Antimicrobial Efficacy of Trifluoro-Anilines Against Vibrio Species
Abstract
1. Introduction
2. Results
2.1. Aniline and Its Derivatives Inhibited Biofilm Formation and Growth in Two Vibrio Species
2.2. Aniline Derivatives Inhibited Dose-Dependent Biofilm, Cell Growth, and Virulence Factors
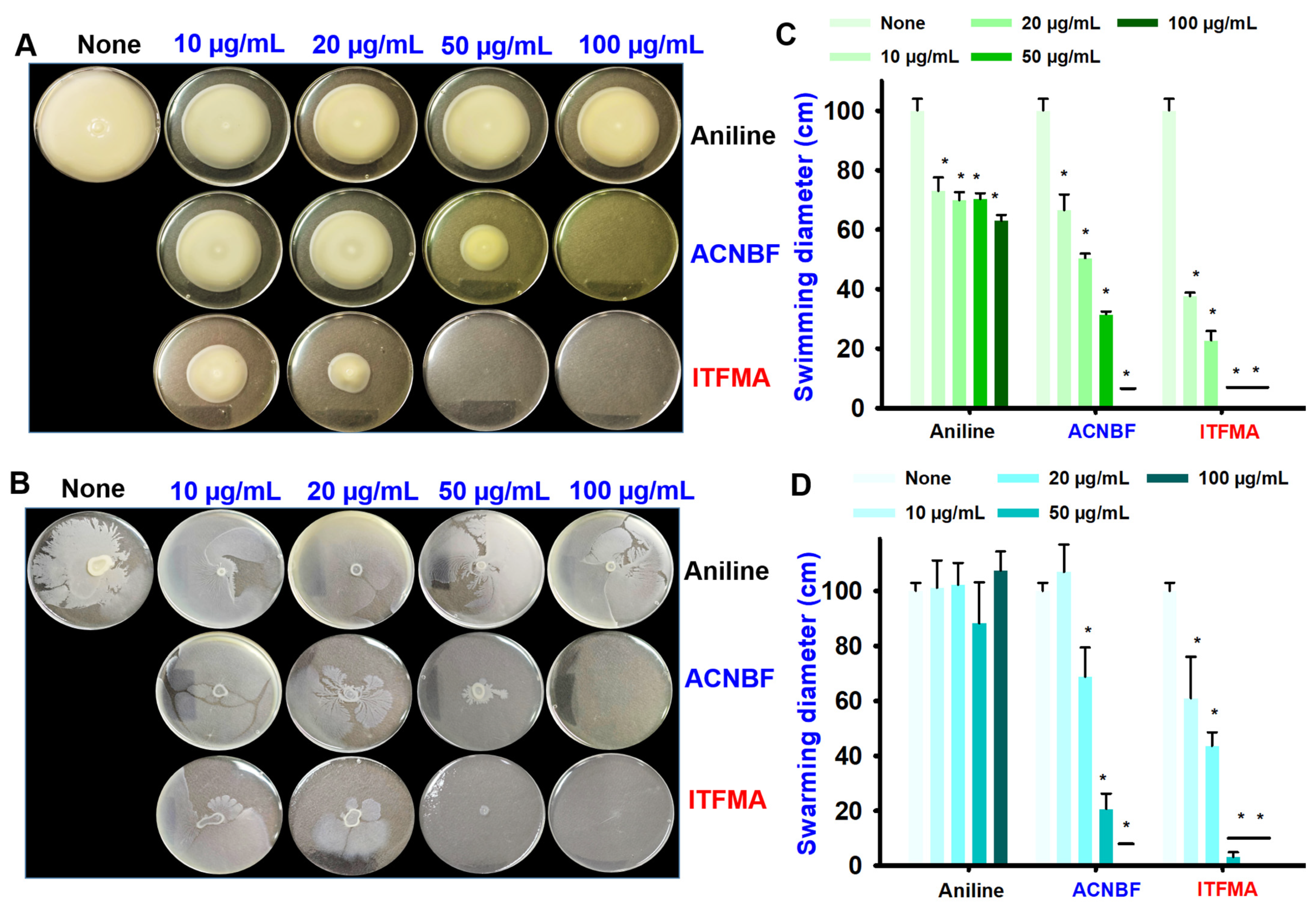
2.3. Aniline and Its Derivatives Suppressed Motility of V. parahaemolyticus
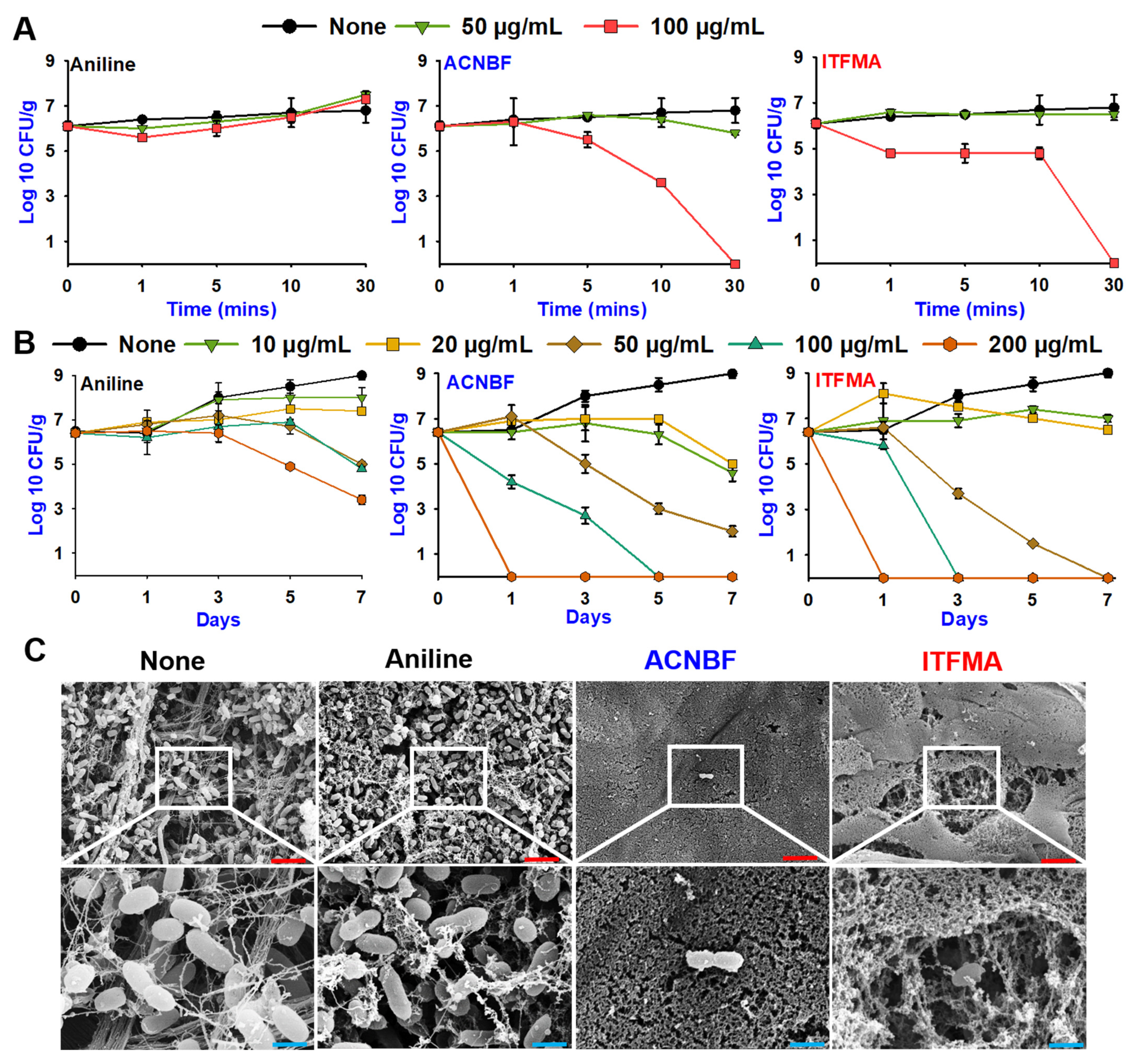
2.4. Aniline Derivatives Inhibited Biofilm Formation in Microscopy and SEM Analyses
2.5. Aniline Derivatives Led Rapid Killing and Inhibited Biofilms on Shrimp and Squid
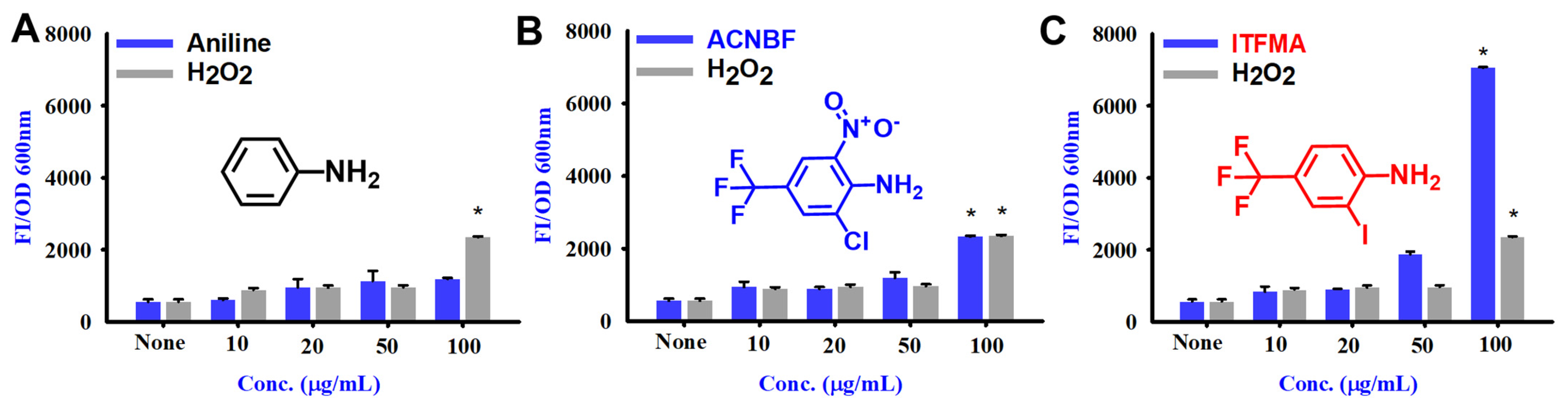
2.6. Aniline and Its Derivatives for ROS Production
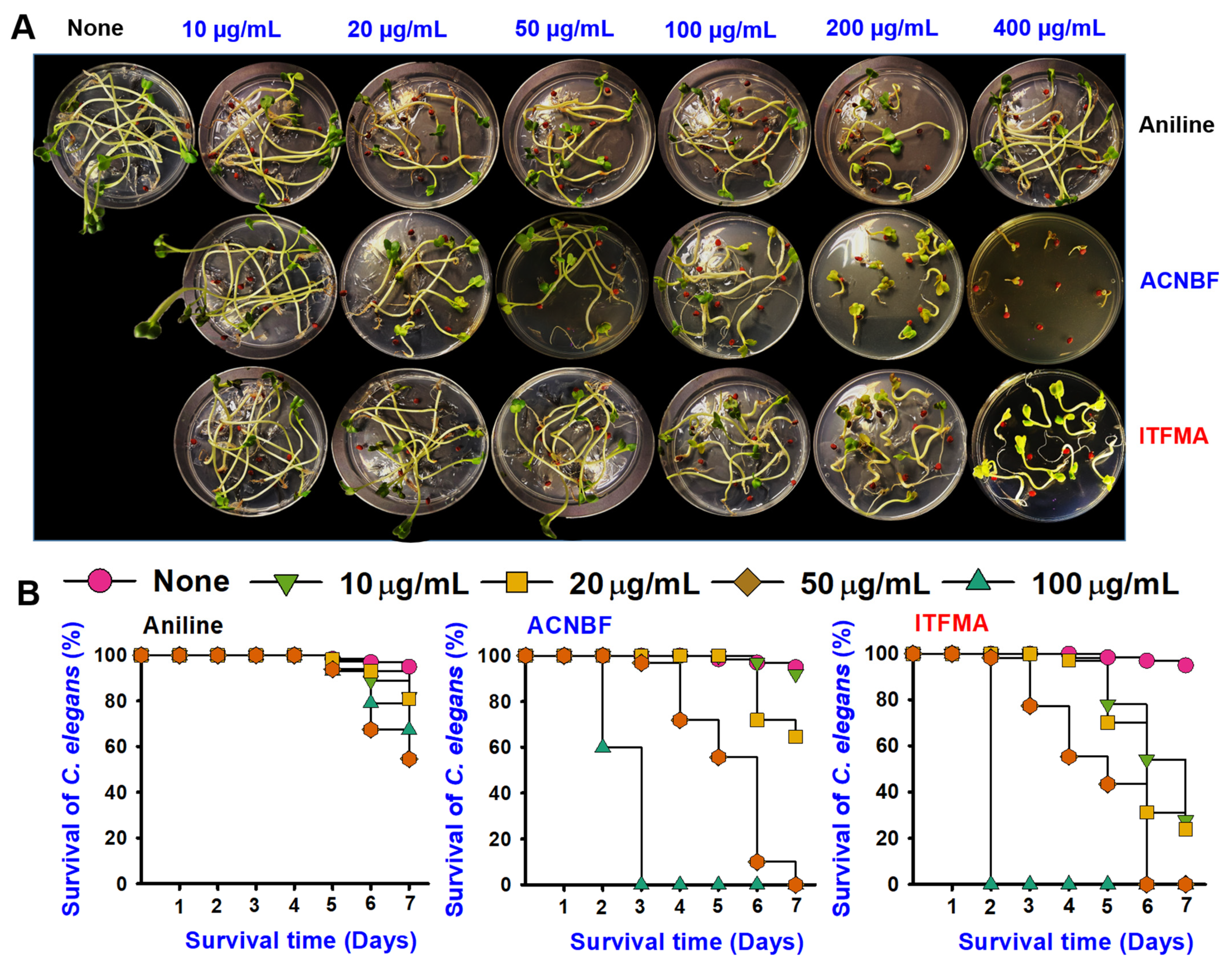
2.7. Aniline Derivatives Exhibited Differential Toxicity
2.8. Analysis of Absorption, Distribution, Metabolism, Excretion, and Toxicity (ADMET)
3. Discussion
4. Materials and Methods
4.1. Microbial Strains and Chemical Reagents
4.2. Growth of V. parahaemolyticus Cells and Evaluation of Minimum Inhibitory Concentration (MIC)
4.3. Assessment of Biofilm Inhibition
4.4. Analysis of Protease Production
4.5. Indole Assay at Different pH
4.6. Hemolysis Assay
4.7. Movement Through Swimming and Swarming
4.8. Live Imaging Microscopy of V. parahaemolyticus
4.9. Scanning Electron Microscope (SEM)
4.10. Rapid-Killing Assay
4.11. Characterization of the Biological Surfaces of Shrimp and Squid
4.12. ROS Production
4.13. Seed Germination with Aniline Derivatives
4.14. Cytotoxicity Evaluation with Caenorhabditis elegans
4.15. ADMET Profile
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baker-Austin, C.; Trinanes, J.A.; Taylor, N.G.H.; Hartnell, R.; Siitonen, A.; Martinez-Urtaza, J. Emerging Vibrio risk at high latitudes in response to ocean warming. Nat. Clim. Change 2013, 3, 73–77. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, X.; Lin, L.; Yao, D.; Sun, J.; Du, X.; Li, X.; Zhang, Y. Passive immune-protection of Litopenaeus vannamei against Vibrio harveyi and Vibrio parahaemolyticus infections with anti-Vibrio egg yolk (IgY)-encapsulated feed. Int. J. Mol. Sci. 2016, 17, 723. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, A.; Silva, V.; Poeta, P.; Aonofriesei, F. Vibrio spp.: Life strategies, ecology, and risks in a changing environment. Diversity 2022, 14, 97. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wuertz, S. Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef]
- Wang, D.; Fletcher, G.C.; On, S.L.W.; Palmer, J.S.; Gagic, D.; Flint, S.H. Biofilm formation, sodium hypochlorite susceptibility and genetic diversity of Vibrio parahaemolyticus. Int. J. Food Microbiol. 2023, 385, 110011. [Google Scholar] [CrossRef]
- Colwell, R.R. Global microbial ecology of Vibrio cholerae. In Oceans and Health: Pathogens in the Marine Environment; Springer: Berlin/Heidelberg, Germany, 2005; pp. 297–305. [Google Scholar] [CrossRef]
- Ayyappan, M.V.; Kishore, P.; Panda, S.K.; Kumar, A.; Uchoi, D.; Nadella, R.K.; Priyadarshi, H.; Obaiah, M.C.; George, D.; Hamza, M.; et al. Emergence of multidrug resistant, ctx negative seventh pandemic Vibrio cholerae O1 El Tor sequence type (ST) 69 in coastal water of Kerala, India. Sci. Rep. 2024, 14, 2031. [Google Scholar] [CrossRef]
- Diban, F.; Di Lodovico, S.; Di Fermo, P.; D’Ercole, S.; D’Arcangelo, S.; Di Giulio, M.; Cellini, L. Biofilms in chronic wound infections: Innovative antimicrobial approaches using the in vitro Lubbock chronic wound biofilm model. Int. J. Mol. Sci. 2023, 24, 1004. [Google Scholar] [CrossRef]
- Kralles, Z.T.; Deherikar, P.K.; Werner, C.A.; Hu, X.; Kolodziej, E.P.; Dai, N. Halogenation of Anilines: Formation of Haloacetonitriles and Large-Molecule Disinfection Byproducts. Environ. Sci. Technol. 2024, 58, 17497–17509. [Google Scholar] [CrossRef]
- Mary, A.; Kanagathara, N.; Baby Suganthi, A.R. A brief review on aniline and its derivatives. Mater. Today 2020, 33, 4751–4755. [Google Scholar] [CrossRef]
- Saleh, I.; Kc, H.R.; Roy, S.; Abugazleh, M.K.; Ali, H.; Gilmore, D.; Alam, M.A. Design, synthesis, and antibacterial activity of N-(trifluoromethyl) phenyl substituted pyrazole derivatives. RSC Med. Chem. 2021, 12, 1690–1697. [Google Scholar] [CrossRef]
- Hansa, R.K.C.; Khan, M.M.K.; Frangie, M.M.; Gilmore, D.F.; Shelton, R.S.; Savenka, A.V.; Basnakian, A.G.; Shuttleworth, S.L.; Smeltzer, M.S.; Alam, M.A. 4-4-(Anilinomethyl)-3-[4-(trifluoromethyl)phenyl]-1H-pyrazol-1-ylbenzoic acid derivatives as potent anti-gram-positive bacterial agents. Eur. J. Med. Chem. 2021, 219, 113402. [Google Scholar] [CrossRef] [PubMed]
- Kovvuri, J.; Nagaraju, B.; Ganesh Kumar, C.; Sirisha, K.; Chandrasekhar, C.; Alarifi, A.; Kamal, A. Catalyst-free synthesis of pyrazole-aniline linked coumarin derivatives and their antimicrobial evaluation. J. Saudi Chem. Soc. 2018, 22, 665–677. [Google Scholar] [CrossRef]
- Gizdavic-Nikolaidis, M.R.; Pagnon, J.C.; Ali, N.; Sum, R.; Davies, N.; Roddam, L.F.; Ambrose, M. Functionalized polyanilines disrupt Pseudomonas aeruginosa and Staphylococcus aureus biofilms. Colloids Surf. B Biointerfaces 2015, 136, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Üstükarcı, H.; Ozyilmaz, G.; Ozyilmaz, A.T. Marine antifouling properties of enzyme modified polyaniline coated stainless steel surface. Enzym. Microb. Technol. 2024, 172, 110340. [Google Scholar] [CrossRef] [PubMed]
- Su, L.-M.; Huang, R.-T.; Hsiao, H.-I. Biofilm formation comparison of Vibrio parahaemolyticus on stainless steel and polypropylene while minimizing environmental impacts and transfer to grouper fish fillets. Int. J. Food Microbiol. 2025, 426, 110913. [Google Scholar] [CrossRef]
- Büyükkıdan, N.; Turgut, S.B.; İlkimen, H.; Sarı, M.; Gülbandılar, A. Aniline-2,5-disulfonic acid based new proton transfer salt and new Co(II) and Cu(II) coordination polymers: Synthesis, structural and antimicrobial studies. Chem. Pap. 2024, 78, 6405–6416. [Google Scholar] [CrossRef]
- Jose, A.; Bansal, M.; Svirskis, D.; Swift, S.; Gizdavic-Nikolaidis, M.R. Synthesis and characterization of antimicrobial colloidal polyanilines. Colloids Surf. B Biointerfaces 2024, 238, 113912. [Google Scholar] [CrossRef]
- Osei-Adjei, G.; Huang, X.; Zhang, Y. The extracellular proteases produced by Vibrio parahaemolyticus. World J. Microbiol. Biotechnol. 2018, 34, 68. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, J. Indole as an intercellular signal in microbial communities. FEMS Microbiol. Rev. 2010, 34, 426–444. [Google Scholar] [CrossRef]
- Mueller, R.S.; Beyhan, S.; Saini, S.G.; Yildiz, F.H.; Bartlett, D.H. Indole acts as an extracellular cue regulating gene expression in Vibrio cholerae. J. Bacteriol. 2009, 191, 3504–3516. [Google Scholar] [CrossRef]
- Zha, F.; Pang, R.; Huang, S.; Zhang, J.; Wang, J.; Chen, M.; Xue, L.; Ye, Q.; Wu, S.; Yang, M.; et al. Evaluation of the pathogenesis of non-typical strain with α-hemolysin, Vibrio parahaemolyticus 353, isolated from Chinese seafood through comparative genome and transcriptome analysis. Mar. Pollut. Bull. 2023, 186, 114276. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Sun, L.; Wang, Y.; Deng, Y.; Fang, Z.; Liu, Y.; Deng, Q.; Sun, D.; Gooneratne, R. Influence of food matrix type on extracellular products of Vibrio parahaemolyticus. BMC Microbiol. 2018, 18, 65. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, P.W.; Tang, Y. AdmetSAR: A Comprehensive source and free tool for assessment of chemical ADMET properties. J. Chem. Inf. Model. 2012, 52, 3099–3105. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef]
- Faleye, O.S.; Boya, B.R.; Lee, J.-H.; Choi, I.; Lee, J. Halogenated antimicrobial agents to combat drug-resistant pathogens. Pharmacol. Rev. 2024, 76, 90–141. [Google Scholar] [CrossRef]
- Grau, B.W.; Kohlbauer, S.; Gu, Y.; Hahn, F.; Lösing, J.; Wangen, C.; Stangier, M.; Ackermann, L.; Marschall, M.; Tsogoeva, S.B. A domino reaction strategy for facile and modular construction of synthetically challenging functionalized ortho-fluoroanilines. Org. Chem. Front. 2024, 11, 6768–6777. [Google Scholar] [CrossRef]
- Bugden, F.E.; Westwood, J.L.; Stone, H.; Xu, Y.; Greenhalgh, M. Synthesis and applications of fluorinated, polyfluoroalkyl-and polyfluoroaryl-substituted 1, 2, 3-triazoles. Org. Chem. Front. 2024, 11, 5938–5984. [Google Scholar] [CrossRef]
- Defoirdt, T. Indole signaling, a promising target to control vibriosis in aquaculture. Aquaculture 2023, 574, 739692. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, K.; Zhang, L.; Zhang, X.; Zhu, B.; Lv, N.; Mi, K. The impact of global warming on the signature virulence gene, thermolabile hemolysin, of Vibrio parahaemolyticus. Microbiol. Spectr. 2023, 11, e01502-23. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Tabassum, N.; Anand, R.; Kim, Y.-M. Motility of Vibrio spp.: Regulation and controlling strategies. Appl. Microbiol. Biotechnol. 2020, 104, 8187–8208. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, Y.M.; Milo, R. Towards a quantitative view of the global ubiquity of biofilms. Nat. Rev. Microbiol. 2019, 17, 199–200. [Google Scholar] [CrossRef] [PubMed]
- D’Arcangelo, S.; Santonocito, D.; Messina, L.; Greco, V.; Giuffrida, A.; Puglia, C.; Di Giulio, M.; Inturri, R.; Vaccaro, S. Almond hull extract valorization: From waste to food recovery to counteract Staphylococcus aureus and Escherichia coli in formation and mature biofilm. Foods 2024, 13, 3834. [Google Scholar] [CrossRef] [PubMed]
- Dinakarkumar, Y.; Rajabathar, J.R.; Al-Lohedan, H.; Venkatesan, D.; Sankaran, K.; Veera, H.M.; Ramakrishnan, G. Inhibition of Vibrio parahaemolyticus biofilm formation in Squid (Loligo duvauceli) meat by Cymbopogon citratus essential oil and DNase: An investigative study. Food Humanit. 2024, 3, 100392. [Google Scholar] [CrossRef]
- Zheng, H.; Ye, E.; Xia, T.; Tan, J.; Zhao, Y.; Guo, L. Anti-biofilm and antioxidant activities of Sargassum muticum extracts and their preservation effect of Chinese shrimp (Penaeus chinensis). Food Control 2025, 167, 110832. [Google Scholar] [CrossRef]
- Wang, C.; Yao, D.; Zhao, M.; Lu, K.; Lin, Z.; Chen, X.; Zhao, Y.; Zhang, Y. Shrimp lipid droplet protein perilipin involves in the pathogenesis of AHPND-causing Vibrio parahaemolyticus. Int. J. Mol. Sci. 2022, 23, 10520. [Google Scholar] [CrossRef]
- de Souza Santos, M.; Salomon, D.; Orth, K. T3SS effector VopL inhibits the host ROS response, promoting the intracellular survival of Vibrio parahaemolyticus. PLoS Pathog. 2017, 13, e1006438. [Google Scholar] [CrossRef]
- Nair, A.S.; Singh, A.K.; Kumar, A.; Kumar, S.; Sukumaran, S.; Koyiparambath, V.P.; Pappachen, L.K.; Rangarajan, T.M.; Kim, H.; Mathew, B. FDA-approved trifluoromethyl group-containing drugs: A review of 20 years. Processes 2022, 10, 2054. [Google Scholar] [CrossRef]
- Jeon, H.; Boya, B.R.; Kim, G.; Lee, J.-H.; Lee, J. Inhibitory effects of bromoindoles on Escherichia coli O157: H7 biofilms. Biotechnol. Bioprocess Eng. 2024, 29, 579–588. [Google Scholar] [CrossRef]
- Kim, Y.-G.; Lee, J.-H.; Kim, S.; Park, S.; Kim, Y.-J.; Ryu, C.-M.; Seo, H.W.; Lee, J. Inhibition of Biofilm Formation in Cutibacterium acnes, Staphylococcus aureus, and Candida albicans by the Phytopigment Shikonin. Int. J. Mol. Sci. 2024, 25, 2426. [Google Scholar] [CrossRef] [PubMed]
- Song, S.-S.; Lu, Y.-Y.; Zhu, M.-J.; Zuo, Q.-Y.; Zhou, L.-X.; Zhu, G.-Y.; Zhang, Y.-J.; Lu, X.-F.; Gong, J.; Wang, S.-Y.; et al. Anti-biofilm activity and in vivo efficacy of quinoline for the control of Vibrio parahaemolyticus in Chinese white shrimps. Food Control 2024, 156, 110118. [Google Scholar] [CrossRef]
- Sathiyamoorthi, E.; Lee, J.-H.; Lee, J. Antibacterial and antibiofilm activity of halogenated phenylboronic acids against Vibrio parahaemolyticus and Vibrio harveyi. Front. Cell. Infect. Microbiol. 2024, 14, 1340910. [Google Scholar] [CrossRef]
- Sathiyamoorthi, E.; Faleye, O.S.; Lee, J.-H.; Lee, J. Hydroquinone derivatives attenuate biofilm formation and virulence factor production in Vibrio spp. Int. J. Food Microbiol. 2023, 384, 109954. [Google Scholar] [CrossRef]
- Chen, X.; Duan, M.; Chang, Y.; Ye, M.; Wang, Z.; Wu, S.; Duan, N. Assembly of a multivalent aptamer for efficient inhibition of thermostable direct hemolysin toxicity induced by Vibrio parahaemolyticus. J. Hazard. Mater. 2024, 478, 135452. [Google Scholar] [CrossRef]
- Faleye, O.O.; Faleye, O.S.; Lee, J.-H.; Lee, J. Antibacterial and antibiofilm activities of iodinated hydrocarbons against Vibrio parahaemolyticus and Staphylococcus aureus. Sci. Rep. 2024, 14, 9160. [Google Scholar] [CrossRef]
- Park, I.; Kim, Y.-G.; Lee, J.-H.; Lee, J. Antibiofilm and antivirulence potentials of 3, 2′-dihydroxyflavone against Staphylococcus aureus. Int. J. Mol. Sci. 2024, 25, 8059. [Google Scholar] [CrossRef]
- Faleye, O.S.; Sathiyamoorthi, E.; Lee, J.-H.; Lee, J. Inhibitory effects of cinnamaldehyde derivatives on biofilm formation and virulence factors in Vibrio species. Pharmaceutics 2021, 13, 2176. [Google Scholar] [CrossRef]
- Ahmed, B.; Jailani, A.; Lee, J.-H.; Lee, J. Inhibition of growth, biofilm formation, virulence, and surface attachment of Agrobacterium tumefaciens by cinnamaldehyde derivatives. Front. Microbiol. 2022, 13, 1001865. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
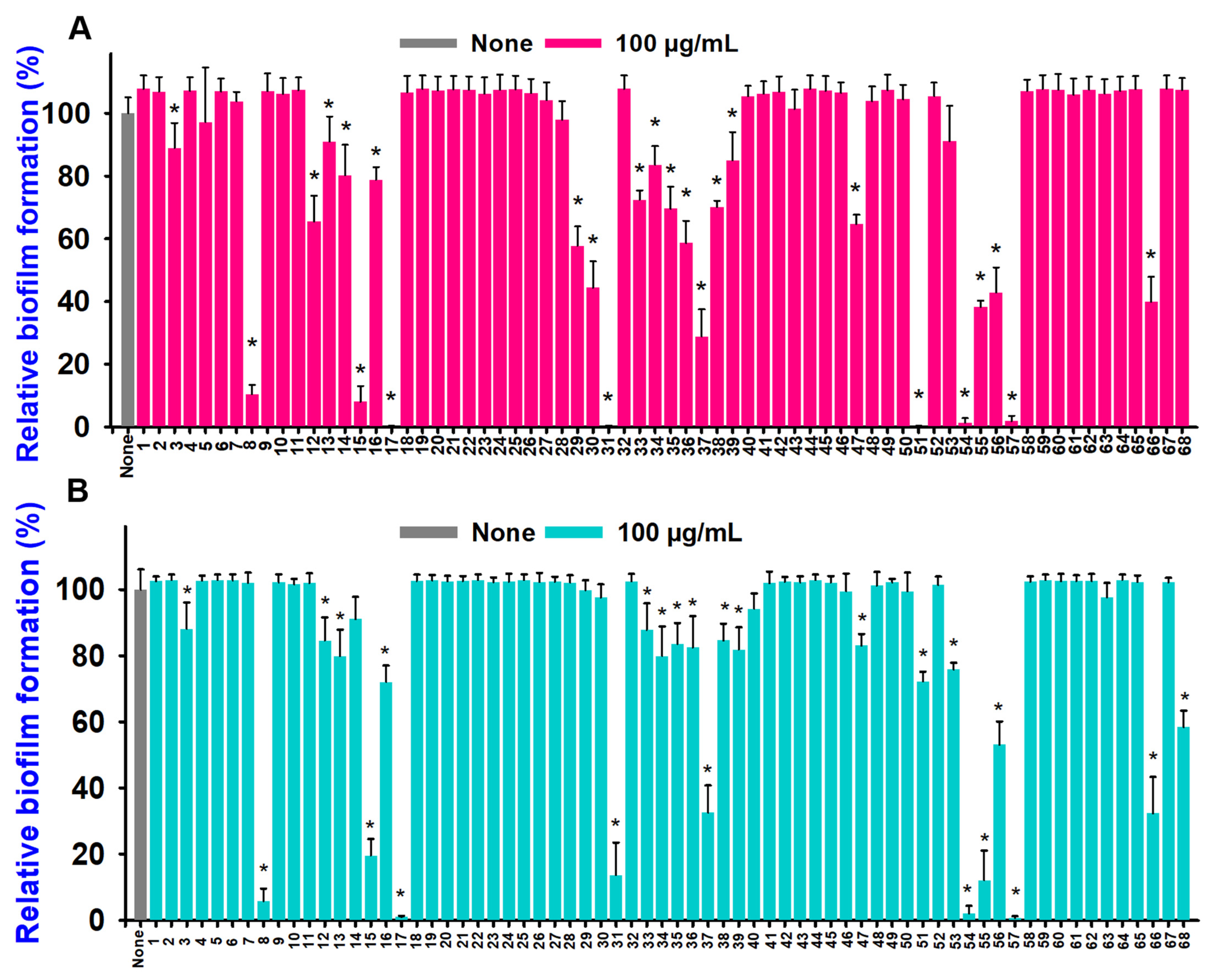


| Compounds | Structure | V. parahaemolyticus MIC (μg/mL) | V. harveyi MIC (μg/mL) | |
|---|---|---|---|---|
| 1. | 3-Chloro-4-methylaniline | 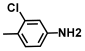 | >500 | 375 |
| 2. | 2-Chloro-4-nitroaniline | 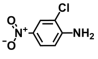 | >500 | 375 |
| 3. | 2-Amino-5-chlorobenzotrifluoride |  | >500 | 375 |
| 4. | 5-Chloro-2,4-dimethoxyaniline | 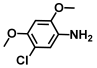 | >500 | >500 |
| 5. | 2-Chloro-5-trifluoromethylaniline | 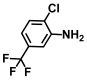 | >500 | >500 |
| 6. | 4-Chloro-2,5-dimethoxyaniline | 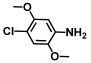 | >500 | >500 |
| 7. | 2-Bromo-4-chloroaniline | 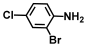 | >500 | 350 |
| 8. | 4-Bromo-3-chloroaniline | 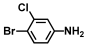 | 125 | 150 |
| 9. | 2-Chloro-6-nitroaniline | 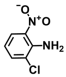 | >500 | >500 |
| 10. | 2,4-Dibromo-6-chloroaniline | 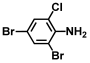 | >500 | >500 |
| 11. | 4-Amino3-chlorobenzonitrile | 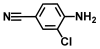 | >500 | >500 |
| 12. | 3-Chloro-2,6-diethylaniline | 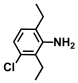 | >500 | 400 |
| 13. | 4-Chloro-2-fluoro-6-iodoaniline |  | >500 | 375 |
| 14. | 2-Amino-4-chlorothiophenol |  | 400 | 250 |
| 15. | 3-Bromo-4-chloroaniline | 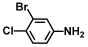 | 175 | 150 |
| 16. | 3-Chloro-4-(trifluoromethoxy)aniline | 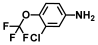 | >500 | 350 |
| 17. | 4-Amino-3-chloro-5-nitrobenzotrifluoride (ACNBF) | 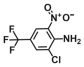 | 100 | 75 |
| 18. | 4-Fluoroaniline | 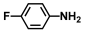 | >500 | >500 |
| 19. | 2- Fluoroaniline |  | >500 | >500 |
| 20. | 2-Amino-5-fluorobenzotrifluoride | 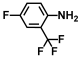 | >500 | >500 |
| 21. | 2-Fluoro-4-(methylsulfonyl)aniline | 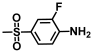 | >500 | >500 |
| 22. | 4-Fluoro-2-methyl-6-nitroaniline | 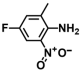 | >500 | >500 |
| 23. | 2-Bromo-4,6-dinitroaniline | 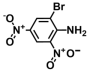 | >500 | >500 |
| 24. | 4-Bromoaniline | 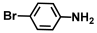 | >500 | >500 |
| 25. | 3-Bromoaniline | 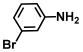 | >500 | 350 |
| 26. | 2-Bromoaniline |  | >500 | >500 |
| 27. | 2-Bromo-4-methylaniline | 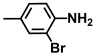 | >500 | >500 |
| 28. | 2-Bromo-4-nitroaniline |  | >500 | 375 |
| 29. | 2,4-Dibromoaniline |  | >500 | 325 |
| 30. | 2-Bromo-3-chloroaniline | 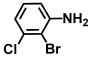 | >500 | 325 |
| 31. | 3,5-Dibromoaniline | 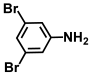 | 100 | 100 |
| 32. | 4-(Difluoromethoxy)aniline | 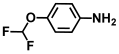 | >500 | >500 |
| 33. | 4-Bromo-2-iodoaniline | 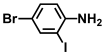 | 150 | 250 |
| 34. | 4-Bromo-2-ethylaniline | 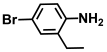 | >500 | 350 |
| 35. | 2-Bromo-4,6-dichloroaniline | 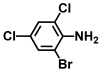 | >500 | >500 |
| 36. | 4-Bromo-2-methyl-6-nitroaniline | 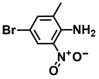 | >500 | >500 |
| 37. | 2-Bromo-4-isopropylaniline | 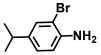 | >500 | 325 |
| 38. | N-Boc-4-bromo-2-fluoroaniline | 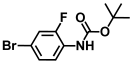 | >500 | >500 |
| 39. | N-Boc-4-bromoaniline | 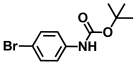 | >500 | >500 |
| 40. | N-Boc-3-bromo-5-trifluoromethylaniline | 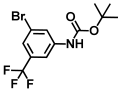 | >500 | >500 |
| 41. | N-Boc-2,6-difluoroaniline |  | >500 | >500 |
| 42. | N-Boc-4-bromo 2-methylaniline | 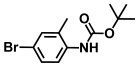 | >500 | >500 |
| 43. | N-Boc-4-bromo N-methylaniline |  | >500 | >500 |
| 44. | N-Boc-4-bromo 2,5-dimethylaniline | 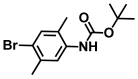 | >500 | >500 |
| 45. | N-Boc-N-isopropyl 4-bromoaniline | 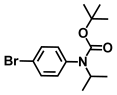 | >500 | >500 |
| 46. | N-Boc-4-fluoro 3-trifluoromethylaniline | 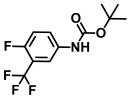 | >500 | >500 |
| 47. | N-Boc-2-fluoro-4-iodoaniline | 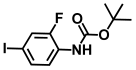 | >500 | >500 |
| 48. | N-Boc-5-bromo 2,3-difluoroaniline | 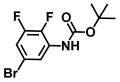 | >500 | >500 |
| 49. | Aniline |  | >1000 | >1000 |
| 50. | 2,6-Difluoro-4-iodoaniline | 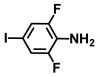 | >500 | >500 |
| 51. | 3,5-Difluoro-4-iodoaniline | 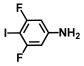 | 150 | 325 |
| 52. | 2-fluoro-4-iodoaniline | 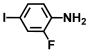 | >500 | 375 |
| 53. | 3-fluoro-4-iodoaniline | 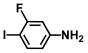 | 300 | 350 |
| 54. | 2-Iodo-4-(trifluoromethyl aniline) (ITFMA) |  | 50 | 20 |
| 55. | 4-Iodo-2-trifluoromethylaniline | 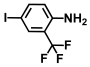 | 50 | 150 |
| 56. | 2-Chloro-4-iodoaniline | 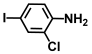 | 300 | 325 |
| 57. | 3-Chloro-4-iodoaniline | 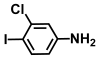 | 125 | 150 |
| 58. | 5-Chloro-2-iodoaniline | 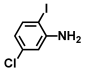 | 200 | 325 |
| 59. | 2-Iodoaniline | 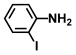 | >500 | >500 |
| 60. | 4-Iodoaniline | 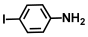 | 400 | 375 |
| 61. | 3-Iodo-4-methoxyaniline |  | >500 | >500 |
| 62. | 3-Iodo-4-methoxyaniline hydrochloride | 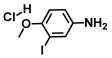 | >500 | >500 |
| 63. | 2-Iodo-4-methylaniline | 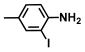 | 400 | 400 |
| 64. | 2-Iodo-5-methylaniline |  | >500 | >500 |
| 65. | 4-Iodo-2-methylaniline | 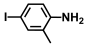 | 400 | 400 |
| 66. | 4-Iodo-3-methylaniline | 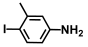 | 250 | 225 |
| 67. | 5-Iodo-2-methylaniline | 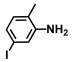 | 400 | 425 |
| 68. | 2-Iodo-4-nitroaniline | 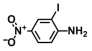 | 200 | 300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sathiyamoorthi, E.; Boya, B.R.; Lee, J.-H.; Lee, J. Antimicrobial Efficacy of Trifluoro-Anilines Against Vibrio Species. Int. J. Mol. Sci. 2025, 26, 623. https://doi.org/10.3390/ijms26020623
Sathiyamoorthi E, Boya BR, Lee J-H, Lee J. Antimicrobial Efficacy of Trifluoro-Anilines Against Vibrio Species. International Journal of Molecular Sciences. 2025; 26(2):623. https://doi.org/10.3390/ijms26020623
Chicago/Turabian StyleSathiyamoorthi, Ezhaveni, Bharath Reddy Boya, Jin-Hyung Lee, and Jintae Lee. 2025. "Antimicrobial Efficacy of Trifluoro-Anilines Against Vibrio Species" International Journal of Molecular Sciences 26, no. 2: 623. https://doi.org/10.3390/ijms26020623
APA StyleSathiyamoorthi, E., Boya, B. R., Lee, J.-H., & Lee, J. (2025). Antimicrobial Efficacy of Trifluoro-Anilines Against Vibrio Species. International Journal of Molecular Sciences, 26(2), 623. https://doi.org/10.3390/ijms26020623







