Vaping in Pregnancy: Unraveling Molecular Drivers of Preeclampsia and Fetal Growth Restriction
Abstract
1. Introduction
2. Results
2.1. Blood Pressure, Proteinuria, and Weight Assessments
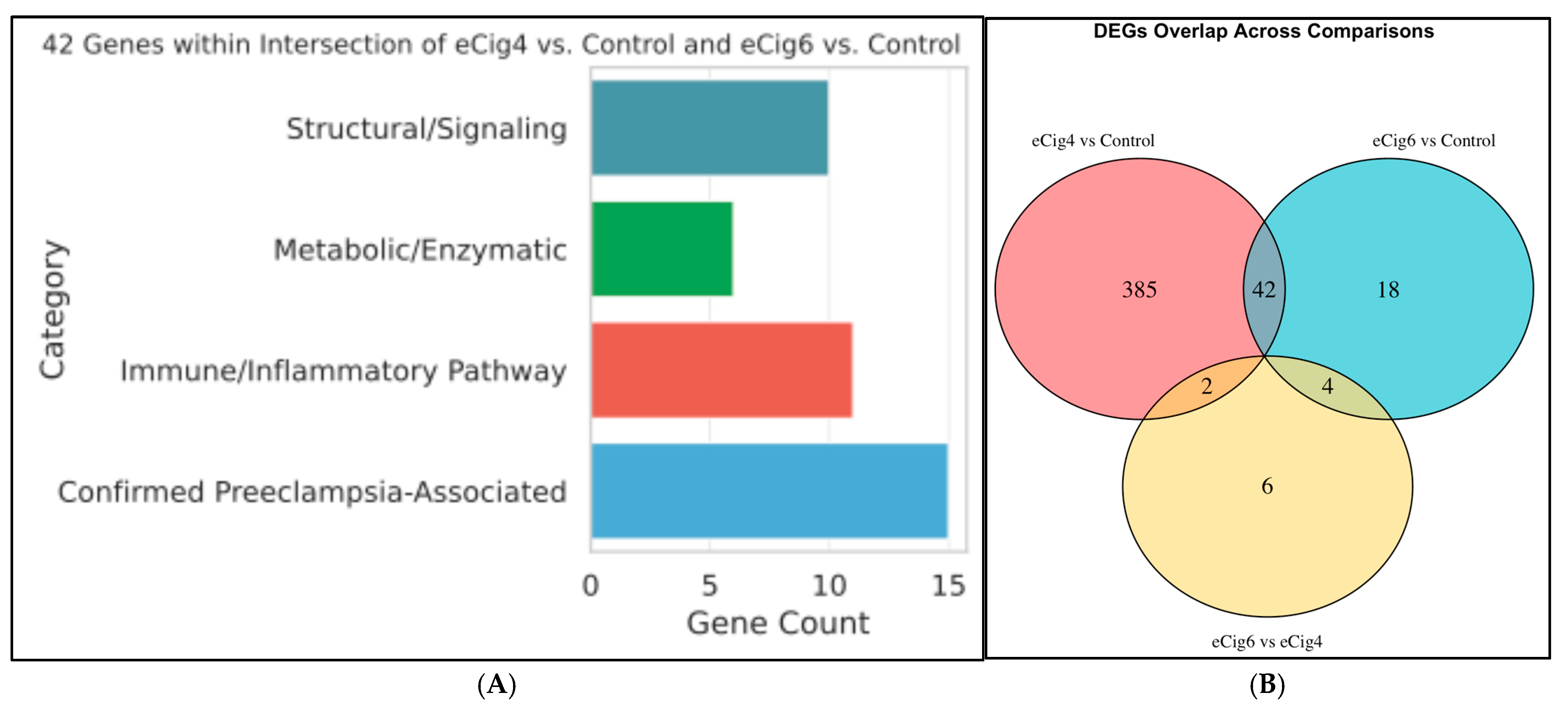
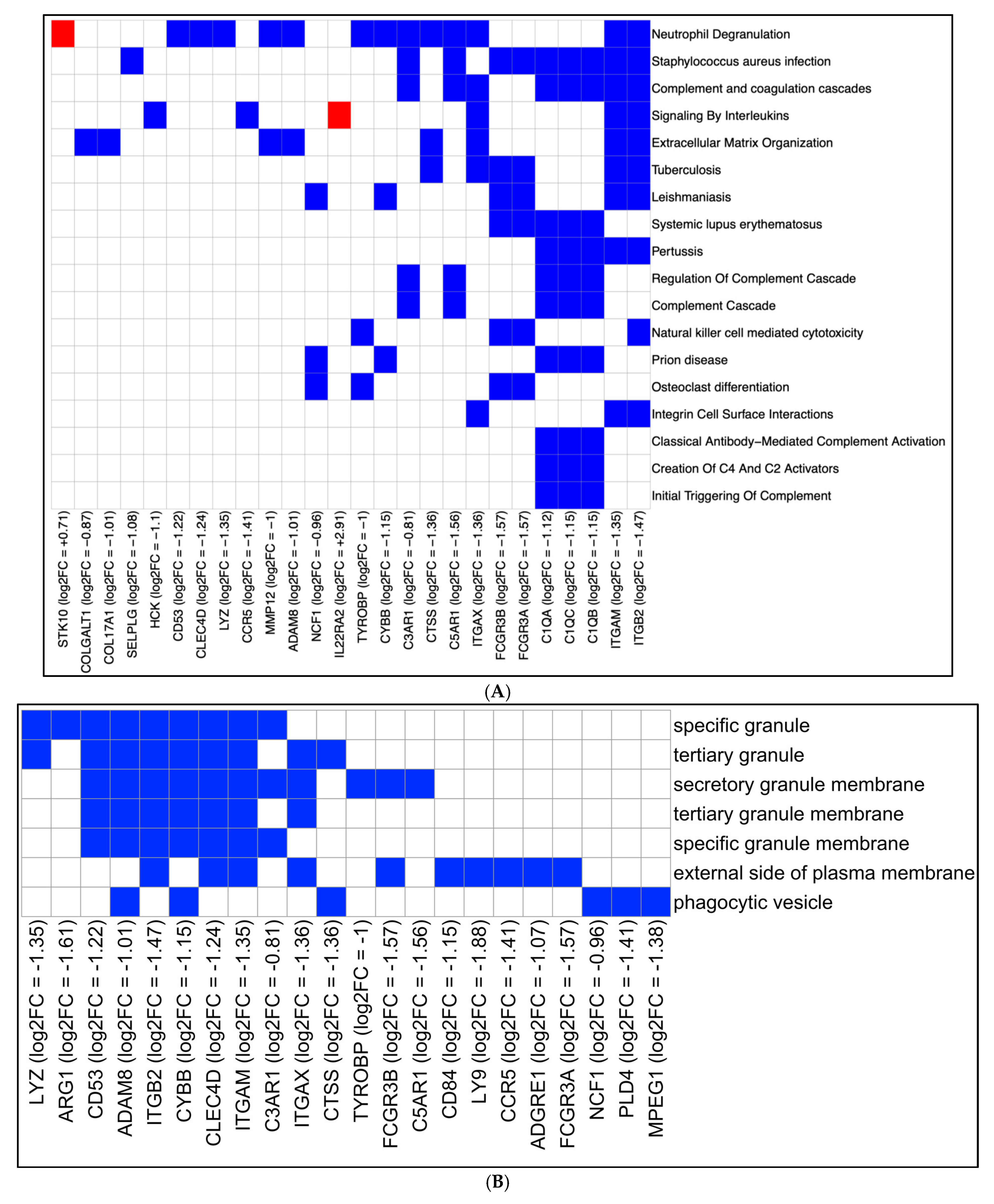
2.2. Differential Gene Expression Analysis
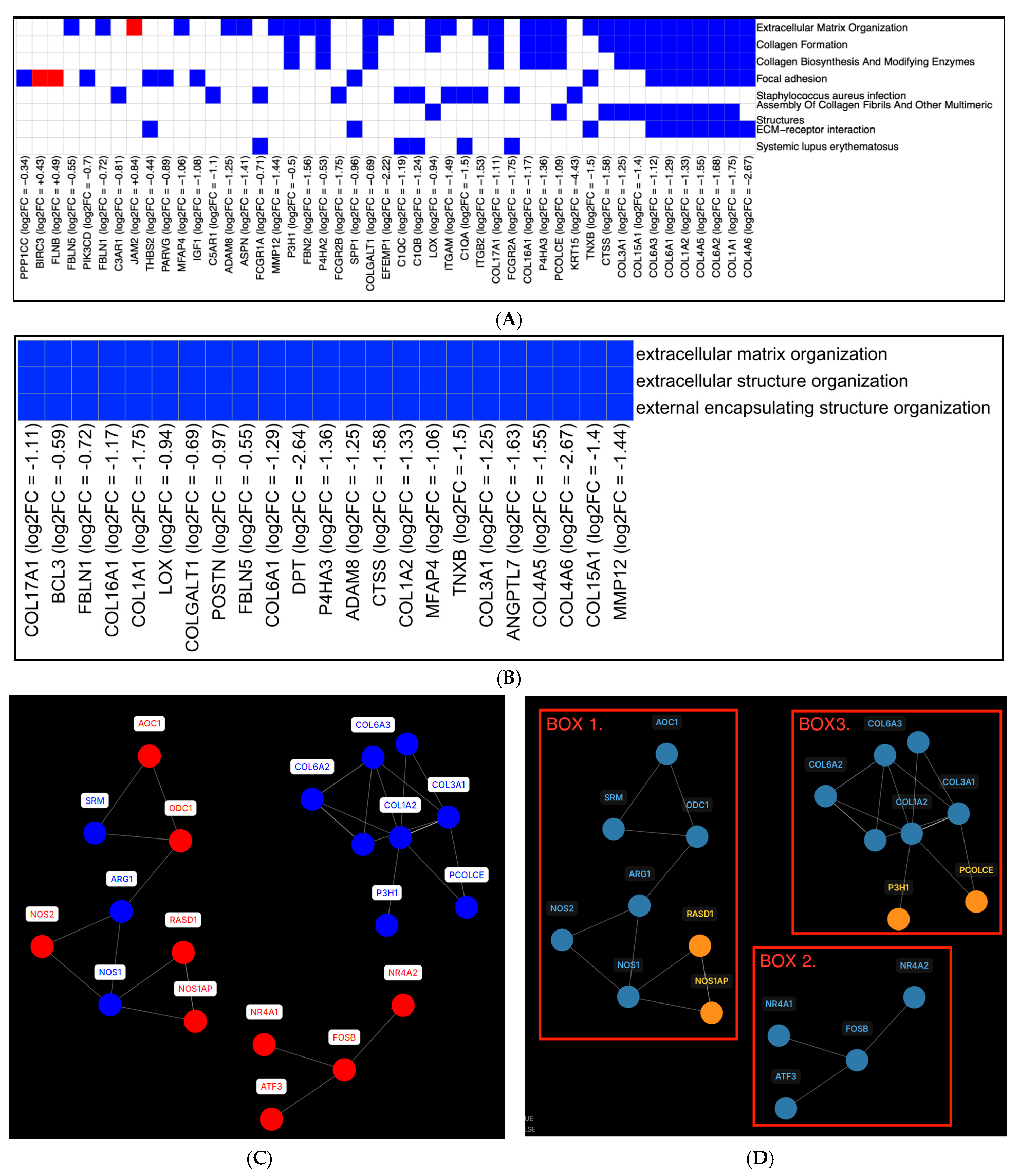
2.3. eCig4-Induced IUGR Model DEG Analysis
2.4. eCig6-Induced IUGR + PE Model
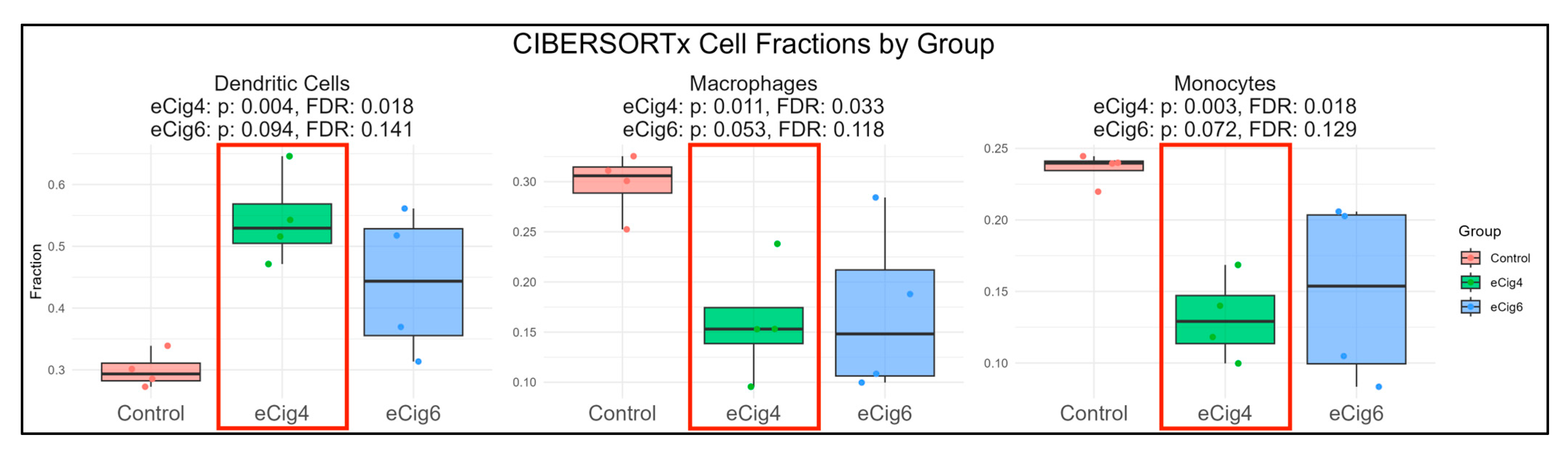
2.5. Immune Infiltration Analysis
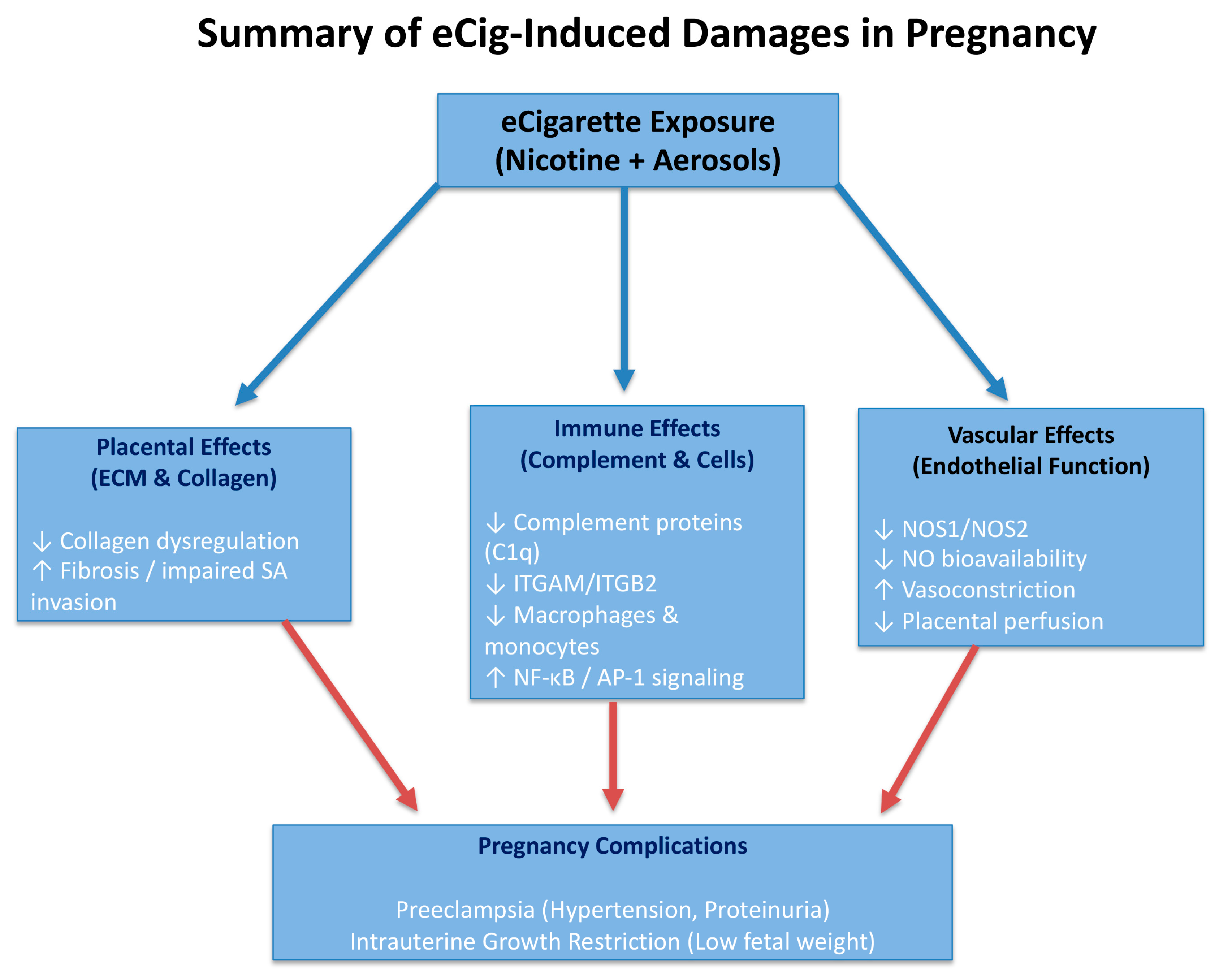
3. Discussion
3.1. DEG Analysis
3.2. eCig4-Induced IUGR Model
3.3. eCig6-Induced IUGR + PE
3.4. Limitations
4. Materials and Methods
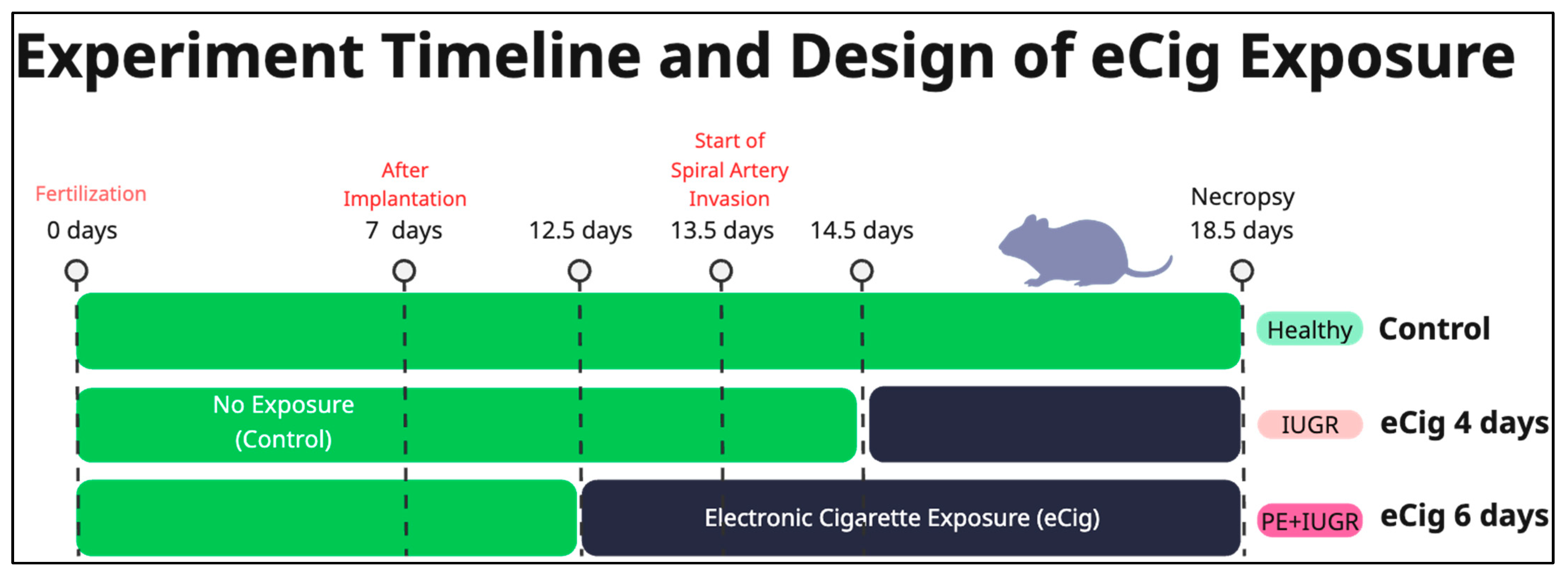
4.1. Animal Husbandry and eCig Exposure Protocol
4.2. Blood Pressure Assessment
4.3. Proteinuria Analysis
4.4. RNA Extraction, Library Preparation, and Sequencing
4.5. Statistical Evaluation
4.6. RNA-Seq Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hypertension in Pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar] [CrossRef]
- World Health Organization International Collaborative Study of Hypertensive Disorders of Pregnancy. Geographic variation in the incidence of hypertension in pregnancy. Am. J. Obstet. Gynecol. 1988, 158, 80–83. [Google Scholar]
- Cresswell, J.A.; Alexander, M.; Chong, M.Y.C.; Link, H.M.; Pejchinovska, M.; Gazeley, U.; Ahmed, S.M.A.; Chou, D.; Moller, A.B.; Simpson, D.; et al. Global and regional causes of maternal deaths 2009-20: A WHO systematic analysis. Lancet Glob Health 2025, 13, e626–e634. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Chaemsaithong, P.; Romero, R.; Shaman, M.; Kim, C.J.; Kim, J.S.; Qureshi, F.; Jacques, S.M.; Ahmed, A.I.; Chaiworapongsa, T.; et al. The frequency of acute atherosis in normal pregnancy and preterm labor, preeclampsia, small-for-gestational age, fetal death and midtrimester spontaneous abortion. J. Matern. Fetal Neonatal Med. 2015, 28, 2001–2009. [Google Scholar] [CrossRef] [PubMed]
- Lawn, J.E.; Ohuma, E.O.; Bradley, E.; Idueta, L.S.; Hazel, E.; Okwaraji, Y.B.; Erchick, D.J.; Yargawa, J.; Katz, J.; Lee, A.C.C.; et al. Small babies, big risks: Global estimates of prevalence and mortality for vulnerable newborns to accelerate change and improve counting. Lancet 2023, 401, 1707–1719. [Google Scholar] [CrossRef]
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef]
- Vahratian, A.; Briones, E.M.; Jamal, A.; Marynak, K.L. Electronic Cigarette Use Among Adults in the United States, 2019–2023. NCHS Data Brief 2025, 524, CS356607. [Google Scholar] [CrossRef]
- Lambers, D.S.; Clark, K.E. The maternal and fetal physiologic effects of nicotine. Semin. Perinatol. 1996, 20, 115–126. [Google Scholar] [CrossRef]
- Luck, W.; Nau, H. Exposure of the fetus, neonate, and nursed infant to nicotine and cotinine from maternal smoking. N. Engl. J. Med. 1984, 311, 672. [Google Scholar] [CrossRef]
- Luck, W.; Nau, H.; Hansen, R.; Steldinger, R. Extent of nicotine and cotinine transfer to the human fetus, placenta and amniotic fluid of smoking mothers. Dev. Pharmacol. Ther. 1985, 8, 384–395. [Google Scholar] [CrossRef]
- Mills, A.; Dakhlallah, D.; Ranpara, A.; Goldsmith, W.T.; Chantler, P.D.; Huang, Y.W.; Boyd, J.; Olfert, I.M. Pregnancy and Postpartum Effects of Electronic Cigarettes on Maternal Health and Vascular Function in the Fourth Trimester. Cardiovasc. Toxicol. 2025, 25, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Raez-Villanueva, S.; Ma, C.; Kleiboer, S.; Holloway, A.C. The effects of electronic cigarette vapor on placental trophoblast cell function. Reprod. Toxicol. 2018, 81, 115–121. [Google Scholar] [CrossRef]
- Lin, S.Y.; Wang, L.; Zhou, W.; Kitsantas, P.; Wen, X.; Xue, H. E-cigarette use during pregnancy and its association with adverse birth outcomes in the US. Prev. Med. 2023, 166, 107375. [Google Scholar] [CrossRef]
- Regan, A.K.; Bombard, J.M.; O’Hegarty, M.M.; Smith, R.A.; Tong, V.T. Adverse Birth Outcomes Associated With Prepregnancy and Prenatal Electronic Cigarette Use. Obstet. Gynecol. 2021, 138, 85–94. [Google Scholar] [CrossRef]
- Al-Sawalha, N.; Alzoubi, K.; Khabour, O.; Karaoghlanian, N.; Ismail, Z.; Shihadeh, A.; Eissenberg, T. Effect of electronic cigarette aerosol exposure during gestation and lactation on learning and memory of adult male offspring rats. Physiol. Behav. 2020, 221, 112911. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Narain, V.; Bondesson, M. E-cigarette vaping liquids and the flavoring chemical cinnamaldehyde perturb bone, cartilage and vascular development in zebrafish embryos. Aquat. Toxicol. 2021, 240, 105995. [Google Scholar] [CrossRef]
- Piechowski, J.M.; Bagatto, B. Cardiovascular function during early development is suppressed by cinnamon flavored, nicotine-free, electronic cigarette vapor. Birth Defects Res. 2021, 113, 1215–1223. [Google Scholar] [CrossRef]
- Kirkham, M.N.; Cooper, C.; Broberg, E.; Robertson, P.; Clarke, D.; Pickett, B.E.; Bikman, B.; Reynolds, P.R.; Arroyo, J.A. Different Lengths of Gestational Exposure to Secondhand Smoke or e-Cigarette Vapor Induce the Development of Placental Disease Symptoms. Cells 2024, 13, 1009. [Google Scholar] [CrossRef]
- Aboaziza, E.; Feaster, K.; Hare, L.; Chantler, P.D.; Olfert, I.M. Maternal electronic cigarette use during pregnancy affects long-term arterial function in offspring. J. Appl. Physiol. 2023, 134, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Puistola, U.; Risteli, L.; Kauppila, A.; Knip, M.; Risteli, J. Markers of type I and type III collagen synthesis in serum as indicators of tissue growth during pregnancy. J. Clin. Endocrinol. Metab. 1993, 77, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Chen, X.; Wang, H.; Chen, X.; Lan, Z.; Li, P.; Cao, Y.; Liu, M.; Lv, J.; Chen, Y.; et al. Collagen I Induces Preeclampsia-Like Symptoms by Suppressing Proliferation and Invasion of Trophoblasts. Front. Endocrinol. 2021, 12, 664766. [Google Scholar] [CrossRef]
- Chou, A.; Davidson, B.; Reynolds, P.R.; Pickett, B.E.; Arroyo, J.A. Reversing Preeclampsia Pathology: AXL Inhibition Restores Mitochondrial Function and ECM Balance. Cells 2025, 14, 1229. [Google Scholar] [CrossRef]
- Bulla, R.; Bossi, F.; Tedesco, F. The complement system at the embryo implantation site: Friend or foe? Front. Immunol. 2012, 3, 55. [Google Scholar] [CrossRef] [PubMed]
- Lokki, A.I.; Heikkinen-Eloranta, J.K.; Laivuori, H. The Immunogenetic Conundrum of Preeclampsia. Front. Immunol. 2018, 9, 2630. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.A.; Green, B.B.; Blair, B.A.; Guerin, D.J.; Litzky, J.F.; Chavan, N.R.; Pearson, K.J.; Marsit, C.J. Maternal smoking during pregnancy is associated with mitochondrial DNA methylation. Environ. Epigenet 2016, 2, dvw020. [Google Scholar] [CrossRef]
- Lepow, I.H.; Naff, G.B.; Todd, E.W.; Pensky, J.; Hinz, C.F. Chromatographic resolution of the first component of human complement into three activities. J. Exp. Med. 1963, 117, 983–1008. [Google Scholar] [CrossRef] [PubMed]
- Shelton, E.; Yonemasu, K.; Stroud, R.M. Ultrastructure of the human complement component, Clq (negative staining-glutamine synthetase-biologically active Clq). Proc. Natl. Acad. Sci. USA 1972, 69, 65–68. [Google Scholar] [CrossRef]
- Liberzon, A.; Subramanian, A.; Pinchback, R.; Thorvaldsdottir, H.; Tamayo, P.; Mesirov, J.P. Molecular signatures database (MSigDB) 3.0. Bioinformatics 2011, 27, 1739–1740. [Google Scholar] [CrossRef]
- Agostinis, C.; Bulla, R.; Tripodo, C.; Gismondi, A.; Stabile, H.; Bossi, F.; Guarnotta, C.; Garlanda, C.; De Seta, F.; Spessotto, P.; et al. An alternative role of C1q in cell migration and tissue remodeling: Contribution to trophoblast invasion and placental development. J. Immunol. 2010, 185, 4420–4429. [Google Scholar] [CrossRef]
- Qing, X.; Redecha, P.B.; Burmeister, M.A.; Tomlinson, S.; D’Agati, V.D.; Davisson, R.L.; Salmon, J.E. Targeted inhibition of complement activation prevents features of preeclampsia in mice. Kidney Int. 2011, 79, 331–339. [Google Scholar] [CrossRef]
- Erlebacher, A. Immunology of the maternal-fetal interface. Annu. Rev. Immunol. 2013, 31, 387–411. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.; Nanan, R.K. Innate and adaptive immune interactions at the fetal-maternal interface in healthy human pregnancy and pre-eclampsia. Front. Immunol. 2014, 5, 125. [Google Scholar] [CrossRef] [PubMed]
- Alahakoon, T.I.; Medbury, H.; Williams, H.; Fewings, N.; Wang, X.M.; Lee, V.W. Distribution of monocyte subsets and polarization in preeclampsia and intrauterine fetal growth restriction. J. Obstet. Gynaecol. Res. 2018, 44, 2135–2148. [Google Scholar] [CrossRef]
- Alahakoon, T.I.; Medbury, H.; Williams, H.; Fewings, N.; Wang, X.M.; Lee, V.W. Characterization of fetal monocytes in preeclampsia and fetal growth restriction. J. Perinat. Med. 2019, 47, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Geraci, F.; Leoncini, M.; Montangero, M.; Pellegrini, M.; Renda, M.E. K-Boost: A scalable algorithm for high-quality clustering of microarray gene expression data. J. Comput. Biol. 2009, 16, 859–873. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Milacic, M.; Beavers, D.; Conley, P.; Gong, C.; Gillespie, M.; Griss, J.; Haw, R.; Jassal, B.; Matthews, L.; May, B.; et al. The Reactome Pathway Knowledgebase 2024. Nucleic Acids Res. 2024, 52, D672–D678. [Google Scholar] [CrossRef]
- Newman, A.M.; Steen, C.B.; Liu, C.L.; Gentles, A.J.; Chaudhuri, A.A.; Scherer, F.; Khodadoust, M.S.; Esfahani, M.S.; Luca, B.A.; Steiner, D.; et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat. Biotechnol. 2019, 37, 773–782. [Google Scholar] [CrossRef]
- Steen, C.B.; Liu, C.L.; Alizadeh, A.A.; Newman, A.M. Profiling Cell Type Abundance and Expression in Bulk Tissues with CIBERSORTx. Methods Mol. Biol. 2020, 2117, 135–157. [Google Scholar] [CrossRef]
- Beck, L.; Kirkham, M.N.; Shin, M.; Bikman, B.T.; Reynolds, P.R.; Arroyo, J.A. Impact of Secondhand Smoke and E-Cigarette Exposure on Placental Apoptotic and Growth-Regulatory Proteins in Mouse Pregnancy. Cells 2025, 14, 453. [Google Scholar] [CrossRef]
- Dobbs Spendlove, M.; T, M.G.; McCain, S.; Stone, B.C.; Gill, T.; Pickett, B.E. Pathway2Targets: An open-source pathway-based approach to repurpose therapeutic drugs and prioritize human targets. PeerJ 2023, 11, e16088. [Google Scholar] [CrossRef]
- Xie, Z.; Rahman, I.; Goniewicz, M.L.; Li, D. Perspectives on Epigenetics Alterations Associated with Smoking and Vaping. Function 2021, 2, zqab022. [Google Scholar] [CrossRef]
- Andersen, A.; Reimer, R.; Dawes, K.; Becker, A.; Hutchens, N.; Miller, S.; Dogan, M.; Hundley, B.; J, A.M.; J, D.L.; et al. DNA methylation differentiates smoking from vaping and non-combustible tobacco use. Epigenetics 2022, 17, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Lyytinen, G.; Brynedal, A.; Anesater, E.; Antoniewicz, L.; Blomberg, A.; Wallen, H.; Bosson, J.A.; Hedman, L.; Mobarrez, F.; Tehrani, S.; et al. Electronic Cigarette Vaping with Nicotine Causes Increased Thrombogenicity and Impaired Microvascular Function in Healthy Volunteers: A Randomised Clinical Trial. Cardiovasc. Toxicol. 2023, 23, 255–264. [Google Scholar] [CrossRef]
- de Vasconcellos, A.C.; Frazzon, J.; Zapata Norena, C.P. Phenolic Compounds Present in Yerba Mate Potentially Increase Human Health: A Critical Review. Plant Foods Hum. Nutr. 2022, 77, 495–503. [Google Scholar] [CrossRef]
- Everson, T.M.; Vives-Usano, M.; Seyve, E.; Cardenas, A.; Lacasana, M.; Craig, J.M.; Lesseur, C.; Baker, E.R.; Fernandez-Jimenez, N.; Heude, B.; et al. Placental DNA methylation signatures of maternal smoking during pregnancy and potential impacts on fetal growth. Nat. Commun. 2021, 12, 5095. [Google Scholar] [CrossRef]
- Ducsay, C.A.; Goyal, R.; Pearce, W.J.; Wilson, S.; Hu, X.Q.; Zhang, L. Gestational Hypoxia and Developmental Plasticity. Physiol. Rev. 2018, 98, 1241–1334. [Google Scholar] [CrossRef]
- Sober, S.; Reiman, M.; Kikas, T.; Rull, K.; Inno, R.; Vaas, P.; Teesalu, P.; Marti, J.M.L.; Mattila, P.; Laan, M. Extensive shift in placental transcriptome profile in preeclampsia and placental origin of adverse pregnancy outcomes. Sci. Rep. 2015, 5, 13336. [Google Scholar] [CrossRef] [PubMed]
- Leavey, K.; Bainbridge, S.A.; Cox, B.J. Large scale aggregate microarray analysis reveals three distinct molecular subclasses of human preeclampsia. PLoS ONE 2015, 10, e0116508. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Quan, L.; Huang, A.; Zhao, Q.; Yuan, Y.; Yuan, X.; Shen, Q.; Shang, J.; Ben, Y.; Qin, F.X.; et al. seq-ImmuCC: Cell-Centric View of Tissue Transcriptome Measuring Cellular Compositions of Immune Microenvironment From Mouse RNA-Seq Data. Front. Immunol. 2018, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- de Alwis, N.; Beard, S.; Binder, N.K.; Pritchard, N.; Kaitu’u-Lino, T.J.; Walker, S.P.; Stock, O.; Groom, K.M.; Petersen, S.; Henry, A.; et al. NR4A2 expression is not altered in placentas from cases of growth restriction or preeclampsia, but is reduced in hypoxic cytotrophoblast. Sci. Rep. 2021, 11, 20670. [Google Scholar] [CrossRef]
- Pei, L.; Castrillo, A.; Chen, M.; Hoffmann, A.; Tontonoz, P. Induction of NR4A orphan nuclear receptor expression in macrophages in response to inflammatory stimuli. J. Biol. Chem. 2005, 280, 29256–29262. [Google Scholar] [CrossRef]
- Dai, Y.; Diao, Z.; Sun, H.; Li, R.; Qiu, Z.; Hu, Y. MicroRNA-155 is involved in the remodelling of human-trophoblast-derived HTR-8/SVneo cells induced by lipopolysaccharides. Hum. Reprod. 2011, 26, 1882–1891. [Google Scholar] [CrossRef]
- Vaughan, J.E.; Walsh, S.W. Activation of NF-kappaB in placentas of women with preeclampsia. Hypertens. Pregnancy 2012, 31, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Wang, T.; Zhou, S.; Han, J.; Wu, W. USP17 regulates preeclampsia by modulating the NF-kappaB signaling pathway via deubiquitinating HDAC2. Placenta 2024, 145, 9–18. [Google Scholar] [CrossRef]
- Melgert, B.N.; Spaans, F.; Borghuis, T.; Klok, P.A.; Groen, B.; Bolt, A.; de Vos, P.; van Pampus, M.G.; Wong, T.Y.; van Goor, H.; et al. Pregnancy and preeclampsia affect monocyte subsets in humans and rats. PLoS ONE 2012, 7, e45229. [Google Scholar] [CrossRef] [PubMed]
- Rukosuev, V.S.; Nanaev, A.K.; Milovanov, A.P. Participation of collagen types I, III, IV, V, and fibronectin in the formation of villi fibrosis in human term placenta. Acta Histochem. 1990, 89, 11–16. [Google Scholar] [CrossRef]
- Juusela, A.; Naik, V.D.; Carabulea, A.L.; Janeski, J.D.; Jiang, H.; Venkatachalam, S.; Ramadoss, J. Altered uterine artery protein signature and function following E-cigarette exposure in pregnancy. Am. J. Transl. Res. 2025, 17, 1662–1678. [Google Scholar] [CrossRef]
- von Mandach, U.; Lauth, D.; Huch, R. Maternal and fetal nitric oxide production in normal and abnormal pregnancy. J. Matern. Fetal Neonatal Med. 2003, 13, 22–27. [Google Scholar] [CrossRef]
- Bubols, G.B.; Zielinsky, P.; Piccoli, A.L., Jr.; Nicoloso, L.H.; Vian, I.; Moro, A.M.; Charao, M.F.; Brucker, N.; Bulcao, R.P.; Nascimento, S.N.; et al. Nitric oxide and reactive species are modulated in the polyphenol-induced ductus arteriosus constriction in pregnant sheep. Prenat. Diagn. 2014, 34, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.O.S.; Elias-Oliveira, J.; Pastore, M.R.; Fontanari, C.; Rodrigues, V.F.; Rodriguez, V.; Gardinassi, L.G.; Faccioli, L.H. CD18 controls the development and activation of monocyte-to-macrophage axis during chronic schistosomiasis. Front. Immunol. 2022, 13, 929552. [Google Scholar] [CrossRef]
- Vorup-Jensen, T.; Jensen, R.K. Structural Immunology of Complement Receptors 3 and 4. Front. Immunol. 2018, 9, 2716. [Google Scholar] [CrossRef]
- Teirila, L.; Heikkinen-Eloranta, J.; Kotimaa, J.; Meri, S.; Lokki, A.I. Regulation of the complement system and immunological tolerance in pregnancy. Semin. Immunol. 2019, 45, 101337. [Google Scholar] [CrossRef] [PubMed]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014, 9, 181–218. [Google Scholar] [CrossRef]
- Sengelov, H.; Follin, P.; Kjeldsen, L.; Lollike, K.; Dahlgren, C.; Borregaard, N. Mobilization of granules and secretory vesicles during in vivo exudation of human neutrophils. J. Immunol. 1995, 154, 4157–4165. [Google Scholar] [CrossRef]
- Kaplan, M.J. Role of neutrophils in systemic autoimmune diseases. Arthritis Res. Ther. 2013, 15, 219. [Google Scholar] [CrossRef]
- Singh, J.; Ahmed, A.; Girardi, G. Role of complement component C1q in the onset of preeclampsia in mice. Hypertension 2011, 58, 716–724. [Google Scholar] [CrossRef]
- Azar, M.; Oatey, M.E.; Moniz, M.H.; Bailey, B.A. Intrapartum Electronic Cigarette Use and Birth Outcomes: Evidence from a Population-Based Study. Int. J. Environ. Res. Public. Health 2024, 21, 1449. [Google Scholar] [CrossRef] [PubMed]
- Gambadauro, A.; Galletta, F.; Andrenacci, B.; Foti Randazzese, S.; Patria, M.F.; Manti, S. Impact of E-Cigarettes on Fetal and Neonatal Lung Development: The Influence of Oxidative Stress and Inflammation. Antioxidants 2025, 14, 262. [Google Scholar] [CrossRef]
- Knopik, V.S.; Maccani, M.A.; Francazio, S.; McGeary, J.E. The epigenetics of maternal cigarette smoking during pregnancy and effects on child development. Dev. Psychopathol. 2012, 24, 1377–1390. [Google Scholar] [CrossRef] [PubMed]
- Sha, H.; Ma, Y.; Tong, Y.; Zhao, J.; Qin, F. Apocynin inhibits placental TLR4/NF-kappaB signaling pathway and ameliorates preeclampsia-like symptoms in rats. Pregnancy Hypertens. 2020, 22, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Encinosa, W.; Valdez, R.B. Menthol Cigarettes and Maternal Health: 2004–2022. J. Womens Health (Larchmt.) 2025, 34, 460–468. [Google Scholar] [CrossRef]
- Soulet, S.; Constans, L.; Quinty, V. Physical and chemical characterizations of a reference e-cigarette used in animal testing. Sci. Rep. 2023, 13, 16624. [Google Scholar] [CrossRef]
- Waker, C.A.; Kaufman, M.R.; Brown, T.L. Current State of Preeclampsia Mouse Models: Approaches, Relevance, and Standardization. Front. Physiol. 2021, 12, 681632. [Google Scholar] [CrossRef]
- Zhang, X.; Jonassen, I. RASflow: An RNA-Seq analysis workflow with Snakemake. BMC Bioinformatics 2020, 21, 110. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Lun, A.T.L.; Baldoni, P.L.; Smyth, G.K. edgeR v4: Powerful differential analysis of sequencing data with expanded functionality and improved support for small counts and larger datasets. Nucleic Acids Res. 2025, 53, gkaf018. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]



| Gene | Description | log2FC | FDR |
|---|---|---|---|
| ARG1 | Arginase 1 | −2.45 | 1.39 × 10−6 |
| C1QA | Complement c1q a chain | −1.5 | 4.10 × 10−13 |
| C1QC | Complement c1q c chain | −1.19 | 4.77 × 10−8 |
| C5AR1 | Complement c5a receptor 1 | −1.1 | 1.47 × 10−3 |
| COL15A1 | Collagen type xv alpha 1 chain | −1.4 | 4.24 × 10−7 |
| COL1A1 | Collagen type i alpha 1 chain | −1.75 | 4.12 × 10−4 |
| COL1A2 | Collagen type i alpha 2 chain | −1.33 | 3.46 × 10−3 |
| COL3A1 | Collagen type iii alpha 1 chain | −1.25 | 1.13 × 10−5 |
| COL4A5 | Collagen type iv alpha 5 chain | −1.55 | 8.32 × 10−4 |
| COL4A6 | Collagen type iv alpha 6 chain | −2.67 | 1.51 × 10−4 |
| COL6A1 | Collagen type vi alpha 1 chain | −1.29 | 3.25 × 10−3 |
| COL6A3 | Collagen type vi alpha 3 chain | −1.12 | 0.014 |
| FOSB | Fosb proto-oncogene, ap-1 transcription factor | 0.94 | 3.25 × 10−3 |
| HCK | Hck proto-oncogene, src family tyrosine kinase | −1.14 | 1.71 × 10−3 |
| ITGAM | Integrin subunit alpha m | −1.49 | 1.80 × 10−7 |
| ITGB2 | Integrin subunit beta 2 | −1.53 | 1.75 × 10−4 |
| NOS1 | Nitric oxide synthase 1 | −0.74 | 0.011 |
| NOS1AP | Nitric oxide synthase 1 adaptor protein | 1.12 | 0.021 |
| NOS2 | Nitric oxide synthase 2 | 0.63 | 0.042 |
| NR4A1 | Nuclear receptor subfamily 4 group a member 1 | 1.36 | 0.037 |
| NR4A2 | Nuclear receptor subfamily 4 group a member 2 | 1.49 | 1.71 × 10−3 |
| TYROBP | Transmembrane immune signaling adaptor | −0.98 | 8.49 × 10−5 |
| Gene | Description | log2FC | FDR |
|---|---|---|---|
| C1QA | Complement c1q a chain | −1.12 | 9.75 × 10−6 |
| C1QB | Complement c1q b chain | −1.15 | 3.94 × 10−7 |
| C1QC | Complement c1q c chain | −1.15 | 2.26 × 10−7 |
| C3AR1 | Complement c3a receptor 1 | −0.81 | 0.029 |
| C5AR1 | Complement c5a receptor 1 | −1.56 | 1.12 × 10−6 |
| ITGAM | Integrin subunit alpha m | −1.35 | 3.94 × 10−6 |
| ITGAX | Integrin subunit alpha x | −1.36 | 0.010 |
| ITGB2 | Integrin subunit beta 2 | −1.47 | 1.92 × 10−9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, A.; Hiatt, O.; Davidson, B.; Reynolds, P.R.; Pickett, B.E.; Arroyo, J.A. Vaping in Pregnancy: Unraveling Molecular Drivers of Preeclampsia and Fetal Growth Restriction. Int. J. Mol. Sci. 2025, 26, 10009. https://doi.org/10.3390/ijms262010009
Chou A, Hiatt O, Davidson B, Reynolds PR, Pickett BE, Arroyo JA. Vaping in Pregnancy: Unraveling Molecular Drivers of Preeclampsia and Fetal Growth Restriction. International Journal of Molecular Sciences. 2025; 26(20):10009. https://doi.org/10.3390/ijms262010009
Chicago/Turabian StyleChou, Archarlie, Olivia Hiatt, Benjamin Davidson, Paul R. Reynolds, Brett E. Pickett, and Juan A. Arroyo. 2025. "Vaping in Pregnancy: Unraveling Molecular Drivers of Preeclampsia and Fetal Growth Restriction" International Journal of Molecular Sciences 26, no. 20: 10009. https://doi.org/10.3390/ijms262010009
APA StyleChou, A., Hiatt, O., Davidson, B., Reynolds, P. R., Pickett, B. E., & Arroyo, J. A. (2025). Vaping in Pregnancy: Unraveling Molecular Drivers of Preeclampsia and Fetal Growth Restriction. International Journal of Molecular Sciences, 26(20), 10009. https://doi.org/10.3390/ijms262010009








