Vitamin D Supplementation and Testosterone Levels in Breast Cancer Survivors
Abstract
1. Introduction
2. Results
2.1. Baseline Data
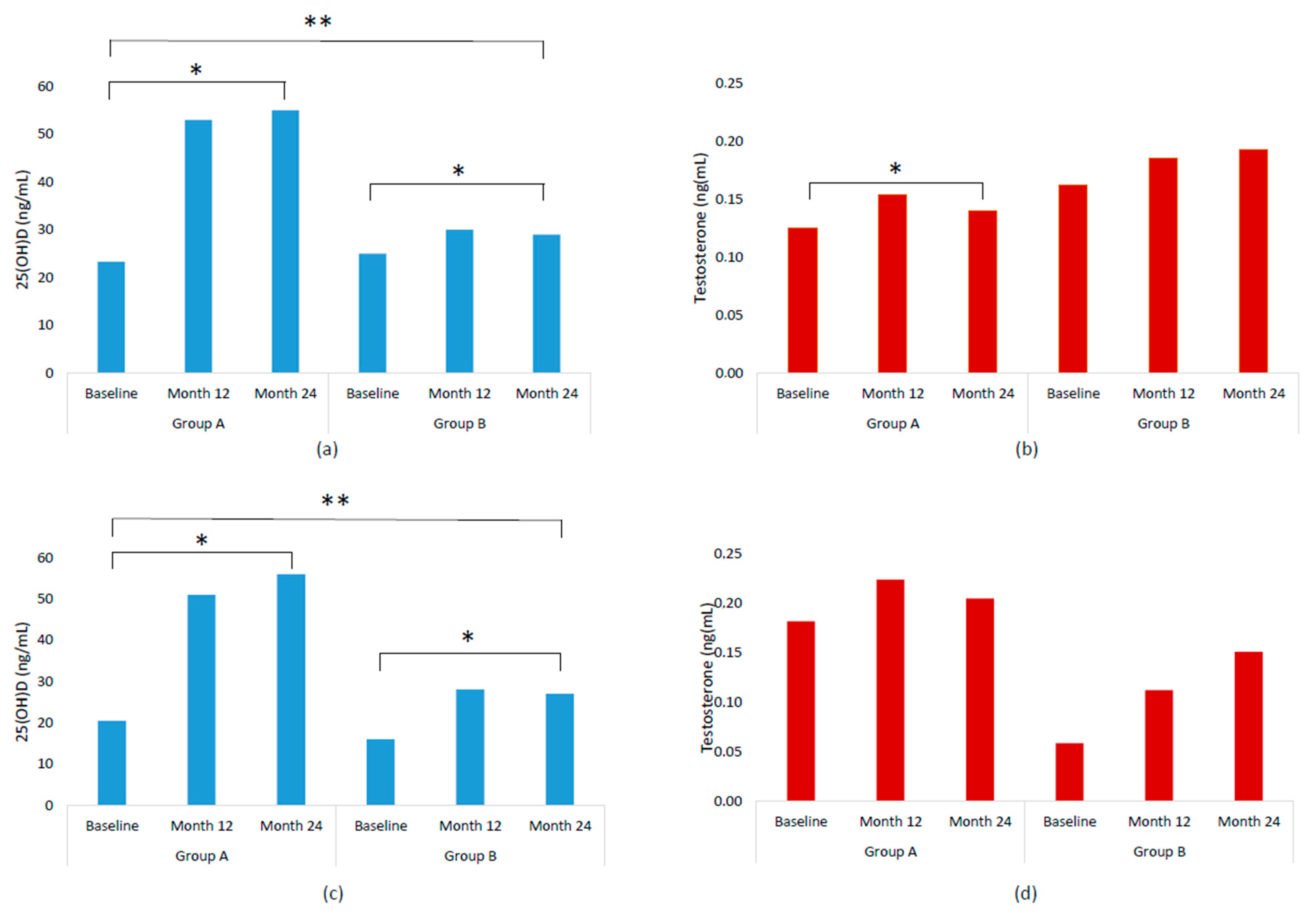
2.2. Serum Concentrations of 25(OH)D and Testosterone by Treatment Groups and Follow-Up Time
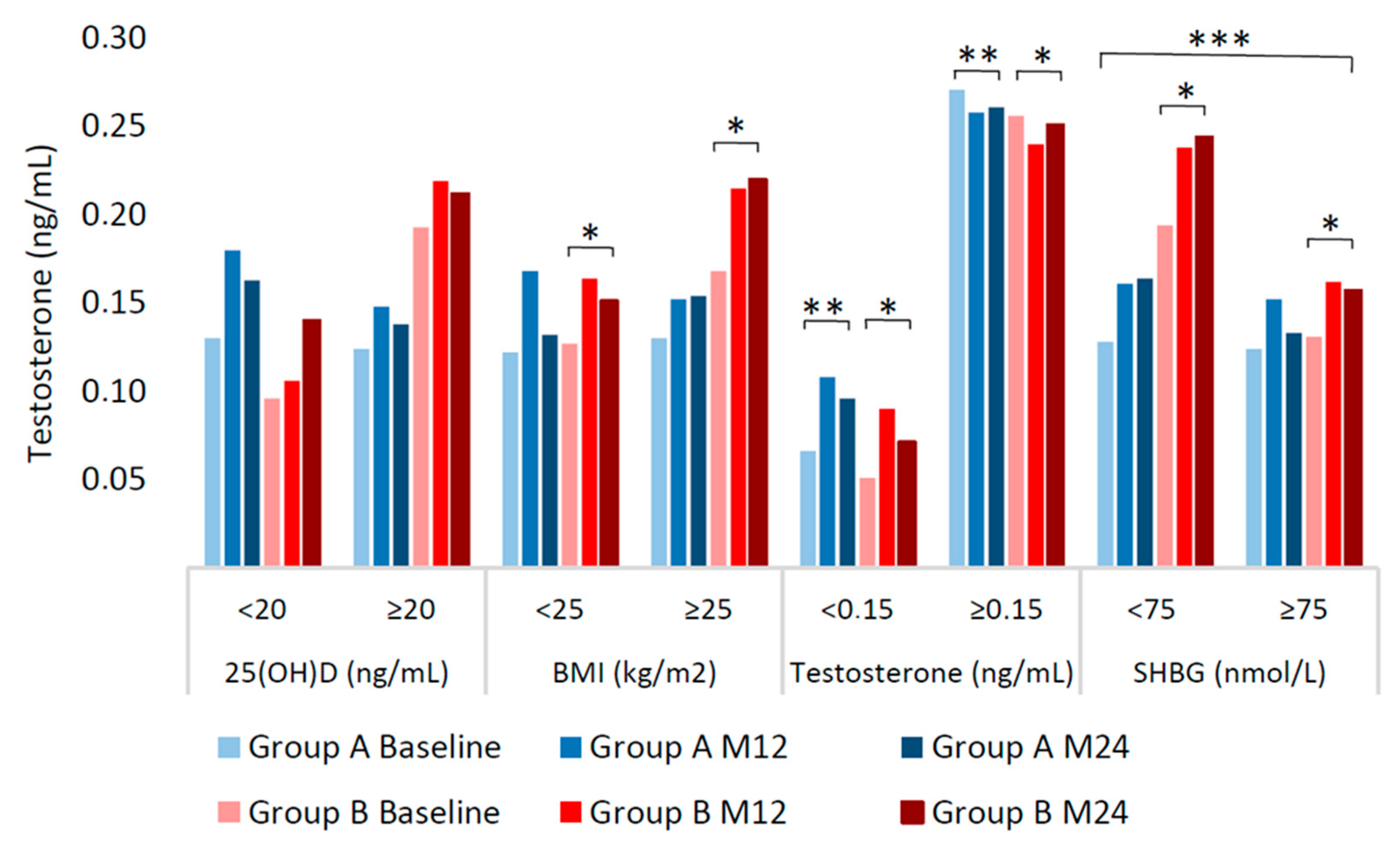
2.3. Multivariable Analysis of Testosterone Variation
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. Anthropometric and Lifestyle Assessments
4.3. Biochemical Analyses
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| MMRM | Mixed Model Repeated Measures |
| SD | Standard Deviation |
| SHBG | Sex Hormone-Binding Globulin |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Danaei, G.; Vander Hoorn, S.; Lopez, A.D.; Murray, C.J.; Ezzati, M. Causes of cancer in the world: Comparative risk assessment of nine behavioural and environmental risk factors. Lancet 2005, 366, 1784–1793. [Google Scholar] [CrossRef]
- Lukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanislawek, A. Breast Cancer-Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies-An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef]
- Ang, B.H.; Teo, S.-H.; Ho, W.-K. Systematic Review and Meta-Analysis of Lifestyle and Reproductive Factors Associated with Risk of Breast Cancer in Asian Women. Cancer Epidemiol. Biomark. Prev. 2024, 33, 1273–1285. [Google Scholar] [CrossRef]
- Lofterod, T.; Frydenberg, H.; Flote, V.; Eggen, A.E.; McTiernan, A.; Mortensen, E.S.; Akslen, L.A.; Reitan, J.B.; Wilsgaard, T.; Thune, I. Exploring the effects of lifestyle on breast cancer risk, age at diagnosis, and survival: The EBBA-Life study. Breast Cancer Res. Treat. 2020, 182, 215–227. [Google Scholar] [CrossRef]
- Zhang, Y.; Lindstrom, S.; Kraft, P.; Liu, Y. Genetic Risk, Health-Associated Lifestyle, and Risk of Early-onset Total Cancer and Breast Cancer. J. Natl. Cancer Inst. 2024, 117, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, I. Vitamin D Metabolism and Guidelines for Vitamin D Supplementation. Clin. Biochem. Rev. 2020, 41, 103–126. [Google Scholar] [CrossRef] [PubMed]
- Dallavalasa, S.; Tulimilli, S.V.; Bettada, V.G.; Karnik, M.; Uthaiah, C.A.; Anantharaju, P.G.; Nataraj, S.M.; Ramashetty, R.; Sukocheva, O.A.; Tse, E.; et al. Vitamin D in Cancer Prevention and Treatment: A Review of Epidemiological, Preclinical, and Cellular Studies. Cancers 2024, 16, 3211. [Google Scholar] [CrossRef]
- Keum, N.; Lee, D.H.; Greenwood, D.C.; Manson, J.E.; Giovannucci, E. Vitamin D supplementation and total cancer incidence and mortality: A meta-analysis of randomized controlled trials. Ann. Oncol. 2019, 30, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Tomas, N.; Balducci, K.; Abar, L.; Aune, D.; Cariolou, M.; Greenwood, D.C.; Markozannes, G.; Nanu, N.; Vieira, R.; Giovannucci, E.L.; et al. Postdiagnosis dietary factors, supplement use and breast cancer prognosis: Global Cancer Update Programme (CUP Global) systematic literature review and meta-analysis. Int. J. Cancer 2023, 152, 616–634. [Google Scholar] [CrossRef]
- Key, T.; Appleby, P.; Barnes, I.; Reeves, G.; Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: Reanalysis of nine prospective studies. J. Natl. Cancer Inst. 2002, 94, 606–616. [Google Scholar]
- Nounu, A.; Kar, S.P.; Relton, C.L.; Richmond, R.C. Sex steroid hormones and risk of breast cancer: A two-sample Mendelian randomization study. Breast Cancer Res. 2022, 24, 66. [Google Scholar] [CrossRef] [PubMed]
- Kaaks, R.; Berrino, F.; Key, T.; Rinaldi, S.; Dossus, L.; Biessy, C.; Secreto, G.; Amiano, P.; Bingham, S.; Boeing, H.; et al. Serum sex steroids in premenopausal women and breast cancer risk within the European Prospective Investigation into Cancer and Nutrition (EPIC). J. Natl. Cancer Inst. 2005, 97, 755–765. [Google Scholar] [CrossRef]
- Tyagi, V.; Scordo, M.; Yoon, R.S.; Liporace, F.A.; Greene, L.W. Revisiting the role of testosterone: Are we missing something? Rev. Urol. 2017, 19, 16–24. [Google Scholar] [PubMed]
- Bianchi, V.E.; Bresciani, E.; Meanti, R.; Rizzi, L.; Omeljaniuk, R.J.; Torsello, A. The role of androgens in women’s health and wellbeing. Pharmacol. Res. 2021, 171, 105758. [Google Scholar] [CrossRef] [PubMed]
- Hammes, S.R.; Levin, E.R. Impact of estrogens in males and androgens in females. J. Clin. Investig. 2019, 129, 1818–1826. [Google Scholar] [CrossRef]
- Ke, B.; Li, C.; Shang, H. Sex hormones in the risk of breast cancer: A two-sample Mendelian randomization study. Am. J. Cancer Res. 2023, 13, 1128–1136. [Google Scholar]
- Venturelli, E.; Orenti, A.; Fabricio, A.S.C.; Garrone, G.; Agresti, R.; Paolini, B.; Bonini, C.; Gion, M.; Berrino, F.; Desmedt, C.; et al. Observational study on the prognostic value of testosterone and adiposity in postmenopausal estrogen receptor positive breast cancer patients. BMC Cancer 2018, 18, 651, Erratum in BMC Cancer 2018, 18, 876. [Google Scholar]
- Dimitrakakis, C.; Bondy, C. Androgens and the breast. Breast Cancer Res. 2009, 11, 212. [Google Scholar] [CrossRef]
- Drummond, A.E.; Swain, C.T.V.; Brown, K.A.; Dixon-Suen, S.C.; Boing, L.; van Roekel, E.H.; Moore, M.M.; Gaunt, T.R.; Milne, R.L.; English, D.R.; et al. Linking Physical Activity to Breast Cancer via Sex Steroid Hormones, Part 2: The Effect of Sex Steroid Hormones on Breast Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2022, 31, 28–37. [Google Scholar] [CrossRef]
- Kotsopoulos, J.; Shafrir, A.L.; Rice, M.; Hankinson, S.E.; Eliassen, A.H.; Tworoger, S.S.; Narod, S.A. The relationship between bilateral oophorectomy and plasma hormone levels in postmenopausal women. Horm. Cancer 2015, 6, 54–63. [Google Scholar] [CrossRef]
- Janssen, I.; Powell, L.H.; Kazlauskaite, R.; Dugan, S.A. Testosterone and visceral fat in midlife women: The Study of Women’s Health Across the Nation (SWAN) fat patterning study. Obesity 2010, 18, 604–610. [Google Scholar] [CrossRef]
- Sieri, S.; Krogh, V.; Bolelli, G.; Abagnato, C.A.; Grioni, S.; Pala, V.; Evangelista, A.; Allemani, C.; Micheli, A.; Tagliabue, G.; et al. Sex hormone levels, breast cancer risk, and cancer receptor status in postmenopausal women: The ORDET cohort. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 169–176. [Google Scholar] [CrossRef]
- Kaaks, R.; Rinaldi, S.; Key, T.J.; Berrino, F.; Peeters, P.H.; Biessy, C.; Dossus, L.; Lukanova, A.; Bingham, S.; Khaw, K.T.; et al. Postmenopausal serum androgens, oestrogens and breast cancer risk: The European prospective investigation into cancer and nutrition. Endocr. Relat. Cancer 2005, 12, 1071–1082. [Google Scholar] [CrossRef]
- Berrino, F.; Pasanisi, P.; Bellati, C.; Venturelli, E.; Krogh, V.; Mastroianni, A.; Berselli, E.; Muti, P.; Secreto, G. Serum testosterone levels and breast cancer recurrence. Int. J. Cancer 2005, 113, 499–502. [Google Scholar] [CrossRef]
- Endogenous Hormones and Breast Cancer Collaborative Group; Key, T.J.; Appleby, P.N.; Reeves, G.K.; Roddam, A.W.; Helzlsouer, K.J.; Alberg, A.J.; Rollison, D.E.; Dorgan, J.F.; Brinton, L.A.; et al. Circulating sex hormones and breast cancer risk factors in postmenopausal women: Reanalysis of 13 studies. Br. J. Cancer 2011, 105, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Tin, S.T.; Reeves, G.K.; Key, T.J. Endogenous hormones and risk of invasive breast cancer in pre- and post-menopausal women: Findings from the UK Biobank. Br. J. Cancer 2021, 125, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, X.; Yu, X.; He, W.; Czene, K.; Yang, H. Hematological and biochemical markers influencing breast cancer risk and mortality: Prospective cohort study in the UK Biobank by multi-state models. Breast 2024, 73, 103603. [Google Scholar] [CrossRef] [PubMed]
- Bleach, R.; McIlroy, M. The Divergent Function of Androgen Receptor in Breast Cancer; Analysis of Steroid Mediators and Tumor Intracrinology. Front. Endocrinol. 2018, 9, 594. [Google Scholar] [CrossRef]
- Ravaioli, S.; Maltoni, R.; Pasculli, B.; Parrella, P.; Giudetti, A.M.; Vergara, D.; Tumedei, M.M.; Pirini, F.; Bravaccini, S. Androgen receptor in breast cancer: The “5W” questions. Front. Endocrinol. 2022, 13, 977331. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Dupuis, M.L.; Pagano, M.T.; Pierdominici, M.; Ortona, E. The role of vitamin D in autoimmune diseases: Could sex make the difference? Biol. Sex Differ. 2021, 12, 12. [Google Scholar] [CrossRef]
- Zhao, D.; Ouyang, P.; de Boer, I.H.; Lutsey, P.L.; Farag, Y.M.; Guallar, E.; Siscovick, D.S.; Post, W.S.; Kalyani, R.R.; Billups, K.L.; et al. Serum vitamin D and sex hormones levels in men and women: The Multi-Ethnic Study of Atherosclerosis (MESA). Maturitas 2017, 96, 95–102. [Google Scholar] [CrossRef]
- Lerchbaum, E.; Trummer, C.; Theiler-Schwetz, V.; Kollmann, M.; Wolfler, M.; Heijboer, A.C.; Pilz, S.; Obermayer-Pietsch, B. Effects of vitamin D supplementation on androgens in men with low testosterone levels: A randomized controlled trial. Eur. J. Nutr. 2019, 58, 3135–3146. [Google Scholar] [CrossRef]
- Yeo, J.K.; Park, S.G.; Park, M.G. Effects of Vitamin D Supplementation on Testosterone, Prostate, and Lower Urinary Tract Symptoms: A Prospective, Comparative Study. World J. Mens Health 2023, 41, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.; Yahyavi, S.K.; Kooij, I.; Poulsen, N.N.; Juul, A.; Jørgensen, N.; Jensen, M.B. Effects of vitamin D on sex steroids, luteinizing hormone, and testosterone to luteinizing hormone ratio in 307 infertile men. Andrology 2024, 12, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Cheshire, J.; Kolli, S. Vitamin A deficiency due to chronic malabsorption: An ophthalmic manifestation of a systemic condition. Case Rep. 2017, 2017, bcr2017220024, Erratum in Case Rep. 2017, 2017, bcr2017220024corr1. [Google Scholar] [CrossRef]
- Anic, G.M.; Albanes, D.; Rohrmann, S.; Kanarek, N.; Nelson, W.G.; Bradwin, G.; Rifai, N.; McGlynn, K.A.; Platz, E.A.; Mondul, A.M. Association between serum 25-hydroxyvitamin D and serum sex steroid hormones among men in NHANES. Clin. Endocrinol. 2016, 85, 258–266. [Google Scholar] [CrossRef]
- Tak, Y.J.; Lee, J.G.; Kim, Y.J.; Park, N.C.; Kim, S.S.; Lee, S.; Cho, B.M.; Kong, E.H.; Jung, D.W.; Yi, Y.H. Serum 25-hydroxyvitamin D levels and testosterone deficiency in middle-aged Korean men: A cross-sectional study. Asian J. Androl. 2015, 17, 324–328. [Google Scholar] [PubMed]
- Endogenous Hormones and Breast Cancer Collaborative Group; Key, T.J.; Appleby, P.N.; Reeves, G.K.; Travis, R.C.; Alberg, A.J.; Barricarte, A.; Berrino, F.; Krogh, V.; Sieri, S.; et al. Sex hormones and risk of breast cancer in premenopausal women: A collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol. 2013, 14, 1009–1019. [Google Scholar]
- Tirabassi, G.; Sudano, M.; Salvio, G.; Cutini, M.; Muscogiuri, G.; Corona, G.; Balercia, G. Vitamin D and Male Sexual Function: A Transversal and Longitudinal Study. Int. J. Endocrinol. 2018, 2018, 3720813. [Google Scholar] [CrossRef]
- Cheng, M.; Song, Z.; Guo, Y.; Luo, X.; Li, X.; Wu, X.; Gong, Y. 1alpha,25-Dihydroxyvitamin D(3) Improves Follicular Development and Steroid Hormone Biosynthesis by Regulating Vitamin D Receptor in the Layers Model. Curr. Issues Mol. Biol. 2023, 45, 4017–4034. [Google Scholar] [CrossRef]
- Wang, N.; Zhai, H.; Zhu, C.; Li, Q.; Han, B.; Chen, Y.; Zhu, C.; Chen, Y.; Xia, F.; Lin, D.; et al. Combined Association of Vitamin D and Sex Hormone Binding Globulin With Nonalcoholic Fatty Liver Disease in Men and Postmenopausal Women: A Cross-Sectional Study. Medicine 2016, 95, e2621. [Google Scholar] [CrossRef]
- Somboonporn, W.; Davis, S.R.; National Health and Medical Research Council. Testosterone effects on the breast: Implications for testosterone therapy for women. Endocr. Rev. 2004, 25, 374–388. [Google Scholar] [CrossRef]
- Farhat, G.N.; Cummings, S.R.; Chlebowski, R.T.; Parimi, N.; Cauley, J.A.; Rohan, T.E.; Huang, A.J.; Vitolins, M.; Hubbell, F.A.; Manson, J.E.; et al. Sex hormone levels and risks of estrogen receptor-negative and estrogen receptor-positive breast cancers. J. Natl. Cancer Inst. 2011, 103, 562–570. [Google Scholar] [CrossRef]
- Henn, M.; Martin-Gorgojo, V.; Martin-Moreno, J.M. Vitamin D in Cancer Prevention: Gaps in Current Knowledge and Room for Hope. Nutrients 2022, 14, 4512. [Google Scholar] [CrossRef]
- Torres, A.; Cameselle, C.; Otero, P.; Simal-Gandara, J. The Impact of Vitamin D and Its Dietary Supplementation in Breast Cancer Prevention: An Integrative Review. Nutrients 2024, 16, 573. [Google Scholar] [CrossRef]
- Augustin, L.S.; Libra, M.; Crispo, A.; Grimaldi, M.; De Laurentiis, M.; Rinaldo, M.; D’Aiuto, M.; Catalano, F.; Banna, G.; Ferrau, F.; et al. Low glycemic index diet, exercise and vitamin D to reduce breast cancer recurrence (DEDiCa): Design of a clinical trial. BMC Cancer 2017, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, M.; Shah, N.; Khan, I.; Shah, M.M.; Saleem, M.N. Association Between Body Mass Index (BMI), Vitamin D, and Testosterone Levels. Cureus 2024, 16, e71509. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Gallos, I.; Tobias, A.; Tan, B.; Eapen, A.; Coomarasamy, A. Vitamin D and assisted reproductive treatment outcome: A systematic review and meta-analysis. Hum. Reprod. 2018, 33, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, F.A.; Grzechocinska, B.; Wielgos, M. The role of vitamin D in reproductive health–A Trojan Horse or the Golden Fleece? Nutrients 2015, 7, 4139–4153. [Google Scholar] [CrossRef]
- Grundmann, M.; von Versen-Höynck, F. Vitamin D—Roles in women’s reproductive health? Reprod. Biol. Endocrinol. 2011, 9, 146. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, L.M.; Catov, J.M.; Simhan, H.N.; Holick, M.F.; Powers, R.W.; Roberts, J.M. Maternal vitamin D deficiency increases the risk of preeclampsia. J. Clin. Endocrinol. Metab. 2007, 92, 3517–3522. [Google Scholar] [CrossRef]
- Jensen, M.B. Vitamin D metabolism, sex hormones, and male reproductive function. Reproduction 2012, 144, 135–152, Erratum in Reproduction 2012, 144, 647. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Tsuprykov, O.; Chen, X.; Elitok, S.; Kramer, B.K.; Hocher, B. Relationship Between Vitamin D and Hormones Important for Human Fertility in Reproductive-Aged Women. Front. Endocrinol. 2021, 12, 666687. [Google Scholar] [CrossRef]
- Davison, S.L.; Bell, R.; Donath, S.; Montalto, J.G.; Davis, S.R. Androgen levels in adult females: Changes with age, menopause, and oophorectomy. J. Clin. Endocrinol. Metab. 2005, 90, 3847–3853. [Google Scholar] [CrossRef] [PubMed]
- Van Londen, G.J.; Perera, S.; Vujevich, K.; Rastogi, P.; Lembersky, B.; Brufsky, A.; Vogel, V.; Greenspan, S.L. The impact of an aromatase inhibitor on body composition and gonadal hormone levels in women with breast cancer. Breast Cancer Res. Treat. 2011, 125, 441–446. [Google Scholar] [CrossRef]
- Williams, A.; Babu, J.R.; Wadsworth, D.D.; Burnett, D.; Geetha, T. The Effects of Vitamin D on Metabolic Profiles in Women with Polycystic Ovary Syndrome: A Systematic Review. Horm. Metab. Res. 2020, 52, 485–491. [Google Scholar] [CrossRef]
- Chang, E.M.; Kim, Y.S.; Won, H.J.; Yoon, T.K.; Lee, W.S. Association between sex steroids, ovarian reserve, and vitamin D levels in healthy nonobese women. J. Clin. Endocrinol. Metab. 2014, 99, 2526–2532. [Google Scholar] [CrossRef]
- Li, H.W.; Brereton, R.E.; Anderson, R.A.; Wallace, A.M.; Ho, C.K. Vitamin D deficiency is common and associated with metabolic risk factors in patients with polycystic ovary syndrome. Metabolism 2011, 60, 1475–1481. [Google Scholar] [CrossRef]
- Ciavattini, A.; Serri, M.; Delli Carpini, G.; Morini, S.; Clemente, N. Ovarian endometriosis and vitamin D serum levels. Gynecol. Endocrinol. 2017, 33, 164–167. [Google Scholar] [CrossRef]
- Lerchbaum, E.; Obermayer-Pietsch, B. Mechanisms in endocrinology: Vitamin D and fertility: A systematic review. Eur. J. Endocrinol. 2012, 166, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Foroozanfard, F.; Rahmani, E.; Talebi, M.; Bahmani, F.; Asemi, Z. Effect of Two Different Doses of Vitamin D Supplementation on Metabolic Profiles of Insulin-Resistant Patients with Polycystic Ovary Syndrome. Nutrients 2017, 9, 1280. [Google Scholar] [CrossRef]
- Marsh, K.A.; Steinbeck, K.S.; Atkinson, F.S.; Petocz, P.; Brand-Miller, J.C. Effect of a low glycemic index compared with a conventional healthy diet on polycystic ovary syndrome. Am. J. Clin. Nutr. 2010, 92, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Zapala, B.; Marszalec, P.; Piwowar, M.; Chmura, O.; Milewicz, T. Reduction in the Free Androgen Index in Overweight Women After Sixty Days of a Low Glycemic Diet. Exp. Clin. Endocrinol. Diabetes 2024, 132, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Hayes, L.D.; Herbert, P.; Sculthorpe, N.F.; Grace, F.M. Exercise training improves free testosterone in lifelong sedentary aging men. Endocr. Connect. 2017, 6, 306–310. [Google Scholar] [CrossRef]
- Hiruntrakul, A.; Nanagara, R.; Emasithi, A.; Borer, K.T. Effect of once a week endurance exercise on fitness status in sedentary subjects. J. Med. Assoc. Thai 2010, 93, 1070–1074. [Google Scholar]
- Shahid, W.; Noor, R. Effects of integrated exercise approach on total testosterone levels in eumenorrheic women: A randomized controlled trial. Sci. Rep. 2025, 15, 15692. [Google Scholar] [CrossRef]
- Hogervorst, E.; Matthews, F.E.; Brayne, C. Are optimal levels of testosterone associated with better cognitive function in healthy older women and men? Biochim. Biophys. Acta BBA Gen. Subj. 2010, 1800, 1145–1152. [Google Scholar] [CrossRef]
- Klingberg, E.; Olerod, G.; Konar, J.; Petzold, M.; Hammarsten, O. Seasonal variations in serum 25-hydroxy vitamin D levels in a Swedish cohort. Endocrine 2015, 49, 800–808. [Google Scholar] [CrossRef]
- Sachs, M.C.; Shoben, A.; Levin, G.P.; Robinson-Cohen, C.; Hoofnagle, A.N.; Swords-Jenny, N.; Ix, J.H.; Budoff, M.; Lutsey, P.L.; Siscovick, D.S.; et al. Estimating mean annual 25-hydroxyvitamin D concentrations from single measurements: The Multi-Ethnic Study of Atherosclerosis. Am. J. Clin. Nutr. 2013, 97, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
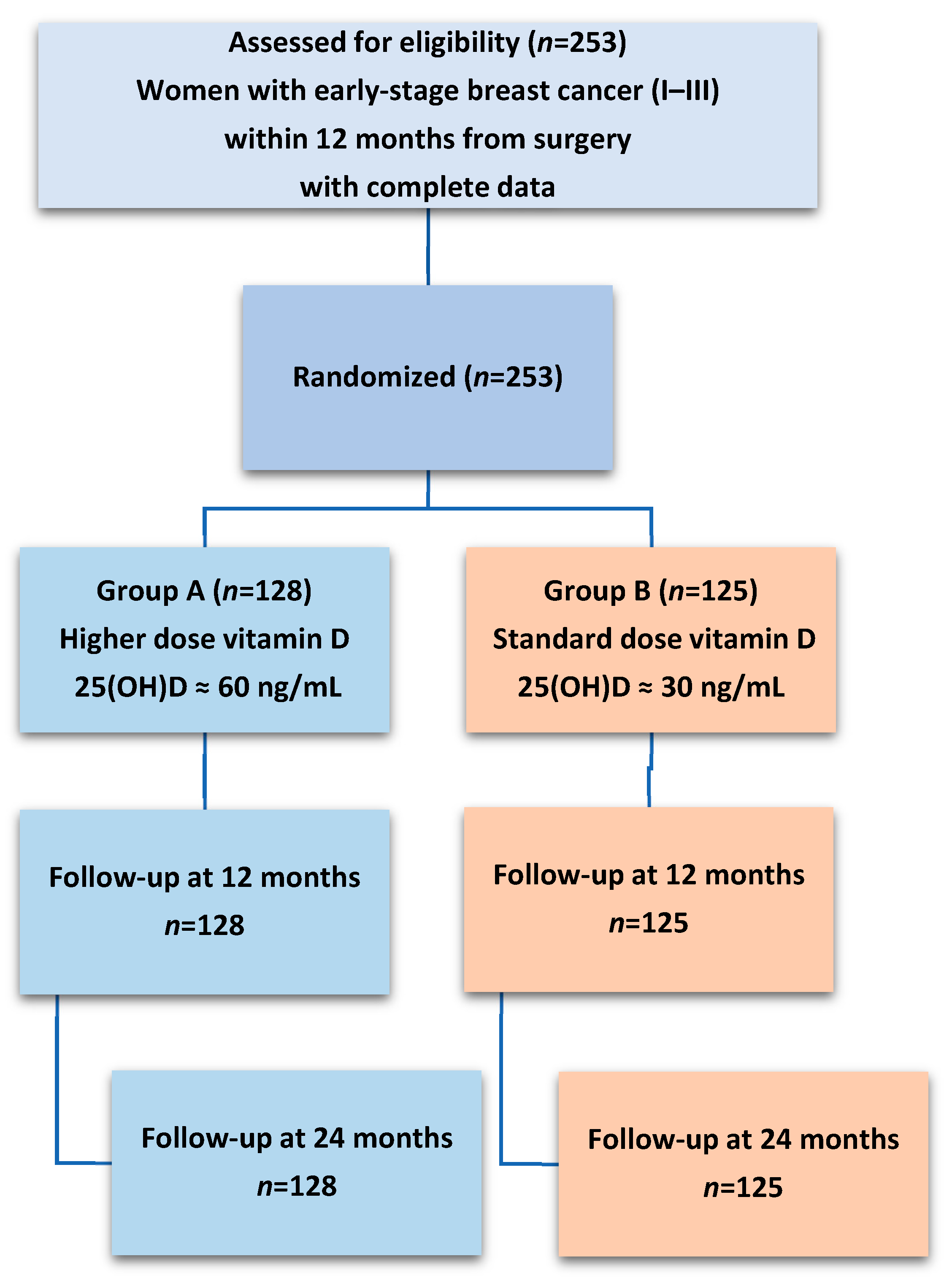
| Overall | Group A | Group B | p | |
|---|---|---|---|---|
| Age (years), mean ± SD | 52 ± 9 | 52 ± 9 | 52 ± 9 | 0.703 a |
| Waist circ. (cm), mean ± SD | 94 ± 13 | 92 ± 13 | 95 ± 13 | 0.155 a |
| BMI (kg/m2), mean ± SD BMI (kg/m2) | 27 ± 5 | 27 ± 5 | 27 ± 5 | 0.390 a 0.866 b |
| <25 | 112 (44.3%) | 56 (43.7%) | 56 (44.8%) | |
| ≥25 | 141 (55.7%) | 72 (56.3%) | 69 (55.2%) | |
| 25(OH)D ng/mL | 0.516 b | |||
| ≤10 | 31 (12.3%) | 14 (10.9%) | 17 (13.6%) | |
| >10 to 20 | 63 (24.9%) | 32 (25.0%) | 31 (24.8%) | |
| >20 to 30 | 77 (30.4%) | 44 (34.4%) | 33 (26.4%) | |
| >30 | 82 (32.4%) | 38 (29.7%) | 44 (35.2%) | |
| Menopausal status | 0.864 c | |||
| Post-menopause | 230 (90.9%) | 116 (90.6%) | 114 (91.2%) | |
| Pre-menopause | 21 (8.3%) | 11 (8.6%) | 10 (8.0%) | |
| (Missing) | 2 (0.8%) | 1 (0.8%) | 1 (0.8%) | |
| Smoking status | 0.852 b | |||
| Never | 133 (52.6%) | 66 (51.6%) | 67 (53.6%) | |
| Current | 43 (17.0%) | 21 (16.4%) | 22 (17.6%) | |
| Past | 77 (30.4%) | 41 (32.0%) | 36 (28.8%) | |
| Type of surgery | 0.341 c | |||
| Mastectomy | 57 (22.5%) | 32 (25.0%) | 25 (20.0%) | |
| Quadrantectomy | 194 (76.7%) | 95 (74.2%) | 99 (79.2%) | |
| (Missing) | 2 (0.8%) | 1 (0.8%) | 1 (0.8%) | |
| Cancer stage at surgery | 0.445 c | |||
| I | 81 (32.0%) | 47 (36.7%) | 34 (27.2%) | |
| IIA | 115 (45.4%) | 53 (41.4%) | 62 (49.6%) | |
| IIB | 29 (11.5%) | 13 (10.2%) | 16 (12.8%) | |
| IIIA | 22 (8.7%) | 11 (8.6%) | 11 (8.8%) | |
| IIIC | 6 (2.4%) | 4 (3.1%) | 2 (1.6%) | |
| Molecular subtypes | 0.156 c | |||
| HER2+ | 11 (4.3%) | 3 (2.3%) | 8 (6.4%) | |
| Luminal A | 83 (32.8%) | 38 (29.7%) | 45 (36%) | |
| Luminal B | 127 (50.2%) | 72 (56.3%) | 55 (44%) | |
| Triple Negative | 32 (12.7%) | 15 (11.7%) | 17 (13.6%) | |
| Hormonal therapy use | 207 (82%) | 108 (84%) | 99 (79%) | 0.286 b |
| Group A | Group B | ||||||
|---|---|---|---|---|---|---|---|
| Variables | Baseline | Month 12 | Month 24 | Baseline | Month 12 | Month 24 | p a |
| n = 128 | n = 128 | n = 128 | n = 125 | n = 125 | n = 125 | ||
| 25(OH)D (ng/mL) | 23.3 (16.8–32.5) | 53.0 (43.8–59.3) | 55.0 (46.8–62.0) * | 25.0 (15.0–33.0) | 30.0 (25.0–34.0) | 29.0 (26.0–35.0) * | <0.001 |
| Testosterone (ng/mL) | 0.125 (0.05–0.24) | 0.154 (0.07–0.26) | 0.140 (0.07–0.31) * | 0.162 (0.06–0.26) | 0.185 (0.08–0.28) | 0.193 (0.08–0.28) | 0.682 |
| Patients without hormone suppressive therapy | |||||||
| n = 20 | n = 20 | n = 20 | n = 26 | n = 26 | n = 26 | ||
| 25(OH)D (ng/mL) | 20.5 (13.3–26.0) | 51 (42.5–57.3) | 56 (45–63.3) * | 16 (12–24) | 28 (24–32) | 27 (25–30.5) * | <0.001 |
| Testosterone (ng/mL) | 0.182 (0.06–0.24) | 0.224 (0.08–0.26) | 0.205 (0.11–0.28) | 0.059 (0.03–0.17) | 0.112 (0.04–0.23) | 0.151 (0.04–0.26) | 0.359 |
| Group A | Group B | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | M12 | M24 | Baseline | M12 | M24 | ||||||
| n (%) | n = 128 | n = 128 | n = 128 | p a | n (%) | n = 125 | n = 125 | n = 125 | p a | p b | |
| 25(OH)D (ng/mL) | 0.900 | 0.099 | 0.211 | ||||||||
| <20 | 42 (32.8) | 0.126 (0.03–0.22) | 0.180 (0.08–0.25) | 0.163 (0.07–0.31) | 46 (36.8) | 0.096 (0.03–0.19) | 0.106 (0.03–0.23) | 0.140 (0.06–0.24) | |||
| ≥20 | 86 (67.2) | 0.124 (0.07–0.25) | 0.148 (0.06–0.3) | 0.138 (0.06–0.28) | 79 (63.2) | 0.193 (0.1–0.28) | 0.219 (0.14–0.3) | 0.212 (0.1–0.29) | |||
| BMI (kg/m2) | 0.987 | 0.007 | 0.056 | ||||||||
| <25 | 56 (43.8) | 0.122 (0.06–0.27) | 0.168 (0.05–0.31) | 0.132 (0.06–0.26) | 56 (44.8) | 0.127 (0.04–0.25) | 0.164 (0.06–0.24) | 0.151 (0.06–0.24) | |||
| ≥25 | 72 (56.2) | 0.130 (0.05–0.23) | 0.152 (0.08–0.25) | 0.154 (0.08–0.31) | 69 (55.2) | 0.168 (0.09–0.26) | 0.215 (0.11–0.3) | 0.220 (0.13–0.34) | |||
| Testosterone (ng/mL) | <0.001 | <0.001 | 0.902 | ||||||||
| <0.150 | 74 (57.8) | 0.066 (0.03–0.12) | 0.108 (0.04–0.17) | 0.096 (0.04–0.17) | 57 (45.6) | 0.051 (0.03–0.1) | 0.090 (0.03–0.18) | 0.071 (0.04–0.19) | |||
| ≥0.150 | 54 (42.2) | 0.271 (0.22–0.32) | 0.258 (0.17–0.37) | 0.261 (0.12–0.38) | 68 (54.4) | 0.256 (0.19–0.36) | 0.240 (0.18–0.32) | 0.251 (0.19–0.34) | |||
| SHBG (nmol/L) | 0.615 | 0.002 | 0.006 | ||||||||
| <75 | 58 (45.3) | 0.128 (0.07–0.27) | 0.161 (0.09–0.25) | 0.164 (0.08–0.31) | 68 (54.4) | 0.194 (0.09–0.33) | 0.238 (0.11–0.32) | 0.244 (0.11–0.35) | |||
| ≥75 | 70 (54.7) | 0.124 (0.05–0.22) | 0.152 (0.06–0.27) | 0.133 (0.07–0.26) | 57 (45.6) | 0.131 (0.04–0.19) | 0.162 (0.07–0.22) | 0.157 (0.06–0.22) | |||
| All Subjects (n = 253) | |||
|---|---|---|---|
| Beta | 95% CI | p | |
| Baseline levels | |||
| 25(OH)D | 0.02 | −0.11, 0.14 | 0.776 |
| Testosterone | −0.37 | −0.46, −0.28 | <0.001 |
| SHBG | −0.03 | −0.07, 0.00 | 0.042 |
| Baseline changes | |||
| 25(OH)D | 0.01 | −0.06, 0.09 | 0.704 |
| SHBG | −0.03 | −0.06, 0.00 | 0.025 |
| BMI | 0.69 | 0.03, 1.3 | 0.039 |
| HOMA-IR | 0.145 | ||
| ≤2.5 | Ref | ||
| >2.5 | −1.7 | −3.9, 0.58 | |
| Follow-up | 0.634 | ||
| Month 12 | Ref | ||
| Month 24 | 0.30 | −0.95, 1.6 | |
| Age | −0.04 | −0.19, 0.11 | 0.626 |
| Hormone suppressive therapy ** | 0.121 | ||
| After baseline (n = 35) | Ref | ||
| Before baseline (n = 170) | −4.3 | −8.5, −0.07 | |
| Never (n = 46) | −2.5 | −7.3, 2.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minopoli, A.; Di Gennaro, P.; Porciello, G.; Palumbo, E.; Vitale, S.; Grimaldi, M.; Pica, R.; Falzone, L.; Montagnese, C.; de Falco, R.; et al. Vitamin D Supplementation and Testosterone Levels in Breast Cancer Survivors. Int. J. Mol. Sci. 2025, 26, 10030. https://doi.org/10.3390/ijms262010030
Minopoli A, Di Gennaro P, Porciello G, Palumbo E, Vitale S, Grimaldi M, Pica R, Falzone L, Montagnese C, de Falco R, et al. Vitamin D Supplementation and Testosterone Levels in Breast Cancer Survivors. International Journal of Molecular Sciences. 2025; 26(20):10030. https://doi.org/10.3390/ijms262010030
Chicago/Turabian StyleMinopoli, Anita, Piergiacomo Di Gennaro, Giuseppe Porciello, Elvira Palumbo, Sara Vitale, Maria Grimaldi, Rosa Pica, Luca Falzone, Concetta Montagnese, Renato de Falco, and et al. 2025. "Vitamin D Supplementation and Testosterone Levels in Breast Cancer Survivors" International Journal of Molecular Sciences 26, no. 20: 10030. https://doi.org/10.3390/ijms262010030
APA StyleMinopoli, A., Di Gennaro, P., Porciello, G., Palumbo, E., Vitale, S., Grimaldi, M., Pica, R., Falzone, L., Montagnese, C., de Falco, R., Crispo, A., Giannascoli, D., Di Capua, L., Meola, S., Pinto, M., De Laurentiis, M., Di Lauro, V., Ferraù, F., Catalano, F., ... Augustin, L. S. A. (2025). Vitamin D Supplementation and Testosterone Levels in Breast Cancer Survivors. International Journal of Molecular Sciences, 26(20), 10030. https://doi.org/10.3390/ijms262010030












