Role of Inflammation Following An Acute Myocardial Infarction: Design of INFINITY. Biomark. Med. 2023, 17, 971–981. [Google Scholar] [CrossRef]
- Mezzaroma, E.; Toldo, S.; Farkas, D.; Seropian, I.M.; Van Tassell, B.W.; Salloum, F.N.; Kannan, H.R.; Menna, A.C.; Voelkel, N.F.; Abbate, A. The Inflammasome Promotes Adverse Cardiac Remodeling Following Acute Myocardial Infarction in the Mouse. Proc. Natl. Acad. Sci. USA 2011, 108, 19725–19730. [Google Scholar] [CrossRef]
- Van Den Borne, S.W.M.; Diez, J.; Blankesteijn, W.M.; Verjans, J.; Hofstra, L.; Narula, J. Myocardial Remodeling after Infarction: The Role of Myofibroblasts. Nat. Rev. Cardiol. 2010, 7, 30–37. [Google Scholar] [CrossRef]
- Liang, C.; Li, Q.; Wang, K.; Du, Y.; Wang, W.; Zhang, H. Mechanisms of Ventricular Arrhythmias Elicited by Coexistence of Multiple Electrophysiological Remodeling in Ischemia: A Simulation Study. PLoS Comput. Biol. 2022, 18, e1009388. [Google Scholar] [CrossRef] [PubMed]
- Mill, J.G.; Stefanon, I.; Dos Santos, L.; Baldo, M.P. Remodeling in the Ischemic Heart: The Stepwise Progression for Heart. Braz. J. Med. Biol. Res. 2011, 44, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Yalta, K.; Yilmaz, M.B.; Yalta, T.; Palabiyik, O.; Taylan, G.; Zorkun, C. Late Versus Early Myocardial Remodeling After Acute Myocardial Infarction: A Comparative Review on Mechanistic Insights and Clinical Implications. J. Cardiovasc. Pharmacol. Ther. 2020, 25, 15–26. [Google Scholar] [CrossRef]
- Zhang, N.; Aiyasiding, X.; Li, W.-J.; Liao, H.-H.; Tang, Q.-Z. Neutrophil Degranulation and Myocardial Infarction. Cell Commun. Signal. 2022, 20, 50. [Google Scholar] [CrossRef] [PubMed]
- Karakas, M.; Koenig, W. Sympathetic Nervous System: A Crucial Player Modulating Residual Cardiovascular Risk. Circ. Res. 2013, 112, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.S.; Ambrosy, A.P.; Velazquez, E.J. Adverse Remodeling and Reverse Remodeling After Myocardial Infarction. Curr. Cardiol. Rep. 2017, 19, 71. [Google Scholar] [CrossRef]
- Mitsis, A.; Myrianthefs, M.; Sokratous, S.; Karmioti, G.; Kyriakou, M.; Drakomathioulakis, M.; Tzikas, S.; Kadoglou, N.P.E.; Karagiannidis, E.; Nasoufidou, A.; et al. Emerging Therapeutic Targets for Acute Coronary Syndromes: Novel Advancements and Future Directions. Biomedicines 2024, 12, 1670. [Google Scholar] [CrossRef]
- Jin, S.; Kang, P.M. A Systematic Review on Advances in Management of Oxidative Stress-Associated Cardiovascular Diseases. Antioxidants 2024, 13, 923. [Google Scholar] [CrossRef]
- Xiang, M.; Lu, Y.; Xin, L.; Gao, J.; Shang, C.; Jiang, Z.; Lin, H.; Fang, X.; Qu, Y.; Wang, Y.; et al. Role of Oxidative Stress in Reperfusion Following Myocardial Ischemia and Its Treatments. Oxid. Med. Cell. Longev. 2021, 2021, 6614009. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G.; Kleinbongard, P. Is Metoprolol More Cardioprotective than Other Beta-Blockers? Eur. Heart J. 2020, 41, 4441–4443. [Google Scholar] [CrossRef]
- Stevens, J.L.; Feelisch, M.; Martin, D.S. Perioperative Oxidative Stress: The Unseen Enemy. Anesth. Analg. 2019, 129, 1749–1760. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Koppenol, W.H. The Centennial of the Fenton Reaction. Free Radic. Biol. Med. 1993, 15, 645–651. [Google Scholar] [CrossRef]
- Kaneko, M.; Beamish, R.E.; Dhalla, N.S. Depression of Heart Sarcolemmal Ca2+-Pump Activity by Oxygen Free Radicals. Am. J. Physiol. 1989, 256, H368–H374. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. ESC Scientific Document Group Fourth Universal Definition of Myocardial Infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef]
- Kurian, G.A.; Rajagopal, R.; Vedantham, S.; Rajesh, M. The Role of Oxidative Stress in Myocardial Ischemia and Reperfusion Injury and Remodeling: Revisited. Oxid. Med. Cell. Longev. 2016, 2016, 1656450. [Google Scholar] [CrossRef]
- Oliveira, P.J.; Gonçalves, L.; Monteiro, P.; Providencia, L.A.; Moreno, A.J. Are the Antioxidant Properties of Carvedilol Important for the Protection of Cardiac Mitochondria? Curr. Vasc. Pharmacol. 2005, 3, 147–158. [Google Scholar] [CrossRef]
- Asanuma, H.; Minamino, T.; Sanada, S.; Takashima, S.; Ogita, H.; Ogai, A.; Asakura, M.; Liao, Y.; Asano, Y.; Shintani, Y.; et al. β-Adrenoceptor Blocker Carvedilol Provides Cardioprotection via an Adenosine-Dependent Mechanism in Ischemic Canine Hearts. Circulation 2004, 109, 2773–2779. [Google Scholar] [CrossRef]
- Park, M.; Steinberg, S.F. Carvedilol Prevents Redox Inactivation of Cardiomyocyte Β1-Adrenergic Receptors. JACC: Basic Transl. Sci. 2018, 3, 521–532. [Google Scholar] [CrossRef]
- Khaper, N.; Rigatto, C.; Seneviratne, C.; Li, T.; Singal, P.K. Chronic Treatment with Propranolol Induces Antioxidant Changes and Protects Against Ischemia–Reperfusion Injury. J. Mol. Cell. Cardiol. 1997, 29, 3335–3344. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, S.; Yamada, Y.; Ichihara, G.; Kanazawa, H.; Hashimoto, K.; Kato, Y.; Matsushita, A.; Oikawa, S.; Yokota, M.; Iwase, M. Attenuation of Oxidative Stress and Cardiac Dysfunction by Bisoprolol in an Animal Model of Dilated Cardiomyopathy. Biochem. Biophys. Res. Commun. 2006, 350, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, M.; Chikura, S.; Watari, K.; Mizuno, N.; Mochinaga, K.; Mangmool, S.; Koyanagi, S.; Ohdo, S.; Sato, Y.; Ide, T.; et al. Induction of Cardiac Fibrosis by β-Blocker in G Protein-Independent and G Protein-Coupled Receptor Kinase 5/β-Arrestin2-Dependent Signaling Pathways. J. Biol. Chem. 2012, 287, 35669–35677. [Google Scholar] [CrossRef]
- Sheridan, F.M.; Dauber, I.M.; McMurtry, I.F.; Lesnefsky, E.J.; Horwitz, L.D. Role of Leukocytes in Coronary Vascular Endothelial Injury Due to Ischemia and Reperfusion. Circ. Res. 1991, 69, 1566–1574. [Google Scholar] [CrossRef]
- Kupatt, C.; Wichels, R.; Horstkotte, J.; Krombach, F.; Habazettl, H.; Boekstegers, P. Molecular Mechanisms of Platelet-Mediated Leukocyte Recruitment during Myocardial Reperfusion. J. Leukoc. Biol. 2002, 72, 455–461. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, B.M.; Vink, H.; Spaan, J.A.E. The Endothelial Glycocalyx Protects against Myocardial Edema. Circ. Res. 2003, 92, 592–594. [Google Scholar] [CrossRef] [PubMed]
- Becker, B.F.; Chappell, D.; Jacob, M. Endothelial Glycocalyx and Coronary Vascular Permeability: The Fringe Benefit. Basic Res. Cardiol. 2010, 105, 687–701. [Google Scholar] [CrossRef]
- Maroko, P.R.; Kjekshus, J.K.; Sobel, B.E.; Watanabe, T.; Covell, J.W.; Ross, J.; Braunwald, E. Factors Influencing Infarct Size Following Experimental Coronary Artery Occlusions. Circulation 1971, 43, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Moragón, A.; Gómez, M.; Villena-Gutiérrez, R.; Lalama, D.V.; García-Prieto, J.; Martínez, F.; Sánchez-Cabo, F.; Fuster, V.; Oliver, E.; Ibáñez, B. Metoprolol Exerts a Non-Class Effect against Ischaemia-Reperfusion Injury by Abrogating Exacerbated Inflammation. Eur. Heart J. 2020, 41, 4425–4440. [Google Scholar] [CrossRef]
- García-Prieto, J.; Villena-Gutiérrez, R.; Gómez, M.; Bernardo, E.; Pun-García, A.; García-Lunar, I.; Crainiciuc, G.; Fernández-Jiménez, R.; Sreeramkumar, V.; Bourio-Martínez, R.; et al. Neutrophil Stunning by Metoprolol Reduces Infarct Size. Nat. Commun. 2017, 8, 14780. [Google Scholar] [CrossRef]
- Ricci, F.; Di Credico, A.; Gaggi, G.; Iannetti, G.; Ghinassi, B.; Gallina, S.; Olshansky, B.; Di Baldassarre, A. Metoprolol Disrupts Inflammatory Response of Human Cardiomyocytes via β-Arrestin2 Biased Agonism and NF-κB Signaling Modulation. Biomed. Pharmacother. 2023, 168, 115804. [Google Scholar] [CrossRef]
- Dunzendorfer, S.; Wiedermann, C.J. Modulation of Neutrophil Migration and Superoxide Anion Release by Metoprolol. J. Mol. Cell. Cardiol. 2000, 32, 915–924. [Google Scholar] [CrossRef]
- Djanani, A.; Kaneider, N.C.; Meierhofer, C.; Sturn, D.; Dunzendorfer, S.; Allmeier, H.; Wiedermann, C.J. Inhibition of Neutrophil Migration and Oxygen Free Radical Release by Metipranolol and Timolol. Pharmacology 2003, 68, 198–203. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Maddock, H.L.; Baxter, G.F.; Yellon, D.M. Inhibiting Mitochondrial Permeability Transition Pore Opening: A New Paradigm for Myocardial Preconditioning? Cardiovasc. Res. 2002, 55, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, M.S.; Halestrap, A.P.; Griffiths, E.J. Mitochondria: A Target for Myocardial Protection. Pharmacol. Ther. 2001, 89, 29–46. [Google Scholar] [CrossRef]
- Kaiser, R.A.; Liang, Q.; Bueno, O.; Huang, Y.; Lackey, T.; Klevitsky, R.; Hewett, T.E.; Molkentin, J.D. Genetic Inhibition or Activation of JNK1/2 Protects the Myocardium from Ischemia-Reperfusion-Induced Cell Death in Vivo*. J. Biol. Chem. 2005, 280, 32602–32608. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.L.; Ma, X.L.; Wang, X.; Romanic, A.M.; Liu, G.L.; Louden, C.; Gu, J.L.; Kumar, S.; Poste, G.; Ruffolo, R.R.; et al. Possible Involvement of Stress-Activated Protein Kinase Signaling Pathway and Fas Receptor Expression in Prevention of Ischemia/Reperfusion-Induced Cardiomyocyte Apoptosis by Carvedilol. Circ. Res. 1998, 82, 166–174. [Google Scholar] [CrossRef]
- Xu, C.; Hu, Y.; Hou, L.; Ju, J.; Li, X.; Du, N.; Guan, X.; Liu, Z.; Zhang, T.; Qin, W.; et al. β-Blocker Carvedilol Protects Cardiomyocytes against Oxidative Stress-Induced Apoptosis by up-Regulating miR-133 Expression. J. Mol. Cell. Cardiol. 2014, 75, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, X.; Li, X.; Li, Z.; Diao, H.; Liu, L.; Zhang, J.; Ju, J.; Wen, L.; Liu, X.; et al. MicroRNA-1 Downregulation Induced by Carvedilol Protects Cardiomyocytes against Apoptosis by Targeting Heat Shock Protein 60. Mol. Med. Rep. 2019, 19, 3527–3536. [Google Scholar] [CrossRef]
- Yeh, C.-H.; Chen, T.-P.; Wang, Y.-C.; Lin, Y.-M.; Fang, S.-W. Carvedilol Treatment after Myocardial Infarct Decreases Cardiomyocytic Apoptosis in the Peri-Infarct Zone during Cardioplegia-Induced Cardiac Arrest. Shock 2013, 39, 343–352. [Google Scholar] [CrossRef]
- Sezer, M.; van Royen, N.; Umman, B.; Bugra, Z.; Bulluck, H.; Hausenloy, D.J.; Umman, S. Coronary Microvascular Injury in Reperfused Acute Myocardial Infarction: A View From an Integrative Perspective. J. Am. Heart Assoc. 2018, 7, e009949. [Google Scholar] [CrossRef]
- Niccoli, G.; Scalone, G.; Lerman, A.; Crea, F. Coronary Microvascular Obstruction in Acute Myocardial Infarction. Eur. Heart J. 2016, 37, 1024–1033. [Google Scholar] [CrossRef]
- Niccoli, G.; Burzotta, F.; Galiuto, L.; Crea, F. Myocardial No-Reflow in Humans. J. Am. Coll. Cardiol. 2009, 54, 281–292. [Google Scholar] [CrossRef]
- Dauber, I.M.; VanBenthuysen, K.M.; McMurtry, I.F.; Wheeler, G.S.; Lesnefsky, E.J.; Horwitz, L.D.; Weil, J.V. Functional Coronary Microvascular Injury Evident as Increased Permeability Due to Brief Ischemia and Reperfusion. Circ. Res. 1990, 66, 986–998. [Google Scholar] [CrossRef]
- Brandt, M.M.; Cheng, C.; Merkus, D.; Duncker, D.J.; Sorop, O. Mechanobiology of Microvascular Function and Structure in Health and Disease: Focus on the Coronary Circulation. Front. Physiol. 2021, 12, 771960. [Google Scholar] [CrossRef]
- Silva, I.V.G.; de Figueiredo, R.C.; Rios, D.R.A. Effect of Different Classes of Antihypertensive Drugs on Endothelial Function and Inflammation. Int. J. Mol. Sci. 2019, 20, 3458. [Google Scholar] [CrossRef] [PubMed]
- Toblli, J.E.; DiGennaro, F.; Giani, J.F.; Dominici, F.P. Nebivolol: Impact on Cardiac and Endothelial Function and Clinical Utility. Vasc. Health Risk Manag. 2012, 8, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Brehm, B.R.; Bertsch, D.; von Fallois, J.; Wolf, S.C. Beta-Blockers of the Third Generation Inhibit Endothelin-1 Liberation, mRNA Production and Proliferation of Human Coronary Smooth Muscle and Endothelial Cells. J. Cardiovasc. Pharmacol. 2000, 36, S401–S403. [Google Scholar] [CrossRef] [PubMed]
- Brehm, B.R.; Wolf, S.C.; Bertsch, D.; Klaussner, M.; Wesselborg, S.; Schüler, S.; Schulze-Osthoff, K. Effects of Nebivolol on Proliferation and Apoptosis of Human Coronary Artery Smooth Muscle and Endothelial Cells. Cardiovasc. Res. 2001, 49, 430–439. [Google Scholar] [CrossRef]
- Wolf, S.C.; Sauter, G.; Jobst, J.; Kempf, V.A.; Risler, T.; Brehm, B.R. Major Differences in Gene Expression in Human Coronary Smooth Muscle Cells after Nebivolol or Metoprolol Treatment. Int. J. Cardiol. 2008, 125, 4–10. [Google Scholar] [CrossRef]
- Virdis, A.; Ghiadoni, L.; Taddei, S. Effects of Antihypertensive Treatment on Endothelial Function. Curr. Hypertens. Rep. 2011, 13, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Taddei, S.; Virdis, A.; Ghiadoni, L.; Magagna, A.; Favilla, S.; Pompella, A.; Salvetti, A. Restoration of Nitric Oxide Availability after Calcium Antagonist Treatment in Essential Hypertension. Hypertension 2001, 37, 943–948. [Google Scholar] [CrossRef]
- Taddei, S.; Virdis, A.; Ghiadoni, L.; Sudano, I.; Salvetti, A. Effects of Antihypertensive Drugs on Endothelial Dysfunction: Clinical Implications. Drugs 2002, 62, 265–284. [Google Scholar] [CrossRef]
- Schiffrin, E.L.; Deng, L.Y. Structure and Function of Resistance Arteries of Hypertensive Patients Treated with a Beta-Blocker or a Calcium Channel Antagonist. J. Hypertens. 1996, 14, 1247–1255. [Google Scholar] [CrossRef]
- Landstrom, A.P.; Dobrev, D.; Wehrens, X.H.T. Calcium Signaling and Cardiac Arrhythmias. Circ. Res. 2017, 120, 1969–1993. [Google Scholar] [CrossRef]
- Hadri, L.; Hajjar, R.J. Calcium Cycling Proteins and Their Association with Heart Failure. Clin. Pharmacol. Ther. 2011, 90, 620–624. [Google Scholar] [CrossRef]
- Shaw, R.M.; Colecraft, H.M. L-Type Calcium Channel Targeting and Local Signalling in Cardiac Myocytes. Cardiovasc. Res. 2013, 98, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M. Calcium Cycling and Signaling in Cardiac Myocytes. Annu. Rev. Physiol. 2008, 70, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Steenbergen, C. Mechanisms Underlying Acute Protection from Cardiac Ischemia-Reperfusion Injury. Physiol. Rev. 2008, 88, 581–609. [Google Scholar] [CrossRef] [PubMed]
- Bourdillon, P.D.; Poole-Wilson, P.A. Commentary on “Effects of Ischaemia and Reperfusion on Calcium Exchange and Mechanical Function in Isolated Rabbit Myocardium”. Cardiovasc. Res. 2000, 45, 95–99. [Google Scholar] [CrossRef]
- Fiolet, J.W.; Baartscheer, A. Cellular Calcium Homeostasis during Ischemia; a Thermodynamic Approach. Cardiovasc. Res. 2000, 45, 100–106. [Google Scholar] [CrossRef]
- Zile, M.R.; Gaasch, W.H. Abnormal Calcium Homeostasis: One Mechanism in Diastolic Heart Failure. J. Am. Coll. Cardiol. 2011, 58, 155–157. [Google Scholar] [CrossRef]
- Reyes Gaido, O.E.; Nkashama, L.J.; Schole, K.L.; Wang, Q.; Umapathi, P.; Mesubi, O.O.; Konstantinidis, K.; Luczak, E.D.; Anderson, M.E. CaMKII as a Therapeutic Target in Cardiovascular Disease. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 249–272. [Google Scholar] [CrossRef]
- Wang, R.; Wang, M.; He, S.; Sun, G.; Sun, X. Targeting Calcium Homeostasis in Myocardial Ischemia/Reperfusion Injury: An Overview of Regulatory Mechanisms and Therapeutic Reagents. Front. Pharmacol. 2020, 11, 872. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.V.; O’Donoghue, M.L.; Ruel, M.; Rab, T.; Tamis-Holland, J.E.; Alexander, J.H.; Baber, U.; Baker, H.; Cohen, M.G.; Cruz-Ruiz, M.; et al. 2025 ACC/AHA/ACEP/NAEMSP/SCAI Guideline for the Management of Patients with Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2025, 151, 2135–2237, Erratum in Circulation 2025, 151, e1098. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.M.; Pan, H.C.; Chen, Y.P.; Peto, R.; Collins, R.; Jiang, L.X.; Xie, J.X.; Liu, L.S. COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group Early Intravenous Then Oral Metoprolol in 45,852 Patients with Acute Myocardial Infarction: Randomised Placebo-Controlled Trial. Lancet 2005, 366, 1622–1632. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Žlahtič, T.; Mrak, M.; Žižek, D. Complexities of Treating Co-Morbidities in Heart Failure with Preserved Ejection Fraction. ESC Heart Fail. 2024, 11, 3425–3429. [Google Scholar] [CrossRef]
- The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): A Randomised Trial. Lancet 1999, 353, 9–13. [CrossRef]
- Hjalmarson, Å.; Goldstein, S.; Fagerberg, B.; Wedel, H.; Waagstein, F.; Kjekshus, J.; Wikstrand, J.; El Allaf, D.; Vítovec, J.; Aldershvile, J.; et al. Effects of Controlled-Release Metoprolol on Total Mortality, Hospitalizations, and Well-Being in Patients with Heart Failure: The Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF). JAMA 2000, 283, 1295. [Google Scholar] [CrossRef]
- Packer, M.; Fowler, M.B.; Roecker, E.B.; Coats, A.J.S.; Katus, H.A.; Krum, H.; Mohacsi, P.; Rouleau, J.L.; Tendera, M.; Staiger, C.; et al. Effect of Carvedilol on the Morbidity of Patients with Severe Chronic Heart Failure: Results of the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study. Circulation 2002, 106, 2194–2199. [Google Scholar] [CrossRef]
- Ibanez, B.; Latini, R.; Rossello, X.; Dominguez-Rodriguez, A.; Fernández-Vazquez, F.; Pelizzoni, V.; Sánchez, P.L.; Anguita, M.; Barrabés, J.A.; Raposeiras-Roubín, S.; et al. Beta-Blockers after Myocardial Infarction without Reduced Ejection Fraction. N. Engl. J. Med. 2025, 2025, NEJMoa2504735. [Google Scholar] [CrossRef]
- Munkhaugen, J.; Kristensen, A.M.D.; Halvorsen, S.; Holmager, T.; Olsen, M.H.; Bakken, A.; Sehested, T.S.G.; Ruddox, V.; Mæng, M.; Vikenes, K.; et al. Beta-Blockers after Myocardial Infarction in Patients without Heart Failure. N. Engl. J. Med. 2025, 2025, NEJMoa2505985. [Google Scholar] [CrossRef]
- Watanabe, H.; Ozasa, N.; Morimoto, T.; Shiomi, H.; Bingyuan, B.; Suwa, S.; Nakagawa, Y.; Izumi, C.; Kadota, K.; Ikeguchi, S.; et al. Long-Term Use of Carvedilol in Patients with ST-Segment Elevation Myocardial Infarction Treated with Primary Percutaneous Coronary Intervention. PLoS ONE 2018, 13, e0199347. [Google Scholar] [CrossRef]
- Bangalore, S.; Bhatt, D.L.; Steg, P.G.; Weber, M.A.; Boden, W.E.; Hamm, C.W.; Montalescot, G.; Hsu, A.; Fox, K.A.A.; Lincoff, A.M. β-Blockers and Cardiovascular Events in Patients with and without Myocardial Infarction: Post Hoc Analysis from the CHARISMA Trial. Circ. Cardiovasc. Qual. Outcomes 2014, 7, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Colombo, G.; Biering-Sorensen, T.; Ferreira, J.P.; Lombardi, C.M.; Bonelli, A.; Garascia, A.; Metra, M.; Inciardi, R.M. Cardiac Remodelling in the Era of the Recommended Four Pillars Heart Failure Medical Therapy. ESC Heart Fail. 2025, 12, 1029–1044. [Google Scholar] [CrossRef]
- Udelson, J.E. Ventricular Remodeling in Heart Failure and the Effect of Beta-Blockade. Am. J. Cardiol. 2004, 93, 43–48. [Google Scholar] [CrossRef]
- Nasoufidou, A.; Bantidos, M.G.; Stachteas, P.; Moysidis, D.V.; Mitsis, A.; Fyntanidou, B.; Kouskouras, K.; Karagiannidis, E.; Karamitsos, T.; Kassimis, G.; et al. The Role of Landiolol in Coronary Artery Disease: Insights into Acute Coronary Syndromes, Stable Coronary Artery Disease and Computed Tomography Coronary Angiography. J. Clin. Med. 2025, 14, 5216. [Google Scholar] [CrossRef]
- Izumida, T.; Imamura, T.; Onoda, H.; Tanaka, S.; Ushijima, R.; Ueno, H.; Kinugawa, K. Effect of Optimal Heart Rate on Left Ventricular Remodeling in Patients with Systolic Heart Failure Following Acute Coronary Syndrome. Int. Heart J. 2024, 65, 833–840. [Google Scholar] [CrossRef]
- Colucci, W.S.; Kolias, T.J.; Adams, K.F.; Armstrong, W.F.; Ghali, J.K.; Gottlieb, S.S.; Greenberg, B.; Klibaner, M.I.; Kukin, M.L.; Sugg, J.E. Metoprolol Reverses Left Ventricular Remodeling in Patients with Asymptomatic Systolic Dysfunction. Circulation 2007, 116, 49–56. [Google Scholar] [CrossRef]
- Senior, R.; Basu, S.; Kinsey, C.; Schaeffer, S.; Lahiri, A. Carvedilol Prevents Remodeling in Patients with Left Ventricular Dysfunction after Acute Myocardial Infarction. Am. Heart J. 1999, 137, 646–652. [Google Scholar] [CrossRef]
- Doughty, R.N.; Whalley, G.A.; Walsh, H.A.; Gamble, G.D.; López-Sendón, J.; Sharpe, N. Effects of Carvedilol on Left Ventricular Remodeling After Acute Myocardial Infarction. Circulation 2004, 109, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Galves, R.; Da Costa, A.; Pierrard, R.; Bayard, G.; Guichard, J.B.; Isaaz, K. Impact of β-Blocker Therapy on Right Ventricular Function in Heart Failure Patients with Reduced Ejection Fraction. A Prospective Evaluation. Echocardiography 2020, 37, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Enzan, N.; Matsushima, S.; Ide, T.; Tohyama, T.; Funakoshi, K.; Higo, T.; Tsutsui, H. Beta-Blockers Are Associated with Reverse Remodeling in Patients with Dilated Cardiomyopathy and Mid-Range Ejection Fraction. Am. Heart J. Plus 2021, 11, 100053. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Tanimoto, S.; Aoki, J.; Nakajima, H.; Hara, K.; Tanabe, K. Effects of β-Blockers on Left Ventricular Remodeling in Patients with Preserved Ejection Fraction after Acute Myocardial Infarction. Int. J. Cardiol. 2016, 221, 765–769. [Google Scholar] [CrossRef]
- Zhu, T.; Song, Y. Efficacy of Sacubitril/Valsartan Combined with Metoprolol on Cardiac Function, Cardiac Remodeling, and Endothelial Function in Patients with Coronary Heart Disease and Heart Failure. Br. J. Hosp. Med. 2025, 86, 1–16. [Google Scholar] [CrossRef]
- Palatini, P.; Faria-Neto, J.R.; Santos, R.D. The Clinical Value of β-Blockers in Patients with Stable Angina. Curr. Med. Res. Opin. 2024, 40, 33–41. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Chronic Coronary Syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Le, D.E.; Alkayed, N.J.; Cao, Z.; Chattergoon, N.N.; Garcia-Jaramillo, M.; Thornburg, K.; Kaul, S. Metabolomics of Repetitive Myocardial Stunning in Chronic Multivessel Coronary Artery Stenosis: Effect of Non-Selective and Selective Β1-Receptor Blockers. J. Physiol. 2024, 602, 3423–3448. [Google Scholar] [CrossRef]
- McCafferty, K.; Forbes, S.; Thiemermann, C.; Yaqoob, M.M. The challenge of translating ischemic conditioning from animal models to humans: The role of comorbidities. Dis. Model. Mech. 2014, 7, 1321–1333. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Shin, H.H.; Shudo, Y. Current Status and Limitations of Myocardial Infarction Large Animal Models in Cardiovascular Translational Research. Front. Bioeng. Biotechnol. 2021, 9, 673683. [Google Scholar] [CrossRef]
- Beta-Blocker Interruption or Continuation After Myocardial Infarction | New England Journal of Medicine. Available online: https://www.nejm.org/doi/full/10.1056/NEJMoa2404204 (accessed on 5 September 2025).
- Drygała, S.; Radzikowski, M.; Maciejczyk, M. β-Blockers and Metabolic Modulation: Unraveling the Complex Interplay with Glucose Metabolism, Inflammation and Oxidative Stress. Front. Pharmacol. 2024, 15, 1489657. [Google Scholar] [CrossRef] [PubMed]
- Rossello, X.; Prescott, E.I.B.; Kristensen, A.M.D.; Latini, R.; Fuster, V.; Fagerland, M.W.; Pocock, S.J.; Halvorsen, S.; Dominguez-Rodriguez, A.; Holmager, T.L.F.; et al. β blockers after myocardial infarction with mildly reduced ejection fraction: An individual patient data meta-analysis of randomised controlled trials. Lancet 2025, 406, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Farzam, K.; Jan, A. Beta Blockers. [Updated 2023 Aug 22]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532906/ (accessed on 1 October 2025).
- Argulian, E.; Bangalore, S.; Messerli, F.H. Misconceptions and Facts About Beta-Blockers. Am. J. Med. 2019, 132, 816–819. [Google Scholar] [CrossRef] [PubMed]
| Gen | Agent | Redox Homeostasis | Neutrophil/ Inflammation | Mitochondria and Apoptosis | Microcirculation/ Endothelium | Ca2+ Handling/ECC |
|---|---|---|---|---|---|---|
| F I R S T | Propranolol | + (chronic pre-conditioning: ↓lipid peroxidation; catalase/GPx ↑; β-blockade-independent) | −/± (limited; context-specific) | − (no clear signal) | − (no clear signal) | + (class β1 effect) |
| S E C O N D | Atenolol | − (no clear signal) | − (no neutrophil effects in comparisons) | − (no clear signal) | − (neutral on endothelial function) | + (class β1 effect) |
| Bisoprolol | + (GSH/GSSG normalization; 4-HNE/3-NT ↓; MnSOD recovery) | − (no clear signal) | + (Bcl-2↑; cytochrome c↓) | − (no clear signal) | + (class β1 effect) | |
| Metoprolol | −/± (context-dependent bias) | ++ (β1/β-arrestin–biased neutrophil “stunning”; ↓NETs/adhesion/migration) | − (no consistent evidence) | − (no consistent endothelial gain) | + (attenuates reperfusion Ca2+ overload; listed among Ca2+-pathway agents) | |
| T H I R D | Carvedilol | ++ (direct scavenging; prevents β1 “redox inactivation”; adenosine restoration) | − (no clear signal) | ++ (↓SAPK/Fas; miR-133↑/miR-1↓; ↓caspase-9/-3; Bcl-2/Bax→survival) | +/++ (↑NO bioavailability; ↓ET-1; flow-mediated dilation ↑) | + (β1 block; stabilizes SR leak indirectly) |
| Nebivolol | + (NOX restraint, indirect) | − (no clear signal) | − (no clear signal) | ++ (eNOS/NO–cGMP ↑; ET-1 ↓; anti-proliferative) | + (class β1 effect) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasoufidou, A.; Bantidos, M.G.; Fyntanidou, B.; Kofos, C.; Stachteas, P.; Arvanitaki, A.; Karakasis, P.; Sagris, M.; Kassimis, G.; Fragakis, N.; et al. Cardioprotective Mechanisms of Beta-Blockers in Myocardial Ischemia and Reperfusion: From Molecular Targets to Clinical Implications. Int. J. Mol. Sci. 2025, 26, 9843. https://doi.org/10.3390/ijms26209843
Nasoufidou A, Bantidos MG, Fyntanidou B, Kofos C, Stachteas P, Arvanitaki A, Karakasis P, Sagris M, Kassimis G, Fragakis N, et al. Cardioprotective Mechanisms of Beta-Blockers in Myocardial Ischemia and Reperfusion: From Molecular Targets to Clinical Implications. International Journal of Molecular Sciences. 2025; 26(20):9843. https://doi.org/10.3390/ijms26209843
Chicago/Turabian StyleNasoufidou, Athina, Marios G. Bantidos, Barbara Fyntanidou, Christos Kofos, Panagiotis Stachteas, Alexandra Arvanitaki, Paschalis Karakasis, Marios Sagris, George Kassimis, Nikolaos Fragakis, and et al. 2025. "Cardioprotective Mechanisms of Beta-Blockers in Myocardial Ischemia and Reperfusion: From Molecular Targets to Clinical Implications" International Journal of Molecular Sciences 26, no. 20: 9843. https://doi.org/10.3390/ijms26209843
APA StyleNasoufidou, A., Bantidos, M. G., Fyntanidou, B., Kofos, C., Stachteas, P., Arvanitaki, A., Karakasis, P., Sagris, M., Kassimis, G., Fragakis, N., & Karagiannidis, E. (2025). Cardioprotective Mechanisms of Beta-Blockers in Myocardial Ischemia and Reperfusion: From Molecular Targets to Clinical Implications. International Journal of Molecular Sciences, 26(20), 9843. https://doi.org/10.3390/ijms26209843










