The SGLT2 Inhibitor Dapagliflozin Disrupts the Cell Cycle at High Concentrations Without Altering Glycosphingolipid (De Novo)Biosynthesis
Abstract
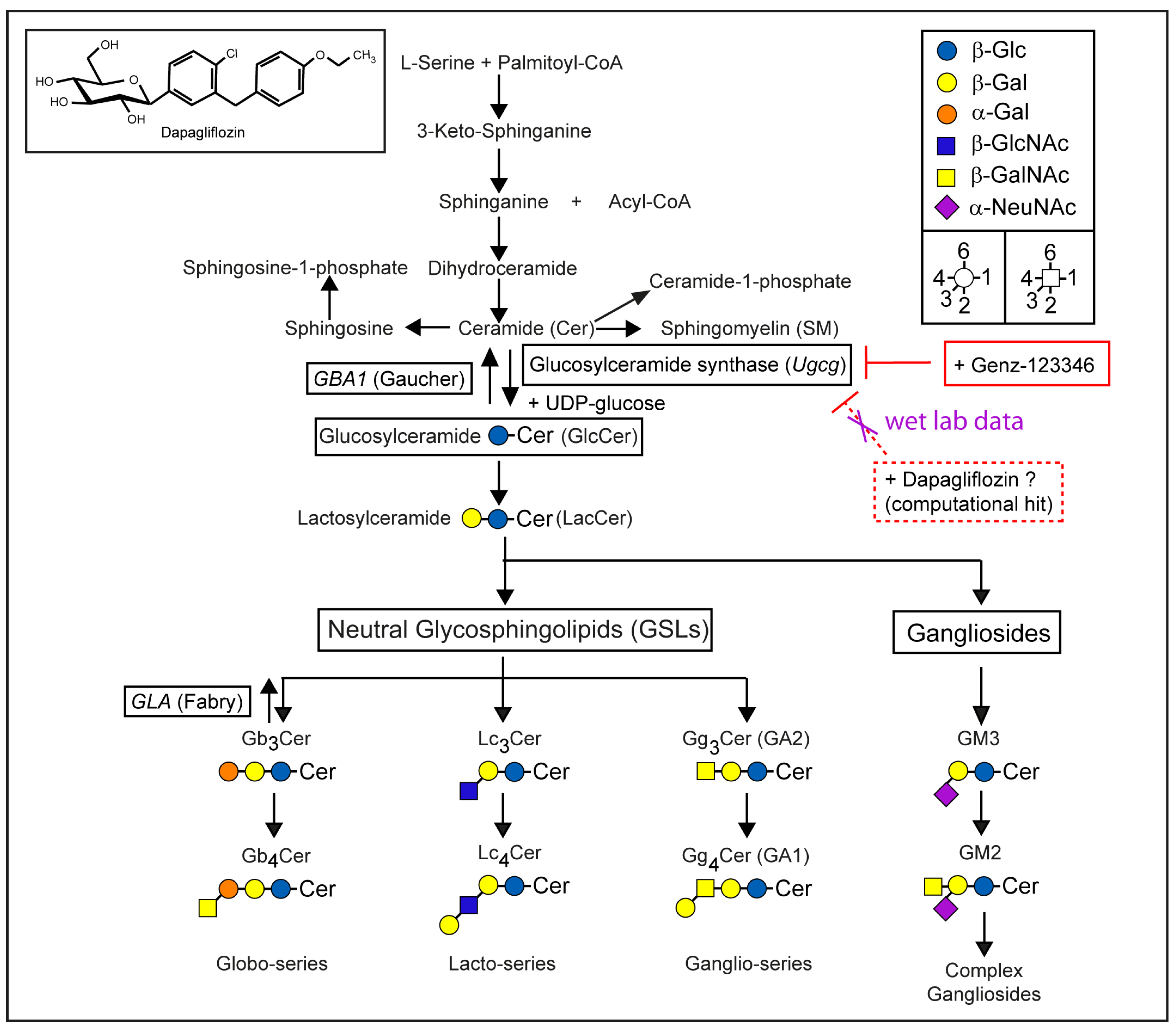
1. Introduction
2. Results
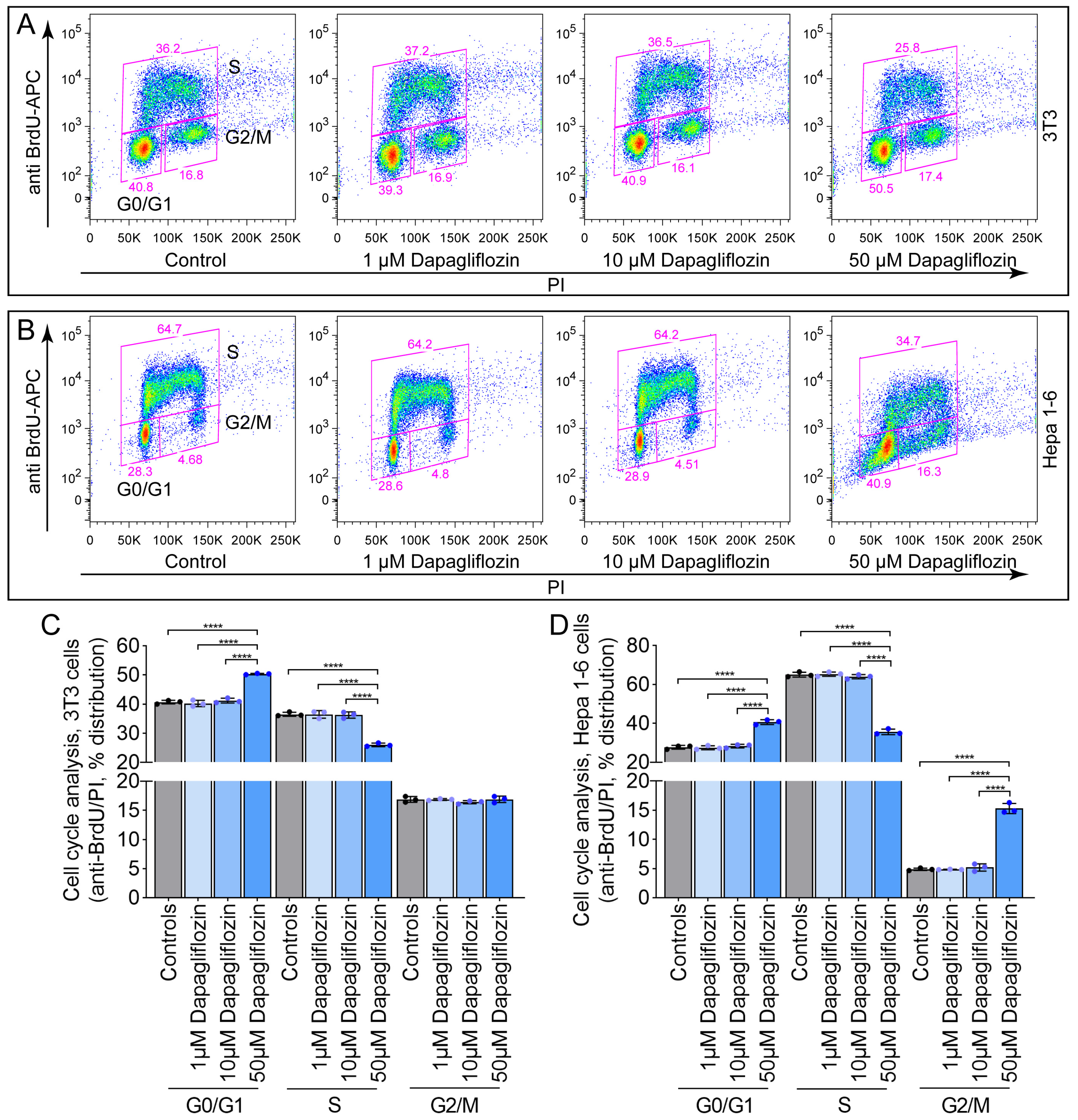
2.1. Dapagliflozin Induces Cell Cycle Arrest in 3T3 and Hepa 1-6 Cells at High Concentrations
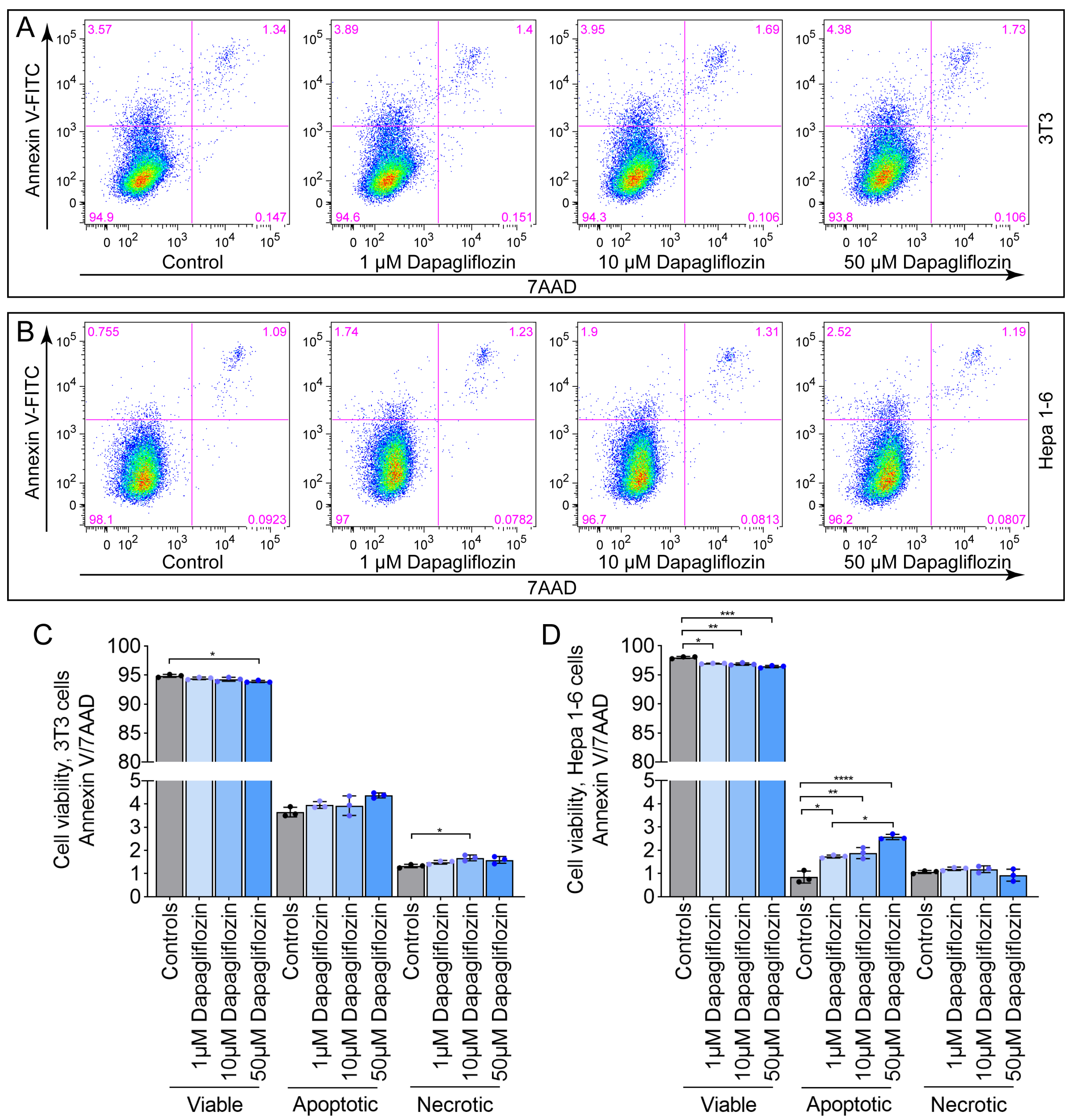
2.2. Dapagliflozin Does Not Induce Pronounced Apoptosis or Necrosis in 3T3 and Hepa 1-6 Cells
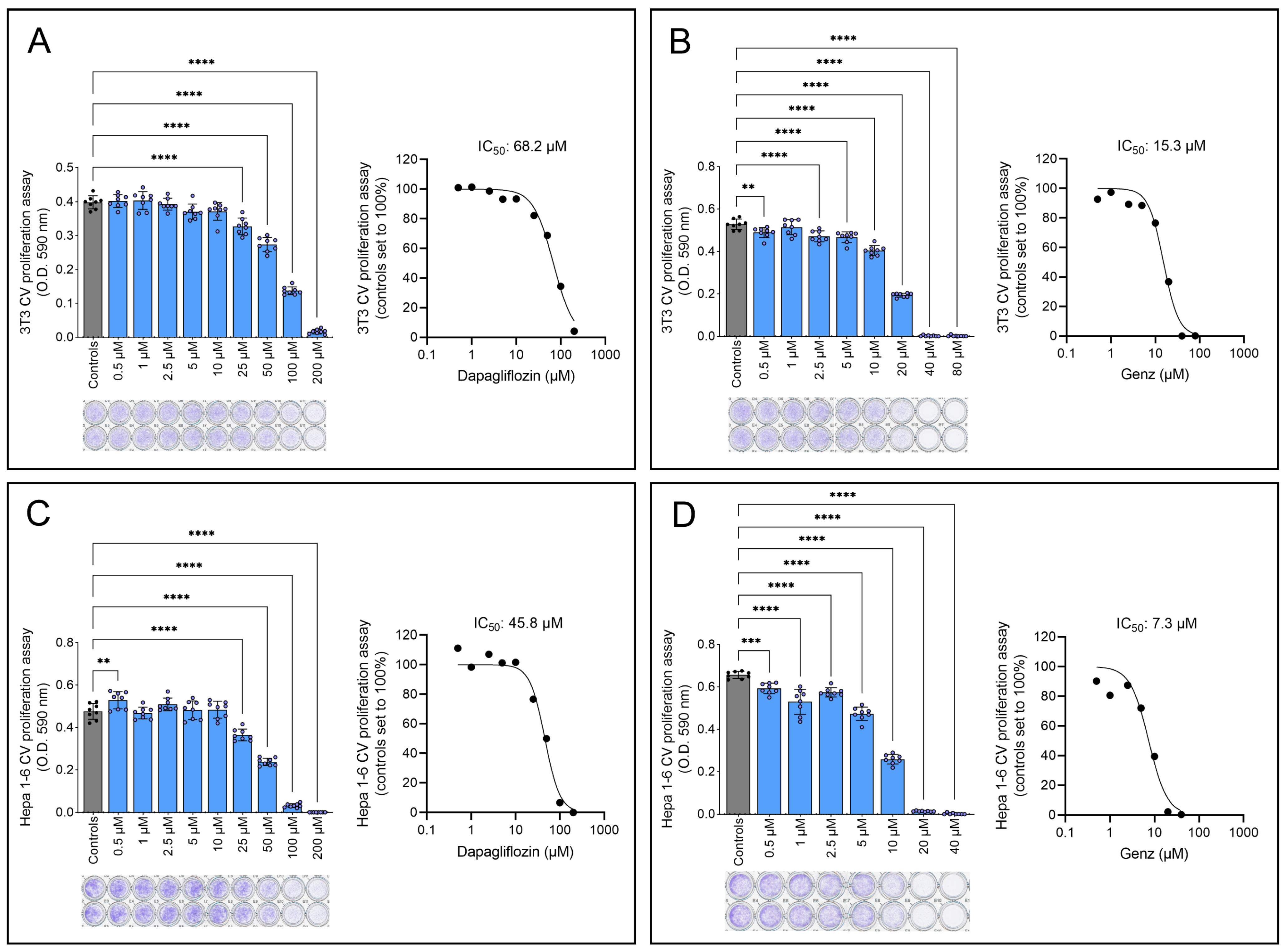
2.3. Genz-123346 Is More Potent than Dapagliflozin in Inhibiting Cell Proliferation
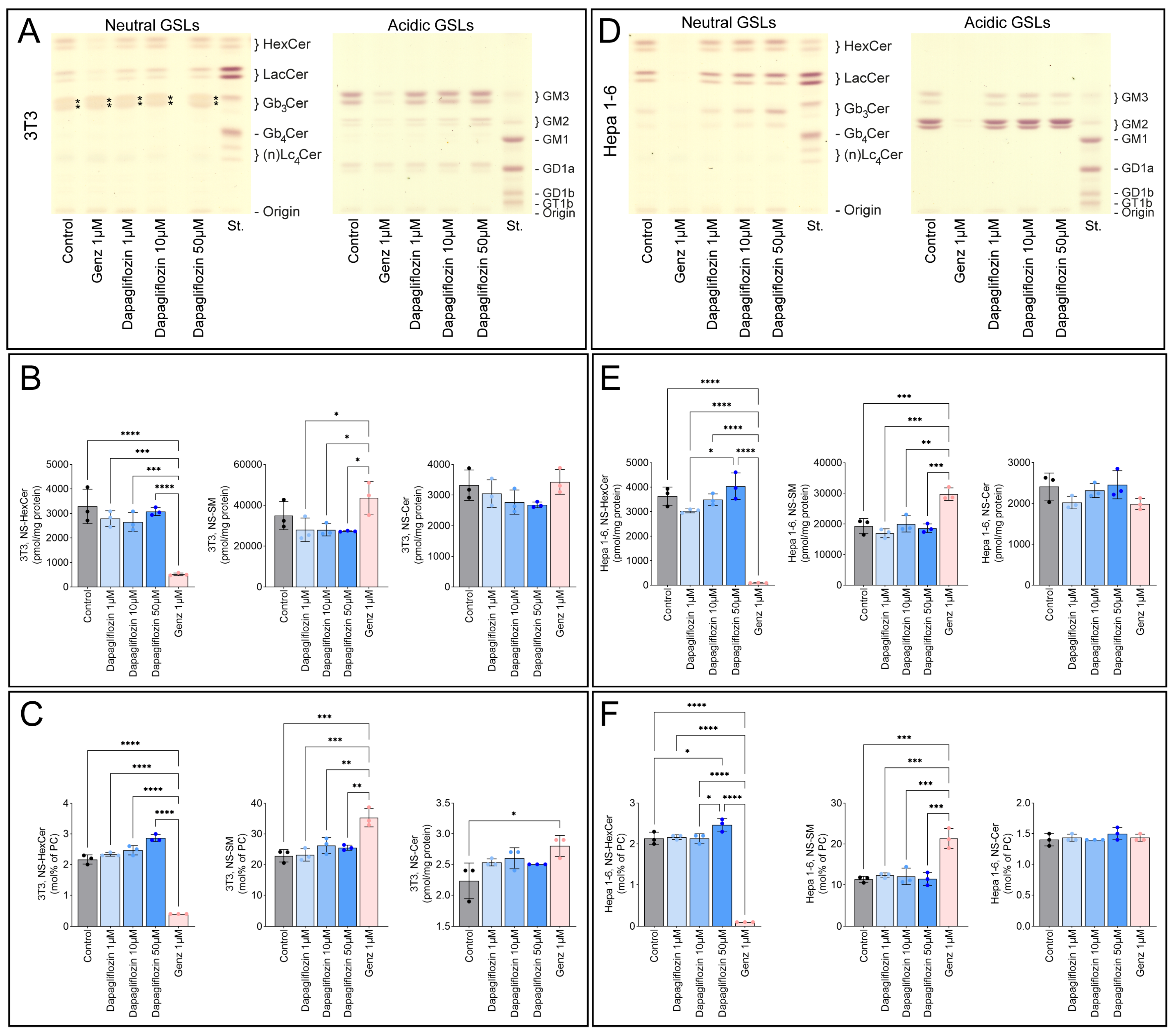
2.4. Dapagliflozin Does Not Inhibit GSL Synthesis
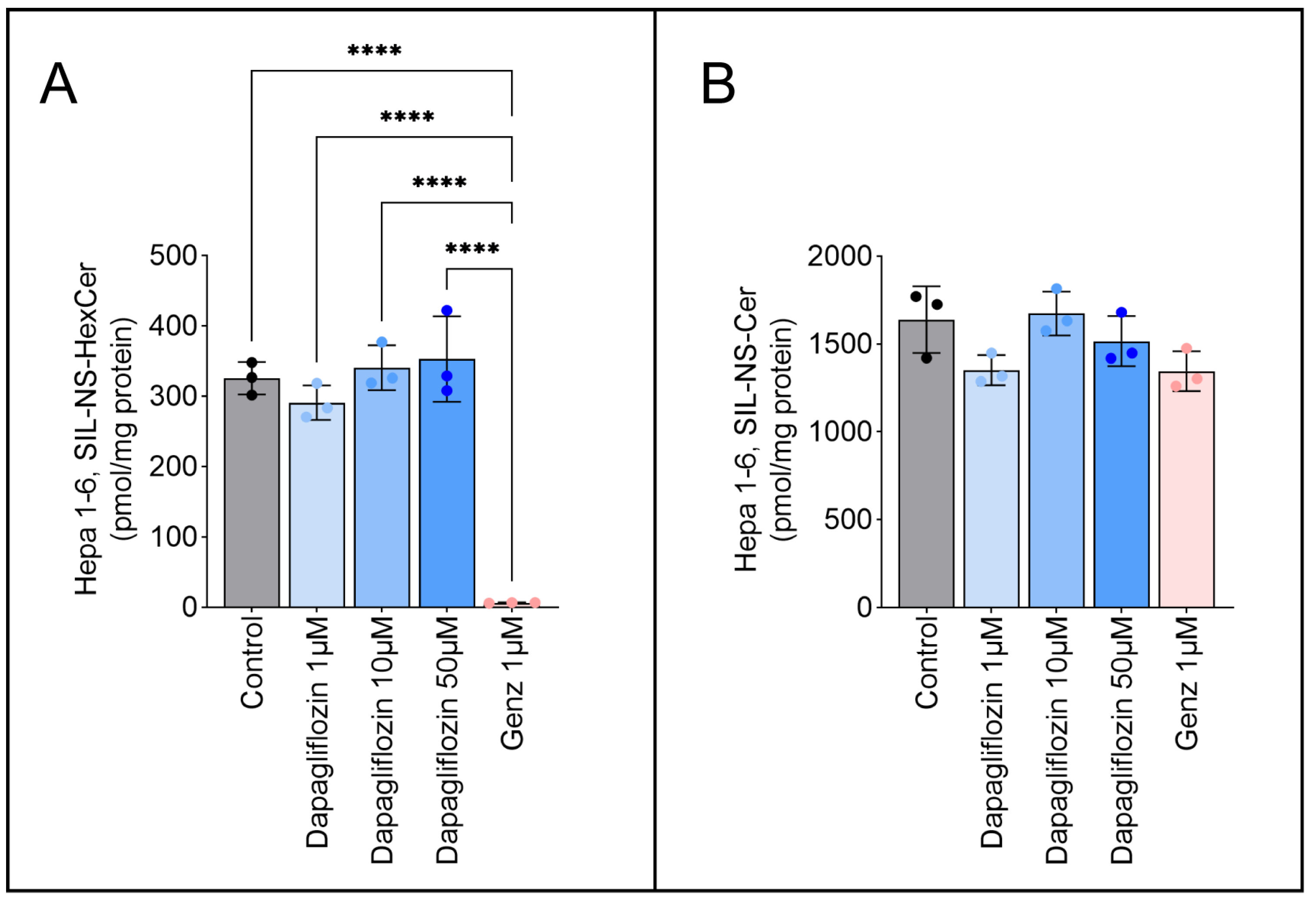
2.5. Dapagliflozin Does Not Inhibit GSL Neo-Biosynthesis
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. BrdU Incorporation Assay
4.4. Cell Viability Assay
4.5. Crystal Violet Proliferation Assay
4.6. GSL Analysis
4.7. Pulse Experiment for GSL Neobiosynthesis
4.8. Statistics
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SGLT2 | Sodium-glucose cotransporter 2 |
| GCS | Glucosylceramide synthase |
| GSL | Glycosphingolipid |
| GlcCer | Glucosylceramide |
| ERT | Enzyme replacement therapy |
| SRT | Substrate reduction therapy |
| BrdU | 5-Bromo-2′-deoxyuridine |
| PI | Propidium iodide |
| TLC | Thin layer chromatography |
| MS | Mass spectrometry |
| (NS)-HexCer | (Non-hydroxylated and sphingosin-containing)-hexosylceramide |
| (NS)-SM | (Non-hydroxylated and sphingosin-containing)-sphingomyelin |
| (NS)-Cer | (Non-hydroxylated and sphingosin-containing)-ceramide |
| PC | Phosphatidylcholine |
References
- Abdul-Ghani, M.A.; Norton, L.; DeFronzo, R.A. Renal sodium-glucose cotransporter inhibition in the management of type 2 diabetes mellitus. Am. J. Physiol. Renal Physiol. 2015, 309, F889–F900. [Google Scholar] [CrossRef]
- Khan, U.; Majeed, Z.; Khan, M.H.; Elsnhory, A.B.; Amin, A.M.; Nawaz, A.; Raza, A.; Siddque, H.M.W.; Turkmani, M.; Abuelazm, M. Efficacy and Safety of Pioglitazone Add-On in Patients with Type 2 Diabetes Mellitus Inadequately Controlled with Metformin and Dapagliflozin: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Endocrinol. Diabetes Metab. 2025, 8, e70061. [Google Scholar] [CrossRef]
- List, J.F.; Woo, V.; Morales, E.; Tang, W.; Fiedorek, F.T. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 2009, 32, 650–657. [Google Scholar] [CrossRef]
- Pagel, P.S.; Hang, D.; Freed, J.K.; Crystal, G.J. Advances in Cardiovascular Pharmacotherapy. IV. Sodium-Glucose Cotransporter Type 2 Inhibitors, Part 2: Mechanisms for Myocardial Protection, Adverse Effects, and Perioperative Implications. J. Cardiothorac. Vasc. Anesth. 2025; ahead of print. [Google Scholar] [CrossRef]
- Pagel, P.S.; Hang, D.; Freed, J.K.; Crystal, G.J. Advances in Cardiovascular Pharmacotherapy. III. Sodium-Glucose Cotransporter Type 2 Inhibitors, Part 1: Efficacy in Heart Failure and Myocardial Infarction. J. Cardiothorac. Vasc. Anesth. 2025, 39, 2147–2171. [Google Scholar] [CrossRef]
- Canini, G.; Mazzinelli, E.; Nocca, G.; Lattanzi, W.; Arcovito, A. Targeting Glucosylceramide Synthase: Innovative Drug Repurposing Strategies for Lysosomal Diseases. Int. J. Mol. Sci. 2025, 26, 2195. [Google Scholar] [CrossRef]
- D’Angelo, G.; Capasso, S.; Sticco, L.; Russo, D. Glycosphingolipids: Synthesis and functions. FEBS J. 2013, 280, 6338–6353. [Google Scholar] [CrossRef]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Mullen, T.D.; Hannun, Y.A.; Obeid, L.M. Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem. J. 2012, 441, 789–802. [Google Scholar] [CrossRef]
- Funakoshi, T.; Yasuda, S.; Fukasawa, M.; Nishijima, M.; Hanada, K. Reconstitution of ATP- and cytosol-dependent transport of de novo synthesized ceramide to the site of sphingomyelin synthesis in semi-intact cells. J. Biol. Chem. 2000, 275, 29938–29945. [Google Scholar] [CrossRef]
- Hanada, K.; Kumagai, K.; Yasuda, S.; Miura, Y.; Kawano, M.; Fukasawa, M.; Nishijima, M. Molecular machinery for non-vesicular trafficking of ceramide. Nature 2003, 426, 803–809. [Google Scholar] [CrossRef]
- Sandhoff, K.; Kolter, T. Topology of glycosphingolipid degradation. Trends Cell Biol. 1996, 6, 98–103. [Google Scholar] [CrossRef]
- Platt, F.M.; d’Azzo, A.; Davidson, B.L.; Neufeld, E.F.; Tifft, C.J. Lysosomal storage diseases. Nat. Rev. Dis. Primers 2018, 4, 27. [Google Scholar] [CrossRef]
- Stirnemann, J.; Belmatoug, N.; Camou, F.; Serratrice, C.; Froissart, R.; Caillaud, C.; Levade, T.; Astudillo, L.; Serratrice, J.; Brassier, A.; et al. A Review of Gaucher Disease Pathophysiology, Clinical Presentation and Treatments. Int. J. Mol. Sci. 2017, 18, 441. [Google Scholar] [CrossRef]
- Elstein, D.; Dweck, A.; Attias, D.; Hadas-Halpern, I.; Zevin, S.; Altarescu, G.; Aerts, J.F.; van Weely, S.; Zimran, A. Oral maintenance clinical trial with miglustat for type I Gaucher disease: Switch from or combination with intravenous enzyme replacement. Blood 2007, 110, 2296–2301. [Google Scholar] [CrossRef]
- Schiffmann, R.; Fitzgibbon, E.J.; Harris, C.; DeVile, C.; Davies, E.H.; Abel, L.; van Schaik, I.N.; Benko, W.; Timmons, M.; Ries, M.; et al. Randomized, controlled trial of miglustat in Gaucher’s disease type 3. Ann. Neurol. 2008, 64, 514–522. [Google Scholar] [CrossRef]
- Torralba-Cabeza, M.A.; Morado-Arias, M.; Pijierro-Amador, A.; Fernandez-Canal, M.C.; Villarrubia-Espinosa, J. Recommendations for oral treatment for adult patients with type 1 Gaucher disease. Rev. Clin. Esp. 2022, 222, 529–542. [Google Scholar] [CrossRef]
- Jennemann, R.; Federico, G.; Mathow, D.; Rabionet, M.; Rampoldi, F.; Popovic, Z.V.; Volz, M.; Hielscher, T.; Sandhoff, R.; Grone, H.J. Inhibition of hepatocellular carcinoma growth by blockade of glycosphingolipid synthesis. Oncotarget 2017, 8, 109201–109216. [Google Scholar] [CrossRef]
- Jennemann, R.; Volz, M.; Bestvater, F.; Schmidt, C.; Richter, K.; Kaden, S.; Muthing, J.; Grone, H.J.; Sandhoff, R. Blockade of Glycosphingolipid Synthesis Inhibits Cell Cycle and Spheroid Growth of Colon Cancer Cells In Vitro and Experimental Colon Cancer Incidence In Vivo. Int. J. Mol. Sci. 2021, 22, 10539. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Hill, R.A.; Li, Y.T. Ceramide glycosylation catalyzed by glucosylceramide synthase and cancer drug resistance. Adv. Cancer Res. 2013, 117, 59–89. [Google Scholar]
- Mostaq, M.S.; Kang, L.; Patwardhan, G.A.; Zhao, Y.; Shi, R.; Liu, Y.Y. Glucosylceramide Synthase, a Key Enzyme in Sphingolipid Metabolism, Regulates Expression of Genes Accounting for Cancer Drug Resistance. Int. J. Mol. Sci. 2025, 26, 5112. [Google Scholar] [CrossRef]
- Kuter, D.J.; Mehta, A.; Hollak, C.E.; Giraldo, P.; Hughes, D.; Belmatoug, N.; Brand, M.; Muller, A.; Schaaf, B.; Giorgino, R.; et al. Miglustat therapy in type 1 Gaucher disease: Clinical and safety outcomes in a multicenter retrospective cohort study. Blood Cells Mol. Dis. 2013, 51, 116–124. [Google Scholar] [CrossRef]
- Hartwig, P.; Hoglinger, D. The Glucosylceramide Synthase Inhibitor PDMP Causes Lysosomal Lipid Accumulation and mTOR Inactivation. Int. J. Mol. Sci. 2021, 22, 7065. [Google Scholar] [CrossRef]
- Jennemann, R.; Volz, M.; Frias-Soler, R.C.; Schulze, A.; Richter, K.; Kaden, S.; Sandhoff, R. Glucosylceramide Synthase Inhibition in Combination with Aripiprazole Sensitizes Hepatocellular Cancer Cells to Sorafenib and Doxorubicin. Int. J. Mol. Sci. 2024, 26, 304. [Google Scholar] [CrossRef]
- Fujii, T.; Tanaka, Y.; Oki, H.; Sato, S.; Shibata, S.; Maru, T.; Tanaka, Y.; Tanaka, M.; Onishi, T. A new brain-penetrant glucosylceramide synthase inhibitor as potential Therapeutics for Gaucher disease. J. Neurochem. 2021, 159, 543–553. [Google Scholar] [CrossRef]
- Babcock, M.; Zheng, J.; Gail Shurr, J.; Li, L.; Wang, B.; Huertas, P.; Ryan, P.J.; Shen, Y.; Garovoy, M. Phase 1 Healthy Volunteer Study of AL01211, an Oral, Non-brain Penetrant Glucosylceramide Synthase Inhibitor, to Treat Fabry Disease and Type 1 Gaucher Disease. Clin. Pharmacol. Drug Dev. 2024, 13, 696–709. [Google Scholar] [CrossRef]
- Arab, H.H.; Eid, A.H.; Alsufyani, S.E.; Ashour, A.M.; El-Sheikh, A.A.K.; Darwish, H.W.; Sabry, F.M. Targeting Autophagy, Apoptosis, and Oxidative Perturbations with Dapagliflozin Mitigates Cadmium-Induced Cognitive Dysfunction in Rats. Biomedicines 2023, 11, 3000. [Google Scholar] [CrossRef]
- Han, X.; Liu, X.; Zhao, X.; Wang, X.; Sun, Y.; Qu, C.; Liang, J.; Yang, B. Dapagliflozin ameliorates sepsis-induced heart injury by inhibiting cardiomyocyte apoptosis and electrical remodeling through the PI3K/Akt pathway. Eur. J. Pharmacol. 2023, 955, 175930. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, Q.; Li, H.; Meng, Z.; Hao, M.; Ma, X.; Lin, W.; Kuang, H. Dapagliflozin Reduces Apoptosis of Diabetic Retina and Human Retinal Microvascular Endothelial Cells Through ERK1/2/cPLA2/AA/ROS Pathway Independent of Hypoglycemic. Front. Pharmacol. 2022, 13, 827896. [Google Scholar] [CrossRef]
- Kasichayanula, S.; Liu, X.; Lacreta, F.; Griffen, S.C.; Boulton, D.W. Clinical pharmacokinetics and pharmacodynamics of dapagliflozin, a selective inhibitor of sodium-glucose co-transporter type 2. Clin. Pharmacokinet. 2014, 53, 17–27. [Google Scholar] [CrossRef]
- Greco, A.; Canale, M.L.; Quagliariello, V.; Oliva, S.; Tedeschi, A.; Inno, A.; De Biasio, M.; Bisceglia, I.; Tarantini, L.; Maurea, N.; et al. SGLT2 Inhibitors in Cancer Patients: A Comprehensive Review of Clinical, Biochemical, and Therapeutic Implications in Cardio-Oncology. Int. J. Mol. Sci. 2025, 26, 4780. [Google Scholar] [CrossRef]
- Choi, M.K.; Nam, S.J.; Ji, H.Y.; Park, M.J.; Choi, J.S.; Song, I.S. Comparative Pharmacokinetics and Pharmacodynamics of a Novel Sodium-Glucose Cotransporter 2 Inhibitor, DWP16001, with Dapagliflozin and Ipragliflozin. Pharmaceutics 2020, 12, 268. [Google Scholar] [CrossRef] [PubMed]
- Therapeutic Goods Administration. Australian Public Assessment Report for Dapagliflozin propanediol monohydrate. In Ageing; Department of Health and Ageing, Australian Government: Canberra, Australia, 2013. [Google Scholar]
- Pawel, B.; Balwierz, R.; Mieszko, D.; Mikolaj, K.; Micewicz, E.; Dawid, B.; Pogorzelec, L. Assessment of basic pharmacokinetic parameters of dapagliflozin in TTS formulations in male minipigs. Sci. Rep. 2024, 14, 23504. [Google Scholar] [CrossRef]
- Sadybekov, A.V.; Katritch, V. Computational approaches streamlining drug discovery. Nature 2023, 616, 673–685. [Google Scholar] [CrossRef]
- Cern, A.; Barenholz, Y.; Tropsha, A.; Goldblum, A. Computer-aided design of liposomal drugs: In silico prediction and experimental validation of drug candidates for liposomal remote loading. J. Control. Release 2014, 173, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ma, S.; Sandhoff, R.; Ming, Y.; Hotz-Wagenblatt, A.; Timmerman, V.; Bonello-Palot, N.; Schlotter-Weigel, B.; Auer-Grumbach, M.; Seeman, P.; et al. Loss of Neurological Disease HSAN-I-Associated Gene SPTLC2 Impairs CD8(+) T Cell Responses to Infection by Inhibiting T Cell Metabolic Fitness. Immunity 2019, 50, 1218–1231. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jennemann, R.; Sandhoff, R. The SGLT2 Inhibitor Dapagliflozin Disrupts the Cell Cycle at High Concentrations Without Altering Glycosphingolipid (De Novo)Biosynthesis. Int. J. Mol. Sci. 2025, 26, 9811. https://doi.org/10.3390/ijms26199811
Jennemann R, Sandhoff R. The SGLT2 Inhibitor Dapagliflozin Disrupts the Cell Cycle at High Concentrations Without Altering Glycosphingolipid (De Novo)Biosynthesis. International Journal of Molecular Sciences. 2025; 26(19):9811. https://doi.org/10.3390/ijms26199811
Chicago/Turabian StyleJennemann, Richard, and Roger Sandhoff. 2025. "The SGLT2 Inhibitor Dapagliflozin Disrupts the Cell Cycle at High Concentrations Without Altering Glycosphingolipid (De Novo)Biosynthesis" International Journal of Molecular Sciences 26, no. 19: 9811. https://doi.org/10.3390/ijms26199811
APA StyleJennemann, R., & Sandhoff, R. (2025). The SGLT2 Inhibitor Dapagliflozin Disrupts the Cell Cycle at High Concentrations Without Altering Glycosphingolipid (De Novo)Biosynthesis. International Journal of Molecular Sciences, 26(19), 9811. https://doi.org/10.3390/ijms26199811





