Ocular Melanoma: A Comprehensive Review with a Focus on Molecular Biology
Abstract
1. Introduction
2. Uveal Melanoma
2.1. Epidemiology
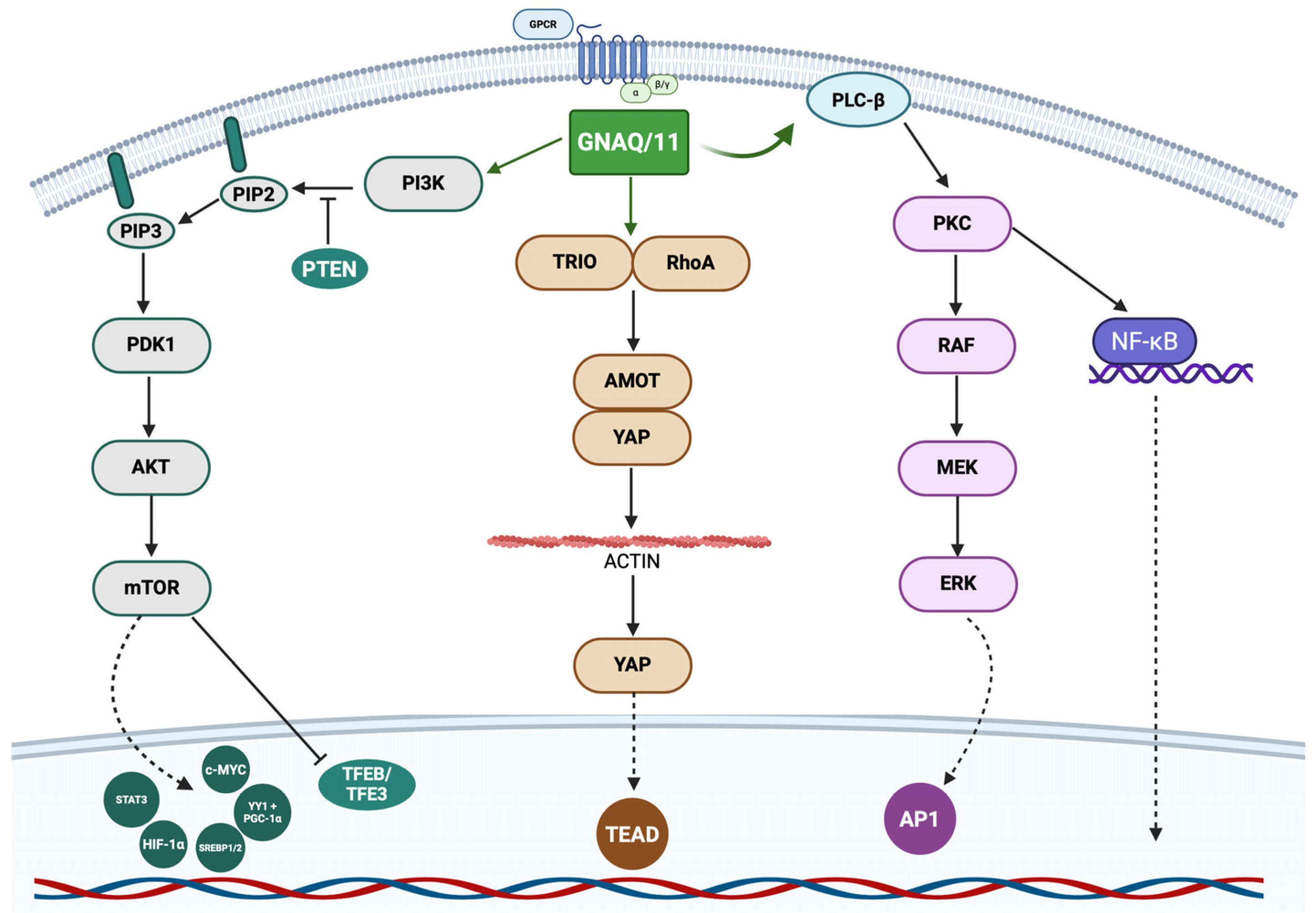
2.2. Molecular Biology
2.3. Histological Features
2.4. Prognosis
3. Conjunctival Melanoma
3.1. Epidemiology
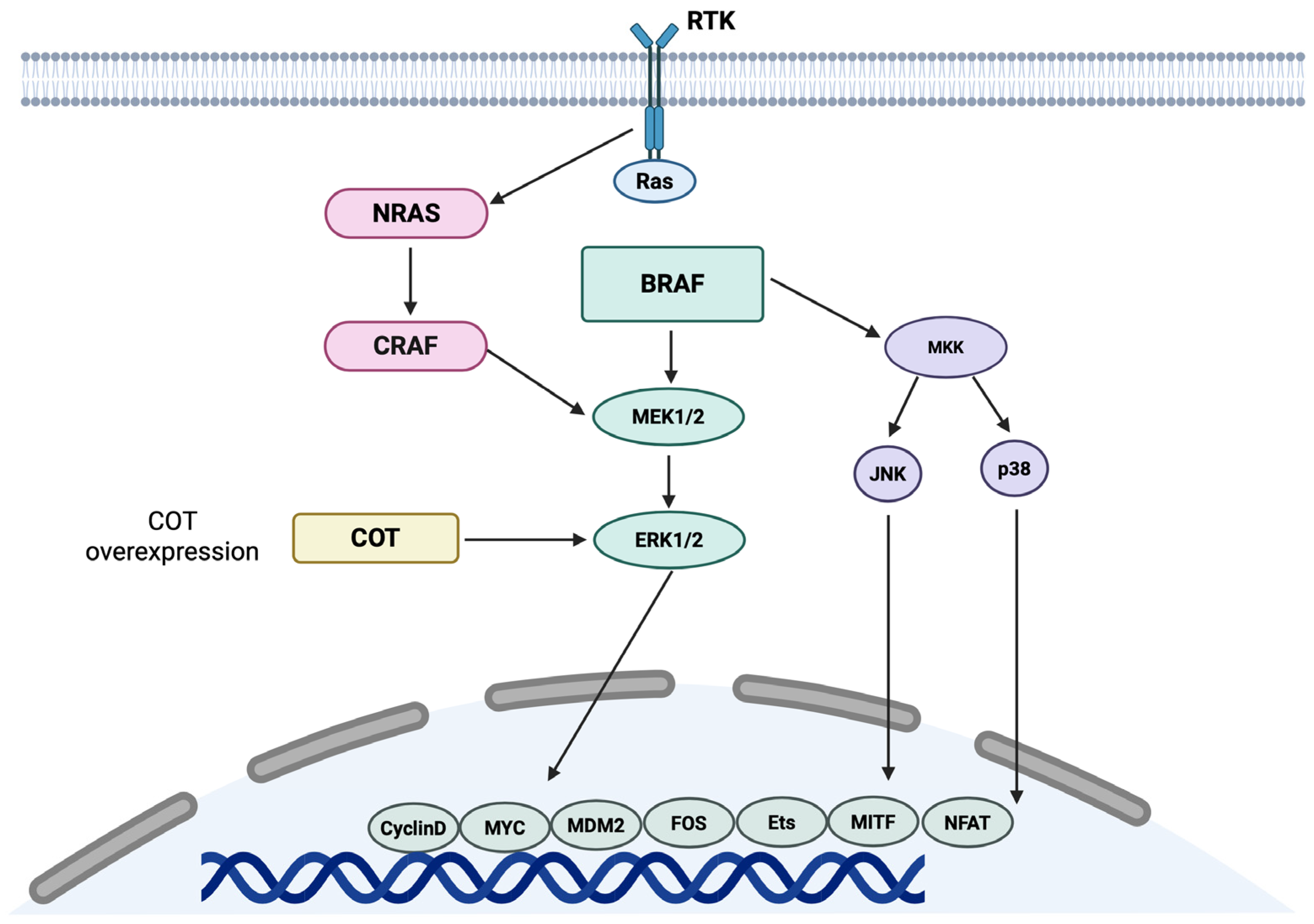
3.2. Molecular Biology
3.3. Histological Features
3.4. Prognosis
4. Eyelid Melanoma
4.1. Epidemiology
4.2. Molecular Biology
4.3. Histological Features
4.4. Prognosis
5. Molecular Distinctions Between Ocular and Cutaneous Melanomas
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Poppelen, N.M.; de Bruyn, D.P.; Bicer, T.; Verdijk, R.; Naus, N.; Mensink, H.; Paridaens, D.; de Klein, A.; Brosens, E.; Kiliç, E. Genetics of ocular melanoma: Insights into genetics, inheritance and testing. Int. J. Mol. Sci. 2020, 22, 336. [Google Scholar] [CrossRef] [PubMed]
- Van Raamsdonk, C.D.; Bezrookove, V.; Green, G.; Bauer, J.; Gaugler, L.; O’Brien, J.M.; Simpson, E.M.; Barsh, G.S.; Bastian, B.C. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 2009, 457, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Van Raamsdonk, C.D.; Griewank, K.G.; Crosby, M.B.; Garrido, M.C.; Vemula, S.; Wiesner, T.; Obenauf, A.C.; Wackernagel, W.; Green, G.; Bouvier, N.; et al. Mutations in GNA11 in uveal melanoma. N. Engl. J. Med. 2010, 363, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Besaratinia, A.; Pfeifer, G.P. Uveal melanoma and GNA11 mutations: A new piece added to the puzzle. Pigment. Cell Melanoma Res. 2011, 24, 18–20. [Google Scholar] [CrossRef]
- Shoushtari, A.N.; Carvajal, R.D. GNAQ and GNA11 mutations in uveal melanoma. Melanoma Res. 2014, 24, 525–534. [Google Scholar] [CrossRef]
- Onken, M.D.; Worley, L.A.; Long, M.D.; Duan, S.; Council, M.L.; Bowcock, A.M.; Harbour, J.W. Oncogenic mutations in GNAQ occur early in uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5230–5234. [Google Scholar] [CrossRef]
- Griewank, K.G.; Westekemper, H.; Murali, R.; Mach, M.; Schilling, B.; Wiesner, T.; Schimming, T.; Livingstone, E.; Sucker, A.; Grabellus, F.; et al. Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas. Clin. Cancer Res. 2013, 19, 3143–3152. [Google Scholar] [CrossRef]
- van Ipenburg, J.A.; Naus, N.C.; Dubbink, H.J.; van Ginderdeuren, R.; Missotten, G.S.; Paridaens, D.; Verdijk, R.M. Prognostic value of TERT promoter mutations in conjunctival melanomas in addition to clinicopathological features. Br. J. Ophthalmol. 2021, 105, 1454–1461. [Google Scholar] [CrossRef]
- Singh, A.D.; Turell, M.E.; Topham, A.K. Uveal melanoma: Trends in incidence, treatment, and survival. Ophthalmology 2011, 118, 1881–1885. [Google Scholar] [CrossRef]
- Hou, X.; Rokohl, A.C.; Li, X.; Guo, Y.; Ju, X.; Fan, W.; Heindl, L.M. Global incidence and prevalence in uveal melanoma. Adv. Ophthalmol. Pract. Res. 2024, 4, 226–232. [Google Scholar] [CrossRef]
- Virgili, G.; Gatta, G.; Ciccolallo, L.; Capocaccia, R.; Biggeri, A.; Crocetti, E.; Lutz, J.M.; Paci, E.; Group, E.W. Incidence of uveal melanoma in Europe. Ophthalmology 2007, 114, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Houtzagers, L.E.; Wierenga, A.P.A.; Ruys, A.A.M.; Luyten, G.P.M.; Jager, M.J. Iris colour and the risk of developing uveal melanoma. Int. J. Mol. Sci. 2020, 21, 7172. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Woods, M.; Calotti, R.; Evans, H.; Medina, R.; Mina, S.; Pena, J.E.; Khan, A.; Nguyen, H.; Bansal, R.; et al. Tumor location of uveal melanoma and impact on metastasis-free survival in 1001 cases. Semin. Ophthalmol. 2025, 40, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Kulbay, M.; Marcotte, E.; Remtulla, R.; Lau, T.H.A.; Paez-Escamilla, M.; Wu, K.Y.; Burnier, M.N., Jr. Uveal melanoma: Comprehensive review of its pathophysiology, diagnosis, treatment, and future perspectives. Biomedicines 2024, 12, 1758. [Google Scholar] [CrossRef]
- Rantala, E.S.; Hernberg, M.; Kivela, T.T. Overall survival after treatment for metastatic uveal melanoma: A systematic review and meta-analysis. Melanoma Res. 2019, 29, 561–568. [Google Scholar] [CrossRef]
- Stålhammar, G.; Herrspiegel, C. Long-term relative survival in uveal melanoma: A systematic review and meta-analysis. Commun. Med. 2022, 2, 18. [Google Scholar] [CrossRef]
- Smidt-Nielsen, I.; Bagger, M.; Heegaard, S.; Andersen, K.K.; Kiilgaard, J.F. Posterior uveal melanoma incidence and survival by AJCC tumour size in a 70-year nationwide cohort. Acta Ophthalmol. 2021, 99, e1474–e1482. [Google Scholar] [CrossRef]
- Aronow, M.E.; Topham, A.K.; Singh, A.D. Uveal Melanoma: 5-year update on incidence, treatment, and survival (SEER 1973–2013). Ocul. Oncol. Pathol. 2018, 4, 145–151. [Google Scholar] [CrossRef]
- Silva-Rodríguez, P.; Fernández-Díaz, D.; Bande, M.; Pardo, M.; Loidi, L.; Blanco-Teijeiro, M.J. GNAQ and GNA11 genes: A comprehensive review on oncogenesis, prognosis and therapeutic opportunities in uveal melanoma. Cancers 2022, 14, 3066. [Google Scholar] [CrossRef]
- Hodis, E.; Watson, I.R.; Kryukov, G.V.; Arold, S.T.; Imielinski, M.; Theurillat, J.P.; Nickerson, E.; Auclair, D.; Li, L.; Place, C.; et al. A landscape of driver mutations in melanoma. Cell 2012, 150, 251–263. [Google Scholar] [CrossRef]
- Pašalić, D.; Nikuševa-Martić, T.; Sekovanić, A.; Kaštelan, S. Genetic and epigenetic features of uveal melanoma-an overview and clinical implications. Int. J. Mol. Sci. 2023, 24, 12807. [Google Scholar] [CrossRef] [PubMed]
- Griewank, K.G.; van de Nes, J.; Schilling, B.; Moll, I.; Sucker, A.; Kakavand, H.; Haydu, L.E.; Asher, M.; Zimmer, L.; Hillen, U.; et al. Genetic and clinico-pathologic analysis of metastatic uveal melanoma. Mod. Pathol. 2014, 27, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, A.E.; Vaarwater, J.; Paridaens, D.; Naus, N.C.; Kilic, E.; de Klein, A.; Rotterdam Ocular Melanoma Study Group. Patient survival in uveal melanoma is not affected by oncogenic mutations in GNAQ and GNA11. Br. J. Cancer 2013, 109, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Populo, H.; Vinagre, J.; Lopes, J.M.; Soares, P. Analysis of GNAQ mutations, proliferation and MAPK pathway activation in uveal melanomas. Br. J. Ophthalmol. 2011, 95, 715–719. [Google Scholar] [CrossRef]
- Schneider, B.; Riedel, K.; Zhivov, A.; Huehns, M.; Zettl, H.; Guthoff, R.F.; Junemann, A.; Erbersdobler, A.; Zimpfer, A. Frequent and yet unreported GNAQ and GNA11 mutations are found in uveal melanomas. Pathol. Oncol. Res. 2019, 25, 1319–1325. [Google Scholar] [CrossRef]
- Silva-Rodriguez, P.; Bande, M.; Fernandez-Diaz, D.; Lago-Baameiro, N.; Pardo, M.; Jose Blanco-Teijeiro, M.; Dominguez, F.; Loidi, L.; Pineiro, A. Role of somatic mutations and chromosomal aberrations in the prognosis of uveal melanoma in a Spanish patient cohort. Acta Ophthalmol. 2021, 99, e1077–e1089. [Google Scholar] [CrossRef]
- Fuentes-Rodriguez, A.; Mitchell, A.; Guérin, S.L.; Landreville, S. Recent advances in molecular and genetic research on uveal melanoma. Cells 2024, 13, 1023. [Google Scholar] [CrossRef]
- Johansson, P.; Aoude, L.G.; Wadt, K.; Glasson, W.J.; Warrier, S.K.; Hewitt, A.W.; Kiilgaard, J.F.; Heegaard, S.; Isaacs, T.; Franchina, M.; et al. Deep sequencing of uveal melanoma identifies a recurrent mutation in PLCB4. Oncotarget 2016, 7, 4624–4631. [Google Scholar] [CrossRef]
- Prescher, G.; Bornfeld, N.; Hirche, H.; Horsthemke, B.; Jockel, K.H.; Becher, R. Prognostic implications of monosomy 3 in uveal melanoma. Lancet 1996, 347, 1222–1225. [Google Scholar] [CrossRef]
- Kilic, E.; van Gils, W.; Lodder, E.; Beverloo, H.B.; van Til, M.E.; Mooy, C.M.; Paridaens, D.; de Klein, A.; Luyten, G.P. Clinical and cytogenetic analyses in uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3703–3707. [Google Scholar] [CrossRef]
- Harbour, J.W.; Onken, M.D.; Roberson, E.D.; Duan, S.; Cao, L.; Worley, L.A.; Council, M.L.; Matatall, K.A.; Helms, C.; Bowcock, A.M. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010, 330, 1410–1413. [Google Scholar] [CrossRef]
- Fallico, M.; Raciti, G.; Longo, A.; Reibaldi, M.; Bonfiglio, V.; Russo, A.; Caltabiano, R.; Gattuso, G.; Falzone, L.; Avitabile, T. Current molecular and clinical insights into uveal melanoma (Review). Int. J. Oncol. 2021, 58, 10. [Google Scholar] [CrossRef]
- Lalonde, E.; Ewens, K.; Richards-Yutz, J.; Ebrahimzedeh, J.; Terai, M.; Gonsalves, C.F.; Sato, T.; Shields, C.L.; Ganguly, A. Improved uveal melanoma copy number subtypes including an ultra-high-risk group. Ophthalmol. Sci. 2022, 2, 100121. [Google Scholar] [CrossRef]
- Martin, M.; Masshofer, L.; Temming, P.; Rahmann, S.; Metz, C.; Bornfeld, N.; van de Nes, J.; Klein-Hitpass, L.; Hinnebusch, A.G.; Horsthemke, B.; et al. Exome sequencing identifies recurrent somatic mutations in EIF1AX and SF3B1 in uveal melanoma with disomy 3. Nat. Genet. 2013, 45, 933–936. [Google Scholar] [CrossRef]
- Ewens, K.G.; Kanetsky, P.A.; Richards-Yutz, J.; Purrazzella, J.; Shields, C.L.; Ganguly, T.; Ganguly, A. Chromosome 3 status combined with BAP1 and EIF1AX mutation profiles are associated with metastasis in uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5160–5167. [Google Scholar] [CrossRef]
- Amaro, A.; Gangemi, R.; Piaggio, F.; Angelini, G.; Barisione, G.; Ferrini, S.; Pfeffer, U. The biology of uveal melanoma. Cancer Metastasis Rev. 2017, 36, 109–140. [Google Scholar] [CrossRef]
- Yavuzyigitoglu, S.; Koopmans, A.E.; Verdijk, R.M.; Vaarwater, J.; Eussen, B.; van Bodegom, A.; Paridaens, D.; Kilic, E.; de Klein, A.; Rotterdam Ocular Melanoma Study Group. Uveal melanomas with SF3B1 mutations: A distinct subclass associated with late-onset metastases. Ophthalmology 2016, 123, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F., 3rd; Rubin, B.P.; Wilson, D.; Town, A.; Schroeder, A.; Haley, A.; Bainbridge, T.; Heinrich, M.C.; Corless, C.L. Absence of BRAF and NRAS mutations in uveal melanoma. Cancer Res. 2003, 63, 5761–5766. [Google Scholar] [PubMed]
- Yu, F.X.; Luo, J.; Mo, J.S.; Liu, G.; Kim, Y.C.; Meng, Z.; Zhao, L.; Peyman, G.; Ouyang, H.; Jiang, W.; et al. Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell 2014, 25, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Q.; Dang, K.; Ma, S.; Cotton, J.L.; Yang, S.; Zhu, L.J.; Deng, A.C.; Ip, Y.T.; Johnson, R.L.; et al. YAP/TAZ activation drives uveal melanoma initiation and progression. Cell Rep. 2019, 29, 3200–3211. [Google Scholar] [CrossRef]
- Ronchi, A.; Montella, M.; Zito Marino, F.; Argenziano, G.; Moscarella, E.; Brancaccio, G.; Ferraro, G.; Nicoletti, G.F.; Troiani, T.; Franco, R.; et al. Cytologic diagnosis of metastatic melanoma by FNA: A practical review. Cancer Cytopathol. 2022, 130, 18–29. [Google Scholar] [CrossRef]
- Stålhammar, G.; Gill, V.T. Digital morphometry and cluster analysis identifies four types of melanocyte during uveal melanoma progression. Commun. Med. 2023, 3, 60. [Google Scholar] [CrossRef]
- Jones, H.; Kalirai, H.; Taktak, A.; Chen, K.; Coupland, S.E. Vascular lakes in uveal melanoma and their association with outcome. Transl. Vis. Sci. Technol. 2022, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Donizy, P.; Spytek, M.; Krzyziński, M.; Kotowski, K.; Markiewicz, A.; Romanowska-Dixon, B.; Biecek, P.; Hoang, M.P. Ki67 is a better marker than PRAME in risk stratification of BAP1-positive and BAP1-loss uveal melanomas. Br. J. Ophthalmol. 2024, 108, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, S.S.; Dryden, I.J.; Naranjo, A.; Toland, A.; Cayrol, R.A.; Born, D.E.; Egbert, P.S.; Brown, R.A.; Mruthyunjaya, P.; Lin, J.H. Preferentially Expressed Antigen in Melanoma immunohistochemistry labeling in uveal melanomas. Ocul. Oncol. Pathol. 2022, 8, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Schefler, A.C.; Koca, E.; Bernicker, E.H.; Correa, Z.M. Relationship between clinical features, GEP class, and PRAME expression in uveal melanoma. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1541–1545. [Google Scholar] [CrossRef]
- Mallone, F.; Sacchetti, M.; Lambiase, A.; Moramarco, A. Molecular insights and emerging strategies for treatment of metastatic uveal melanoma. Cancers 2020, 12, 2761. [Google Scholar] [CrossRef]
- Rodrigues, M.; Ait Rais, K.; Salviat, F.; Algret, N.; Simaga, F.; Barnhill, R.; Gardrat, S.; Servois, V.; Mariani, P.; Piperno-Neumann, S.; et al. Association of partial chromosome 3 deletion in uveal melanomas with metastasis-free survival. JAMA Ophthalmol. 2020, 138, 182–188. [Google Scholar] [CrossRef]
- See, T.R.O.; Stålhammar, G.; Phillips, S.S.; Grossniklaus, H.E. BAP1 Immunoreactivity correlates with gene expression class in uveal melanoma. Ocular Oncol. Pathol. 2020, 6, 129–137. [Google Scholar] [CrossRef]
- Durgham, R.A.; Nassar, S.I.; Gun, R.; Nguyen, S.A.; Asarkar, A.A.; Nathan, C.O. The prognostic value of the 31-gene expression profile test in cutaneous melanoma: A systematic review and meta-analysis. Cancers 2024, 16, 3714. [Google Scholar] [CrossRef]
- Podlipnik, S.; Martin, B.J.; Morgan-Linnell, S.K.; Bailey, C.N.; Siegel, J.J.; Petkov, V.I.; Puig, S. The 31-gene expression profile test outperforms AJCC in stratifying risk of recurrence in patients with stage I cutaneous melanoma. Cancers 2024, 16, 287. [Google Scholar] [CrossRef]
- Martin, B.J.; Covington, K.R.; Quick, A.P.; Cook, R.W. Risk stratification of patients with stage I cutaneous melanoma using 31-gene expression profiling. J. Clin. Aesthet. Dermatol. 2021, 14, E61–E63. [Google Scholar]
- Kaštelan, S.; Gverović Antunica, A.; Beketić Orešković, L.; Salopek Rabatić, J.; Kasun, B.; Bakija, I. Conjunctival melanoma-epidemiological trends and features. Pathol. Oncol. Res. 2018, 24, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, R.R.; Yaghy, A.; Dalvin, L.A.; Vaidya, S.; Perez, A.L.; Lally, S.E.; Shields, J.A.; Shields, C.L. Conjunctival melanoma: Outcomes based on tumour origin in 629 patients at a single ocular oncology centre. Eye 2022, 36, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Mudhar, H.S.; Krishna, Y.; Cross, S.; Auw-Haedrich, C.; Barnhill, R.; Cherepanoff, S.; Eagle, R.; Farmer, J.; Folberg, R.; Grossniklaus, H.; et al. A multicenter study validates the WHO 2022 classification for conjunctival melanocytic intraepithelial lesions with clinical and prognostic relevance. Lab. Investig. 2024, 104, 100281. [Google Scholar] [CrossRef] [PubMed]
- Virgili, G.; Parravano, M.; Gatta, G.; Capocaccia, R.; Mazzini, C.; Mallone, S.; Botta, L.; RARECAREnet Working Group. Incidence and survival of patients with conjunctival melanoma in Europe. JAMA Ophthalmol. 2020, 138, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Liao, Y.L.; Chu, Y.C.; Tsai, Y.J. Conjunctival melanoma: A 20-year survey in a comprehensive medical center. J. Formos Med. Assoc. 2021, 120 Pt 1, 250–255. [Google Scholar] [CrossRef]
- Weppelmann, T.A.; Zimmerman, K.T.; Rashidi, V. Trends in incidence of conjunctival melanoma in the US. JAMA Netw. Open 2022, 5, e2237229. [Google Scholar] [CrossRef]
- Lodde, G.C.; Jansen, P.; Möller, I.; Sucker, A.; Hassel, J.C.; Forschner, A.; Eckardt, J.; Meier, F.; Reinhardt, L.; Kähler, K.C.; et al. Genetic characterization of advanced conjunctival melanoma and response to systemic treatment. Eur. J. Cancer 2022, 166, 60–72. [Google Scholar] [CrossRef]
- Kaštelan, S.; Pavičić, A.D.; Pašalić, D.; Nikuševa-Martić, T.; Čanović, S.; Kovačević, P.; Konjevoda, S. Biological characteristics and clinical management of uveal and conjunctival melanoma. Oncol. Res. 2024, 32, 1265–1285. [Google Scholar] [CrossRef]
- Brouwer, N.J.; Verdijk, R.M.; Heegaard, S.; Marinkovic, M.; Esmaeli, B.; Jager, M.J. Conjunctival melanoma: New insights in tumour genetics and immunology, leading to new therapeutic options. Prog. Retin. Eye Res. 2022, 86, 100971. [Google Scholar] [CrossRef] [PubMed]
- Valentín-Bravo, F.J.; Pérez-Rodríguez, Á.; García-Álvarez, C.; García-Lagarto, E.; Saornil-Álvarez, M.A. BRAF and NRAS prognostic values in conjunctival melanoma: Analysis and literature review. Arq. Bras. Oftalmol. 2023, 86, e20230071. [Google Scholar] [CrossRef] [PubMed]
- Randic, T.; Kozar, I.; Margue, C.; Utikal, J.; Kreis, S. NRAS mutant melanoma: Towards better therapies. Cancer Treat. Rev. 2021, 99, 102238. [Google Scholar] [CrossRef]
- Rossi, E.; Schinzari, G.; Maiorano, B.A.; Pagliara, M.M.; Di Stefani, A.; Bria, E.; Peris, K.; Blasi, M.A.; Tortora, G. Conjunctival melanoma: Genetic and epigenetic insights of a distinct type of melanoma. Int. J. Mol. Sci. 2019, 20, 5447. [Google Scholar] [CrossRef] [PubMed]
- Verdijk, R.M. Advances in molecular understanding of ocular adnexal disease. Int. J. Mol. Sci. 2024, 25, 6896. [Google Scholar] [CrossRef]
- Chang, E.; Demirci, H.; Demirci, F.Y. Genetic aspects of conjunctival melanoma: A review. Genes 2023, 14, 1668. [Google Scholar] [CrossRef]
- van Poppelen, N.M.; van Ipenburg, J.A.; van den Bosch, Q.; Vaarwater, J.; Brands, T.; Eussen, B.; Magielsen, F.; Dubbink, H.J.; Paridaens, D.; Brosens, E.; et al. Molecular genetics of conjunctival melanoma and prognostic value of TERT promoter mutation analysis. Int. J. Mol. Sci. 2021, 22, 5784. [Google Scholar] [CrossRef]
- Kenawy, N.; Kalirai, H.; Sacco, J.J.; Lake, S.L.; Heegaard, S.; Larsen, A.C.; Finger, P.T.; Milman, T.; Chin, K.; Mosci, C.; et al. Conjunctival melanoma copy number alterations and correlation with mutation status, tumor features, and clinical outcome. Pigment. Cell Melanoma Res. 2019, 32, 564–575. [Google Scholar] [CrossRef]
- Papaoikonomou, M.A.; Pavlidis, L.; Apalla, Z.; Papas, A. Conjunctival melanoma: A narrative review of current knowledge. Pigment. Cell Melanoma Res. 2025, 38, e70006. [Google Scholar] [CrossRef]
- Esposito, E.; Zoroquiain, P.; Mastromonaco, C.; Morales, M.C.; Belfort Neto, R.; Burnier, M., Jr. Epithelial inclusion cyst in conjunctival melanoma. Int. J. Surg. Pathol. 2016, 24, 562–567. [Google Scholar] [CrossRef]
- Bresler, S.C.; Simon, C.; Shields, C.L.; McHugh, J.B.; Stagner, A.M.; Patel, R.M. Conjunctival melanocytic lesions. Arch. Pathol. Lab. Med. 2022, 146, 632–646. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Hrycaj, S.M.; Chan, M.P.; Stagner, A.M.; Patel, R.M.; Bresler, S.C. PRAME expression in junctional melanocytic proliferations of the conjunctiva: A potential biomarker for primary acquired melanosis/conjunctival melanocytic intraepithelial lesions. Am. J. Dermatopathol. 2022, 44, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Zhu, T.; Shi, H.; Zong, C.; Bao, Y.; Wen, X.; Ge, S.; Ruan, J.; Xu, S.; Jia, R.; et al. American Joint Committee on Cancer Tumor Staging System predicts the outcome and metastasis pattern in conjunctival melanoma. Ophthalmology 2022, 129, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Finger, P.T.; Filì, M.; Damato, B.; Coupland, S.E.; Heimann, H.; Kenawy, N.; Brouwer, N.J.; Marinkovic, M.; van Duinen, S.; et al. Metastatic conjunctival melanoma: A multicentre international study. Br. J. Ophthalmol. 2025, 109, 652–659. [Google Scholar] [CrossRef]
- Esmaeli, B.; Rubin, M.L.; Xu, S.; Goepfert, R.P.; Curry, J.L.; Prieto, V.G.; Ning, J.; Tetzlaff, M.T. Greater tumor thickness, ulceration, and positive sentinel lymph node are associated with worse prognosis in patients with conjunctival melanoma: Implications for future AJCC classifications. Am. J. Surg. Pathol. 2019, 43, 1701–1710. [Google Scholar] [CrossRef]
- Baum, S.H.; Westekemper, H.; Bechrakis, N.E.; Mohr, C. Conjunctival and uveal melanoma: Survival and risk factors following orbital exenteration. Eur. J. Ophthalmol. 2022, 32, 612–619. [Google Scholar] [CrossRef]
- Shields, C.L.; Shields, J.A.; Gündüz, K.; Cater, J.; Mercado, G.V.; Gross, N.; Lally, B. Conjunctival melanoma: Risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Arch. Ophthalmol. 2000, 118, 1497–1507. [Google Scholar] [CrossRef]
- Jain, P.; Finger, P.T.; Fili, M.; Damato, B.; Coupland, S.E.; Heimann, H.; Kenawy, N.; Brouwer, N.J.; Marinkovic, M.; Van Duinen, S.G.; et al. Conjunctival melanoma treatment outcomes in 288 patients: A multicentre international data-sharing study. Br. J. Ophthalmol. 2021, 105, 1358–1364. [Google Scholar] [CrossRef]
- Shan, Y.; Xu, Y.; Lu, Y.; Chen, M.; Cao, J.; Wang, Y.; Lin, X.; Ye, J. Epidemiology and survival outcomes for eyelid primary malignant melanoma: An analysis of 1397 cases in the SEER database. J. Ophthalmol. 2020, 2020, 4858636. [Google Scholar] [CrossRef]
- Brunetti, P.; Margo, C.E.; French, D.D. Incidence of cutaneous melanoma of eyelid analysis of the surveillance, epidemiology, and end results database. Ocul. Oncol. Pathol. 2021, 7, 66–69. [Google Scholar] [CrossRef]
- Oliver, J.D.; Boczar, D.; Sisti, A.; Huayllani, M.T.; Restrepo, D.J.; Spaulding, A.C.; Gabriel, E.; Bagaria, S.; Rinker, B.D.; Forte, A.J. Eyelid melanoma in the United States: A national cancer database analysis. J. Craniofac Surg. 2019, 30, 2412–2415. [Google Scholar] [CrossRef]
- Mukarram, M.; Khachemoune, A. Upper and lower eyelid malignancies: Differences in clinical presentation, metastasis, and treatment. Arch. Dermatol. Res. 2024, 316, 429. [Google Scholar] [CrossRef]
- Mancera, N.; Smalley, K.S.M.; Margo, C.E. Melanoma of the eyelid and periocular skin: Histopathologic classification and molecular pathology. Surv. Ophthalmol. 2019, 64, 272–288. [Google Scholar] [CrossRef]
- Milman, T.; McCormick, S.A. The molecular genetics of eyelid tumors: Recent advances and future directions. Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Grossniklaus, H.E.; McLean, I.W. Cutaneous melanoma of the eyelid. Clinicopathologic features. Ophthalmology 1991, 98, 1867–1873. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, M.; Buffam, F.V.; Martinka, M.; Oryschak, A.; Dhaliwal, H.; White, V.A. Clinicopathologic features and behavior of cutaneous eyelid melanoma. Ophthalmology 2002, 109, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S. Benign eyelid lesions and its histopathological picture: A case-series. J. Family Med. Prim. Care. 2024, 13, 5903–5907. [Google Scholar] [CrossRef]
- Dini, F.; Susini, P.; Zuccaro, B.; Nisi, G.; Cuomo, R.; Grimaldi, L.; Perillo, G.; Tinunin, L.; Antonini, P.; Innocenti, A.; et al. Head and neck melanoma: The eyelid region has a better prognosis and easier management. A retrospective survey and systematic review. Melanoma Res. 2024, 34, 429–438. [Google Scholar] [CrossRef]
- Robbins, J.O.; Huck, N.A.; Khosravi, P.; Torabi, S.J.; Woodward, J.A.; Kuan, E.C.; Dermarkarian, C.R. Trends in demographic, clinical, socioeconomic, and facility-specific factors linked to eyelid melanoma survival: A national cancer database analysis. Ophthalmic Plast. Reconstr. Surg. 2025, 41, 456–464. [Google Scholar] [CrossRef]
- Boulos, P.R.; Rubin, P.A. Cutaneous melanomas of the eyelid. Semin. Ophthalmol. 2006, 21, 195–206. [Google Scholar] [CrossRef]
- Pisano, C.E.; Trager, M.H.; Fan, W.; Samie, F.H. Surgical margins and outcomes for eyelid melanoma: A systematic review and meta-analysis. Arch. Dermatol. Res. 2024, 316, 106. [Google Scholar] [CrossRef]
- Cazzato, G.; Colagrande, A.; Cimmino, A.; Caporusso, C.; Candance, P.M.V.; Trabucco, S.M.R.; Zingarelli, M.; Lorusso, A.; Marrone, M.; Stellacci, A.; et al. Urological melanoma: A comprehensive review of a rare subclass of mucosal melanoma with emphasis on differential diagnosis and therapeutic approaches. Cancers 2021, 13, 4424. [Google Scholar] [CrossRef]
- Carvajal, R.; Maniar, R. Extracutaneous melanoma. Hematol. Oncol. Clin. North. Am. 2021, 35, 85–98. [Google Scholar] [CrossRef]
- Gutiérrez-Castañeda, L.D.; Nova, J.A.; Tovar-Parra, J.D. Frequency of mutations in BRAF, NRAS, and KIT in different populations and histological subtypes of melanoma: A systemic review. Melanoma Res. 2020, 30, 62–70. [Google Scholar] [CrossRef]
| Histological Feature | Melanoma | Nevus |
|---|---|---|
| Presence of epithelioid cells | Possible | Rare or absent |
| Mitotic activity | Present | Absent |
| Nuclear atypia | Marked nuclear enlargement, irregular contours, coarse chromatin, and prominent nucleoli | Mild or absent atypia |
| Growth pattern | Infiltrative margins with extension into adjacent ocular tissues (e.g., sclera, optic nerve) | Well circumscribed |
| Closed loops and vascular networks | Present | Absent |
| Necrosis | May be focally present | Absent |
| Feature | Cutaneous Melanoma | Uveal Melanoma | Conjunctival Melanoma | Eyelid Melanoma |
|---|---|---|---|---|
| Main Driver Mutations | BRAF (V600E), NRAS, NF1 | GNAQ, GNA11, BAP1, SF3B1, EIF1AX | BRAF, NRAS, occasional KIT | BRAF, NRAS |
| Pathways Involved | MAPK, PI3K-AKT | MAPK, YAP/TEAD, splicing alterations | MAPK, PI3K-AKT | MAPK, PI3K-AKT |
| Mutational Burden | High (UV-signature mutations) | Low | Intermediate | High |
| Chromosomal Alterations | Variable (chromothripsis, amplifications) | Monosomy 3, chr 8q gain, chr 6p gain | Less defined, some similarities to CM | Variable |
| Therapeutic Implications | BRAF/MEK inhibitors, immunotherapy | Limited response to immunotherapy; trials with YAP/PKC inhibitors | Potential use of BRAF/MEK inhibitors | BRAF/MEK inhibitors, immunotherapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iavarone, L.; Franco, R.; Zito Marino, F.; D’Abbronzo, G.; Argenziano, G.; Scharf, C.; Nucci, G.; Ronchi, A.; Cazzato, G. Ocular Melanoma: A Comprehensive Review with a Focus on Molecular Biology. Int. J. Mol. Sci. 2025, 26, 9799. https://doi.org/10.3390/ijms26199799
Iavarone L, Franco R, Zito Marino F, D’Abbronzo G, Argenziano G, Scharf C, Nucci G, Ronchi A, Cazzato G. Ocular Melanoma: A Comprehensive Review with a Focus on Molecular Biology. International Journal of Molecular Sciences. 2025; 26(19):9799. https://doi.org/10.3390/ijms26199799
Chicago/Turabian StyleIavarone, Lucia, Renato Franco, Federica Zito Marino, Giuseppe D’Abbronzo, Giuseppe Argenziano, Camila Scharf, Grazia Nucci, Andrea Ronchi, and Gerardo Cazzato. 2025. "Ocular Melanoma: A Comprehensive Review with a Focus on Molecular Biology" International Journal of Molecular Sciences 26, no. 19: 9799. https://doi.org/10.3390/ijms26199799
APA StyleIavarone, L., Franco, R., Zito Marino, F., D’Abbronzo, G., Argenziano, G., Scharf, C., Nucci, G., Ronchi, A., & Cazzato, G. (2025). Ocular Melanoma: A Comprehensive Review with a Focus on Molecular Biology. International Journal of Molecular Sciences, 26(19), 9799. https://doi.org/10.3390/ijms26199799









