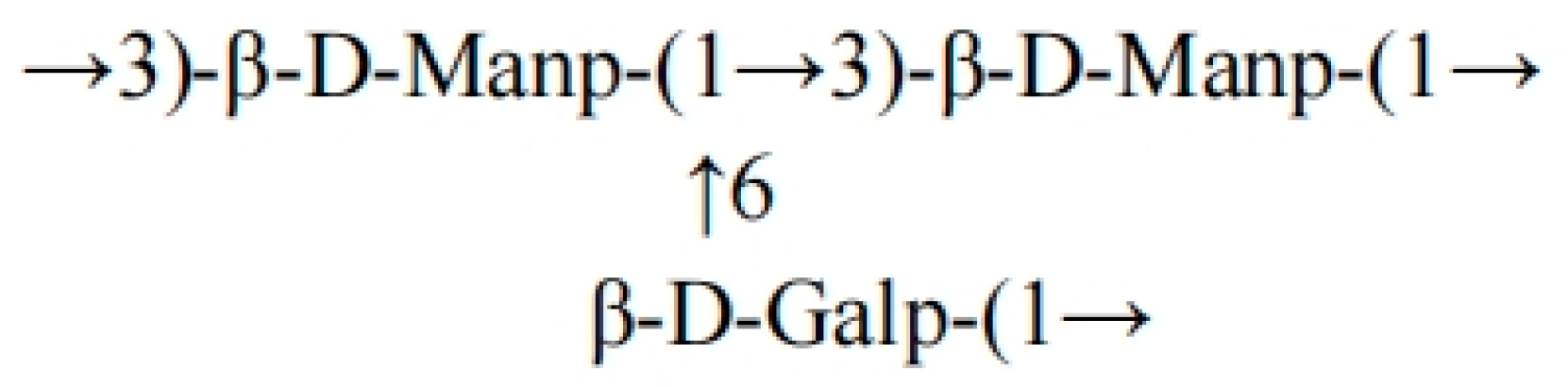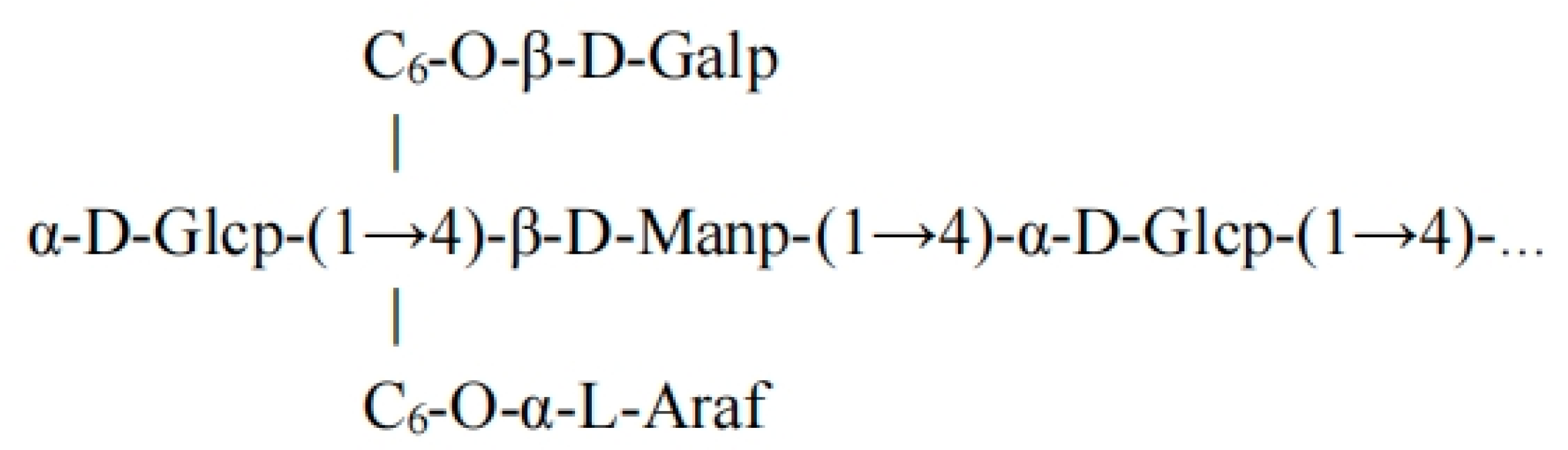1. Introduction
Structure–Activity Relationship and Potential Bioactivities of Fresh Coconut Meat Polysaccharides (FCMPs): As an important class of biomacromolecules, FCMPs hold significant application value in the food, pharmaceutical, and health product industries. In recent years, polysaccharides isolated from natural resources—particularly plant-derived polysaccharides—have attracted considerable attention due to their structural diversity and multifunctionality, demonstrating remarkable biological activities such as immunomodulation, anti-inflammatory effects, and antioxidant properties [
1].
Recent studies have revealed that Polygonatum sibiricum water extracts exhibit a broad spectrum of bioactivities, including hepatoprotection, antioxidant effects, and anti-inflammatory activity [
2,
3,
4,
5,
6]. Pharmacological investigations have shown that polysaccharides derived from Cordyceps sinensis possess immunomodulatory, antitumor, hypoglycemic, and antifibrotic effects [
7]. Furthermore, modern pharmacological research has demonstrated that Sargassum pallidum polysaccharides exhibit a wide range of health-promoting properties, such as antioxidant, anticancer, hypolipidemic, and immunomodulatory activities [
8,
9,
10,
11]. However, due to the intricate molecular weight hierarchy and unresolved mechanisms of action—particularly regarding how polysaccharides and oligosaccharides with polydisperse molecular weights exert their bioactivities—the structure–activity relationship remains to be further elucidated [
12].
FCMPs, as bioactive components derived from coconut processing by-products, a tropical specialty resource, have been reported to possess various biological activities, including immunomodulation, anti-inflammation, and antioxidant effects [
13,
14,
15]. Nevertheless, the fine structural characteristics of FCMP extracts and their relationship with bioactivities have not been systematically investigated. The polysaccharide components of FCMPs are complex, with a broad molecular weight distribution, and different fractions may exhibit distinct biological activities and mechanisms of action [
16].
In this study, we employed modern separation and purification techniques to fractionate FCMPs and applied multiple structural characterization methods (e.g., infrared spectroscopy, nuclear magnetic resonance, and methylation analysis) to elucidate their fine structures. Additionally, molecular docking simulations were performed to predict the interactions between FCMP fractions and immune-related targets (e.g., TLR4/MD-2 complex, DC-SIGN receptor, AQP1), aiming to explore their potential bioactivities. The findings of this study will provide new insights into the structure–function relationship of FCMPs and lay a foundation for their application in immunomodulation, anti-inflammatory therapy, and antioxidant interventions.
2. Results and Analysis
2.1. Molecular Weight Determination Results
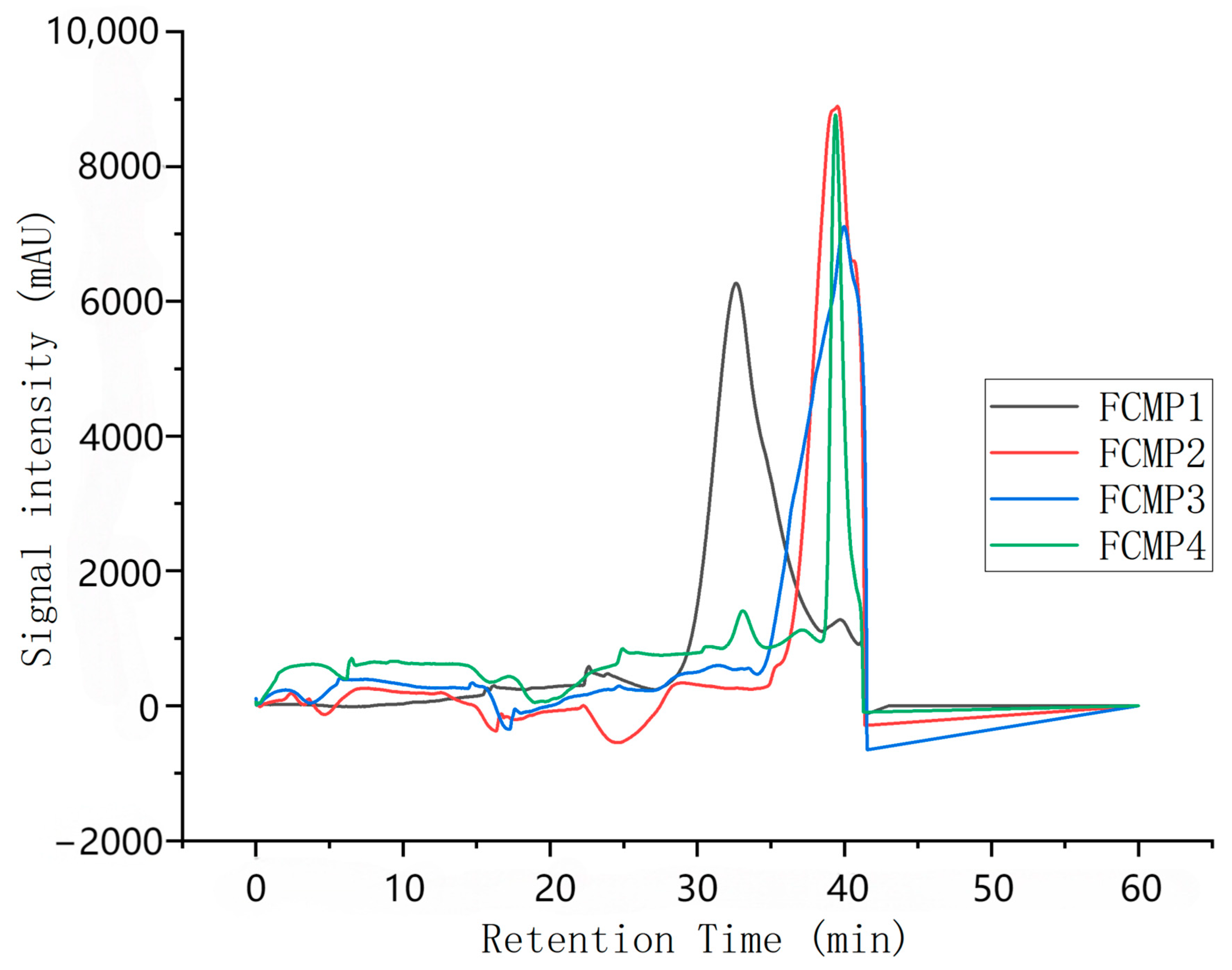
Based on the results obtained using the dextran standard, the standard logarithmic curve is plotted as y = −3.0907x + 49.748, R2 = 0.9978.
As shown in
Figure 1, the retention times of FCMP 1–4 are 32.64 min, 39.37 min, 40.06 min, and 39.40 min, respectively. Substituting these values into the standard curve, the calculated molecular weights are 343,016.9, 2279.4, 1363.2, and 2228.9, respectively.
These molecular weight data indicate that FCMP 1 has a significantly higher molecular weight than FCMP 2, FCMP 3, and FCMP 4, which may suggest that FCMP 1 has a more complex structure or contains more long-chain polysaccharide molecules. The molecular weights of FCMP 2, FCMP 3, and FCMP 4 are relatively close, which may indicate that they share structural similarities or originate from similar polysaccharide components.
2.2. Infrared Spectroscopy Results
Infrared spectroscopy analysis employs the KBr pellet method, and each absorption peak in the functional group region of the infrared spectrum corresponds to a specific functional group. As shown in
Figure 2, the FT-IR spectra of FCMP 1–4 samples all exhibit typical characteristic absorption peaks of polysaccharides. The characteristic peak of polysaccharides at 3300–3400 cm
−1 corresponds to the stretching vibration of O-H, indicating the presence of abundant hydroxyl (-OH) groups in the samples, which is a core characteristic of polysaccharide structure. The absorption peak at 2920–2930 cm
−1 corresponds to the stretching vibration of C-H bonds, further confirming the presence of C-H bonds in the polysaccharide sugar rings [
17].
The characteristic absorption peaks at 1710–1750 cm
−1 and 1649–1690 cm
−1 can be attributed to C=O stretching vibrations, indicating that the sample may contain ester groups (-COOR) or carboxyl groups (-COOH), suggesting that these polysaccharides possess acidic properties [
18]. The absorption peak near 1400–1420 cm
−1 may be related to N-H bending vibrations, suggesting the presence of acetylamino groups (e.g., N-acetylglucosamine) in the polysaccharides, commonly found in polysaccharides such as chitin [
19].
The absorption peak at 1100–1280 cm
−1 corresponds to C-O stretching vibrations, indicating the presence of glycosidic bonds (C-O-C) or hydroxyl groups (C-OH) in the polysaccharides. The characteristic peak at 1038.5 cm
−1 further supports C-O vibrations, consistent with the C-O-C bonds in the pyranose monosaccharide ring. The absorption peak at 818.6 cm
−1 can be attributed to C-H bending vibrations, consistent with the typical configuration of D-pyranose glucose. These characteristic peaks collectively confirm the presence of pyranose rings in FCMP 1–4 samples and indicate their structural characteristics as acidic polysaccharides [
20].
2.3. Results of Monosaccharide Composition Analysis
In
Figure 3, the monosaccharide composition diagram shows that standards 1–7 are, in order, mannose, rhamnose, galacturonic acid, glucose, sorbitol, galactose, and arabinose. From this diagram, it can be seen that FCMP 1 consists of mannose, rhamnose, glucose, and galactose; FCMP 2 consists of mannose and galactose; FCMP 3 consists of mannose, rhamnose, galactose, and arabinose; and FCMP 4 consists of mannose, glucose, galactose, and arabinose.
2.4. Methylation Experiment Results
2.4.1. Methylation Results of FCMP 1
The methylation results of FCMP 1 shown in
Table 1 indicate that the monosaccharide composition consists of D-mannose (approximately 40%), D-glucose (approximately 30%), D-galactose (approximately 20%), and L-rhamnose (approximately 10%). The main chain is composed of alternating β-1→4-linked mannose and glucose, branches are formed by galactose linked via β-1→6 bonds, and the terminal end is modified by α-L-rhamnose via 1→2/1→3 bonds.
2.4.2. Methylation Results of FCMP 2
As shown in
Table 2, the FCMP 2 methylation results indicate that the molar ratio of the sugar residues in FCMP 2 is main chain mannose/branch galactose/terminal mannose = 2:1:1, with a molecular weight of 2279.40 Da. Combining the molecular weights of the methylated monosaccharides (both mannose and galactose are 273.25 Da after methylation) and the dehydration weight of the glycosidic bonds (18.02 Da per bond), it can be inferred that this polysaccharide is composed of eight monosaccharide units, with the main chain containing five β-(1→3)-Manp units (5 × 273.25 = 1366.25 Da), the branches containing two β-(1→6)-Galp units (2 × 273.25 = 546.50 Da), the terminal containing one Manp unit (273.25 Da), and the glycosidic bonds undergoing seven dehydration steps (7 × 18.02 = 126.14 Da), with total molecular weight of 1366.25 + 546.50 + 273.25 − 126.14 ≈ 2060 Da. The actual molecular weight is slightly higher, possibly due to incomplete coverage of some methylation sites or the presence of trace impurities, but the overall structure is consistent with the experimental data [
21].
2.4.3. Methylation Results of FCMP 3
As shown in
Table 3, the total molar ratio of FCMP 3 sugar residues is D-mannose/D-galactose/L-rhamnose/L-arabinose = 3.16:1.63:4.8:1.60. The main chain is primarily composed of D-mannose and D-galactose, the linkage patterns include 1→3 and 1→6; and the side chains include 1→4 linkages of L-rhamnose and terminal structures of L-arabinose [
22].
2.4.4. Methylation Results of FCMP 4
As shown in
Table 4 FCMP 4 methylation results, glucose (Glc) forms the main chain through 1→4 glycosidic bonds (retention time 6.899 min, characteristic peak
m/
z 126.06, and molar ratio 25.05%), with some containing 1→4,6 branches (retention time 14.069 min,
m/
z 199, and molar ratio 8.73%), and terminal Glc (retention time 27.464 min and
m/
z 218) indicates the terminal end of the linear chain. Mannose (Man) is primarily present as 1→6 linkages (retention time 6.925 min,
m/
z 175.13, and molar ratio 15.34%) and 1→3,6 branches (retention time 17.788 min,
m/
z 207, and molar ratio 7.98%). Galactose (Gal) is present in 1→3 linkages (retention time 11.769 min,
m/
z 156.1, and molar ratio 10.31%) and 1→2 linkages (retention time 19.038 min,
m/
z 154.08, and molar ratio 6.15%). Arabinose (Ara) is primarily present in a 1→5 linkage (retention time 12.947 min,
m/
z 149.05, and molar ratio 9.82%) and a 1→3,5 branch (retention time 20.006 min,
m/
z 227, and molar ratio 5.21%) [
23].
2.5. Nuclear Magnetic Resonance Analysis Results
2.5.1. FCMP 1 Magnetic Resonance Analysis Results
Figure S1a shows the
13C NMR characteristics: C1 (δ100.17) combined with the H1 coupling constant (J = 8.7 Hz) in
1H NMR confirms that the main chain is a β-1→4-linked D-mannose/D-glucose. C4 (δ68.38) and the H1 long-range coupling in HMBC verify the 1→4 bond, C6 (δ61.21) indicates an unsubstituted primary alcohol group, and the branch point C6 (δ68.38) is associated with a β-1→6-linked D-galactose. The terminal α-L-rhamnose is confirmed by the methyl signal (δ1.09/1.66) and the hetero proton (δ4.94, single peak). HMBC shows that it is linked to the main chain via 1→2/1→3 connections [
24,
25].
1H NMR data in
Figure S1b: δ4.94 (s, 1H) corresponds to α-L-rhamnose H1 (J ~ 1–2 Hz); δ3.90 (d, J = 8.7 Hz, 2H) is assigned to β-D-glucose/mannose H1; δ3.84 (d, J = 17.7 Hz, 5H) corresponds to the C6-H6a/H6b trans coupling; and δ3.73 (d, J = 9.8 Hz, 4H) corresponds to the β-1→4-linked H4/H5 trans coupling [
26,
27].
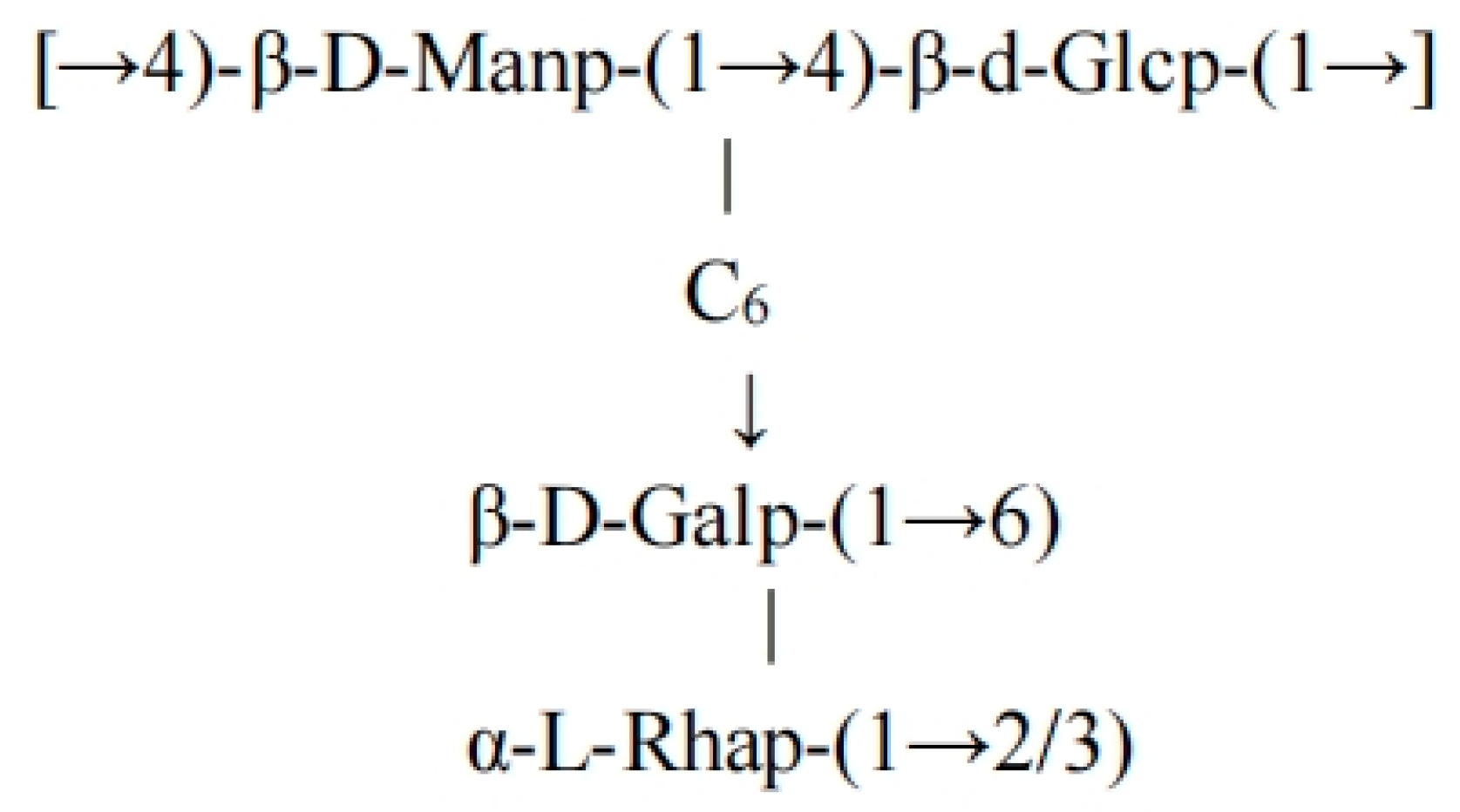
Based on the methylation analysis and NMR data, FCMP 1 consists of ~40% D-mannose, ~30% D-glucose, ~20% D-galactose, and ~10% L-rhamnose, with the main chain consisting of alternately linked β-1→4 mannose/glucose units, β-1→6 galactose branches, and terminal α-L-rhamnose modified via 1→2/1→3 linkages. The highly branched structure (molecular weight > 300 kDa) exhibits one branch point every 5–6 main chain units [
28,
29]. In summary, the polysaccharide possesses a complex topology characterized by a β-1→4 glycosidic backbone, β-1→6 glycosidic side chains, and α-L-rhamnose terminal units. The structure of the backbone is depicted in
Figure 4.
2.5.2. FCMP 2 Nuclear Magnetic Resonance Analysis Results
Figure S2 shows the NMR (
1H/
13C/COSY/HSQC) spectrum: the main chain is connected by β-D-Manp (1→3), with C1 (δ99.91) strongly correlated with H1 (δ5.11, dd, J = 16.8 Hz) and the de-shielding effect of C3 (δ74.68) confirming the β-configuration; the branch is connected to the main chain Man by β-D-Galp (1→6), and C1 (δ97.32, H1 δ4.91, J≈7.5 Hz) and Man C6 (δ60.44, H6 δ3.48) chemical shifts support this structure. In the HSQC spectrum, the carbon–hydrogen correlations of Man C2 (δ76.62, H2 δ4.05), C4 (δ72.74, H4 δ3.73), and Gal C6 (δ65.62, H6 δ3.55) in the HSQC spectrum are consistent with the methylation data (
m/
z 199.10/232.09), confirming the Man-(1→3)-Man main chain and Gal-(1→6)-Man branch [
30,
31]. The terminal Man signal (C1 δ93.44, H1 δ4.70) and trace Gal signal (C1 δ98.62) suggest chain termination or incomplete linkage. In summary, FCMP 2 is a heteropolysaccharide with a β-(1→3)-mannan main chain containing β-(1→6)-galactose branches [
32,
33]. The structural formula of the FCMP 2 backbone is shown in
Figure 5.
2.5.3. FCMP 3 Nuclear Magnetic Resonance Analysis Results
Through comprehensive methylation analysis and systematic interpretation of the FCMP 3 nuclear magnetic resonance (NMR) spectra (including
1H,
13C, COSY, HSQC, and HMBC) shown in
Figure S3, the sugar residue composition, linkage patterns, and structural characteristics of this polysaccharide were determined based on known data, as listed in
Table 5 [
34,
35]. The main chain of this polysaccharide is composed of alternating β-D-mannose (1→3) and β-D-galactose (1→6) linkages, with side chains containing short branches of α-L-rhamnose (1→4) and terminal α-L-arabinose and β-D-mannose. The COSY spectrum confirmed the proton connectivity order within the sugar rings (e.g., mannose H1 (5.24)—H2 (3.85)—H3 (3.73)). The HSQC spectrum clarified the C-H correlations (e.g., galactose C6 (68.21 ppm)/H6 (3.55 ppm)). The HMBC spectrum confirmed the long-range connections between sugar residues (e.g., mannose C1 (98.71 ppm)→galactose H6 (3.66 ppm)).
The structural formula of the FCMP 3 backbone is shown in
Figure 6.
2.5.4. FCMP 4 Nuclear Magnetic Resonance Analysis Results
Combining infrared spectroscopy, NMR (
1H/
13C/COSY) (
Figure S4), and methylation analysis, it was determined that FCMP 4 is mainly composed of glucose (Glc) and mannose (Man) (containing small amounts of galactose (Gal) and arabinose (Ara)). The coupling of H1 (4.60 ppm) with H2 (4.35 ppm) and the C4 (75.97/75.32 ppm) signal in the COSY spectrum indicate that the main chain is connected by 1→4 glycosidic bonds; the coupling of H6 (3.83 ppm) with H5 (4.11 ppm) suggests a small number of 1→6 branches. Infrared spectroscopy at 3400–3200 cm
−1 (O–H) and 1100–1000 cm
−1 (C–O–C) confirms the presence of hydroxyl groups and glycosidic bonds [
36,
37].
Structural model: The main chain consists of α-D-Glcp (heterotopic H 5.16 ppm) and β-D-Manp (heterotopic H 4.76 ppm) alternately connected via 1→4 bonds (→4)-α-D-Glcp-(1→4)-β-D-Manp-(1→4)-α-D-Glcp-(1→). Some Glc molecules are branched at the C6 position via a 1→6 bond to β-D-Galp (heterotopic H 4.60 ppm) or α-L-Araf (heterotopic H 4.30 ppm) (→6)-β-D-Galp-(1→) or →6)-α-L-Araf-(1→). The minimum repeating unit contains three main chain residues + one branch residue, with a branch frequency of approximately once every two Glc residues. Methylation data (m/z 126.06/175.13 corresponding to the 1→4 bond and m/z 190.18 corresponding to the 1→6 bond) and 13C NMR (C4 75.32 ppm, C6 60.44 ppm) further validate this structure.
The structural formula of the FCMP 4 backbone is shown in
Figure 7.
2.6. Molecular Docking Prediction Results
As shown in
Table 6 and
Figures SS1–SS4, ① FCMP 1 polysaccharides are polysaccharides with a β-1→4 main chain, β-1→6 branches, and α-L-rhamnose terminals. They exert immune regulatory effects through TLR4 and DC-SIGN receptors; by specifically targeting AQP4, they are applied to cerebral edema (such as post-stroke edema) or glioma (dependent on high AQP4 expression). ② FCMP 2 polysaccharides possess anti-edema β-(1→3)-mannan-β-(1→6)-galactose heteropolysaccharides and their characteristics. They specifically bind to AQP1 aquaporin proteins, exhibiting therapeutic activity for AQP1-mediated diseases, including brain edema, pulmonary edema, and glaucoma. ③ FCMP 3 polysaccharides are characterized by a main chain composed of alternating β-D-mannose (1→3) and β-D-galactose (1→6) units, with side chains containing α-L-rhamnose (1→4) and terminal α-L-arabinose and β-D-mannose units. Through molecular docking predictions, FCMP 3 polysaccharides exhibit significant TLR4 activation, CD44 inhibition, and pancreatic lipase inhibition activities, making them widely applicable in immunomodulation, antitumor, and lipid-lowering fields. They are also predicted to efficiently inhibit water permeability, with potential applications in edema treatment or water metabolism regulation. ④ FCMP 4 polysaccharide samples showed strong binding affinity with target proteins such as TLR4, CD44, and PDL1 through molecular docking prediction, indicating their potential activity in immune regulation and anti-inflammatory and antitumor effects. FCMP 4 significantly enhances water channel activity (water flow +35%) by specifically binding to AQP1, making it a potential AQP1 modulator and a candidate for development as an edema treatment drug.
FCMP 1 polysaccharides have the following structural characteristics, which may be related to their biological activity:
β-1→4 main chain: The linear main chain may participate in binding with immune receptors (such as TLR4 or Dectin-1) to activate immune responses.
β-1→6 branches: The branched structure may enhance multivalent binding with cell surface receptors, thereby increasing biological activity.
- (1)
FCMP 2 docking with target molecules
Based on the structural characteristics of FCMP 2 polysaccharides (β-(1→3)-mannan main chain and β-(1→6)-galactose branches), it is speculated that they may have immunomodulatory, antitumor, or anti-inflammatory activities, as shown in
Supplementary Material S1: Figure SS2A,B.
- (2)
FCMP 3 docking with target molecules
Based on the structural characteristics of FCMP 3 polysaccharides (main chain consisting of alternating β-D-mannose and β-D-galactose and side chains containing α-L-rhamnose and terminal monosaccharides), molecular docking was performed, as shown in
Supplementary Material S1: Figure SS3A–F.
- (3)
FCMP 4 docking with target molecules
The main chain of FCMP 4 polysaccharide samples consists of glucose (Glc) and mannose (Man) linked by 1→4 bonds, while the branch structure includes galactose (Gal) and arabinose (Ara) linked by 1→6 bonds. Molecular docking and activity prediction were performed on these samples, as shown in
Supplementary Material S1: Figure SS4A–F.
3. Materials and Methods
3.1. Materials and Reagents
Fresh coconut meat (5–7 mm) was sourced from Wenchang, Hainan. KBr (7758-02-3) was obtained from Merck (Rahway, NJ, USA); trifluoroacetic acid (T103294) was obtained from Aladdin (Riverside, CA, USA); sodium boron deuteride (98 atom% D) was obtained from Schick (Vaihingen an der Enz, Germany); and fucose (B25632), Rhamnose (B50770), arabinose (B24220), galactose (B21893), glucose (B21882), xylose (B21880), mannose (B21895), galacturonic acid (B21894), and glucuronic acid (B25302) were purchased from Shanghai Yuanye Technology Co., Ltd. (Shanghai, China).
3.2. Methods
3.2.1. Extraction and Purification of Polysaccharides from Fresh Coconut Meat
A total of 35 g of fresh coconut meat was weighed and passed through a 60-mesh sieve. The solid-to-liquid (fresh coconut meat to pure water) ratio was 1:12 (g/mL). The mixture was extracted in a water bath at 75 °C, the extraction was repeated twice, the filtrates were combined and cooled, anhydrous ethanol was added three times, and the mixture was precipitated at 4 °C for 24 h. The mixture was centrifuged at 4000 rpm for 15 min, the supernatant was discarded, and the precipitate was collected. The supernatant was freeze-dried to obtain crude polysaccharides [
38]. Petroleum ether was added to the crude polysaccharides for defatting [
39], then the Sevag method was used to remove proteins from the defatted polysaccharides, yielding crude FCMPs.
An appropriate amount of the crude FCMP sample was taken and dissolved in pure water. The solution was sampled onto a DEAE-52 cellulose anion exchange chromatography column (20 mm × 1100 mm) and eluted sequentially with pure water and 0.1, 0.3, and 0.5 mol/L NaCl solutions at a flow rate of 1 mL/min, collecting 8 mL elution per tube. According to the accompanying detection method, the four polysaccharide components showed only one main peak in the gel filtration column. Therefore, FCMPs were further purified using a Sephadex dextran gel G-100 column (20 mm × 900 mm). Subsequent detection was performed using the phenol–sulfuric acid method. According to the results from the phenol–sulfuric acid assay, the same fractions were combined, rotary-evaporated at 60 °C, and dialyzed with dialysis membranes (500–1000 Da) at 4 °C.
3.2.2. Molecular Weight Determination
The average molecular weights of dextran (1.000, 2.000, 4.000, 8.000, and 500.000) were used as standards. HPLC conditions: a SHIMADZU LC-20A coupled with an RI detector (RID-10A) was used for analysis. Separation was performed using Shodex OHpak SB-803 HQ columns (8.0 × 300 mm) and Shodex OHpak SB-806 HQ columns (8.0 × 300 mm) (Shodex, Tokyo, Japan), with moderate elution in a 0.1 M sodium dihydrogen phosphate solution (pH 7.2) at a flow rate of 0.5 mL/min. The column and detector temperatures were maintained at 40 °C. The total runtime was 60 min, with an injection volume of 80 μL. A standard curve was plotted with the retention time of the standard sample as the x-axis and signal intensity as the y-axis. The molecular weights of FCMP 1, FCMP 2, FCMP 3, and FCMP 4 were calculated based on the retention times of the samples [
40] (Shimadzu Corporation, Shanghai, China).
3.2.3. Infrared Spectroscopy (FT-IR) Analysis
Appropriate amounts of polysaccharide FCMP 1, FCMP 2, FCMP 3, and FCMP 4 samples were taken and mixed with dry KBr. The samples were ground evenly, pressed into thin sheets using a tablet press, and scanned using a Fourier transform infrared spectrometer. The scanning range was 4000–400 cm
−1 [
41] (Thermo Nicolet iS50, Thermo Scientific, Shanghai, China).
3.2.4. Monosaccharide Composition Analysis
Following the PMP-derived method established by Tu et al. [
42], the monosaccharide composition of FCMP 1–FCMP 4 was determined. A total of 5 mg of each polysaccharide sample was weighed and dissolved in 2 mL of 2 M TFA. The tube was sealed and hydrolyzed at 110 °C for 2 h. TFA was removed by rotary evaporation with methanol (3 times), then 0.5 mL of 0.5 M PMP and 0.5 mL of 0.3 M NaOH were added for dissolution. Next, 0.1 mL of the derivatized solution was taken and reacted at 70 °C for 30 min. The solution was cooled and centrifuged to collect the supernatant. Then, 0.05 mL of 0.3 M HCl was added and neutralized with ultra-pure water. Extraction was performed with chloroform, and the supernatant was collected and filtered through a 0.22 μm membrane for analysis. Monosaccharide reference standards were treated using the same method. Chromatographic conditions: Phosphate buffer (pH 6.8, A)—acetonitrile (B) gradient elution (0–10 min, 10%→17% B; 10–18 min, 17%→22% B; 18.5–20 min, 22%→23.5% B; 20–32 min, 23.5%→31% B), Zorbax SB-C18 column (4.6 × 150 mm, 5 μm), flow rate 0.8 mL/min, column temperature 30 °C, detection wavelength 250 nm, injection volume 10 μL.
3.2.5. Methylation Experiment
The methylation analysis was conducted as previously reported [
43]. Fresh coconut meat polysaccharides were dried with P
2O
5, and 5 mg was dissolved in DMSO (2 mL) dried with a 3A molecular sieve. A total of 2 mg of NaOH was added and sonicated for 0.5 h. The mixture was reacted with methylated iodine in the dark at low temperature for 1 h. Sequential extraction with H
2O (1 mL) and CHCl
3 (2 mL) (×3) was performed, then the organic phase was freeze-dried to obtain the methylated polysaccharides. Then, 2 mol/L TFA (100 μL) was added, followed by hydrolyzation at 121 °C for 1.5 h then evaporation to dryness at 50 °C.
Thermal degradation: First, 3 mL of 2 mol/L TFA was added to the methylated sample and reacted at 110 °C for 3 h. The sample was evaporated under reduced pressure, and TFA was removed by rotary evaporation with methanol (×3–5). Next, 2 mL of H2O and 25 mg of NaBD4 were added to the degradation products and reduced at room temperature with shaking for 2 h. The pH was adjusted to 5.0 with acetic acid and concentrated under reduced pressure. Methanol (2 mL) and acetic acid (1 drop) were added sequentially and evaporated under reduced pressure (×5, without adding acetic acid in the last two steps) to remove boron deuteride. Acetic anhydride (2 mL) and acetylate were added at 100 °C for 1 h. The sample was concentrated under reduced pressure, and then methanol rotary evaporation (×3) was performed to obtain polymethylacrylic acid (PMAAs). The PMAAs were dissolved in chloroform (4 mL), washed with water (×3), dried with a 3A molecular sieve, filtered, and analyzed by GC-MS.
GC-MS conditions: InertCap 5 MS column (30 m × 0.25 mm × 0.25 μm); injection volume 1 μL; temperature program: 120 °C (1 min)→3 °C/min→210 °C (2 min)→10 °C/min→260 °C (4 min); carrier gas He (99.999%); transfer line temperature 280 °C; ion trap temperature 220 °C; full scan mode (43–500 m/z), NIST147.LIB spectral library search.
3.2.6. Nuclear Magnetic Resonance (NMR) Analysis
First, 25 mg of FCMP 1, FCMP 2, FCMP 3, and FCMP 4 powder was separately dissolved in 1 mL of heavy water (D2O) then transferred to a nuclear magnetic resonance tube and analyzed using a Bruker BioSpin AG (Billerica, MA, USA) 400HZ nuclear magnetic resonance instrument (Bruker, Billerica, MA, USA).
3.2.7. Molecular Docking Methods and Target Selection
AutoDock Vina software Version 1.2.0 was used for molecular docking.
The following targets were selected:
TLR4/MD-2 complex: The binding ability of polysaccharides to TLR4 was predicted, and their immunomodulatory activity was evaluated.
DC-SIGN receptor: The binding of terminal rhamnose to DC-SIGN was studied, and its immunomodulatory potential was evaluated.
AQP1 (PDB ID: 1J4N): A widely expressed classical aquaporin.
AQP4 (PDB ID: 3GD8): Highly expressed in the central nervous system and associated with cerebral edema.
AQP5 (PDB ID: 6F7H): Enriched in glands and lungs and involved in secretion regulation.
4. Conclusions
Four major fractions (FCMP 1–FCMP 4) were isolated and purified from fresh coconut meat polysaccharides, with molecular weights of 343,016.9, 2279.4, 1363.2, and 2228.9 Da, respectively. Fourier-transform infrared (FT-IR) spectroscopy analysis revealed that all fractions exhibited characteristic polysaccharide absorption peaks, indicative of functional groups including hydroxyl, C–H, C=O, and glycosidic bonds. Monosaccharide composition analysis demonstrated that CMP1 consisted of mannose, glucose, galactose, and rhamnose; CMP2 comprised mannose and galactose; CMP3 contained mannose, rhamnose, galactose, and arabinose; and CMP4 was composed of mannose, glucose, galactose, and arabinose. Methylation analysis and nuclear magnetic resonance (NMR) spectroscopy collectively elucidated the detailed structural features of each fraction: CMP1: The backbone consisted of an alternating β-1→4-linked mannose and glucose chain, with β-1→6-linked galactose branches and α-L-rhamnose as the terminal residue, forming a complex topological structure characterized by a β-1→4 backbone, β-1→6 branches, and an α-L-rhamnose terminus. CMP2: The backbone was a β-(1→3)-linked mannan, with β-(1→6)-linked galactose branches. CMP3: The backbone comprised an alternating β-D-mannose and β-D-galactose chain, with side chains containing α-L-rhamnose, along with terminal α-L-arabinose and β-D-mannose residues. CMP4: The backbone primarily consisted of 1→4-linked glucose and mannose, with 1→6-linked branches at the C6 position of some glucose or mannose residues.
Molecular docking studies revealed the specific bioactive mechanisms of each fraction: CMP1 exerted immunomodulatory effects by interacting with TLR4 and DC-SIGN receptors and exhibited therapeutic potential for brain edema or glioma via targeted binding to AQP4. CMP2 specifically bound to the AQP1 water channel protein, demonstrating potential for treating AQP1-mediated diseases. CMP3 significantly activated TLR4, inhibited CD44 and pancreatic lipase, and showed applicability in immunomodulation, antitumor therapy, and lipid-lowering. CMP4 exhibited strong binding affinity to target proteins such as TLR4, CD44, and PDL1, conferring immunomodulatory, anti-inflammatory, and antitumor activities. Additionally, its binding to AQP1 enhanced water channel activity, indicating potential as an edema treatment agent.
In conclusion, this study systematically elucidated the fine structural characteristics and multidimensional bioactive mechanisms of CMP, providing a theoretical basis for its application in immunomodulation, anti-inflammation, antioxidant therapy, and edema treatment. These findings hold significant scientific value and practical application prospects.
Author Contributions
Conceptualization, Y.R. and J.H.; methodology, M.M. and J.H.; software, M.M.; validation, M.Q. and X.L.; formal analysis, J.H.; investigation, J.H.; resources, Y.R.; data curation, J.H.; writing—original draft preparation, J.H.; writing—review and editing, Y.R.; visualization, M.M.; supervision, Y.R.; project administration, Y.R.; funding acquisition, Y.R. All authors have read and agreed to the published version of the manuscript.
Funding
Hainan University research fund XJ2300002971.
Data Availability Statement
The original contributions presented in this study are included in the article/
Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Guo, J.; Kang, Y. Future prospect of polysaccharide as a potential therapy in hepatocellular carcinoma: A review. Int. J. Biol. Macromol. 2024, 270, 132300. [Google Scholar] [CrossRef] [PubMed]
- Maity, P.; Maity, K.K.; Chakraborty, I.; Sen, I.K.; Mondal, S.; Maity, G.N.; Patra, S. Polysaccharides from Ganoderma: Extraction, Chemical Features, and Bioactivity. In Ganoderma; CRC Press: Boca Raton, FL, USA, 2024; pp. 1–19. [Google Scholar]
- Khaytmetova, S.B.; Turaev, A.S.; Khalilova, G.A.; Muhitdinov, B.I. Isolation, Physico-Chemical Characteristics, and Acute Toxicity Evaluation of Water-Soluble Polysaccharide from Basidial Raw Material Ganoderma lucidum. Russ. J. Bioorganic Chem. 2024, 50, 95–105. [Google Scholar] [CrossRef]
- Zangeneh, M.; Derakhshankhah, H.; Modarresi, M.; Haghshenas, B.; Foroozanfar, Z.; Izadi, Z. In vitro evaluation of antioxidant, probiotic, and antiproliferative activity of Ganoderma lucidum-extracted polysaccharides for the prevention of complication associated with gastrointestinal inflammatory diseases. Heliyon 2025, 11, e42936. [Google Scholar] [CrossRef]
- Salehi, M.; Rashidinejad, A. Multifaceted roles of plant-derived bioactive polysaccharides: A review of their biological functions, delivery, bioavailability, and applications within the food and pharmaceutical sectors. Int. J. Biol. Macromol. 2025, 290, 138855. [Google Scholar] [CrossRef]
- Nie, S.; Cui, S.W.; Xie, M.; Phillips, A.O.; Phillips, G.O. Bioactive polysaccharides from Cordyceps sinensis: Isolation, structure features and bioactivities. Bioact. Carbohydr. Diet. Fibre 2013, 1, 38–52. [Google Scholar] [CrossRef]
- Li, L.J.; Li, M.Y.; Li, Y.T.; Feng, J.J.; Hao, F.Q.; Zhang, L. Adjuvant activity of Sargassum pallidum polysaccharides against combined Newcastle disease, infectious bronchitis and avian influenza inactivated vaccines. Mar. Drugs 2012, 10, 2648–2660. [Google Scholar] [CrossRef]
- Ceaser, R.; Chimphango, A.F. A strategy for co-extracting hydroxycinnamic acids with hemicellulose, cellulose and lignin from wheat residues in an integrated biorefinery. Int. J. Biol. Macromol. 2025, 290, 138925. [Google Scholar] [CrossRef]
- Spínola, M.P.; Mendes, A.R.; Prates, J.A. Chemical composition, bioactivities, and applications of Spirulina (Limnospira platensis) in food, feed, and medicine. Foods 2024, 13, 3656. [Google Scholar] [CrossRef]
- Saetan, U.; Nontasak, P.; Palasin, K.; Saelim, H.; Wonglapsuwan, M.; Mayakun, J.; Pongparadon, S.; Chotigeat, W. Potential health benefits of fucoidan from the brown seaweeds Sargassum plagiophyllum and Sargassum polycystum. J. Appl. Phycol. 2021, 33, 3357–3364. [Google Scholar] [CrossRef]
- Hu, Y.; He, Y.; Niu, Z.; Shen, T.; Zhang, J.; Wang, X.; Hu, W.; Cho, J.Y. A review of the immunomodulatory activities of polysaccharides isolated from Panax species. J. Ginseng Res. 2022, 46, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.; Sathuvan, M.; Lorenzo, J.M.; Boateng, E.F.; Brohi, S.A.; Zhang, W. Insight into the effects of coconut kernel fiber on the functional and microstructural properties of myofibrillar protein gel system. LWT 2021, 138, 110745. [Google Scholar] [CrossRef]
- Srinivasan, S.; Jayaraman, A.; Ramasamy, A.; Mohanraj, K.; Charan Raja, M.R.; Kar Mahapatra, S. Isolation and characterization of glycoprotein (CNP) isolated from Cocos nucifera L. nutshell and its immunomodulatory role on macrophage activation. J. Funct. Foods 2023, 100, 105380. [Google Scholar] [CrossRef]
- Zhu, Q.; Chen, H.; Chen, W.; Zhang, M.; Zhong, Q.; Chen, Z.; Pei, J.; Chen, W. Effects of glycation method on the emulsifying performance and interfacial behavior of coconut globulins-fucoidan complexes. Food Chem. 2024, 430, 137033. [Google Scholar] [CrossRef]
- DebMandal, M.; Mandal, S. Coconut (Cocos nucifera L.: Arecaceae): In health promotion and disease prevention. Asian Pac. J. Trop. Med. 2011, 4, 241–247. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Pękala, P.; Siemińska-Kuczer, A.; Zdunek, A. A determination of the composition and structure of the polysaccharides fractions isolated from apple cell wall based on FT-IR and FT-Raman spectra supported by PCA analysis. Food Hydrocoll. 2024, 150, 109688. [Google Scholar] [CrossRef]
- Qiu, Y.; Batool, Z.; Liu, R.; Sui, G.; Sheng, B.; Zheng, X.; Xu, D. Characterization and immunological activity of polysaccharides from Potentilla chinensis. Int. J. Biol. Macromol. 2020, 165, 683–690. [Google Scholar] [CrossRef]
- Huo, J.; Lu, Y.; Xia, L.; Chen, D. Structural characterization and anticomplement activities of three acidic homogeneous polysaccharides from Artemisia annua. J. Ethnopharmacol. 2020, 247, 112281. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, T.; Chen, Z.; Guo, K.; Zhao, P. Structural characterization of Corydalis yanhusuo polysaccharides and its bioactivity in vitro. Int. J. Biol. Macromol. 2025, 306, 141575. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, H.; Zhao, Y.; Huang, J.; Zhu, D.; Wang, S.; Zhu, L.; Chen, L.; Xu, X.; Liu, H. Chemical structure, chain conformation and rheological properties of pectic polysaccharides from soy hulls. Int. J. Biol. Macromol. 2020, 148, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Shen, C.-Y.; Jiang, J.-G. Structural characterization of novel arabinoxylan and galactoarabinan from citron with potential antitumor and immunostimulatory activities. Carbohydr. Polym. 2021, 269, 118331. [Google Scholar] [CrossRef]
- Khawas, S.; Sivová, V.; Anand, N.; Bera, K.; Ray, B.; Nosáľová, G.; Ray, S. Chemical profile of a polysaccharide from Psidium guajava leaves and it’s in vivo antitussive activity. Int. J. Biol. Macromol. 2018, 109, 681–686. [Google Scholar] [CrossRef]
- Ding, H.H.; Cui, S.W.; Goff, H.D.; Chen, J.; Guo, Q.; Wang, Q. Xyloglucans from flaxseed kernel cell wall: Structural and conformational characterisation. Carbohydr. Polym. 2016, 151, 538–545. [Google Scholar] [CrossRef]
- Jiang, S.; Ma, J.; Li, Y.; Lu, B.; Du, J.; Xu, J.; Qin, Z.; Ning, T.; Dong, C. A polysaccharide from native Curcuma kwangsiensis and its mechanism of reversing MDSC-induced suppressive function. Carbohydr. Polym. 2022, 297, 120020. [Google Scholar] [CrossRef]
- Hoang, T.-V.; Alshiekheid, M.A.; K, P. A study on anticancer and antioxidant ability of selected brown algae biomass yielded polysaccharide and their chemical and structural properties analysis by FT-IR and NMR analyses. Environ. Res. 2024, 260, 119567. [Google Scholar] [CrossRef]
- Shi, X.; Li, O.; Yin, J.; Nie, S. Structure identification of α-glucans from Dictyophora echinovolvata by methylation and 1D/2D NMR spectroscopy. Food Chem. 2019, 271, 338–344. [Google Scholar] [CrossRef]
- Meng, F.; Li, Q.; Qi, Y.; He, C.; Wang, C.; Zhang, Q. Characterization and immunoregulatory activity of two polysaccharides from the root of Ilex asprella. Carbohydr. Polym. 2018, 197, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Shakhmatov, E.G.; Toukach, P.V.; Makarova, E.N. Structural studies of the pectic polysaccharide from fruits of Punica granatum. Carbohydr. Polym. 2020, 235, 115978. [Google Scholar] [CrossRef] [PubMed]
- Makarova, E.N.; Shakhmatov, E.G. Characterization of pectin-xylan-glucan-arabinogalactan proteins complex from Siberian fir Abies sibirica Ledeb. Carbohydr. Polym. 2021, 260, 117825. [Google Scholar] [CrossRef]
- Zhuang, Y.; Huan, X.-Y.; Sun, L.; Wang, W.; Zhou, M.-J.; Zhao, M.; Chen, P.-D.; Yan, H.; Pang, P.; Shi, X.-Q.; et al. Structural characterization, immunomodulatory effect and immune-mediated antitumor activity of a novel polysaccharide from the rhizome of Atractylodis macrocephala Koidz. Results Chem. 2024, 7, 101397. [Google Scholar] [CrossRef]
- Xiao, Z.; Yan, C.; Jia, C.; Li, Y.; Li, Y.; Li, J.; Yang, X.; Zhan, X.; Ma, C. Structural characterization of chia seed polysaccharides and evaluation of its immunomodulatory and antioxidant activities. Food Chem. X 2023, 20, 101011. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, L.; Yan, A.; Feng, L.; Wan, Y. Fractionation, structure and conformation characterization of polysaccharides from Anoectochilus roxburghii. Carbohydr. Polym. 2020, 231, 115688. [Google Scholar] [CrossRef]
- Bu, Y.; Yin, B.; Qiu, Z.; Li, L.; Zhang, B.; Zheng, Z.; Li, M. Structural characterization and antioxidant activities of polysaccharides extracted from Polygonati rhizoma pomace. Food Chem. X 2024, 23, 101778. [Google Scholar] [CrossRef]
- Zheng, X.; Sun, H.; Wu, L.; Kong, X.; Song, Q.; Zhu, Z. Structural characterization and inhibition on α-glucosidase of the polysaccharides from fruiting bodies and mycelia of Pleurotus eryngii. Int. J. Biol. Macromol. 2020, 156, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Zhang, L.; Black, I.; Glushka, J.; Urbanowicz, B.; Heiss, C.; Azadi, P. Most of the rhamnogalacturonan-I from cultured Arabidopsis cell walls is covalently linked to arabinogalactan-protein. Carbohydr. Polym. 2023, 301, 120340. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Q.; Wu, G.; Ma, J.; Luo, L.; Yin, W.; Wu, M. Structural analysis and adjuvant activity of a polysaccharide from Urtica macrorrhiza. Int. J. Biol. Macromol. 2024, 283, 137433. [Google Scholar] [CrossRef]
- Shao, K.; Sun, X.; Zhang, W.; Cao, Y.; Li, H.; Dai, J. Structural characterisation and evaluation of antioxidant activity of coffee pericarp polysaccharides. Carbohydr. Polym. Technol. Appl. 2025, 11, 100884. [Google Scholar] [CrossRef]
- Rahmi, M.H.; Metusalach, M.; Rahim, S.W.; Heryanto, H.; Tahir, D. Characteristics of lipid content from the analysis of structural and optical properties of brown seaweed (Sargassum polycystum) after defatting by petroleum ether: Preliminary studies. J. Phys. Conf. Ser. 2021, 1763, 012089. [Google Scholar] [CrossRef]
- Li, L.; Qiu, Z.; Dong, H.; Ma, C.; Qiao, Y.; Zheng, Z. Structural characterization and antioxidant activities of one neutral polysaccharide and three acid polysaccharides from the roots of Arctium lappa L.: A comparison. Int. J. Biol. Macromol. 2021, 182, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. Applications of infrared spectroscopy in polysaccharide structural analysis: Progress, challenge and perspective. Food Chem. X 2021, 12, 100168. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Wang, Y.; Liu, J.; Li, Y.; Lu, Y.; Zhao, C.; Li, Z. Determination of the composition and monosaccharide content of atractylodes polysaccharides using pre-column derivatization and a quantitative analysis of multicomponents by a single marker method. J. Chromatogr. Open 2025, 7, 100224. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, J.; Chen, Y.; Ma, Y.; Yang, Q.; Fan, Y.; Fu, C.; Limsila, B.; Li, R.; Liao, W. Extraction, structural characterization and antioxidant activity of turmeric polysaccharides. LWT 2022, 154, 112805. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).