ARGOS Genes in Cauliflower: Genome-Wide Identification and Functional Validation of BobARL2 Under Abiotic Stresses
Abstract
1. Introduction
2. Results
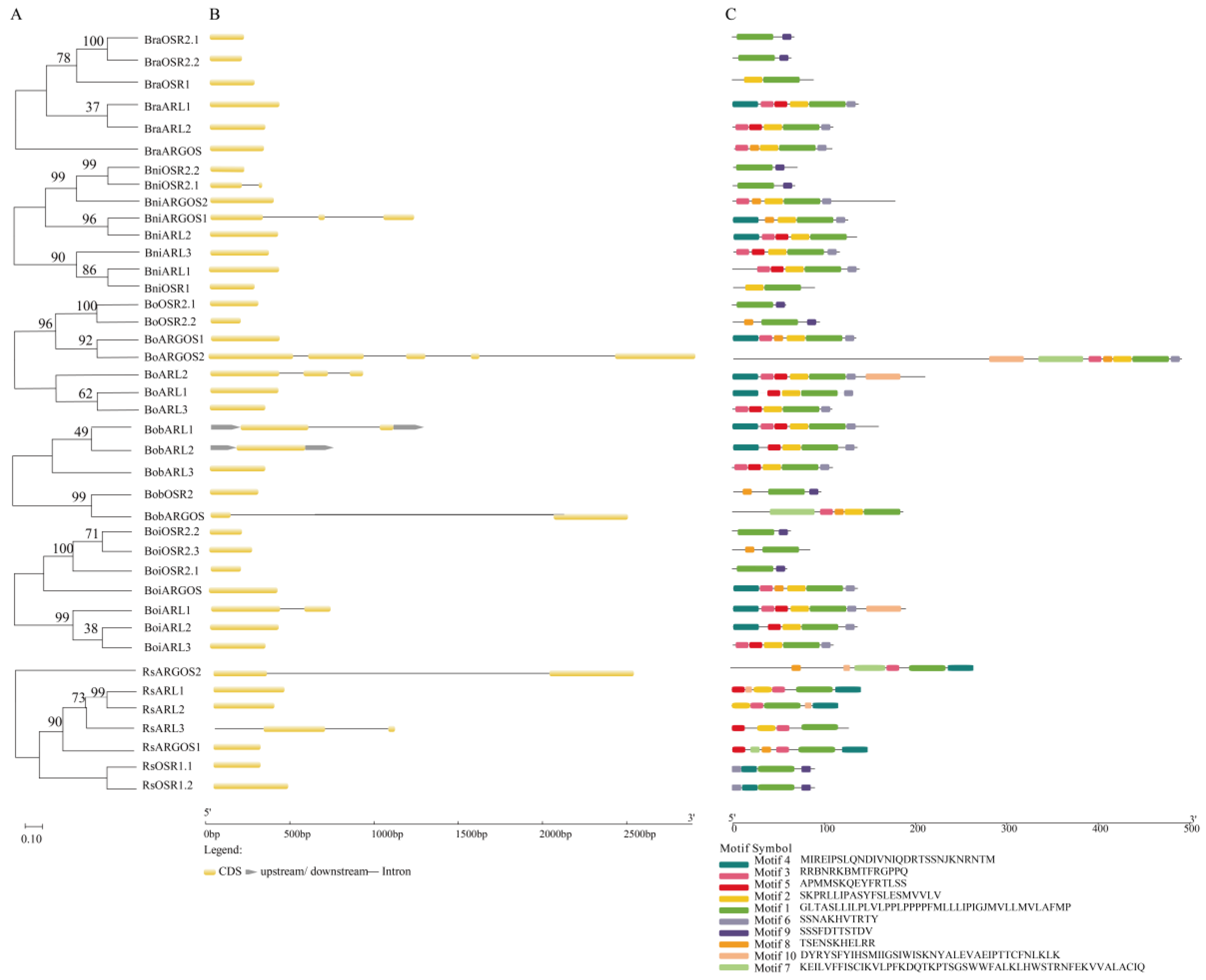
2.1. Genome-Wide Identification and Characterization of ARGOS Genes
2.2. Structural Analysis of ARGOS Genes
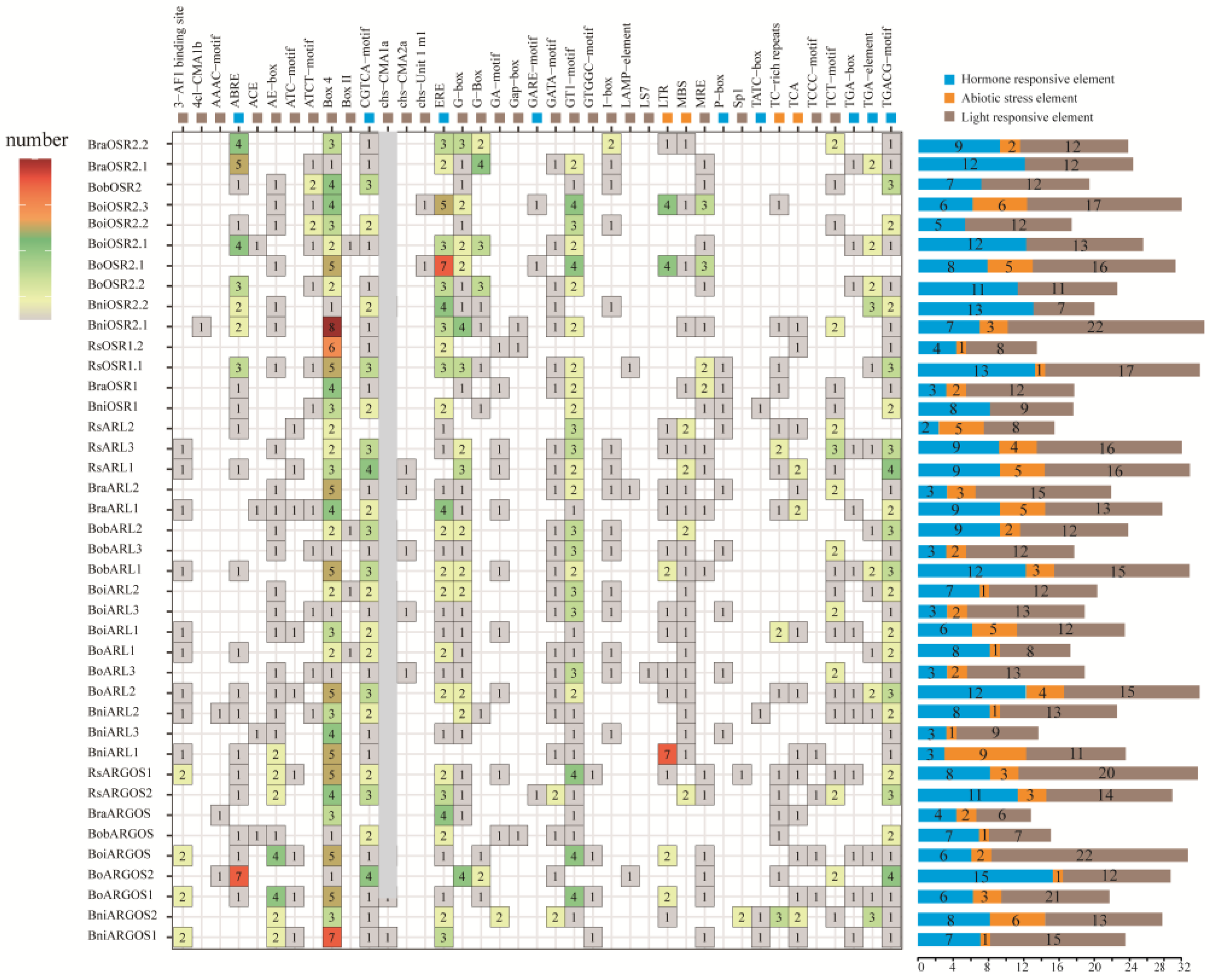
2.3. Promoter Cis-Acting Elements in ARGOS Genes
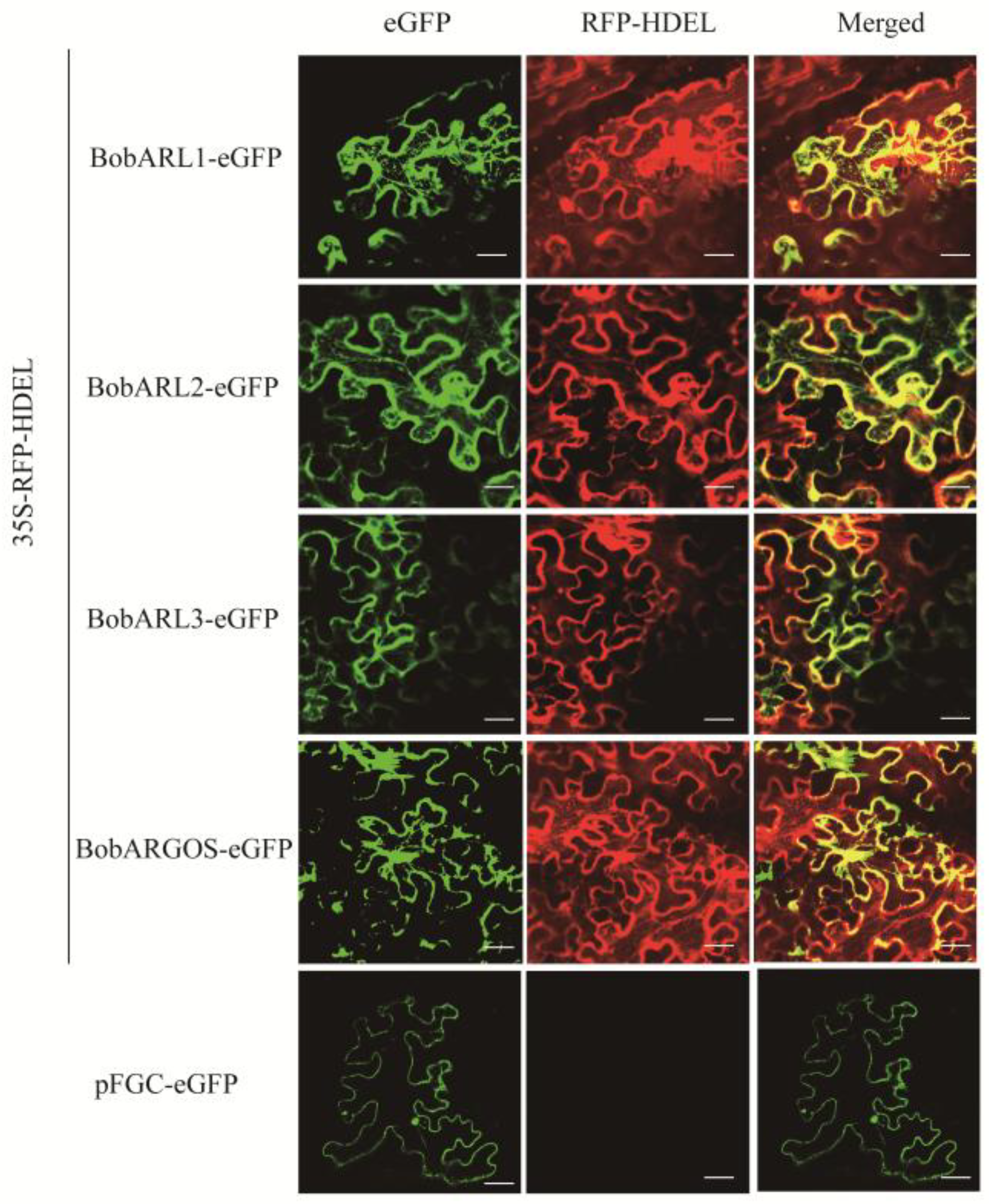
2.4. BobARGOS Endoplasmic Reticulum Localization
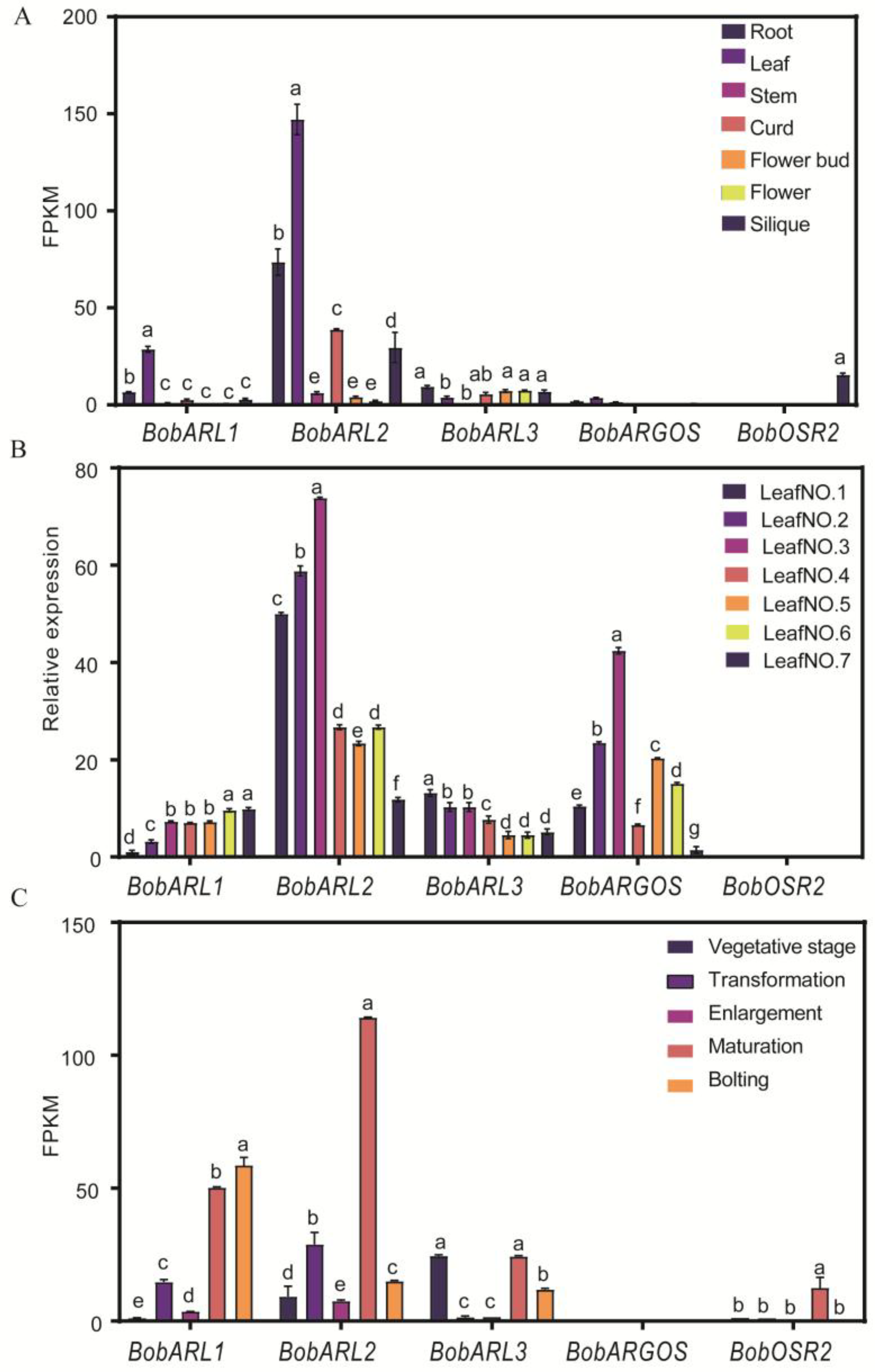
2.5. ARGOS Gene Expression in Various Cauliflower Organs
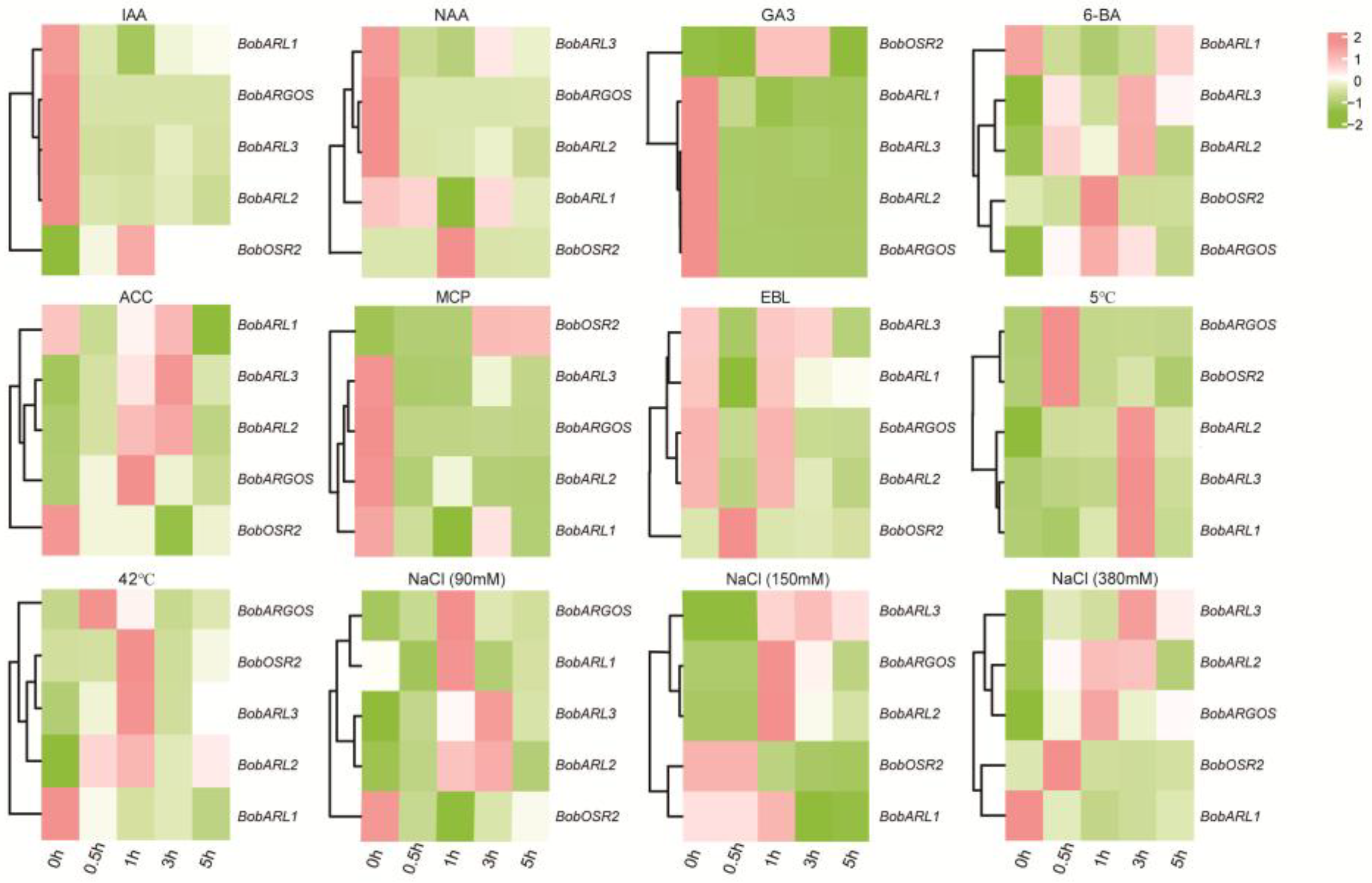
2.6. Cauliflower ARGOS Gene Expression in Response to Different Abiotic Stresses
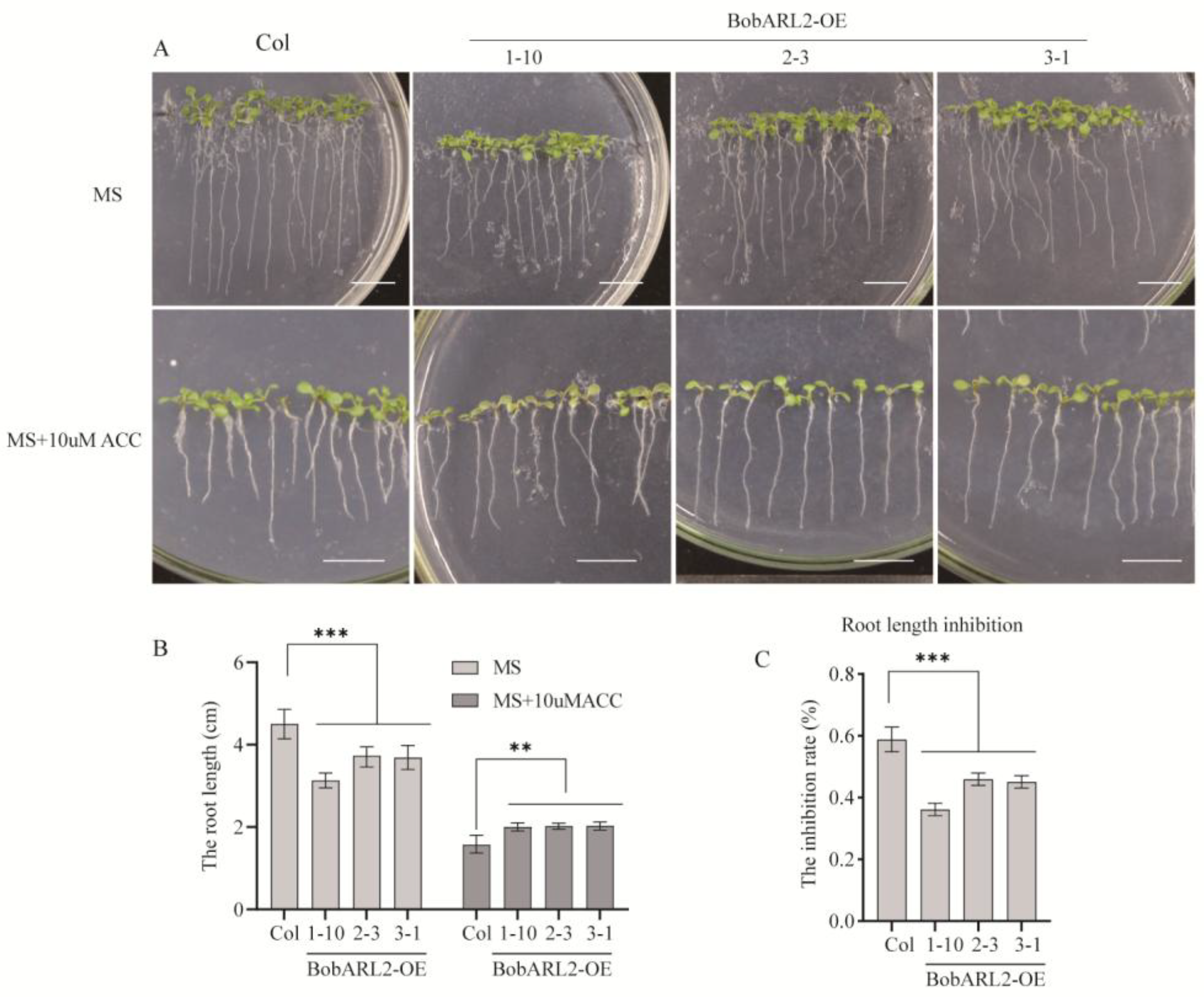
2.7. Regulatory Effects of BobARL2 on Arabidopsis Root Growth Under ACC and NaCl Stress Conditions
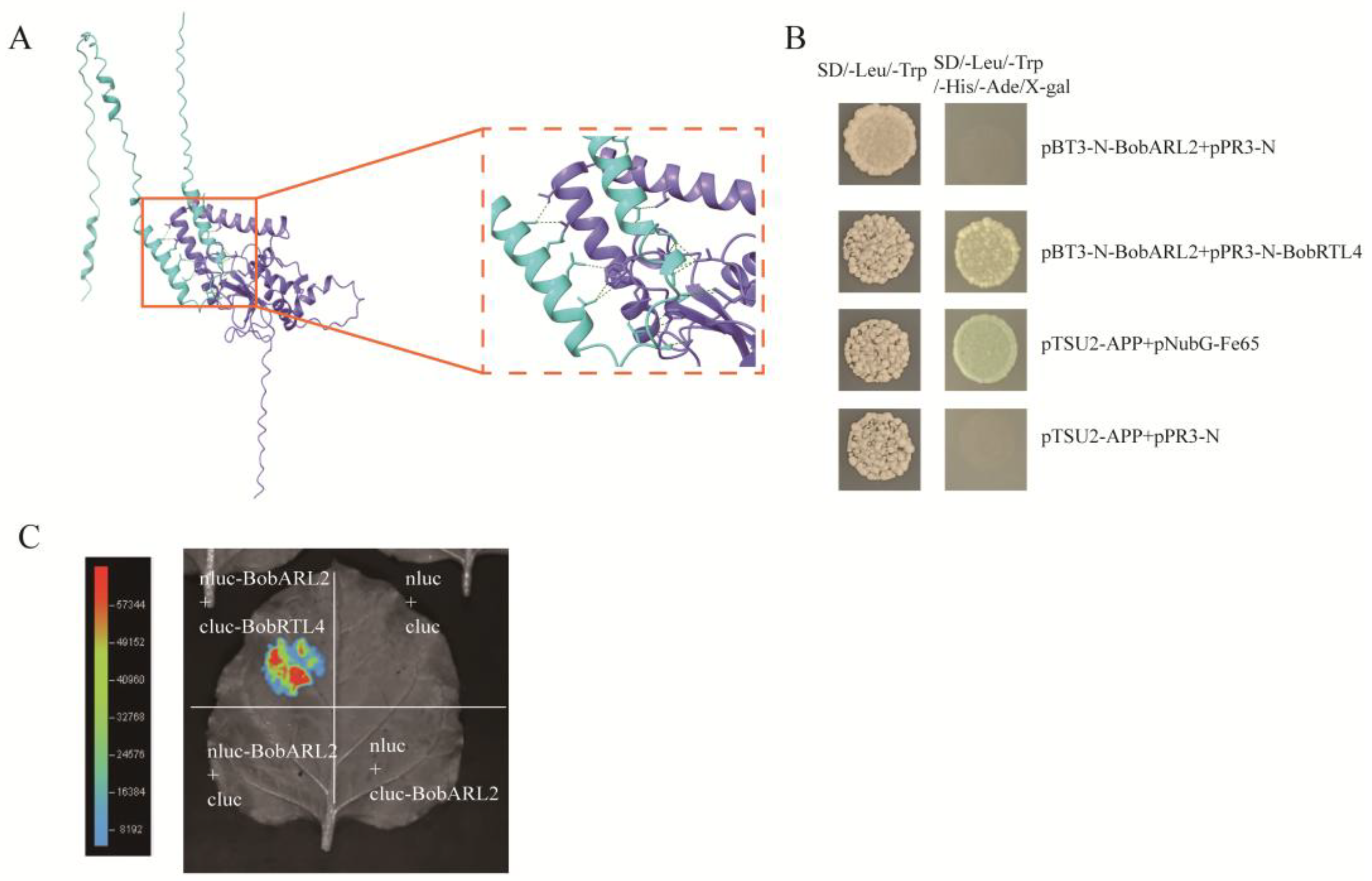
2.8. Interaction Between BobARL2 and BobRTL4
3. Discussion
4. Materials and Methods
4.1. Sequence Acquisition and Genome-Wide Identification of ARGOS Genes in Different Species
4.2. Sequence Alignment and Phylogenetic Analyses of ARGOS Genes
4.3. Synteny Analysis of OSR1 in Brassica oleracea and Arabidopsis
4.4. Analyses of ARGOS Gene Structures and Conserved Motifs
4.5. Analysis of ARGOS Promoter Cis-Acting Elements
4.6. Plant Materials and Treatments
4.7. qRT-PCR Analysis
4.8. Subcellular Localization
4.9. Vector Construction and Plant Transformation
4.10. ACC and NaCl Treatments of Transgenic Arabidopsis
4.11. Predicting the Interaction Between BobARL2 and BobRTL4
4.12. Protein Interaction Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARGOS | Auxin-Regulated Gene Involved in Organ Size |
| BobRTL4 | Reversion-to-ethylene sensitivity Like4 |
| OSR | Organ size-related |
| ANT | Aintegumenta |
| BRI1 | Br insensitive 1 |
| RTE1 | Reversion-to-ethylene sensitivity1 |
| qRT-PCR | quantitative real-time polymerase chain reaction |
| IAA | including indole-3-acetic acid |
| NAA | 1-naphthaleneacetic acid |
| 6-BA | 6-benzylaminopurine |
| 1-MCP | 1-methylcyclopropene |
| GA3 | gibberellin |
| ACC | 1-aminocyclopropane-1-carboxylic acid |
| EBL | 24-epibrassinolide |
| RTA1 | Responsive-to-antagonist1 |
| ETR1 | Ethylene response1 |
| WAT1 | Walls Are Thin1 |
| LCI | luciferase complementation imaging |
| Cyc D3;1 | Cyclin D3;1 |
| RsCBF2 | C-repeat Binding Factor 2 |
| RsERF18 | C-repeat Binding Factor 2 |
| MS | Murashige and Skoog |
| Y2H | yeast two-hybrid assay |
| GeBP3 | GLABROUS1 enhancer-binding protein |
References
- Guo, N.; Wang, S.; Wang, T.; Duan, M.; Zong, M.; Miao, L.; Han, S.; Wang, G.; Liu, X.; Zhang, D.; et al. A graph-based pan-genome of Brassica oleracea provides new insights into its domestication and morphotype diversification. Plant Commun. 2024, 5, 100791. [Google Scholar] [CrossRef]
- Jiang, Q.; Wu, X.; Zhang, X.; Ji, Z.; Cao, Y.; Duan, Q.; Huang, J. Genome-Wide Identification and Expression Analysis of AS2 Genes in Brassica rapa Reveal Their Potential Roles in Abiotic Stress. Int. J. Mol. Sci. 2023, 24, 10611. [Google Scholar] [CrossRef]
- Karamat, U.; Guo, J.; Jiang, S.; Khan, I.; Lu, M.; Fu, M.; Li, G. Comprehensive, Genome-Wide Identification and Expression Analyses of Phenylalanine Ammonia-Lyase Family under Abiotic Stresses in Brassica oleracea. Int. J. Mol. Sci. 2024, 25, 10378. [Google Scholar] [CrossRef]
- Bian, X.; Cao, Y.; Zhi, X.; Ma, N. Genome-Wide Identification and Analysis of the Plant Cysteine Oxidase (PCO) Gene Family in Brassica napus and Its Role in Abiotic Stress Response. Int. J. Mol. Sci. 2023, 24, 11242. [Google Scholar] [CrossRef]
- Cheng, F.; Sun, R.; Hou, X.; Zheng, H.; Zhang, F.; Zhang, Y.; Liu, B.; Liang, J.; Zhuang, M.; Liu, Y.; et al. Subgenome parallel selection is associated with morphotype diversification and convergent crop domestication in Brassica rapa and Brassica oleracea. Nat. Genet. 2016, 48, 1218–1224. [Google Scholar] [CrossRef]
- Guo, N.; Wang, S.; Gao, L.; Liu, Y.; Wang, X.; Lai, E.; Duan, M.; Wang, G.; Li, J.; Yang, M.; et al. Genome sequencing sheds light on the contribution of structural variants to Brassica oleracea diversification. BMC Biol. 2021, 19, 93. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Chen, K.; Yao, X.; Zhang, X.; Yang, Y.; Su, X.; Lyu, M.; Wang, Q.; Zhang, G.; Wang, M.; et al. Genomic analyses reveal the stepwise domestication and genetic mechanism of curd biogenesis in cauliflower. Nat. Genet. 2024, 56, 1235–1244. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Wang, J.; Sun, R.; Wu, J.; Liu, S.; Bai, Y.; Mun, J.H.; Bancroft, I.; Cheng, F.; et al. The genome of the mesopolyploid crop species Brassica rapa. Nat. Genet. 2011, 43, 1035–1039. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Cai, C.; Ji, J.; Han, F.; Zhang, L.; Chen, S.; Zhang, L.; Yang, Y.; Tang, Q.; et al. Large-scale gene expression alterations introduced by structural variation drive morphotype diversification in Brassica oleracea. Nat. Genet. 2024, 56, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Qin, Z.; Yan, J.; Zhang, X.; Hu, Y. Arabidopsis ORGAN SIZE RELATED1 regulates organ growth and final organ size in orchestration with ARGOS and ARL. New Phytol. 2011, 191, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Zhang, X.; Zhang, X.; Feng, G.; Hu, Y. The Arabidopsis ORGAN SIZE RELATED 2 is involved in regulation of cell expansion during organ growth. BMC Plant Biol. 2014, 14, 349. [Google Scholar] [CrossRef]
- Shi, J.; Gao, H.; Wang, H.; Lafitte, H.R.; Archibald, R.L.; Yang, M.; Hakimi, S.M.; Mo, H.; Habben, J.E. ARGOS8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant Biotechnol. J. 2017, 15, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Drummond, B.; Wang, H.; Archibald, R.L.; Habben, J.E. Maize and Arabidopsis ARGOS proteins interact with ethylene receptor signaling complex, supporting a regulatory role for ARGOS in ethylene signal transduction. Plant Physiol. 2016, 171, 2783–2797. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Habben, J.E.; Archibald, R.L.; Drummond, B.J.; Chamberlin, M.A.; Williams, R.W.; Lafitte, H.R.; Weers, B.P. Overexpression of ARGOS genes modifies plant sensitivity to ethylene, leading to improved drought tolerance in both Arabidopsis and Maize. Plant Physiol. 2015, 169, 266–282. [Google Scholar] [CrossRef]
- Hu, Y.; Xie, Q.; Chua, N.H. The Arabidopsis Auxin-Inducible Gene ARGOS Controls Lateral Organ Size. Plant Cell. 2003, 15, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.I.; Wang, X.; Thibault, D.M.; Kim, H.J.; Schaller, G.E. The ARGOS gene family functions in a negative feedback loop to desensitize plants to ethylene. BMC Plant Biol. 2015, 15, 157. [Google Scholar] [CrossRef]
- Wang, B.; Sang, Y.; Song, J.; Gao, X.Q.; Zhang, Q. Expression of a rice OsARGOS gene in Arabidopsis promotes cell division and expansion and increases organ size. J. Genet. Genom. 2009, 36, 31–40. [Google Scholar] [CrossRef]
- Guo, M.; Rupe, M.A.; Wei, J.; Winkler, C.; Goncalves-Butruille, M.; Weers, B.P.; Cerwick, S.F.; Dieter, J.A.; Duncan, K.E.; Howard, R.J.; et al. Maize ARGOS1 (ZAR1) transgenic alleles increase hybrid maize yield. J. Exp. Bot. 2014, 65, 249–260. [Google Scholar] [CrossRef]
- Kuluev, B.; Mikhaylova, E.; Ermoshin, A.; Veselova, S.; Tugbaeva, A.; Gumerova, G.; Gainullina, K.; Zaikina, E. The ARGOS-LIKE genes of Arabidopsis and tobacco as targets for improving plant productivity and stress tolerance. J. Plant Physiol. 2019, 234–235, 9–17. [Google Scholar] [CrossRef]
- Mizukami, Y.; Fischer, R.L. Plant organ size control: AINTEGUMENTA regulates growth and cell numbers during organogenesis. Proc. Natl. Acad. Sci. USA 2000, 97, 942–947. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, G.; Zhang, G.; Sun, Q.; Liu, C.; Chu, J.; Jing, Y.; Niu, S.; Fu, C.; Lew, T.T.S.; et al. PagARGOS Promotes Low-Lignin Wood Formation in Poplar. Plant Biotechnol. J. 2024, 22, 2201–2215. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Poh, H.M.; Chua, N.H. The Arabidopsis ARGOS-LIKE gene regulates cell expansion during organ growth. Plant J. 2006, 47, 1–9, Erratum in Plant J. 2006, 47, 490. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, X.; Li, Y.; Zhang, L.; Guan, P.; Kou, X.; Wang, X.; Xin, M.; Hu, Z.; Yao, Y.; et al. Molecular and Functional Characterization of Wheat ARGOS Genes Influencing Plant Growth and Stress Tolerance. Front. Plant Sci. 2017, 8, 1704. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Yang, X.; Tong, C.; Edwards, D.; Parkin, I.A.; Zhao, M.; Ma, J.; Yu, J.; Huang, S.; et al. The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat. Commun. 2014, 5, 3930. [Google Scholar] [CrossRef]
- Parkin, I.A.; Koh, C.; Tang, H.; Robinson, S.J.; Kagale, S.; Clarke, W.E.; Town, C.D.; Nixon, J.; Krishnakumar, V.; Bidwell, S.L.; et al. Transcriptome and methylome profiling reveals relics of genome dominance in the mesopolyploid Brassica oleracea. Genome Biol. 2014, 15, R77. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Q.; Yu, X.; Chen, X.; Cao, F.; Wu, Z.; Sun, Y.; Jing, Y.; Liu, B.; Shen, D.; et al. A de novo Genome of a Chinese Radish Cultivar. Hortic. Plant J. 2015, 1, 155–164. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, X.; Wu, J.; Liu, M.; Grob, S.; Cheng, F.; Liang, J.; Cai, C.; Liu, Z.; Liu, B.; et al. Improved Brassica rapa reference genome by single-molecule sequencing and chromosome conformation capture technologies. Hortic. Res. 2018, 5, 50, Erratum in Hortic. Res. 2019, 13, 124. [Google Scholar] [CrossRef]
- Yang, J.; Liu, D.; Wang, X.; Ji, C.; Cheng, F.; Liu, B.; Hu, Z.; Chen, S.; Pental, D.; Ju, Y.; et al. The genome sequence of allopolyploid Brassica juncea and analysis of differential homoeolog gene expression influencing selection. Nat. Genet. 2016, 48, 1225–1232, Erratum in Nat. Genet. 2018, 50, 1616. [Google Scholar] [CrossRef] [PubMed]
- Belser, C.; Istace, B.; Denis, E.; Dubarry, M.; Baurens, F.C.; Falentin, C.; Genete, M.; Berrabah, W.; Chèvre, A.M.; Delourme, R.; et al. Chromosome-scale assemblies of plant genomes using nanopore long reads and optical maps. Nat. Plants. 2018, 4, 879–887. [Google Scholar] [CrossRef]
- Hillis, D.M. Phylogenetic analysis. Curr. Biol. 1997, 7, R129–R131. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Mandáková, T.; Wu, J.; Xie, Q.; Lysak, M.A.; Wang, X. Deciphering the Diploid Ancestral Genome of the Mesohexaploid Brassica rapa. Plant Cell 2013, 25, 1541–1554. [Google Scholar] [CrossRef]
- Zou, C.; Sun, K.; Mackaluso, J.D.; Seddon, A.E.; Jin, R.; Thomashow, M.F.; Shiu, S.H. Cis-regulatory code of stress-responsive transcription in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2011, 108, 14992–14997. [Google Scholar] [CrossRef]
- Molina, C.; Grotewold, E. Genome wide analysis of Arabidopsis core promoters. BMC Genom. 2005, 6, 25. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, J.; Jiang, D.; Hong, Y.; Xu, J.; Zheng, S.; Yang, J.; Chen, W. The miR157-SPL-CNR module acts upstream of bHLH101 to negatively regulate iron deficiency responses in tomato. J. Integr. Plant Biol. 2022, 64, 1059–1075. [Google Scholar] [CrossRef]
- Yoshida, T.; Fujita, Y.; Sayama, H.; Kidokoro, S.; Maruyama, K.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. Plant J. 2010, 61, 672–685. [Google Scholar] [CrossRef]
- Wu, R.; Duan, L.; Pruneda-Paz, J.L.; Oh, D.H.; Pound, M.; Kay, S.; Dinneny, J.R. The 6xABRE Synthetic Promoter Enables the Spatiotemporal Analysis of ABA-Mediated Transcriptional Regulation. Plant Physiol. 2018, 177, 1650–1665. [Google Scholar] [CrossRef]
- Montgomery, J.; Goldman, S.; Deikman, J.; Margossian, L.; Fischer, R.L. Identification of an ethylene-responsive region in the promoter of a fruit ripening gene. Proc. Natl. Acad. Sci. USA 1993, 90, 5939–5943. [Google Scholar] [CrossRef]
- Licausi, F.; Ohme-Takagi, M.; Perata, P. APETALA2/Ethylene Responsive Factor (AP2/ERF) transcription factors: Mediators of stress responses and developmental programs. New Phytol. 2013, 199, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Shabbir, G.; Ahmed, M.; Noor, S.; Mohi Ud Din, A.; Qamar, M.; Rehman, N. Expression profiling of TaARGOS homoeologous drought responsive genes in bread wheat. Sci. Rep. 2022, 12, 3595. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Yuan, X.; Zhang, M.; Qin, T.; He, Y.; Ying, J.; Wang, H.; Xu, L.; Liu, L.; Wang, Y. Ethylene-Mediated RsCBF2 and RsERF18 Enhance Salt Tolerance by Directly Regulating Aquaporin Gene RsPIP2-1 in Radish (Raphanus sativus L.). Plant Cell Environ. 2025, 48, 5740–5752. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, M.; Kim, J.H.; Kim, S.H.; Park, S.Y.; Lee, S.I. BrPP5.2 Overexpression Confers Heat Shock Tolerance in Transgenic Brassica rapa through Inherent Chaperone Activity, Induced Glucosinolate Biosynthesis, and Differential Regulation of Abiotic Stress Response Genes. Int. J. Mol. Sci. 2021, 22, 6437. [Google Scholar] [CrossRef]
- Luo, J.; Tang, S.; Mei, F.; Peng, X.; Li, J.; Li, X.; Yan, X.; Zeng, X.; Liu, F.; Wu, Y.; et al. BnSIP1-1, a Trihelix Family Gene, Mediates Abiotic Stress Tolerance and ABA Signaling in Brassica napus. Front. Plant Sci. 2017, 8, 44. [Google Scholar] [CrossRef]
- Binder, B.M.; Rodríguez, F.I.; Bleecker, A.B. The copper transporter RAN1 is essential for biogenesis of ethylene receptors in Arabidopsis. J. Biol. Chem. 2010, 285, 37263–37270. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Kwok, S.F.; Bleecker, A.B.; Meyerowitz, E.M. Arabidopsis ethylene-response gene ETR1: Similarity of product to two-component regulators. Science 1993, 262, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Meyerowitz, E.M. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 1998, 94, 261–271. [Google Scholar] [CrossRef]
- Resnick, J.S.; Rivarola, M.; Chang, C. Involvement of RTE1 in conformational changes promoting ETR1 ethylene receptor signaling in Arabidopsis. Plant J. 2008, 56, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.H.; Rivarola, M.; Resnick, J.S.; Maggin, B.D.; Chang, C. Subcellular co-localization of Arabidopsis RTE1 and ETR1 supports a regulatory role for RTE1 in ETR1 ethylene signaling. Plant J. 2008, 53, 275–286. [Google Scholar] [CrossRef]
- Dong, C.H.; Jang, M.; Scharein, B.; Malach, A.; Rivarola, M.; Liesch, J.; Groth, G.; Hwang, I.; Chang, C. Molecular association of the Arabidopsis ETR1 ethylene receptor and a regulator of ethylene signaling, RTE1. J. Biol. Chem. 2010, 285, 40706–40713. [Google Scholar] [CrossRef]
- Lin, P.-Y.; Huang, S.-C.; Chen, K.-L.; Huang, Y.-C.; Liao, C.-Y.; Lin, G.-J.; Lee, H.; Chen, P.-Y. Analysing Protein Complexes in Plant Science: Insights and Limitations with AlphaFold 3. Bot. Stud. 2025, 66, 14. [Google Scholar] [CrossRef]
- Cao, H.; Wang, D.; Li, X.; Zhang, Y.; Su, D.; Lu, W.; Xu, K.; Li, Z. Genome-Wide Identification and Expression Profiling of SlGeBP Gene Family in Response to Hormone and Abiotic Stresses in Solanum lycopersicum L. Int. J. Mol. Sci. 2025, 26, 6008. [Google Scholar] [CrossRef]
- Kohli, A.; Sreenivasulu, N.; Lakshmanan, P.; Kumar, P.P. The Phytohormone Crosstalk Paradigm Takes Center Stage in Understanding How Plants Respond to Abiotic Stresses. Plant Cell Rep. 2013, 32, 945–957. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Chen, X.; Liu, F. Crosstalk between ABA and ethylene in regulating stomatal behavior in tomato under high CO2 and progressive soil drying. J. Exp. Bot. 2023, 74, 5931–5946. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef]
- Yu, C.S.; Lin, C.J.; Hwang, J.K. Predicting subcellular localization of proteins for Gram-negative bacteria by support vector machines based on n-peptide compositions. Protein Sci. 2004, 13, 1402–1406. [Google Scholar] [CrossRef]
- Rice, P.; Longden, I.; Bleasby, A. EMBOSS: The European Molecular Biology Open Software Suite. Trends Genet. 2000, 16, 276–277. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Corpet, F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988, 16, 10881–10890. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Cheng, F.; Wu, J.; Fang, L.; Wang, X. Syntenic gene analysis between Brassica rapa and other Brassicaceae species. Front. Plant Sci. 2012, 3, 198. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Sheng, X.G.; Zhao, Z.Q.; Yu, H.F.; Wang, J.S.; Zheng, C.F.; Gu, H.H. In-depth analysis of internal control genes for quantitative real-time PCR in Brassica oleracea var. botrytis. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Dang, F.; Wang, Y.; She, J.; Lei, Y.; Liu, Z.; Eulgem, T.; Lai, Y.; Lin, J.; Yu, L.; Lei, D.; et al. Overexpression of CaWRKY27, a subgroup IIe WRKY transcription factor of Capsicum annuum, positively regulates tobacco resistance to Ralstonia solanacearum infection. Physiol. Plant. 2014, 150, 397–411. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, M.; Wang, G.; Zong, M.; Han, S.; Guo, N.; Liu, F. ARGOS Genes in Cauliflower: Genome-Wide Identification and Functional Validation of BobARL2 Under Abiotic Stresses. Int. J. Mol. Sci. 2025, 26, 9810. https://doi.org/10.3390/ijms26199810
Duan M, Wang G, Zong M, Han S, Guo N, Liu F. ARGOS Genes in Cauliflower: Genome-Wide Identification and Functional Validation of BobARL2 Under Abiotic Stresses. International Journal of Molecular Sciences. 2025; 26(19):9810. https://doi.org/10.3390/ijms26199810
Chicago/Turabian StyleDuan, Mengmeng, Guixiang Wang, Mei Zong, Shuo Han, Ning Guo, and Fan Liu. 2025. "ARGOS Genes in Cauliflower: Genome-Wide Identification and Functional Validation of BobARL2 Under Abiotic Stresses" International Journal of Molecular Sciences 26, no. 19: 9810. https://doi.org/10.3390/ijms26199810
APA StyleDuan, M., Wang, G., Zong, M., Han, S., Guo, N., & Liu, F. (2025). ARGOS Genes in Cauliflower: Genome-Wide Identification and Functional Validation of BobARL2 Under Abiotic Stresses. International Journal of Molecular Sciences, 26(19), 9810. https://doi.org/10.3390/ijms26199810




