Endophytic Penicillium oxalicum AUMC 14898 from Opuntia ficus-indica: A Novel Source of Tannic Acid Inhibiting Virulence and Quorum Sensing of Extensively Drug-Resistant Pseudomonas aeruginosa
Abstract
1. Introduction
2. Results
2.1. Endophytic Penicillium oxalicum Isolate EF10 Showed the Highest Anti-QS Activity
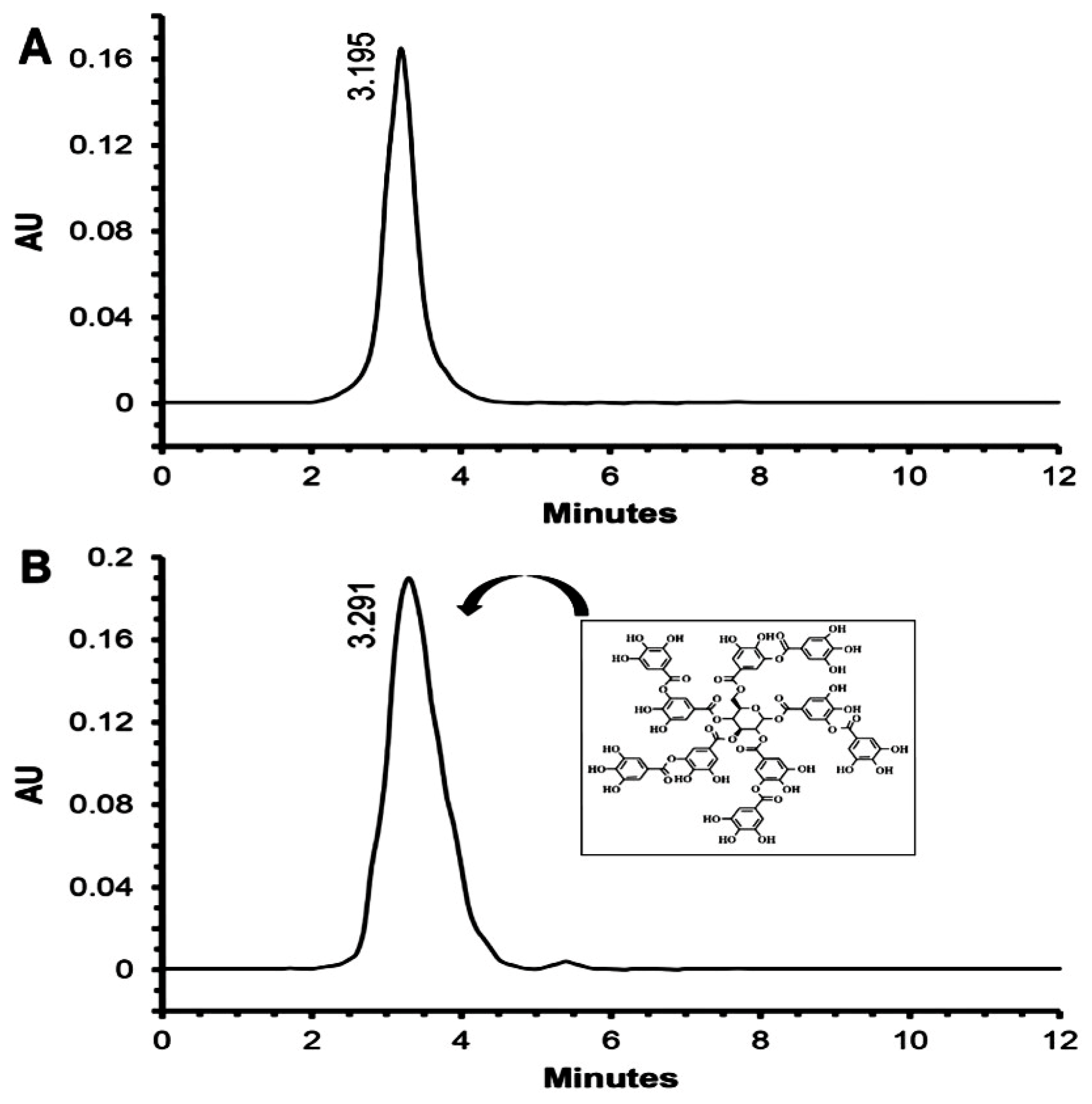
2.2. Tannic Acid as a Bioactive Compound-Inhibiting QS
2.3. Inhibitory Activity of TA and MIC
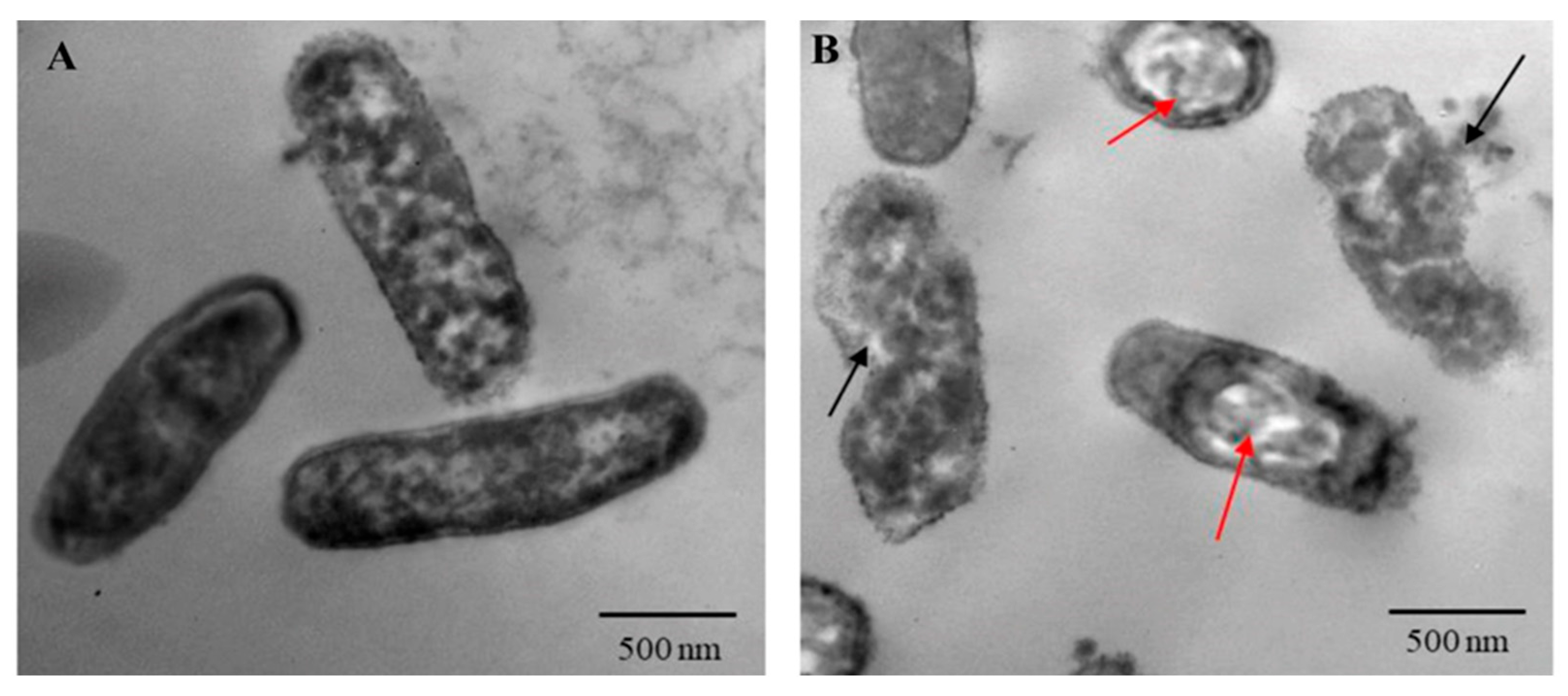
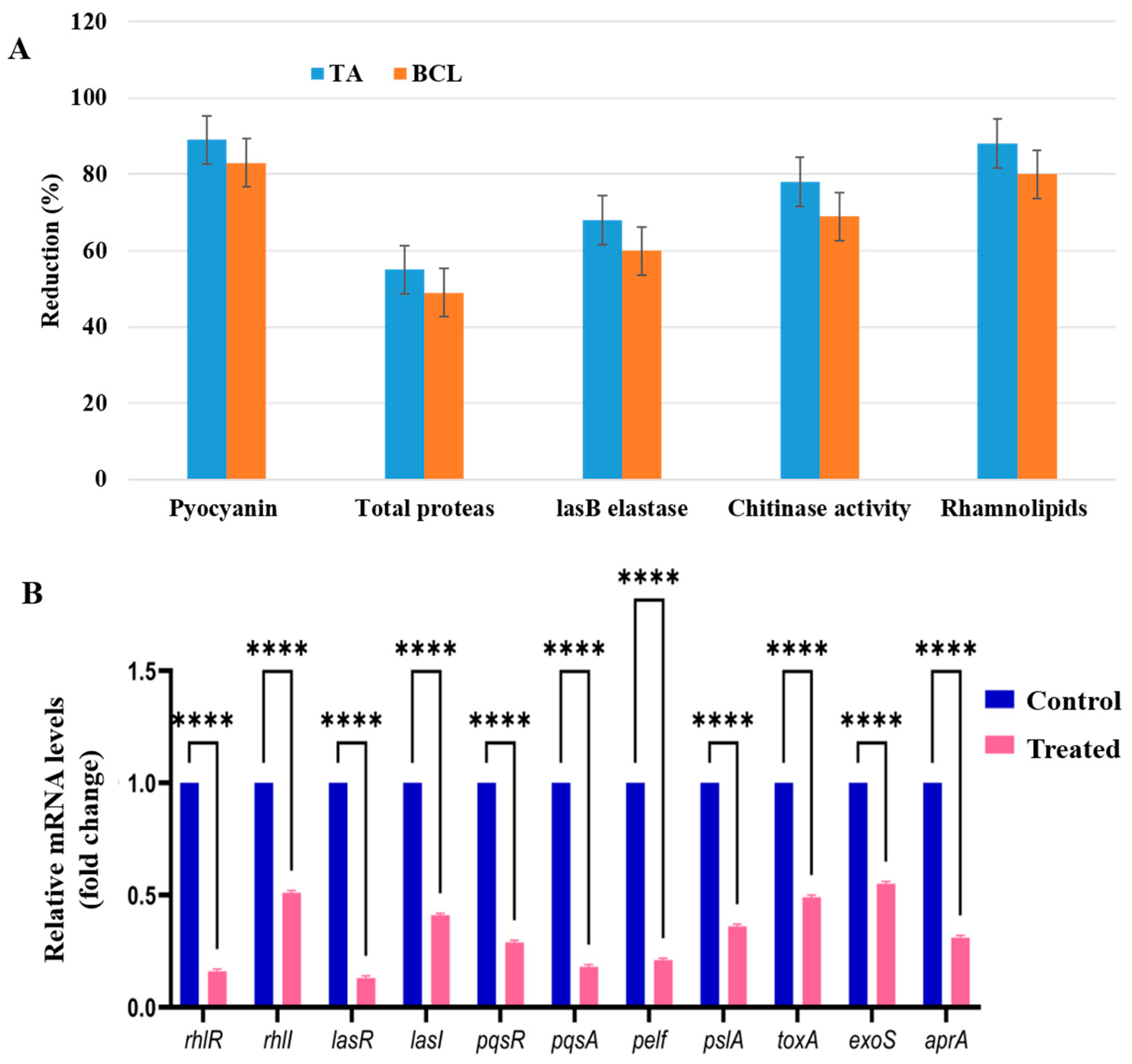
2.4. Virulence Inhibition of PA-05 by TA
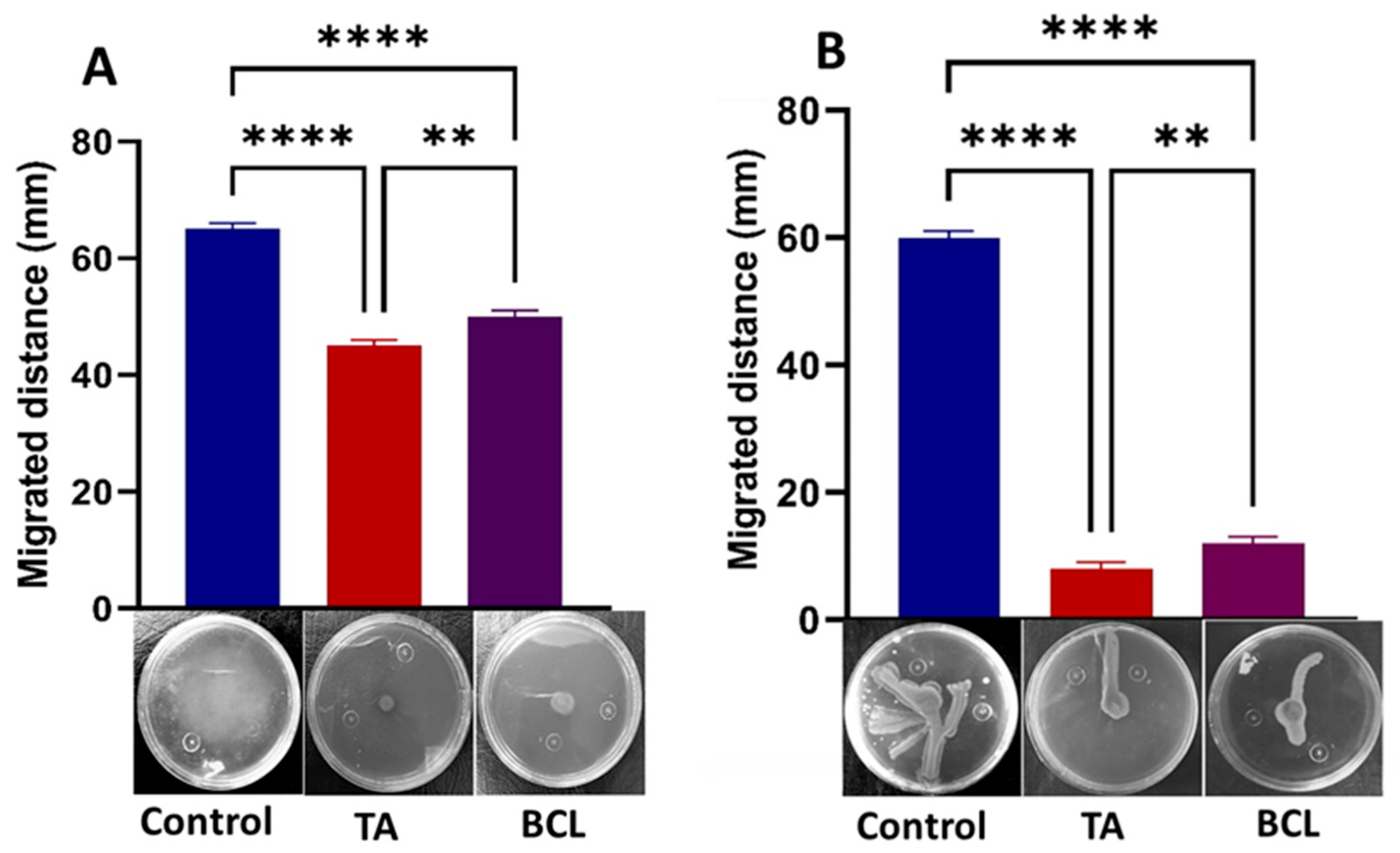
2.5. TA Strongly Inhibits Motility of PA-05
2.6. TA Downregulates QS Genes and Its Relative Genes of PA-05
2.7. Computational Investigations of the Interactions of LasR, RhlR, and PqsR with TA
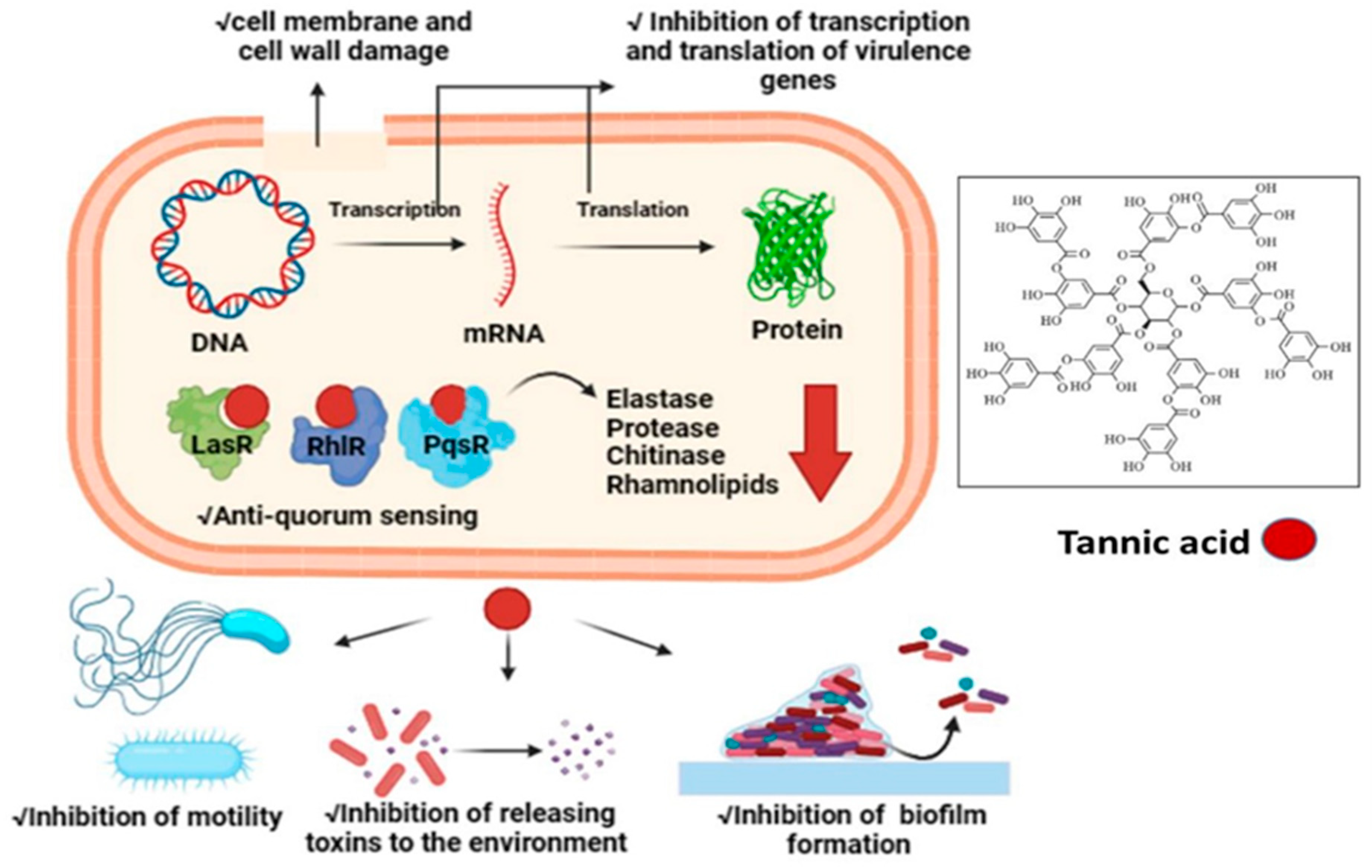
3. Discussion
4. Materials and Methods
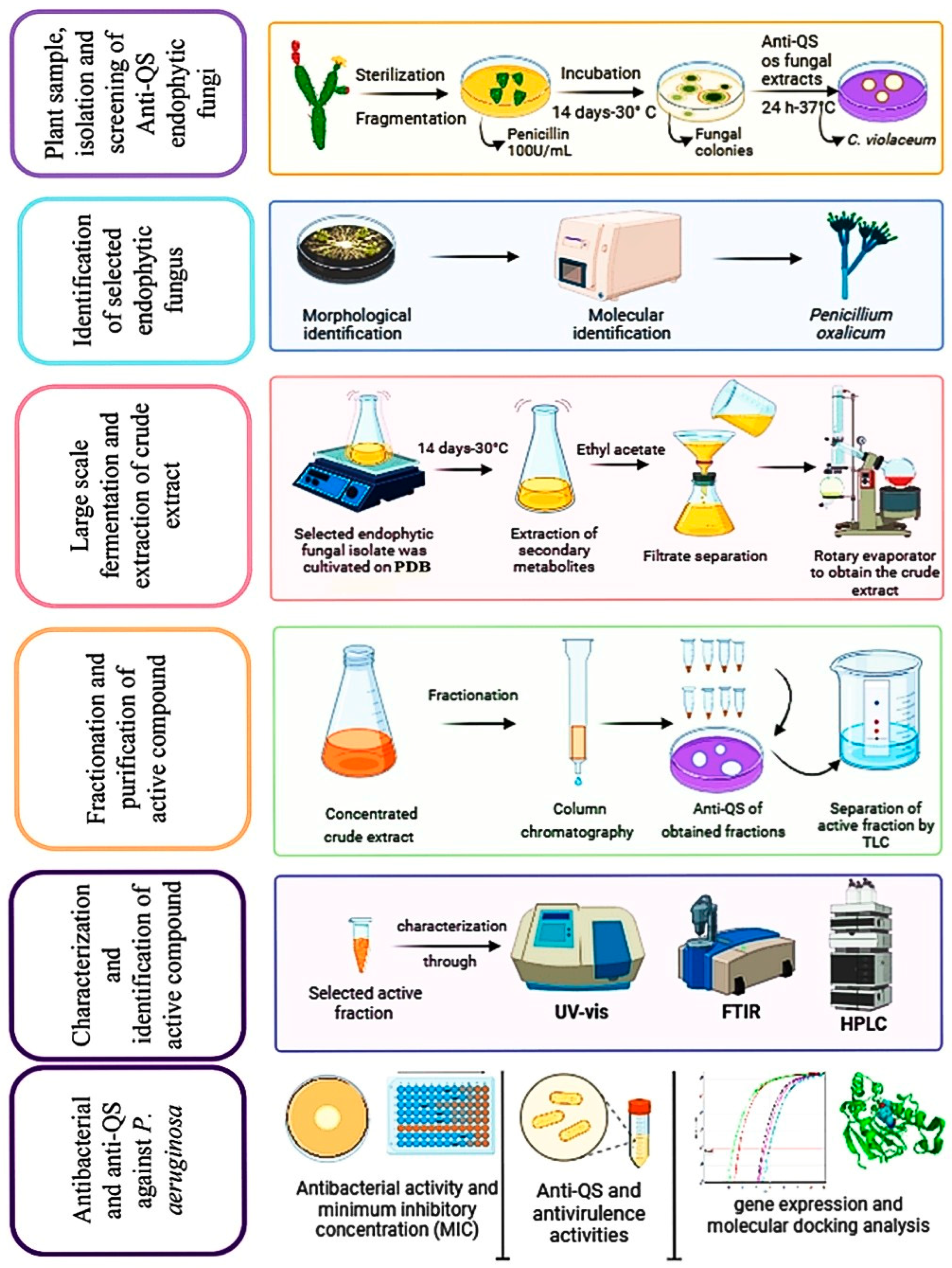
4.1. Isolation of Endophytes and Screening for Their Anti-QS Activity
4.2. Identification of the Chosen Fungal Species
4.3. Obtaining a Crude Extract Form the Culture of the Chosen Fungal Isolate
4.4. Fractionation and Purification of the Active Compound
4.5. Characterization and Identification of the Active Compound
4.6. Antibacterial Activity of the Isolated Active Compound (TA)
4.7. Anti-QS Potential of the Isolated TA
4.8. Assessment of Minimal Inhibitory Concentrations (MICs) and Growth Curves
4.9. Efficacy of TA on Virulence Factors Regulated by QS
4.9.1. Pyocyanin Production Assay
4.9.2. Chitinase Activity Assay
4.9.3. LasA Protease Assay
4.9.4. LasB Elastase Assay
4.9.5. Rhamnolipids Assay
4.10. Motility Behavior of PA-05
4.11. Extraction of RNA and Measurement of Gene Expression Using Quantitative Real-Time PCR (qRT-PCR)
4.12. Molecular Docking
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Zawawy, N.A.; Ali, S.S.; Nouh, H.S. Exploring the potential of Rhizopus oryzae AUMC14899 as a novel endophytic fungus for the production of l-tyrosine and its biomedical applications. Microb. Cell Factories 2023, 22, 31. [Google Scholar] [CrossRef] [PubMed]
- Elkady, W.M.; Raafat, M.M.; Abdel-Aziz, M.M.; Al-Huqail, A.A.; Ashour, M.L.; Fathallah, N. Endophytic Fungus from Opuntia ficus-indica: A Source of Potential Bioactive Antimicrobial Compounds against Multidrug-Resistant Bacteria. Plants 2022, 11, 1070. [Google Scholar] [CrossRef] [PubMed]
- Elkady, W.M.; Bishr, M.M.; Abdel-Aziz, M.M.; Salama, O.M. Identification and isolation of anti-pneumonia bioactive compounds from Opuntia ficus-indica fruit waste peels. Food Funct. 2020, 11, 5275–5283. [Google Scholar] [CrossRef]
- Hashem, A.H.; Attia, M.S.; Kandil, E.K.; Fawzi, M.M.; Abdelrahman, A.S.; Khader, M.S.; Khodaira, M.A.; Emam, A.E.; Goma, M.A.; Abdelaziz, A.M. Bioactive compounds and biomedical applications of endophytic fungi: A recent review. Microb. Cell Factories 2023, 22, 107. [Google Scholar] [CrossRef]
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted interactions between endophytes and plant: Developments and prospects. Front. Microbiol. 2018, 9, 2732. [Google Scholar] [CrossRef]
- World Health Organization Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance. WHO Report. ISBN: 978-92-4-009346-1. Available online: https://www.who.int/publications/i/item/9789240093461 (accessed on 24 May 2024).
- Bjarnsholt, T.; Jensen, P.Ø.; Jakobsen, T.H.; Phipps, R.; Nielsen, A.K.; Rybtke, M.T.; Tolker-Nielsen, T.; Givskov, M.; Høiby, N.; Ciofu, O.; et al. Quorum sensing and virulence of Pseudomonas aeruginosa during lung infection of cystic fibrosis patients. PLoS ONE 2010, 5, e10115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, G.; Xu, K.; Sun, F.; Sun, Y.; Kong, Z.; Fang, B. Risk Factors of Multidrug-Resistant Bacteria in Lower Respiratory Tract Infections: A Systematic Review and Meta-Analysis. Can. J. Infect. Dis. Med Microbiol. 2020, 2020, 7268519. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mishra, R.; Kushveer, J.S.; Khan, M.I.K.; Pagal, S.; Meena, C.K.; Murali, A.; Dhayalan, A.; Sarma, V. 2,4-Di-Tert-Butylphenol Isolated From an Endophytic Fungus, Daldinia eschscholtzii, Reduces Virulence and Quorum Sensing in Pseudomonas aeruginosa. Front. Microbiol. 2020, 11, 1668. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.P.; Passador, L.; Iglewski, B.H.; Greenberg, E.P. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 1995, 92, 1490–1494. [Google Scholar] [CrossRef]
- Schuster, M.; Lostroh, C.P.; Ogi, T.; Greenberg, E.P. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: A transcriptome analysis. J. Bacteriol. 2003, 185, 2066–2079. [Google Scholar] [CrossRef]
- Ahmed, S.A.K.S.; Rudden, M.; Smyth, T.J.; Dooley, J.S.G.; Marchant, R.; Banat, I.M. Natural quorum sensing inhibitors effectively downregulate gene expression of Pseudomonas aeruginosa virulence factors. Appl. Microbiol. Biotechnol. 2019, 103, 3521–3535. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.P.; Feldman, M.; Iglewski, B.H.; Prince, A. Pseudomonas aeruginosa cell-to-cell signaling is required for virulence in a model of acute pulmonary infection. Infect. Immun. 2000, 68, 4331–4334. [Google Scholar] [CrossRef] [PubMed]
- Fong, J.; Mortensen, K.T.; Nørskov, A.; Qvortrup, K.; Yang, L.; Tan, C.H.; Nielsen, T.E.; Givskov, M. Itaconimides as novel quorum sensing inhibitors of Pseudomonas aeruginosa. Front. Cell. Infect. Microbiol. 2019, 8, 443. [Google Scholar] [CrossRef] [PubMed]
- Kalia, M.; Yadav, V.K.; Singh, P.K.; Sharma, D.; Pandey, H.; Narvi, S.S.; Agarwal, V. Effect of cinnamon oil on quorum sensing-controlled virulence factors and biofilm formation in Pseudomonas aeruginosa. PLoS ONE 2015, 10, e0135495. [Google Scholar] [CrossRef]
- Kalia, V.C.; Patel, S.K.S.; Kang, Y.C.; Lee, J.K. Quorum sensing inhibitors as antipathogens: Biotechnological applications. Biotechnol. Adv. 2018, 37, 68–90. [Google Scholar] [CrossRef]
- Meena, H.; Mishra, R.; Ranganathan, S.; Sarma, V.V.; Ampasala, D.R.; Kalia, V.C.; Lee, J.-K.; Siddhardha, B. Phomopsis tersa as inhibitor of quorum sensing system and biofilm forming ability of Pseudomonas aeruginosa. Indian J. Microbiol. 2020, 60, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, M.; Meena, H.; Meena, C.; Kushveer, J.S.; Busi, S.; Murali, A.; Sarma, V. Anti-quorum sensing and antibiofilm potential of Alternaria alternata, a foliar endophyte of Carica papaya, evidenced by QS assays and in-silico analysis. Fungal Biol. 2018, 122, 998–1012. [Google Scholar] [CrossRef]
- Bauer, A.; Brönstrup, M. Industrial natural product chemistry for drug discovery and development. Nat. Prod. Rep. 2014, 31, 35–60. [Google Scholar] [CrossRef]
- Mishra, S.; Priyanka; Sharma, S. Metabolomic insights into endophyte-derived bioactive compounds. Front. Microbiol. 2022, 13, 835931. [Google Scholar] [CrossRef]
- Rashmi, M.; Kushveer, J.S.; Sarma, V.V. A worldwide list of endophytic fungi with notes on ecology and diversity. Mycosphere 2019, 10, 798–1079. [Google Scholar] [CrossRef]
- Wright, G.D. Mechanisms of resistance to antibiotics. Curr. Opin. Chem. Biol. 2003, 7, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Shaibani, A.B.A.; Al-Shakarchi, F.I.; Ameen, R.S. Extraction and characterization of antibacterial compound from Aspergillus niger. J. Al-Nahrain Univ. 2013, 16, 167–174. [Google Scholar] [CrossRef]
- Selim, K.; El-Beih, A.; AbdEl-Rahman, T.; El-Diwany, A. Biodiversity and antimicrobial activity of endophytes associated with Egyptian medicinal plants. Mycosphere 2011, 2, 669–678. [Google Scholar] [CrossRef]
- Fathallah, N.; Raafat, M.M.; Issa, M.Y.; Abdel-Aziz, M.M.; Bishr, M.; Abdelkawy, M.A.; Salama, O. Bio-Guided Fractionation of Prenylated Benzaldehyde Derivatives as Potent Antimicrobial and Antibiofilm from Ammi majus L. Fruits-Associated Aspergillus amstelodami. Molecules 2019, 24, 4118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adebayo, E.A.; Ibikunle, J.B.; Oke, A.M.; Lateef, A.; Azeez, M.A.; Oluwatoyin, A.O.; AyanfeOluwa, A.V.; Blessing, O.T.; Comfort, O.O.; Adekunle, O.O.; et al. Antimicrobial and antioxidant activity of silver, gold and silver-gold alloy nanoparticles phytosynthesized using extract of Opuntia ficus-indica. Rev. Adv. Mater. Sci. 2019, 58, 313–326. [Google Scholar] [CrossRef]
- Blando, F.; Russo, R.; Negro, C.; De Bellis, L.; Frassinetti, S. Antimicrobial and Antibiofilm Activity against Staphylococcus aureus of Opuntia ficus-indica (L.) Mill. Cladode Polyphenolic Extracts. Antioxidants 2019, 8, 117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akinyemi, A. Antimicrobial activities of secondary metabolites from fungal endophytes. IOSR J. Pharm. Biol. Sci. 2017, 12, 13–17. [Google Scholar]
- Orfali, R.; Perveen, S.; AlAjmI, M.F.; Ghaffar, S.; Rehman, M.T.; AlanzI, A.R.; Gamea, S.B.; Khwayri, M. Antimicrobial Activity of Dihydroisocoumarin Isolated from Wadi Lajab Sediment-Derived Fungus Penicillium chrysogenum: In Vitro and In Silico Study. Molecules 2022, 27, 3630. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- EL-Zawawy, N.A.; Abou-Zeid, A.M.; Beltagy, D.M.; Hantera, N.H.; Nouh, H.S. Mycosynthesis of silver nanoparticles from endophytic Aspergillus flavipes AUMC 15772: Ovat-statistical optimization, characterization and biological activities. Microb. Cell Factories 2023, 22, 228. [Google Scholar] [CrossRef]
- Marinho, A.M.R.; Rodrigues-Filho, E.; Moitinho, M.D.L.R.; Santos, L.S. Biologically active polyketides produced by Penicillium janthinellum isolated as an endophytic fungus from fruits of Melia azedarach. J. Brazilian Chem. Soc. 2005, 16, 280–283. [Google Scholar] [CrossRef]
- Devi, P.; D’Souza, L.; Kamat, T.; Rodrigues, C.; Naik, C.G. Batch culture fermentation of Penicillium chrysogenum and a report on the isolation, purification, identification and antibiotic activity of citrinin. Indian J Mar Sci. 2009, 38, 38–44. [Google Scholar]
- Espina, A.; Sanchez-Cortes, S.; Jurašeková, Z. Vibrational Study (Raman, SERS, and IR) of Plant Gallnut Polyphenols Related to the Fabrication of Iron Gall Inks. Molecules 2022, 27, 279. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stauff, D.L.; Bassler, B.L. Quorum sensing in Chromobacterium violaceum: DNA recognition and gene regulation by the CviR receptor. J. Bacteriol. 2011, 193, 3871–3878. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, M.E.; Liu, J.; Wallace, J.; Akhlaghi, F.; Rowley, D.C. Secondary metabolites produced by the marine bacterium Halobacillus salinus that inhibit quorum sensing-controlled phenotypes in gram-negative bacteria. Appl. Environ. Microbiol. 2009, 75, 567–572. [Google Scholar] [CrossRef]
- Strompfová, V.; Štempelová, L.; Wolaschka, T. Antibacterial activity of plant-derived compounds and cream formulations against canine skin bacteria. Veter-Res. Commun. 2024, 48, 1459–1470. [Google Scholar] [CrossRef]
- Reyes, A.W.B.; Hong, T.G.; Hop, H.T.; Arayan, L.T.; Huy, T.X.N.; Min, W.; Lee, H.J.; Lee, K.S.; Kim, S. The in vitro and in vivo protective effects of tannin derivatives against Salmonella enterica serovar Typhimurium infection. Microb. Pathog. 2017, 109, 86–93. [Google Scholar] [CrossRef]
- Luo, J.; Kong, J.L.; Dong, B.-Y.; Huang, H.; Wang, K.; Wu, L.H.; Hou, C.C.; Liang, Y.; Li, B.; Chen, Y.Q. Baicalein attenuates the quorum sensing-controlled virulence factors of Pseudomonas aeruginosa and relieves the inflammatory response in P. aeruginosa-infected macrophages by downregulating the MAPK and NFκB signal-transduction pathways. Drug Des. Dev. Ther. 2016, 10, 183–203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bratu, S.; Gupta, J.; Quale, J. Expression of the las and rhl quorum-sensing systems in clinical isolates of Pseudomonas aeruginosa does not correlate with efflux pump expression or antimicrobial resistance. J. Antimicrob. Chemother. 2006, 58, 1250–1253. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, C.Y.; Xie, J.; Dai, J.H.; He, S.L.; Tian, Y. Identification of Potential Dipeptidyl Peptidase (DPP)-IV Inhibitors among Moringa oleifera Phytochemicals by Virtual Screening, Molecular Docking Analysis, ADME/T-Based Prediction, and In Vitro Analyses. Molecules 2020, 25, 189. [Google Scholar] [CrossRef]
- Kessler, E.; Safrin, M.; Olson, J.C.; Ohman, D.E. Secreted LasA of Pseudomonas aeruginosa is a staphylolytic protease. J. Biol. Chem. 1993, 268, 7503–7508. [Google Scholar] [CrossRef] [PubMed]
- Musthafa, K.S.; Saroja, V.; Pandian, S.K.; Ravi, A.V. Antipathogenic potential of marine Bacillus sp. SS4 on N-acyl-homoserine-lactone-mediated virulence factors production in Pseudomonas aeruginosa (PAO1). J. Biosci. 2011, 36, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Gooday, G.W. The ecology of chitin degradation. In Advances in Microbial Ecology II; Springer: Boston, MA, USA, 1990; pp. 387–430. [Google Scholar]
- Salunkhe, P.; Smart, C.H.M.; Morgan, J.A.W.; Panagea, S.; Walshaw, M.J.; Hart, C.A.; Geffers, R.; Tümmler, B.; Winstanley, C. A cystic fibrosis epidemic strain of Pseudomonas aeruginosa displays enhanced virulence and antimicrobial resistance. J. Bacteriol. 2005, 187, 4908–4920. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Ravichandran, V.; Zhang, N.; Wang, H.; Bian, X.; Zhang, Y.; Li, A. Attenuation of Pseudomonas aeruginosa Quorum Sensing by Natural Products: Virtual Screening, Evaluation and Biomolecular Interactions. Int. J. Mol. Sci. 2020, 21, 2190. [Google Scholar] [CrossRef]
- Husain, F.M.; Ahmad, I.; Asif, M.; Tahseen, Q. Influence of clove oil on certain quorum-sensing-regulated functions and biofilm of Pseudomonas aeruginosa and Aeromonas hydrophila. J. Biosci. 2013, 38, 835–844. [Google Scholar] [CrossRef]
- O′Toole, G.A.; Kolter, R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 1998, 30, 295–304. [Google Scholar] [CrossRef]
- Murphy, T.F. Pseudomonas aeruginosa in adults with chronic obstructive pulmonary disease. Curr. Opin. Pulm. Med. 2009, 15, 138–142. [Google Scholar] [CrossRef]
- El-Mowafy, S.A.; El Galil, K.H.A.; El-Messery, S.M.; Shaaban, M.I. Aspirin is an efficient inhibitor of quorum sensing, virulence and toxins in Pseudomonas aeruginosa. Microb. Pathog. 2014, 74, 25–32. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, Q.; Defoirdt, T. Does quorum sensing interference affect the fitness of bacterial pathogens in the real world? Environ. Microbiol. 2018, 20, 3918–3926. [Google Scholar] [CrossRef] [PubMed]
- Köhler, T.; Guanella, R.; Carlet, J.; Van Delden, C. Quorum sensing-dependent virulence during Pseudomonas aeruginosa colonisation and pneumonia in mechanically ventilated patients. Thorax 2010, 65, 703–710. [Google Scholar] [CrossRef][Green Version]
- Rajkumari, J.; Borkotoky, S.; Murali, A.; Suchiang, K.; Mohanty, S.K.; Busi, S. Cinnamic acid attenuates quorum sensing associated virulence factors and biofilm formation in Pseudomonas aeruginosa PAO1. Biotechnol. Lett. 2018, 40, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Moni, J.N.R.; Adnan, M.; Tareq, A.M.; Kabir, M.; Reza, A.; Nasrin, M.; Chowdhury, K.H.; Sayem, S.A.J.; Rahman, M.A.; Alam, A.; et al. Therapeutic potentials of Syzygium fruticosum fruit (seed) reflect into an array of pharmacological assays and prospective receptors-mediated pathways. Life 2021, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- El-Sapagh, S.; El-Shenody, R.; Pereira, L.; Elshobary, M. Unveiling the Potential of Algal Extracts as Promising Antibacterial and Antibiofilm Agents against Multidrug-Resistant Pseudomonas aeruginosa: In Vitro and In Silico Studies including Molecular Docking. Plants 2023, 12, 3324. [Google Scholar] [CrossRef] [PubMed]
- Anju, V.; Busi, S.; Mohan, M.S.; Ranganathan, S.; Ampasala, D.R.; Kumavath, R.; Dyavaiah, M. In vivo, in vitro and molecular docking studies reveal the anti-virulence property of hispidulin against Pseudomonas aeruginosa through the modulation of quorum sensing. Int. Biodeterior. Biodegradation 2022, 174, 105487. [Google Scholar] [CrossRef]
- Alsohaili, S.A.; Bani-hasan, B.M. Morphological and molecular identifcation of fungi isolated from diferent environmental sources in the northern eastern desert of Jordan. JJBS 2018, 11, 329–337. [Google Scholar]
- Tapfuma, K.I.; Uche-Okereafor, N.; Sebola, T.E.; Hussan, R.; Mekuto, L.; Makatini, M.M.; Green, E.; Mavumengwana, V. Cytotoxic activity of crude extracts from Datura stramonium’s fungal endophytes against A549 lung carcinoma and UMG87 glioblastoma cell lines and LC-QTOF-MS/MS based metabolite profling. BMC Complement Altern Med. 2019, 19, 330. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplifcation and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A guide to Methods and Applications; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Supaphon, P.; Phongpaichit, S.; Rukachaisirikul, V.; Sakayaroj, J. Antimicrobial potential of endophytic fungi derived from three seagrass species: Cymodocea serrulata, Halophila ovalis and Thalassia hemprichii. PLoS ONE 2013, 8, e72520. [Google Scholar] [CrossRef]
- Sharma, A.; Bhot, M.; Varghese, J.; Chandra, N. Separation and Quantification of Tannic Acid in Bryophyllum pinnatum (Lam.) Kurz. by High Performance Thin Layer Chromatography. Asian J. Chem. 2013, 25, 9097–9100. [Google Scholar] [CrossRef]
- El-Zawawy, N.A.; Ali, S.S.; Khalil, M.A.; Sun, J.; Nouh, H.S. Exploring the potential of benzoic acid derived from the endophytic fungus strain Neurospora crassa SSN01 as a promising antimicrobial agent in wound healing. Microbiol. Res. 2022, 262, 127108. [Google Scholar] [CrossRef]
- Kenawy, E.R.; Ali, S.S.; Al-Etewy, M.; Sun, J.; Wu, J.; El-Zawawy, N. Synthesis, characterization and biomedical applications of a novel Schif base on methyl acrylate-functionalized chitosan bearing p-nitrobenzaldehyde groups. Int. J. Biol. Macromol. 2019, 122, 833–843. [Google Scholar] [CrossRef]
- Panicker, C.Y.; Varghese, H.T.; Philip, D. FT-IR, FT-raman and SERS spectra of vitamin C. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 65, 802–804. [Google Scholar] [CrossRef] [PubMed]
- Durgawale, T.P.; Durgawale, P.P.; Khanwelkar, C.C. Quantitative estimation of tannins by HPLC. Der Pharm. Lett. 2016, 8, 123–126. [Google Scholar]
- Allam, N.G.; Shabana, S.A.; Osman, Y.A.; Nouh, H.S. Prevalence of some virulence factors among Gram negative bacteria isolated from patients with lung infection and their antimicrobial susceptibility patterns. Egypt. J. Bot. 2019, 59, 633–643. [Google Scholar]
- El Zawawy, N.; El Shafay, S.; Abomohra, A.E.F. Macroalgal activity against fungal urinary tract infections, in vitro screening and evaluation study. Rendiconti Lince-Sci. Fis. Nat. 2020, 31, 165–175. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Saad-Allah, K.; Fareed, M.; Ali, A.; El-Badry, A.; El-Zawawy, N.A.; Wuam, J.; Sun, J.; Maof, G.; et al. Phytochemical analysis and assessment of antioxidant and antimicrobial activities of some medicinal plant species from Egyptian fora. J. Appl. Biomed. 2018, 16, 289–300. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. CLSI Suppl. 2017, M100, 106–112. [Google Scholar]
- Bala, A.; Kumar, R.; Harjai, K. Inhibition of quorum sensing in Pseudomonas aeruginosa by azithromycin and its effectiveness in urinary tract infections. J. Med Microbiol. 2011, 60, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, P.S.; Rai, R.V. Inhibition of quorum-sensing-controlled virulence factors of Pseudomonas aeruginosa by Murraya koenigii essential oil: A study in a Caenorhabditis elegans infectious model. J. Med. Microbiol. 2016, 65, 1528–1535. [Google Scholar] [CrossRef]
- Hentzer, M.; Riedel, K.; Rasmussen, T.B.; Heydorn, A.; Andersen, J.B.; Parsek, M.R.; Rice, S.A.; Eberl, L.; Molin, S.; Høiby, N.; et al. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology 2002, 148, 87–102. [Google Scholar] [CrossRef]
- Ohman, D.E.; Cryz, S.J.; Iglewski, B.H. Isolation and characterization of Pseudomonas aeruginosa PAO mutant that produces altered elastase. J. Bacteriol. 1980, 142, 836–842. [Google Scholar] [CrossRef]
- Luo, J.; Dong, B.; Wang, K.; Cai, S.; Liu, T.; Cheng, X.; Lei, D.; Chen, Y.; Li, Y.; Kong, J.; et al. Baicalin inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances Pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model. PLoS ONE 2017, 12, e0176883. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lee, B.; Yang, L.; Wang, H.; Givskov, M.; Molin, S.; Høiby, N.; Song, Z. Effects of ginseng on Pseudomonas aeruginosa motility and biofilm formation. FEMS Immunol. Med Microbiol. 2011, 62, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, J.H.; Zi, S.F.; Liu, F.R.; Deng, C.; Ao, X.; Zhang, P. AgNP combined with quorum sensing inhibitor increased the antibiofilm effect on Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2019, 103, 6195–6204. [Google Scholar] [CrossRef] [PubMed]
- Anju, V.T.; Busi, S.; Ranganathan, S.; Ampasala, D.R.; Kumar, S.; Suchiang, K.; Kumavath, R.; Dyavaiah, M. Sesamin and sesamolin rescues Caenorhabditis elegans from Pseudomonas aeruginosa infection through the attenuation of quorum sensing regulated virulence factors. Microb. Pathog. 2021, 155, 104912. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. Wiley Online Library. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


| Fungal Extract | Diameter Zone (mm) |
|---|---|
| EF1 | 17 bc ± 0.6 |
| EF2 | 18 bc ± 0.1 |
| EF3 | 10 e ± 0.1 |
| EF4 | 19 b ± 0.1 |
| EF5 | 14 d ± 0.0 |
| EF6 | 16 c ± 0.6 |
| EF7 | 0 f |
| EF8 | 0 f |
| EF9 | 19 b ± 0.3 |
| EF10 | 23 a ± 0.0 |
| Positive control (baicalein) | 18 bc ±0.0 |
| Isolate Code | Diameter of the Inhibition Zone (mm) in a Test with Tannic Acid Applied in the Following Concentrations: | |||
|---|---|---|---|---|
| 125 µg/mL | 250 µg/mL | 500 µg/mL | 1000 µg/mL | |
| PA-01 | 6 f ± 0.0 | 14 f ± 0.1 | 23 e ± 0.6 | 30 e ± 0.0 |
| PA-02 | 7 e ± 0.2 | 16 d ± 0.0 | 26 c ± 0.0 | 33 b ± 0.1 |
| PA-03 | 11 a ± 0.1 | 18 b ± 0.0 | 27 b ± 0.3 | 33 b ± 0.1 |
| PA-04 | 7 e ± 0.3 | 14 f ± 0.1 | 21 h ± 0.0 | 30 e ± 0.0 |
| PA-05 | 12 a ± 0.3 | 19 a ± 0.6 | 28 a ± 0.0 | 35 a ± 0.3 |
| PA-06 | 8 d ± 0.0 | 15 c ± 0.7 | 24 e ± 0.1 | 31 d ± 0.0 |
| PA-07 | 7 e ± 0.0 | 13 g ± 0.0 | 24 d ± 0.6 | 31 d ± 0.1 |
| PA-08 | 7 e ± 0.5 | 15 d ± 0.9 | 22 f ± 0.1 | 29 f ± 0.0 |
| PA-09 | 7 e ± 0.3 | 15 e ± 0.5 | 21 g ± 0.0 | 30 e ± 0.5 |
| PA-10 | 8 d ± 0.3 | 16 d ± 0.5 | 23 f ± 0.0 | 33 b ± 0.1 |
| PA-11 | 9 c ± 0.0 | 16 d ± 0.0 | 22 g ± 0.1 | 30 e ± 0.0 |
| PA-12 | 9 c ± 0.1 | 16 c ± 0.9 | 21 g ± 0.9 | 30 e ± 0.7 |
| PA-13 | 8 d ± 0.1 | 15 e ± 0.2 | 22 f ± 0.3 | 29 f ± 0.5 |
| PA-14 | 10 b ± 0.0 | 17 c ± 0.3 | 25 d ± 0.0 | 32 c ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nouh, H.S.; El-Zawawy, N.A.; Halawa, M.; Shalamesh, E.M.; Ali, S.S.; Korbecka-Glinka, G.; Shala, A.Y.; El-Sapagh, S. Endophytic Penicillium oxalicum AUMC 14898 from Opuntia ficus-indica: A Novel Source of Tannic Acid Inhibiting Virulence and Quorum Sensing of Extensively Drug-Resistant Pseudomonas aeruginosa. Int. J. Mol. Sci. 2024, 25, 11115. https://doi.org/10.3390/ijms252011115
Nouh HS, El-Zawawy NA, Halawa M, Shalamesh EM, Ali SS, Korbecka-Glinka G, Shala AY, El-Sapagh S. Endophytic Penicillium oxalicum AUMC 14898 from Opuntia ficus-indica: A Novel Source of Tannic Acid Inhibiting Virulence and Quorum Sensing of Extensively Drug-Resistant Pseudomonas aeruginosa. International Journal of Molecular Sciences. 2024; 25(20):11115. https://doi.org/10.3390/ijms252011115
Chicago/Turabian StyleNouh, Hoda S., Nessma A. El-Zawawy, Mohamed Halawa, Ebrahim M. Shalamesh, Sameh Samir Ali, Grażyna Korbecka-Glinka, Awad Y. Shala, and Shimaa El-Sapagh. 2024. "Endophytic Penicillium oxalicum AUMC 14898 from Opuntia ficus-indica: A Novel Source of Tannic Acid Inhibiting Virulence and Quorum Sensing of Extensively Drug-Resistant Pseudomonas aeruginosa" International Journal of Molecular Sciences 25, no. 20: 11115. https://doi.org/10.3390/ijms252011115
APA StyleNouh, H. S., El-Zawawy, N. A., Halawa, M., Shalamesh, E. M., Ali, S. S., Korbecka-Glinka, G., Shala, A. Y., & El-Sapagh, S. (2024). Endophytic Penicillium oxalicum AUMC 14898 from Opuntia ficus-indica: A Novel Source of Tannic Acid Inhibiting Virulence and Quorum Sensing of Extensively Drug-Resistant Pseudomonas aeruginosa. International Journal of Molecular Sciences, 25(20), 11115. https://doi.org/10.3390/ijms252011115






