Foliar Nano-Selenium Modulates Metabolic and Antioxidant Responses in Alfalfa (Medicago sativa L.): Integration of Pot and Field Evidence
Abstract
1. Introduction
2. Results
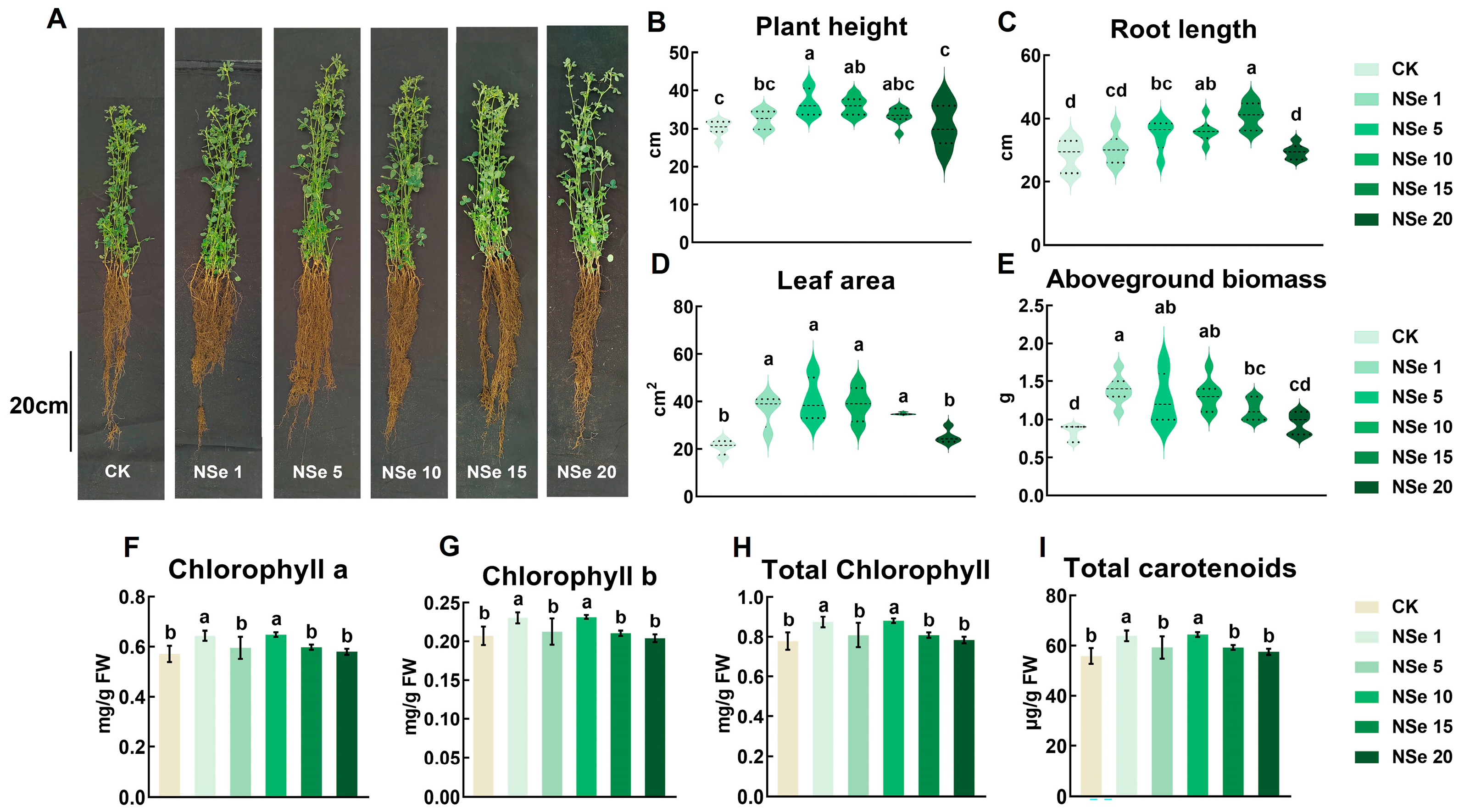
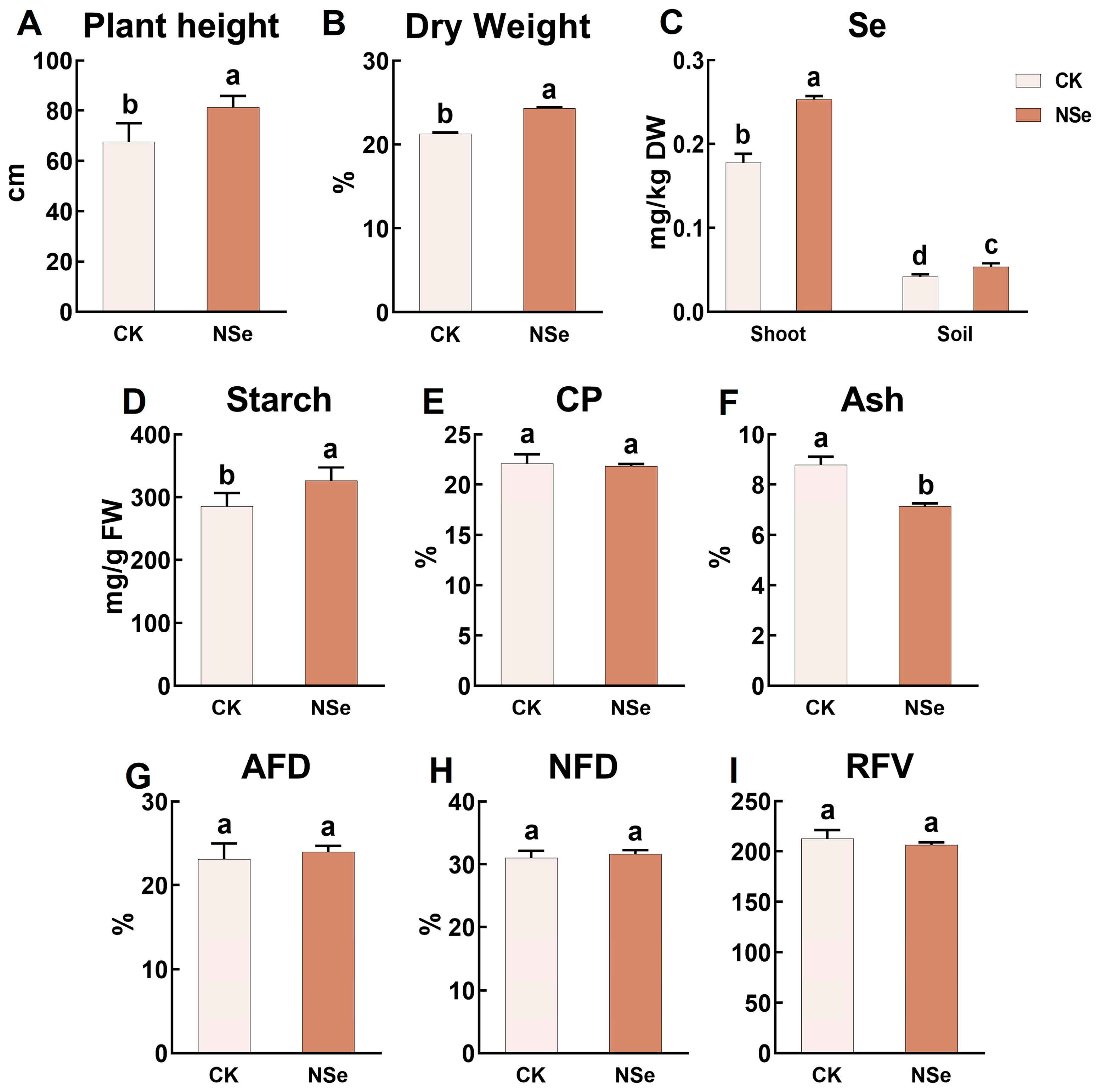
2.1. Effect on Alfalfa Growth
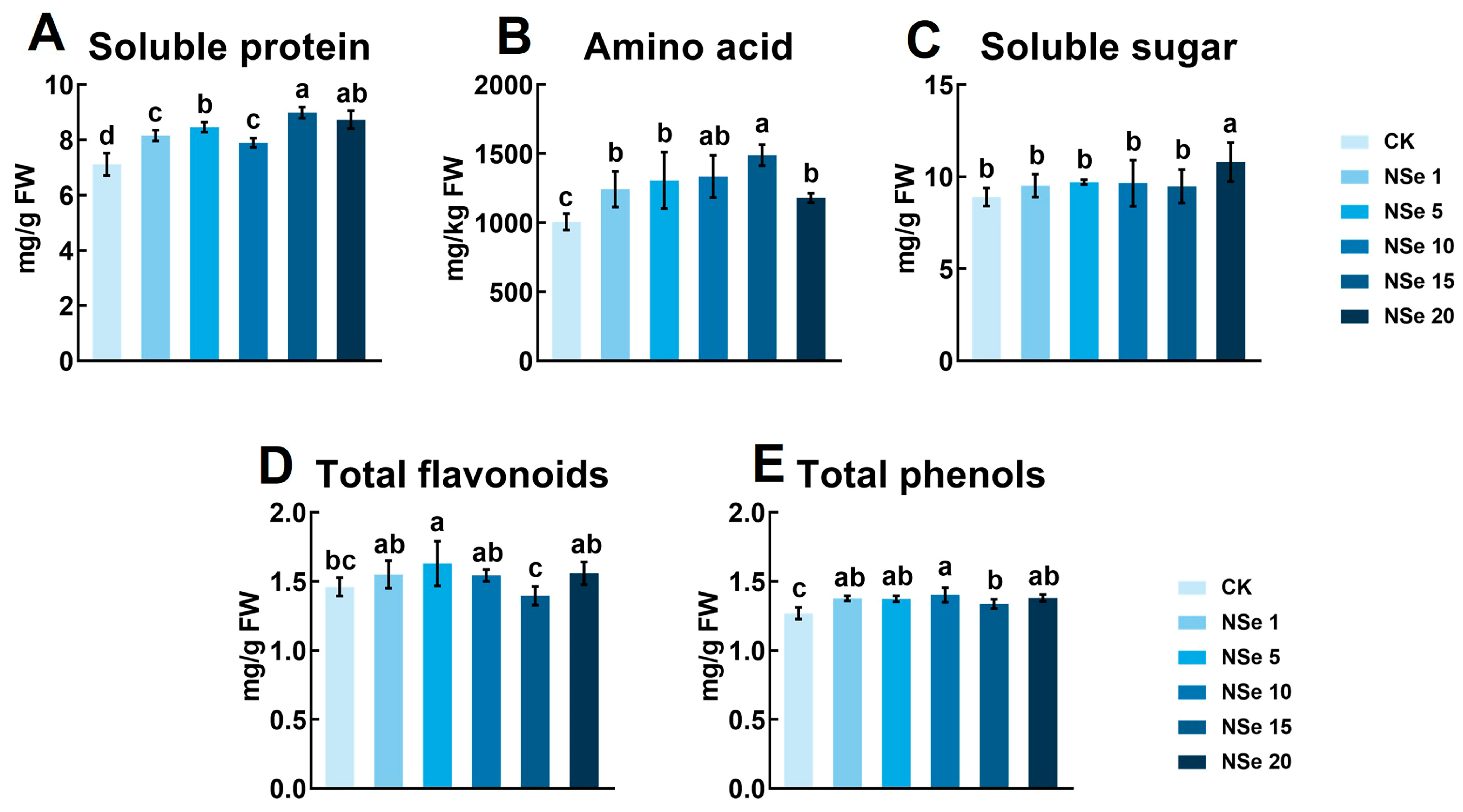
2.2. Effects on Alfalfa Quality Components
2.3. Effects on Alfalfa Free Amino Acids
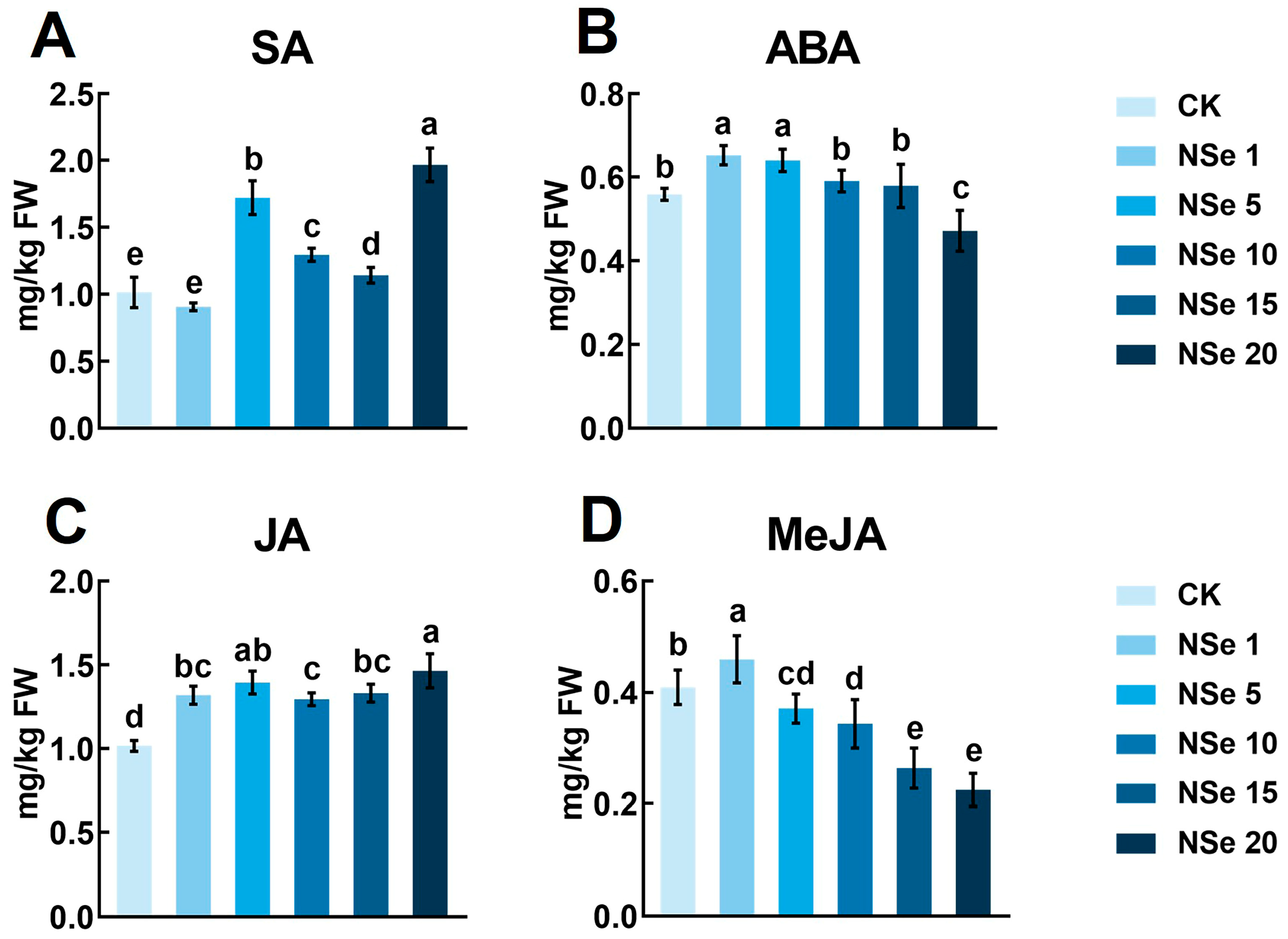
2.4. Effect on Phytohormones
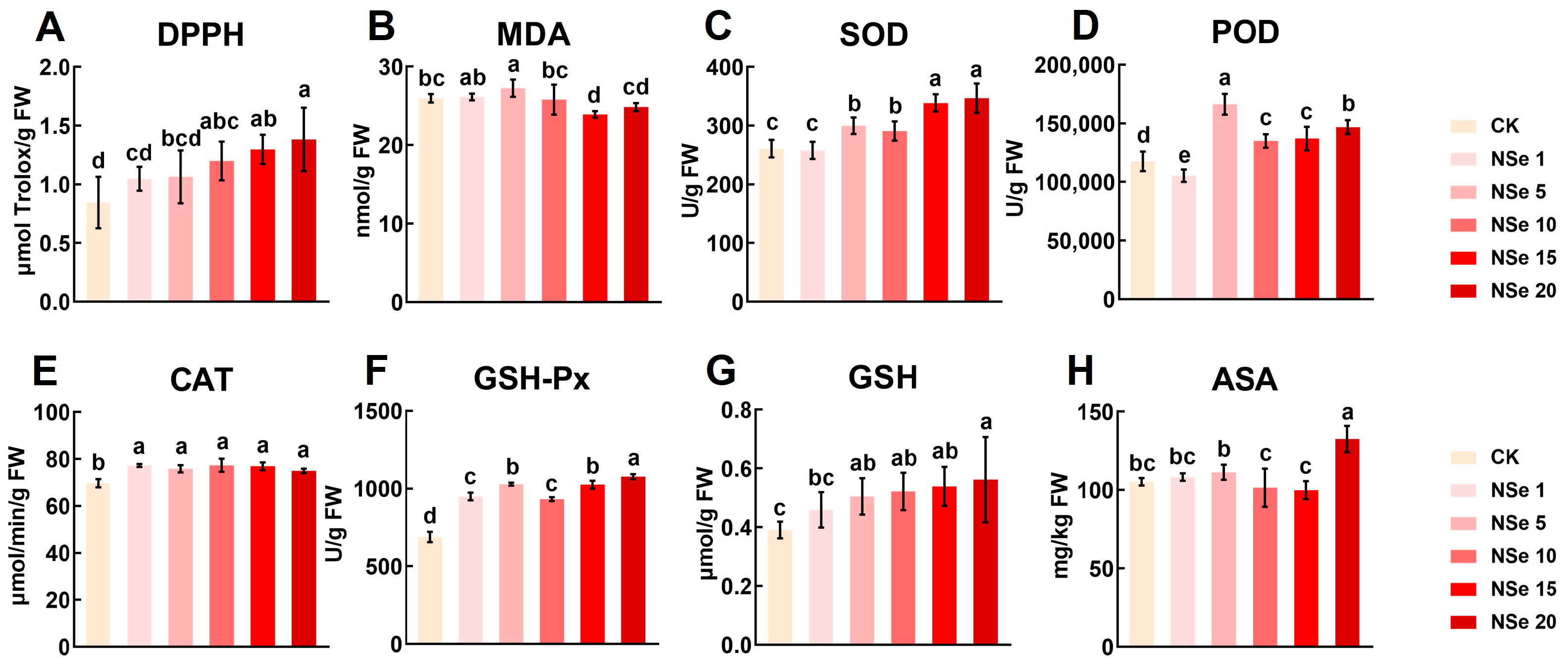
2.5. Effect on Antioxidant System
2.6. Effect on Field Trial: Yield and Quality
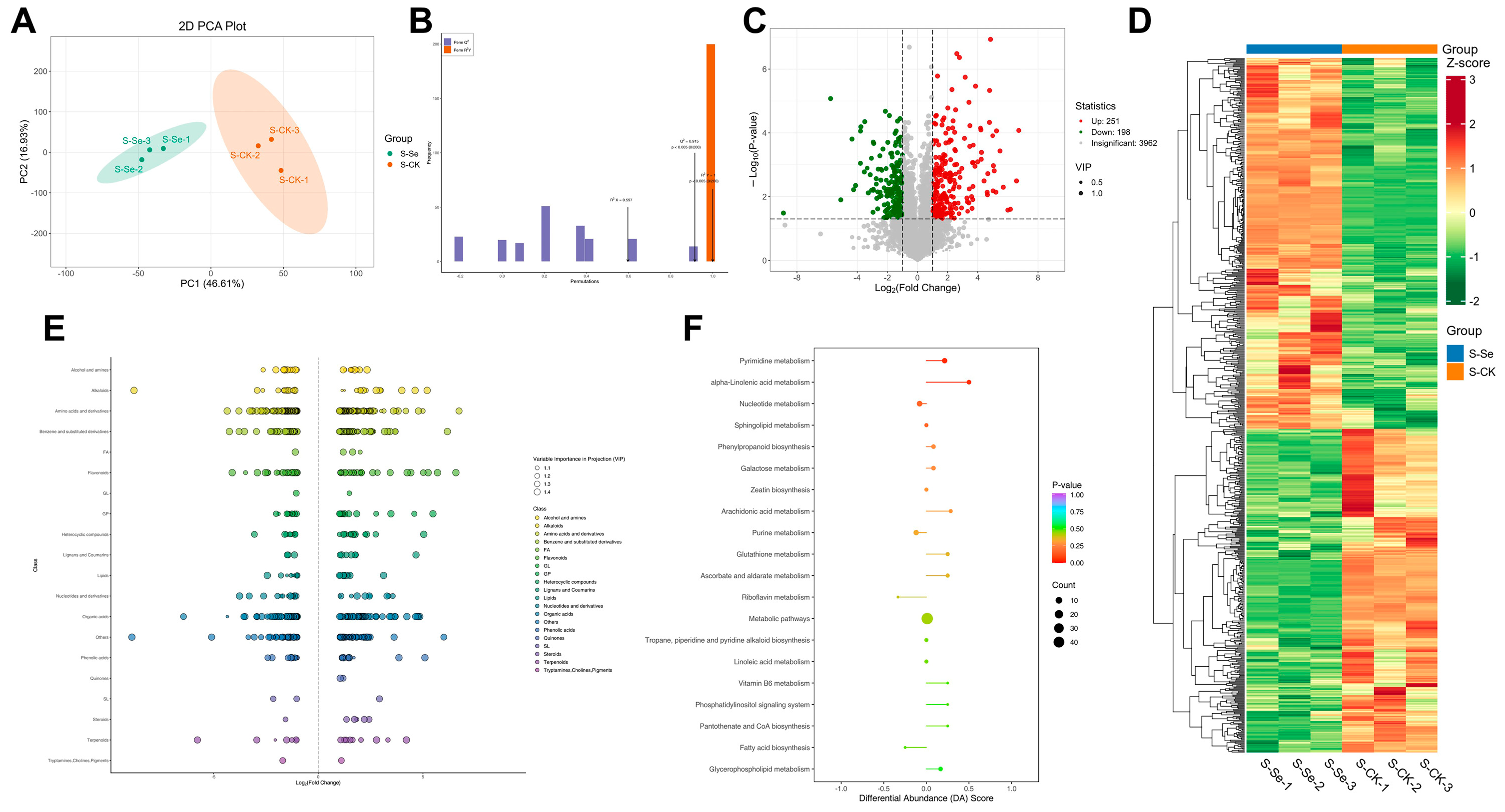
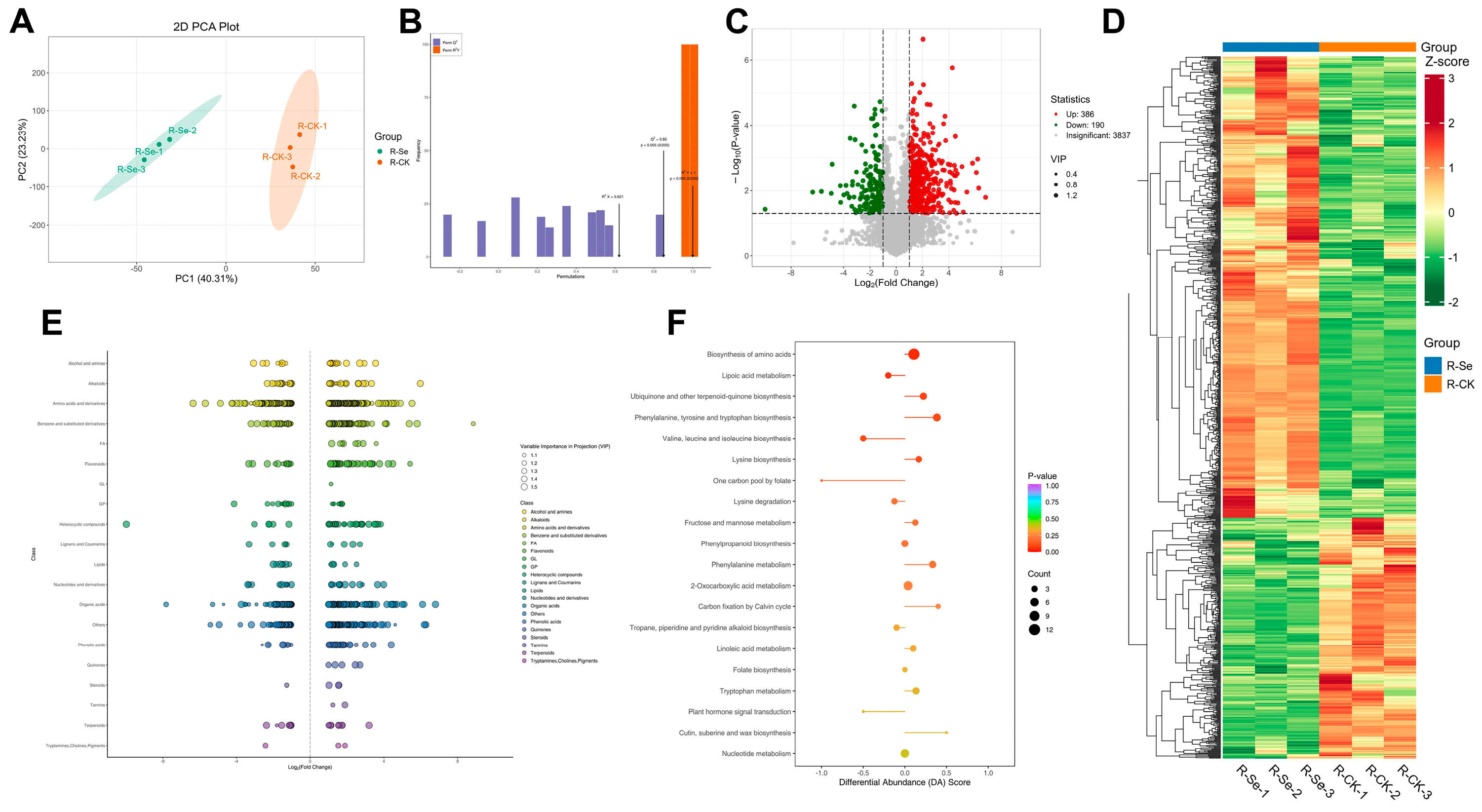
2.7. Metabolite Analysis
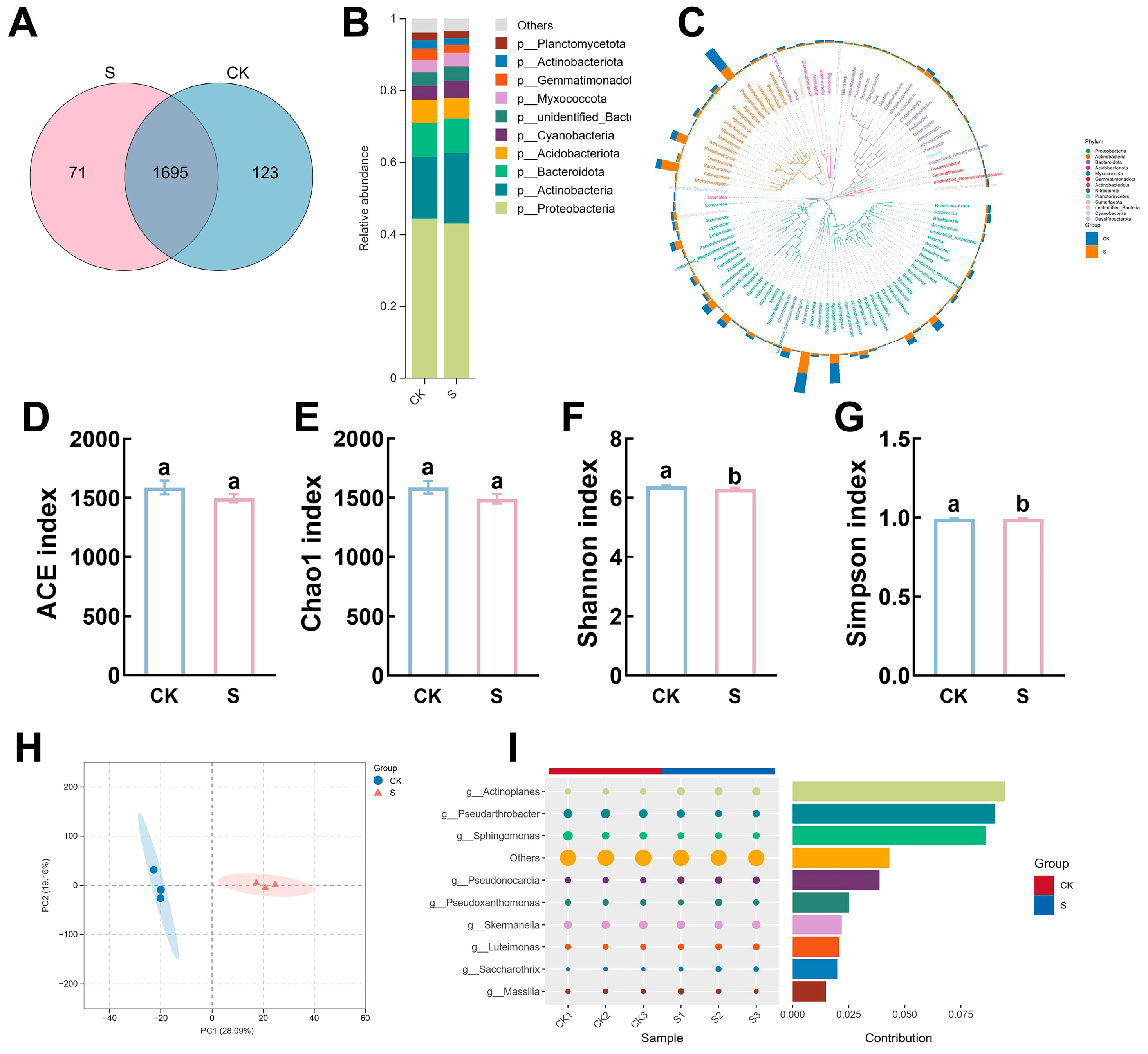
2.8. Soil Microbial Analysis
2.9. Correlation Analysis
3. Discussion
3.1. Nutrient Metabolism in Alfalfa Under Foliar NSe
3.2. Antioxidant Defense and Stress Hormones in Response to Foliar NSe
3.3. Rhizosphere Microenvironment Alterations Under Foliar NSe
4. Materials and Methods
4.1. Plant Cultivation
4.2. Alfalfa Feeding Value Analysis
4.3. Total Selenium Content Analysis
4.4. Photosynthetic Pigment Analysis
4.5. Enzyme Activity Analysis
4.6. Amino Acid Analysis
4.7. Phytohormone Analysis
4.8. Metabolomics Analysis
4.9. Microbiome Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Y.; Duan, X.; Wu, Y.; Huang, H.; Fu, T.; Chu, H.; Xue, S. Carbon sequestration potential and its main drivers in soils under alfalfa (Medicago sativa L.). Sci. Total Environ. 2024, 935, 173338. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, J.; Dong, C.; Wang, H.; Liu, B.; Li, D.; Cui, Y.; Wang, Z.; Ma, S.; Shi, Y.; et al. Physiological and biochemical characteristics and microbial responses of Medicago sativa (Fabales: Fabaceae) varieties with different resistance to atrazine stress. Front. Microbiol. 2024, 15, 1447348. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, G.; Shehzad, M.A.; Tahir, M.H.N.; Nawaz, F.; Akhtar, G.; Bashir, M.A.; Ghaffar, A. Pretreatment with Chitosan Arbitrates Physiological Processes and Antioxidant Defense System to Increase Drought Tolerance in Alfalfa (Medicago sativa L.). J. Soil Sci. Plant Nutr. 2022, 22, 2169–2186. [Google Scholar] [CrossRef]
- Das, U.; Rahman, M.A.; Ela, E.J.; Lee, K.-W.; Kabir, A.H. Sulfur triggers glutathione and phytochelatin accumulation causing excess Cd bound to the cell wall of roots in alleviating Cd-toxicity in alfalfa. Chemosphere 2021, 262, 128361. [Google Scholar] [CrossRef]
- Khan, Z.; Thounaojam, T.C.; Chowdhury, D.; Upadhyaya, H. The role of selenium and nano selenium on physiological responses in plant: A review. Plant Growth Regul. 2023, 100, 409–433. [Google Scholar] [CrossRef]
- Cunha, M.L.O.; de Oliveira, L.C.A.; Mendes, N.A.C.; Silva, V.M.; Vicente, E.F.; dos Reis, A.R. Selenium Increases Photosynthetic Pigments, Flavonoid Biosynthesis, Nodulation, and Growth of Soybean Plants (Glycine max L.). J. Soil Sci. Plant Nutr. 2023, 23, 1397–1407. [Google Scholar] [CrossRef]
- Jia, Y.; Kang, L.; Wu, Y.; Zhou, C.; Cai, R.; Zhang, H.; Li, J.; Chen, Z.; Kang, D.; Zhang, L.; et al. Nano-selenium foliar intervention-induced resistance of cucumber to by activating jasmonic acid biosynthesis and regulating phenolic acid and cucurbitacin. Pest Manage. Sci. 2024, 80, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Tu, S.; Dai, Z.; Huang, W.; Jia, W.; Xu, Z.; Shao, H. Comparison of selenite and selenate in alleviation of drought stress in Nicotiana tabacum L. Chemosphere 2022, 287, 132136. [Google Scholar] [CrossRef]
- Taha, R.S.; Seleiman, M.F.; Shami, A.; Alhammad, B.A.; Mahdi, A.H.A. Integrated Application of Selenium and Silicon Enhances Growth and Anatomical Structure, Antioxidant Defense System and Yield of Wheat Grown in Salt-Stressed Soil. Plants 2021, 10, 1040. [Google Scholar] [CrossRef]
- Jiang, S.; Du, B.; Wu, Q.; Zhang, H.; Deng, Y.; Tang, X.; Zhu, J. Selenium Decreases the Cadmium Content in Brown Rice: Foliar Se Application to Plants Grown in Cd-contaminated Soil. J. Soil Sci. Plant Nutr. 2022, 22, 1033–1043. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, K.S. Role of nano-selenium in health and environment. J. Biotechnol. 2021, 325, 152–163. [Google Scholar] [CrossRef]
- Li, D.; Zhou, C.; Zou, N.; Wu, Y.; Zhang, J.; An, Q.; Li, J.-Q.; Pan, C. Nanoselenium foliar application enhances biosynthesis of tea leaves in metabolic cycles and associated responsive pathways. Environ. Pollut. 2021, 273, 116503. [Google Scholar] [CrossRef]
- Zhou, C.; Miao, P.; Dong, Q.; Li, D.; Pan, C. Multiomics Explore the Detoxification Mechanism of Nanoselenium and Melatonin on Bensulfuron Methyl in Wheat Plants. J. Agric. Food Chem. 2024, 72, 3958–3972. [Google Scholar] [CrossRef]
- Xia, Q.; Yang, Z.; Shui, Y.; Liu, X.; Chen, J.; Khan, S.; Wang, J.; Gao, Z. Methods of Selenium Application Differentially Modulate Plant Growth, Selenium Accumulation and Speciation, Protein, Anthocyanins and Concentrations of Mineral Elements in Purple-Grained Wheat. Front. Plant Sci. 2020, 11, 1114. [Google Scholar] [CrossRef]
- Polić Pasković, M.; Herak Ćustić, M.; Lukić, I.; Marcelić, Š.; Žurga, P.; Vidović, N.; Major, N.; Goreta Ban, S.; Pecina, M.; Ražov, J.; et al. Foliar Nutrition Strategies for Enhancing Phenolic and Amino Acid Content in Olive Leaves. Plants 2024, 13, 3514. [Google Scholar] [CrossRef]
- Hu, H.; Hu, J.; Wang, Q.; Xiang, M.; Zhang, Y. Transcriptome analysis revealed accumulation-assimilation of selenium and physio-biochemical changes in alfalfa (Medicago sativa L.) leaves. J. Sci. Food Agric. 2022, 102, 4577–4588. [Google Scholar] [CrossRef] [PubMed]
- Holík, M.; Kunzová, E.; Ludvíková, V.; Hakl, J. Impact of Long-Term Manure and Mineral Fertilization on Accumulation of Non-Structural Carbohydrates in Lucerne Forage. Agronomy 2022, 12, 639. [Google Scholar] [CrossRef]
- Xu, Z.; Heuschele, D.J.; Lamb, J.F.S.; Jung, H.-J.G.; Samac, D.A. Improved Forage Quality in Alfalfa (Medicago sativa L.) via Selection for Increased Stem Fiber Digestibility. Agronomy 2023, 13, 770. [Google Scholar] [CrossRef]
- Wang, K.; Wang, F.; Sun, S.; Zou, Y.; Gao, Z.; Hua, Y.; Qin, L.; Hu, G. Ensiling Characteristics, Bacterial Community Structure, Co-Occurrence Networks, and Their Predicted Functionality in Alfalfa Haylage Silage with or Without Foliar Selenium Application. Agronomy 2024, 14, 2709. [Google Scholar] [CrossRef]
- Wang, F.; Yang, J.; Hua, Y.; Wang, K.; Guo, Y.; Lu, Y.; Zhu, S.; Zhang, P.; Hu, G. Transcriptome and Metabolome Analysis of Selenium Treated Alfalfa Reveals Influence on Phenylpropanoid Biosynthesis to Enhance Growth. Plants 2023, 12, 2038. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Ge, G.; Sun, L.; Bao, J.; Zhao, M.; Hao, J.; Zhang, Y.; Liu, G.; Wang, Z.; Jia, Y. Metabolomics combined with physiology and transcriptomics reveal the regulation of key nitrogen metabolic pathways in alfalfa by foliar spraying with nano-selenium. J. Nanobiotechnol. 2025, 23, 7. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Johnson, G.A.; Satterfield, M.C.; Washburn, S.E. Metabolism and Nutrition of L-Glutamate and L-Glutamine in Ruminants. Animals 2024, 14, 1788. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Li, Y.; Zhao, X.; Liang, H.; Li, K.; Qu, M.; Qiu, Q.; Ouyang, K. Glutamate Supplementation Improves Growth Performance, Rumen Fermentation, and Serum Metabolites in Heat-Stressed Hu Sheep. Front. Nutr. 2022, 9, 851386. [Google Scholar] [CrossRef]
- Danso Ofori, A.; Su, W.; Zheng, T.; Datsomor, O.; Titriku, J.K.; Xiang, X.; Kandhro, A.G.; Ahmed, M.I.; Mawuli, E.W.; Awuah, R.T.; et al. Jasmonic Acid (JA) Signaling Pathway in Rice Defense Against Chilo suppressalis Infestation. Rice 2025, 18, 7. [Google Scholar] [CrossRef]
- Tarkowski, Ł.P.; Signorelli, S.; Höfte, M. γ-Aminobutyric acid and related amino acids in plant immune responses: Emerging mechanisms of action. Plant Cell Environ. 2020, 43, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Huang, K.; Batool, M.; Idrees, F.; Afzal, R.; Haroon, M.; Noushahi, H.A.; Wu, W.; Hu, Q.; Lu, X.; et al. Versatile roles of polyamines in improving abiotic stress tolerance of plants. Front. Plant Sci. 2022, 13, 1003155. [Google Scholar] [CrossRef] [PubMed]
- Renzetti, M.; Funck, D.; Trovato, M. Proline and ROS: A Unified Mechanism in Plant Development and Stress Response? Plants 2025, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Hosseinifard, M.; Stefaniak, S.; Ghorbani Javid, M.; Soltani, E.; Wojtyla, Ł.; Garnczarska, M. Contribution of Exogenous Proline to Abiotic Stresses Tolerance in Plants: A Review. Int. J. Mol. Sci. 2022, 23, 5186. [Google Scholar] [CrossRef]
- Yan, S.; Zhan, M.; Liu, Z.; Zhang, X. Insight into the transcriptional regulation of key genes involved in proline metabolism in plants under osmotic stress. Biochimie 2025, 228, 8–14. [Google Scholar] [CrossRef]
- Mavrommatis, A.; Mitsiopoulou, C.; Christodoulou, C.; Kariampa, P.; Simoni, M.; Righi, F.; Tsiplakou, E. Effects of Supplementing Rumen-Protected Methionine and Lysine on Milk Performance and Oxidative Status of Dairy Ewes. Antioxidants 2021, 10, 654. [Google Scholar] [CrossRef]
- Li, N.; Euring, D.; Cha, J.Y.; Lin, Z.; Lu, M.; Huang, L.-J.; Kim, W.Y. Plant Hormone-Mediated Regulation of Heat Tolerance in Response to Global Climate Change. Front. Plant Sci. 2021, 11, 627969. [Google Scholar] [CrossRef]
- Nie, R.; Chen, D.; Hu, T.; Zhang, S.; Qu, G. A Review: The Role of Jasmonic Acid in Tomato Flower and Fruit Development. Plant Mol. Biol. Rep. 2024, 43, 474–483. [Google Scholar] [CrossRef]
- Shuang, S.; Huo, X.; Chen, Q.; Dai, R.; Li, J.; Yan, J.; Jiang, X.; Tan, Y.; Zhang, Z. Exogenous methyl jasmonate-mediated physiological and transcriptomic network improves thrips tolerance in alfalfa (Medicago sativa). J. Pest Sci. 2025, 98, 1655–1674. [Google Scholar] [CrossRef]
- Fonseca, S.; Chico, J.M.; Solano, R. The jasmonate pathway: The ligand, the receptor and the core signalling module. Curr. Opin. Plant Biol. 2009, 12, 539–547. [Google Scholar] [CrossRef]
- Singh, S.; Kaur, I.; Kariyat, R. The Multifunctional Roles of Polyphenols in Plant-Herbivore Interactions. Int. J. Mol. Sci. 2021, 22, 1442. [Google Scholar] [CrossRef]
- Bin, D.; Reymick, O.O.; Liu, Z.; Zhou, Y.; Wang, X.; Feng, Z.; Tao, N. Citral enhances disease resistance in postharvest citrus fruit through inducing jasmonic acid pathway and accumulating phenylpropanoid compounds. Postharvest Biol. Technol. 2024, 207, 112633. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, C.; Zhao, Y.; Duan, X.; Yang, Z.; Zhu, J. Salicylic acid alleviates Zn-induced inhibition of growth via enhancing antioxidant system and glutathione metabolism in alfalfa. Ecotoxicol. Environ. Saf. 2023, 265, 115500. [Google Scholar] [CrossRef]
- Zhou, C.; Li, D.; Shi, X.; Zhang, J.; An, Q.; Wu, Y.; Kang, L.; Li, J.-Q.; Pan, C. Nanoselenium Enhanced Wheat Resistance to Aphids by Regulating Biosynthesis of DIMBOA and Volatile Components. J. Agric. Food Chem. 2021, 69, 14103–14114. [Google Scholar] [CrossRef]
- Li, D.; Zhou, C.; Zhang, J.; An, Q.; Wu, Y.; Li, J.-Q.; Pan, C. Nanoselenium Foliar Applications Enhance the Nutrient Quality of Pepper by Activating the Capsaicinoid Synthetic Pathway. J. Agric. Food Chem. 2020, 68, 9888–9895. [Google Scholar] [CrossRef]
- He, S.-X.; Liu, Y.-W.; Zhou, Q.-Y.; Liu, C.-J.; Li, W.; Ma, L.Q. Selenium increases antimony uptake in As-hyperaccumulators Pteris vittata and Pteris cretica by promoting antimonate reduction: GSH-GSSG cycle and arsenate reductases HAC1/ACR2. J. Hazard. Mater. 2024, 480, 135875. [Google Scholar] [CrossRef]
- Li, L.; Lu, X.; Ma, H.; Lyu, D. Jasmonic acid regulates the ascorbate–glutathione cycle in Malus baccata Borkh. roots under low root-zone temperature. Acta Physiol. Plant. 2017, 39, 174. [Google Scholar] [CrossRef]
- Wang, M.; Ali, F.; Wang, M.; Dinh, Q.T.; Zhou, F.; Bañuelos, G.S.; Liang, D. Understanding boosting selenium accumulation in Wheat (Triticum aestivum L.) following foliar selenium application at different stages, forms, and doses. Environ. Sci. Pollut. Res. 2020, 27, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Riahi, H.S.; Heidarieh, P.; Fatahi-Bafghi, M. Genus Pseudonocardia: What we know about its biological properties, abilities and current application in biotechnology. J. Appl. Microbiol. 2022, 132, 890–906. [Google Scholar] [CrossRef]
- Moreira, A.; Fageria, N.K. Liming influence on soil chemical properties, nutritional status and yield of alfalfa grown in acid soil. Rev. Bras. Cienc. Solo 2010, 34, 1231–1239. [Google Scholar] [CrossRef]
- Neina, D. The Role of Soil pH in Plant Nutrition and Soil Remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Li, J.; Tang, W.; Lu, S.; Wang, Y.; Kuang, Z.; Yuan, J. Application of Selenocysteine Increased Soil Nitrogen Content, Enzyme Activity, and Microbial Quantity in Camellia oleifera Abel. Forests. Forests 2023, 14, 982. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, J.; Miao, P.; Dong, Q.; Lin, Y.; Li, D.; Pan, C. Novel Finding on How Melatonin and Nanoselenium Alleviate 2,4-D Butylate Stress in Wheat Plants. J. Agric. Food Chem. 2023, 71, 12943–12957. [Google Scholar] [CrossRef]
- Banerjee, M.; Chakravarty, D.; Kalwani, P.; Ballal, A. Voyage of selenium from environment to life: Beneficial or toxic? J. Biochem. Mol. Toxicol. 2022, 36, e23195. [Google Scholar] [CrossRef]
- Yang, C.; Yao, H.; Wu, Y.; Sun, G.; Yang, W.; Li, Z.; Shang, L. Status and risks of selenium deficiency in a traditional selenium-deficient area in Northeast China. Sci. Total Environ. 2021, 762, 144103. [Google Scholar] [CrossRef]
- Li, D.; An, Q.; Wu, Y.; Li, J.-Q.; Pan, C. Foliar Application of Selenium Nanoparticles on Celery Stimulates Several Nutrient Component Levels by Regulating the α-Linolenic Acid Pathway. ACS Sustain. Chem. Eng. 2020, 8, 10502–10510. [Google Scholar] [CrossRef]
- GB 5009.268-2016; National Food Safety Standard-Determination of Multiple Elements in Foods. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
- Yu, H.; Li, D.; Tang, S.; Cheng, H.; Miao, P.; Zhou, C.; Wan, X.; Dong, Q.; Zhao, Y.; Liu, Z.; et al. Balancing Growth and Defense: Nanoselenium and Melatonin in Tea (Camellia sinensis) Protection against Glufosinate. ACS Nano 2024, 18, 32145–32161. [Google Scholar] [CrossRef] [PubMed]
- GB/T 30987-2020; Determination of Free Amino Acids in Plants. State Administration for Market Regulation: Beijing, China, 2020.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.; Yu, H.; Dong, Q.; Zhou, C.; Huang, T.; Fang, X.; Pan, C. Foliar Nano-Selenium Modulates Metabolic and Antioxidant Responses in Alfalfa (Medicago sativa L.): Integration of Pot and Field Evidence. Int. J. Mol. Sci. 2025, 26, 9013. https://doi.org/10.3390/ijms26189013
Cheng H, Yu H, Dong Q, Zhou C, Huang T, Fang X, Pan C. Foliar Nano-Selenium Modulates Metabolic and Antioxidant Responses in Alfalfa (Medicago sativa L.): Integration of Pot and Field Evidence. International Journal of Molecular Sciences. 2025; 26(18):9013. https://doi.org/10.3390/ijms26189013
Chicago/Turabian StyleCheng, Haiyan, Huan Yu, Qinyong Dong, Chunran Zhou, Tingjie Huang, Xun Fang, and Canping Pan. 2025. "Foliar Nano-Selenium Modulates Metabolic and Antioxidant Responses in Alfalfa (Medicago sativa L.): Integration of Pot and Field Evidence" International Journal of Molecular Sciences 26, no. 18: 9013. https://doi.org/10.3390/ijms26189013
APA StyleCheng, H., Yu, H., Dong, Q., Zhou, C., Huang, T., Fang, X., & Pan, C. (2025). Foliar Nano-Selenium Modulates Metabolic and Antioxidant Responses in Alfalfa (Medicago sativa L.): Integration of Pot and Field Evidence. International Journal of Molecular Sciences, 26(18), 9013. https://doi.org/10.3390/ijms26189013




