Unravelling the Viral Hypothesis of Schizophrenia: A Comprehensive Review of Mechanisms and Evidence
Abstract
1. Introduction
2. Herpes Simplex Virus Types 1 and 2 (HSV-1 and HSV-2)
3. Varicella-Zoster Virus (VZV)
4. Epstein–Barr Virus (EBV)
5. Cytomegalovirus (CMV)
6. Human Herpesvirus-6 (HHV-6)
7. Human Herpesvirus-8 (HHV-8)
8. Influenza Virus
9. Hepatitis B and C Viruses (HBV and HCV)
10. Human Immunodeficiency Virus (HIV)
11. Human Endogenous Retroviruses (HERVs)
12. Zika Virus (ZIKV)
13. Borna Disease Virus (BoDV)
14. Human Coronavirus Infection and SARS-CoV-2 (COVID-19)
15. Other Viruses Associated with Schizophrenia
16. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wahbeh, M.H.; Avramopoulos, D. Gene-Environment Interactions in Schizophrenia: A Literature Review. Genes 2021, 12, 1850. [Google Scholar] [CrossRef] [PubMed]
- Fellerhoff, B.; Laumbacher, B.; Mueller, N.; Gu, S.; Wank, R. Associations between Chlamydophila infections, schizophrenia and risk of HLA-A10. Mol. Psychiatry 2007, 12, 264–272. [Google Scholar] [CrossRef]
- Anderson, G.; Maes, M. Schizophrenia: Linking Prenatal Infection to Cytokines, the Tryptophan Catabolite (TRYCAT) Pathway, NMDA Receptor Hypofunction, Neurodevelopment and Neuroprogression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 42, 5–19. [Google Scholar] [CrossRef]
- Jmii, H.; Fisson, S.; Aouni, M.; Jaidane, H. Type B Coxsackieviruses and Central Nervous System Disorders: Critical Review of Reported Associations. Rev. Med. Virol. 2021, 31, e2191. [Google Scholar] [CrossRef]
- Rantala, M.J.; Luoto, S.; Borráz-León, J.I.; Krams, I. Schizophrenia: The New Etiological Synthesis. Neurosci. Biobehav. Rev. 2022, 142, 104894. [Google Scholar] [CrossRef]
- Severance, E.G.; Dickerson, F.B.; Viscidi, R.P.; Bossis, I.; Stallings, C.R.; Origoni, A.E.; Sullens, A.; Yolken, R.H. Coronavirus Immunoreactivity in Individuals with a Recent Onset of Psychotic Symptoms. Schizophr. Bull. 2011, 37, 101–107. [Google Scholar] [CrossRef]
- Pearce, B.D. Modeling the Role of Infections in the Etiology of Mental Illness. Clin. Neurosci. Res. 2003, 3, 271–282. [Google Scholar] [CrossRef]
- Anders, S.; Kinney, D.K. Abnormal Immune System Development and Function in Schizophrenia Helps Reconcile Diverse Findings and Suggests New Treatment and Prevention Strategies. Brain Res. 2015, 1617, 93–112. [Google Scholar] [CrossRef] [PubMed]
- Jansen van Vuren, E.; Steyn, S.F.; Brink, C.B.; Möller, M.; Viljoen, F.P.; Harvey, B.H. The Neuropsychiatric Manifestations of COVID-19: Interactions with Psychiatric Illness and Pharmacological Treatment. Biomed. Pharmacother. 2021, 135, 111200. [Google Scholar] [CrossRef]
- Meyer, U.; Feldon, J. Neural Basis of Psychosis-Related Behaviour in the Infection Model of Schizophrenia. Behav. Brain Res. 2009, 204, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Canuti, M.; van Beveren, N.J.; Farsani, S.M.J.; de Vries, M.; Deijs, M.; Jebbink, M.F.; Zaaijer, H.L.; van Schaik, B.D.; van Kampen, A.H.; van der Kuyl, A.C.; et al. Viral Metagenomics in Drug-Naïve, First-Onset Schizophrenia Patients with Prominent Negative Symptoms. Psychiatry Res. 2015, 229, 678–684. [Google Scholar] [CrossRef]
- Davis, J.; Eyre, H.; Jacka, F.N.; Dodd, S.; Dean, O.; McEwen, S.; Debnath, M.; McGrath, J.; Maes, M.; Amminger, P.; et al. A Review of Vulnerability and Risks for Schizophrenia: Beyond the Two Hit Hypothesis. Neurosci. Biobehav. Rev. 2016, 65, 185–194. [Google Scholar] [CrossRef]
- Anacker, C.; Cattaneo, A.; Luoni, A.; Musaelyan, K.; Zunszain, P.A.; Milanesi, E.; Rybka, J.; Berry, A.; Cirulli, F.; Thuret, S.; et al. Glucocorticoid-Related Molecular Signaling Pathways Regulating Hippocampal Neurogenesis. Neuropsychopharmacology 2013, 38, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Sanders, V.J.; Felisan, S.; Waddell, A.; Tourtellotte, W.W. Detection of Herpesviridae in Postmortem Multiple Sclerosis Brain Tissue and Controls by Polymerase Chain Reaction. J. Neurovirol. 1996, 2, 249–258. [Google Scholar] [CrossRef]
- Labouesse, M.A.; Langhans, W.; Meyer, U. Long-Term Pathological Consequences of Prenatal Infection: Beyond Brain Disorders. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R1–R12. [Google Scholar] [CrossRef] [PubMed]
- Yolken, R.H.; Torrey, E.F. Are Some Cases of Psychosis Caused by Microbial Agents? A Review of the Evidence. Mol. Psychiatry 2008, 13, 470–479. [Google Scholar] [CrossRef]
- Moises, H.W.; Rüger, R.; Reynolds, G.P.; Fleckenstein, B. Human Cytomegalovirus DNA in the Temporal Cortex of a Schizophrenic Patient. Eur. Arch. Psychiatry Neurol. Sci. 1988, 238, 110–113. [Google Scholar] [CrossRef]
- Nunes, S.O.; Itano, E.N.; Amarante, M.K.; Reiche, E.M.; Miranda, H.C.; de Oliveira, C.E.; Matsuo, T.; Vargas, H.O.; Watanabe, M.A. RNA from Borna Disease Virus in Patients with Schizophrenia, Schizoaffective Patients, and in Their Biological Relatives. J. Clin. Lab. Anal. 2008, 22, 314–320. [Google Scholar] [CrossRef]
- Faustmann, T.J.; Kamp, D.; Räuber, S.; Dukart, J.; Melzer, N.; Schilbach, L. Social Interaction, Psychotic Disorders and Inflammation: A Triangle of Interest. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 122, 110697. [Google Scholar] [CrossRef]
- Pollak, T.A.; Drndarski, S.; Stone, J.M.; David, A.S.; McGuire, P.; Abbott, N.J. The Blood-Brain Barrier in Psychosis. Lancet Psychiatry 2018, 5, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.H. Potential Microbial Origins of Schizophrenia and Their Treatments. Drugs Today 2009, 45, 305–314. [Google Scholar] [CrossRef][Green Version]
- Ratnayake, U.; Quinn, T.A.; Castillo-Melendez, M.; Dickinson, H.; Walker, D.W. Behaviour and Hippocampus-Specific Changes in Spiny Mouse Neonates after Treatment of the Mother with the Viral-Mimetic Poly I:C at Mid-Pregnancy. Brain Behav. Immun. 2012, 26, 1288–1299. [Google Scholar] [CrossRef]
- Lukasz, B.; O’Sullivan, N.C.; Loscher, J.S.; Pickering, M.; Regan, C.M.; Murphy, K.J. Peripubertal viral-like challenge and social isolation mediate overlapping but distinct effects on behaviour and brain interferon regulatory factor 7 expression in the adult Wistar rat. Brain Behav. Immun. 2013, 27, 71–80. [Google Scholar] [CrossRef]
- Nudel, R.; Wang, Y.; Appadurai, V.; Schork, A.J.; Buil, A.; Agerbo, E.; Bybjerg-Grauholm, J.; Børglum, A.D.; Daly, M.J.; Mors, O.; et al. A large-scale genomic investigation of susceptibility to infection and its association with mental disorders in the Danish population. Transl. Psychiatry 2019, 9, 283. [Google Scholar] [CrossRef]
- Carter, C.J. Schizophrenia susceptibility genes directly implicated in the life cycles of pathogens: Cytomegalovirus, influenza, herpes simplex, rubella, and Toxoplasma gondii. Schizophr. Bull. 2009, 35, 1163–1182. [Google Scholar] [CrossRef] [PubMed]
- Deverman, B.E.; Patterson, P.H. Cytokines and CNS development. Neuron 2009, 64, 61–78. [Google Scholar] [CrossRef]
- Asor, E.; Ben-Shachar, D. Platelets: A possible glance into brain biological processes in schizophrenia. World J. Psychiatry 2012, 2, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Benros, M.E.; Mortensen, P.B.; Eaton, W.W. Autoimmune diseases and infections as risk factors for schizophrenia. Ann. N. Y. Acad. Sci. 2012, 1262, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Tomasik, J.; Rahmoune, H.; Guest, P.C.; Bahn, S. Neuroimmune biomarkers in schizophrenia. Schizophr. Res. 2016, 176, 3–13. [Google Scholar] [CrossRef]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511, 421–427. [Google Scholar] [CrossRef]
- Romeo, B.; Brunet-Lecomte, M.; Martelli, C.; Benyamina, A. Kinetics of cytokine levels during antipsychotic treatment in schizophrenia: A meta-analysis. Int. J. Neuropsychopharmacol. 2018, 21, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef]
- Canetta, S.; Sourander, A.; Surcel, H.M.; Hinkka-Yli-Salomäki, S.; Leiviskä, J.; Kellendonk, C.; McKeague, I.W.; Brown, A.S. Elevated maternal C-reactive protein and increased risk of schizophrenia in a national birth cohort. Am. J. Psychiatry 2014, 171, 960–968. [Google Scholar] [CrossRef]
- Bechter, K. Updating the mild encephalitis hypothesis of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 42, 71–91. [Google Scholar] [CrossRef]
- Sommer, I.E.; de Witte, L.; Begemann, M.; Kahn, R.S. Nonsteroidal anti-inflammatory drugs in schizophrenia: Ready for practice or a good start? A meta-analysis. J. Clin. Psychiatry 2012, 73, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, B.; Heiber, G.; Torres-González, F.; Cervilla, J.A. The role of microbial agents in the etiology of schizophrenia: An infectious hypothesis for psychosis? Curr. Immunol. Rev. 2011, 7, 57–63. [Google Scholar] [CrossRef]
- Prasad, K.M.; Shirts, B.H.; Yolken, R.H.; Keshavan, M.S.; Nimgaonkar, V.L. Brain morphological changes associated with exposure to HSV1 in first-episode schizophrenia. Mol. Psychiatry 2007, 12, 105–113. [Google Scholar] [CrossRef]
- Brown, A.S.; Schaefer, C.A.; Quesenberry, C.P., Jr.; Shen, L.; Susser, E.S. No evidence of relation between maternal exposure to herpes simplex virus type 2 and risk of schizophrenia? Am. J. Psychiatry 2006, 163, 2178–2180. [Google Scholar] [CrossRef]
- Carter, C.J. Susceptibility genes are enriched in those of the herpes simplex virus 1/host interactome in psychiatric and neurological disorders. Pathog. Dis. 2013, 69, 240–261. [Google Scholar] [CrossRef]
- Demontis, D.; Nyegaard, M.; Buttenschøn, H.N.; Hedemand, A.; Pedersen, C.B.; Grove, J.; Flint, T.J.; Nordentoft, M.; Werge, T.; Hougaard, D.M.; et al. Association of GRIN1 and GRIN2A-D with schizophrenia and genetic interaction with maternal herpes simplex virus-2 infection affecting disease risk. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011, 156B, 913–922. [Google Scholar] [CrossRef]
- Jonker, I.; Doorduin, J.; Knegtering, H.; Van’t Hag, E.; Dierckx, R.A.; de Vries, E.F.J.; Schoevers, R.A.; Klein, H.C. Antiviral treatment in schizophrenia: A randomized pilot PET study on the effects of valaciclovir on neuroinflammation. Psychol. Med. 2023, 53, 7087–7095. [Google Scholar] [CrossRef]
- Cheslack-Postava, K.; Brown, A.S.; Chudal, R.; Suominen, A.; Huttunen, J.; Surcel, H.M.; Sourander, A. Maternal exposure to sexually transmitted infections and schizophrenia among offspring. Schizophr. Res. 2015, 166, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Desforges, M.; Le Coupanec, A.; Dubeau, P.; Bourgouin, A.; Lajoie, L.; Dubé, M.; Talbot, P.J. Human Coronaviruses and Other Respiratory Viruses: Underestimated Opportunistic Pathogens of the Central Nervous System? Viruses 2019, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.M.; Eack, S.M.; Goradia, D.; Pancholi, K.M.; Keshavan, M.S.; Yolken, R.H.; Nimgaonkar, V.L. Progressive gray matter loss and changes in cognitive functioning associated with exposure to herpes simplex virus 1 in schizophrenia: A longitudinal study. Am. J. Psychiatry 2011, 168, 822–830. [Google Scholar] [CrossRef]
- Mohagheghi, M.; Eftekharian, M.M.; Taheri, M.; Alikhani, M.Y. Determining the IgM and IgG antibodies titer against HSV1, HSV2 and CMV in the serum of schizophrenia patients. Hum. Antibodies 2018, 26, 87–93. [Google Scholar] [CrossRef]
- Bolu, A.; Oznur, T.; Tok, D.; Balikci, A.; Sener, K.; Celik, C.; Gulsun, M. Seropositivity of neurotropic infectious agents in first-episode schizophrenia patients and the relationship with positive and negative symptoms. Psychiatr. Danub. 2016, 28, 132–138. [Google Scholar] [PubMed]
- Yong, S.J.; Yong, M.H.; Teoh, S.L.; Soga, T.; Parhar, I.; Chew, J.; Lim, W.L. The Hippocampal Vulnerability to Herpes Simplex Virus Type I Infection: Relevance to Alzheimer’s Disease and Memory Impairment. Front. Cell. Neurosci. 2021, 15, 695738. [Google Scholar] [CrossRef]
- Breier, A.; Buchanan, R.W.; D’Souza, D.; Nuechterlein, K.; Marder, S.; Dunn, W.; Preskorn, S.; Macaluso, M.; Wurfel, B.; Maguire, G.; et al. Herpes simplex virus 1 infection and valacyclovir treatment in schizophrenia: Results from the VISTA study. Schizophr. Res. 2019, 206, 291–299. [Google Scholar] [CrossRef]
- Mortensen, P.B.; Pedersen, C.B.; Hougaard, D.M.; Nørgaard-Petersen, B.; Mors, O.; Børglum, A.D.; Yolken, R.H. A Danish National Birth Cohort study of maternal HSV-2 antibodies as a risk factor for schizophrenia in their offspring. Schizophr. Res. 2010, 122, 257–263. [Google Scholar] [CrossRef]
- Margolis, T.P.; Imai, Y.; Yang, L.; Vallas, V.; Krause, P.R. Herpes simplex virus type 2 (HSV-2) establishes latent infection in a different population of ganglionic neurons than HSV-1: Role of latency-associated transcripts. J. Virol. 2007, 81, 1872–1878. [Google Scholar] [CrossRef]
- Buka, S.L.; Tsuang, M.T.; Torrey, E.F.; Klebanoff, M.A.; Bernstein, D.; Yolken, R.H. Maternal infections and subsequent psychosis among offspring. Arch. Gen. Psychiatry 2001, 58, 1032–1037. [Google Scholar] [CrossRef]
- Mortensen, P.B.; Nørgaard-Pedersen, B.; Waltoft, B.L.; Sørensen, T.L.; Hougaard, D.; Torrey, E.F.; Yolken, R.H. Toxoplasma gondii as a risk factor for early-onset schizophrenia: Analysis of filter paper blood samples obtained at birth. Biol. Psychiatry 2007, 61, 688–693. [Google Scholar] [CrossRef]
- Roberts, J.A.; Elkind, M.S.V.; Liu, M.; Wright, C.B.; Rundek, T.; Gutierrez, J. Herpes simplex virus 2 serology is associated with thinner whole-brain cortex in community-dwelling older adults. J. Neurol. Sci. 2023, 454, 120856. [Google Scholar] [CrossRef]
- Otth, C.; Acuña-Hinrichsen, F.; Leyton, L.; Martin, C.; Concha, M.I. Herpes Simplex Virus Type 1 at the Central Nervous System. In Herpesviridae; InTech: Rijeka, Croatia, 2016. [Google Scholar]
- Dosa, S.; Castellanos, K.; Bacsa, S.; Gagyi, E.; Kovacs, S.K.; Valyi-Nagy, K.; Shukla, D.; Dermody, T.S.; Valyi-Nagy, T. Chronic Progressive Deficits in Neuron Size, Density and Number in the Trigeminal Ganglia of Mice Latently Infected with Herpes Simplex Virus. Brain Pathol. 2011, 21, 583–593. [Google Scholar] [CrossRef]
- Engel, J.A.; Zhang, J.; Bergström, T.; Conradi, N.; Forkstam, C.; Liljeroth, A.; Svensson, L.; Svensson, T.H. Neonatal Herpes Simplex Virus Type 1 Brain Infection Affects the Development of Sensorimotor Gating in Rats. Brain Res. 2000, 863, 233–240. [Google Scholar] [CrossRef]
- Braff, D.L.; Geyer, M.A. Sensorimotor Gating and Schizophrenia: Human and Animal Model Studies. Arch. Gen. Psychiatry 1990, 47, 181–188. [Google Scholar] [CrossRef]
- Andreou, D.; Jørgensen, K.N.; Nerland, S.; Ueland, T.; Vaskinn, A.; Haukvik, U.K.; Yolken, R.H.; Andreassen, O.A.; Agartz, I. Herpes Simplex Virus 1 Infection on Grey Matter and General Intelligence in Severe Mental Illness. Transl. Psychiatry 2022, 12, 276. [Google Scholar] [CrossRef]
- Horváth, S.; Prandovszky, E.; Kis, Z.; Krummenacher, C.; Eisenberg, R.J.; Cohen, G.H.; Janka, Z.; Toldi, J. Spatiotemporal Changes of the Herpes Simplex Virus Entry Receptor Nectin-1 in Murine Brain during Postnatal Development. J. Neurovirol. 2006, 12, 161–170. [Google Scholar] [CrossRef]
- Nimgaonkar, V.L.; Yolken, R.H. Neurotropic Infectious Agents and Cognitive Impairment in Schizophrenia. Schizophr. Bull. 2012, 38, 1135–1136. [Google Scholar] [CrossRef]
- Duarte, L.F.; Farias, M.A.; Alvarez, D.M.; Bueno, S.M.; Riedel, C.A.; Gonzalez, P.A. Herpes Simplex Virus Type 1 Infection of the Central Nervous System: Insights into Proposed Interrelationships with Neurodegenerative Disorders. Front. Cell. Neurosci. 2019, 13, 46. [Google Scholar] [CrossRef]
- Harris, S.A.; Harris, E.A. Molecular Mechanisms for Herpes Simplex Virus Type 1 Pathogenesis in Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 48. [Google Scholar] [CrossRef]
- Prasad, K.M.; Watson, A.M.; Dickerson, F.B.; Yolken, R.H.; Nimgaonkar, V.L. Exposure to Herpes Simplex Virus Type 1 and Cognitive Impairments in Individuals with Schizophrenia. Schizophr. Bull. 2012, 38, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Bhatia, T.; Gauba, D.; Wood, J.; Long, C.; Prasad, K.; Dickerson, F.B.; Gur, R.E.; Gur, R.C.; Yolken, R.H.; et al. Exposure to herpes simplex virus, type 1 and reduced cognitive function. J. Psychiatr. Res. 2013, 47, 1680–1685. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Neto, N.F.; Caixeta, R.A.V.; Zerbinati, R.M.; Zarpellon, A.C.; Caetano, M.W.; Pallos, D.; Junges, R.; Costa, A.L.F.; Aitken-Saavedra, J.; Giannecchini, S.; et al. The Emergence of Saliva as a Diagnostic and Prognostic Tool for Viral Infections. Viruses 2024, 16, 1759. [Google Scholar] [CrossRef]
- Klein, H.C.; Doorduin, J.; de Witte, L.; de Vries, E.F.J. Microglia Activation, Herpes Infection, and NMDA Receptor Inhibition: Common Pathways to Psychosis? In Immunology and Psychiatry: From Basic Research to Therapeutic Interventions; Müller, N., Myint, A.M., Schwarz, M.J., Eds.; Springer: Cham, Switzerland, 2015; Volume 8, pp. 243–254. [Google Scholar][Green Version]
- Fruchter, E.; Goldberg, S.; Fenchel, D.; Grotto, I.; Ginat, K.; Weiser, M. The impact of Herpes simplex virus type 1 on cognitive impairments in young, healthy individuals—A historical prospective study. Schizophr. Res. 2015, 168, 292–296. [Google Scholar] [CrossRef]
- Dickerson, F.; Stallings, C.; Sullens, A.; Origoni, A.; Leister, F.; Krivogorsky, B.; Yolken, R. Association between cognitive functioning, exposure to Herpes Simplex Virus type 1, and the COMT Val158Met genetic polymorphism in adults without a psychiatric disorder. Brain Behav. Immun. 2008, 22, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Strandberg, T.E.; Pitkala, K.H.; Linnavuori, K.H.; Tilvis, R.S. Impact of viral and bacterial burden on cognitive impairment in elderly persons with cardiovascular diseases. Stroke 2003, 34, 2126–2131. [Google Scholar] [CrossRef]
- Watson, A.M.M.; Prasad, K.M.; Klei, L.; Wood, J.A.; Yolken, R.H.; Gur, R.C.; Bradford, L.D.; Calkins, M.E.; Richard, J.A.; Edwards, N.; et al. Persistent infection with neurotropic herpes viruses and cognitive impairment. Psychol. Med. 2013, 43, 1023–1031. [Google Scholar] [CrossRef]
- Jonker, I.; Klein, H.C.; Duivis, H.E.; Yolken, R.H.; Rosmalen, J.G.M.; Schoevers, R.A. Association between exposure to HSV1 and cognitive functioning in a general population of adolescents. The TRAILS study. PLoS ONE 2014, 9, e101549. [Google Scholar] [CrossRef]
- Schretlen, D.J.; Vannorsdall, T.D.; Winicki, J.M.; Mushtaq, Y.; Hikida, T.; Sawa, A.; Dickinson, D.; Barta, P.; Pearlson, G.D. Neuroanatomic and Cognitive Abnormalities Related to Herpes Simplex Virus Type 1 in Schizophrenia. Schizophr. Res. 2010, 118, 224–231. [Google Scholar] [CrossRef]
- Pandurangi, A.K.; Pelonero, A.L.; Nadel, L.; Calabrese, V.P. Brain Structure Changes in Schizophrenics with High Serum Titers of Antibodies to Herpes Virus. Schizophr. Res. 1994, 11, 245–250. [Google Scholar] [CrossRef]
- Shirts, B.H.; Wood, J.; Yolken, R.H.; Nimgaonkar, V.L. Comprehensive Evaluation of Positional Candidates in the IL-18 Pathway Reveals Suggestive Associations with Schizophrenia and Herpes Virus Seropositivity. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008, 147, 343–350. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, J.; Wang, Y.; Li, F.; Song, X.; Qin, S.; Wang, Z.; Kitazato, K.; Wang, Y. Roles of HSV-1 infection-induced microglial immune responses in CNS diseases: Friends or foes? Crit. Rev. Microbiol. 2019, 45, 581–594. [Google Scholar] [CrossRef]
- Prasad, K.M.; Bamne, M.N.; Shirts, B.H.; Goradia, D.; Mannali, V.; Pancholi, K.M.; Xue, B.; McClain, L.; Yolken, R.H.; Keshavan, M.S.; et al. Grey matter changes associated with host genetic variation and exposure to Herpes Simplex Virus 1 (HSV1) in first episode schizophrenia. Schizophr. Res. 2010, 118, 232–239. [Google Scholar] [CrossRef] [PubMed]
- James, L.M.; Charonis, S.A.; Georgopoulos, A.P. Schizophrenia, Human Leukocyte Antigen (HLA), and Herpes Viruses: Immunogenetic Associations at the Population Level. Neurosci. Insights 2023, 18, 26331055231166411. [Google Scholar] [CrossRef]
- Huang, Y.C.; Ping, L.Y.; Hsu, S.H.; Tsai, H.Y.; Cheng, M.C. Indicators of HSV1 Infection, ECM-Receptor Interaction, and Chromatin Modulation in a Nuclear Family with Schizophrenia. J. Pers. Med. 2023, 13, 1392. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, F.; Jones-Brando, L.; Ford, G.; Genovese, G.; Stallings, C.; Origoni, A.; O’Dushlaine, C.; Katsafanas, E.; Sweeney, K.; Khushalani, S.; et al. Schizophrenia is associated with an aberrant immune response to Epstein-Barr virus. Schizophr. Bull. 2019, 45, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Houenou, J.; d’Albis, M.A.; Daban, C.; Hamdani, N.; Delavest, M.; Lepine, J.P.; Vederine, F.E.; Carde, S.; Lajnef, M.; Cabon, C.; et al. Cytomegalovirus seropositivity and serointensity are associated with hippocampal volume and verbal memory in schizophrenia and bipolar disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 48, 142–148. [Google Scholar] [CrossRef]
- Torrey, E.F.; Yolken, R.; Albrecht, P. Cytomegalovirus as a possible etiological agent in schizophrenia. Adv. Biol. Psychiatry 1983, 12, 150–160. [Google Scholar]
- van den Pol, A.N. Viral infections in the developing and mature brain. Trends Neurosci. 2006, 29, 398–406. [Google Scholar] [CrossRef]
- Khandaker, G.M.; Stochl, J.; Zammit, S.; Lewis, G.; Jones, P.B. Childhood Epstein-Barr Virus infection and subsequent risk of psychotic experiences in adolescence: A population-based prospective serological study. Schizophr. Res. 2014, 158, 19–24. [Google Scholar] [CrossRef]
- Kennelly, S.P. Cognitive dysfunction: An important extrahepatic manifestation of hepatitis C infection? Postgrad. Med. J. 2013, 89, 431–432. [Google Scholar] [CrossRef] [PubMed]
- Blomström, Å.; Karlsson, H.; Wick, S.; Yang, S.; Yolken, R.H.; Dalman, C. Maternal antibodies to infectious agents and risk for non-affective psychoses in the offspring—A matched case-control study. Schizophr. Res. 2012, 140, 25–30. [Google Scholar] [CrossRef]
- Zheng, H.; Webster, M.J.; Weickert, C.S.; Beasley, C.L.; Paulus, M.P.; Yolken, R.H.; Savitz, J. Cytomegalovirus antibodies are associated with mood disorders, suicide, markers of neuroinflammation, and microglia activation in postmortem brain samples. Mol. Psychiatry 2023, 28, 5282–5292. [Google Scholar] [CrossRef] [PubMed]
- Sutkowski, N.; Chen, G.; Calderon, G.; Huber, B.T. Epstein-Barr virus latent membrane protein LMP-2A is sufficient for transactivation of the human endogenous retrovirus HERV-K18 superantigen. J. Virol. 2004, 78, 7852–7860. [Google Scholar] [CrossRef]
- D’Aiuto, L.; Prasad, K.M.; Upton, C.H.; Viggiano, L.; Milosevic, J.; Raimondi, G.; McClain, L.; Chowdari, K.; Tischfield, J.; Sheldon, M. Persistent infection by HSV-1 is associated with changes in functional architecture of iPSC-derived neurons and brain activation patterns underlying working memory performance. Schizophr. Bull. 2015, 41, 123–132. [Google Scholar] [CrossRef]
- Cheeran, M.C.; Lokensgard, J.R.; Schleiss, M.R. Neuropathogenesis of congenital cytomegalovirus infection: Disease mechanisms and prospects for intervention. Clin. Microbiol. Rev. 2009, 22, 99–126. [Google Scholar] [CrossRef]
- Arai, Y.; Ishiwata, M.; Baba, S.; Kawasaki, H.; Kosugi, I.; Li, R.Y.; Tsuchida, T.; Miura, K.; Tsutsui, Y. Neuron-specific activation of murine cytomegalovirus early gene e1 promoter in transgenic mice. Am. J. Pathol. 2003, 163, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yolken, R.H.; Hoekstra, P.J.; Burger, H.; Klein, H.C. Antibodies to infectious agents and the positive symptom dimension of subclinical psychosis: The TRAILS study. Schizophr. Res. 2011, 129, 47–51. [Google Scholar] [CrossRef]
- Levite, M. Glutamate receptor antibodies in neurological diseases: Anti-AMPA-GluR3 antibodies, anti-NMDA-NR1 antibodies, anti-NMDA-NR2A/B antibodies, anti-mGluR1 antibodies or anti-mGluR5 antibodies are present in subpopulations of patients with various neurological disorders. J. Neural Transm. 2014, 121, 1029–1075. [Google Scholar]
- Segundo, M. Direct evidence of viral infection and mitochondrial alterations in the brain of fetuses at high risk for schizophrenia. In Proceedings of the 5th World Congress on Health Economics, Health Policy and Healthcare Management, Copenhagen, Denmark, 14–15 October 2019. [Google Scholar]
- Reeves, M.B.; Compton, T. Inhibition of inflammatory interleukin-6 activity via extracellular signal-regulated kinase–mitogen-activated protein kinase signaling antagonizes human cytomegalovirus reactivation from dendritic cells. J. Virol. 2011, 85, 12750–12758. [Google Scholar] [CrossRef] [PubMed]
- Lucchese, G.; Flöel, A.; Stahl, B. A Peptide Link Between Human Cytomegalovirus Infection, Neuronal Migration, and Psychosis. Front. Psychiatry 2020, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Doorduin, J.; de Vries, E.F.J.; Willemsen, A.T.M.; de Groot, J.C.; Dierckx, R.A.; Klein, H.C. Neuroinflammation in schizophrenia-related psychosis: A PET study. J. Nucl. Med. 2009, 50, 1801–1807. [Google Scholar] [CrossRef]
- Lokensgard, J.R.; Cheeran, M.C.; Hu, S.; Gekker, G.; Peterson, P.K. Glial Cell Responses to Herpesvirus Infections: Role in Defense and Immunopathogenesis. J. Infect. Dis. 2002, 186, S171–S179. [Google Scholar] [CrossRef]
- Dickerson, F.; Stallings, C.; Origoni, A.; Vaughan, C.; Khushalani, S.; Yolken, R. Additive Effects of Elevated C-Reactive Protein and Exposure to Herpes Simplex Virus Type 1 on Cognitive Impairment in Individuals with Schizophrenia. Schizophr. Res. 2012, 134, 83–88. [Google Scholar] [CrossRef]
- Severance, E.G.; Leister, F.; Lea, A.; Yang, S.; Dickerson, F.; Yolken, R.H. Complement C4 associations with altered microbial biomarkers exemplify gene-by-environment interactions in schizophrenia. Schizophr. Res. 2021, 234, 87–93. [Google Scholar] [CrossRef]
- Børglum, A.D.; Demontis, D.; Grove, J.; Pallesen, J.; Hollegaard, M.V.; Pedersen, C.B.; Hedemand, A.; Mattheisen, M.; Uitterlinden, A.; Nyegaard, M.; et al. Genome-wide study of association and interaction with maternal cytomegalovirus infection suggests new schizophrenia loci. Mol. Psychiatry 2014, 19, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Lathe, R.; St Clair, D. From conifers to cognition: Microbes, brain and behavior. Genes Brain Behav. 2020, 19, e12680. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Folsom, T.D.; Rooney, R.J.; Mori, S.; Kornfield, T.E.; Reutiman, T.J.; Kneeland, R.E.; Liesch, S.B.; Hua, K.; Hsu, J.; et al. The Viral Theory of Schizophrenia Revisited: Abnormal Placental Gene Expression and Structural Changes with Lack of Evidence for H1N1 Viral Presence in Placentae of Infected Mice or Brains of Exposed Offspring. Neuropharmacology 2012, 62, 1290–1298. [Google Scholar] [CrossRef]
- Sparkman, N.L.; Buchanan, J.B.; Heyen, J.R.; Chen, J.; Beverly, J.L.; Johnson, R.W. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J. Neurosci. 2006, 26, 10709–10716. [Google Scholar] [CrossRef]
- Yao, H.W.; Ling, P.; Tung, Y.Y.; Hsu, S.M.; Chen, S.H. In vivo reactivation of latent herpes simplex virus 1 in mice can occur in the brain before occurring in the trigeminal ganglion. J. Virol. 2014, 88, 11264–11270. [Google Scholar] [CrossRef]
- Whitford, T.J.; Wood, S.J.; Yung, A.; Cocchi, L.; Berger, G.; Shenton, M.E.; Kubicki, M.; Phillips, L.; Velakoulis, D.; Yolken, R.H.; et al. Structural Abnormalities in the Cuneus Associated with Herpes Simplex Virus (Type 1) Infection in People at Ultra High Risk of Developing Psychosis. Schizophr. Res. 2012, 135, 175–180. [Google Scholar] [CrossRef]
- Cagnin, A.; Myers, R.; Gunn, R.N.; Lawrence, A.D.; Stevens, T.; Kreutzberg, G.W.; Jones, T.; Banati, R.B. In Vivo Visualization of Activated Glia by 11C-PK11195-PET Following Herpes Encephalitis Reveals Projected Neuronal Damage Beyond the Primary Focal Lesion. Brain 2001, 124, 2014–2027. [Google Scholar] [CrossRef]
- Yolken, R.H.; Torrey, E.F.; Lieberman, J.A.; Yang, S.; Dickerson, F.B. Serological Evidence of Exposure to Herpes Simplex Virus Type 1 Is Associated with Cognitive Deficits in the CATIE Schizophrenia Sample. Schizophr. Res. 2011, 128, 61–65. [Google Scholar] [CrossRef]
- Dickerson, F.; Schroeder, J.R.; Nimgaonkar, V.; Gold, J.; Yolken, R. The Association Between Exposure to Herpes Simplex Virus Type 1 (HSV-1) and Cognitive Functioning in Schizophrenia: A Meta-Analysis. Psychiatry Res. 2020, 291, 113157. [Google Scholar] [CrossRef]
- Bhatia, T.; Wood, J.; Iyengar, S.; Narayanan, S.S.; Beniwal, R.P.; Prasad, K.M.; Chen, K.; Yolken, R.H.; Dickerson, F.; Gur, R.C.; et al. Emotion discrimination in humans: Its association with HSV-1 infection and its improvement with antiviral treatment. Schizophr. Res. 2018, 193, 161–167. [Google Scholar] [CrossRef]
- Schneider, F.; Gur, R.C.; Koch, K.; Backes, V.; Amunts, K.; Shah, N.J.; Bilker, W.; Gur, R.E.; Habel, U. Impairment in the specificity of emotion processing in schizophrenia. Am. J. Psychiatry 2006, 163, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Couture, S.M.; Penn, D.L.; Roberts, D.L. The functional significance of social cognition in schizophrenia: A review. Schizophr. Bull. 2006, 32, S44–S63. [Google Scholar] [CrossRef]
- Becker, Y. HSV-1 brain infection by the olfactory nerve route and virus latency and reactivation may cause learning and behavioral deficiencies and violence in children and adults: A point of view. Virus Genes 1995, 10, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Karatas, H.; Gurer, G.; Pinar, A.; Soylemezoglu, F.; Tezel, G.G.; Hascelik, G.; Akalan, N.; Tuncer, S.; Ciger, A.; Saygi, S. Investigation of HSV-1, HSV-2, CMV, HHV-6 and HHV-8 DNA by real-time PCR in surgical resection materials of epilepsy patients with mesial temporal lobe sclerosis. J. Neurol. Sci. 2008, 264, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Shirts, B.H.; Kim, J.J.; Reich, S.; Dickerson, F.B.; Yolken, R.H.; Devlin, B.; Nimgaonkar, V.L. Polymorphisms in MICB are associated with human herpes virus seropositivity and schizophrenia risk. Schizophr. Res. 2007, 94, 342–353. [Google Scholar] [CrossRef]
- Meshreky, K.M.; Wood, J.; Chowdari, K.V.; Hall, M.H.; Wilckens, K.A.; Yolken, R.; Buysse, D.J.; Nimgaonkar, V.L. Infection with Herpes Simplex Virus Type 1 (HSV-1) and Sleep: The Dog That Did Not Bark. Psychiatry Res. 2019, 280, 112502. [Google Scholar] [CrossRef]
- Nissen, J.; Trabjerg, B.; Pedersen, M.G.; Banasik, K.; Pedersen, O.B.; Sørensen, E.; Nielsen, K.R.; Erikstrup, C.; Petersen, M.S.; Paarup, H.M.; et al. Herpes Simplex Virus Type 1 Infection Is Associated with Suicidal Behavior and First Registered Psychiatric Diagnosis in a Healthy Population. Psychoneuroendocrinology 2019, 108, 150–154. [Google Scholar] [CrossRef]
- Koyuncu, O.O.; Hogue, I.B.; Enquist, L.W. Virus infections in the nervous system. Cell Host Microbe 2013, 13, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Abdoli, A.; Taghipour, A.; Pirestani, M.; Mofazzal Jahromi, M.A.; Roustazadeh, A.; Mir, H.; Ardakani, H.M.; Kenarkoohi, A.; Falahi, S.; Karimi, M. Infections, inflammation, and risk of neuropsychiatric disorders: The neglected role of “co-infection”. Heliyon 2020, 6, e05645. [Google Scholar] [CrossRef]
- Sawtell, N.M.; Thompson, R.L. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J. Virol. 1992, 66, 2150–2156. [Google Scholar] [CrossRef] [PubMed]
- Kleinschmidt-DeMasters, B.K.; DeBiasi, R.L.; Tyler, K.L. Polymerase chain reaction as a diagnostic adjunct in herpesvirus infections of the nervous system. Brain Pathol. 2001, 11, 452–464. [Google Scholar] [CrossRef]
- Agner, S.C.; Klein, R.S. Viruses have multiple paths to central nervous system pathology. Curr. Opin. Neurol. 2018, 31, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Prüss, H. Autoantibodies in neurological disease. Nat. Rev. Immunol. 2021, 21, 798–813. [Google Scholar] [CrossRef]
- Coughlin, S.S. Anxiety and Depression: Linkages with Viral Diseases. Public Health Rev. 2012, 34, 7. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Lei, Y.; Liu, X.; Zhou, X.; Liu, Y.; Wang, M.; Yang, L.; Zhang, L.; Fan, S.; et al. Meta-analysis of infectious agents and depression. Sci. Rep. 2014, 4, 4530. [Google Scholar] [CrossRef]
- Yolken, R.H.; Torrey, E.F. Viruses, schizophrenia, and bipolar disorder. Clin. Microbiol. Rev. 1995, 8, 131–145. [Google Scholar] [CrossRef]
- Pang, D.; Syed, S.; Fine, P.; Jones, P.B. No association between prenatal viral infection and depression in later life—A long-term cohort study of 6152 subjects. Can. J. Psychiatry 2009, 54, 565–570. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, B.P.; Tan, C.T.; Pan, F.; Larbi, A.; Ng, T.P. Lifetime pathogen burden, inflammatory markers, and depression in community-dwelling older adults. Brain Behav. Immun. 2022, 102, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Depino, A.M. Perinatal inflammation and adult psychopathology: From preclinical models to humans. Semin. Cell Dev. Biol. 2018, 77, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Torrey, E.F.; Rawlings, R.; Waldman, I.N. Schizophrenic births and viral diseases in two states. Schizophr. Res. 1988, 1, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Arias, I.; Sorlozano, A.; Villegas, E.; Luna, J.D.; McKenney, K.; Cervilla, J.; Gutierrez, B.; Gutierrez, J. Infectious agents associated with schizophrenia: A meta-analysis. Schizophr. Res. 2012, 136, 128–136. [Google Scholar] [CrossRef]
- Gutiérrez-Fernández, J.; de Dios Luna Del Castillo, J.; Mañanes-González, S.; Carrillo-Ávila, J.A.; Gutiérrez, B.; Cervilla, J.A.; Sorlózano-Puerto, A. Different presence of Chlamydia pneumoniae, herpes simplex virus type 1, human herpes virus 6, and Toxoplasma gondii in schizophrenia: Meta-analysis and analytical study. Neuropsychiatr. Dis. Treat. 2015, 11, 843–852. [Google Scholar] [CrossRef]
- Lycke, E.; Norrby, R.; Roos, B.E. A serological study on mentally ill patients with particular reference to the prevalence of herpesvirus infections. Br. J. Psychiatry 1974, 124, 273–279. [Google Scholar] [CrossRef]
- Leweke, F.M.; Gerth, C.W.; Koethe, D.; Klosterkötter, J.; Ruslanova, I.; Krivogorsky, B.; Torrey, E.F.; Yolken, R.H. Antibodies to infectious agents in individuals with recent onset schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2004, 254, 4–8. [Google Scholar] [CrossRef]
- Fukuda, R.; Sasaki, T.; Kunugi, H.; Nanko, S. No changes in paired viral antibody titers during the course of acute schizophrenia. Neuropsychobiology 1999, 40, 57–62. [Google Scholar] [CrossRef]
- Burkhardt, E.; Berger, M.; Yolken, R.H.; Lin, A.; Yuen, H.P.; Wood, S.J.; Francey, S.M.; Thompson, A.; McGorry, P.D.; Nelson, B.; et al. Toxoplasma gondii, Herpesviridae and long-term risk of transition to first-episode psychosis in an ultra high-risk sample. Schizophr. Res. 2021, 233, 24–30. [Google Scholar] [CrossRef]
- Alexander, R.C.; Cabirac, G.; Lowenkopf, T.; Casanova, M.; Kleinman, J.; Wyatt, R.J.; Kirch, D.G. Search for evidence of herpes simplex virus, type 1, or varicella-zoster virus infection in postmortem brain tissue from schizophrenic patients. Acta Psychiatr. Scand. 1992, 86, 418–420. [Google Scholar] [CrossRef]
- Taller, A.M.; Asher, D.M.; Pomeroy, K.L.; Eldadah, B.A.; Godec, M.S.; Falkai, P.G.; Bogert, B.; Kleinman, J.E.; Stevens, J.R.; Torrey, E.F. Search for viral nucleic acid sequences in brain tissues of patients with schizophrenia using nested polymerase chain reaction. Arch. Gen. Psychiatry 1996, 53, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.I.; Taylor, G.R.; Crow, T.J. Search for viral nucleic acid sequences in the post mortem brains of patients with schizophrenia and individuals who have committed suicide. J. Neurol. Neurosurg. Psychiatry 1987, 50, 247–251. [Google Scholar] [CrossRef]
- Nicolson, G.L.; Haier, J. Role of Chronic Bacterial and Viral Infections in Neurodegenerative, Neurobehavioral, Psychiatric, Autoimmune and Fatiguing Illnesses: Part 2. Br. J. Med. Pract. 2010, 3, e301. [Google Scholar]
- Lünemann, J.D.; Münz, C. Epstein–Barr Virus and Multiple Sclerosis. Curr. Neurol. Neurosci. Rep. 2007, 7, 253–258. [Google Scholar] [CrossRef]
- Ambinder, R.F. Epstein-Barr Virus-Associated Lymphoproliferative Disorders. Rev. Clin. Exp. Hematol. 2003, 7, 362–374. [Google Scholar]
- Ambinder, R.F.; Lin, L. Mononucleosis in the Laboratory. J. Infect. Dis. 2005, 192, 1503–1504. [Google Scholar] [CrossRef]
- Niederman, J.C.; Miller, G.; Pearson, H.A.; Pagano, J.S.; Dowaliby, J.M. Infectious Mononucleosis: Epstein-Barr Virus Shedding in Saliva and the Oropharynx. N. Engl. J. Med. 1976, 294, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Martelius, T.; Lappalainen, M.; Palomäki, M.; Anttila, V.J. Clinical Characteristics of Patients with Epstein-Barr Virus in Cerebrospinal Fluid. BMC Infect. Dis. 2011, 11, 281. [Google Scholar] [CrossRef]
- Tzartos, J.S.; Khan, G.; Vossenkämper, A.; Cruz-Sadaba, M.; Lonardi, S.; Sefia, E.; Meager, A.; Elia, A.; Middeldorp, J.M.; Clemens, M.; et al. Association of Innate Immune Activation with Latent Epstein-Barr Virus in Active MS Lesions. Neurology 2012, 78, 15–23. [Google Scholar] [CrossRef]
- Mechawar, N.; Savitz, J. Neuropathology of Mood Disorders: Do We See the Stigmata of Inflammation? Transl. Psychiatry 2016, 6, e946. [Google Scholar] [CrossRef]
- Montoya, J.G.; Kogelnik, A.M.; Bhangoo, M.; Lunn, M.R.; Flamand, L.; Merrihew, L.E.; Watt, T.; Kubo, S.; Ruiz, M.I.; Aden, B.; et al. Randomized Clinical Trial to Evaluate the Efficacy and Safety of Valganciclovir in a Subset of Patients with Chronic Fatigue Syndrome. J. Med. Virol. 2013, 85, 2101–2109. [Google Scholar] [CrossRef] [PubMed]
- Hassani, A.; Corboy, J.R.; Al-Salam, S.; Khan, G. Epstein-Barr Virus Is Present in the Brain of Most Cases of Multiple Sclerosis and May Engage More Than Just B Cells. PLoS ONE 2018, 13, e0192109. [Google Scholar] [CrossRef] [PubMed]
- Farina, A.; Cirone, M.; York, M.; Lenna, S.; Padilla, C.; Mclaughlin, S.; Faggioni, A.; Lafyatis, R.; Trojanowska, M.; Farina, G.A. Epstein-Barr Virus Infection Induces Aberrant TLR Activation Pathway and Fibroblast–Myofibroblast Conversion in Scleroderma. J. Investig. Dermatol. 2014, 134, 954–964. [Google Scholar] [CrossRef]
- Mészáros, Z.S.; Perl, A.; Faraone, S.V. Psychiatric Symptoms in Systemic Lupus Erythematosus: A Systematic Review. J. Clin. Psychiatry 2012, 73, 993–1001. [Google Scholar] [CrossRef]
- Chiewthanakul, P.; Sawanyawisuth, K.; Foocharoen, C.; Tiamkao, S. Clinical Features and Predictive Factors in Neuropsychiatric Lupus. Asian Pac. J. Allergy Immunol. 2012, 30, 55–60. [Google Scholar] [PubMed]
- Weisbrot, D.; Charvet, L.; Serafin, D.; Milazzo, M.; Preston, T.E.; Cleary, R.E.; Krupp, L.B. Psychiatric Diagnoses and Cognitive Impairment in Pediatric Multiple Sclerosis. Mult. Scler. 2014, 20, 588–593. [Google Scholar] [CrossRef]
- Brenner, P.; Alexanderson, K.; Björkenstam, C.; Jokinen, J.; Hillert, J.; Friberg, E.; Tinghög, P. Psychiatric Diagnoses, Medication and Risk for Disability Pension in Multiple Sclerosis Patients: A Population-Based Register Study. PLoS ONE 2014, 9, e104165. [Google Scholar] [CrossRef]
- Kosmidis, M.H.; Giannakou, M.; Messinis, L.; Papathanasopoulos, P. Psychotic Features Associated with Multiple Sclerosis. Int. Rev. Psychiatry 2010, 22, 55–66. [Google Scholar] [CrossRef]
- Bayturan, S.; Sapmaz, Ş.Y.; Uzun, A.D.; Kandemir, H.; Ecemiş, T. Relationship of herpesvirus (HSV1, EBV, CMV, HHV6) seropositivity with depressive disorder and its clinical aspects: The first study in children. J. Med. Virol. 2022, 94, 5484–5491. [Google Scholar] [CrossRef]
- Markkula, N.; Lindgren, M.; Yolken, R.H.; Suvisaari, J. Association of exposure to Toxoplasma gondii, Epstein-Barr Virus, Herpes Simplex virus Type 1 and Cytomegalovirus with new-onset depressive and anxiety disorders: An 11-year follow-up study. Brain Behav. Immun. 2020, 87, 238–242. [Google Scholar] [CrossRef]
- Haeri, S.; Johnson, N.; Baker, A.M.; Stuebe, A.M.; Raines, C.; Barrow, D.A.; Boggess, K.A. Maternal depression and Epstein-Barr virus reactivation in early pregnancy. Obstet. Gynecol. 2011, 117, 862–866. [Google Scholar] [CrossRef]
- Zhu, P.; Chen, Y.J.; Hao, J.H.; Ge, J.F.; Huang, K.; Tao, R.X.; Jiang, X.M.; Tao, F.B. Maternal depressive symptoms related to Epstein-Barr virus reactivation in late pregnancy. Sci. Rep. 2013, 3, 3096. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.L.; Stowe, R.P. Depressive symptoms are associated with salivary shedding of Epstein-Barr virus in female adolescents: The role of sex differences. Psychoneuroendocrinology 2017, 86, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Gotlieb-Stematsky, T.; Zonis, J.; Arlazoroff, A.; Mozes, T.; Sigal, M.; Szekely, A.G. Antibodies to Epstein–Barr virus, herpes simplex type 1, cytomegalovirus and measles virus in psychiatric patients. Arch. Virol. 1981, 67, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Delisi, L.E.; Smith, S.B.; Hamovit, J.R.; Maxwell, M.E.; Goldin, L.R.; Dingman, C.W.; Gershon, E.S. Herpes simplex virus, cytomegalovirus and Epstein–Barr virus antibody titres in sera from schizophrenic patients. Psychol. Med. 1986, 16, 757–763. [Google Scholar] [CrossRef]
- Torrey, E.F.; Leweke, M.F.; Schwarz, M.J.; Mueller, N.; Bachmann, S.; Schroeder, J.; Dickerson, F.; Yolken, R.H. Cytomegalovirus and schizophrenia. CNS Drugs 2006, 20, 879–885. [Google Scholar] [CrossRef]
- Stefansson, H.; Ophoff, R.A.; Steinberg, S.; Andreassen, O.A.; Cichon, S.; Rujescu, D.; Werge, T.; Pietiläinen, O.P.H.; Mors, O.; Mortensen, P.B.; et al. Common variants conferring risk of schizophrenia. Nature 2009, 460, 744–747. [Google Scholar] [CrossRef]
- Poulton, R.; Caspi, A.; Moffitt, T.E.; Cannon, M.; Murray, R.; Harrington, H. Children’s self-reported psychotic symptoms and adult schizophreniform disorder: A 15-year longitudinal study. Arch. Gen. Psychiatry 2000, 57, 1053–1058. [Google Scholar] [CrossRef]
- Zammit, S.; Kounali, D.; Cannon, M.; David, A.S.; Gunnell, D.; Heron, J.; Jones, P.B.; Lewis, S.; Sullivan, S.; Wolke, D.; et al. Psychotic experiences and psychotic disorders at age 18 in relation to psychotic experiences at age 12 in a longitudinal population-based cohort study. Am. J. Psychiatry 2013, 170, 742–750. [Google Scholar] [CrossRef]
- Wingerchuk, D.M. Environmental factors in multiple sclerosis: Epstein-Barr virus, vitamin D, and cigarette smoking. Mt. Sinai J. Med. 2011, 78, 221–230. [Google Scholar] [CrossRef]
- Buka, S.L.; Tsuang, M.T.; Torrey, E.F.; Klebanoff, M.A.; Wagner, R.L.; Yolken, R.H. Maternal cytokine levels during pregnancy and adult psychosis. Brain Behav. Immun. 2001, 15, 411–420. [Google Scholar] [CrossRef]
- Brown, A.S.; Hooton, J.; Schaefer, C.A.; Zhang, H.; Petkova, E.; Babulas, V.; Perrin, M.; Gorman, J.M.; Susser, E.S. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am. J. Psychiatry 2004, 161, 889–895. [Google Scholar] [CrossRef]
- Miller, B.J.; Buckley, P.; Seabolt, W.; Mellor, A.; Kirkpatrick, B. Meta-analysis of cytokine alterations in schizophrenia: Clinical status and antipsychotic effects. Biol. Psychiatry 2011, 70, 663–671. [Google Scholar] [CrossRef] [PubMed]
- van Berckel, B.N.M.; Bossong, M.G.; Boellaard, R.; Kloet, R.; Schuitemaker, A.; Caspers, E.; Luurtsema, G.; Windhorst, A.D.; Cahn, W.; Lammertsma, A.A.; et al. Microglia activation in recent-onset schizophrenia: A quantitative (R)-[11C]PK11195 positron emission tomography study. Biol. Psychiatry 2008, 64, 820–822. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U.; Feldon, J. Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog. Neurobiol. 2010, 90, 285–326. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, F.; Katsafanas, E.; Origoni, A.; Squire, A.; Khushalani, S.; Newman, T.; Rowe, K.; Stallings, C.; Savage, C.L.G.; Sweeney, K.; et al. Exposure to Epstein Barr virus and cognitive functioning in individuals with schizophrenia. Schizophr. Res. 2021, 228, 193–197. [Google Scholar] [CrossRef]
- de Witte, L.D.; van Mierlo, H.C.; Litjens, M.; Klein, H.C.; Bahn, S.; Osterhaus, A.D.; GROUP Investigators. The association between antibodies to neurotropic pathogens and schizophrenia: A case-control study. NPJ Schizophr. 2015, 1, 15041. [Google Scholar] [CrossRef]
- Jog, N.R.; McClain, M.T.; Heinlen, L.D.; Gross, T.; Towner, R.; Guthridge, J.M.; Axtell, R.C.; Pardo, G.; Harley, J.B.; James, J.A. Epstein Barr virus nuclear antigen 1 (EBNA-1) peptides recognized by adult multiple sclerosis patient sera induce neurologic symptoms in a murine model. J. Autoimmun. 2019, 102, 102332. [Google Scholar] [CrossRef]
- Lindsey, J.W.; deGannes, S.L.; Pate, K.A.; Zhao, X. Antibodies specific for Epstein-Barr virus nuclear antigen-1 cross-react with human heterogeneous nuclear ribonucleoprotein L. Mol. Immunol. 2016, 69, 7–12. [Google Scholar]
- Jones-Brando, L.; Dickerson, F.; Ford, G.; Stallings, C.; Origoni, A.; Katsafanas, E.; Sweeney, K.; Squire, A.; Khushalani, S.; Yolken, R. Atypical immune response to Epstein-Barr virus in major depressive disorder. J. Affect. Disord. 2020, 264, 221–226. [Google Scholar] [CrossRef]
- Kachuri, L.; Francis, S.S.; Morrison, M.; Bossé, Y.; Cavazos, T.B.; Rashkin, S.R.; Ziv, E.; Witte, J.S. The landscape of host genetic factors involved in infection to common viruses and SARS-CoV-2. Genome Med. 2020, 12, 93. [Google Scholar] [CrossRef]
- Vistarop, A.; Jimenez, O.; Cohen, M.; De Matteo, E.; Preciado, M.V.; Chabay, P. Differences in Epstein-Barr virus characteristics and viral-related microenvironment could be responsible for lymphomagenesis in children. Pathogens 2020, 9, 68. [Google Scholar] [CrossRef]
- Steel, A.J.; Eslick, G.D. Herpes viruses increase the risk of Alzheimer’s disease: A meta-analysis. J. Alzheimer’s Dis. 2015, 47, 351–364. [Google Scholar] [CrossRef]
- Dickerson, F.; Stallings, C.; Origoni, A.; Katsafanas, E.; Schweinfurth, L.A.; Savage, C.L.; Yolken, R. Association between cytomegalovirus antibody levels and cognitive functioning in non-elderly adults. PLoS ONE 2014, 9, e95510. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, F.B.; Boronow, J.J.; Stallings, C.; Origoni, A.E.; Ruslanova, I.; Yolken, R.H. Association of serum antibodies to herpes simplex virus 1 with cognitive deficits in individuals with schizophrenia. Arch. Gen. Psychiatry 2003, 60, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Torniainen-Holm, M.; Suvisaari, J.; Lindgren, M.; Harkanen, T.; Dickerson, F.; Yolken, R.H. Association of cytomegalovirus and Epstein-Barr virus with cognitive functioning and risk of dementia in the general population: 11-year follow-up study. Brain Behav. Immun. 2018, 69, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Vanyukov, M.M.; Nimgaonkar, V.L.; Kirisci, L.; Kirillova, G.P.; Reynolds, M.D.; Prasad, K.; Tarter, R.E.; Yolken, R.H. Association of cognitive function and liability to addiction with childhood herpesvirus infections: A prospective cohort study. Dev. Psychopathol. 2018, 30, 143–152. [Google Scholar] [CrossRef]
- Runge, K.; Balla, A.; Fiebich, B.L.; Maier, S.J.; Pankratz, B.; Schlump, A.; Nickel, K.; Dersch, R.; Domschke, K.; van Elst, L.T.; et al. Antibody indices of infectious pathogens from serum and cerebrospinal fluid in patients with schizophrenia spectrum disorders. Fluids Barriers CNS 2022, 19, 61. [Google Scholar] [CrossRef] [PubMed]
- Amminger, G.P.; McGorry, P.D.; Berger, G.E.; Wade, D.; Yung, A.R.; Phillips, L.J.; Harrigan, S.M.; Francey, S.M.; Yolken, R.H. Antibodies to infectious agents in individuals at ultra-high risk for psychosis. Biol. Psychiatry 2007, 61, 1215–1217. [Google Scholar] [CrossRef]
- de Witte, L.D.; Snijders, G.; Litjens, M.; Kamperman, A.M.; Kushner, S.A.; Kahn, R.S.; Bergink, V. Are infectious agents involved in the pathogenesis of postpartum psychosis? J. Affect. Disord. 2018, 229, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, A.; Pender, M.P.; Khanna, R.; Steinman, L.; Hartung, H.P.; Maniar, T.; Croze, E.; Aftab, B.T.; Giovannoni, G.; Joshi, M.A. Epstein-Barr virus in multiple sclerosis: Theory and emerging immunotherapies. Trends Mol. Med. 2020, 26, 296–310. [Google Scholar] [CrossRef]
- Anderson, A.G.; Gaffy, C.B.; Weseli, J.R.; Gorres, K.L. Inhibition of Epstein-Barr virus lytic reactivation by the atypical antipsychotic drug clozapine. Viruses 2019, 11, 450. [Google Scholar] [CrossRef]
- Dupont, L.; Reeves, M.B. Cytomegalovirus latency and reactivation: Recent insights into an age old problem. Rev. Med. Virol. 2016, 26, 75–89. [Google Scholar] [CrossRef]
- Wills, M.R.; Poole, E.; Lau, B.; Krishna, B.; Sinclair, J.H. The immunology of human cytomegalovirus latency: Could latent infection be cleared by novel immunotherapeutic strategies? Cell. Mol. Immunol. 2015, 12, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Kosugi, I.; Meguro, S.; Iwashita, T. Pathogenesis of developmental anomalies of the central nervous system induced by congenital cytomegalovirus infection. Pathol. Int. 2017, 67, 72–82. [Google Scholar] [CrossRef]
- Teissier, N.; Fallet-Bianco, C.; Delezoide, A.L.; Laquerriere, A.; Marcorelles, P.; Khung-Savatovsky, S.; Nardelli, J.; Cipriani, S.; Csaba, Z.; Picone, O.; et al. Cytomegalovirus-induced brain malformations in fetuses. J. Neuropathol. Exp. Neurol. 2014, 73, 143–158. [Google Scholar] [CrossRef]
- Hoffmann, C.; Grossman, R.; Bokov, I.; Lipitz, S.; Biegon, A. Effect of cytomegalovirus infection on temporal lobe development in utero: Quantitative MRI studies. Eur. Neuropsychopharmacol. 2010, 20, 848–854. [Google Scholar] [CrossRef]
- Dickerson, F.; Kirkpatrick, B.; Boronow, J.; Stallings, C.; Origoni, A.; Yolken, R. Deficit Schizophrenia: Association with Serum Antibodies to Cytomegalovirus. Schizophr. Bull. 2006, 32, 396–400. [Google Scholar] [CrossRef]
- Krause, D.; Matz, J.; Weidinger, E.; Wagner, J.; Wildenauer, A.; Obermeier, M.; Riedel, M.; Müller, N. The association of infectious agents and schizophrenia. World J. Biol. Psychiatry 2010, 11, 739–743. [Google Scholar] [CrossRef]
- Kneeland, R.E.; Fatemi, S.H. Viral infection, inflammation and schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 42, 35–45. [Google Scholar] [CrossRef]
- Toroudi, H.P.; Nazer, Y.; Shamsabadi, S. The Potential Link between Cytomegalovirus Infection and the Onset and Symptoms of Schizophrenia. Clin. Schizophr. Relat. Psychoses 2024, 18. [Google Scholar] [CrossRef]
- Shirts, B.H.; Prasad, K.M.; Pogue-Geile, M.F.; Dickerson, F.; Yolken, R.H.; Nimgaonkar, V.L. Antibodies to Cytomegalovirus and Herpes Simplex Virus 1 Associated with Cognitive Function in Schizophrenia. Schizophr. Res. 2008, 106, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, P.; Torrey, E.F.; Boone, E.; Hicks, J.T.; Daniel, N. Raised Cytomegalovirus-Antibody Level in Cerebrospinal Fluid of Schizophrenic Patients. Lancet 1980, 316, 769–772. [Google Scholar] [CrossRef] [PubMed]
- Rimón, R.; Ahokas, A.; Palo, J. Serum and Cerebrospinal Fluid Antibodies to Cytomegalovirus in Schizophrenia. Acta Psychiatr. Scand. 1986, 73, 642–644. [Google Scholar] [CrossRef]
- Torrey, E.F.; Yolken, R.H.; Winfrey, C.J. Cytomegalovirus Antibody in Cerebrospinal Fluid of Schizophrenic Patients Detected by Enzyme Immunoassay. Science 1982, 216, 892–894. [Google Scholar] [CrossRef]
- Kaufmann, C.A.; Weinberger, D.; Yolken, R.; Torrey, E.; Potkin, S. Viruses and Schizophrenia. Lancet 1983, 322, 1136–1137. [Google Scholar] [CrossRef]
- Andreou, D.; Jørgensen, K.N.; Wortinger, L.A.; Engen, K.; Vaskinn, A.; Ueland, T.; Yolken, R.H.; Andreassen, O.A.; Agartz, I. Cytomegalovirus Infection and IQ in Patients with Severe Mental Illness and Healthy Individuals. Psychiatry Res. 2021, 300, 113929. [Google Scholar] [CrossRef]
- Krech, U. Complement-Fixing Antibodies Against Cytomegalovirus in Different Parts of the World. Bull. World Health Organ. 1973, 49, 103–106. [Google Scholar]
- Dickerson, F.; Wilcox, H.C.; Adamos, M.; Katsafanas, E.; Khushalani, S.; Origoni, A.; Savage, C.; Schweinfurth, L.; Stallings, C.; Sweeney, K.; et al. Suicide attempts and markers of immune response in individuals with serious mental illness. J. Psychiatr. Res. 2017, 87, 37–43. [Google Scholar] [CrossRef]
- Foiselle, M.; Lajnef, M.; Hamdani, N.; Boukouaci, W.; Wu, C.L.; Naamoune, S.; Chami, L.; Mezoued, E.; Richard, J.R.; Bouassida, J.; et al. Immune cell subsets in patients with bipolar disorder or schizophrenia with history of childhood maltreatment. Brain Behav. Immun. 2023, 112, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Solana, C.; Pereira, D.; Tarazona, R. Early Senescence and Leukocyte Telomere Shortening in SCHIZOPHRENIA: A Role for Cytomegalovirus Infection? Brain Sci. 2018, 8, 188. [Google Scholar] [CrossRef]
- Dickerson, F.B.; Boronow, J.J.; Stallings, C.R.; Origoni, A.E.; Yolken, R.H. Reduction of symptoms by valacyclovir in cytomegalovirus-seropositive individuals with schizophrenia. Am. J. Psychiatry 2003, 160, 2234–2236. [Google Scholar] [CrossRef]
- Prasad, K.M.; Eack, S.M.; Keshavan, M.S.; Yolken, R.H.; Iyengar, S.; Nimgaonkar, V.L. Antiherpes virus–specific treatment and cognition in schizophrenia: A test-of-concept randomized double-blind placebo-controlled trial. Schizophr. Bull. 2013, 39, 857–866. [Google Scholar] [CrossRef]
- Müller, N.; Riedel, M.; Scheppach, C.; Brandstätter, B.; Sokullu, S.; Krampe, K.; Ulmschneider, M.; Engel, R.R.; Möller, H.J.; Schwarz, M.J. Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. Am. J. Psychiatry 2002, 159, 1029–1034. [Google Scholar] [CrossRef]
- Martelius, T.J.; Wolff, H.; Bruggeman, C.A.; Krogerus, L.A.; Hayry, P.J. Induction of cyclo-oxygenase-2 by acute liver allograft rejection and cytomegalovirus infection in the rat. Transpl. Int. 2002, 15, 610–614. [Google Scholar] [CrossRef]
- Zhu, H.; Cong, J.P.; Yu, D.; Bresnahan, W.A.; Shenk, T.E. Inhibition of cyclooxygenase 2 blocks human cytomegalovirus replication. Proc. Natl. Acad. Sci. USA 2002, 99, 3932–3937. [Google Scholar] [CrossRef] [PubMed]
- Craighead, J.E. Cytomegalovirus. In Pathology and Pathogenesis of Human Viral Disease; Academic Press: San Diego, CA, USA, 2000; pp. 87–115. [Google Scholar]
- Bailey, O.T.; Wolf, A.; Schneck, S.A.; Hurst, E.W.; Craig, J.M. Iatrogenic modification of tissue responses to infectious agents in the central nervous system. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 1968, 44, 445–462. [Google Scholar] [PubMed]
- Dorfman, L.J. Cytomegalovirus encephalitis in adults. Neurology 1973, 23, 136–144. [Google Scholar] [CrossRef]
- Kim, J.J.; Shirts, B.H.; Dayal, M.; Bacanu, S.A.; Wood, J.; Xie, W.; Zhang, X.; Chowdari, K.V.; Yolken, R.; Devlin, B.; et al. Are exposure to cytomegalovirus and genetic variation on chromosome 6p joint risk factors for schizophrenia? Ann. Med. 2007, 39, 145–153. [Google Scholar] [CrossRef]
- Torkamani, A.; Dean, B.; Schork, N.J.; Thomas, E.A. Coexpression network analysis of neural tissue reveals perturbations in developmental processes in schizophrenia. Genome Res. 2010, 20, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Hagihara, H.; Ohira, K.; Takao, K.; Miyakawa, T. Transcriptomic evidence for immaturity of the prefrontal cortex in patients with schizophrenia. Mol. Brain 2014, 7, 41. [Google Scholar] [CrossRef]
- Komaroff, A.L.; Rizzo, R.; Ecker, J.L. Human Herpesviruses 6A and 6B in Reproductive Diseases. Front. Immunol. 2021, 12, 648945. [Google Scholar] [CrossRef]
- Santpere, G.; Telford, M.; Andrés-Benito, P.; Navarro, A.; Ferrer, I. The Presence of Human Herpesvirus 6 in the Brain in Health and Disease. Biomolecules 2020, 10, 1520. [Google Scholar] [CrossRef]
- Prusty, B.K.; Gulve, N.; Govind, S.; Krueger, G.R.F.; Feichtinger, J.; Larcombe, L.; Aspinall, R.; Ablashi, D.V.; Toro, C.T. Active HHV-6 Infection of Cerebellar Purkinje Cells in Mood Disorders. Front. Microbiol. 2018, 9, 1955. [Google Scholar] [CrossRef]
- Kristensson, K. Microbes’ roadmap to neurons. Nat. Rev. Neurosci. 2011, 12, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Arbuckle, J.H.; Medveczky, M.M.; Luka, J.; Hadley, S.H.; Luegmayr, A.; Ablashi, D.; Lund, T.C.; Tolar, J.; De Meirleir, K.; Montoya, J.G.; et al. The latent human herpesvirus-6A genome specifically integrates in telomeres of human chromosomes in vivo and in vitro. Proc. Natl. Acad. Sci. USA 2010, 107, 5563–5568. [Google Scholar] [CrossRef] [PubMed]
- Hogestyn, J.M.; Mock, D.J.; Mayer-Proschel, M. Contributions of neurotropic human herpesviruses herpes simplex virus 1 and human herpesvirus 6 to neurodegenerative disease pathology. Neural Regen. Res. 2018, 13, 211–221. [Google Scholar] [CrossRef]
- Tiwari, S.; Akyuz, E.; Das, A. Recurrent Catatonia: Infection and Immunity in an Idiopathic Illness. J. Psychiatr. Pract. 2023, 29, 82–89. [Google Scholar] [CrossRef]
- Li, Y.; Weber, N.S.; Fisher, J.A.; Yolken, R.H.; Cowan, D.N.; Larsen, R.A.; Niebuhr, D.W. Association between antibodies to multiple infectious and food antigens and new onset schizophrenia among US military personnel. Schizophr. Res. 2013, 151, 36–42. [Google Scholar] [CrossRef]
- Niebuhr, D.W.; Millikan, A.M.; Cowan, D.N.; Yolken, R.; Li, Y.; Weber, N.S. Selected infectious agents and risk of schizophrenia among U.S. military personnel. Am. J. Psychiatry 2008, 165, 99–106. [Google Scholar] [CrossRef]
- Niebuhr, D.W.; Millikan, A.M.; Yolken, R.; Li, Y.; Weber, N.S. Results from a hypothesis generating case-control study: Herpes family viruses and schizophrenia among military personnel. Schizophr. Bull. 2008, 34, 1182–1188. [Google Scholar] [CrossRef]
- Conejero-Goldberg, C.; Torrey, E.F.; Yolken, R.H. Herpesviruses and Toxoplasma gondii in orbital frontal cortex of psychiatric patients. Schizophr. Res. 2003, 60, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.; Herrmann, Z.; Hsu, J.; Rush, A.J. HHV-6 and schizophrenia: An unusual presentation or an unproven etiology? Prim. Care Companion CNS Disord. 2022, 24, 21cr02944. [Google Scholar] [CrossRef] [PubMed]
- Hannachi, N.; El Kissi, Y.; Samoud, S.; Nakhli, J.; Letaief, L.; Gaabout, S.; Ali, B.B.; Boukadida, J. High prevalence of Human Herpesvirus 8 in schizophrenic patients. Psychiatry Res. 2014, 216, 192–197. [Google Scholar] [CrossRef]
- Carneiro, V.C.S.; Pereira, J.G.; de Paula, V.S. Family Herpesviridae and neuroinfections: Current status and research in progress. Mem. Inst. Oswaldo Cruz 2022, 117, e220200. [Google Scholar] [CrossRef]
- Opsahl, M.L.; Kennedy, P.G. Investigating the presence of human herpesvirus 7 and 8 in multiple sclerosis and normal control brain tissue. J. Neurol. Sci. 2006, 240, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Edelman, D.C. Human herpesvirus 8—A novel human pathogen. Virol. J. 2005, 2, 78. [Google Scholar] [CrossRef] [PubMed]
- Potvin, S.; Stip, E.; Sepehry, A.A.; Gendron, A.; Bah, R.; Kouassi, E. Inflammatory cytokine alterations in schizophrenia: A systematic quantitative review. Biol. Psychiatry 2008, 63, 801–808. [Google Scholar] [CrossRef]
- Schwarz, M.J.; Müller, N.; Riedel, M.; Ackenheil, M. The Th2-hypothesis of schizophrenia: A strategy to identify a subgroup of schizophrenia caused by immune mechanisms. Med. Hypotheses 2001, 56, 483–486. [Google Scholar] [CrossRef]
- Suthaus, J.; Adam, N.; Grötzinger, J.; Scheller, J.; Rose-John, S. Viral Interleukin-6: Structure, pathophysiology and strategies of neutralization. Eur. J. Cell Biol. 2011, 90, 495–504. [Google Scholar] [CrossRef]
- Deng, H.; Chu, J.T.; Rettig, M.B.; Martinez-Maza, O.; Sun, R. Rta of the human herpesvirus 8/Kaposi sarcoma-associated herpesvirus up-regulates human interleukin-6 gene expression. Blood 2002, 100, 1919–1921. [Google Scholar] [CrossRef]
- Zhao, J.; Punj, V.; Matta, H.; Mazzacurati, L.; Schamus, S.; Yang, Y.; Yang, T.; Hong, Y.; Chaudhary, P.M. K13 blocks KSHV lytic replication and deregulates vIL6 and hIL6 expression: A model of lytic replication induced clonal selection in viral oncogenesis. PLoS ONE 2007, 2, e1067. [Google Scholar] [CrossRef]
- Nakashima, K.; Taga, T. Mechanisms underlying cytokine-mediated cell-fate regulation in the nervous system. Mol. Neurobiol. 2002, 25, 233–244. [Google Scholar] [PubMed]
- Brown, A.S. The environment and susceptibility to schizophrenia. Prog. Neurobiol. 2011, 93, 23–58. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, E.; Gibson, T.; Colohan, H.A.; Walshe, D.; Buckley, P.; Larkin, C.; Waddington, J.L. Season of birth in schizophrenia. Evidence for confinement of an excess of winter births to patients without a family history of mental disorder. Br. J. Psychiatry 1991, 158, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S.; Derkits, E.J. Prenatal infection and schizophrenia: A review of epidemiologic and translational studies. Am. J. Psychiatry 2010, 167, 261–280. [Google Scholar] [CrossRef]
- Kępińska, A.P.; Iyegbe, C.O.; Vernon, A.C.; Yolken, R.; Murray, R.M.; Pollak, T.A. Schizophrenia and influenza at the centenary of the 1918–1919 Spanish influenza pandemic: Mechanisms of psychosis risk. Front. Psychiatry 2020, 11, 72. [Google Scholar] [CrossRef]
- Mednick, S.A.; Machon, R.A.; Huttunen, M.O.; Bonett, D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch. Gen. Psychiatry 1988, 45, 189–192. [Google Scholar] [CrossRef]
- Limosin, F.; Rouillon, F.; Payan, C.; Cohen, J.-M.; Strub, N. Prenatal exposure to influenza as a risk factor for adult schizophrenia. Acta Psychiatr. Scand. 2003, 107, 331–335. [Google Scholar] [CrossRef]
- Schwartz, P.J. Season of birth in schizophrenia: A maternal-fetal chronobiological hypothesis. Med. Hypotheses 2011, 76, 785–793. [Google Scholar] [CrossRef]
- Schwartz, P.J. Can the season of birth risk factor for schizophrenia be prevented by bright light treatment for the second trimester mother around the winter solstice? Med. Hypotheses 2014, 83, 809–815. [Google Scholar] [CrossRef]
- Brown, A.S.; Begg, M.D.; Gravenstein, S.; Schaefer, C.A.; Wyatt, R.J.; Bresnahan, M.; Babulas, V.P.; Susser, E.S. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch. Gen. Psychiatry 2004, 61, 774–780. [Google Scholar] [CrossRef]
- Shi, L.; Fatemi, S.H.; Sidwell, R.W.; Patterson, P.H. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J. Neurosci. 2003, 23, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Li, J.; Garbett, K.; Mirnics, K.; Patterson, P.H. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 2007, 27, 10695–10702. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U.; Murray, P.J.; Urwyler, A.; Yee, B.K.; Schedlowski, M.; Feldon, J. Adult behavioral and pharmacological dysfunctions following disruption of the fetal brain balance between pro-inflammatory and IL-10-mediated anti-inflammatory signaling. Mol. Psychiatry 2008, 13, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Beraki, S.; Aronsson, F.; Karlsson, H.; Ögren, S.O.; Kristensson, K. Influenza A virus infection causes alterations in expression of synaptic regulatory genes combined with changes in cognitive and emotional behaviors in mice. Mol. Psychiatry 2005, 10, 299–308. [Google Scholar] [CrossRef]
- Du, Y.; Li, X.-S.; Chen, L.; Chen, G.-Y.; Cheng, Y. A Network Analysis of Epigenetic and Transcriptional Regulation in a Neurodevelopmental Rat Model of Schizophrenia with Implications for Translational Research. Schizophr. Bull. 2020, 46, 612–622. [Google Scholar] [CrossRef]
- Andreau, F.; Galeano, P.; Caltana, L.R.; Masciotra, L.; Chertcoff, A.; Pontoriero, A.; Baumeister, E.; Amoroso, M.; Brusco, H.A.; Tous, M.I.; et al. Effects of Two Commonly Found Strains of Influenza A Virus on Developing Dopaminergic Neurons, in Relation to the Pathophysiology of Schizophrenia. PLoS ONE 2012, 7, e51068. [Google Scholar]
- Kepinska, A.; Pollak, T.; Iyegbe, C. SU116: Peptide sharing between schizophrenia-related proteins and the Influenza A virus may offer a link between immunity and psychotic disorders. Eur. Neuropsychopharmacol. 2019, 29, S1329. [Google Scholar] [CrossRef]
- Iyegbe, C.; Kepinska, A.; Pollak, T. 55. Multi-modal evidence that pandemic influenza influences schizophrenia risk. Eur. Neuropsychopharmacol. 2021, 51, e70. [Google Scholar] [CrossRef]
- Marreiros, R.; Prikulis, I.; Müller-Schiffmann, A.; Moreira, A.R.; Sahu, S.; Soloviev, I.; Selvarajah, S.; Lingappa, V.; Korth, C. Molecular linking of influenza infection to cellular pathology of protein misassembly: The case of Disrupted-in-Schizophrenia 1 (DISC1). Eur. Neuropsychopharmacol. 2018, 28, S15. [Google Scholar] [CrossRef]
- Holtze, M.; Asp, L.; Schwieler, L.; Engberg, G.; Karlsson, H. Induction of the kynurenine pathway by neurotropic influenza A virus infection. J. Neurosci. Res. 2008, 86, 3674–3683. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, S.; Olsson, S.K.; Engberg, G. Pharmacological manipulation of kynurenic acid: Potential in the treatment of psychiatric disorders. CNS Drugs 2009, 23, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Abi-Dargham, A.; Gil, R.; Krystal, J.; Baldwin, R.M.; Seibyl, J.P.; Bowers, M.; van Dyck, C.H.; Charney, D.S.; Innis, R.B.; Laruelle, M. Increased striatal dopamine transmission in schizophrenia: Confirmation in a second cohort. Am. J. Psychiatry 1998, 155, 761–767. [Google Scholar] [CrossRef]
- Selten, J.P.; Frissen, A.; Lensvelt-Mulders, G.; Morgan, V.A. Schizophrenia and 1957 pandemic of influenza: Meta-analysis. Schizophr. Bull. 2010, 36, 219–228. [Google Scholar] [CrossRef]
- Morgan, V.; Castle, D.; Page, A.; Fazio, S.; Gurrin, L.; Burton, P.; Montgomery, P.; Jablensky, A. Influenza epidemics and incidence of schizophrenia, affective disorders and mental retardation in Western Australia: No evidence of a major effect. Schizophr. Res. 1997, 26, 25–39. [Google Scholar] [CrossRef]
- Fung, S.G.; Fakhraei, R.; Condran, G.; Regan, A.K.; Dimanlig-Cruz, S.; Ricci, C.; Foo, D.; Sarna, M.; Török, E.; Fell, D.B. Neuropsychiatric outcomes in offspring after fetal exposure to maternal influenza infection during pregnancy: A systematic review. Reprod. Toxicol. 2022, 113, 155–169. [Google Scholar] [CrossRef]
- Gu, J.; Xie, Z.; Gao, Z.; Liu, J.; Korteweg, C.; Ye, J.; Lau, L.T.; Lu, J.; Gao, Z.; Zhang, B.; et al. H5N1 infection of the respiratory tract and beyond: A molecular pathology study. Lancet 2007, 370, 1137–1145. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Folsom, T.D.; Reutiman, T.J.; Sidwell, R.W. Viral regulation of aquaporin 4, connexin 43, microcephalin and nucleolin. Schizophr. Res. 2008, 98, 163–177. [Google Scholar] [CrossRef]
- Takei, N.; O’Callaghan, E.; Sham, P.C.; Glover, G.; Murray, R.M. Does prenatal influenza divert susceptible females from later affective psychosis to schizophrenia? Acta Psychiatr. Scand. 1993, 88, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Tochigi, M.; Okazaki, Y.; Kato, N.; Sasaki, T. What causes seasonality of birth in schizophrenia? Neurosci. Res. 2004, 48, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.J. Hyperthermia in utero due to maternal influenza is an environmental risk factor for schizophrenia. Congenit. Anom. 2007, 47, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Jurgens, H.A.; Amancherla, K.; Johnson, R.W. Influenza infection induces neuroinflammation, alters hippocampal neuron morphology, and impairs cognition in adult mice. J. Neurosci. 2012, 32, 3958–3968. [Google Scholar] [CrossRef]
- Hosseini, S.; Wilk, E.; Michaelsen-Preusse, K.; Gerhauser, I.; Baumgärtner, W.; Geffers, R.; Schughart, K.; Korte, M. Long-term neuroinflammation induced by influenza A virus infection and the impact on hippocampal neuron morphology and function. J. Neurosci. 2018, 38, 3060–3080. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Pearce, D.A.; Brooks, A.I.; Sidwell, R.W. Prenatal viral infection in mouse causes differential expression of genes in brains of mouse progeny: A potential animal model for schizophrenia and autism. Synapse 2005, 57, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.L.; Kurita, M.; Holloway, T.; López, J.; Cadagan, R.; Martínez-Sobrido, L.; García-Sastre, A.; González-Maeso, J. Maternal influenza viral infection causes schizophrenia-like alterations of 5-HT2A and mGlu2 receptors in the adult offspring. J. Neurosci. 2011, 31, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Suschana, E.; Anderson, T.; Hong, C.; Narikatte, A.; Silverberg, J.; Sharma, M.S. The Role of Anti-Inflammatory Diets and Supplementation in Metabolic Syndrome and Symptom Remission in Adults with Schizophrenia: A Systematic Review. Front. Psychiatry 2025, 15, 1506353. [Google Scholar] [CrossRef]
- Cheng, J.S.; Hu, J.H.; Chang, M.Y.; Lin, M.S.; Ku, H.P.; Chien, R.N.; Chang, M.L. Hepatitis C-associated late-onset schizophrenia: A nationwide, population-based cohort study. J. Psychiatry Neurosci. 2021, 46, E583–E591. [Google Scholar] [CrossRef]
- Shehata, G.A.; Ahmed, G.K.; Hassan, E.A.; Abdel Rehim, A.S.E.D.; Mahmoud, S.Z.; Masoud, N.A.; Seifeldein, G.S.; Hassan, W.A.; Aboshaera, K.O. Impact of direct-acting antivirals on neuropsychiatric and neurocognitive dysfunction in chronic hepatitis C patients. Egypt. J. Neurol. Psychiatry Neurosurg. 2022, 58, 143. [Google Scholar] [CrossRef]
- Adinolfi, L.E.; Nevola, R.; Lus, G.; Restivo, L.; Guerrera, B.; Romano, C.; Zampino, R.; Rinaldi, L.; Sellitto, A.; Giordano, M.; et al. Chronic hepatitis C virus infection and neurological and psychiatric disorders: An overview. World J. Gastroenterol. 2015, 21, 2269–2280. [Google Scholar] [CrossRef]
- Benrós, M.E.; Mortensen, P.B. The Role of Infections and Autoimmune Diseases for Schizophrenia and Depression: Findings from Large-Scale Epidemiological Studies. In Immunology and Psychiatry; Müller, N., Ed.; Springer: Cham, Switzerland, 2015; pp. 107–135. [Google Scholar]
- Karim, S.; Mirza, Z.; Kamal, M.A.; Abuzenadah, A.M.; Azhar, E.I.; Al-Qahtani, M.H.; Damanhouri, G.A.; Ahmad, F.; Gan, S.H.; Sohrab, S.S. The role of viruses in neurodegenerative and neurobehavioral diseases. CNS Neurol. Disord. Drug Targets 2014, 13, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Fuller, B.E.; Rodriguez, V.L.; Linke, A.; Sikirica, M.; Dirani, R.; Hauser, P. Prevalence of liver disease in veterans with bipolar disorder or schizophrenia. Gen. Hosp. Psychiatry 2011, 33, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, G.M.; Cousins, L.; Deakin, J.; Lennox, B.R.; Yolken, R.; Jones, P.B. Inflammation and immunity in schizophrenia: Implications for pathophysiology and treatment. Lancet Psychiatry 2015, 2, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Himelhoch, S.; McCarthy, J.F.; Ganoczy, D.; Medoff, D.; Kilbourne, A.; Goldberg, R.; Dixon, L.; Blow, F.C. Understanding associations between serious mental illness and hepatitis C virus among veterans: A national multivariate analysis. Psychosomatics 2009, 50, 30–37. [Google Scholar] [CrossRef]
- Hung, C.; Loh, E.; Hu, T.; Chiu, H.; Hsieh, H.; Chan, C.; Lan, T. Prevalence of hepatitis B and hepatitis C in patients with chronic schizophrenia living in institutions. J. Chin. Med. Assoc. 2012, 75, 275–280. [Google Scholar] [CrossRef]
- De Hert, M.; Franic, T.; Vidovic, D.; Wampers, M.; Van Eyck, D.; Van Herck, K.; Van Damme, P.; Peuskens, J. Prevalence of HIV and hepatitis C infection among patients with schizophrenia. Schizophr. Res. 2009, 108, 307–308. [Google Scholar] [CrossRef]
- Lluch, E.; Miller, B.J. Rates of hepatitis B and C in patients with schizophrenia: A meta-analysis. Gen. Hosp. Psychiatry 2019, 61, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Leucht, S.; Burkard, T.; Henderson, J.; Maj, M.; Sartorius, N. Physical illness and schizophrenia: A review of the literature. Acta Psychiatr. Scand. 2007, 116, 317–333. [Google Scholar] [CrossRef]
- Nakamura, Y.; Koh, M.; Miyoshi, E.; Ida, O.; Morikawa, M.; Tokuyama, A.; Nagano, T.; Honda, Y.; Iida, J.; Yamamoto, K.; et al. High prevalence of the hepatitis C virus infection among the inpatients of schizophrenia and psychoactive substance abuse in Japan. Prog. Neuropsychopharmacol. Biol. Psychiatry 2004, 28, 591–597. [Google Scholar] [CrossRef]
- Freudenreich, O.; Gandhi, R.T.; Walsh, J.P.; Henderson, D.C.; Goff, D.C. Hepatitis C in schizophrenia: Screening experience in a community-dwelling clozapine cohort. Psychosomatics 2007, 48, 405–411. [Google Scholar] [CrossRef]
- Mohd Hanafiah, K.; Groeger, J.; Flaxman, A.D.; Wiersma, S.T. Global epidemiology of hepatitis C virus infection: New estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013, 57, 1333–1342. [Google Scholar] [CrossRef]
- Chiu, Y.L.; Lin, H.C.; Kao, N.W.; Kao, S.; Lee, H.C. Increased risk of concurrent hepatitis C among male patients with schizophrenia. Psychiatry Res. 2017, 258, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Ramrakha, S.; Caspi, A.; Dickson, N.; Moffitt, T.E.; Paul, C. Psychiatric disorders and risky sexual behaviour in young adulthood: Cross sectional study in birth cohort. BMJ 2000, 321, 263–266. [Google Scholar] [CrossRef]
- Abdeljaber, M.H.; Nair, M.P.; Schork, M.A.; Schwartz, S.A. Depressed natural killer cell activity in schizophrenic patients. Immunol. Investig. 1994, 23, 259–268. [Google Scholar] [CrossRef]
- Helleberg, M.; Pedersen, M.G.; Pedersen, C.B.; Mortensen, P.B.; Obel, N. Associations between HIV and schizophrenia and their effect on HIV treatment outcomes: A nationwide population-based cohort study in Denmark. Lancet HIV 2015, 2, 344–350. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Valuev-Elliston, V.T.; Ivanova, O.N.; Kochetkov, S.N.; Starodubova, E.S.; Bartosch, B.; Isaguliants, M.G. Oxidative Stress during HIV Infection: Mechanisms and Consequences. Oxid. Med. Cell. Longev. 2016, 2016, 8910396. [Google Scholar] [CrossRef] [PubMed]
- Keshavan, M.S.; Kaneko, Y. Secondary psychoses: An update. World Psychiatry 2013, 12, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Misiak, B.; Frydecka, D.; Zawadzki, M.; Krefft, M.; Kiejna, A. Refining and integrating schizophrenia pathophysiology—Relevance of the allostatic load concept. Neurosci. Biobehav. Rev. 2014, 45, 183–201. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.J.; Heath, R.G.; Sautter, F.J.J.; Schwartz, B.D.; Garry, R.F.; Choi, B.; Beilke, M.A.; Hart, L.K. Antiretroviral antibodies: Implications for schizophrenia, schizophrenia spectrum disorders, and bipolar disorder. Biol. Psychiatry 1999, 45, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Gurling, H.M. Testing the retrovirus hypothesis of manic depression and schizophrenia with molecular genetic techniques. J. R. Soc. Med. 1988, 81, 332–334. [Google Scholar] [CrossRef]
- Bechter, K.; Reiber, H.; Herzog, S.; Fuchs, D.; Tumani, H.; Maxeiner, H.G. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: Identification of subgroups with immune responses and blood-CSF barrier dysfunction. J. Psychiatr. Res. 2010, 44, 321–330. [Google Scholar] [CrossRef]
- Hornig, M.; Lipkin, W.I. Infectious and immune factors in the pathogenesis of neurodevelopmental disorders: Epidemiology, hypotheses, and animal models. Ment. Retard. Dev. Disabil. Res. Rev. 2001, 7, 200–210. [Google Scholar] [CrossRef]
- Laskaris, L.E.; Di Biase, M.A.; Everall, I.; Chana, G.; Christopoulos, A.; Skafidas, E.; Cropley, V.L.; Pantelis, C. Microglial activation and progressive brain changes in schizophrenia. Br. J. Pharmacol. 2016, 173, 666–680. [Google Scholar] [CrossRef]
- Pérez-De La Cruz, V.; Königsberg, M.; Santamaría, A. Kynurenine pathway and disease: An overview. CNS Neurol. Disord. Drug Targets 2007, 6, 398–410. [Google Scholar]
- Atlas, A.; Gisslén, M.; Nordin, C.; Lindström, L.; Schwieler, L. Acute psychotic symptoms in HIV-1 infected patients are associated with increased levels of kynurenic acid in cerebrospinal fluid. Brain Behav. Immun. 2007, 21, 86–91. [Google Scholar] [CrossRef]
- Johansson, A.S.; Owe-Larsson, B.; Asp, L.; Kocki, T.; Adler, M.; Hetta, J.; Gardner, R.; Lundkvist, G.B.; Urbanska, E.M.; Karlsson, H. Activation of kynurenine pathway in ex vivo fibroblasts from patients with bipolar disorder or schizophrenia: Cytokine challenge increases production of 3-hydroxykynurenine. J. Psychiatr. Res. 2013, 47, 1815–1823. [Google Scholar] [CrossRef]
- Launay, J.M.; Copel, L.; Callebert, J.; Corvaia, N.; Bricaire, F.; Laplanche, J.L.; Saal, F.; Peries, J. Serotonin and human immunodeficiency viruses. Nouvelle Rev. Fr. Hematol. 1989, 31, 159–161. [Google Scholar]
- Sierra-Honigmann, A.M.; Carbone, K.M.; Yolken, R.H. Polymerase chain reaction (PCR) search for viral nucleic acid sequences in schizophrenia. Br. J. Psychiatry 1995, 166, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Leboyer, M.; Tamouza, R.; Charron, D.; Faucard, R.; Perron, H. Human Endogenous Retrovirus Type W (HERV-W) in Schizophrenia: A New Avenue of Research at the Gene–Environment Interface. World J. Biol. Psychiatry 2013, 14, 80–90. [Google Scholar] [CrossRef]
- Huang, W.; Li, S.; Hu, Y.; Yu, H.; Luo, F.; Zhang, Q.; Zhu, F. Implication of the env Gene of the Human Endogenous Retrovirus W Family in the Expression of BDNF and DRD3 and Development of Recent-Onset Schizophrenia. Schizophr. Bull. 2011, 37, 988–1000. [Google Scholar] [CrossRef]
- Weis, S.; Llenos, I.C.; Sabunciyan, S.; Dulay, J.R.; Isler, L.; Yolken, R.; Perron, H. Reduced Expression of Human Endogenous Retrovirus (HERV)-W GAG Protein in the Cingulate Gyrus and Hippocampus in Schizophrenia, Bipolar Disorder, and Depression. J. Neural Transm. 2007, 114, 645–655. [Google Scholar] [CrossRef]
- Aftab, A.; Shah, A.A.; Hashmi, A.M. Pathophysiological Role of HERV-W in Schizophrenia. J. Neuropsychiatry Clin. Neurosci. 2016, 28, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Slokar, G.; Hasler, G. Human Endogenous Retroviruses as Pathogenic Factors in the Development of Schizophrenia. Front. Psychiatry 2016, 6, 183. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Sabunciyan, S.; Yolken, R.H.; Lee, D.; Kim, S.; Karlsson, H. Transcription of Human Endogenous Retroviruses in Human Brain by RNA-Seq Analysis. PLoS ONE 2019, 14, e0207353. [Google Scholar] [CrossRef]
- Karlsson, H.; Bachmann, S.; Schröder, J.; McArthur, J.; Torrey, E.F.; Yolken, R.H. Retroviral RNA Identified in the Cerebrospinal Fluids and Brains of Individuals with Schizophrenia. Proc. Natl. Acad. Sci. USA 2001, 98, 4634–4639. [Google Scholar] [CrossRef]
- Yao, Y.; Schröder, J.; Nellåker, C.; Bottmer, C.; Bachmann, S.; Yolken, R.H.; Karlsson, H. Elevated Levels of Human Endogenous Retrovirus-W Transcripts in Blood Cells from Patients with First Episode Schizophrenia. Genes Brain Behav. 2008, 7, 103–112. [Google Scholar] [CrossRef]
- Huang, W.J.; Liu, Z.C.; Wei, W.; Wang, G.H.; Wu, J.G.; Zhu, F. Human Endogenous Retroviral pol RNA and Protein Detected and Identified in the Blood of Individuals with Schizophrenia. Schizophr. Res. 2006, 83, 193–199. [Google Scholar] [CrossRef]
- Dickerson, F.; Lillehoj, E.; Stallings, C.; Wiley, M.; Origoni, A.; Vaughan, C.; Khushalani, S.; Sabunciyan, S.; Yolken, R. Antibodies to Retroviruses in Recent Onset Psychosis and Multi-Episode Schizophrenia. Schizophr. Res. 2012, 138, 198–205. [Google Scholar] [CrossRef]
- Mak, M.; Samochowiec, J.; Frydecka, D.; Pełka-Wysiecka, J.; Szmida, E.; Karpiński, P.; Sąsiadek, M.M.; Piotrowski, P.; Samochowiec, A.; Misiak, B. First-Episode Schizophrenia Is Associated with a Reduction of HERV-K Methylation in Peripheral Blood. Psychiatry Res. 2019, 271, 459–463. [Google Scholar] [CrossRef]
- Christensen, T. Human Endogenous Retroviruses in Neurologic Disease. APMIS 2016, 124, 116–126. [Google Scholar] [CrossRef]
- Li, F.; Karlsson, H. Expression and Regulation of Human Endogenous Retrovirus W Elements. APMIS 2016, 124, 52–66. [Google Scholar] [CrossRef]
- Suntsova, M.; Gogvadze, E.V.; Salozhin, S.; Gaifullin, N.; Eroshkin, F.; Dmitriev, S.E.; Martynova, N.; Kulikov, K.; Malakhova, G.; Tukhbatova, G.; et al. Human-Specific Endogenous Retroviral Insert Serves as an Enhancer for the Schizophrenia-Linked Gene PRODH. Proc. Natl. Acad. Sci. USA 2013, 110, 19472–19477. [Google Scholar] [CrossRef]
- Iturrieta-Zuazo, I.; Alelú-Paz, R. Human Endogenous Retroviruses, Hormones and APOBEC3G: A Connection to Explore in Schizophrenia. Biosci. Hypotheses 2009, 2, 236–239. [Google Scholar] [CrossRef]
- Sharma, R.P.; Rosen, C.; Melbourne, J.K.; Feiner, B.; Chase, K.A. Activated Phosphorylated STAT1 Levels as a Biologically Relevant Immune Signal in Schizophrenia. Neuroimmunomodulation 2016, 23, 224–229. [Google Scholar] [CrossRef]
- Song, X.Q.; Lv, L.X.; Li, W.Q.; Hao, Y.H.; Zhao, J.P. The Interaction of Nuclear Factor-Kappa B and Cytokines Is Associated with Schizophrenia. Biol. Psychiatry 2009, 65, 481–488. [Google Scholar] [CrossRef]
- Küry, P.; Nath, A.; Créange, A.; Dolei, A.; Marche, P.; Gold, J.; Giovannoni, G.; Hartung, H.P.; Perron, H. Human Endogenous Retroviruses in Neurological Diseases. Trends Mol. Med. 2018, 24, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Perron, H.; Mekaoui, L.; Bernard, C.; Veas, F.; Stefas, I.; Leboyer, M. Endogenous Retrovirus Type W GAG and Envelope Protein Antigenemia in Serum of Schizophrenic Patients. Biol. Psychiatry 2008, 64, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Balestrieri, E.; Matteucci, C.; Cipriani, C.; Grelli, S.; Ricceri, L.; Calamandrei, G.; Vallebona, P.S. Endogenous Retroviruses Activity as a Molecular Signature of Neurodevelopmental Disorders. Int. J. Mol. Sci. 2019, 20, 6050. [Google Scholar] [CrossRef]
- Wu, X.; Yan, Q.; Liu, L.; Xue, X.; Yao, W.; Li, X.; Li, W.; Ding, S.; Xia, Y.; Zhang, D.; et al. Domesticated HERV-W env Contributes to the Activation of the Small Conductance Ca2+-Activated K+ Type 2 Channels via Decreased 5-HT4 Receptor in Recent-Onset Schizophrenia. Virol. Sin. 2023, 38, 9–22. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, Q.; Zhou, P.; Li, S.; Zhu, F. HERV-W env Regulates Calcium Influx via Activating TRPC3 Channel Together with Depressing DISC1 in Human Neuroblastoma Cells. J. Neurovirol. 2019, 25, 101–113. [Google Scholar] [CrossRef]
- Li, S.; Liu, Z.C.; Yin, S.J.; Chen, Y.T.; Yu, H.L.; Zeng, J.; Zhang, Q.; Zhu, F. Human Endogenous Retrovirus W Family Envelope Gene Activates the Small Conductance Ca2+-Activated K+ Channel in Human Neuroblastoma Cells through CREB. Neuroscience 2013, 247, 164–174. [Google Scholar] [CrossRef]
- Grube, S.; Gerchen, M.F.; Adamcio, B.; Pardo, L.A.; Martin, S.; Malzahn, D.; Papiol, S.; Begemann, M.; Ribbe, K.; Friedrichs, H.; et al. A CAG Repeat Polymorphism of KCNN3 Predicts SK3 Channel Function and Cognitive Performance in Schizophrenia. EMBO Mol. Med. 2011, 3, 309–319. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, X.; Xue, X.; Li, W.; Zhou, P.; Lv, Z.; Zhao, K.; Zhu, F. Ancient Dormant Virus Remnant ERVW-1 Drives Ferroptosis via Degradation of GPX4 and SLC3A2 in Schizophrenia. Virol. Sin. 2024, 39, 31–43. [Google Scholar] [CrossRef]
- Radkowski, M.; Wilkinson, J.; Nowicki, M.; Adair, D.; Vargas, H.; Ingui, C.; Rakela, J.; Laskus, T. Search for Hepatitis C Virus Negative-Strand RNA Sequences and Analysis of Viral Sequences in the Central Nervous System: Evidence of Replication. J. Virol. 2002, 76, 600–608. [Google Scholar] [CrossRef]
- Powell, T.; Duarte, R.R.; Pain, O.; Bendall, M.; de Mulder Rougvie, M.; Marston, J.; Selvackadunco, S.; Troakes, C.; O’Reilly, P.; Srivastava, D.; et al. Integrating Human Endogenous Retroviruses in Transcriptome-Wide Association Studies Highlights Novel Risk Factors for Major Psychiatric Conditions. Eur. Neuropsychopharmacol. 2023, 75, S84. [Google Scholar] [CrossRef]
- Frank, O.; Giehl, M.; Zheng, C.; Hehlmann, R.; Leib-Mösch, C.; Seifarth, W. Human Endogenous Retrovirus Expression Profiles in Samples from Brains of Patients with Schizophrenia and Bipolar Disorders. J. Virol. 2005, 79, 10890–10901. [Google Scholar] [CrossRef]
- Canli, T. A Model of Human Endogenous Retrovirus (HERV) Activation in Mental Health and Illness. Med. Hypotheses 2019, 133, 109404. [Google Scholar] [CrossRef]
- Nogueira, C.O.; Lopes da Silva, M.O.; de Lima, E.V.; Christoff, R.R.; Gavino-Leopoldino, D.; Lemos, F.S.; da Silva, N.E.; Da Poian, A.T.; Assunção-Miranda, I.; Figueiredo, C.P.; et al. Immunosuppression-induced Zika virus reactivation causes brain inflammation and behavioral deficits in mice. iScience 2024, 27, 110178. [Google Scholar] [CrossRef]
- Elgueta, D.; Murgas, P.; Riquelme, E.; Yang, G.; Cancino, G.I. Consequences of Viral Infection and Cytokine Production During Pregnancy on Brain Development in Offspring. Front. Immunol. 2022, 13, 816619. [Google Scholar] [CrossRef]
- Kung, P.L.; Chou, T.W.; Lindman, M.; Chang, N.P.; Estevez, I.; Buckley, B.D.; Atkins, C.; Daniels, B.P. Zika virus-induced TNF-α signaling dysregulates expression of neurologic genes associated with psychiatric disorders. J. Neuroinflamm. 2022, 19, 100. [Google Scholar] [CrossRef] [PubMed]
- Pierson, J.; Yeruva, R.R.; El-Mallakh, R.S. Can in utero Zika virus exposure be a risk factor for schizophrenia in the offspring? World J. Biol. Psychiatry 2020, 21, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Klase, Z.A.; Khakhina, S.; Schneider, A.D.B.; Callahan, M.V.; Glasspool-Malone, J.; Malone, R. Zika fetal neuropathogenesis: Etiology of a viral syndrome. PLoS Negl. Trop. Dis. 2016, 10, e0004877. [Google Scholar] [CrossRef]
- Souza, I.N.; Frost, P.S.; França, J.V.; Nascimento-Viana, J.B.; Neris, R.L.S.; Freitas, L.; Pinheiro, D.J.L.L.; Nogueira, C.O.; Neves, G.; Chimelli, L.; et al. Acute and chronic neurological consequences of early-life Zika virus infection in mice. Sci. Transl. Med. 2018, 10, eaar2749. [Google Scholar] [CrossRef] [PubMed]
- Kapogiannis, B.G.; Chakhtoura, N.; Hazra, R.; Spong, C.Y. Bridging knowledge gaps to understand how Zika virus exposure and infection affect child development. JAMA Pediatr. 2017, 171, 478–485. [Google Scholar] [CrossRef]
- Janssens, S.; Schotsaert, M.; Karnik, R.; Balasubramaniam, V.; Dejosez, M.; Meissner, A.; García-Sastre, A.; Zwaka, T.P. Zika Virus Alters DNA Methylation of Neural Genes in an Organoid Model of the Developing Human Brain. mSystems 2018, 3, e00217–e00219. [Google Scholar] [CrossRef]
- Corrêa-Oliveira, G.E.; do Amaral, J.L.; da Fonseca, B.A.L.; Del-Ben, C.M. Zika virus infection followed by a first episode of psychosis: Another flavivirus leading to pure psychiatric symptomatology. Rev. Bras. Psiquiatr. 2017, 39, 381–382. [Google Scholar] [CrossRef]
- Zucker, J.; Neu, N.; Chiriboga, C.A.; Hinton, V.J.; Leonardo, M.; Sheikh, A.; Thakur, K. Zika virus-associated cognitive impairment in adolescent, 2016. Emerg. Infect. Dis. 2017, 23, 1047–1048. [Google Scholar] [CrossRef]
- Carteaux, G.; Maquart, M.; Bedet, A.; Contou, D.; Brugieres, P.; Fourati, S.; de Langavant, L.C.; de Broucker, T.; Brun-Buisson, C.; Leparc-Goffart, I.; et al. Zika virus associated with meningoencephalitis. N. Engl. J. Med. 2016, 374, 1595–1596. [Google Scholar] [CrossRef]
- Aguilar Ticona, J.P.; Nery, N.; Ladines-Lim, J.B.; Gambrah, C.; Sacramento, G.; de Paula Freitas, B.; Bouzon, J.; Oliveira-Filho, J.; Borja, A.; Adhikarla, H.; et al. Developmental outcomes in children exposed to Zika virus in utero from a Brazilian urban slum cohort study. PLoS Negl. Trop. Dis. 2021, 15, e0009162. [Google Scholar] [CrossRef]
- Stringer, E.M.; Martinez, E.; Blette, B.; Toval Ruiz, C.E.; Boivin, M.; Zepeda, O.; Stringer, J.S.A.; Morales, M.; Ortiz-Pujols, S.; Familiar, I.; et al. Neurodevelopmental outcomes of children following in utero exposure to Zika in Nicaragua. Clin. Infect. Dis. 2021, 73, e14–e16. [Google Scholar] [CrossRef]
- Grant, R.; Fléchelles, O.; Tressières, B.; Dialo, M.; Elenga, N.; Mediamolle, N.; Mallard, A.; Hebert, J.C.; Lachaume, N.; Couchy, E.; et al. In utero Zika virus exposure and neurodevelopment at 24 months in toddlers normocephalic at birth: A cohort study. BMC Med. 2021, 19, 12. [Google Scholar] [CrossRef]
- Sheffield, J.M.; Karcher, N.R.; Barch, D.M. Cognitive deficits in psychotic disorders: A lifespan perspective. Neuropsychol. Rev. 2018, 28, 509–533. [Google Scholar] [CrossRef]
- Zimmer, A.; Youngblood, A.; Adnane, A.; Miller, B.J.; Goldsmith, D.R. Prenatal exposure to viral infection and neuropsychiatric disorders in offspring: A review of the literature and recommendations for the COVID-19 pandemic. Brain Behav. Immun. 2021, 91, 756–770. [Google Scholar] [CrossRef]
- Ebinger, A.; Santos, P.D.; Pfaff, F.; Dürrwald, R.; Kolodziejek, J.; Schlottau, K.; Ruf, V.; Liesche-Starnecker, F.; Ensser, A.; Korn, K.; et al. Lethal Borna disease virus 1 infections of humans and animals—In-depth molecular epidemiology and phylogeography. Nat. Commun. 2024, 15, 7908. [Google Scholar] [CrossRef]
- van den Pol, A.N. Viral infection leading to brain dysfunction: More prevalent than appreciated? Neuron 2009, 64, 17–20. [Google Scholar] [CrossRef]
- Pörtner, K.; Wilking, H.; Frank, C.; Stark, K.; Wunderlich, S.; Tappe, D. Clinical analysis of Bornavirus Encephalitis cases demonstrates a small time window for etiological diagnostics and treatment attempts, a large case series from Germany 1996–2022. Infection 2025, 53, 155–164. [Google Scholar] [CrossRef]
- Nawa, H.; Takei, N. Recent progress in animal modeling of immune inflammatory processes in schizophrenia: Implication of specific cytokines. Neurosci. Res. 2006, 56, 2–13. [Google Scholar] [CrossRef]
- Boksa, P. Animal models of obstetric complications in relation to schizophrenia. Brain Res. Brain Res. Rev. 2004, 45, 1–17. [Google Scholar] [CrossRef]
- Bilzer, T.; Planz, O.; Lipkin, W.I.; Stitz, L. Presence of CD4+ and CD8+ T cells and expression of MHC class I and MHC class II antigen in horses with Borna disease virus-induced encephalitis. Brain Pathol. 1995, 5, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Smith, S.E.; Malkova, N.; Tse, D.; Su, Y.; Patterson, P.H. Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav. Immun. 2009, 23, 116–123. [Google Scholar] [CrossRef]
- Williams, B.L.; Yaddanapudi, K.; Hornig, M.; Lipkin, W.I. Spatiotemporal analysis of Purkinje cell degeneration relative to parasagittal expression domains in a model of neonatal viral infection. J. Virol. 2007, 81, 2675–2687. [Google Scholar] [CrossRef]
- Kim, Y.K.; Maes, M. The role of the immune system in the pathophysiology of schizophrenia. Brain Behav. Immun. 2005, 19, 1–12. [Google Scholar]
- Miranda, H.C.; Nunes, S.O.; Calvo, E.S.; Suzart, S.; Itano, E.N.; Watanabe, M.A. Detection of Borna disease virus p24 RNA in peripheral blood cells from Brazilian mood and psychotic disorder patients. J. Affect. Disord. 2006, 90, 43–47. [Google Scholar] [CrossRef]
- Bode, L.; Ludwig, H. Borna disease virus infection, a human mental-health risk. Clin. Microbiol. Rev. 2003, 16, 534–545. [Google Scholar] [CrossRef]
- Schneider, U.; Schwemmle, M.; Staeheli, P. Genome trimming: A unique strategy for replication control employed by Borna disease virus. Proc. Natl. Acad. Sci. USA 2005, 102, 3441–3446. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, A.; Adamaszek, M. Anti-Borna disease virus antibody responses in psychiatric patients: Long-term follow up. Psychiatry Clin. Neurosci. 2010, 64, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Corrado, S.; Marchesi, F.; Bottazzoli, M. Clinical Features of BoDV-1 Encephalitis: A Systematic Review. Zoonotic Dis. 2023, 3, 279–300. [Google Scholar] [CrossRef]
- Matsunaga, H.; Fukumori, A.; Mori, K.; Morihara, T.; Sato, S.; Kitauchi, K.; Yanagida, K.; Taguchi, K.; Honda, T.; Tomonaga, K. Ribavirin Treatment for Severe Schizophrenia with Anti-Borna Disease Virus 1 Antibodies 30 Years after Onset. Case Rep. Psychiatry 2023, 2023, 4899364. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Sawada, T.; Naraki, T.; Igata-Yi, R.; Shiraki, H.; Horii, Y.; Ishii, T.; Ikeda, K.; Asou, N.; Okabe, H.; et al. Detection of Borna Disease Virus-reactive antibodies from patients with psychiatric disorders and from horses by electrochemiluminescence immunoassay. Clin. Diagn. Lab. Immunol. 1999, 6, 696–700. [Google Scholar] [CrossRef]
- Azami, M.; Jalilian, F.A.; Khorshidi, A.; Mohammadi, Y.; Tardeh, Z. The association between Borna Disease Virus and schizophrenia: A systematic review and meta-analysis. Asian J. Psychiatr. 2018, 34, 67–73. [Google Scholar] [CrossRef]
- Karakose, A.R.; Yuksel, P.; Turan, N.; Ziver, T.; Saribas, S.; Alpay, N.; Balcıoğlu, İ.; Aslan, M.; Helps, C.R.; Yilmaz, H.; et al. Does borna disease virus (BDV) have a role in the etiopathogenesis of schizophrenia? Afr. J. Microbiol. Res. 2011, 5, 1062–1069. [Google Scholar] [CrossRef]
- Waltrip, R.W., II; Buchanan, R.W.; Summerfelt, A.; Breier, A.; Carpenter, W.T.J.; Bryant, N.L.; Rubin, S.A.; Carbone, K.M. Borna disease virus and schizophrenia. Psychiatry Res. 1995, 56, 33–44. [Google Scholar] [CrossRef]
- Iwahashi, K.; Watanabe, M.; Nakamura, K.; Suwaki, H.; Nakaya, T.; Nakamura, Y.; Takahashi, H.; Ikuta, K. Clinical investigation of the relationship between Borna disease virus (BDV) infection and schizophrenia in 67 patients in Japan. Acta Psychiatr. Scand. 1997, 96, 412–415. [Google Scholar] [CrossRef]
- Ludwig, H.; Bode, L. The neuropathogenesis of Borna disease virus infections. Intervirology 1997, 40, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Strous, R.D.; Shoenfeld, Y. Schizophrenia, autoimmunity and immune system dysregulation: A comprehensive model updated and revisited. J. Autoimmun. 2006, 27, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Ledgerwood, L.G.; Ewald, P.W.; Cochran, G.M. Genes, germs, and schizophrenia: An evolutionary perspective. Perspect. Biol. Med. 2003, 46, 317–348. [Google Scholar] [CrossRef] [PubMed]
- Bechter, K.; Herzog, S.; Estler, H.C.; Schüttler, R. Increased psychiatric comorbidity in Borna disease virus seropositive psychiatric patients. Acta Psychiatr. Belg. 1998, 98, 190–204. [Google Scholar]
- Bechter, K.; Herzog, S.; Schreiner, V.; Wollinsky, K.H.; Schüttler, R. Cerebrospinal fluid filtration in a case of schizophrenia related to “subclinical” Borna disease virus encephalitis. Key Topics in Brain Research. In Psychiatry, Psychoimmunology, and Viruses; Müller, N., Ed.; Springer-Verlag: Wien, Austria, 1999; pp. 19–35. [Google Scholar]
- Terayama, H.; Nishino, Y.; Kishi, M.; Ikuta, K.; Itoh, M.; Iwahashi, K. Detection of anti-Borna disease virus (BDV) antibodies from patients with schizophrenia and mood disorders in Japan. Psychiatry Res. 2003, 120, 201–206. [Google Scholar] [CrossRef]
- Fukuda, K.; Takahashi, K.; Iwata, Y.; Mori, N.; Gonda, K.; Ogawa, T.; Osonoe, K.; Sato, M.; Ogata, S.-I.; Horimoto, T.; et al. Immunological and PCR analyses for Borna Disease Virus in psychiatric patients and blood donors in Japan. J. Clin. Microbiol. 2001, 39, 419–429. [Google Scholar] [CrossRef]
- Chen, C.H.; Chiu, Y.L.; Shaw, C.K.; Tsai, M.T.; Hwang, A.L.; Hsiao, K.J. Detection of Borna Disease Virus RNA from peripheral blood cells in schizophrenic patients and mental health workers. Mol. Psychiatry 1999, 4, 566–571. [Google Scholar] [CrossRef]
- Chen, C.H.; Chiu, Y.L.; Wei, F.C.; Koong, F.J.; Liu, H.C.; Shaw, C.K.; Hwu, H.G.; Hsiao, K.J. High seroprevalence of Borna Virus infection in schizophrenic patients, family members and mental health workers in Taiwan. Mol. Psychiatry 1999, 4, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Na, K.S.; Tae, S.H.; Song, J.W.; Kim, Y.K. Failure to detect Borna Disease Virus antibody and RNA from peripheral blood mononuclear cells of psychiatric patients. Psychiatry Investig. 2009, 6, 306–312. [Google Scholar] [CrossRef]
- Yang, A.Y.; Zhang, F.M.; Li, J.H.; Li, G.M.; Ma, P.L.; Gu, H.X.; Ikuta, K. Detection of Borna disease virus-p24 specific antibody in the sera of schizophrenic patients of China by means of Western-blot. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 2003, 17, 85–87. [Google Scholar] [PubMed]
- Iwata, Y.; Takahashi, K.; Peng, X.; Fukuda, K.; Ohno, K.; Ogawa, T.; Gonda, K.; Mori, N.; Niwa, S.; Shigeta, S. Detection and sequence analysis of Borna disease virus p24 RNA from peripheral blood mononuclear cells of patients with mood disorders or schizophrenia and of blood donors. J. Virol. 1998, 72, 10044–10049. [Google Scholar] [CrossRef]
- Selten, J.P.; Slaets, J.; Kahn, R. Prenatal exposure to influenza and schizophrenia in Surinamese and Dutch Antillean immigrants to The Netherlands. Schizophr. Res. 1998, 30, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Lipkin, W.I.; Hornig, M.; Briese, T. Borna disease virus and neuropsychiatric disease—A reappraisal. Trends Microbiol. 2001, 9, 295–298. [Google Scholar] [CrossRef]
- Soltani, H.; Mohammadzadeh, S.; Makvandi, M.; Pakseresht, S.; Samarbaf-Zadeh, A. Detection of Borna Disease Virus (BDV) in Patients with First Episode of Schizophrenia. Iran. J. Psychiatry 2016, 11, 257–261. [Google Scholar]
- Selten, J.; van Loon, A.M.; van Vliet, K.; Pleyte, W.; Hoek, H.; Kahn, R. Borna Disease Virus in Caribbean immigrants to the Netherlands, diagnosed with schizophrenia. Schizophr. Res. 1998, 1, 19. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, S.H.; Han, C.S.; Lee, H.J.; Kim, H.S.; Yoon, S.C.; Kim, D.J.; Song, K.J.; Maes, M.; Song, J.W. Borna disease virus and deficit schizophrenia. Acta Neuropsychiatr. 2003, 15, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Hornig, M.; Briese, T.; Licinio, J.; Khabbaz, R.F.; Altshuler, L.L.; Potkin, S.G.; Schwemmle, M.; Siemetzki, U.; Mintz, J.; Honkavuori, K.; et al. Absence of evidence for bornavirus infection in schizophrenia, bipolar disorder and major depressive disorder. Mol. Psychiatry 2012, 17, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Ovanesov, M.V.; Ayhan, Y.; Wolbert, C.; Moldovan, K.; Sauder, C.; Pletnikov, M.V. Astrocytes play a key role in activation of microglia by persistent Borna disease virus infection. J. Neuroinflamm. 2008, 5, 50. [Google Scholar] [CrossRef]
- Harrison, P.J.; Weinberger, D.R. Schizophrenia genes, gene expression, and neuropathology: On the matter of their convergence. Mol. Psychiatry 2005, 10, 40–68. [Google Scholar] [CrossRef]
- Nagy, M.A.; Mahmoud, M.M. Schizophrenia and Viruses: A Focus on Coronavirus Disease (COVID-19). Clin. Med. Rev. Rep. 2022, 4. [Google Scholar] [CrossRef]
- Butler, M.; Cross, B.; Hafeez, D.; Lim, M.F.; Morrin, H.; Rengasamy, E.R.; Pollak, T.; Nicholson, T.R. Emerging Knowledge of the Neurobiology of COVID-19. Psychiatr. Clin. N. Am. 2022, 45, 29–43. [Google Scholar] [CrossRef]
- Kulaga, S.S.; Miller, C.W.T. Viral Respiratory Infections and Psychosis: A Review of the Literature and the Implications of COVID-19. Neurosci. Biobehav. Rev. 2021, 127, 520–530. [Google Scholar] [CrossRef]
- Goldsmith, D.R.; Haroon, E.; Miller, A.H.; Strauss, G.P.; Buckley, P.F.; Miller, B.J. TNF-α and IL-6 Are Associated with the Deficit Syndrome and Negative Symptoms in Patients with Chronic Schizophrenia. Schizophr. Res. 2018, 199, 281–284. [Google Scholar] [CrossRef]
- Canham, L.J.W.; Staniaszek, L.E.; Mortimer, A.M.; Nouri, L.F.; Kane, N.M. Electroencephalographic (EEG) Features of Encephalopathy in the Setting of COVID-19: A Case Series. Clin. Neurophysiol. Pract. 2020, 5, 199–205. [Google Scholar] [CrossRef]
- DeLisi, L.E. A Commentary Revisiting the Viral Hypothesis of Schizophrenia: Onset of a Schizophreniform Disorder Subsequent to SARS-CoV-2 Infection. Psychiatry Res. 2021, 295, 113573. [Google Scholar] [CrossRef] [PubMed]
- Pallanti, S.; Di Ponzio, M.; Gavazzi, G.; Gasic, G.; Besteher, B.; Heller, C.; Kikinis, R.; Makris, N.; Kikinis, Z. From ‘Mental Fog’ to Post-Acute COVID-19 Syndrome’s Executive Function Alteration: Implications for Clinical Approach. J. Psychiatr. Res. 2023, 167, 10–15. [Google Scholar] [CrossRef]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-Month Neurological and Psychiatric Outcomes in 236,379 Survivors of COVID-19: A Retrospective Cohort Study Using Electronic Health Records. Lancet Psychiatry 2021, 8, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Zandifar, A.; Badrfam, R. COVID-19: Considering the Prevalence of Schizophrenia in the Coming Decades. Psychiatry Res. 2020, 288, 112982. [Google Scholar] [CrossRef]
- Watson, C.J.; Thomas, R.H.; Solomon, T.; Michael, B.D.; Nicholson, T.R.; Pollak, T.A. COVID-19 and Psychosis Risk: Real or Delusional Concern? Neurosci. Lett. 2021, 741, 135491. [Google Scholar] [CrossRef]
- Baranova, A.; Cao, H.; Zhang, F. Severe COVID-19 Increases the Risk of Schizophrenia. Psychiatry Res. 2022, 317, 114809. [Google Scholar] [CrossRef]
- Amin, M.M.; Futrawan, R.; Husada, M.S. Correlation Between Schizophrenia and Coronavirus Disease in North Sumatera, Indonesia: A Correlative Analytical Study. Front. Psychiatry 2022, 13, 896623. [Google Scholar] [CrossRef]
- Pourfridoni, M.; Askarpour, H. COVID-19 and the Increase in Schizophrenia Incidence in the Future: A Hypothesis and a Serious Warning. Health Sci. Rep. 2022, 6, e978. [Google Scholar] [CrossRef]
- Hu, W.; Su, L.; Li, D.; Zhou, Y.; Zhu, J. Risk of First-Episode Schizophrenia in Aged Adults Increased During COVID-19 Outbreak. Int. J. Ment. Health Addict. 2021, 21, 1455–1465. [Google Scholar] [CrossRef]
- Smith, C.M.; Gilbert, E.B.; Riordan, P.A.; Helmke, N.; von Isenburg, M.; Kincaid, B.R.; Shirey, K.G. COVID-19-Associated Psychosis: A Systematic Review of Case Reports. Gen. Hosp. Psychiatry 2021, 73, 84–100. [Google Scholar] [CrossRef] [PubMed]
- Varatharaj, A.; Thomas, N.; Ellul, M.A.; Davies, N.W.S.; Pollak, T.A.; Tenorio, E.L.; Sultan, M.; Easton, A.; Breen, G.; Zandi, M.S.; et al. Neurological and Neuropsychiatric Complications of COVID-19 in 153 Patients: A UK-Wide Surveillance Study. Lancet Psychiatry 2020, 7, 875–882. [Google Scholar] [CrossRef]
- Alquisiras-Burgos, I.; Peralta-Arrieta, I.; Alonso-Palomares, L.A.; Zacapala-Gómez, A.E.; Salmerón-Bárcenas, E.G.; Aguilera, P. Neurological Complications Associated with the Blood-Brain Barrier Damage Induced by the Inflammatory Response During SARS-CoV-2 Infection. Mol. Neurobiol. 2021, 58, 520–535. [Google Scholar] [CrossRef] [PubMed]
- Quincozes-Santos, A.; Rosa, R.L.; Tureta, E.F.; Bobermin, L.D.; Berger, M.; Guimarães, J.A.; Santi, L.; Beys-da-Silva, W.O. COVID-19 Impacts the Expression of Molecular Markers Associated with Neuropsychiatric Disorders. Brain Behav. Immun. Health 2021, 11, 100196. [Google Scholar] [CrossRef] [PubMed]
- Kotsiri, I.; Resta, P.; Spyrantis, A.; Panotopoulos, C.; Chaniotis, D.; Beloukas, A.; Magiorkinis, E. Viral Infections and Schizophrenia: A Comprehensive Review. Viruses 2023, 15, 1345. [Google Scholar] [CrossRef] [PubMed]
- Patlola, S.R.; Donohoe, G.; McKernan, D.P. Counting the Toll of Inflammation on Schizophrenia—A Potential Role for Toll-like Receptors. Biomolecules 2023, 13, 1188. [Google Scholar] [CrossRef]
- Kowalski, K.; Misiak, B. Schizophrenia and the COVID-19 Pandemic: A Narrative Review from the Biomedical Perspective. Span. J. Psychiatry Ment Health 2025, 18, 141–148. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Namkung, H.; Smith, A.; Sakamoto, S.; Zhu, X.; Ishizuka, K.; Lane, A.P.; Sawa, A.; Kamiya, A. Causal Impact of Local Inflammation in the Nasal Cavity on Higher Brain Function and Cognition. Neurosci. Res. 2021, 172, 110–115. [Google Scholar] [CrossRef]
- Yang, K.; Hua, J.; Etyemez, S.; Paez, A.; Prasad, N.; Ishizuka, K.; Sawa, A.; Kamath, V. Volumetric Alteration of Olfactory Bulb and Immune-Related Molecular Changes in Olfactory Epithelium in First Episode Psychosis Patients. Schizophr. Res. 2021, 235, 9–11. [Google Scholar] [CrossRef]
- Hobbs, J.A. Detection of adeno-associated virus 2 and parvovirus B19 in the human dorsolateral prefrontal cortex. J. Neurovirol. 2006, 12, 190–199. [Google Scholar] [CrossRef]
- Cavalcante, M.B.; Cavalcante, C.T.M.B.; Sarno, M.; Barini, R.; Kwak-Kim, J. Maternal immune responses and obstetrical outcomes of pregnant women with COVID-19 and possible health risks of offspring. J. Reprod. Immunol. 2021, 143, 103250. [Google Scholar] [CrossRef]
- Dickerson, F.; Stallings, C.; Origoni, A.; Copp, C.; Khushalani, S.; Yolken, R. Antibodies to measles in individuals with recent onset psychosis. Schizophr. Res. 2010, 119, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Eagles, J.M. Are polioviruses a cause of schizophrenia? Br. J. Psychiatry 1992, 160, 598–600. [Google Scholar] [CrossRef] [PubMed]
- Rantakallio, P.; Lapinleimu, K.; Mäntyjärvi, R. Coxsackie B5 outbreak in a newborn nursery with 17 cases of serous meningitis. Scand. J. Infect. Dis. 1970, 2, 17–23. [Google Scholar] [CrossRef]
- Brown, A.S.; Cohen, P.; Harkavy-Friedman, J.; Babulas, V.; Malaspina, D.; Gorman, J.M.; Susser, E.S. Prenatal rubella, premorbid abnormalities, and adult schizophrenia. Biol. Psychiatry 2001, 49, 473–486. [Google Scholar] [CrossRef]
- Mahato, K.; Maben, A.J.; Sharma, A. Psychosis and catatonia associated with West Nile virus. Prim. Care Companion CNS Disord. 2023, 25, 22cr03379. [Google Scholar] [CrossRef]
- Chaudhury, S.; Jagtap, B.; Ghosh, D.K. Psychosis in dengue fever. Med. J. Dr. D.Y. Patil Univ. 2017, 10, 202–204. [Google Scholar] [CrossRef]
- Elavia, Z.; Patra, S.S.; Kumar, S.; Inban, P.; Yousuf, M.A.; Thassu, I.; Chaudhry, H.A. Acute Psychosis and Mania: An Uncommon Complication of Dengue Fever. Cureus 2023, 15, e47425. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.G.; Galvão-Phileto, A.V.; Lima, N.S.; Jesus, R.S.; Galvão-Castro, B.; Lima, M.G. Frequency of mental disturbances in HTLV-1 patients in the state of Bahia, Brazil. Braz. J. Infect. Dis. 2009, 13, 5–8. [Google Scholar] [CrossRef]
- Hammond, C.J.; Hobbs, J.A. Parvovirus B19 infection of brain: Possible role of gender in determining mental illness and autoimmune thyroid disorders. Med. Hypotheses 2007, 69, 113–116. [Google Scholar] [CrossRef]
- Torrey, E.F.; Peterson, M.R. Slow and latent viruses in schizophrenia. Lancet 1973, 2, 22–24. [Google Scholar] [CrossRef]
- Vostrikov, V.M.; Oifa, A.I. Paramyxoviruses in the Brain in Febrile Schizophrenia. In Viruses, Immunity, and Mental Disorders; Kurstak, E., Lipowski, Z.J., Morozov, P.V., Eds.; Springer: Boston, MA, USA, 1987; pp. 157–168. [Google Scholar]
- McQuillan, G.M.; Kruszon-Moran, D.; Hyde, T.B.; Forghani, B.; Bellini, W.; Dayan, G.H. Seroprevalence of measles antibody in the US population, 1999–2004. J. Infect. Dis. 2007, 196, 1459–1464. [Google Scholar] [CrossRef]
- Hutchins, S.S.; Redd, S.C.; Schrag, S.; Kruszon-Moran, D.; Wooten, K.; McQuillan, G.M.; Bellini, W.; Meyer, P.A.; Hadler, S. National serologic survey of measles immunity among persons 6 years of age or older, 1988–1994. MedGenMed 2001, 3, E5. [Google Scholar]
- Stoler, M.; Meshulam, B.; Zoldan, J.; Sirota, P. Schizophreniform episode following measles infection. Br. J. Psychiatry 1987, 150, 861–862. [Google Scholar] [CrossRef]
- Suvisaari, J.; Haukka, J.; Tanskanen, A.; Hovi, T.; Lönnqvist, J. Association between prenatal exposure to poliovirus infection and adult schizophrenia. Am. J. Psychiatry 1999, 156, 1100–1102. [Google Scholar] [CrossRef]
- Cahill, M.; Chant, D.; Welham, J.; McGrath, J. No significant association between prenatal exposure to poliovirus epidemics and psychosis. Aust. N. Z. J. Psychiatry 2002, 36, 373–375. [Google Scholar] [CrossRef]
- Tanskanen, A.; Taipale, H.; Cannon, M.; Cotter, D.; Tiihonen, J. Incidence of schizophrenia and influence of prenatal and infant exposure to viral infectious diseases. Acta Psychiatr. Scand. 2021, 143, 487–494. [Google Scholar] [CrossRef]
- Brown, A.S.; Cohen, P.; Greenwald, S.; Susser, E. Nonaffective psychosis after prenatal exposure to rubella. Am. J. Psychiatry 2000, 157, 438–443. [Google Scholar] [CrossRef]
- Lim, K.; Beal, M.; Harvey, R.; Myers, T.; Lane, B.; Sullivan, E.V.; Faustman, W.O.; Pfefferbaum, A. Brain dysmorphology in adults with congenital rubella plus schizophrenialike symptoms. Biol. Psychiatry 1995, 37, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Najjar, S.; Pearlman, D.M.; Alper, K.; Najjar, A.; Devinsky, O. Neuroinflammation and psychiatric illness. J. Neuroinflamm. 2013, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Rantakallio, P.; Jones, P.; Moring, J.; von Wendt, L. Association between central nervous system infections during childhood and adult onset schizophrenia and other psychoses: A 28-year follow-up. Int. J. Epidemiol. 1997, 26, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Koponen, H.; Rantakallio, P.; Veijola, J.; Jones, P.; Jokelainen, J.; Isohanni, M. Childhood central nervous system infections and risk for schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2004, 254, 9–13. [Google Scholar] [CrossRef] [PubMed]
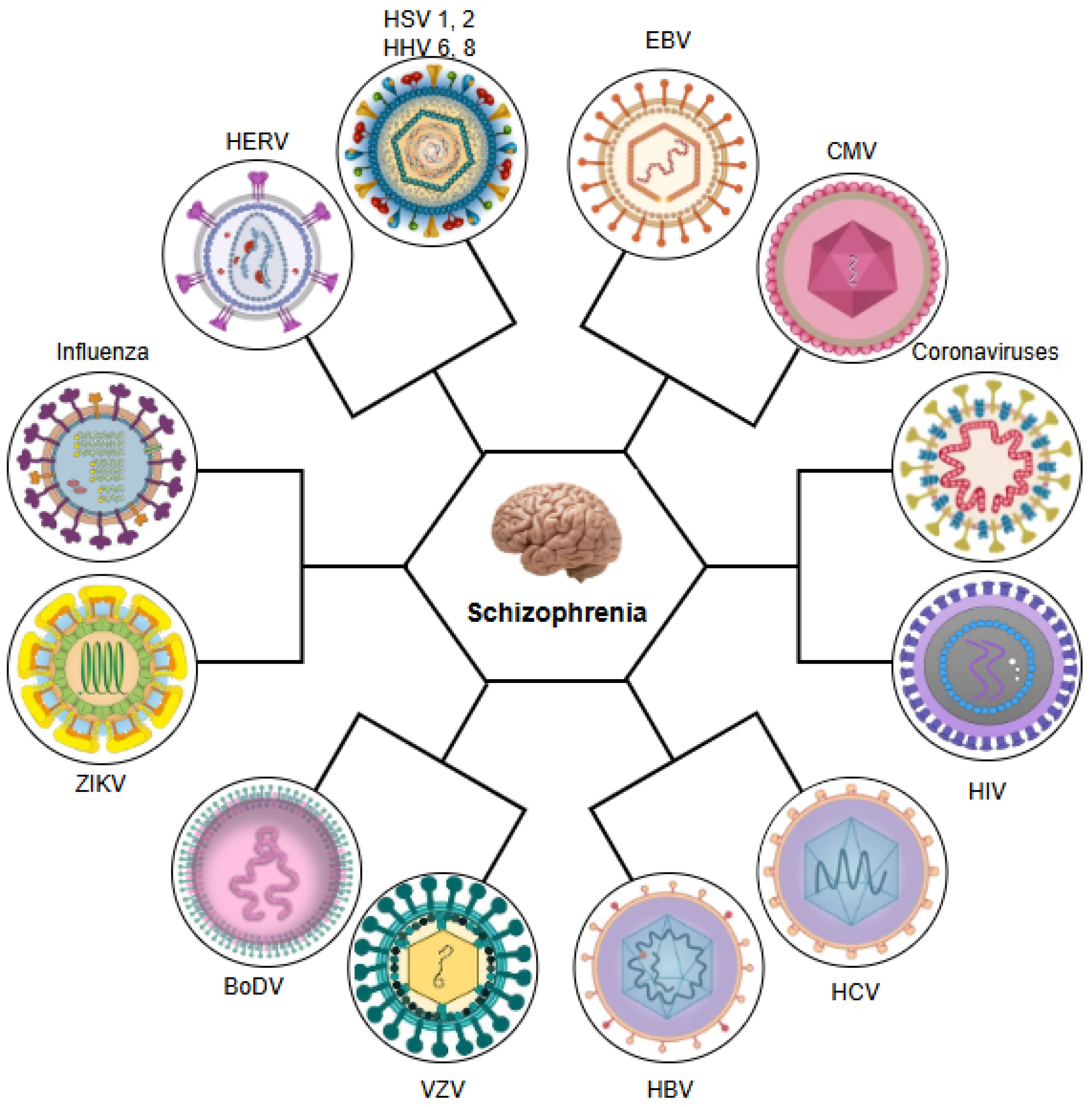
 ): inhibition.
): inhibition.
 ): inhibition.
): inhibition.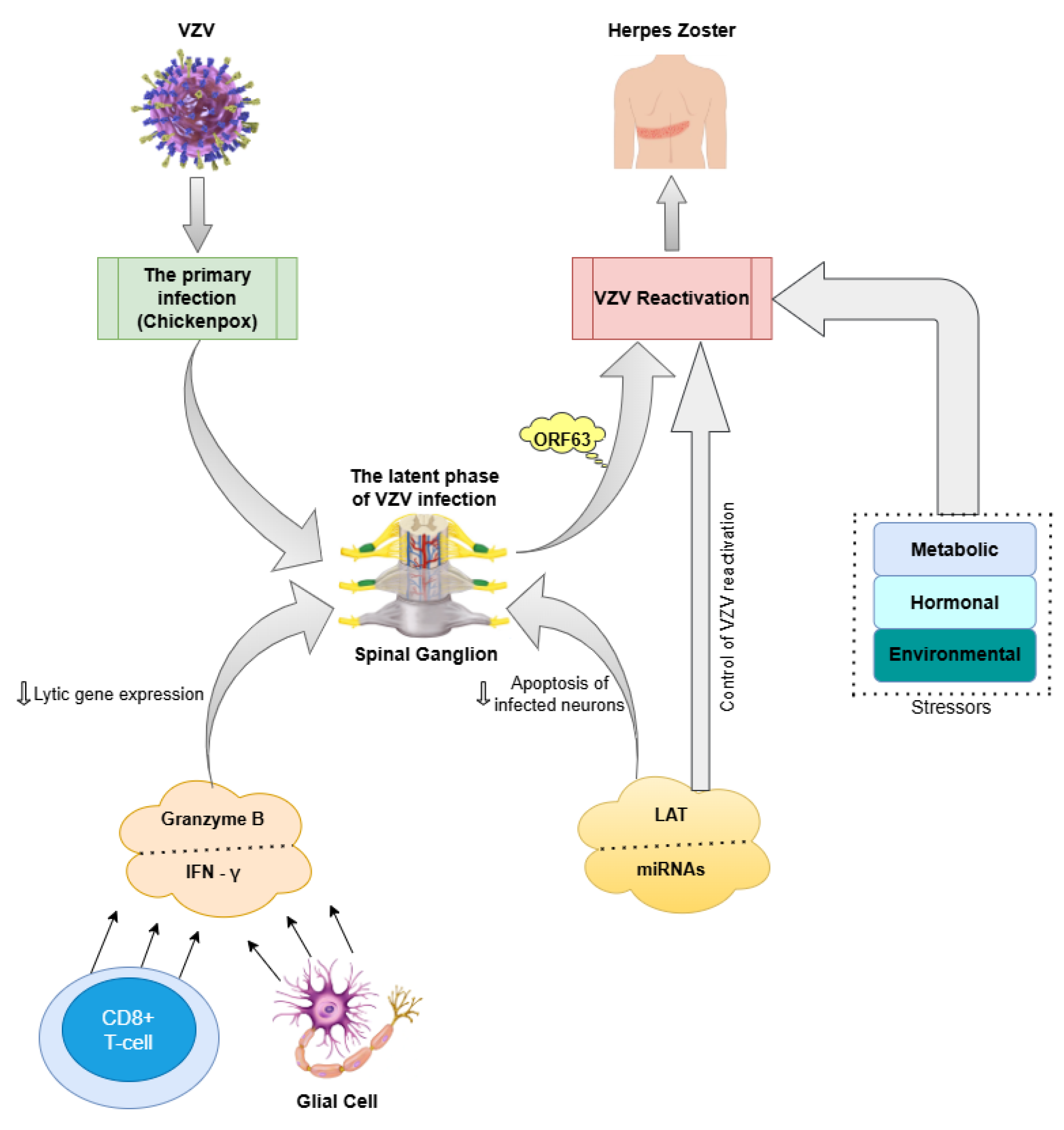
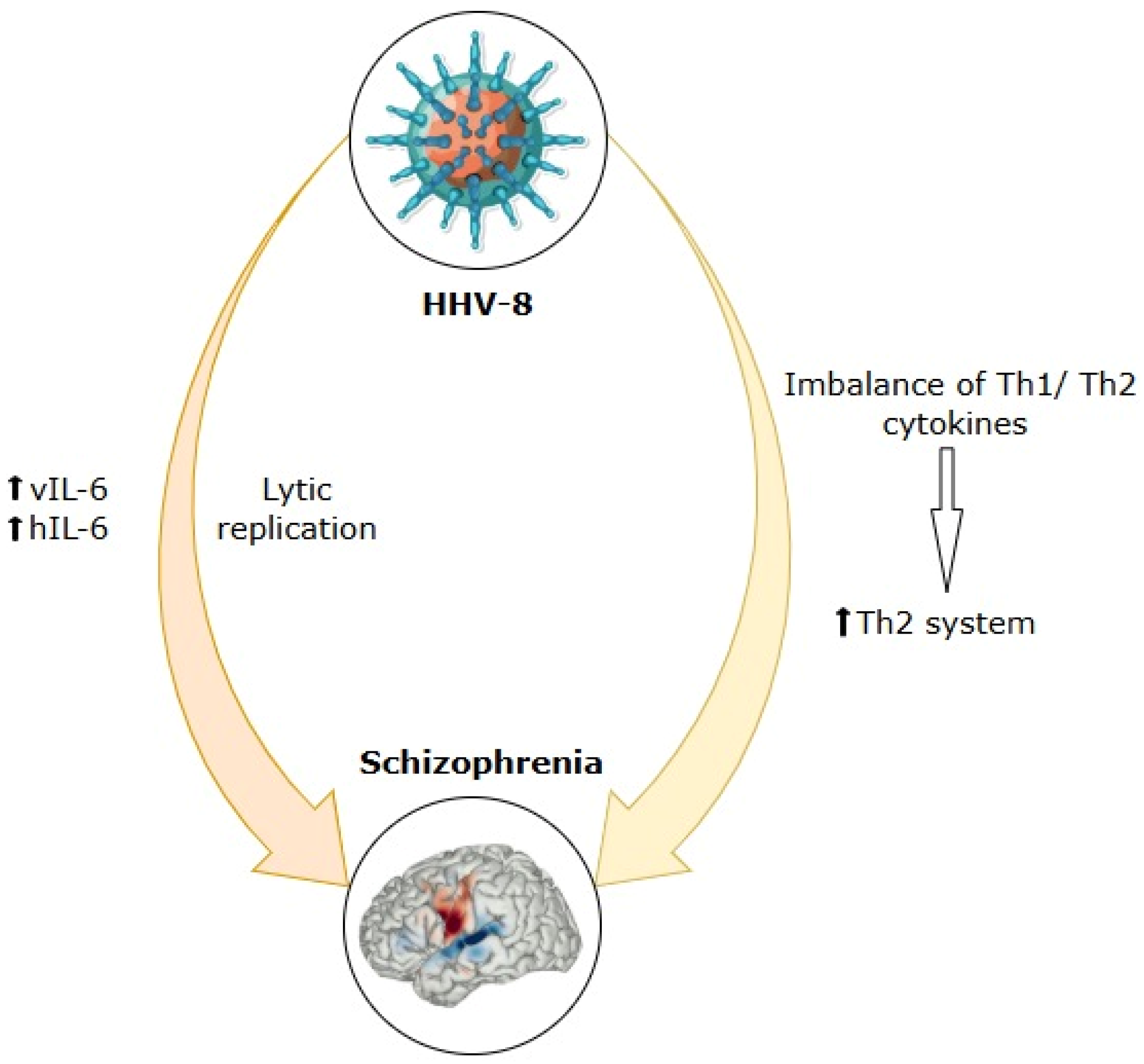
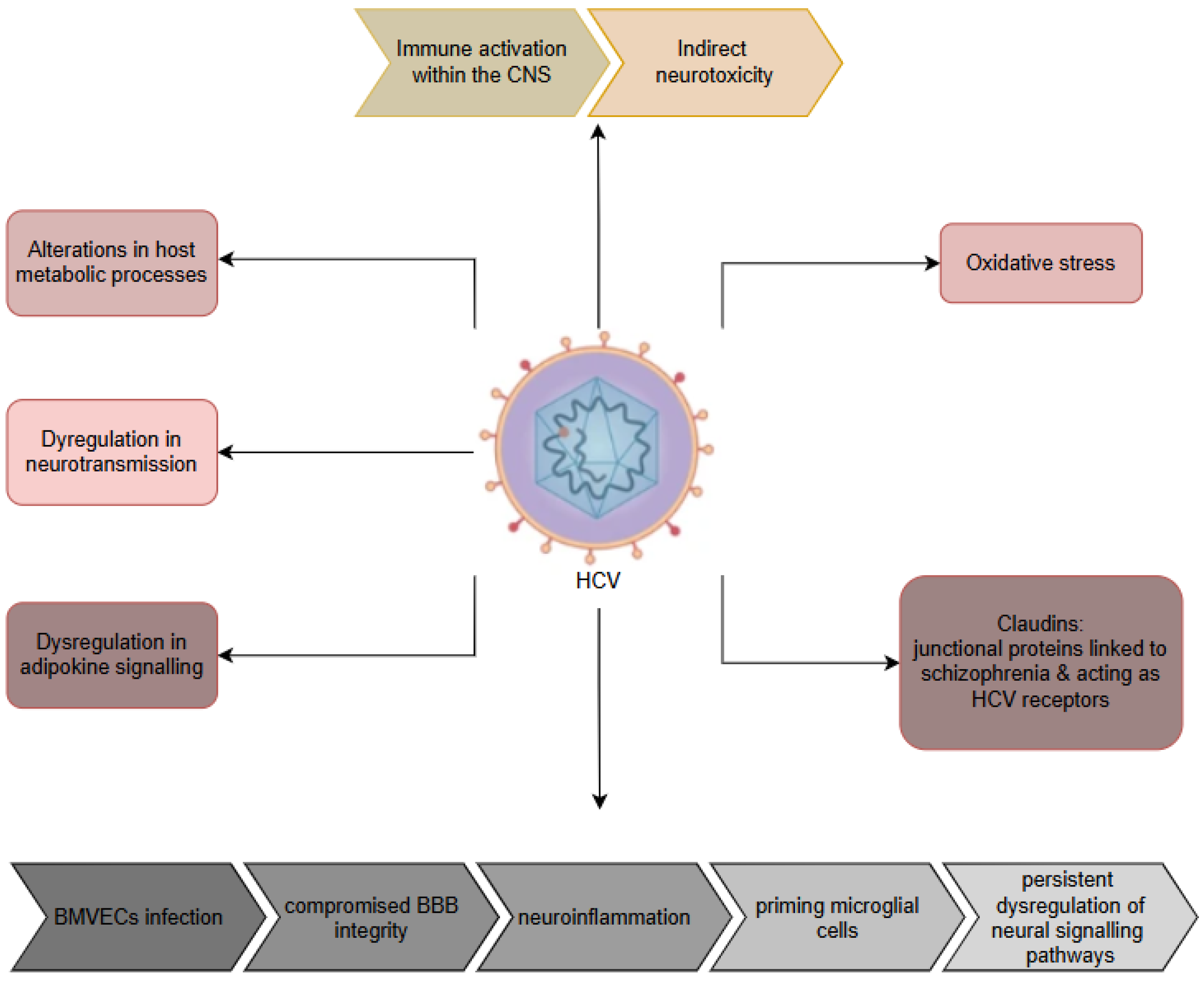
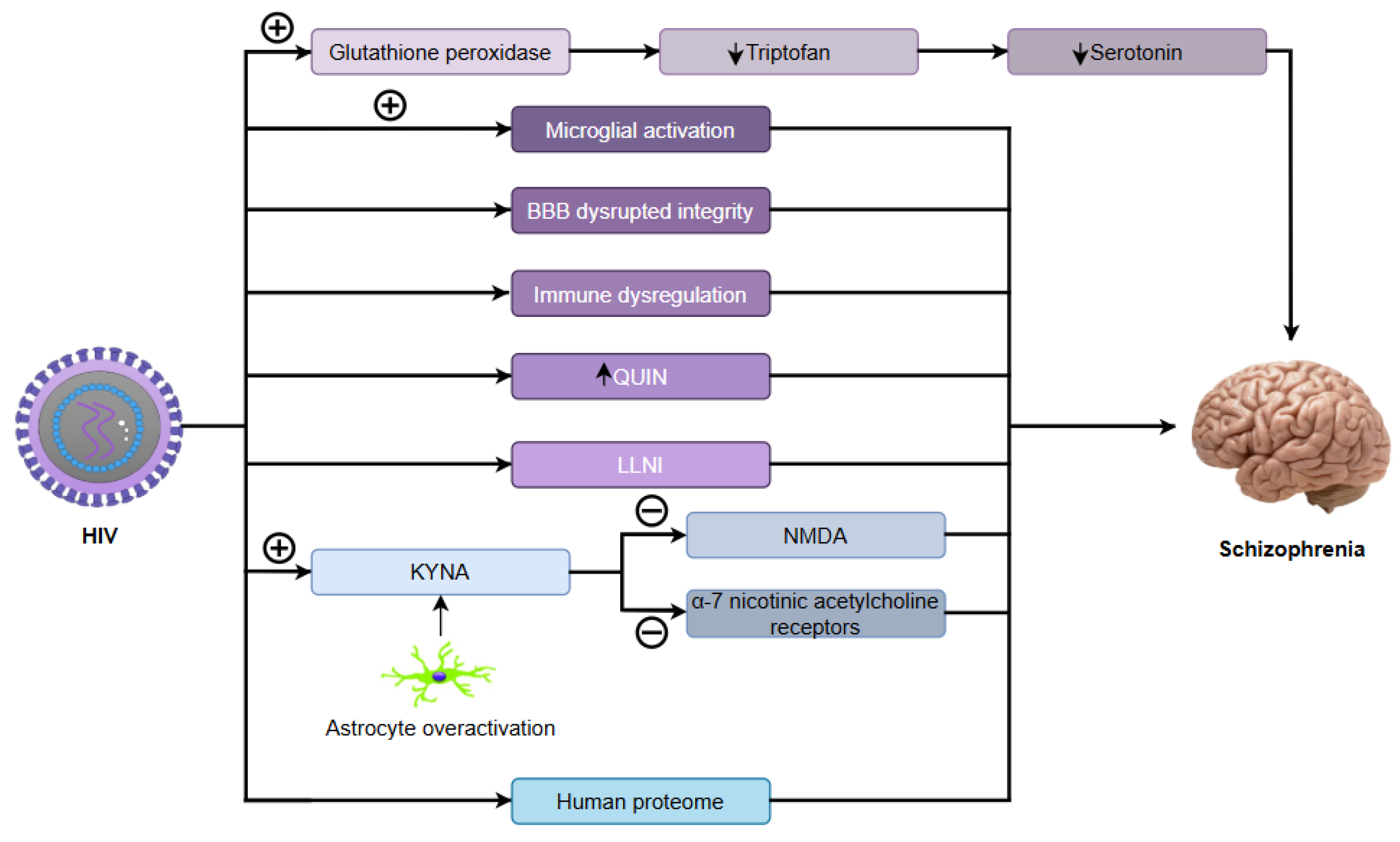
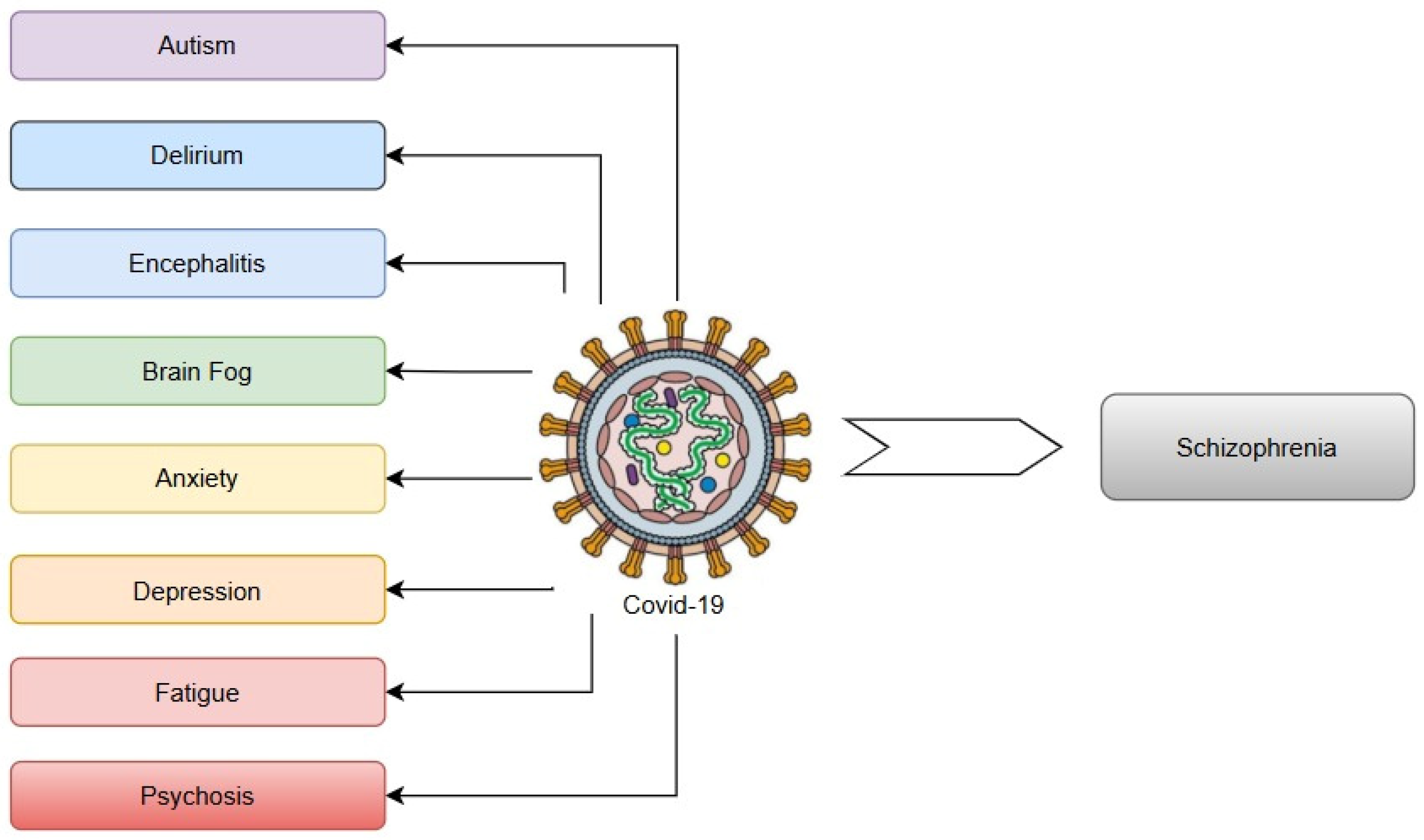
| Feature | HSV-1 | HSV-2 | References |
|---|---|---|---|
| Epidemiological Association with Schizophrenia | Stronger and more consistent association with schizophrenia risk and progression | Mixed findings; weaker and less consistent association | [37,38,39,40,41,44] |
| Primary Transmission Route | Oral (via saliva, close contact) | Sexual (genital) | [50,65] |
| Neuronal Tropism | Preferential infection of the trigeminal ganglia; tropism for frontal/temporal cortices, hippocampus, and limbic system | Lumbosacral ganglia; less neurotropic than HSV-1 | [47,50,55,58,59] |
| Mechanisms of Neuropathology | Direct neuronal damage, chronic neuroinflammation, oxidative stress, mitochondrial dysfunction, apoptosis, and disruption of the NMDA receptor | Primarily immune-mediated (maternal immune activation during pregnancy) | [37,40,41,44,61,62,63,64,66] |
| Cognitive and Symptomatic Effects | Strongly linked to cognitive deficits (working memory, executive function), negative symptoms, and progressive cortical atrophy | Less clear; possible association with accelerated cortical thinning, the neuroanatomical substrate for cognitive decline, when combined with other infections (e.g., C. pneumoniae). | [37,44,46,53,67,68,69,70,71] |
| Neuroimaging Findings | Diminished grey matter observed in the prefrontal and anterior cingulate cortices, as well as the hippocampus; progressive posterior cingulate atrophy | Cortical thinning and reduction in hippocampal volume (when combined with other infections) | [37,44,53,72,73] |
| Immunological Impact | Activates neuroinflammation via TLR signalling pathways, involving cytokines including IL-6, IL-1β, and TNF-α, leading to microglial activation | Maternal IgG/IgM antibodies may disrupt foetal neurodevelopment through immune dysregulation | [41,49,51,73,74,75] |
| Genetic Interactions | Overlap with schizophrenia genome-wide association studies loci (e.g., NRP1, HLA alleles); MICB polymorphism modifies risk | Gene–environment interactions (e.g., GRIN2B variants) in offspring of HSV-2-positive mothers | [39,40,76,77,78] |
| Therapeutic Implications | Antivirals (e.g., valacyclovir) show modest cognitive benefits in trials; immunomodulation strategies under investigation | Limited evidence; potential focus on maternal–foetal interventions | [41,48,63] |
| No. | EBV | HSV-1 | CMV |
|---|---|---|---|
| 1 | Genetic predisposition to developing neuropsychiatric effects following EBV infection [79] | Direct neuronal damage via hippocampal and frontotemporal tropism, disrupting neurodevelopment and synaptic function [37,47,58,59] | Congenital infection causing neurodevelopmental disruptions (microcephaly, polymicrogyria) [80,81,82] |
| 2 | Impairment of neuronal survival and function through microglial priming, leading to a dysregulated response to subsequent infections [83] | Chronic neuroinflammation triggered by microglial activation (TLR2/3/9) and elevated pro-inflammatory cytokines (IL-6, TNF-α, IL-1β) [41,75,84] | Immune dysregulation through chronic inflammation (elevated TNF-α, IL-10) [16,85,86] |
| 3 | Transactivation of endogenous retroviruses by EBV, influencing the regulation of host gene expression [87] | NMDA receptor dysfunction exacerbating glutamatergic hypofunction [66,88] | Neural progenitor cell infection disrupting proliferation/migration [16,80,89,90] |
| 4 | Indirect effects via activation of systemic cytokine responses and stress-related pathways, resulting in neuroinflammatory processes within the brain [91,92] | Epigenetic dysregulation in neural progenitor cells (e.g., viral thymidine kinase-mediated interference with differentiation) [60,93] | Latency/reactivation in neural cells causing neuroinflammation [90,94] |
| 5 | Modulation of the inflammatory response following EBV infection by immune-related genes, resulting in altered brain development and function [83] | Structural brain abnormalities (grey matter loss in prefrontal cortex, anterior cingulate, and hippocampus) correlating with cognitive deficits [37,44,72,73] | Molecular mimicry triggering autoimmune responses (e.g., against GAD) [95] |
| 6 | Dysregulated immune responses to EBV infection [79] | Disrupted neurodevelopmental pathways via nectin-1 receptor-mediated adhesion and connectivity impairments [59,60] | Hippocampal volume reduction and dentate gyrus abnormalities [80,96,97] |
| 7 | Disruptions in neurotransmitter receptor interactions [92] | Maternal immune activation (elevated IL-18, CRP) during pregnancy, altering foetal brain development [41,74,98] | Genetic susceptibility via immune-related polymorphisms (TNF-α, IL-10, C4, CTNNA3) [16,99,100] |
| 8 | The interaction between complement receptor 2, used by EBV for cellular entry, and complement component 4 (C4), which participates in viral inactivation and is also implicated in schizophrenia via allelic variations in the C4 gene [101] | Genetic interactions (e.g., HLA alleles, MICB rs1051788, Fyn kinase) modulating infection susceptibility and neuroinflammation [39,76,77,102] | |
| 9 | Latent central nervous system infection with periodic reactivation, causing cumulative neuronal damage and oxidative stress [44,63,103,104] |
| No. | Mechanism | Description of the Mechanism | References |
|---|---|---|---|
| 1 | Genetic predisposition | - HERV-K elements may enhance PRODH, a schizophrenia susceptibility gene involved in glutamatergic transmission. - The 22q13 region harbours APOBEC3G, an inhibitor of retroviral replication, and may represent a potential locus of genetic vulnerability to HERV activation. | [318,320,321] |
| 2 | Epigenetic dysregulation | - Hypomethylation of HERV-W and HERV-K loci. - Chromatin remodelling at long terminal repeats (LTRs) facilitating transcriptional activation. | [317,318,319,320] |
| 3 | Gene–environment interaction | Toxoplasma gondii and viral infections (e.g., influenza, HSV-1, HIV, EBV) can transcriptionally activate HERVs, particularly HERV-W, through inflammatory signalling pathways (e.g., NF-κB) or through direct activation by viral proteins such as Tat or Tax. | [311,316,324,332,333] |
| 4 | Neuroimmune mechanisms and neuroinflammation | - Upregulation of inflammatory mediators including TNF-α, IL-6, and IL-1β via NF-κB and JAK-STAT1 signalling pathways, which are triggered by the envelope protein (env) of HERV-W, a potent activator of TLR4. - Induction of oxidative stress and nitric oxide-mediated neurotoxicity via enhanced expression of iNOS, which is triggered by HERV-W Env. - Disruption of the blood–brain barrier. - Microglial activation. | [9,307,322,324,325,329] |
| 5 | Neurodevelopmental and neurotransmission disruption | - Disruption of synaptic development, neuronal differentiation, and dopaminergic signalling through HERV-W Env, which has been demonstrated to modulate the expression of critical neuroplasticity genes associated with these processes, such as BDNF, NTRK2/TrkB, and DRD3. - Leading to an excitatory/inhibitive imbalance through the Env protein, which also interacts with sodium-dependent amino acid transporters hASCT1 and hASCT2, disrupting glutamate uptake. - Impaired neuronal excitability and hippocampal long-term potentiation through HERV-W Env, which downregulates 5-HT4 receptors and activates SK2 and SK3 potassium channels. | [308,309,326,327,328,329,330] |
| 6 | Ferroptosis | HERV-W ENV downregulates neuroprotective genes such as GPX4 and SLC3A2, resulting in iron accumulation, lipid peroxidation, and mitochondrial dysfunction. | [331] |
| 7 | HERV-W activation via NF-κB and viral protein interactions | - HERV-W Env protein binds to the TLR4 receptor, activating NF-κB → releases TNF-α, IL-6, and IL-1β → chronic neuroinflammation and neuronal damage. - Viral protein synergy: HSV-1 (ICP0), HIV (Tat), and EBV (LMP-1) activate NF-κB, which binds to HERV-W promoters → HERV-W transcriptional reactivation. - Epigenetic priming: viral infections cause DNA hypomethylation of HERV-W LTRs, making them responsive to NF-κB → loss of HERV silencing → persistent expression. | [9,307,316,317,319,322,332,333] |
| No. | Mechanism | References |
|---|---|---|
| 1 | Maternal immune activation, which interferes with foetal neurodevelopment. | [392,394] |
| 2 | Immune-mediated mechanisms. | [401,403,404] |
| 3 | Direct neural damage by targeting the brain’s microvascular endothelial cells. | [408] |
| 4 | Triggering molecular markers associated with schizophrenia: interleukin-6 (IL-6), Apolipoprotein L2 (APOL2), Apolipoprotein L4 (APOL4), Chitinase 3-like 1 (CHI3L1), Synapsin II (SYN2), and methylenetetrahydrofolate reductase (MTHFR). | [409,410] |
| 5 | Downregulation of angiotensin-converting enzyme 2 (ACE2), leading to disruption of the dopaminergic and serotonergic systems. | [394] |
| 6 | Upregulation of Toll-like receptor (TLR) mRNA expression, resulting in elevated levels of cytokines and chemokines. | [411] |
| 7 | Disruption of the peripheral olfactory system. | [413] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sighencea, M.G.; Trifu, S.C. Unravelling the Viral Hypothesis of Schizophrenia: A Comprehensive Review of Mechanisms and Evidence. Int. J. Mol. Sci. 2025, 26, 7429. https://doi.org/10.3390/ijms26157429
Sighencea MG, Trifu SC. Unravelling the Viral Hypothesis of Schizophrenia: A Comprehensive Review of Mechanisms and Evidence. International Journal of Molecular Sciences. 2025; 26(15):7429. https://doi.org/10.3390/ijms26157429
Chicago/Turabian StyleSighencea, Mădălina Georgeta, and Simona Corina Trifu. 2025. "Unravelling the Viral Hypothesis of Schizophrenia: A Comprehensive Review of Mechanisms and Evidence" International Journal of Molecular Sciences 26, no. 15: 7429. https://doi.org/10.3390/ijms26157429
APA StyleSighencea, M. G., & Trifu, S. C. (2025). Unravelling the Viral Hypothesis of Schizophrenia: A Comprehensive Review of Mechanisms and Evidence. International Journal of Molecular Sciences, 26(15), 7429. https://doi.org/10.3390/ijms26157429







