The Etiological Role of Impaired Neurogenesis in Schizophrenia: Interactions with Inflammatory, Microbiome and Hormonal Signaling
Abstract
1. Introduction
2. Neurogenesis and Neurodevelopment
3. Immune Dysregulation in Schizophrenia
4. Gut Microbiome and Schizophrenia
5. Cortisol and the HPA Axis
6. Sex Steroids and Sexual Dimorphism
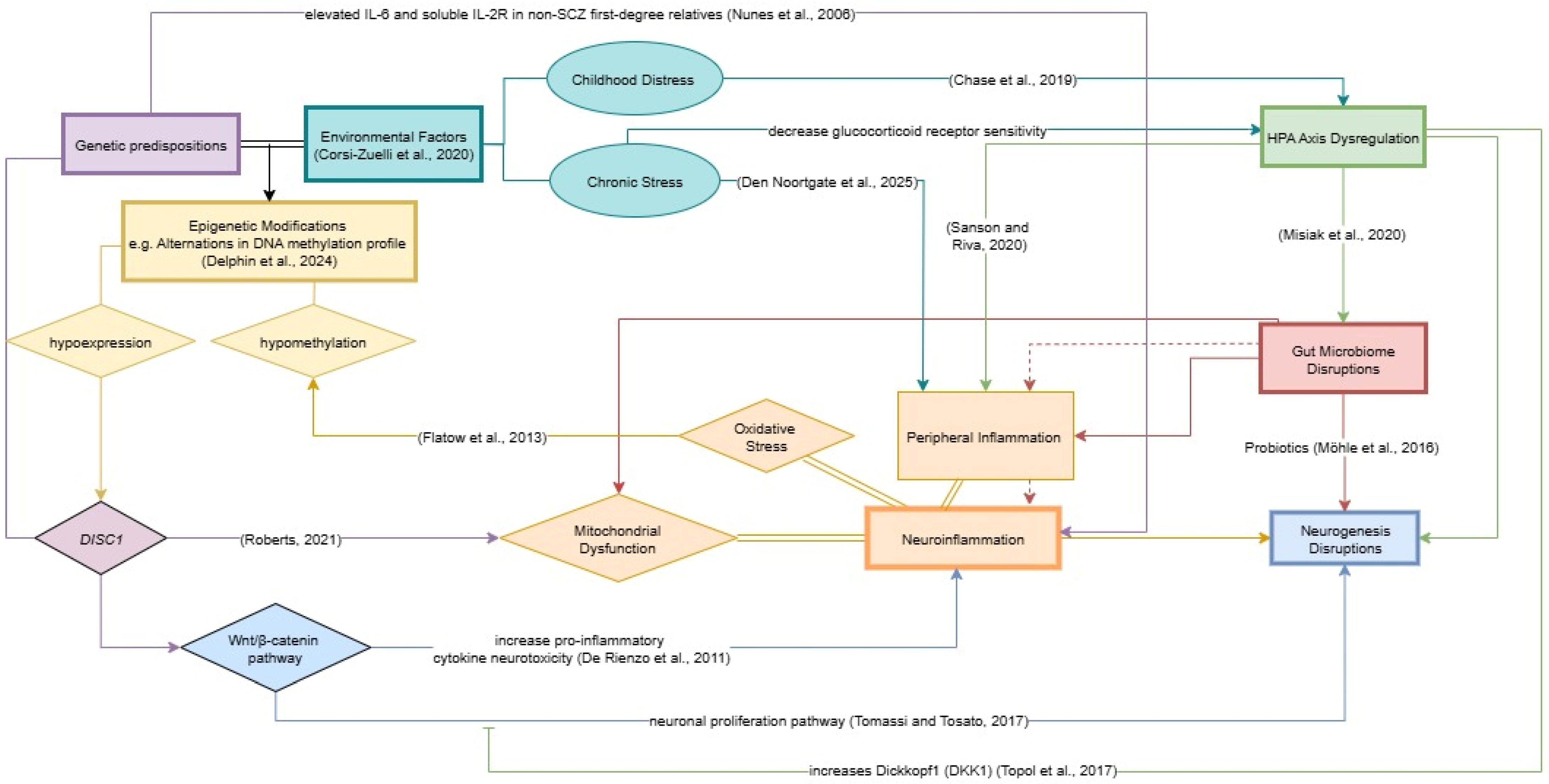
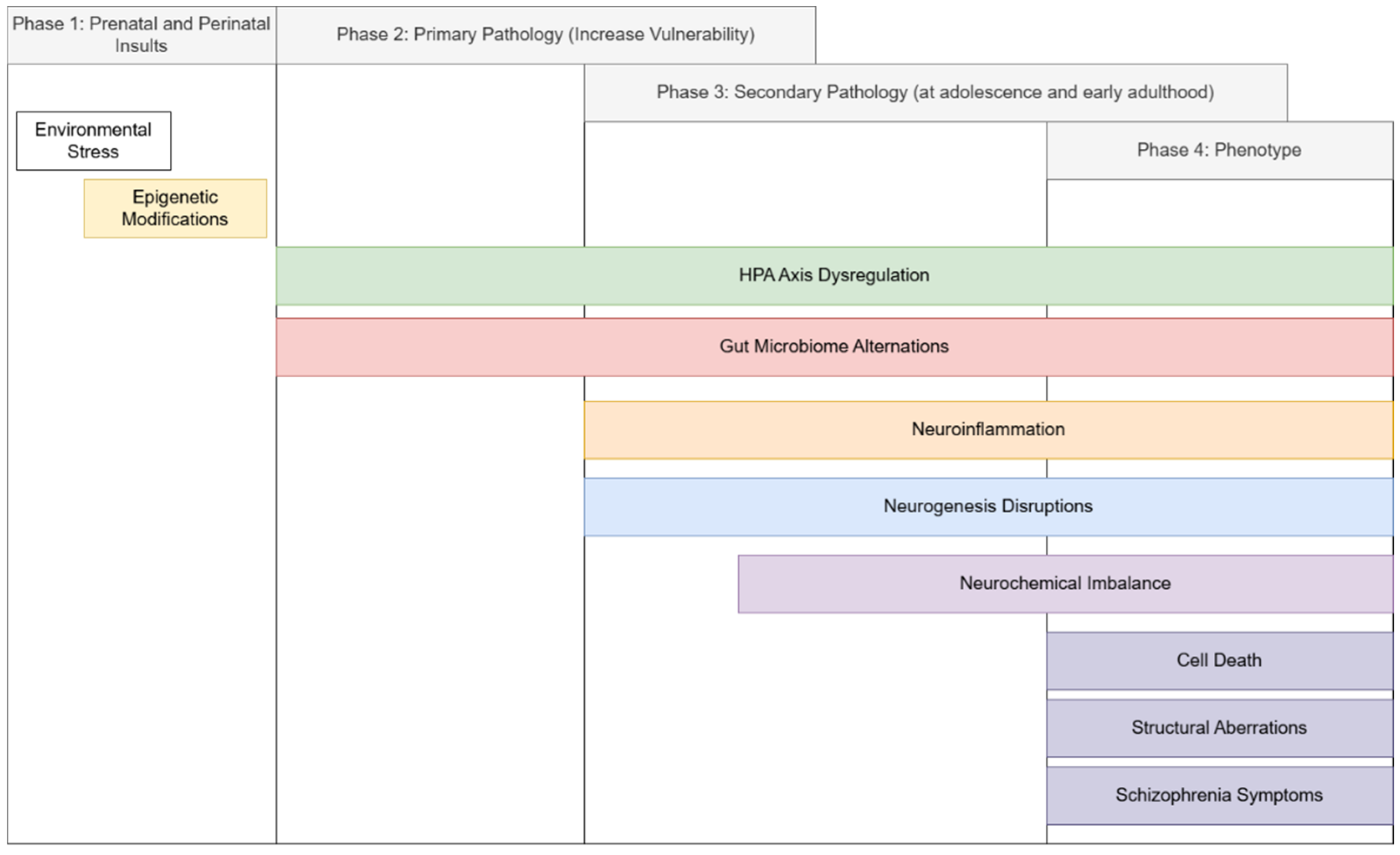
7. Hypothesis: The Neurogenesis-Inflammatory-Gut Dysbiosis Framework of Schizophrenia
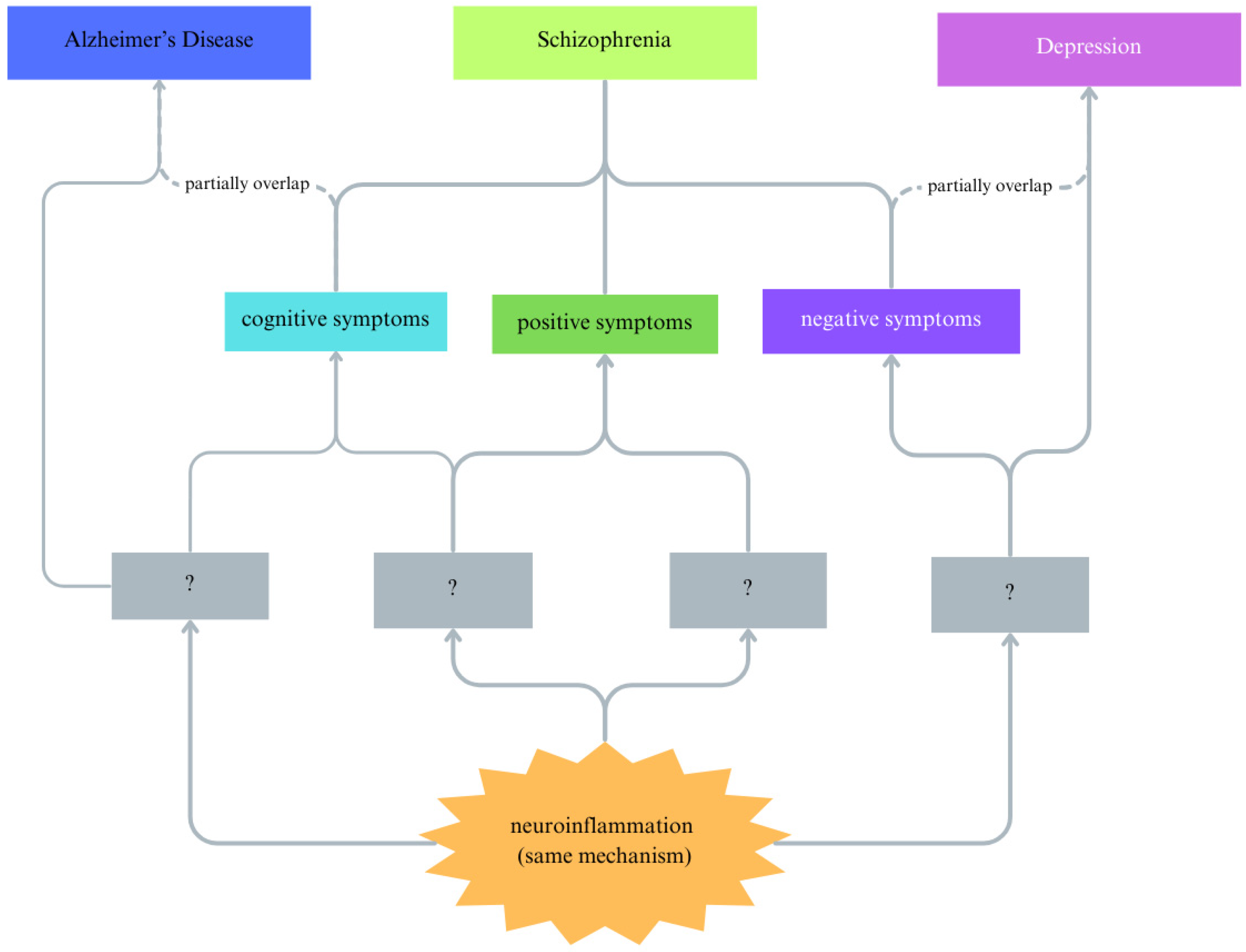
8. Future Interventions
8.1. Atypical Antipsychotics
8.2. Exogenous Stem Cells
8.3. Anti-Inflammatory Agents
| (a) | |||||
|---|---|---|---|---|---|
| Study | Study Methods | Durations | Subjects | Outcome | Interpretation |
| [162] | DB, RCT, Adjunct MC (200 mg/day) | 8 weeks | SCZ patients (n = 94) | Consistent improvement in negative symptoms in the MC group, significantly higher than that of the placebo group. | Negative symptoms |
| [152] | DB, RCT. Adjunct MC (200 mg/day) | 12 months | SCZ patients (n = 24) | Significant improvement in CGI scores of the MC group only. Significant reduction in the PANSS total score and positive, negative, and general psychopathology subscales in the MC group only. Reduced tracer intake in fronto-temporal areas when compared with control. | MC may prevent brain alterations observed in early stages of disorder. Protective against gray matter reduction. |
| [153] | DB, RCT. Adjunct MC with RIS (200 mg/day) | 8 weeks | SCZ patients (n = 35) | From baseline to week 4, no significant differences. At week 8, a significant reduction in negative symptom scores was observed. | MC can be used to treat negative symptoms. |
| [154] | DB, RCT. Adjunct MC with RIS (200 mg/day) | 8 weeks | SCZ patients (n = 38) | Time x treatment interaction for negative, general psychopathology, positive subscales, and total PANSS scores is significant. The MC group predicts negative and positive symptoms significantly. | MC is a tolerable short-term add-on for RIS. |
| [163] | DB, RCT. Adjunct MC (200 mg/day) | 6 months | SCZ patients (n = 54) | Minocycline was well tolerated, with few adverse events. It showed a beneficial effect on negative symptoms and general outcome (evident in SANS and CGI). A similar pattern was found for cognitive functioning, mainly in executive functions (working memory, cognitive shifting, and cognitive planning). | Overall, the findings support the beneficial effect of MC add-on therapy in early-phase schizophrenia. |
| [164] | DB, RCT. Adjunct MC (200 mg/day) with RIS | 16 weeks | SCZ patients (n = 63) | Significant improvements on total scores, negative subscale scores, and attention domain compared to the placebo group. Better treatment response than placebo. | Considerable negative symptom adjunct treatment |
| [165] | DB, RCT. Adjunct MC (200 mg/day for 2 weeks, then 300 mg/day for 12 months) | 12 months | SCZ patients (n = 207) | No effects on any symptom scores, gray matter volume, IL-6, or CRP levels. | MC has shown no effects on SCZ treatment. |
| [166] | DB, RCT. Adjunct MC (100 mg and 200 mg/day) with RIS | 3 months | SCZ patients (n = 57) | Significant improvement of cognitive domains (information processing speed, vigilance, working memory, verbal learning and memory, reasoning and problem solving); significant decrease in IL-1β, IL-6, and TNF-α in the high-dosage group compared with the two other groups, but not in the low-dosage group compared with the control. A decrease in IL-1β and IL-6 in the high-dosage group correlated with improvements in cognitive symptoms. | Cognitive deficits ameliorated after adjunct MC, with greater anti-inflammatory properties in high-dosage group. |
| [138] | DB, RCT. Adjunct MC (200 mg/day) with RIS | 16 weeks | SCZ patients (n = 55) | significant decreases in the SANS total score, the PANSS total score, and the PANSS negative symptoms score at week 16 compared to the placebo group. In addition, the minocycline group had a significant decrease in plasma levels of nitric oxide metabolites but no significant difference in changes in plasma levels of IL-1β or TNF-α compared to the placebo group at week 16. | The nitric oxide pathway may correlate with negative symptom improvements. |
| [170] | DB, CT. Adjunct MC (100 mg twice daily) with CLZ | 10 weeks | SCZ and Schizoaffective (n = 52) | BPRS psychosis factor, total score NS. Global cognitive function NS. Working memory improvement, avolition and the BPRS anxiety/depression factor are significant (p < 0.05). Fewer headaches and less constipation. | Cognitive subdomains, side effects, negative symptoms, but not overall. |
| [171] | DB, RCT. Adjunct MC (200 mg/day) with RIS | 16 weeks | SCZ patients (n = 55) | No improvements on body metabolism, e.g., fasting insulin, lipids, glucose, BMI, waist circumference, and body weight. | |
| (b) | |||||
| Study | Outcome | ||||
| Symptom Domains | |||||
| [138,152,154] | Total score improvement | ||||
| [152,154] | +ve symptom improvement | ||||
| [138,152,153,154,162,163,164] | −ve symptom improvement | ||||
| [170]: Avolition | Improvements in some domains of −ve symptom | ||||
| [163,166] | Cognitive symptom improvement | ||||
| [164,170] | Improvement in some domains of the cognitive domain | ||||
| Inflammatory Markers | |||||
| [166] | Significant reduction in pro-inflammatory cytokines | ||||
| [165,171] | No differences in pro-inflammatory cytokines | ||||
8.4. Exercise and Schizophrenia
9. Limitations
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-HT | 5-Hydroxytryptamine |
| ACTH | Adrenocorticosteroid Hormone |
| AD | Alzheimer’s Disease |
| AD/HD | Attention-deficit Hyperactivity Disorder |
| Akt | Protein kinase A |
| APP | Atypical Antipsychotics |
| AR | Androgen receptor |
| BBB | Blood–brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| BPRS | Brief Psychiatric Rating Scale |
| CAR | Cortisol awakening response |
| CNS | Central nervous system |
| CRH | Corticosteroid-releasing hormone |
| DA | Dopamine |
| DHEA | Dehydroepiandrosterone |
| DISC1 | Disrupted in Schizophrenia 1 |
| DKK1 | Dickkopf 1 |
| EPS | Extrapyramidal symptoms |
| ERK | Extracellular signal-regulated kinase |
| FEP | First-episode psychosis |
| hiPSC | Human-induced pluripotent stem cell |
| HPA | Hypothalamus–pituitary–adrenal |
| GABA | Γ-aminobutyric acid |
| GPER | G-protein estrogen receptor |
| GSK-3β | Glycogen-synthetic kinase 3β |
| IFN-γ | Interferon gamma |
| IGF-1 | Insulin growth factor-1 |
| IL | Interleukin |
| LTP | Long-term potentiation |
| MAPK | Mitogen-activated protein kinase |
| MD | Mitochondrial dysfunction |
| MDD | Major Depressive Disorder |
| MR | Mendelian Randomization |
| MSC | Mesenchymal stem cell |
| NF-κB | Necrosis factor kappa B |
| NMDAR | N-methyl-D-aspartate glutamatergic receptor |
| NSAID | Non-steroid anti-inflammatory drug |
| NSC | Neural stem cell |
| OS | Oxidative stress |
| PDGF | Platelet-derived growth factor |
| PKA | Protein kinase A |
| PI3K | Phosphoinositide 3-kinases |
| ROS | Reactive oxidative species |
| RNS | Reactive nitrogen species |
| SCZ | Schizophrenia |
| TAS | Total antioxidant capacity |
| TGF | Transforming Growth Factor |
| TH2 | T helper 2 cells |
| TNF-α | Tumor necrosis factor alpha |
| TrkB | Tropomyosin receptor kinase B |
| Wnt | Wingless-type MMTV integration site family |
References
- Murray, R.M.; Lewis, S.W. Is Schizophrenia a Neurodevelopmental Disorder? Br. Med. J. (Clin. Res. Ed.) 1987, 295, 681–682. [Google Scholar] [CrossRef]
- Bayer, B.; Falkai, P.; Maier, W. Genetic and Non-Genetic Vulnerability Factors in Schizophrenia: The Basis of the “Two Hit Hypothesis”. J. Psychiatr. Res. 1999, 33, 543–548. [Google Scholar] [CrossRef]
- Guerrin, C.G.J.; Doorduin, J.; Sommer, I.E.; de Vries, E.F.J. The Dual Hit Hypothesis of Schizophrenia: Evidence from Animal Models. Neurosci. Biobehav. Rev. 2021, 131, 1150–1168. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Fan, F.; Tan, S.; Huang, J.; Chen, S.; Fan, H.; Wang, Z.; Li, C.-S.R.; Tan, Y. Functional Disconnection Between Subsystems of the Default Mode Network in Schizophrenia. Psychol. Med. 2022, 52, 2270–2280. [Google Scholar] [CrossRef] [PubMed]
- Torres, U.S.; Duran, F.L.S.; Schaufelberger, M.S.; Crippa, J.A.S.; Louzã, M.R.; Sallet, P.C.; Kanegusuku, C.Y.O.; Elkis, H.; Gattaz, W.F.; Bassitt, D.P.; et al. Patterns of Regional Gray Matter Loss at Different Stages of Schizophrenia: A Multisite, Cross-Sectional VBM Study in First-Episode and Chronic Illness. Neuroimage Clin. 2016, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Hollander, P.; Raucher-Chéné, D.; Lepage, M.; Lavigne, K.M. Structural Brain Correlates of Cognitive Function in Schizophrenia: A Meta-Analysis. Neurosci. Biobehav. Rev. 2022, 132, 37–49. [Google Scholar] [CrossRef]
- Littrell, R.A.; Schneiderhan, M. The Neurobiology of Schizophrenia. Pharmacotherapy 1996, 16, 143S–147S. [Google Scholar] [CrossRef]
- Laruelle, M.; Abi-Dargham, A. Dopamine as the Wind of the Psychotic Fire: New Evidence from Brain Imaging Studies. J. Psychopharmacol. 1999, 13, 358–371. [Google Scholar] [CrossRef]
- Schmack, K.; Bosc, M.; Ott, T.; Sturgill, J.F.; Kepecs, A. Striatal Dopamine Mediates Hallucination-like Perception in Mice. Science 2021, 372, eabf4740. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Yang, B.; Anair, J.D.; Martin, M.M.; Fleps, S.W.; Pamukcu, A.; Yeh, N.-H.; Contractor, A.; Kennedy, A.; Parker, J.G. Antipsychotic Drug Efficacy Correlates with the Modulation of D1 Rather than D2 Receptor-Expressing Striatal Projection Neurons. Nat. Neurosci. 2023, 26, 1417–1428. [Google Scholar] [CrossRef]
- Liu, Y.; Ouyang, P.; Zheng, Y.; Mi, L.; Zhao, J.; Ning, Y.; Guo, W. A Selective Review of the Excitatory-Inhibitory Imbalance in Schizophrenia: Underlying Biology, Genetics, Microcircuits, and Symptoms. Front. Cell Dev. Biol. 2021, 9, 664535. [Google Scholar] [CrossRef]
- Lewis, D.A.; Hashimoto, T. Deciphering the Disease Process of Schizophrenia: The Contribution of Cortical GABA Neurons. Int. Rev. Neurobiol. 2007, 78, 109–131. [Google Scholar] [CrossRef]
- Funk, A.J.; Rumbaugh, G.; Harotunian, V.; McCullumsmith, R.E.; Meador-Woodruff, J.H. Decreased Expression of NMDA Receptor-Associated Proteins in Frontal Cortex of Elderly Patients with Schizophrenia. Neuroreport 2009, 20, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Accogli, A.; Addour-Boudrahem, N.; Srour, M. Neurogenesis, neuronal migration, and axon guidance. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 173, pp. 25–42. [Google Scholar] [CrossRef]
- Schoenfeld, T.J.; Cameron, H.A. Adult Neurogenesis and Mental Illness. Neuropsychopharmacology 2015, 40, 113–128. [Google Scholar] [CrossRef]
- Hong, S.; Yi, J.H.; Lee, S.; Park, C.-H.; Ryu, J.H.; Shin, K.S.; Kang, S.J. Defective Neurogenesis and Schizophrenia-like Behavior in PARP-1-Deficient Mice. Cell Death Dis. 2019, 10, 943. [Google Scholar] [CrossRef] [PubMed]
- Reif, A.; Fritzen, S.; Finger, M.; Strobel, A.; Lauer, M.; Schmitt, A.; Lesch, K.-P. Neural Stem Cell Proliferation Is Decreased in Schizophrenia, but Not in Depression. Mol. Psychiatry 2006, 11, 514–522. [Google Scholar] [CrossRef]
- Weissleder, C.; North, H.F.; Bitar, M.; Fullerton, J.M.; Sager, R.; Barry, G.; Piper, M.; Halliday, G.M.; Webster, M.J.; Shannon Weickert, C. Reduced Adult Neurogenesis Is Associated with Increased Macrophages in the Subependymal Zone in Schizophrenia. Mol. Psychiatry 2021, 26, 6880–6895. [Google Scholar] [CrossRef] [PubMed]
- Iannitelli, A.; Quartini, A.; Tirassa, P.; Bersani, G. Schizophrenia and Neurogenesis: A Stem Cell Approach. Neurosci. Biobehav. Rev. 2017, 80, 414–442. [Google Scholar] [CrossRef]
- Unzueta-Larrinaga, P.; Cuesta-Vega, E.; Barrena-Barbadillo, R.; Olabarrieta, E.; Recio-Barbero, M.; Horrillo, I.; Mentxaka, O.; Segarra, R.; Meana, J.J.; Nacher, J.; et al. Extracellular matrix dysfunction and synaptic alterations in schizophrenia. Mol. Psychiatry 2025. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.B.; Sun, Z.; Brundu, F.; Chen, Y.; Sun, Y.; Zhu, H.; Shprintzen, R.J.; Tomer, R.; Rabadan, R.; Leong, K.W.; et al. Aberrant pace of cortical neuron development in brain organoids from patients with 22q11.2 deletion syndrome-associated schizophrenia. Nat. Commun. 2025, 16, 6986. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Habela, C.W.; Liu, S.; Taga, A.; Oddoye, S.; Dastgheyb, R.; Haughey, N.; Bergles, D.E.; Song, H.; Ming, G.L.; Maragakis, N.J. Altered development and network connectivity in a human neuronal model of 15q11.2 deletion-related neurodevelopmental disorders. Transl. Psychiatry 2025, 15, 329. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smith, R.S.; Maes, M. The Macrophage-T-Lymphocyte Theory of Schizophrenia: Additional Evidence. Med. Hypotheses 1995, 45, 135–141. [Google Scholar] [CrossRef]
- Maes, M.; Delange, J.; Ranjan, R.; Meltzer, H.Y.; Desnyder, R.; Cooremans, W.; Scharpé, S. Acute Phase Proteins in Schizophrenia, Mania and Major Depression: Modulation by Psychotropic Drugs. Psychiatry Res. 1997, 66, 1–11. [Google Scholar] [CrossRef]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammation Response CNS: Friend or Foe? Mol. Neurobiol. 2016, 54, 8071–8089. [Google Scholar] [CrossRef]
- Goldsmith, D.R.; Rapaport, M.H.; Miller, B.J. A Meta-Analysis of Blood Cytokine Network Alterations in Psychiatric Patients: Comparisons Between Schizophrenia, Bipolar Disorder and Depression. Mol. Psychiatry 2016, 21, 1696–1709. [Google Scholar] [CrossRef]
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation Pathways: A General Review. Int. J. Neurosci. 2017, 127, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, L.; Paul, E.R.; Wåhlén, K.; Haring, L.; Vasar, E.; Vaheri, A.; Lindholm, D. Multivariate Analyses of Immune Markers Reveal Increases in Plasma EN-RAGE in First-Episode Psychosis Patients. Transl. Psychiatry 2023, 13, 326. [Google Scholar] [CrossRef] [PubMed]
- Petrikis, P.; Voulgari, P.V.; Tzallas, A.T.; Archimandriti, D.T.; Skapinakis, P.; Mavreas, V. Cytokine Profile in Drug-Naïve, First Episode Patients with Psychosis. J. Psychosom. Res. 2015, 79, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Gaughran, F.; O’Neill, E.; Sham, P.; Daly, R.J.; Shanahan, F. Soluble Interleukin 2 Receptor Levels in Families of People with Schizophrenia. Schizophr. Res. 2002, 56, 235–239. [Google Scholar] [CrossRef]
- Nunes, S.O.V.; Matsuo, T.; Kaminami, M.S.; Watanabe, M.A.E.; Reiche, E.M.V.; Itano, E.N. An Autoimmune or an Inflammatory Process in Patients with Schizophrenia, Schizoaffective Disorder, and in Their Biological Relatives. Schizophr. Res. 2006, 84, 180–182. [Google Scholar] [CrossRef]
- Weng, J.; Zhu, X.; Ouyang, Y.; Liu, Y.; Lu, H.; Yao, J.; Pan, B. Identification of Immune-Related Biomarkers of Schizophrenia in the Central Nervous System Using Bioinformatic Methods and Machine Learning Algorithms. Mol. Neurobiol. 2025, 62, 3226–3243. [Google Scholar] [CrossRef] [PubMed]
- Corsi-Zuelli, F.; Loureiro, C.M.; Shuhama, R.; Fachim, H.A.; Menezes, P.R.; Louzada-Junior, P.; Mondelli, V.; Del-Ben, C.M. Cytokine Profile in First-Episode Psychosis, Unaffected Siblings and Community-Based Controls: The Effects of Familial Liability and Childhood Maltreatment. Psychol. Med. 2020, 50, 1139–1147. [Google Scholar] [CrossRef]
- Delphin, N.; Aust, C.; Griffiths, L.; Fernandez, F. Epigenetic Regulation in Schizophrenia: Focus on Methylation and Histone Modifications in Human Studies. Genes 2024, 15, 272. [Google Scholar] [CrossRef]
- Tomassi, S.; Tosato, S. Epigenetics and Gene Expression Profile in First-Episode Psychosis: The Role of Childhood Trauma. Neurosci. Biobehav. Rev. 2017, 83, 226–237. [Google Scholar] [CrossRef]
- De Rienzo, G.; Bishop, J.A.; Mao, Y.; Pan, L.; Ma, T.P.; Moens, C.B.; Tsai, L.-H.; Sive, H. Disc1 Regulates Both β-Catenin-Mediated and Noncanonical Wnt Signaling During Vertebrate Embryogenesis. FASEB J. 2011, 25, 4184–4197. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Plaimas, K.; Suratanee, A.; Noto, C.; Kanchanatawan, B. First Episode Psychosis and Schizophrenia Are Systemic Neuro-Immune Disorders Triggered by a Biotic Stimulus in Individuals with Reduced Immune Regulation and Neuroprotection. Cells 2021, 10, 2929. [Google Scholar] [CrossRef]
- Flatow, J.; Buckley, P.; Miller, B.J. Meta-Analysis of Oxidative Stress in Schizophrenia. Biol. Psychiatry 2013, 74, 400–409. [Google Scholar] [CrossRef]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roberts, R.C. Mitochondrial dysfunction in schizophrenia: With a focus on postmortem studies. Mitochondrion 2021, 56, 91–101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ni, P.; Chung, S. Mitochondrial Dysfunction in Schizophrenia. Bioessays 2020, 42, e1900202. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, V.; Muthuramamoorthy, M.; Arasu, M.V.; Arockiaraj, J. Gut microbiome and mitochondrial crosstalk in Schizophrenia, a mental disability: Emerging mechanisms and therapeutic targets. Neurosci. Biobehav. Rev. 2025, 8, 106371. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, Q.; Liu, X. The Microbiota—Gut—Brain Axis and Neurodevelopmental Disorders. Protein Cell 2023, 14, 762–775. [Google Scholar] [CrossRef]
- Iannone, L.F.; Preda, A.; Blottière, H.M.; Clarke, G.; Albani, D.; Belcastro, V.; Carotenuto, M.; Cattaneo, A.; Citraro, R.; Ferraris, C.; et al. Microbiota-Gut Brain Axis Involvement in Neuropsychiatric Disorders. Expert Rev. Neurother. 2019, 19, 1037–1050. [Google Scholar] [CrossRef]
- McGuinness, A.J.; Davis, J.A.; Dawson, S.L.; Loughman, A.; Collier, F.; O’Hely, M.; Simpson, C.A.; Green, J.; Marx, W.; Hair, C.; et al. A Systematic Review of Gut Microbiota Composition in Observational Studies of Major Depressive Disorder, Bipolar Disorder and Schizophrenia. Mol. Psychiatry 2022, 27, 1920–1935. [Google Scholar] [CrossRef] [PubMed]
- Regenold, W.T.; Phatak, P.; Marano, C.M.; Sassan, A.; Conley, R.R.; Kling, M.A. Elevated Cerebrospinal Fluid Lactate Concentrations in Patients with Bipolar Disorder and Schizophrenia: Implications for the Mitochondrial Dysfunction Hypothesis. Biol. Psychiatry 2009, 65, 489–494. [Google Scholar] [CrossRef]
- Bicknell, B.; Liebert, A.; Borody, T.; Herkes, G.; McLachlan, C.; Kiat, H. Neurodegenerative and Neurodevelopmental Diseases and the Gut-Brain Axis: The Potential of Therapeutic Targeting of the Microbiome. Int. J. Mol. Sci. 2023, 24, 9577. [Google Scholar] [CrossRef]
- Li, S.; Song, J.; Ke, P.; Kong, L.; Lei, B.; Zhou, J.; Huang, Y.; Li, H.; Li, G.; Chen, J.; et al. The Gut Microbiome Is Associated with Brain Structure and Function in Schizophrenia. Sci. Rep. 2021, 11, 9743. [Google Scholar] [CrossRef]
- Ju, S.; Shin, Y.; Han, S.; Kwon, J.; Choi, T.G.; Kang, I.; Kim, S.S. The Gut–Brain Axis in Schizophrenia: The Implications of the Gut Microbiome and SCFA Production. Nutrients 2023, 15, 4391. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Z.; Yang, T.; Lin, F.; Ye, S.; Yan, J.; Li, T.; Chen, J. Sodium Butyrate Facilitates CRHR2 Expression to Alleviate HPA Axis Hyperactivity in Autism-like Rats Induced by Prenatal Lipopolysaccharides Through Histone Deacetylase Inhibition. mSystems 2023, 8, e0041523, Erratum in mSystems 2023, 8, e00915-23. [Google Scholar] [CrossRef] [PubMed]
- van de Wouw, M.; Boehme, M.; Lyte, J.M.; Wiley, N.; Strain, C.; O’Sullivan, O.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Short-Chain Fatty Acids: Microbial Metabolites That Alleviate Stress-Induced Brain—Gut Axis Alterations. J. Physiol. 2018, 596, 4923–4944. [Google Scholar] [CrossRef]
- Peng, H.; Ouyang, L.; Li, D.; Li, Z.; Yuan, L.; Fan, L.; Liao, A.; Li, J.; Wei, Y.; Yang, Z.; et al. Short-Chain Fatty Acids in Patients with Schizophrenia and Ultra-High Risk Population. Front. Psychiatry 2022, 13, 977538. [Google Scholar] [CrossRef]
- Lai, Z.; Shan, W.; Li, J.; Min, J.; Zeng, X.; Zuo, Z. Appropriate Exercise Level Attenuates Gut Dysbiosis and Valeric Acid Increase to Improve Neuroplasticity and Cognitive Function After Surgery in Mice. Mol. Psychiatry 2021, 26, 7167–7187. [Google Scholar] [CrossRef]
- Jayaraj, R.L.; Beiram, R.; Azimullah, S.; Mf, N.M.; Ojha, S.K.; Adem, A.; Jalal, F.Y. Valeric Acid Protects Dopaminergic Neurons by Suppressing Oxidative Stress, Neuroinflammation and Modulating Autophagy Pathways. Int. J. Mol. Sci. 2020, 21, 7670. [Google Scholar] [CrossRef]
- Celorrio, M.; Abellanas, M.A.; Rhodes, J.; Goodwin, V.; Moritz, J.; Vadivelu, S.; Wang, L.; Rodgers, R.; Xiao, S.; Anabayan, I.; et al. Gut Microbial Dysbiosis After Traumatic Brain Injury Modulates the Immune Response and Impairs Neurogenesis. Acta Neuropathol. Commun. 2021, 9, 40. [Google Scholar] [CrossRef]
- Möhle, L.; Mattei, D.; Heimesaat, M.M.; Bereswill, S.; Fischer, A.; Alutis, M.; French, T.; Hambardzumyan, D.; Matzinger, P.; Dunay, I.R.; et al. Ly6Chi Monocytes Provide a Link Between Antibiotic-Induced Changes in Gut Microbiota and Adult Hippocampal Neurogenesis. Cell Rep. 2016, 15, 1945–1956. [Google Scholar] [CrossRef]
- Williams, N.T. Probiotics. Am. J. Health Syst. Pharm. 2010, 67, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Romero-Ferreiro, V.; García-Fernández, L.; Romero, C.; De la Fuente, M.; Diaz-Del Cerro, E.; Scala, M.; González-Soltero, R.; Álvarez-Mon, M.A.; Peñuelas-Calvo, I.; Rodriguez-Jimenez, R. Impact of Probiotic Treatment on Clinical Symptom Reduction in Schizophrenia: A Systematic Review and Meta-Analysis. J. Psychiatr. Res. 2025, 182, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Romero-Ferreiro, V.; García-Fernández, L.; Biscaia, J.M.; Romero, C.; González-Soltero, R.; De la Fuente, M.; Álvarez-Mon, M.A.; Wynn, R.; Rodriguez-Jimenez, R. Effect of Probiotics on C-Reactive Protein Levels in Schizophrenia: Evidence from a Systematic Review and Meta-Analysis. Complement. Ther. Med. 2025, 89, 103126. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Du, F.; Liu, X.; Song, M.; Grosso, G.; Battino, M.; Boesch, C.; Li, H.; Liu, X. Effect of Supplementation with Probiotics in Patients with Schizophrenia: Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. Foods 2025, 14, 1773. [Google Scholar] [CrossRef]
- Brown, R.E. An Introduction to Neuroendocrinology; Cambridge University Press: Cambridge, UK, 1994; ISBN 978-0-521-42665-7. [Google Scholar]
- Ratnayake, U.; Quinn, T.; Walker, D.W.; Dickinson, H. Cytokines and the Neurodevelopmental Basis of Mental Illness. Front. Neurosci. 2013, 7, 180. [Google Scholar] [CrossRef]
- Sanson, A.; Riva, M.A. Anti-Stress Properties of Atypical Antipsychotics. Pharmaceuticals 2020, 13, 322. [Google Scholar] [CrossRef]
- Antelman, S.M.; Chiodo, L.A. Stress: Its Effect on Interactions Among Biogenic Amines and Role in the Induction and Treatment of Disease. In Drugs, Neurotransmitters, and Behavior; Iversen, L.L., Iversen, S.D., Snyder, S.H., Eds.; Springer: Boston, MA, USA, 1984; pp. 279–341. ISBN 978-1-4615-7178-0. [Google Scholar]
- Walker, E.F.; Diforio, D. Schizophrenia: A Neural Diathesis-Stress Model. Psychol. Rev. 1997, 104, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Chambers, R. Cognitive Effects of Neonatal Hippocampal Lesions in a Rat Model of Schizophrenia. Neuropsychopharmacology 1996, 15, 587–594. [Google Scholar] [CrossRef]
- Mokrani, M.C.; Duval, F.; Crocq, M.A.; Bailey, P.E.; Macher, J.P. Multihormonal Responses to Apomorphine in Mental Illness. Psychoneuroendocrinology 1995, 20, 365–375. [Google Scholar] [CrossRef]
- Anacker, C.; Cattaneo, A.; Luoni, A.; Musaelyan, K.; Zunszain, P.A.; Milanesi, E.; Rybka, J.; Berry, A.; Cirulli, F.; Thuret, S.; et al. Glucocorticoid-Related Molecular Signaling Pathways Regulating Hippocampal Neurogenesis. Neuropsychopharmacology 2013, 38, 872–883. [Google Scholar] [CrossRef]
- Fuchs, E.; Flügge, G.; Ohl, F.; Lucassen, P.; Vollmann-Honsdorf, G.K.; Michaelis, T. Psychosocial Stress, Glucocorticoids, and Structural Alterations in the Tree Shrew Hippocampus. Physiol. Behav. 2001, 73, 285–291. [Google Scholar] [CrossRef]
- Topol, A.; Zhu, S.; Tran, N.; Simone, A.; Fang, G.; Brennand, K.J. Altered WNT Signaling in Human Induced Pluripotent Stem Cell Neural Progenitor Cells Derived from Four Schizophrenia Patients. Biol. Psychiatry 2015, 78, e29–e34. [Google Scholar] [CrossRef]
- Odaka, H.; Adachi, N.; Numakawa, T. Impact of Glucocorticoid on Neurogenesis. Neural Regen. Res. 2017, 12, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Raciti, M.; Ong, J.; Weis, L.; Edoff, K.; Battagli, C.; Falk, A.; Ceccatelli, S. Glucocorticoids Alter Neuronal Differentiation of Human Neuroepithelial-like Cells by Inducing Long-Lasting Changes in the Reactive Oxygen Species Balance. Neuropharmacology 2016, 107, 422–431. [Google Scholar] [CrossRef]
- Bose, R.; Moors, M.; Tofighi, R.; Cascante, A.; Hermanson, O.; Ceccatelli, S. Glucocorticoids Induce Long-Lasting Effects in Neural Stem Cells Resulting in Senescence-Related Alterations. Cell Death Dis. 2010, 1, e92. [Google Scholar] [CrossRef] [PubMed]
- Schloesser, R.J.; Manji, H.K.; Martinowich, K. Suppression of Adult Neurogenesis Leads to an Increased HPA Axis Response. Neuroreport 2009, 20, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Pruessner, M.; Béchard-Evans, L.; Boekestyn, L.; Iyer, S.N.; Pruessner, J.C.; Malla, A.K. Attenuated Cortisol Response to Acute Psychosocial Stress in Individuals at Ultra-High Risk for Psychosis. Schizophr. Res. 2013, 146, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Eliwa, H.; Brizard, B.; Le Guisquet, A.-M.; Hen, R.; Belzung, C.; Surget, A. Adult Neurogenesis Augmentation Attenuates Anhedonia and HPA Axis Dysregulation in a Mouse Model of Chronic Stress and Depression. Psychoneuroendocrinology 2021, 124, 105097. [Google Scholar] [CrossRef]
- Culig, L.; Surget, A.; Bourdey, M.; Khemissi, W.; Le Guisquet, A.-M.; Vogel, E.; Sahay, A.; Hen, R.; Belzung, C. Increasing Adult Hippocampal Neurogenesis in Mice After Exposure to Unpredictable Chronic Mild Stress May Counteract Some of the Effects of Stress. Neuropharmacology 2017, 126, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Arinami, H.; Watanabe, Y.; Suzuki, Y.; Tajiri, M.; Tsuneyama, N.; Someya, T. Serum Cortisol and Insulin-like Growth Factor 1 Levels in Major Depressive Disorder and Schizophrenia. Sci. Rep. 2023, 13, 1148. [Google Scholar] [CrossRef]
- Belvederi Murri, M.; Pariante, C.M.; Dazzan, P.; Hepgul, N.; Papadopoulos, A.S.; Zunszain, P.; Di Forti, M.; Murray, R.M.; Mondelli, V. Hypothalamic-Pituitary-Adrenal Axis and Clinical Symptoms in First-Episode Psychosis. Psychoneuroendocrinology 2012, 37, 629–644. [Google Scholar] [CrossRef]
- Kogler, L.; Wang, R.; Luther, T.; Hofer, A.; Frajo-Apor, B.; Derntl, B. Cortisol in Schizophrenia Spectrum Disorders: A Comprehensive Meta-Analysis. Front. Neuroendocr. 2025, 77, 101186. [Google Scholar] [CrossRef]
- Pruessner, J.C.; Wolf, O.T.; Hellhammer, D.H.; Buske-Kirschbaum, A.; Von Auer, K.; Jobst, S.; Kaspers, F.; Kirschbaum, C. Free Cortisol Levels After Awakening: A Reliable Biological Marker for the Assessment of Adrenocortical Activity. Life Sci. 1997, 61, 2539–2549. [Google Scholar] [CrossRef]
- Mondelli, V.; Dazzan, P.; Hepgul, N.; Di Forti, M.; Aas, M.; D’Albenzio, A.; Di Nicola, M.; Fisher, H.; Handley, R.; Marques, T.R.; et al. Abnormal Cortisol Levels During the Day and Cortisol Awakening Response in First-Episode Psychosis: The Role of Stress and of Antipsychotic Treatment. Schizophr. Res. 2010, 116, 234–242. [Google Scholar] [CrossRef]
- Berger, M.; Kraeuter, A.K.; Romanik, D.; Malouf, P.; Amminger, G.P.; Sarnyai, Z. Cortisol Awakening Response in Patients with Psychosis: Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2016, 68, 157–166. [Google Scholar] [CrossRef]
- Gogos, A.; Sbisa, A.M.; Sun, J.; Gibbons, A.; Udawela, M.; Dean, B. A Role for Estrogen in Schizophrenia: Clinical and Preclinical Findings. Int. J. Endocrinol. 2015, 2015, 615356. [Google Scholar] [CrossRef]
- Aleman, A.; Kahn, R.S.; Selten, J.-P. Sex Differences in the Risk of Schizophrenia: Evidence from Meta-Analysis. Arch. Gen. Psychiatry 2003, 60, 565–571. [Google Scholar] [CrossRef]
- Nopoulos, P.; Flaum, M.; Andreasen, N.C. Sex Differences in Brain Morphology in Schizophrenia. Am. J. Psychiatry 1997, 154, 1648–1654. [Google Scholar] [CrossRef]
- Rao, M.L.; Kölsch, H. Effects of Estrogen on Brain Development and Neuroprotection—Implications for Negative Symptoms in Schizophrenia. Psychoneuroendocrinology 2003, 28, 83–96. [Google Scholar] [CrossRef]
- Riecher-Rössler, A.; Häfner, H.; Stumbaum, M.; Maurer, K.; Schmidt, R. Can Estradiol Modulate Schizophrenic Symptomatology? Schizophr. Bull. 1994, 20, 203–214. [Google Scholar] [CrossRef]
- Rubin, L.H.; Carter, C.S.; Drogos, L.; Pournajafi-Nazarloo, H.; Sweeney, J.A.; Maki, P.M. Peripheral Oxytocin Is Associated with Reduced Symptom Severity in Schizophrenia. Schizophr. Res. 2010, 124, 13–21. [Google Scholar] [CrossRef]
- Au, A.; Feher, A.; McPhee, L.; Jessa, A.; Oh, S.; Einstein, G. Estrogens, Inflammation and Cognition. Front. Neuroendocr. 2016, 40, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Lindamer, L.A.; Buse, D.C.; Lohr, J.B.; Jeste, D.V. Hormone Replacement Therapy in Postmenopausal Women with Schizophrenia: Positive Effect on Negative Symptoms? Biol. Psychiatry 2001, 49, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Vahdani, B.; Armani Kian, A.; Esmaeilzadeh, A.; Zenoozian, S.; Yousefi, V.; Mazloomzadeh, S. Adjunctive Raloxifene and Isradipine Improve Cognitive Functioning in Patients with Schizophrenia: A Pilot Study. J. Clin. Psychopharmacol. 2020, 40, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Veenman, L. Raloxifene as Treatment for Various Types of Brain Injuries and Neurodegenerative Diseases: A Good Start. Int. J. Mol. Sci. 2020, 21, 7586. [Google Scholar] [CrossRef]
- Toro-Urrego, N.; Luaces, J.P.; Kobiec, T.; Udovin, L.; Bordet, S.; Otero-Losada, M.; Capani, F. Raloxifene Protects Oxygen-Glucose-Deprived Astrocyte Cells Used to Mimic Hypoxic-Ischemic Brain Injury. Int. J. Mol. Sci. 2024, 25, 12121. [Google Scholar] [CrossRef]
- Hayeems, R.Z.; Seeman, M.V. Puberty and Schizophrenia Onset. In Estrogen Effects in Psychiatric Disorders; Springer: Vienna, Austria, 2005; pp. 95–106. [Google Scholar]
- Bustamante-Barrientos, F.A.; Méndez-Ruette, M.; Ortloff, A.; Luz-Crawford, P.; Rivera, F.J.; Figueroa, C.D.; Molina, L.; Bátiz, L.F. The Impact of Estrogen and Estrogen-like Molecules in Neurogenesis and Neurodegeneration: Beneficial or Harmful? Front. Cell. Neurosci. 2021, 15, 636176. [Google Scholar] [CrossRef]
- Kim, J.; Schalk, J.C.; Koss, W.A.; Gremminger, R.L.; Taxier, L.R.; Gross, K.S.; Frick, K.M. Dorsal Hippocampal Actin Polymerization Is Necessary for Activation of G-Protein-Coupled Estrogen Receptor (GPER) to Increase CA1 Dendritic Spine Density and Enhance Memory Consolidation. J. Neurosci. 2019, 39, 9598–9610. [Google Scholar] [CrossRef]
- Zhang, C.; Niu, J.-G.; Kong, X.-R.; Mi, X.-J.; Liu, Q.; Chen, F.-F.; Rong, W.-F.; Liu, J. G Protein-Coupled Estrogen Receptor 1 Deficiency Impairs Adult Hippocampal Neurogenesis in Mice with Schizophrenia. J. Chem. Neuroanat. 2023, 132, 102319. [Google Scholar] [CrossRef]
- Bazzett, T.J.; Becker, J.B. Sex Differences in the Rapid and Acute Effects of Estrogen on Striatal D2 Dopamine Receptor Binding. Brain Res. 1994, 637, 163–172. [Google Scholar] [CrossRef]
- Pasqualini, C.; Olivier, V.; Guibert, B.; Frain, O.; Leviel, V. Acute Stimulatory Effect of Estradiol on Striatal Dopamine Synthesis. J. Neurochem. 1995, 65, 1651–1657. [Google Scholar] [CrossRef]
- Vandegrift, B.J.; You, C.; Satta, R.; Brodie, M.S.; Lasek, A.W. Estradiol Increases the Sensitivity of Ventral Tegmental Area Dopamine Neurons to Dopamine and Ethanol. PLoS ONE 2017, 12, e0187698. [Google Scholar] [CrossRef]
- Misiak, B.; Frydecka, D.; Loska, O.; Moustafa, A.A.; Samochowiec, J.; Kasznia, J.; Stańczykiewicz, B. Testosterone, DHEA and DHEA-S in Patients with Schizophrenia: A Systematic Review and Meta-Analysis. Psychoneuroendocrinology 2018, 89, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Sisek-Šprem, M.; Križaj, A.; Jukić, V.; Milošević, M.; Petrović, Z.; Herceg, M. Testosterone Levels and Clinical Features of Schizophrenia with Emphasis on Negative Symptoms and Aggression. Nord. J. Psychiatry 2015, 69, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Klemenčić, A.; Herceg, M.; Herceg, D.; Puljić, K.; Sisek-Šprem, M. Influence of Serum Testosterone Level on Aggression in Women with Schizophrenia. Psychiatr. Danub. 2021, 33, 511–517. [Google Scholar] [PubMed]
- Picot, M.; Billard, J.-M.; Dombret, C.; Albac, C.; Karameh, N.; Daumas, S.; Hardin-Pouzet, H.; Mhaouty-Kodja, S. Neural Androgen Receptor Deletion Impairs the Temporal Processing of Objects and Hippocampal CA1-Dependent Mechanisms. PLoS ONE 2016, 11, e0148328. [Google Scholar] [CrossRef]
- Skucas, V.A.; Duffy, A.M.; Harte-Hargrove, L.C.; Magagna-Poveda, A.; Radman, T.; Chakraborty, G.; Schroeder, C.E.; MacLusky, N.J.; Scharfman, H.E. Testosterone Depletion in Adult Male Rats Increases Mossy Fiber Transmission, LTP, and Sprouting in Area CA3 of Hippocampus. J. Neurosci. 2013, 33, 2338–2355. [Google Scholar] [CrossRef]
- Sheng, J.A.; Bales, N.J.; Myers, S.A.; Bautista, A.I.; Roueinfar, M.; Hale, T.M.; Handa, R.J. The Hypothalamic-Pituitary-Adrenal Axis: Development, Programming Actions of Hormones, and Maternal-Fetal Interactions. Front. Behav. Neurosci. 2020, 14, 601939. [Google Scholar] [CrossRef]
- Qi, D.; Wang, W.; Chu, L.; Wu, Y.; Wang, W.; Zhu, M.; Yuan, L.; Gao, W.; Deng, H. Associations of Schizophrenia with the Activities of the HPA and HPG Axes and Their Interactions Characterized by Hair-Based Biomarkers. Psychoneuroendocrinology 2024, 165, 107049. [Google Scholar] [CrossRef]
- Brand, B.A.; de Boer, J.N.; Sommer, I.E.C. Estrogens in Schizophrenia: Progress, Current Challenges and Opportunities. Curr. Opin. Psychiatry 2021, 34, 228–237. [Google Scholar] [CrossRef]
- Handa, R.J.; Weiser, M.J. Gonadal Steroid Hormones and the Hypothalamo–Pituitary–Adrenal Axis. Front. Neuroendocr. 2014, 35, 197–220. [Google Scholar] [CrossRef] [PubMed]
- Pruessner, M.; King, S.; Vracotas, N.; Abadi, S.; Iyer, S.; Malla, A.K.; Shah, J.; Joober, R. Gender Differences in Childhood Trauma in First Episode Psychosis: Association with Symptom Severity over Two Years. Schizophr. Res. 2019, 205, 30–37. [Google Scholar] [CrossRef]
- Chase, K.A.; Melbourne, J.K.; Rosen, C.; McCarthy-Jones, S.; Jones, N.; Feiner, B.M.; Sharma, R.P. Traumagenics: At the Intersect of Childhood Trauma, Immunity and Psychosis. Psychiatry Res. 2019, 273, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Miljevic, C.; Munjiza-Jovanovic, A.; Jovanovic, T. Impact of Childhood Adversity, as Early Life Distress, on Cytokine Alterations in Schizophrenia. Neuropsychiatr. Dis. Treat. 2023, 19, 579–586. [Google Scholar] [CrossRef]
- Den Noortgate, M.V.; Morrens, M.; Foiselle, M.; De Picker, L. Immune Dysregulation in Psychiatric Disorders with and without Exposure to Childhood Maltreatment: A Transdiagnostic Stratified Meta-Analysis. Brain Behav. Immun. 2025, 127, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions Between the Microbiota, Immune and Nervous Systems in Health and Disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef]
- Müller, N.; Weidinger, E.; Leitner, B.; Schwarz, M.J. The Role of Inflammation in Schizophrenia. Front. Neurosci. 2015, 9, 372. [Google Scholar] [CrossRef]
- Misiak, B.; Łoniewski, I.; Marlicz, W.; Frydecka, D.; Szulc, A.; Rudzki, L.; Samochowiec, J. he HPA axis dysregulation in severe mental illness: Can we shift the blame to gut microbiota? Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 102, 109951. [Google Scholar] [CrossRef]
- Sochocka, M.; Donskow-Łysoniewska, K.; Diniz, B.S.; Kurpas, D.; Brzozowska, E.; Leszek, J. The Gut Microbiome Alterations and Inflammation-Driven Pathogenesis of Alzheimer’s Disease—A Critical Review. Mol. Neurobiol. 2019, 56, 1841–1851. [Google Scholar] [CrossRef]
- Caruso, G.; Grasso, M.; Fidilio, A.; Tascedda, F.; Drago, F.; Caraci, F. Antioxidant Properties of Second-Generation Antipsychotics: Focus on Microglia. Pharmaceuticals 2020, 13, 457. [Google Scholar] [CrossRef] [PubMed]
- Dakhale, G.; Khanzode, S.; Saoji, A.; Khobragade, L.; Turankar, A. Oxidative Damage and Schizophrenia: The Potential Benefit by Atypical Antipsychotics. Neuropsychobiology 2004, 49, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Monji, A.; Hashioka, S.; Kanba, S. Risperidone Significantly Inhibits Interferon-γ-Induced Microglial Activation In Vitro. Schizophr. Res. 2007, 92, 108–115. [Google Scholar] [CrossRef]
- Ono, T.; Hashimoto, E.; Ukai, W.; Ishii, T.; Saito, T. The Role of Neural Stem Cells for in Vitro Models of Schizophrenia: Neuroprotection via Akt/ERK Signal Regulation. Schizophr. Res. 2010, 122, 239–247. [Google Scholar] [CrossRef]
- Gobshtis, N.; Tfilin, M.; Fraifeld, V.E.; Turgeman, G. Transplantation of Mesenchymal Stem Cells Causes Long-Term Alleviation of Schizophrenia-like Behaviour Coupled with Increased Neurogenesis. Mol. Psychiatry 2021, 26, 4448–4463. [Google Scholar] [CrossRef] [PubMed]
- Kayir, H.; Jenkins, B.W.; Alural, B.; Khokhar, J.Y. Clozapine Increases Nestin Concentration in the Adult Male Rat Hippocampus: A Preliminary Study. Int. J. Mol. Sci. 2022, 23, 3436. [Google Scholar] [CrossRef]
- Bartzokis, G.; Lu, P.H.; Nuechterlein, K.H.; Gitlin, M.; Doi, C.; Edwards, N.; Lieu, C.; Altshuler, L.L.; Mintz, J. Differential Effects of Typical and Atypical Antipsychotics on Brain Myelination in Schizophrenia. Schizophr. Res. 2007, 93, 13–22. [Google Scholar] [CrossRef][Green Version]
- Seeman, P. Atypical Antipsychotics: Mechanism of Action. Can. J. Psychiatry 2002, 47, 27–38. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, J.; Deng, L.; Johnson, N.R.; Yu, X.; Zhang, N.; Lou, T.; Zhang, Y.; Wei, X.; Chen, Z.; et al. Intravenously Delivered Neural Stem Cells Migrate into Ischemic Brain, Differentiate and Improve Functional Recovery After Transient Ischemic Stroke in Adult Rats. Int. J. Clin. Exp. Pathol. 2015, 8, 2928–2936. [Google Scholar]
- Hou, B.; Ma, J.; Guo, X.; Ju, F.; Gao, J.; Wang, D.; Liu, J.; Li, X.; Zhang, S.; Ren, H. Exogenous Neural Stem Cells Transplantation as a Potential Therapy for Photothrombotic Ischemia Stroke in Kunming Mice Model. Mol. Neurobiol. 2017, 54, 1254–1262. [Google Scholar] [CrossRef]
- Liu, J.; Götherström, C.; Forsberg, M.; Samuelsson, E.-B.; Wu, J.; Calzarossa, C.; Hovatta, O.; Sundström, E.; Åkesson, E. Human Neural Stem/Progenitor Cells Derived from Embryonic Stem Cells and Fetal Nervous System Present Differences in Immunogenicity and Immunomodulatory Potentials In Vitro. Stem Cell Res. 2013, 10, 325–337. [Google Scholar] [CrossRef]
- Song, M.; Paul, S.; Lim, H.; Dayem, A.A.; Cho, S.-G. Induced Pluripotent Stem Cell Research: A Revolutionary Approach to Face the Challenges in Drug Screening. Arch. Pharmacal Res. 2012, 35, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wong, S.; Snyder, E.Y.; Hamblin, M.H.; Lee, J.-P. Human Neural Stem Cells Rapidly Ameliorate Symptomatic Inflammation in Early-Stage Ischemic-Reperfusion Cerebral Injury. Stem Cell Res. Ther. 2014, 5, 129. [Google Scholar] [CrossRef]
- Abeysinghe, H.C.S.; Bokhari, L.; Quigley, A.; Choolani, M.; Chan, J.; Dusting, G.J.; Crook, J.M.; Kobayashi, N.R.; Roulston, C.L. Pre-Differentiation of Human Neural Stem Cells into GABAergic Neurons Prior to Transplant Results in Greater Repopulation of the Damaged Brain and Accelerates Functional Recovery After Transient Ischemic Stroke. Stem Cell Res. Ther. 2015, 6, 186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-A.; Yuan, C.-X.; Liu, K.-F.; Yang, Q.-F.; Zhao, J.; Li, H.; Yang, Q.-H.; Song, D.; Quan, Z.-Z.; Qing, H. Neural Stem Cell Transplantation Alleviates Functional Cognitive Deficits in a Mouse Model of Tauopathy. Neural Regen. Res. 2021, 17, 152–162. [Google Scholar] [CrossRef]
- Patlola, S.R.; Donohoe, G.; McKernan, D.P. Anti-Inflammatory Effects of 2nd Generation Antipsychotics in Patients with Schizophrenia: A Systematic Review and Meta-Analysis. J. Psychiatr. Res. 2023, 160, 126–136. [Google Scholar] [CrossRef]
- Bergemann, N.; Mundt, C.; Parzer, P.; Jannakos, I.; Nagl, I.; Salbach, B.; Klinga, K.; Runnebaum, B.; Resch, F. Plasma Concentrations of Estradiol in Women Suffering from Schizophrenia Treated with Conventional versus Atypical Antipsychotics. Schizophr. Res. 2005, 73, 357–366. [Google Scholar] [CrossRef]
- Bassett, B.; Subramaniyam, S.; Fan, Y.; Varney, S.; Pan, H.; Carneiro, A.M.D.; Chung, C.Y. Minocycline Alleviates Depression-like Symptoms by Rescuing Decrease in Neurogenesis in Dorsal Hippocampus via Blocking Microglia Activation/Phagocytosis. Brain Behav. Immun. 2021, 91, 519–530. [Google Scholar] [CrossRef]
- Miao, H.; Li, R.; Han, C.; Lu, X.; Zhang, H. Minocycline Promotes Posthemorrhagic Neurogenesis via M2 Microglia Polarization via Upregulation of the TrkB/BDNF Pathway in Rats. J. Neurophysiol. 2018, 120, 1307–1317. [Google Scholar] [CrossRef]
- Mohammadi, A.; Sadighi, G.; Nazeri Astaneh, A.; Tajabadi-Ebrahimi, M.; Dejam, T. Co-Administration of Probiotic and Vitamin D Significantly Improves Cognitive Function in Schizophrenic Patients: A Double-Blinded Randomized Controlled Trial. Neuropsychopharmacol. Rep. 2024, 44, 389–398. [Google Scholar] [CrossRef]
- Ghaderi, A.; Banafshe, H.R.; Mirhosseini, N.; Moradi, M.; Karimi, M.-A.; Mehrzad, F.; Bahmani, F.; Asemi, Z. Clinical and Metabolic Response to Vitamin D plus Probiotic in Schizophrenia Patients. BMC Psychiatry 2019, 19, 77. [Google Scholar] [CrossRef]
- Peitl, V.; Silić, A.; Orlović, I.; Vidrih, B.; Crnković, D.; Karlović, D. Vitamin D and Neurotrophin Levels and Their Impact on the Symptoms of Schizophrenia. Neuropsychobiology 2020, 79, 179–185. [Google Scholar] [CrossRef]
- Dauwan, M.; Begemann, M.J.H.; Heringa, S.M.; Sommer, I.E. Exercise Improves Clinical Symptoms, Quality of Life, Global Functioning, and Depression in Schizophrenia: A Systematic Review and Meta-Analysis. Schizophr. Bull. 2016, 42, 588–599. [Google Scholar] [CrossRef]
- Firth, J.; Cotter, J.; Elliott, R.; French, P.; Yung, A.R. A Systematic Review and Meta-Analysis of Exercise Interventions in Schizophrenia Patients. Psychol. Med. 2015, 45, 1343–1361. [Google Scholar] [CrossRef]
- Rosenbaum, S.; Tiedemann, A.; Sherrington, C.; Curtis, J.; Ward, P.B. Physical Activity Interventions for People with Mental Illness: A Systematic Review and Meta-Analysis. J. Sci. Med. Sport 2014, 18, e150. [Google Scholar] [CrossRef]
- Rhodes, J.; Hosack, G.; Girard, I.; Kelley, A.; Mitchell, G.; Garland, T. Differential Sensitivity to Acute Administration of Cocaine, GBR 12909, and Fluoxetine in Mice Selectively Bred for Hyperactive Wheel-Running Behavior. Psychopharmacology 2001, 158, 120–131. [Google Scholar] [CrossRef]
- Barrientos, R.M.; Frank, M.G.; Crysdale, N.Y.; Chapman, T.R.; Ahrendsen, J.T.; Day, H.E.W.; Campeau, S.; Watkins, L.R.; Patterson, S.L.; Maier, S.F. Little Exercise, Big Effects: Reversing Aging and Infection-Induced Memory Deficits, and Underlying Processes. J. Neurosci. 2011, 31, 11578–11586. [Google Scholar] [CrossRef]
- Pajonk, F.-G.; Wobrock, T.; Gruber, O.; Scherk, H.; Berner, D.; Kaizl, I.; Kierer, A.; Müller, S.; Oest, M.; Meyer, T.; et al. Hippocampal Plasticity in Response to Exercise in Schizophrenia. Arch. Gen. Psychiatry 2010, 67, 133–143. [Google Scholar] [CrossRef]
- Damme, K.S.F.; Gupta, T.; Ristanovic, I.; Kimhy, D.; Bryan, A.D.; Mittal, V.A. Exercise Intervention in Individuals at Clinical High Risk for Psychosis: Benefits to Fitness, Symptoms, Hippocampal Volumes, and Functional Connectivity. Schizophr. Bull. 2022, 48, 1394–1405. [Google Scholar] [CrossRef]
- Yi, Y.; Song, Y.; Lu, Y. Parvalbumin Interneuron Activation-Dependent Adult Hippocampal Neurogenesis Is Required for Treadmill Running to Reverse Schizophrenia-Like Phenotypes. Front. Cell Dev. Biol. 2020, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Papiol, S.; Schmitt, A.; Maurus, I.; Rossner, M.J.; Schulze, T.G.; Falkai, P. Association Between Physical Activity and Schizophrenia: Results of a 2-Sample Mendelian Randomization Analysis. JAMA Psychiatry 2020, 78, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Chaves, C.; Marque, C.R.; Maia-de-Oliveira, J.P.; Wichert-Ana, L.; Ferrari, T.B.; Santos, A.C.; Araújo, D.; Machado-de-Sousa, J.P.; Bressan, R.A.; Elkis, H.; et al. Effects of Minocycline Add-on Treatment on Brain Morphometry and Cerebral Perfusion in Recent-Onset Schizophrenia. Schizophr. Res. 2015, 161, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Ghanizadeh, A.; Dehbozorgi, S.; OmraniSigaroodi, M.; Rezaei, Z. Minocycline as Add-on Treatment Decreases the Negative Symptoms of Schizophrenia; a Randomized Placebo-Controlled Clinical Trial. Recent Pat. Inflamm. Allergy Drug Discov. 2014, 8, 211–215. [Google Scholar] [CrossRef]
- Khodaie-Ardakani, M.-R.; Mirshafiee, O.; Farokhnia, M.; Tajdini, M.; Hosseini, S.-M.-R.; Modabbernia, A.; Rezaei, F.; Salehi, B.; Yekehtaz, H.; Ashrafi, M.; et al. Minocycline Add-on to Risperidone for Treatment of Negative Symptoms in Patients with Stable Schizophrenia: Randomized Double-Blind Placebo-Controlled Study. Psychiatry Res. 2014, 215, 540–546. [Google Scholar] [CrossRef]
- Liao, L.-Y.; Lau, B.W.-M.; Sánchez-Vidaña, D.I.; Gao, Q. Exogenous Neural Stem Cell Transplantation for Cerebral Ischemia. Neural Regen. Res. 2019, 14, 1129–1137. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, M.; Shi, L.; Yang, X.; Chen, L.; Cao, N.; Lei, A.; Cao, Y. Neural Stemness Contributes to Cell Tumorigenicity. Cell Biosci. 2021, 11, 21. [Google Scholar] [CrossRef]
- Zhang, Z.; Lei, A.; Xu, L.; Chen, L.; Chen, Y.; Zhang, X.; Gao, Y.; Yang, X.; Zhang, M.; Cao, Y. Similarity in Gene-Regulatory Networks Suggests That Cancer Cells Share Characteristics of Embryonic Neural Cells. J. Biol. Chem. 2017, 292, 12842–12859. [Google Scholar] [CrossRef]
- Mauffrey, P.; Tchitchek, N.; Barroca, V.; Bemelmans, A.-P.; Firlej, V.; Allory, Y.; Roméo, P.-H.; Magnon, C. Progenitors from the Central Nervous System Drive Neurogenesis in Cancer. Nature 2019, 569, 672–678, Correction in Nature 2020, 577, E10.. [Google Scholar] [CrossRef]
- Köhler-Forsberg, O.N.; Lydholm, C.; Hjorthøj, C.; Nordentoft, M.; Mors, O.; Benros, M.E. Efficacy of Anti-Inflammatory Treatment on Major Depressive Disorder or Depressive Symptoms: Meta-Analysis of Clinical Trials. Acta Psychiatr. Scand. 2019, 139, 404–419. [Google Scholar] [CrossRef]
- Plane, J.M.; Shen, Y.; Pleasure, D.E.; Deng, W. Prospects for Minocycline Neuroprotection. Arch. Neurol. 2010, 67, 1442–1448. [Google Scholar] [CrossRef]
- Deakin, B.; Suckling, J.; Barnes, T.R.E.; Byrne, K.; Chaudhry, I.B.; Dazzan, P.; Drake, R.J.; Giordano, A.; Husain, N.; Jones, P.B.; et al. The Benefit of Minocycline on Negative Symptoms of Schizophrenia in Patients with Recent-Onset Psychosis (BeneMin): A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Psychiatry 2018, 5, 885–894, Correction in Lancet Psychiatry 2018, 12, e28.. [Google Scholar] [CrossRef]
- Chaudhry, I.B.; Hallak, J.; Husain, N.; Minhas, F.; Stirling, J.; Richardson, P.; Dursun, S.; Dunn, G.; Deakin, B. Minocycline Benefits Negative Symptoms in Early Schizophrenia: A Randomised Double-Blind Placebo-Controlled Clinical Trial in Patients on Standard Treatment. J. Psychopharmacol. 2012, 26, 1185–1193. [Google Scholar] [CrossRef]
- Levkovitz, Y.; Mendlovich, S.; Riwkes, S.; Braw, Y.; Levkovitch-Verbin, H.; Gal, G.; Fennig, S.; Treves, I.; Kron, S. A Double-Blind, Randomized Study of Minocycline for the Treatment of Negative and Cognitive Symptoms in Early-Phase Schizophrenia. J. Clin. Psychiatry 2010, 71, 138–149. [Google Scholar] [CrossRef]
- Liu, F.; Guo, X.; Wu, R.; Ou, J.; Zheng, Y.; Zhang, B.; Xie, L.; Zhang, L.; Yang, L.; Yang, S.; et al. Minocycline Supplementation for Treatment of Negative Symptoms in Early-Phase Schizophrenia: A Double Blind, Randomized, Controlled Trial. Schizophr. Res. 2014, 153, 169–176. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, H.; Wu, R.; Kosten, T.R.; Zhang, X.-Y.; Zhao, J. The Effect of Minocycline on Amelioration of Cognitive Deficits and Pro-Inflammatory Cytokines Levels in Patients with Schizophrenia. Schizophr. Res. 2019, 212, 92–98. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, B.; Xie, L.; Ruan, Y.; Xu, X.; Zeng, Y.; Messina, L.; Zhao, J.; Fan, X. Changes in Plasma Levels of Nitric Oxide Metabolites and Negative Symptoms After 16-Week Minocycline Treatment in Patients with Schizophrenia. Schizophr. Res. 2018, 199, 390–394. [Google Scholar] [CrossRef]
- Kalejahi, P.; Kheirouri, S.; Noorazar, S.G. A Randomized Controlled Trial of Vitamin D Supplementation in Iranian Patients with Schizophrenia: Effects on Serum Levels of Glycogen Synthase Kinase-3β and Symptom Severity. Int. J. Psychiatry Med. 2023, 58, 559–575. [Google Scholar] [CrossRef]
- Krivoy, A.; Onn, R.; Vilner, Y.; Hochman, E.; Weizman, S.; Paz, A.; Hess, S.; Sagy, R.; Kimhi-Nesher, S.; Kalter, E.; et al. Vitamin D Supplementation in Chronic Schizophrenia Patients Treated with Clozapine: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. BioMedicine 2017, 26, 138–145. [Google Scholar] [CrossRef]
- Mohammadzadeh Honarvar, N.; Samadi, M.; Seyedi Chimeh, M.; Gholami, F.; Bahrampour, N.; Jalali, M.; Effatpanah, M.; Yekaninejad, M.S.; Abdolahi, M.; Chamari, M. Effect of Vitamin D on Paraxonase-1, Total Antioxidant Capacity, and 8-Isoprostan in Children with Attention Deficit Hyperactivity Disorder. Int. J. Clin. Pr. 2022, 2022, 4836731. [Google Scholar] [CrossRef]
- Kelly, D.L.; Sullivan, K.M.; McEvoy, J.P.; McMahon, R.P.; Wehring, H.J.; Gold, J.M.; Liu, F.; Warfel, D.; Vyas, G.; Richardson, C.M.; et al. Adjunctive Minocycline in Clozapine-Treated Schizophrenia Patients with Persistent Symptoms. J. Clin. Psychopharmacol. 2015, 35, 374–381. [Google Scholar] [CrossRef]
- Liu, F.; Xie, L.; Zhang, B.; Ruan, Y.; Zeng, Y.; Xu, X.; Zhao, J.; Fan, X. No Effect of Adjunctive Minocycline Treatment on Body Metabolism in Patients with Schizophrenia. J. Clin. Psychopharmacol. 2018, 38, 125–128, Erratum in J. Clin. Psychopharmacol. 2018, 38, 128.. [Google Scholar] [CrossRef]
- Van Praag, H. Neurogenesis and Exercise: Past and Future Directions. Neuromol. Med. 2008, 10, 128–140. [Google Scholar] [CrossRef]
- Molteni, R.; Ying, Z.; Gómez-Pinilla, F. Differential Effects of Acute and Chronic Exercise on Plasticity-Related Genes in the Rat Hippocampus Revealed by Microarray. Eur. J. Neurosci. 2002, 16, 1107–1116. [Google Scholar] [CrossRef]
- Rhodes, J.S.; van Praag, H.; Jeffrey, S.; Girard, I.; Mitchell, G.S.; Garland, T.; Gage, F.H. Exercise Increases Hippocampal Neurogenesis to High Levels but Does Not Improve Spatial Learning in Mice Bred for Increased Voluntary Wheel Running. Behav. Neurosci. 2003, 117, 1006–1016. [Google Scholar] [CrossRef]
- Dean, D.J.; Bryan, A.D.; Newberry, R.; Gupta, T.; Carol, E.; Mittal, V.A. A Supervised Exercise Intervention for Youth at Risk for Psychosis: An Open-Label Pilot Study. J. Clin. Psychiatry 2017, 78, e1167–e1173. [Google Scholar] [CrossRef]
- Mahalakshmi, B.; Maurya, N.; Lee, S.-D.; Bharath Kumar, V. Possible Neuroprotective Mechanisms of Physical Exercise in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 5895. [Google Scholar] [CrossRef]
- Svensson, M.; Lexell, J.; Deierborg, T. Effects of Physical Exercise on Neuroinflammation, Neuroplasticity, Neurodegeneration, and Behavior: What We Can Learn from Animal Models in Clinical Settings. Neurorehabil. Neural Repair 2015, 29, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, S.; Dohm-Hansen, S.; Lavelle, A.; Bastiaanssen, T.F.S.; English, J.A.; Cryan, J.F.; Nolan, Y.M. Exercise Mitigates a Gut Microbiota-Mediated Reduction in Adult Hippocampal Neurogenesis and Associated Behaviours in Rats. Transl. Psychiatry 2024, 14, 195. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s Disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [PubMed]
| Study | Conclusion | Studies Included |
|---|---|---|
| [47] | No significant differences post-intervention in overall, positive, or negative symptoms | 3 |
| [60] | Reduction in PANSS total score (SMD: −0.608, p = 0.035) | 5 |
| [61] | Reduction in CRP levels (SMD: −0.46, p = 0.001) | 4 |
| [62] | Reduction in total, general, and negative scores | 6 |
| Phase 2: Primary Pathology | Phase 3: Secondary Pathology | Phase 4: Phenotype | ||||
|---|---|---|---|---|---|---|
| Interventions | Primary Target | OS and Neuroinflam | HPA Axis | Neurogenesis | Neurochemistry | Symptomatic Improvements |
| Atypical Antipsychotics | Neurochemistry | ↓ oxidative products [122]; ↓ nitric oxide, IL-1β, IL-6, and TNF-α [123] | ↓ basal cortisol [65] | ↑ survival [124]; ↑ BDNF expression [125]; ↑ newborn neurons [126]; ↑ 17β-estradiol [127] | D2R and 5-HT 2AR antagonist [128] | −ve symptoms [128] |
| Exogenous NSCs | Neurogenesis [129,130,131,132,133] | ↓ symptomatic inflammation [133] | / | ↑ Akt; ↑ survival [124]; ↑ proliferation [134] | / | spatial working memory [135] |
| Exogenous MSCs | ↓ IL-1β; ↑ IL-17 [133] | ↑ hippocampal neurogenesis [125] | / | social and cognitive symptoms [125] | ||
| Minocycline, adjunct (NSAID) | Neuroinflam | ↓ IL-1β, IL-6 and TNF-α, nitric oxide metabolites (mixed results) [124,125,126,127,136,137]; ↑ M2 microglia [138,139] | / | ↑ neurogenesis; ↑ BDNF [138,139] | / | −ve symptoms, depressive behaviours [124,125,126,127,136,137] |
| Vitamin D | Neuroinflam | ↓ CRP [140]; ↓ lipid peroxidation [141]; ↑ TAS [141] | / | / | / | Cognitive symptoms [140], +ve symptoms [142] |
| Exercise | Overall neuroprotective | ↓ free radicals [143]; + TH2 profile [143]; ↑ M2 microglia [144] | / | ↑ proliferation [145]; ↑ survival [145]; ↑ BDNF [146]; ↑ dendritic density and morphology [147]; ↑ connectivity; ↑ maturation of newborn neurons [146] | ↑ glutamatergic [148] | Episodic memory [149], recognition accuracy [149], spatial learning [150], working memory [149], long-term memory [151]; +ve symptoms; −ve symptoms; general symptoms [140,152,153,154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
So, M.T.-W.; Ullah, A.; Waris, A.; Alhumaydhi, F.A. The Etiological Role of Impaired Neurogenesis in Schizophrenia: Interactions with Inflammatory, Microbiome and Hormonal Signaling. Int. J. Mol. Sci. 2025, 26, 9814. https://doi.org/10.3390/ijms26199814
So MT-W, Ullah A, Waris A, Alhumaydhi FA. The Etiological Role of Impaired Neurogenesis in Schizophrenia: Interactions with Inflammatory, Microbiome and Hormonal Signaling. International Journal of Molecular Sciences. 2025; 26(19):9814. https://doi.org/10.3390/ijms26199814
Chicago/Turabian StyleSo, Miu Tsz-Wai, Ata Ullah, Abdul Waris, and Fahad A. Alhumaydhi. 2025. "The Etiological Role of Impaired Neurogenesis in Schizophrenia: Interactions with Inflammatory, Microbiome and Hormonal Signaling" International Journal of Molecular Sciences 26, no. 19: 9814. https://doi.org/10.3390/ijms26199814
APA StyleSo, M. T.-W., Ullah, A., Waris, A., & Alhumaydhi, F. A. (2025). The Etiological Role of Impaired Neurogenesis in Schizophrenia: Interactions with Inflammatory, Microbiome and Hormonal Signaling. International Journal of Molecular Sciences, 26(19), 9814. https://doi.org/10.3390/ijms26199814







