Metabolic Reprogramming at the Edge of Redox: Connections Between Metabolic Reprogramming and Cancer Redox State
Abstract
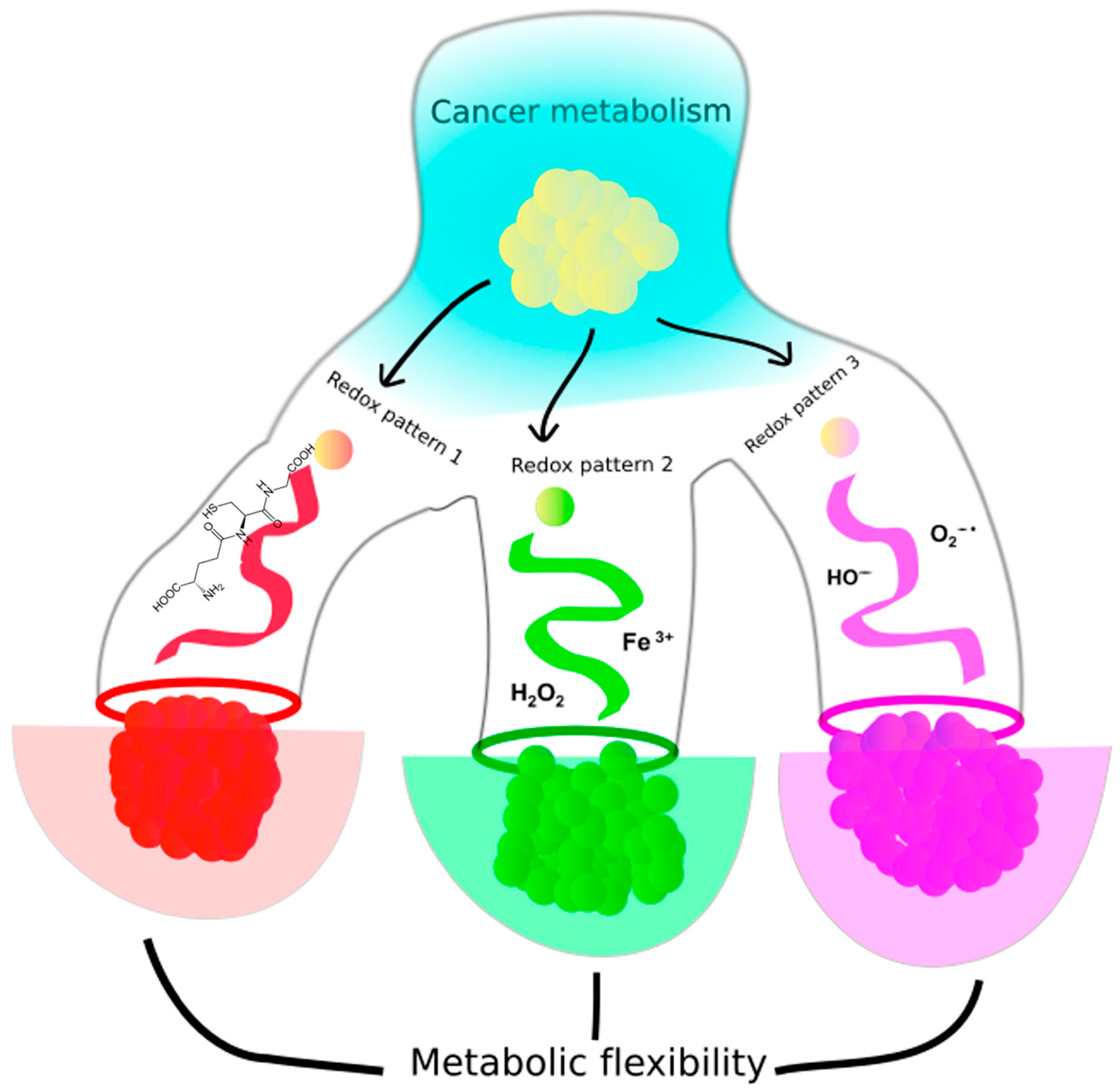
1. Introduction
2. Glucose Metabolism and Cancer Redox State
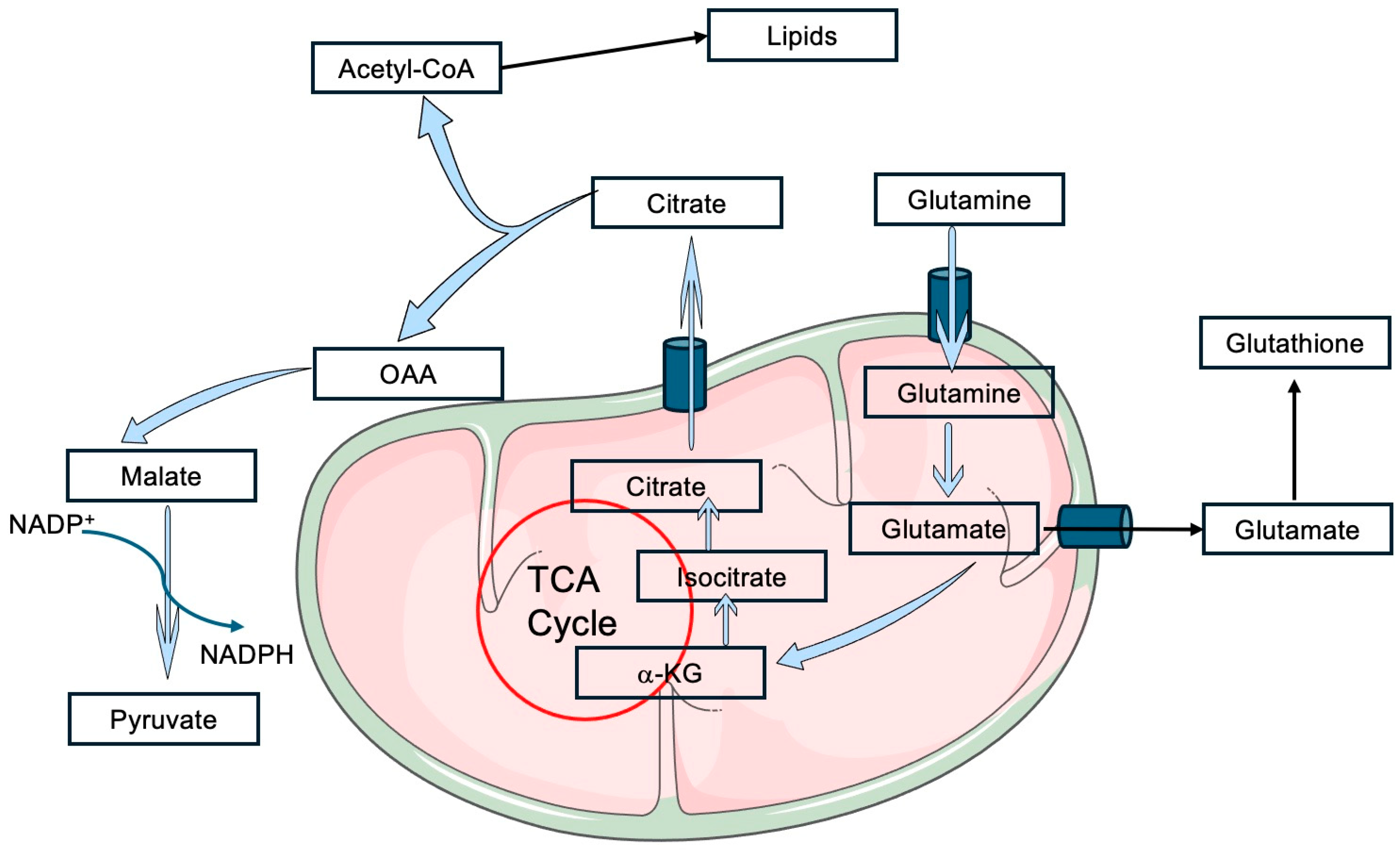
3. Glutamine Metabolism and Cancer Redox State
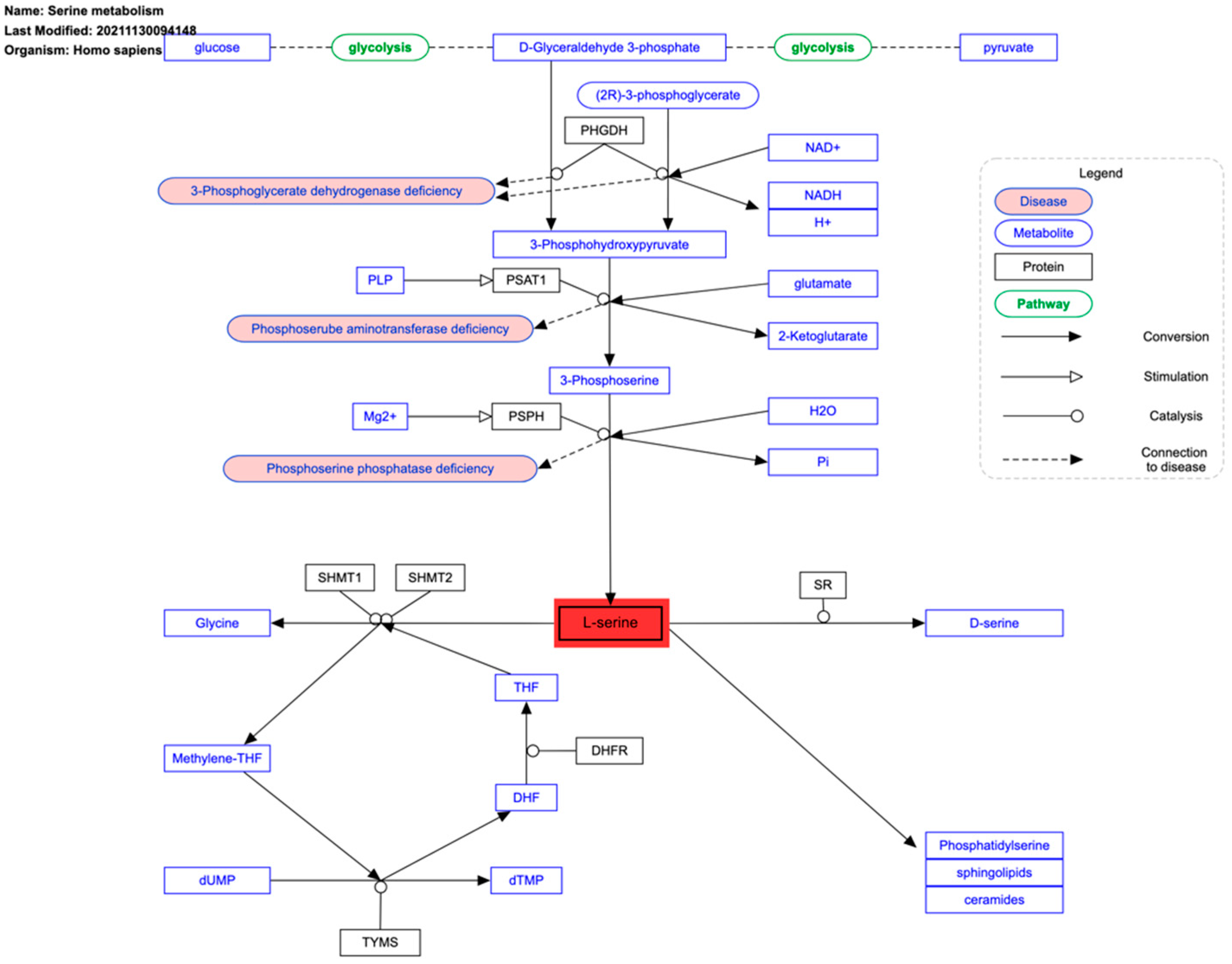
4. Serine Metabolism and Cancer Redox State
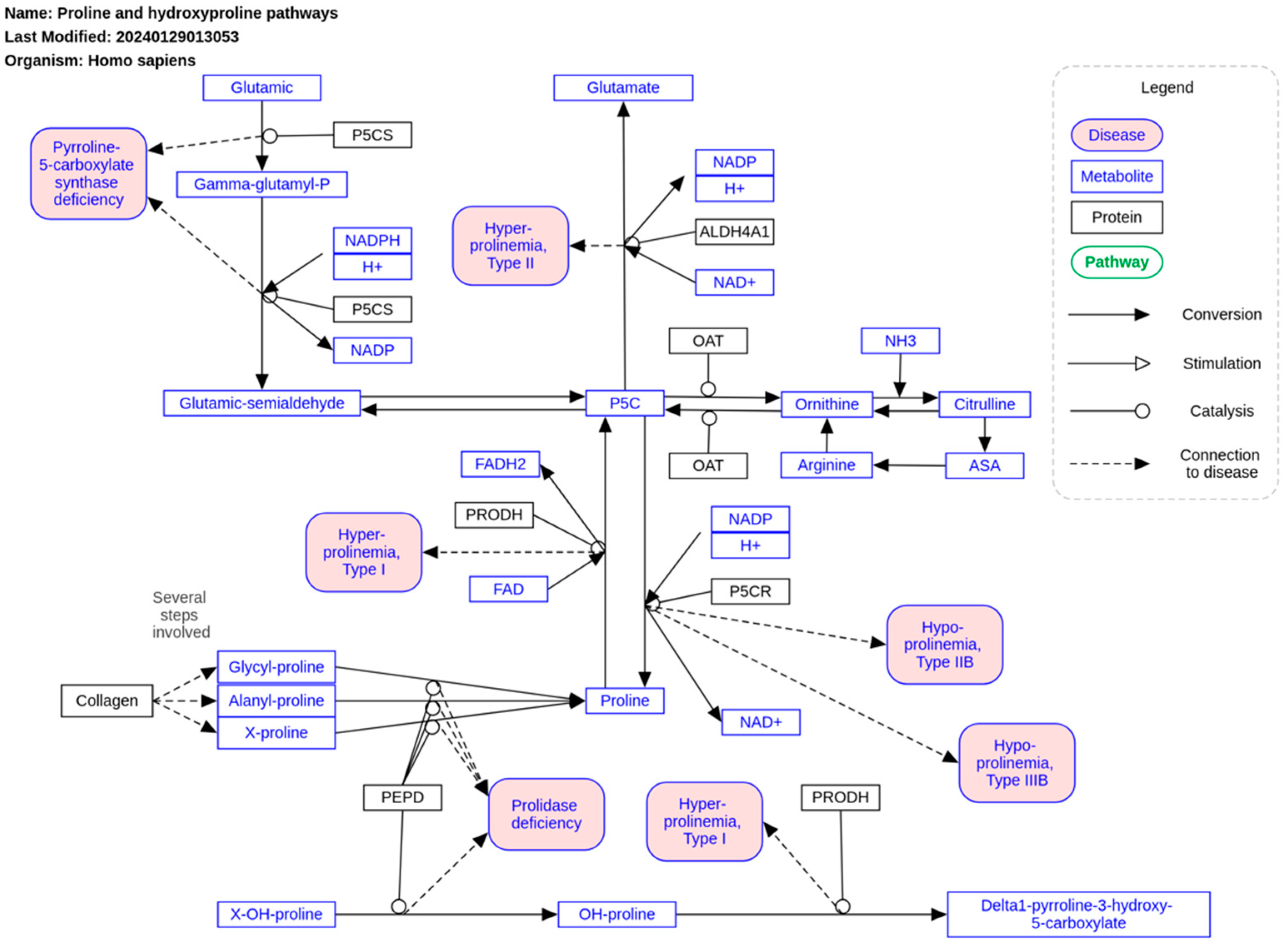
5. Parametabolic Regulation and Proline Metabolism
6. The Krebs Cycle and Cancer Redox State
7. Heme Catabolism and Cancer Redox State
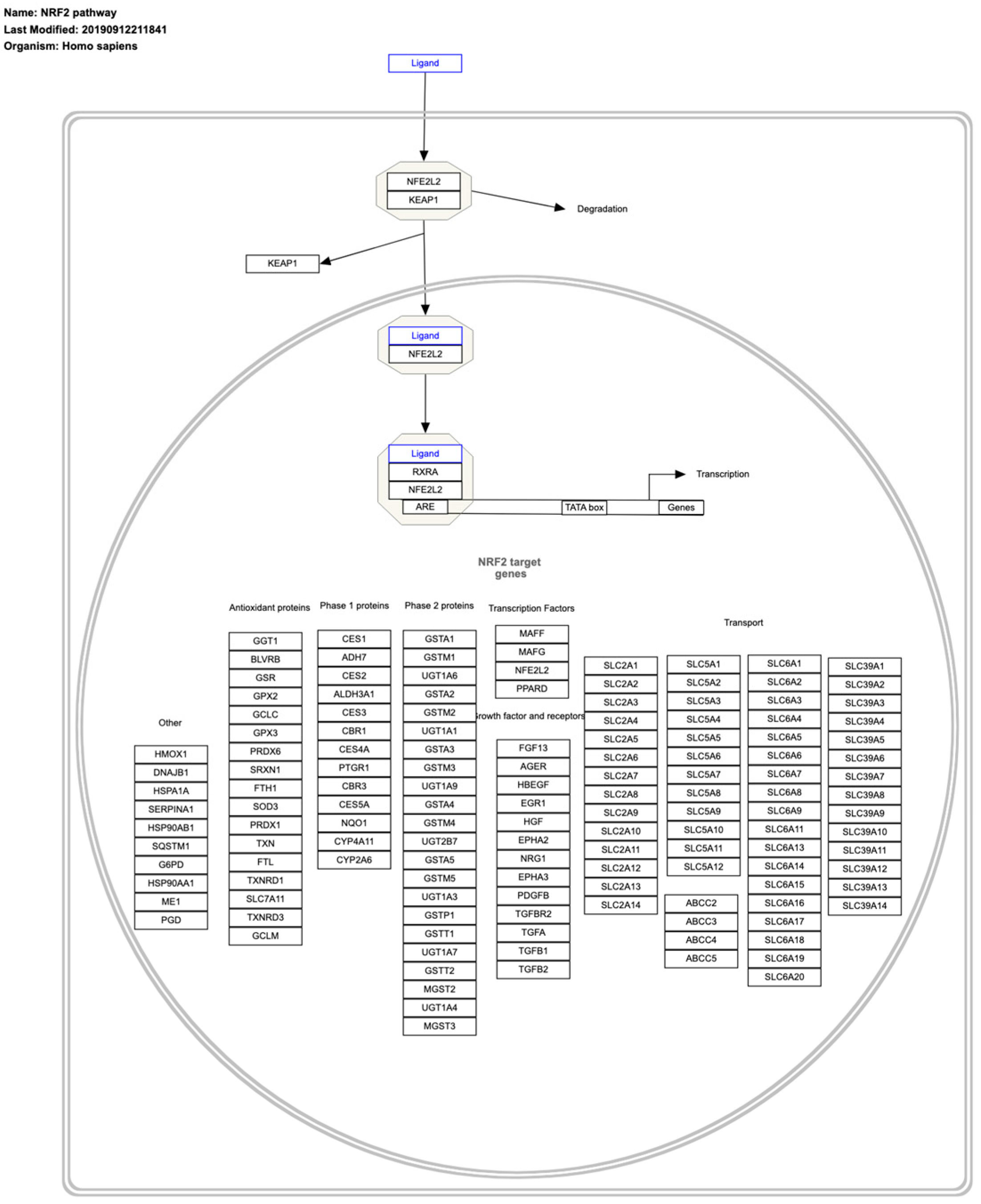
8. Master Regulators of Cancer Redox State
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.; Posener, K.; Negelein, E. Über den Stoffwechsel der Tumoren. Biochem. Zeitschrift 1924, 152, 319–344. [Google Scholar]
- Warburg, O. Über de n Stoffwechsel der Carcinomzelle. Klin. Wochenschr. 1925, 4, 534–536. [Google Scholar] [CrossRef]
- Thompson, C.B.; Vousden, K.H.; Johnson, R.S.; Koppenol, W.H.; Sies, H.; Lu, Z.; Finley, L.W.S.; Frezza, C.; Kim, J.; Hu, Z.; et al. A century of the Warburg effect. Nat. Metab. 2023, 5, 1840–1843. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Balling, R. The Warburg effect reinterpreted 100 yr on: A first principles stoichiometric analysis and interpretation from the perspective of ATP metabolism in cancer cells. Function 2024, 5, zqae008. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, S.; Gottlieb, E. Oncometabolites: Tailoring our genes. FEBS J. 2015, 282, 1796–2805. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wang, Z.; Guo, S.; Lin, C.; Yao, H.; Yang, Q.; Wang, Y.; Yu, X.; He, X.; Sun, W.; et al. Detection, mechanisms, and therapeutic implications of oncometabolites. Trends Endocrinol. Metab. 2023, 34, 849–861. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; DeBefrardinis, R.J. Understanding the intersections between metabolism and cancer biology. Cell 2017, 168, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The emerging hallmarks of cancer metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the origin of cancer cell. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Wenner, C.E.; Weinhouse, S. Metabolism of neoplastic tissue. III. Diphosphopyridine nucleotide requirements for oxidations by mitochondria of neoplastic and non-neoplastic tissues. Cancer Res. 1953, 13, 21–26. [Google Scholar] [PubMed]
- Gentric, G.; Mieulet, V.; Mechta-Grigoriou, F. Heterogeneity in Cancer metabolism: New concepts in an old field. Antiox. Redox Sign. 2017, 26, 462–485. [Google Scholar] [CrossRef]
- Li, L.; Fath, M.A.; Scarbrough, P.M.; Watson, W.H.; Spitz, D.R. Combined inhibition of glycolysis, the pentose cycle, and thioredoxin metabolism selectively increases cytotoxicity and oxidative stress in human breast and prostate cancer. Redox Biol. 2015, 4, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Xu, I.M.J.; Lai, R.K.H.; Lin, S.H.; Tse, A.P.W.; Chiu, D.K.C.; Koh, H.Y.; Law, C.T.; Wong, C.M.; Cai, Z.; Wong, C.C.L.; et al. Transketolase counteracts oxidative stress to drive cancer development. Proc. Natl. Acad. Sci. USA 2016, 113, E725–E734. [Google Scholar] [CrossRef]
- Krüger, A.; Grüning, N.M.; Wamelink, M.M.; Kerck, M.; Kirpy, A.; Parkhomchuk, D.; Bluemlein, K.; Scweiger, M.R.; Soldatov, A.; Lehrach, H.; et al. The pentose phosphate pathway is a metabolic redox sensor and regulates transcription during the antioxidant response. Antiox. Redox Sign. 2011, 15, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Cossu, V.; Bonanomi, M.; Bauckneht, M.; Ravera, S.; Righi, N.; Miceli, A.; Morbelli, S.; Orengo, A.M.; Piccioli, P.; Bruno, S.; et al. Two high-rate pentose-phosphate patways in cancer cells. Sci. Rep. 2020, 10, 22111. [Google Scholar] [CrossRef] [PubMed]
- Sakamaki, T.; Casimiro, M.C.; Ju, X.; Quong, A.A.; Katiyar, S.; Liu, M.; Jiao, X.; Li, A.; Zhang, X.; Lu, Y.; et al. Cyclin D1 determines mitochondrial function in vivo. Mol. Cell Biol. 2006, 26, 5449–5469. [Google Scholar] [CrossRef]
- Gwangwa, M.V.; Joubert, A.M.; Visagie, M.H. Crosstal between the Warburg effect, redox regulation and autophagy induction in tumourigenesis. Cell. Moil. Biol. Lett. 2018, 23, 20. [Google Scholar]
- Ippolito, L.; Marini, A.; Cavallini, L.; Morandi, A.; Pietrovito, L.; Pintus, G.; Giannoni, E.; Schrader, T.; Puhr, M.; Chiarugi, P.; et al. Metabolic shift toward oxidative phosphorylation in docetaxel resistant prostate cancer cells. Oncotarget 2016, 7, 61890–61904. [Google Scholar] [CrossRef]
- Lu, C.L.; Qin, L.; Qiu, H.C.; Candas, D.; Fan, M.; Li, J.J. Tumor cells switch to mitochondrial oxidative phosphorylation under radiation via mTOR-mediated hexokinase II inhibition-A Warburg-reversing effect. PLoS ONE 2015, 10, e0121046. [Google Scholar] [CrossRef] [PubMed]
- Maycotte, P.; Sarmiento-Salinas, F.L.; García-Miranda, A.; Ovando-Ovando, C.I.; Robledo-Cadena, D.X.; Hernández-Esquivel, L.; Jasso-Chávez, R.; Marín-hernández, A. Metabolic and oxidative stress management heterogeneity in a panel of breast cancer cell lines. Metabolites 2024, 14, 435. [Google Scholar] [CrossRef]
- Biondini, M.; Lehuédé, C.; Tabariès, S.; Annis, M.G.; Pacis, A.; Ma, E.H.; Tam, C.; Hsu, B.E.; Audet-Delage, Y.; Abu-Thuraia, A.; et al. Metastatic breast cacner cells are metabolically reprogrammed to maintain redox homeostasis during metastasis. Redox Biol. 2024, 75, 103276. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Lal, S.; FitzGerald, K.E.; Zhang, L. A holistic view of cancer bioenergetics: Mitochondrial function and respiration play fundamental roles in the development and progression of diverse tumors. Clin. Translat. Med. 2016, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Lu, W.; Tian, J.; Qiao, S.; Li, J.; Glorieux, C.; Wen, S.; Zhang, H.; Li, Y.; Huang, P. Reductive TCA cycle catalyzed by wild-type IDH2 promotes acute myeloid leukemia and is a metabolic vulnerability for potential targeted therapy. J. Hematol. Oncol. 2022, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Kamphorst, J.J.; Mathew, R.; Chung, M.K.; White, E.; Shlomi, T.; Rabinowitz, J.D. Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia. Mol. Systems Biol. 2013, 9, 712. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Miwa, H.; Shikami, M.; Tsunekawa-Imai, N.; Suganuma, K.; Mizuno, S.; Takahashi, M.; Mizutani, M.; Hanamura, I.; Nitta, M. Importance of glutamine metabolism in leukemia cells by energy production through TCA cycle and by redox homeostasis. Cancer Investig. 2014, 32, 241–247. [Google Scholar] [CrossRef]
- Son, J.; Lyssiotis, C.A.; Ying, H.; Wang, X.; Hua, S.; Ligorio, M.; Perera, R.M.; Ferrone, C.R.; Mullarky, E.; Shyh-Chang, N.; et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 2013, 496, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Alberghina, L.; Gaglio, D. Redox control of glutamine utilization in cancer. Cell Death Dis. 2014, 5, e1561. [Google Scholar] [CrossRef]
- Alberghina, L. The Warburg effect explained: Integration of enhanced glycolysis with heterogeneous mitochondria to promote cancer cell proliferation. Int. J. Mol. Sci. 2023, 24, 15787. [Google Scholar] [CrossRef]
- Metallo, C.M.; Gameiro, P.A.; Bell, E.L.; Mattaini, K.R.; Yang, J.; Hiller, K.; Jewell, C.M.; Johnson, Z.R.; Irvine, D.; Guarente, L.; et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 2011, 481, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Shestov, A.A.; Swain, P.; Yang, C.; Parker, S.J.; Wang, Q.A.; Terada, L.S.; Adams, N.D.; McCabe, M.T.; Pietrak, B.; et al. Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Nature 2016, 532, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Peng, X.; Li, Y.; Wei, S.; He, G.; Liu, J.; Li, X.; Yang, S.; Li, D.; Lin, W.; et al. Glutamine addiction in tumor cell: Oncogene regulation and clinical treatment. Cell Commun. Signal. 2024, 22, 12. [Google Scholar] [CrossRef]
- Cetinbas, N.M.; Sudderth, J.; Harris, R.C.; Cebeci, A.; Negri, G.L.; Yilmaz, Ö.H.; DeBerardinis, R.J.; Sorensen, P.H. Glucose-dependent anaplerosis in cancer cells is required for cellular redox balance in the absence of glutamine. Sci. Rep. 2016, 6, 32606. [Google Scholar] [CrossRef]
- Sánchez-Aragó, M.; Formentini, L.; Cuezva, J.M. Mitochondria-mediated energy adaption in cancer: The H+-ATP synthase-geared switch of metabolism in human tumors. Antiox. Redox Signal. 2013, 19, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Locasale, J.W. Serine, glycine and one-carbon units: Cancer metabolism in full circle. Nat. Rev. Cancer 2013, 13, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Vousden, K.H. Serene and one-carbon metabolism in cancer. Nat. Rev. Cancer 2016, 16, 650–662. [Google Scholar] [CrossRef]
- Geeraerts, S.L.; Heylen, E.; De Keersmaecker, K.; Kampen, K.R. The ins and outs of serine and glycine metabolism in cancer. Nat. Metab. 2021, 3, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Labuschagne, C.F.; Van Den Broek, N.J.; Mackay, G.M.; Vousden, K.H.; Maddocks, O.D. Serine, but not glycine, supports one-carbon metabolism and proliferation of cancer cells. Cell Rep. 2014, 7, 1248–1258. [Google Scholar] [CrossRef]
- Maddocks, O.D.; Labuschagne, C.F.; Adams, P.D.; Vousden, K.H. Serine metabolism supports the methionine cycle and DNA/R;A methylation through de novo ATP synthesis in cancer cells. Mol. Cell 2016, 61, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Mehrmohamadi, M.; Liu, X.; Shestov, A.A.; Locasale, J.W. Characterization of the usage of the serine metabolic network in human cancer. Cell Rep. 2014, 9, 1507–1519. [Google Scholar] [CrossRef]
- Fan, J.; Ye, J.; Kamphorst, J.J.; Shlomi, T.; Thompson, C.B.; Rabinowitz, J.D. Quantitative flux analysis reveals folate-dependent NADPH production. Nature 2014, 510, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Meiser, J.; Tumanov, S.; Maddocks, O.; Labuschagne, C.F.; Athineos, D.; Van Den Broek, N.; Mackay, G.M.; Gottlieb, E.; Blyth, K.; Vousden, K.; et al. Serine one-carbon catabolism with formate overflow. Sci. Adv. 2016, 2, e1601273. [Google Scholar] [CrossRef] [PubMed]
- Samanta, D.; Park, Y.; Andrabi, S.A.; Shelton, L.M.; Gilkes, D.M.; Semenza, G.L. PHGDH expression is required for mitochondrial redox homeostasis, breast cancer stem cell maintenance, and lung metastasis. Cancer Res. 2016, 76, 4430–4442. [Google Scholar] [CrossRef]
- Samanta, D.; Semenza, G.L. Serine synthesis helps hypoxic cancer stem cells regulate redox. Cancer Res. 2016, 76, 6458–6462. [Google Scholar] [CrossRef] [PubMed]
- Ocaña, M.C.; Bernal, M.; Yang, C.; Caro, C.; Domínguez, A.; Vu, H.S.; Cárdenas, C.; García-Martín, M.L.; DeBerardinis, R.J.; Quesada, A.R.; et al. New insights in the targets of action of dimethyl fumarate in endothelial cells: Effects on energetic metabolism and serine synthesis in vitro and in vivo. Commun. Biol. 2023, 6, 1084. [Google Scholar] [CrossRef]
- Riscal, R.; Schrepfer, E.; Arena, G.; Cissé, M.Y.; Bellvert, F.; Heuillet, M.; Rambow, F.; Bonneil, E.; Sabourdy, F.; Vincent, C.; et al. Chromatin-bound MDM2 regulates serine metabolism and redox homeostasis independently of p53. Mol. Cell 2016, 62, 890–902. [Google Scholar] [CrossRef]
- Filippone, M.G.; Gaglio, D.; Bonfanti, R.; Tucci, F.A.; Ceccacci, E.; Pennisi, R.; Bonanomi, M.; Jodice, G.; Tillhon, M.; Montani, F.; et al. CDK12 promotes tumorigenesis but induces vulnerability to therapies inhibiting folate one-carbon metabolism in breast cancer. Nat. Commun. 2022, 13, 2642. [Google Scholar] [CrossRef] [PubMed]
- Phang, J.M.; Liu, W.; Hancock, C. Bridging epigenetics and metabolism: Role of non-essential amino acids. Epigenetics 2013, 8, 231–236. [Google Scholar] [CrossRef]
- Locasale, J.W.; Cantley, L.C. Metabolic flux and the regulation of mammalian cell growth. Cell Metab. 2011, 14, 443–451. [Google Scholar] [CrossRef]
- Phang, J.M.; Lius, W.; Hancock, C.; Christian, K.J. The proline regulatory axis and cancer. Front. Oncol. 2012, 2, 60. [Google Scholar] [CrossRef] [PubMed]
- Kononczuk, J.; Czyzewska, U.L.; Moczydlowska, J.; Palka, J.; Miltyk, W. Proline oxidase (POX) as a target for cancer therapy. Curr. Drug Targets 2015, 16, 1464–1469. [Google Scholar] [CrossRef]
- Phang, J.M.; Liu, W.; Hancock, C.; Fischer, J.W. Proline metabolism and cnacer: Emerging links to glutamine and collagen. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 71–77. [Google Scholar] [CrossRef]
- Liu, W.; Hancock, C.; Fischer, J.W.; Harman, M.; Phang, J.M. Proline biosynthesis augments tumor cell growth and aerobic glycolysis: Involvement of pyridine nucleotides. Sci. Rep. 2015, 5, 17206. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Le, A.; Hancock, C.; Lane, A.N.; Dang, C.V.; Fan, T.W.M.; Phang, J.M. Reprogramming of proline and glutamine metabolism contributes to the proliferative and metabolic responses regulated by oncogenic transcription factor c-MYC. Proc. Natl. Acad. Sci. USA 2012, 109, 8983–8988. [Google Scholar] [CrossRef] [PubMed]
- Hancock, C.N.; Liu, W.; Alvord, W.G.; Phang, J.M. Co-regulation of mitochondrial respiration by proline dehydrogenase/oxidase and succinate. Amino Acids 2016, 48, 859–872. [Google Scholar] [CrossRef] [PubMed]
- Cardaci, S.; Ciriolo, M.R. TCA cycle defects and cancer: When metabolism tunes redox state. In. J. Cell Biol. 2012, 2012, 161837. [Google Scholar] [CrossRef] [PubMed]
- Matés, J.M.; Segura, J.A.; Campos-Sandoval, J.A.; Lobo, C.; Alonso, L.; Alonso, F.J.; Márquez, J. Glutamine homeostasis and mitochondrial dynamics. Int. J. Biochem. Cell Biol. 2009, 41, 2051–2061. [Google Scholar] [CrossRef] [PubMed]
- Reitman, Z.J.; Jin, G.; Karoly, E.D.; Spasojevic, I.; Yang, J.; Kinzler, K.W.; He, Y.; Bigner, D.D.; Vogelstein, B.; Yan, H. Profiling the effects of isocitrate dehydrogenase 1 and 2 mutations on the cellular metabolome. Proc. Natl. Acad. Sci. USA 2011, 108, 3270–3275. [Google Scholar] [CrossRef]
- Ward, P.S.; Patel, J.; Wise, D.R.; Abdel-Wahab, O.; Bennett, B.D.; Coller, H.A.; Cross, J.R.; Fantin, V.R.; Hedvat, C.V.; Perl, A.E.; et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting α-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 2010, 17, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, M. Oncometabolites in cancer: From cancer cells to the tumor microenvironment. Holistic Integrative Oncol. 2024, 3, 26. [Google Scholar] [CrossRef]
- Selak, M.A.; Durán, R.V.; Gottlieb, E. Redox stress is not essential for the pseudo-hypoxic phenotype of succinate dehydrogenase deficient cells. Biochim, Biophys. Acta 2016, 1757, 567–572. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.S.H.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Däbritz, J.H.M.; Gottlieb, E.; Latorre, I.; et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 2016, 167, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Shestov, A.A.; Worth, A.J.; Nath, K.; Nelson, D.S.; Leeper, D.B.; Glickson, J.D.; Blair, I.A. Inhibition of mitochondrial complex II by the anticancer agent lonidamine. J. Biol. Chem. 2015, 291, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Raimundo, N.; Ahtinen, J.; Fumic, K.; Baric, I.; Remes, A.M.; Renkonen, R.; Lapatto, R.; Suomalainen, A. Differential metabolic consequences of fumarate hydratase and respiratory chain defects. Biochim. Biophys. Acta 2008, 1782, 287–294. [Google Scholar] [CrossRef]
- Zheng, L.; Cardaci, S.; Jerby, L.; MacKenzie, E.D.; Sciacovelli, M.; Johnson, T.I.; Gaude, E.; King, A.; Leach, J.D.G.; Edrada-Eberl, R.A.; et al. Fumarate induces redox-dependent senescence by modifying glutathione metabolism. Nat. Commun. 2015, 6, 6001. [Google Scholar] [CrossRef] [PubMed]
- Torti, S.V.; Torti, F.M. Iron and cancer: More ore to be mined. Nat. Rev. Cancer 2013, 13, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Nitti, M.; Piras, S.; Marinari, U.M.; Moretta, L.; Pronzato, M.A.; Furfaro, A.L. HO-1 Induction in cancer progression: A matter of cell adaptation. Antioxidants 2017, 6, 29. [Google Scholar] [CrossRef]
- Gibbs, P.E.; Miralem, T.; Maines, M.D. Biliverdin reductase: A target for cancer therapy? Front Pharmacol. 2015, 6, 119. [Google Scholar] [CrossRef] [PubMed]
- Moi, P.; Chan, K.; Asunis, I.; Cao, A.; Kan, Y.W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. USA 1994, 91, 9926–9930. [Google Scholar] [CrossRef]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role fo nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Yamamot, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 system: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and oxidative stress: A general overview of mechanisms and implications in human diseases. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef] [PubMed]
- Pillai, R.; Hayashi, M.; Zavitsanou, A.M.; Papagiannakopoulos, T. Nrf2: KEAPing tumors protected. Cancer Discov. 2022, 12, 625–643. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Ohake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef] [PubMed]
- DeNicola, G.M.; Karreth, F.A.; Humpton, T.J.; Gopinathan, A.; Wei, C.; Frese, K.; Mangak, D.; Yu, K.H.; Yeo, C.J.; Calhoun, E.S.; et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 2011, 475, 106–109. [Google Scholar] [CrossRef]
- Homma, S.; Ishii, Y.; Morishima, Y.; Yamadori, T.; Matsuno, Y.; Haraguchi, N.; Kikuchi, N.; Satoh, N.; Sakamoto, T.; Hizawa, N.; et al. Nrf2 enhances cell proliferation and resistance to anticancer drugs in human lung cancer. Clin. Cancer Res. 2009, 15, 3423–3432. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Kokubu, A.; Saito, A.; Narisawa-Saito, M.; Sasaki, H.; Aoyagi, K.; Yoshimatsu, Y.; Tachimori, Y.; Kushima, R.; Kiyono, T.; et al. NRF2 mutation confers malignant potential and resistance to chemoradiation therapy in advanced esophageal squamous cancer. Neoplasia 2011, 13, 864–873. [Google Scholar] [CrossRef]
- Kinkley, M.S.; Jeon, Y.J.; Nesselbush, M.; Moding, E.J.; Nabet, B.Y.; Almanza, D.; Kunder, C.; Stehr, H.; Yoo, C.H.; Rhee, S.; et al. KEAP1/NFE2L2 mutations predict lung cancer radiation resistance that can be targeted by glutaminase inhibition. Cancer Discov. 2020, 10, 1826–1841. [Google Scholar] [CrossRef]
- Liu, Y.; Lang, F.; Yang, C. NRF2 in human neoplasm: Cancer biology and potential therapeutic target. Pharmacol. Therapeutics 2021, 217, 107664. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, S.; Patinen, T.; Deen, A.J.; Pitkänen, S.; Härkönen, J.; Kansanen, E.; Kübleck, J.; Levonen, A.L. The KEAP1-NRF2 pathway: Target for therapy and role in cancer. Redox Biol. 2023, 63, 102726. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.W.; Wakabayashi, N. Nrf2: Friend or foe for chemoprevention? Carcinogenesis 2010, 31, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; McMahon, M.; Chowdhry, S.; Dinkova-Kostova, A.T. Cancer chemoprevention mechanisms mediated through the Keap1–Nrf2 pathway. Antioxid. Redox Signal. 2010, 13, 1713–1748. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, C.; Enriquez, C.; González, C.; Aguirre-Martínez, G.; Calderón, P.C. The multifacted roles of NRF2 in cancer: Friend or foe? Antioxidants 2024, 13, 70. [Google Scholar] [CrossRef] [PubMed]
- Sporn, M.B.; Liby, K.T. NRF2 and cancer: The good, the bad and the importance of context. Nat. Rev. Cancer 2012, 12, 564–571. [Google Scholar] [CrossRef]
- Rojo de la Vega, M.; Chapman, E.; Zhang, D.D. NRF2 and the hallmarks of cancer. Cancer Cell 2018, 34, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Pouremamali, F.; Pouremamali, A.; Dadshpour, M.; Soozangar, N. An update of Nrf2 activators and inhibitors in cancer prevention/promotion. Cell Commun. Signal. 2022, 20, 100. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Xing, S.; Wang, Z.; Zhang, A.; Wang, Q.; Bian, Y.; Pei, Y.; Sun, H.; Chen, Y. NRF2 inhibitors: Recent progress, future design and therapeutic potential. Eur. J. Med. Chem. 2024, 279, 116822. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.K.; Lee, C.S.; Young, P.; Beskow, A.; Chan, J.Y.; Deshaies, R.J. Transcription factor Nrf1 mediates the proteasome recovery pathway after proteasomal inhibition in mammalian cells. Mol. Cell 2010, 38, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, A.; Nakada, S.; Matsumoto, G.; Satoh, K.; Aketa, I.; Watanabe, A.; Hirakawa, T.; Tsujita, T.; Waku, T.; Kobayashi, A. The transcription factor NRF1 (NFE2L1) activates aggrephagy by inducing p62 and GABARAPL1 after proteasome inhibition to maintain proteostasis. Sci. Rep. 2023, 13, 14405. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Snell, A.; Dyka, F.M.; Colvin, E.R.; Ildefonso, C.; Ash, J.D.; Lobanova, E.S. Overexpression of Nfe2l1 increases proteasome activity and delays vision loss in a preclinical model of human blindness. Sci. Adv. 2023, 9, eadd5479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Y.; Zhang, K.; Hong, Z.; Liu, Z.; Liy, Z.; Li, G.; Xu, Y.; Pi, J.; Fu, J.; et al. Understanding the transcription factor NFE2L1/NRF1 from the perspective of hallmarks of cancer. Antioxidants 2024, 13, 758. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Kerins, M.J.; Tian, W.; Neupane, D.; Zhang, D.D.; Ooi, A. Differential and overlapping targets of the transcriptional regulators NRF1, NRF2, and NRF3 in human cells. J. Biol. Chem. 2019, 294, 18131–18149. [Google Scholar] [CrossRef] [PubMed]
- Sekine, H.; Motohashi, H. Unique and overlapping roles of NRF2 and NRF1 in transcriptional regulation. J. Clin. Biochem. Nutr. 2024, 74, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Ito, E.; Toki, T.; Kogame, K.; Takahashi, S.; Igarashi, K.; Hayashi, H.; Yamamoto, M. Molecular cloning and functional characterization of a new Cap’n’collar family transcription factor Nrf3. J. Biol. Chem. 1999, 274, 6443–6452. [Google Scholar] [CrossRef]
- Waku, T.; Nakamura, N.; Koji, M.; Watanabe, H.; Katoh, H.; Tatsumi, C.; Tamura, N.; Hatanaka, A.; Hirose, S.; Katayama, H.; et al. (NRF3-POMP-20S proteasome assembly Axis promotes cancer development via ubiquitin-independent proteolysis of p53 and retinoblastoma protein. Mol. Cell Biol. 2020, 40, e00597-19. [Google Scholar] [CrossRef]
- Kobayashi, A.; Waku, T. New addictions to the NRF2-related factor NRF3 in cancer cells: Ubiquitin-independent proteolysis through the 20S proteasome. Cancer Sci. 2020, 111, 6–14. [Google Scholar] [CrossRef]
- Hirose, S.; Waku, T.; Tani, M.; Masuda, H.; Endo, K.; Ashitani, S.; Aketa, I.; Kitano, H.; Nakafa, S.; Wada, A.; et al. NRF3 activates mTROC1 arginine-dependently for cancer cell viability. IScience 2023, 26, 106405. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Ryan, J.J.; Archer, S.L. The role of redox signaling in epigenetics and cardiovascular disease. Antiox. Redox Signal. 2013, 18, 1920–1936. [Google Scholar] [CrossRef] [PubMed]
- García-Guede, A.; Vera, O.; Ibañez-de-Caceres, I. When oxidative stress meets epigenetics: Implications in cancer development. Antioxidants 2020, 9, 468. [Google Scholar] [CrossRef]
- Giallongo, S.; Rehakova, D.; Raffaele, M.; Lo Re, O.; Koutna, I.; Vinciguerra, M. Redox and epigenetics in human pluripotent stem cells differentiation. Antiox. Redox Signal. 2021, 34, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Morellato, A.; Umansky, C.; Pontel, L.B. The toxic side of one-carbon metabolism and epigenetics. Redox Biol. 2021, 40, 101850. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, Z.; Qin, Y. Connections between metabolism and epigenetics: Mechanisms and novel anti-cancer strategy. Front. Pharmacol. 2022, 13, 935536. [Google Scholar] [CrossRef]
- Phull, A.; Arain, S.Q.; Majid, A.; Fatima, H.; Ahmed, M.; Kim, S.J. Oxidative stress-mediated epigenetic remodeling, metastatic progression and cell signaling in cancer. Oncologie 2024, 26, 493–507. [Google Scholar] [CrossRef]
- Plutynski, A. How is cancer complex? Eur. J. Philosophy Sci. 2021, 11, 55. [Google Scholar] [CrossRef]
- Santolini, J.; Wootton, S.A.; Jackson, A.A.; Feelisch, M. The Redox architecture of physiological function. Curr. Opinion Physiol. 2019, 9, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, M.G.; Margaritelis, N.V. Same redox evidence but different physiological “stories”: The Rashomon effect in biology. BioEssays 2018, 40, 1800041. [Google Scholar] [CrossRef]
- Kim, J.; DeBerardinis, R.J. Mechanisms and implications of metabolic heterogeneity in cancer. Cell Metab. 2019, 30, 434–446. [Google Scholar] [CrossRef]
- Fendt, S.M.; Frezza, C.; Erez, A. Targeting metabolic plasticity and flexibility dynamics for cancer therapy. Cancer Discov. 2020, 10, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Martincorena, I.; Roshan, A.; Gerstung, M.; Ellis, P.; Van Loo, P.; Mclaren, S.; Wedge, D.C.; Fullam, A.; Alexandrov, L.B.; Tubio, J.M.; et al. High burden and pervasive positive selection of somatic mutations in normal human skin. Science 2015, 348, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Martincorena, I.; Fowler, J.C.; Wabik, A.; Lawson, A.R.; Abascal, F.; Hall, M.W.; Cagan, A.; Murai, K.; Mahbubani, K.; Stratton, M.R.; et al. Somatic mutant clones colonize the human esophagus with age. Science 2018, 362, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic reprogramming and cancer progression. Science 2020, 368, eaaw5473. [Google Scholar] [CrossRef]
- Bosque, J.J.; Calvo, G.F.; Molina-García, D.; Pérez-Beteta, J.; Vicente, A.M.G.; Pérez-García, V.M. Metabolic activity grows in human cancers pushed by phenotypic variability. iScience 2023, 26, 106118. [Google Scholar] [CrossRef]
- Attal, R.; Bakkar, A.; Bouillaud, F.; Devin, A.; Henry, M.; Pontié, M.; Schwartz, L. From electrons to cancer: Redox shift as a driving force of tumorigenesis. Adv. Redox Res. 2024, 10, 100087. [Google Scholar] [CrossRef]
- Huang, J.H.; Co, H.K.; Lee, Y.C.; Wu, C.C.; Chen, S.H. Multistability maintains redox homeostasis in human cells. Mol. Syst. Biol. 2021, 17, e10480. [Google Scholar] [CrossRef] [PubMed]
- Juarrero, A. What does the closure of context-sensitive constraints mean for determinism, autonomy, self-determination, and agency? Prog. Biophys. Mol. Biol. 2015, 119, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Wulund, L.; Reddy, A.B. A brief history of circadian time: The emergence of redox oscillations as a novel component of biological rhythms. Perspect. Sci. 2015, 6, 27–37. [Google Scholar] [CrossRef]
- Milev, N.B.; Reddy, A.B. Circadian redox oscillations and metabolism. Trends Endocrinol. Metab. 2015, 26, 430–437. [Google Scholar] [CrossRef]
- Rey, G.; Valekunja, U.K.; Feeney, K.A.; Wulund, L.; Milev, N.B.; Stangherlin, A.; Ansel-Bollepalli, L.; Velagapudi, V.; O’Neill, J.S.; Reddy, A.B. The pentose phosphate pathway regulates circadian clock. Cell Metab. 2016, 24, 462–473. [Google Scholar] [CrossRef]
- Ch, R.; Rey, G.; Ray, S.; Jha, P.K.; Driscoll, P.C.; Dos Santos, M.S.; Malik, D.M.; Lach, R.; Weljie, A.M.; MacRae, J.I.; et al. Rhythmic glucose metabolism regulates redox circadian clockwork in human red blood cells. Nat. Commun. 2021, 12, 377. [Google Scholar] [CrossRef]
- Talwar, D.; Miller, C.G.; Grossmann, J.; Szyrwiel, L.; Schwecke, T.; Demichev, V.; Dick, T.P. The GADPH redox switch safeguards reductive capacity and enables survival of stressed tumour cells. Nat. Metab. 2023, 5, 660–676. [Google Scholar] [CrossRef]
- da Veiga Moreira, J.; Peres, S.; Steyaert, J.M.; Bigan, E.; Paulevé, L.; Nogueira, M.L.; Schwartz, L. Cell cycle progression is regulated by intertwined redox oscillators. Theor. Biol. Med. Model. 2015, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Dyar, K.A.; Lutter, D.; Artati, A.; Ceglia, N.J.; Liu, Y.; Armenta, D.; Sassone-Corsi, P. Atlas of circadian metabolism reveals system-wide coordination and communication between clocks. Cell 2018, 174, 1571–1585. [Google Scholar] [CrossRef] [PubMed]
- Alamoudi, A.A. Why do cancer cells break from host circadian rhythms? Insights from unicellular organisms. BioEssays 2021, 43, 2000205. [Google Scholar] [CrossRef] [PubMed]
- Omer, S.; Karunagaran, D.; Suraishkumar, G.K. The role of circadian redox rhythms in cancer hypoxia. Adv. Redox Res. 2021, 3, 100018. [Google Scholar] [CrossRef]
- Nagy, A.D.; Reddy, A.B. Redox clocks: Time to rethink redox interventions. Free Rad. Biol. Med. 2018, 119, 3–7. [Google Scholar] [CrossRef]
- Sancar, A.; Van Gelder, R.N. Clocks, cancer and chronochemotherapy. Science 2021, 371, eabb0738. [Google Scholar] [CrossRef] [PubMed]
- Kizhuveetil, U.; Omer, S.; Karunagaran, D.; Suraishkumar, G.K. Improved redox anti-cancer treatment efficacy through reactive species rhythm manipulation. Sci. Rep. 2020, 10, 1588. [Google Scholar] [CrossRef] [PubMed]
- Amiama-Roig, A.; Verdugo-Sivianes, E.M.; Carnero, A.; Blanco, J.R. Chronotherapy: Circadian rhythms and their influence in cancer therapy. Cancers 2022, 14, 5071. [Google Scholar] [CrossRef]



| Metabolic/Signaling Pathway | Enzyme 1/Metabolite | Comments |
|---|---|---|
| Aerobic glycolysis | PKM2 | Enhanced in cancer |
| Lactate | Increased production | |
| Pentose phosphate pathway | G6PD TLK | Regulated by cellular ROS through Nrf2 activation |
| Glutamine catabolism | Glutamine | TCA 2 anaplerosis |
| Malic enzyme I | Reductive TCA | |
| mTOR | Translocation to mitochondria | |
| Serine metabolism | PHGDH | Overexpressed in hypoxia |
| Proline/pyrroline-5-carboxylate cycle | In lung cancer, it supports glycolysis | |
| TCA | IDH SDH FH | Its deficiency is pro-oxidative Its deficiency is prooxidative A more reductive cell state Cell |
| Heme catabolism | HO-1 + BVR | It prevents ROS formation through the Fenton reaction |
| Nrf2 | A central regulator of cell redox |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, J.J.; Medina, M.Á. Metabolic Reprogramming at the Edge of Redox: Connections Between Metabolic Reprogramming and Cancer Redox State. Int. J. Mol. Sci. 2025, 26, 498. https://doi.org/10.3390/ijms26020498
Serrano JJ, Medina MÁ. Metabolic Reprogramming at the Edge of Redox: Connections Between Metabolic Reprogramming and Cancer Redox State. International Journal of Molecular Sciences. 2025; 26(2):498. https://doi.org/10.3390/ijms26020498
Chicago/Turabian StyleSerrano, José J., and Miguel Ángel Medina. 2025. "Metabolic Reprogramming at the Edge of Redox: Connections Between Metabolic Reprogramming and Cancer Redox State" International Journal of Molecular Sciences 26, no. 2: 498. https://doi.org/10.3390/ijms26020498
APA StyleSerrano, J. J., & Medina, M. Á. (2025). Metabolic Reprogramming at the Edge of Redox: Connections Between Metabolic Reprogramming and Cancer Redox State. International Journal of Molecular Sciences, 26(2), 498. https://doi.org/10.3390/ijms26020498






