Synthesis of New Phenothiazine/3-cyanoquinoline and Phenothiazine/3-aminothieno[2,3-b]pyridine(-quinoline) Heterodimers
Abstract
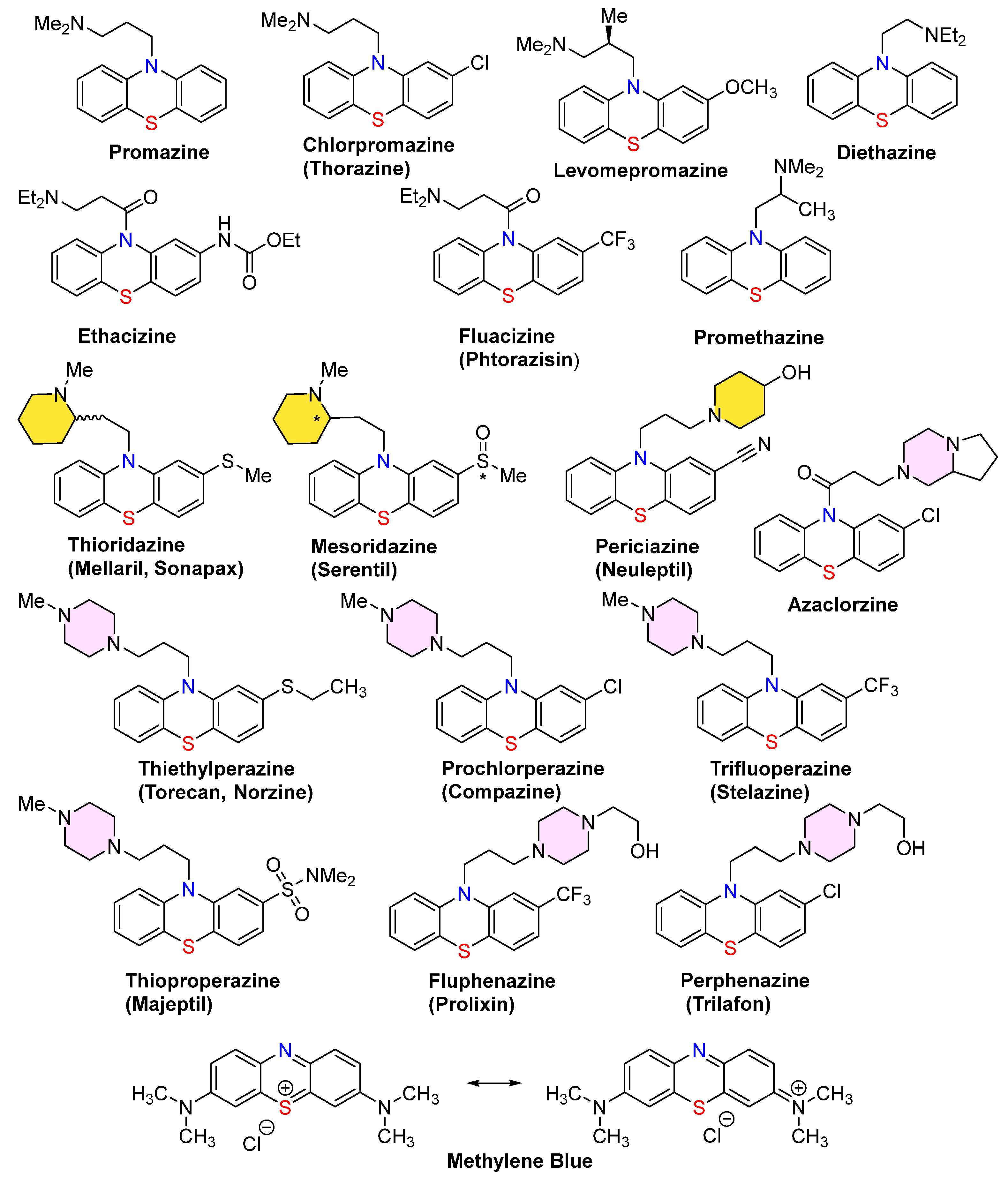
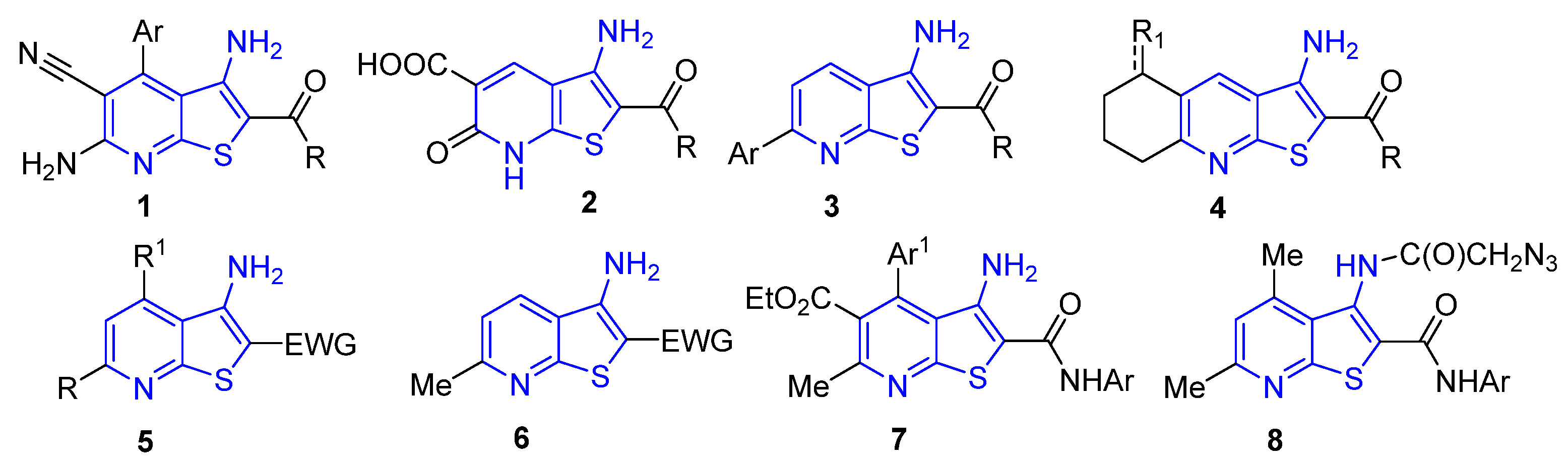
1. Introduction
2. Results and Discussion
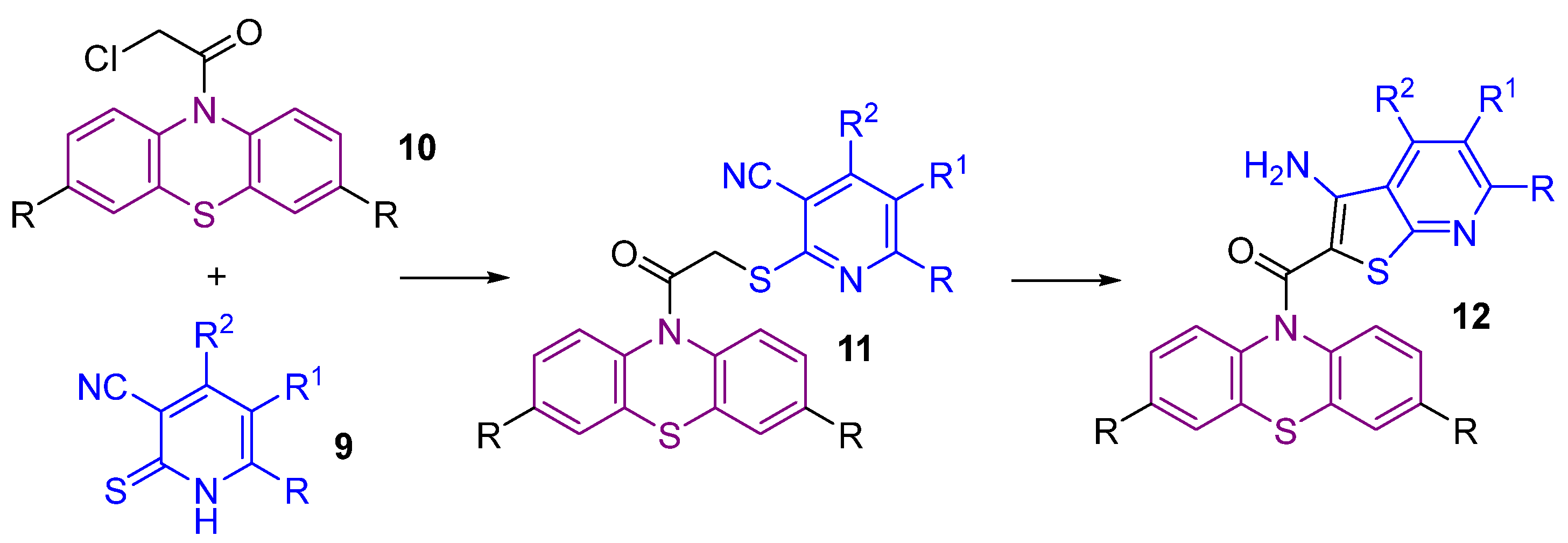
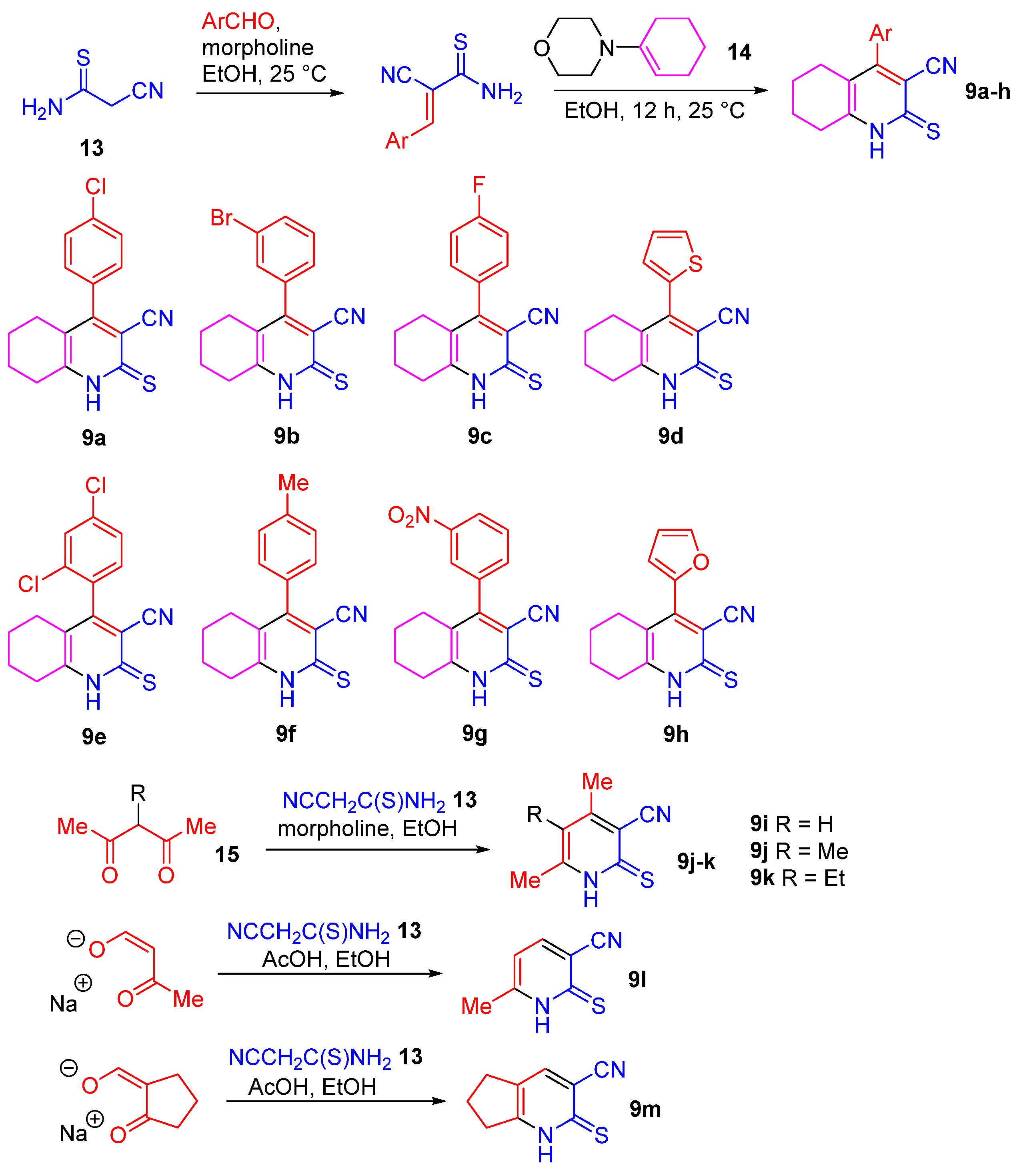
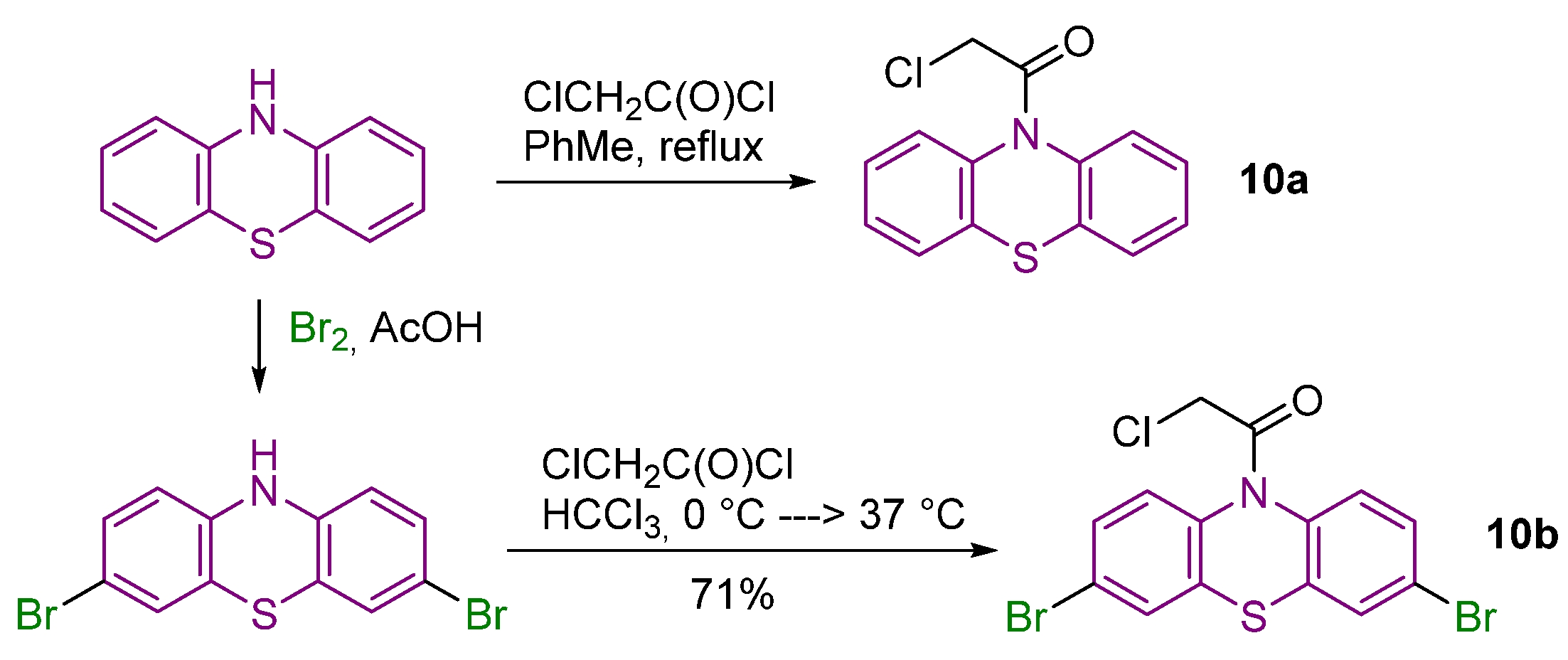
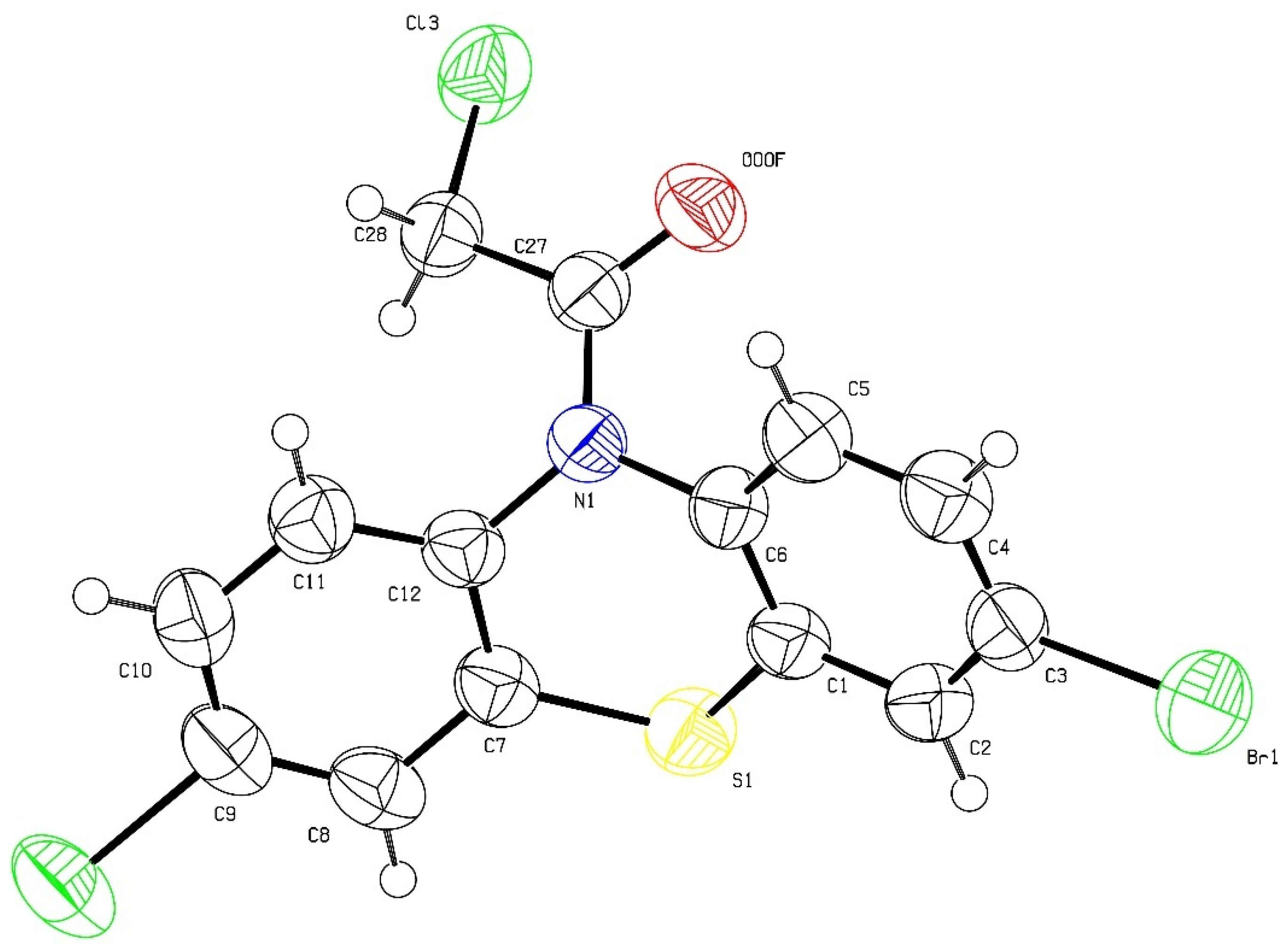
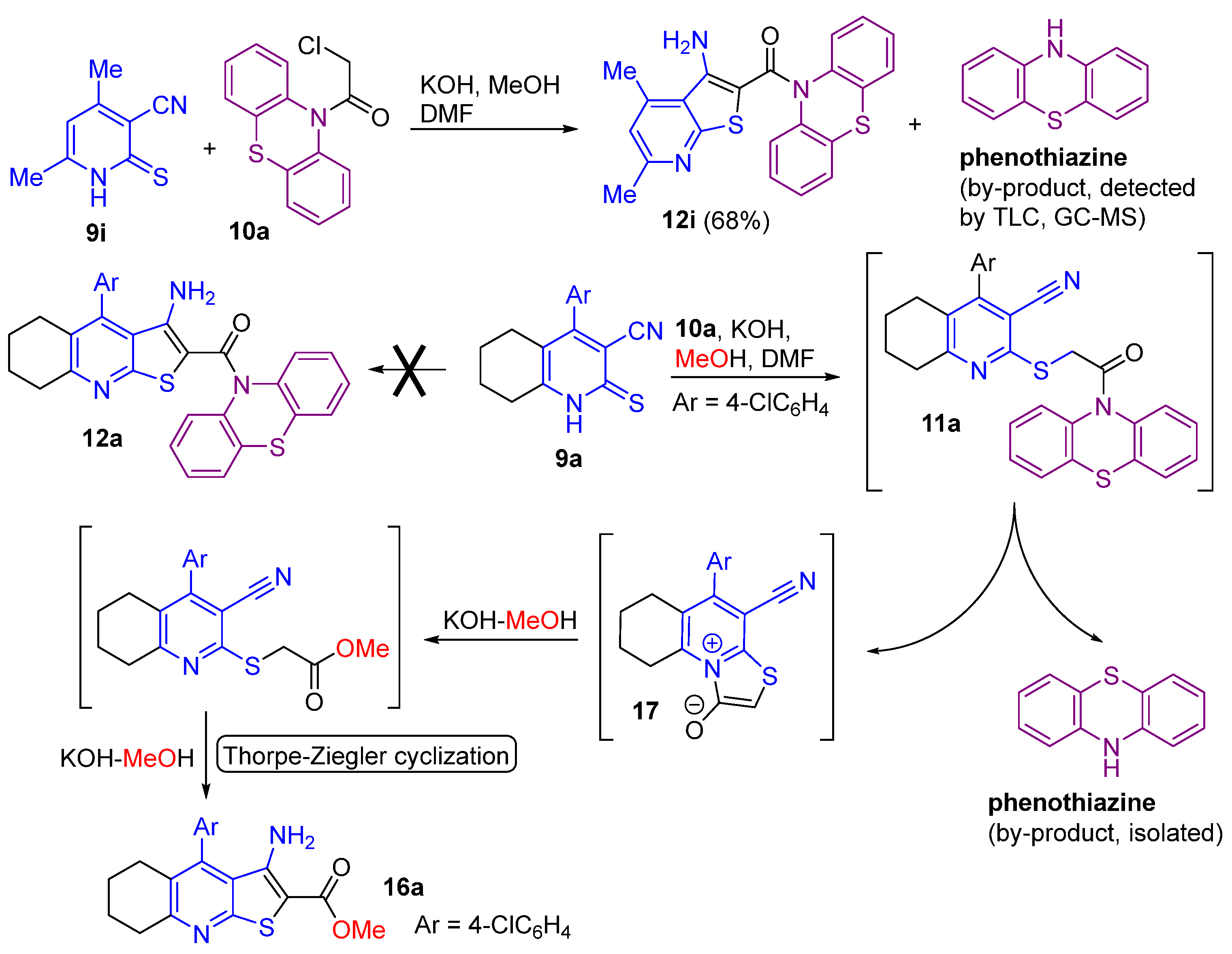
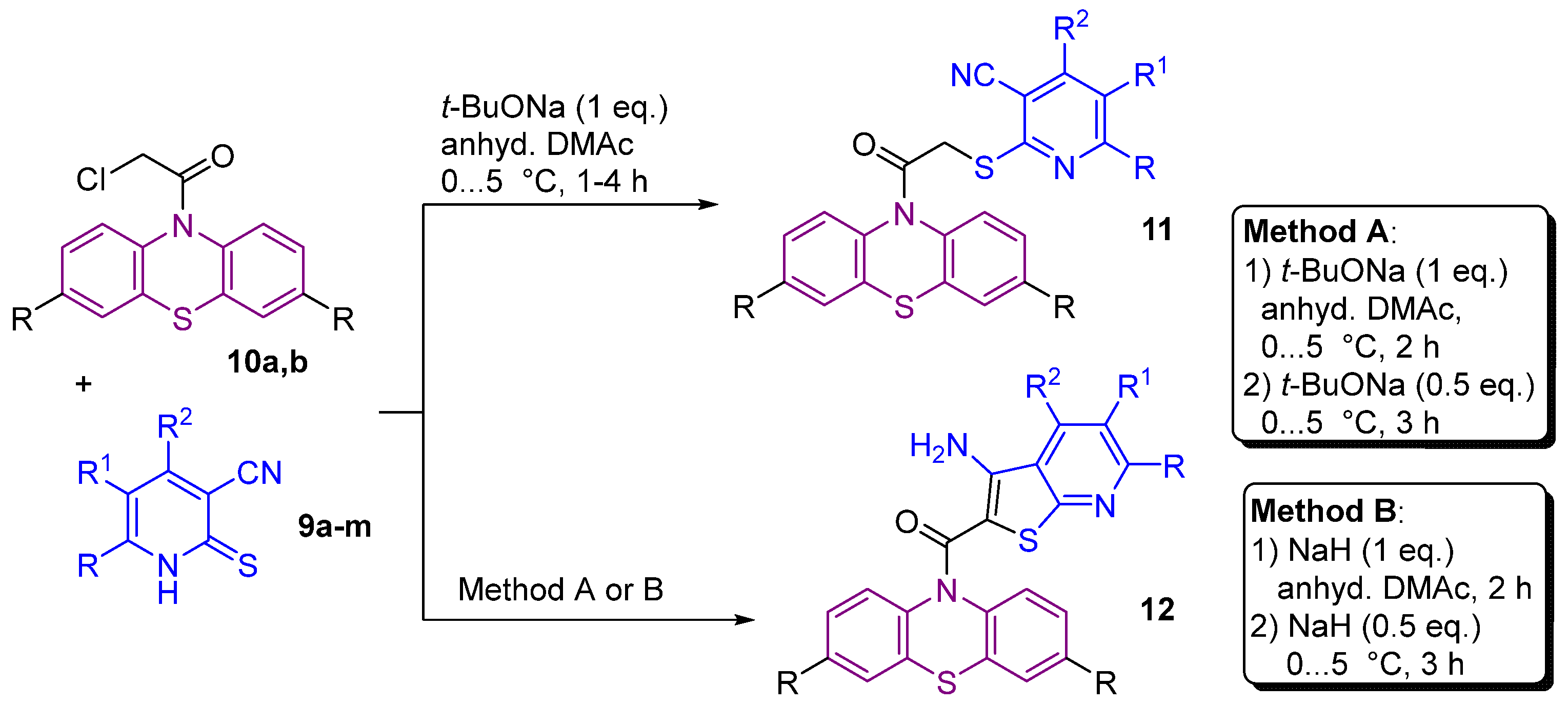
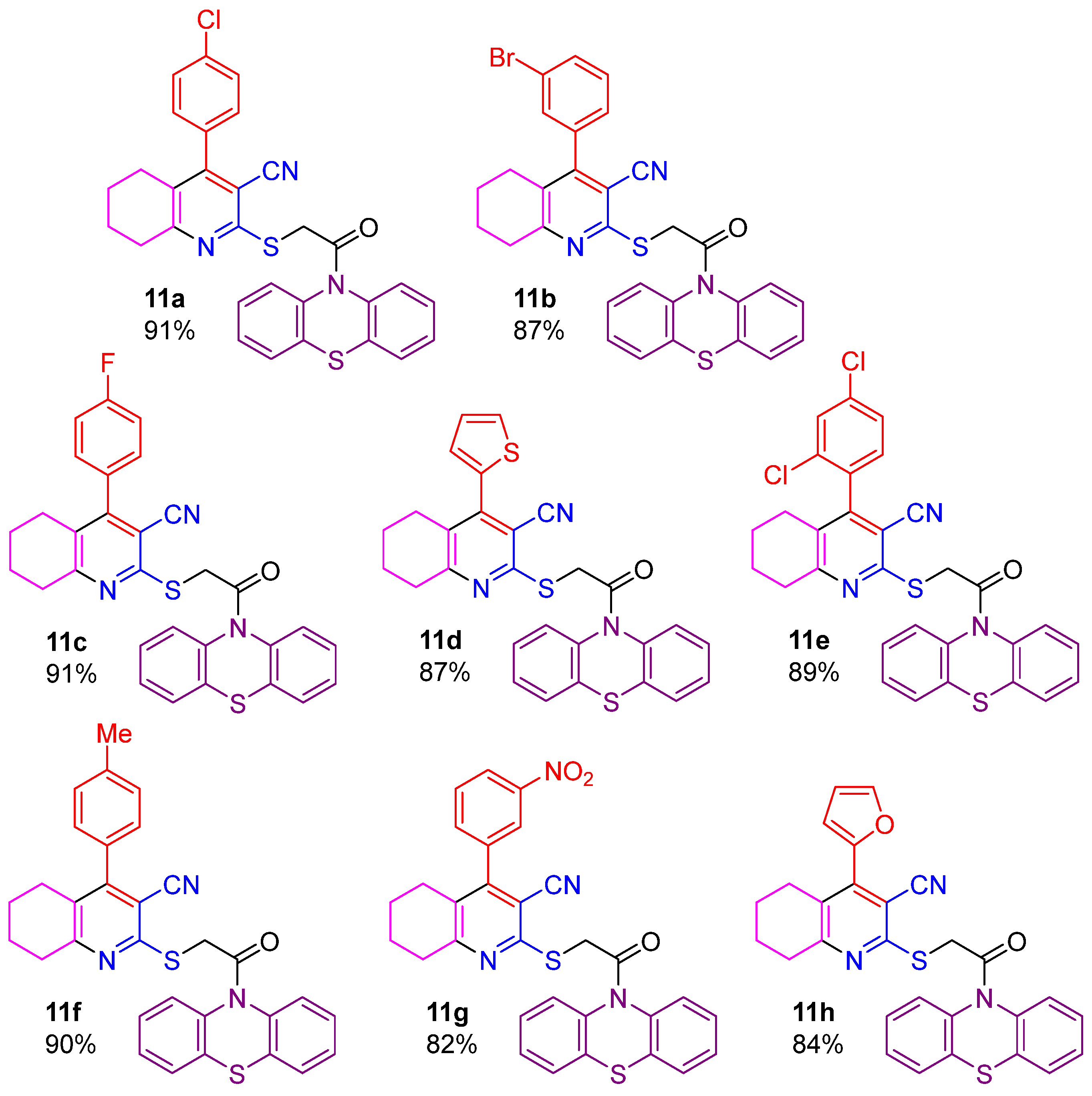
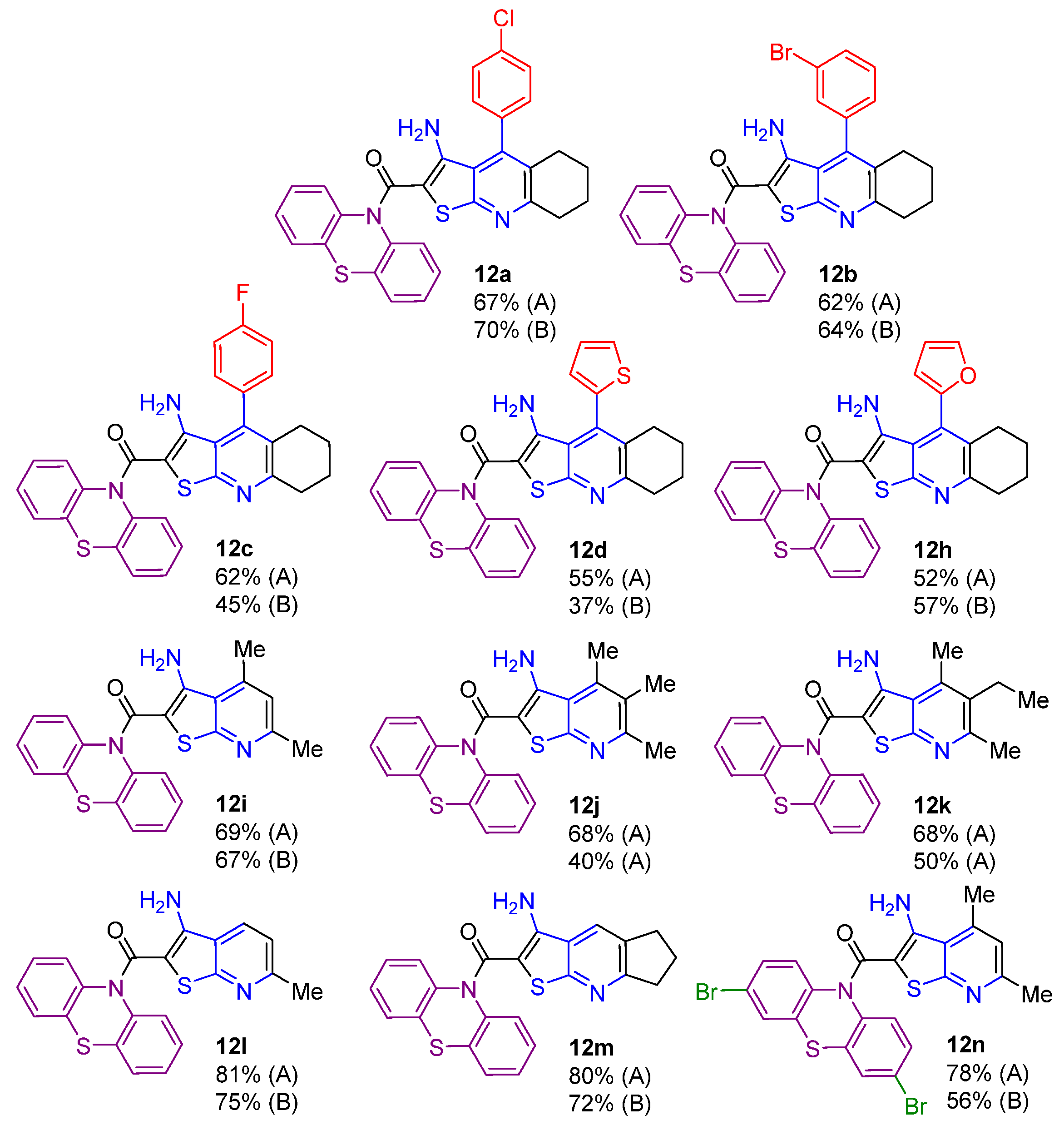
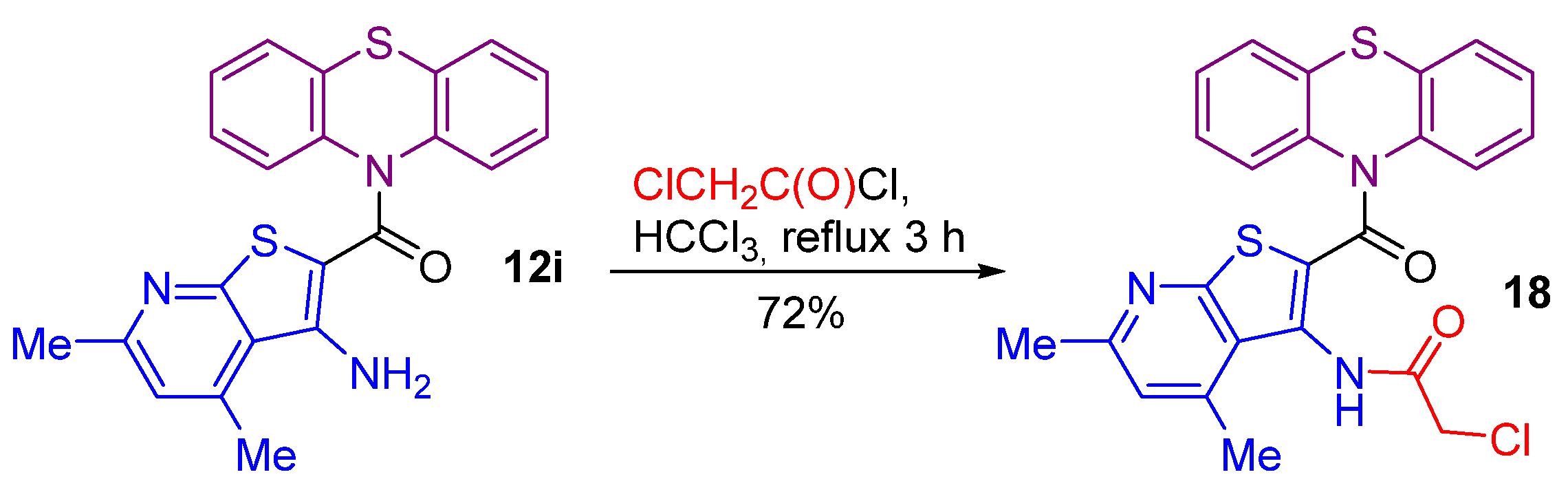
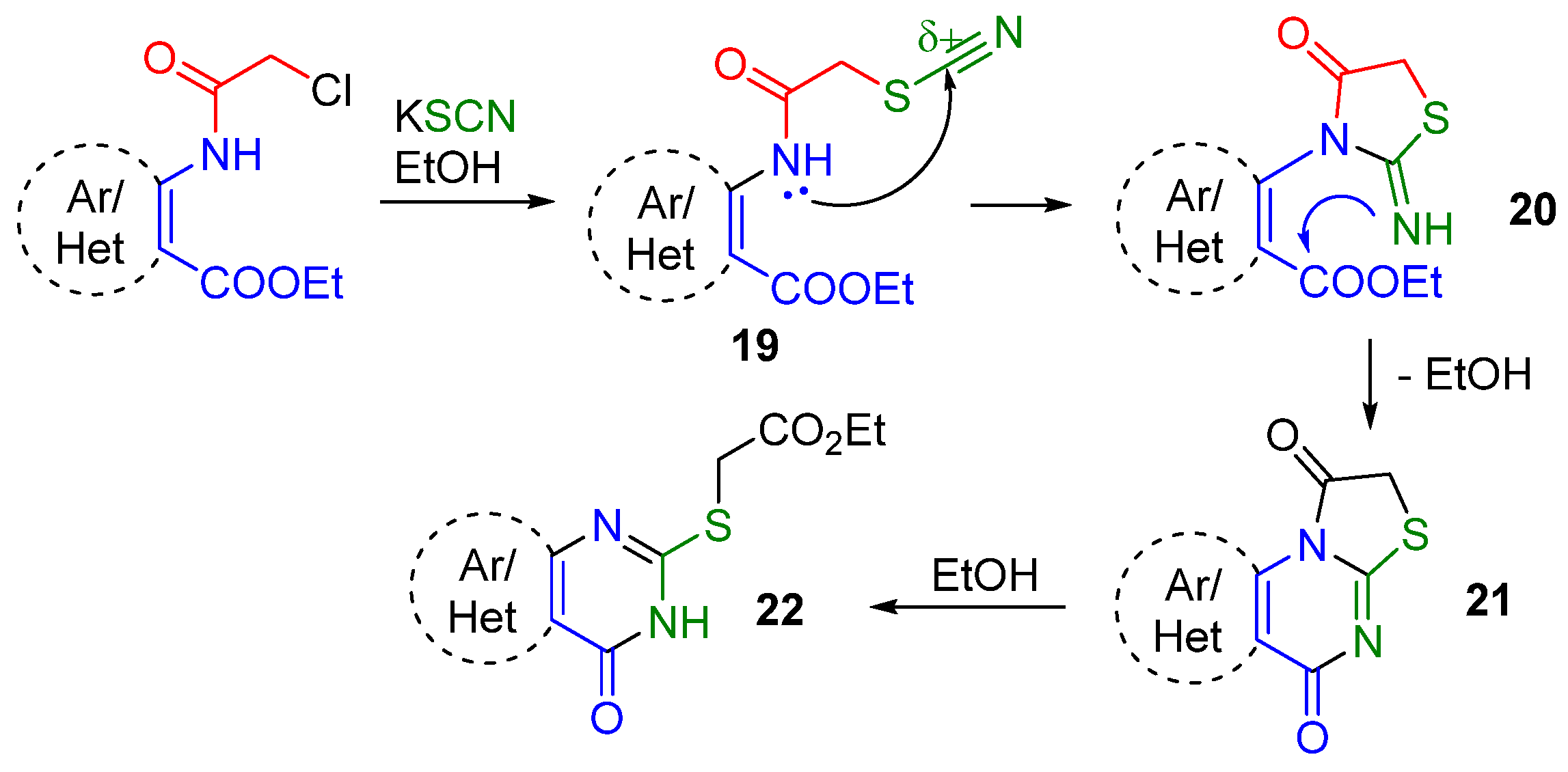
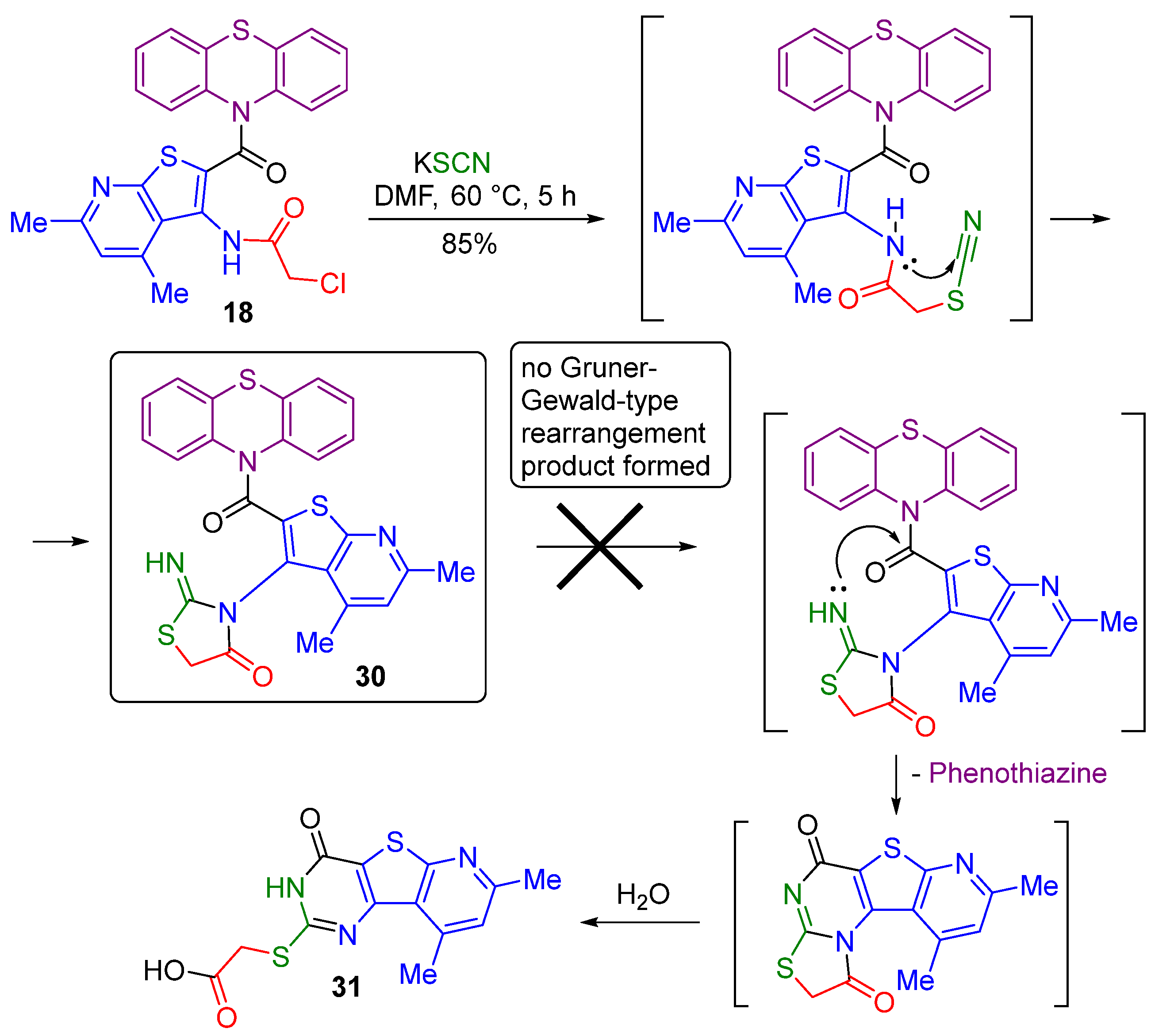
2.1. Synthesis
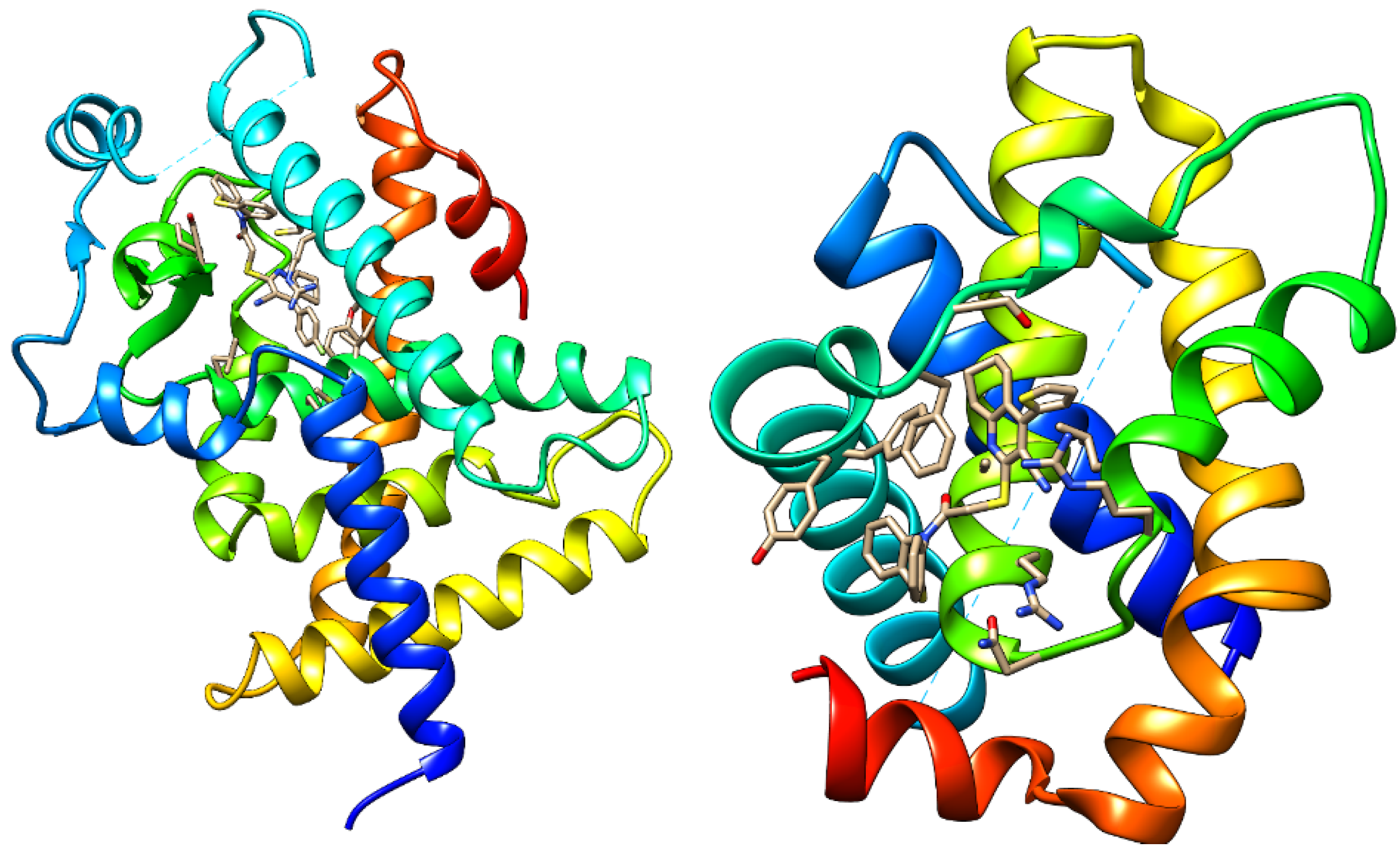
2.2. Drug-Relevant Properties, ADMET, and Docking Studies
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Soltan, O.M.; Shoman, M.E.; Abdel-Aziz, S.A.; Narumi, A.; Konno, H.; Abdel-Aziz, M. Molecular hybrids: A five-year survey on structures of multiple targeted hybrids of protein kinase inhibitors for cancer therapy. Eur. J. Med. Chem. 2021, 225, 113768. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, A.; Singh, H.; Sonawane, P.; Paliwal, H.; Thareja, S.; Pathak, P.; Grishina, M.; Jaremko, M.; Emwas, A.H.; et al. Concept of hybrid drugs and recent advancements in anticancer hybrids. Pharmaceuticals 2022, 15, 1071. [Google Scholar] [CrossRef] [PubMed]
- Alkhzem, A.H.; Woodman, T.J.; Blagbrough, I.S. Design and synthesis of hybrid compounds as novel drugs and medicines. RSC Adv. 2022, 12, 19470–19484. [Google Scholar] [CrossRef]
- Bérubé, G. An overview of molecular hybrids in drug discovery. Expert Opin. Drug Discov. 2016, 11, 281–305. [Google Scholar] [CrossRef]
- Szumilak, M.; Wiktorowska-Owczarek, A.; Stanczak, A. Hybrid drugs—A strategy for overcoming anticancer drug resistance? Molecules 2021, 26, 2601. [Google Scholar] [CrossRef]
- Dong, G.; Jiang, Y.; Zhang, F.; Zhu, F.; Liu, J.; Xu, Z. Recent updates on 1,2,3-, 1,2,4-, and 1,3,5-triazine hybrids (2017–present): The anticancer activity, structure–activity relationships, and mechanisms of action. Arch. Pharm. 2023, 356, e2200479. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, S.J.; Liu, Y. 1,2,3-Triazole-containing hybrids as potential anticancer agents: Current developments, action mechanisms and structure-activity relationships. Eur. J. Med. Chem. 2019, 183, 111700. [Google Scholar] [CrossRef]
- Shagufta; Ahmad, I. Therapeutic significance of molecular hybrids for breast cancer research and treatment. RSC Med. Chem. 2022, 14, 218–238. [Google Scholar] [CrossRef]
- Shalini; Kumar, V. Have molecular hybrids delivered effective anti-cancer treatments and what should future drug discovery focus on? Expert Opin. Drug Discov. 2021, 16, 335–363. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Y. Recent Updates on Anticancer Activity of Betulin and Betulinic Acid Hybrids (A Review). Russ. J. Gen. Chem. 2023, 93, 610–627. [Google Scholar] [CrossRef]
- de Sena Murteira Pinheiro, P.; Franco, L.S.; Montagnoli, T.L.; Fraga, C.A.M. Molecular hybridization: A powerful tool for multitarget drug discovery. Expert Opin. Drug Discov. 2024, 19, 451–470. [Google Scholar] [CrossRef]
- Peter, S.; Alven, S.; Maseko, R.B.; Aderibigbe, B.A. Doxorubicin-based hybrid compounds as potential anticancer agents: A Review. Molecules 2022, 27, 4478. [Google Scholar] [CrossRef]
- Alam, M.M. 1,2,3-Triazole hybrids as anticancer agents: A review. Arch. Pharm. 2022, 355, 2100158. [Google Scholar] [CrossRef]
- Wang, S.; Qian, S.; Wang, S.; Zou, Y. Recent advances on pyrazole-pyrimidine/fused pyrimidine hybrids with anticancer potential (a review). Russ. J. Gen. Chem. 2023, 93, 2090–2112. [Google Scholar] [CrossRef]
- Nakypova, S.; Smolobochkin, A.; Rizbayeva, T.; Turmanov, R.; Gazizov, A.; Akylbekov, N.; Zhapparbergenov, R.; Narmanova, R.; Ibadullayeva, S.; Zalaltdinova, A.; et al. Taurine-based hybrid drugs as potential anticancer therapeutic agents: In vitro, in vivo evaluations. Pharmaceuticals 2025, 18, 1056. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kaur, M.; Chadha, N.; Silakari, O. Hybrids: A new paradigm to treat Alzheimer’s disease. Mol. Divers. 2016, 20, 271–297. [Google Scholar] [CrossRef]
- Khudina, O.G.; Grishchenko, M.V.; Makhaeva, G.F.; Kovaleva, N.V.; Boltneva, N.P.; Rudakova, E.V.; Lushchekina, S.V.; Shchegolkov, E.V.; Borisevich, S.S.; Burgart, Y.V.; et al. Conjugates of amiridine and thiouracil derivatives as effective inhibitors of butyrylcholinesterase with the potential to block β-amyloid aggregation. Arch. Pharm. 2024, 357, 2300447. [Google Scholar] [CrossRef]
- Hatami, M.; Basri, Z.; Sakhvidi, B.K.; Mortazavi, M. Thiadiazole—A promising structure in design and development of Anti-Alzheimer agents. Int. Immunopharmacol. 2023, 118, 110027. [Google Scholar] [CrossRef]
- Bubley, A.; Erofeev, A.; Gorelkin, P.; Beloglazkina, E.; Majouga, A.; Krasnovskaya, O. Tacrine-based hybrids: Past, present, and future. Int. J. Mol. Sci. 2023, 24, 1717. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Kovaleva, N.V.; Rudakova, E.V.; Boltneva, N.P.; Lushchekina, S.V.; Astakhova, T.Y.; Timokhina, E.N.; Serkov, I.V.; Proshin, A.N.; Soldatova, Y.V.; et al. Combining experimental and computational methods to produce conjugates of anticholinesterase and antioxidant pharmacophores with linker chemistries affecting biological activities related to treatment of Alzheimer’s disease. Molecules 2024, 29, 321. [Google Scholar] [CrossRef]
- Pathak, C.; Kabra, U.D. A Comprehensive Review of multi-target directed ligands in the treatment of Alzheimer’s disease. Bioorg. Chem. 2024, 144, 107152. [Google Scholar] [CrossRef]
- Litus, E.A.; Shevelyova, M.P.; Vologzhannikova, A.A.; Deryusheva, E.I.; Chaplygina, A.V.; Rastrygina, V.A.; Machulin, A.V.; Alikova, V.D.; Nazipova, A.A.; Permyakova, M.E.; et al. Interaction between glucagon-like peptide 1 and its analogs with amyloid-β peptide affects its fibrillation and cytotoxicity. Int. J. Mol. Sci. 2025, 26, 4095. [Google Scholar] [CrossRef]
- Jana, A.; Bhattacharjee, A.; Das, S.S.; Srivastava, A.; Choudhury, A.; Bhattacharjee, R.; De, S.; Perveen, A.; Iqbal, D.; Gupta, P.K.; et al. Molecular insights into therapeutic potentials of hybrid compounds targeting Alzheimer’s disease. Mol. Neurobiol. 2022, 59, 3512–3528. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Grishchenko, M.V.; Kovaleva, N.V.; Boltneva, N.P.; Rudakova, E.V.; Astakhova, T.Y.; Timokhina, E.N.; Pronkin, P.G.; Lushchekina, S.V.; Khudina, O.G.; et al. Conjugates of amiridine and salicylic derivatives as promising multifunctional CNS agents for potential treatment of Alzheimer’s Disease. Arch. Pharm. 2025, 358, e2400819. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Kovaleva, N.V.; Rudakova, E.V.; Boltneva, N.P.; Grishchenko, M.V.; Lushchekina, S.V.; Astakhova, T.Y.; Serebryakova, O.G.; Timokhina, E.N.; Zhilina, E.F.; et al. Conjugates of tacrine and salicylic acid derivatives as new promising multitarget agents for Alzheimer’s disease. Int. J. Mol. Sci. 2023, 24, 2285. [Google Scholar] [CrossRef]
- Elkina, N.A.; Grishchenko, M.V.; Shchegolkov, E.V.; Makhaeva, G.F.; Kovaleva, N.V.; Rudakova, E.V.; Boltneva, N.P.; Lushchekina, S.V.; Astakhova, T.Y.; Radchenko, E.V.; et al. New multifunctional agents for potential Alzheimer’s disease Treatment Based on tacrine conjugates with 2-arylhydrazinylidene-1,3-diketones. Biomolecules 2022, 12, 1551. [Google Scholar] [CrossRef] [PubMed]
- Grishchenko, M.V.; Makhaeva, G.F.; Burgart, Y.V.; Rudakova, E.V.; Boltneva, N.P.; Kovaleva, N.V.; Serebryakova, O.G.; Lushchekina, S.V.; Astakhova, T.Y.; Zhilina, E.F.; et al. Conjugates of tacrine with salicylamide as promising multitarget agents for Alzheimer’s disease. ChemMedChem 2022, 17, e202200080. [Google Scholar] [CrossRef] [PubMed]
- Abdul Rahaman, T.A.; Rajendra, T.N.; Suhas, K.P.; Ippagunta, S.K.; Chaudhary, S. 1,2,4,5-Tetraoxane derivatives/hybrids as potent antimalarial endoperoxides: Chronological advancements, structure−activity relationship (SAR) studies and future perspectives. Med. Res. Rev. 2024, 44, 2266–2290. [Google Scholar]
- Robert, A.; Paloque, L.; Augereau, J.M.; Nardella, F.; Nguyen, M.; Meunier, B.; Benoit-Vical, F. Hybrid Molecules As Efficient drugs against multidrug-resistant malaria parasites. ChemMedChem 2025, 20, e202500086. [Google Scholar] [CrossRef]
- Peter, S.; Jama, S.; Alven, S.; Aderibigbe, B.A. Artemisinin and derivatives-based hybrid compounds: Promising therapeutics for the treatment of cancer and malaria. Molecules 2021, 26, 7521. [Google Scholar] [CrossRef]
- Ferreira, V.F.; Graciano, I.A.; de Carvalho, A.S.; de Carvalho da Silva, F. 1,2,3-Triazole- and quinoline-based hybrids with potent antiplasmodial activity. Med. Chem. 2022, 18, 521–535. [Google Scholar] [CrossRef]
- Vinindwa, B.; Dziwornu, G.A.; Masamba, W. Synthesis and Evaluation of chalcone-quinoline based molecular hybrids as potential anti-malarial agents. Molecules 2021, 26, 4093. [Google Scholar] [CrossRef]
- Pacheco, P.A.F.; Santos, M.M.M. Recent Progress in the development of indole-based compounds active against malaria, trypanosomiasis and leishmaniasis. Molecules 2022, 27, 319. [Google Scholar] [CrossRef]
- Ravindar, L.; Hasbullah, S.A.; Rakesh, K.P.; Raheem, S.; Ismail, N.; Ling, L.Y.; Hassan, N.I. Pyridine and pyrimidine hybrids as privileged scaffolds in antimalarial drug discovery: A recent development. Bioorg. Med. Chem. Lett. 2024, 114, 129992. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.; Khambete, M.; Abhyankar, A.; Omri, A. Anti-Tuberculosis Mur Inhibitors: Structural insights and the way ahead for development of novel agents. Pharmaceuticals 2023, 16, 377. [Google Scholar] [CrossRef] [PubMed]
- Hegde, V.; Bhat, R.M.; Budagumpi, S.; Adimule, V.; Keri, R.S. Quinoline hybrid derivatives as effective structural motifs in the treatment of tuberculosis: Emphasis on structure-activity relationships. Tuberculosis 2024, 149, 102573. [Google Scholar] [CrossRef] [PubMed]
- Leite, D.I.; de Castro Bazan Moura, S.; da Conceição Avelino Dias, M.; Costa, C.C.P.; Machado, G.P.; Pimentel, L.C.F.; Branco, F.S.C.; Moreira, R.; Bastos, M.M.; Boechat, N. A Review of the development of multitarget molecules against HIV-TB coinfection pathogens. Molecules 2023, 28, 3342. [Google Scholar] [CrossRef]
- Mishra, S.; Kumar, G.; Singh, P. Isoniazid hybrids as potential antitubercular agents. ChemistrySelect 2024, 9, e202402933. [Google Scholar] [CrossRef]
- Owais, M.; Kumar, A.; Hasan, S.M.; Singh, K.; Azad, I.; Hussain, A.; Suvaiv; Akil, M. Quinoline derivatives as promising scaffolds for antitubercular activity: A comprehensive review. Mini-Rev. Med. Chem. 2024, 24, 1238–1251. [Google Scholar] [CrossRef]
- Reddy, D.S.; Kongot, M.; Kumar, A. Coumarin hybrid derivatives as promising leads to treat tuberculosis: Recent developments and critical aspects of structural design to exhibit anti-tubercular activity. Tuberculosis 2021, 127, 102050. [Google Scholar] [CrossRef]
- Montana, M.; Montero, V.; Khoumeri, O.; Vanelle, P. Quinoxaline moiety: A potential scaffold against Mycobacterium tuberculosis. Molecules 2021, 26, 4742. [Google Scholar] [CrossRef]
- Alghamdi, S.; Qusty, N.F.; Atwah, B.; Alhindi, Z.; Alatawy, R.; Verma, S.; Asif, M. Isoniazid analogs and their biological activities as antitubercular agents (A Review). Russ. J. Gen. Chem. 2024, 94, 2101–2141. [Google Scholar] [CrossRef]
- Liman, W.; Ait Lahcen, N.; Oubahmane, M.; Hdoufane, I.; Cherqaoui, D.; Daoud, R.; El Allali, A. Hybrid molecules as potential drugs for the treatment of HIV: Design and Applications. Pharmaceuticals 2022, 15, 1092. [Google Scholar] [CrossRef] [PubMed]
- Starosotnikov, A.M.; Bastrakov, M.A. Recent Developments in the Synthesis of HIV-1 integrase strand transfer inhibitors incorporating pyridine moiety. Int. J. Mol. Sci. 2023, 24, 9314. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wu, C.; Zhu, J.; Li, E.; Xu, Z. Therapeutic potential of indole derivatives as Anti-HIV Agents: A Mini-Review. Curr. Top. Med. Chem. 2022, 22, 993–1008. [Google Scholar] [CrossRef]
- Feng, L.S.; Zheng, M.J.; Zhao, F.; Liu, D. 1,2,3-Triazole hybrids with anti-HIV-1 activity. Arch. Pharm. 2021, 354, 2000163. [Google Scholar] [CrossRef]
- Suleiman, M.; Almalki, F.A.; Ben Hadda, T.; Kawsar, S.M.A.; Chander, S.; Murugesan, S.; Bhat, A.R.; Bogoyavlenskiy, A.; Jamalis, J. Recent Progress in Synthesis, POM Analyses and SAR of coumarin-hybrids as potential Anti-HIV Agents—A Mini Review. Pharmaceuticals 2023, 16, 1538. [Google Scholar] [CrossRef]
- Deng, C.; Yan, H.; Wang, J.; Liu, B.; Liu, K.; Shi, Y. The Anti-HIV potential of imidazole, oxazole and thiazole hybrids: A mini-review. Arab. J. Chem. 2022, 15, 104242. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, F.; Zhang, J.; Liu, C.; López-Carrobles, N.; Liu, X.; Menéndez-Arias, L.; Zhan, P. Current medicinal chemistry strategies in the discovery of novel HIV-1 ribonuclease h inhibitors. Eur. J. Med. Chem. 2022, 243, 114760. [Google Scholar] [CrossRef]
- Navacchia, M.L.; Cinti, C.; Marchesi, E.; Perrone, D. Insights into SARS-CoV-2: Small-Molecule Hybrids for COVID-19 Treatment. Molecules 2024, 29, 5403. [Google Scholar] [CrossRef]
- Lungu, I.A.; Moldovan, O.L.; Biriș, V.; Rusu, A. Fluoroquinolones hybrid molecules as promising antibacterial agents in the fight against antibacterial resistance. Pharmaceutics 2022, 14, 1749. [Google Scholar] [CrossRef]
- Belakhov, V.V. Polyfunctional drugs: Search, development, use in medical practice, and environmental aspects of preparation and application (A Review). Russ. J. Gen. Chem. 2022, 92, 3030–3055. [Google Scholar] [CrossRef]
- Gao, J.; Hou, H.; Gao, F. Current scenario of quinolone hybrids with potential antibacterial activity against ESKAPE Pathogens. Eur. J. Med. Chem. 2023, 247, 115026. [Google Scholar] [CrossRef] [PubMed]
- Smolobochkin, A.; Gazizov, A.; Appazov, N.; Sinyashin, O.; Burilov, A. Progress in the stereoselective synthesis methods of pyrrolidine-containing drugs and their precursors. Int. J. Mol. Sci. 2024, 25, 11158. [Google Scholar] [CrossRef] [PubMed]
- Chugunova, E.; Gibadullina, E.; Matylitsky, K.; Bazarbayev, B.; Neganova, M.; Volcho, K.; Rogachev, A.; Akylbekov, N.; Nguyen, H.B.T.; Voloshina, A.; et al. Diverse biological activity of benzofuroxan/sterically hindered phenols hybrids. Pharmaceuticals 2023, 16, 499. [Google Scholar] [CrossRef]
- Marinescu, M. Benzimidazole-triazole hybrids as antimicrobial and antiviral agents: A systematic review. Antibiotics 2023, 12, 1220. [Google Scholar] [CrossRef]
- Patel, K.B.; Kumari, P. A Review: Structure-activity relationship and antibacterial activities of quinoline based hybrids. J. Mol. Struct. 2022, 1268, 133634. [Google Scholar] [CrossRef]
- Volynkina, I.A.; Bychkova, E.N.; Karakchieva, A.O.; Tikhomirov, A.S.; Zatonsky, G.V.; Solovieva, S.E.; Martynov, M.M.; Grammatikova, N.E.; Tereshchenkov, A.G.; Paleskava, A.; et al. Hybrid molecules of azithromycin with chloramphenicol and metronidazole: Synthesis and study of antibacterial properties. Pharmaceuticals 2024, 17, 187. [Google Scholar] [CrossRef]
- Wang, L.P.; Tu, Y.; Tian, W. Current scenario of pleuromutilin derivatives with antibacterial potential (A Review). Russ. J. Gen. Chem. 2023, 93, S908–S927. [Google Scholar] [CrossRef]
- Levshin, I.B.; Simonov, A.Y.; Panov, A.A.; Grammatikova, N.E.; Alexandrov, A.I.; Ghazy, E.S.M.O.; Ivlev, V.A.; Agaphonov, M.O.; Mantsyzov, A.B.; Polshakov, V.I. Synthesis and biological evaluation of a series of new hybrid amide derivatives of triazole and thiazolidine-2,4-dione. Pharmaceuticals 2024, 17, 723. [Google Scholar] [CrossRef]
- Khwaza, V.; Aderibigbe, B.A. Antifungal activities of natural products and their hybrid molecules. Pharmaceutics 2023, 15, 2673. [Google Scholar] [CrossRef]
- Gharge, S.; Alegaon, S.G. Recent studies of nitrogen and sulfur containing heterocyclic analogues as novel antidiabetic agents: A Review. Chem. Biodivers. 2024, 21, e202301738. [Google Scholar] [CrossRef]
- Fallah, Z.; Tajbakhsh, M.; Alikhani, M.; Larijani, B.; Faramarzi, M.A.; Hamedifar, H.; Mohammadi-Khanaposhtani, M.; Mahdavi, M. A Review on synthesis, mechanism of action, and structure-activity relationships of 1,2,3-triazole-based α-glucosidase inhibitors as promising anti-diabetic agents. J. Mol. Struct. 2022, 1255, 132469. [Google Scholar] [CrossRef]
- Chawla, G.; Pradhan, T.; Gupta, O. An insight into the combat strategies for the treatment of type 2 Diabetes Mellitus. Mini-Rev. Med. Chem. 2024, 24, 403–430. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Kaushal, R. Nitrogen Containing Heterocyclic Chalcone Hybrids and Their Biological Potential (A Review). Russ. J. Gen. Chem. 2024, 94, 1794–1814. [Google Scholar] [CrossRef]
- Trifonov, R.E.; Ostrovskii, V.A. Tetrazoles and related heterocycles as promising synthetic antidiabetic agents. Int. J. Mol. Sci. 2023, 24, 17190. [Google Scholar] [CrossRef] [PubMed]
- Tretyakova, E.; Smirnova, I.; Kazakova, O.; Nguyen, H.T.T.; Shevchenko, A.; Sokolova, E.; Babkov, D.; Spasov, A. New molecules of diterpene origin with inhibitory properties toward A-Glucosidase. Int. J. Mol. Sci. 2022, 23, 13535. [Google Scholar] [CrossRef]
- Huneif, M.A.; Mahnashi, M.H.; Jan, M.S.; Shah, M.; Almedhesh, S.A.; Alqahtani, S.M.; Alzahrani, M.J.; Ayaz, M.; Ullah, F.; Rashid, U.; et al. New succinimide–thiazolidinedione hybrids as multitarget antidiabetic agents: Design, synthesis, bioevaluation, and molecular modelling studies. Molecules 2023, 28, 1207. [Google Scholar] [CrossRef]
- Mohammad, B.D.; Baig, M.S.; Bhandari, N.; Siddiqui, F.A.; Khan, S.L.; Ahmad, Z.; Khan, F.S.; Tagde, P.; Jeandet, P. Heterocyclic compounds as dipeptidyl peptidase-IV inhibitors with special emphasis on oxadiazoles as potent anti-diabetic agents. Molecules 2022, 27, 6001. [Google Scholar] [CrossRef]
- Farwa, U.; Raza, M.A. Heterocyclic compounds as a magic bullet for diabetes mellitus: A Review. RSC Adv. 2022, 12, 22951–22973. [Google Scholar] [CrossRef]
- Khator, R.; Monga, V. Recent advances in the synthesis and medicinal perspective of pyrazole-based α-amylase inhibitors as antidiabetic agents. Future Med. Chem. 2024, 16, 173–195. [Google Scholar] [CrossRef]
- Ramsis, T.M.; Ebrahim, M.A.; Fayed, E.A. Synthetic coumarin derivatives with anticoagulation and antiplatelet aggregation inhibitory effects. Med. Chem. Res. 2023, 32, 2269–2278. [Google Scholar] [CrossRef]
- Spasov, A.A.; Fedorova, O.V.; Rasputin, N.A.; Ovchinnikova, I.G.; Ishmetova, R.I.; Ignatenko, N.K.; Gorbunov, E.B.; Sadykhov, G.A.; Kucheryavenko, A.F.; Gaidukova, K.A.; et al. Novel substituted azoloazines with anticoagulant activity. Int. J. Mol. Sci. 2023, 24, 15581. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, P.P.; Bansode, T.N. Coumarin derivatives: Pioneering new frontiers in biological applications. Curr. Org. Chem. 2025, 29, 794–813. [Google Scholar] [CrossRef]
- Skoptsova, A.A.; Geronikaki, A.; Novichikhina, N.P.; Sulimov, A.V.; Ilin, I.S.; Sulimov, V.B.; Bykov, G.A.; Podoplelova, N.A.; Pyankov, O.V.; Shikhaliev, K.S. Design, synthesis, and evaluation of new hybrid derivatives of 5,6-dihydro-4h-pyrrolo[3,2,1-ij]quinolin-2(1H)-one as potential dual inhibitors of blood coagulation factors Xa and XIa. Molecules 2024, 29, 373. [Google Scholar] [CrossRef] [PubMed]
- Kouznetsov, V.V. Exploring acetaminophen prodrugs and hybrids: A Review. RSC Adv. 2024, 14, 9691–9715. [Google Scholar] [CrossRef]
- Laev, S.S.; Salakhutdinov, N.F. New small-molecule analgesics. Curr. Med. Chem. 2021, 28, 6234–6273. [Google Scholar] [CrossRef]
- Belyaeva, E.R.; Myasoedova, Y.V.; Ishmuratova, N.M.; Ishmuratov, G.Y. Synthesis and biological activity of N-Acylhydrazones. Russ. J. Bioorg. Chem. 2022, 48, 1123–1150. [Google Scholar] [CrossRef]
- Cheremnykh, K.; Bryzgalov, A.; Baev, D.; Borisov, S.; Sotnikova, Y.; Savelyev, V.; Tolstikova, T.; Sagdullaev, S.; Shults, E. Synthesis, pharmacological evaluation, and molecular modeling of lappaconitine–1,5-benzodiazepine hybrids. Molecules 2023, 28, 4234. [Google Scholar] [CrossRef]
- Zayed, M.F. Medicinal chemistry of quinazolines as analgesic and anti-inflammatory agents. ChemEngineering 2022, 6, 94. [Google Scholar] [CrossRef]
- Baramaki, I.; Altıntop, M.D.; Arslan, R.; Alyu Altınok, F.; Özdemir, A.; Dallali, I.; Hasan, A.; Bektaş Türkmen, N. Design, synthesis, and in vivo evaluation of a new series of indole-chalcone hybrids as analgesic and anti-inflammatory agents. ACS Omega 2024, 9, 12175–12183. [Google Scholar] [CrossRef]
- Ryazantsev, M.N.; Strashkov, D.M.; Nikolaev, D.M.; Shtyrov, A.A.; Panov, M.S. Photopharmacological compounds based on azobenzenes and azoheteroarenes: Principles of molecular design, molecular modelling, and synthesis. Russ. Chem. Rev. 2021, 90, 868–893. [Google Scholar] [CrossRef]
- da Cruz, R.M.D.; Mendonça-Junior, F.J.B.; de Mélo, N.B.; Scotti, L.; de Araújo, R.S.A.; de Almeida, R.N.; de Moura, R.O. Thiophene-based compounds with potential anti-inflammatory activity. Pharmaceuticals 2021, 14, 692. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Bekeschus, S.; Weltmann, K.D.; von Woedtke, T.; Wende, K. Non-Steroidal anti-inflammatory drugs: Recent advances in the use of synthetic COX-2 inhibitors. RSC Med. Chem. 2022, 13, 471–496. [Google Scholar] [CrossRef] [PubMed]
- Bian, M.; Ma, Q.; Wu, Y.; Du, H.; Guo-hua, G. Small molecule compounds with good anti-inflammatory activity reported in the literature from 01/2009 to 05/2021: A Review. J. Enzym. Inhib. Med. Chem. 2021, 36, 2139–2159. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, Y.; Yao, C.; Zhan, J.; Wu, P.; Han, Z.; Zuo, J.; Feng, H.; Qian, Z. Recent advances in small-molecule fluorescent photoswitches with photochromism in diverse states. J. Mater. Chem. C 2023, 11, 15393–15411. [Google Scholar] [CrossRef]
- Khuzin, A.A.; Tuktarov, A.R.; Venidiktova, O.V.; Barachevsky, V.A.; Mullagaliev, I.N.; Salikhov, T.R.; Salikhov, R.B.; Khalilov, L.M.; Khuzina, L.L.; Dzhemilev, U.M. Hybrid molecules based on fullerene C60 and dithienylethenes. synthesis and photochromic properties. optically controlled organic field-effect transistors. Photochem. Photobiol. 2022, 98, 815–822. [Google Scholar] [CrossRef]
- Khuzin, A.A.; Galimov, D.I.; Khuzina, L.L. Photochromic and luminescent properties of a salt of a hybrid molecule based on C60 fullerene and spiropyran—A promising approach to the creation of anticancer drugs. Molecules 2023, 28, 1107. [Google Scholar] [CrossRef]
- Stoikov, I.I.; Antipin, I.S.; Burilov, V.A.; Kurbangalieva, A.R.; Rostovskii, N.V.; Pankova, A.S.; Balova, I.A.; Remizov, Y.O.; Pevzner, L.M.; Petrov, M.L.; et al. Organic Chemistry in Russian Universities. Achievements of Recent Years. Russ. J. Org. Chem. 2024, 60, 1361–1584, Eratum in: Russ. J. Org. Chem. 2024, 60, 2052–2053. [Google Scholar] [CrossRef]
- Charushin, V.N.; Verbitskiy, E.V.; Chupakhin, O.N.; Vorobyeva, D.V.; Gribanov, P.S.; Osipov, S.N.; Ivanov, A.V.; Martynovskaya, S.V.; Sagitova, E.F.; Dyachenko, V.D.; et al. The chemistry of heterocycles in the 21st Century. Russ. Chem. Rev. 2024, 93, RCR5125. [Google Scholar] [CrossRef]
- Larin, A.A.; Fershtat, L.L. Energetic Heterocyclic N-Oxides: Synthesis and performance. Mendeleev Commun. 2022, 32, 703–713. [Google Scholar] [CrossRef]
- Shaferov, A.V.; Ananyev, I.V.; Monogarov, K.A.; Fomenkov, I.V.; Pivkina, A.N.; Fershtat, L.L. Energetic methylene-bridged furoxan-triazole/tetrazole hybrids. ChemPlusChem 2024, 89, e202400496. [Google Scholar] [CrossRef] [PubMed]
- Muravyev, N.V.; Fershtat, L.; Zhang, Q. Synthesis, design and development of energetic materials: Quo vadis? Chem. Eng. J. 2024, 486, 150410. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Frolov, K.A.; Krivokolysko, S.G. Synthesis of partially hydrogenated 1,3,5-thiadiazines by Mannich reaction. Chem. Heterocycl. Compd. 2015, 51, 109–127. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Buryi, D.S.; Lukina, D.Y.; Krivokolysko, S.G. Recent advances in the chemistry of thieno[2,3-b]pyridines. 1. Methods of synthesis of thieno[2,3-b]pyridines. Russ. Chem. Bull. 2020, 69, 1829–1858. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Frolov, K.A.; Chigorina, E.A.; Khrustaleva, A.N.; Bibik, E.Y.; Krivokolysko, S.G. New possibilities of the Mannich reaction in the synthesis of N-, S,N-, and Se,N-Heterocycles. Russ. Chem. Bull. 2019, 68, 691–707. [Google Scholar] [CrossRef]
- Stroganova, T.A.; Vasilin, V.K.; Dotsenko, V.V.; Aksenov, N.A.; Morozov, P.G.; Vassiliev, P.M.; Volynkin, V.A.; Krapivin, G.D. Unusual oxidative dimerization in the 3-aminothieno[2,3-b]pyridine-2-carboxamide series. ACS Omega 2021, 6, 14030–14048. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Muraviev, V.S.; Lukina, D.Y.; Strelkov, V.D.; Aksenov, N.A.; Aksenova, I.V.; Krapivin, G.D.; Dyadyuchenko, L.V. Reaction of 3-amino-4,6-diarylthieno[2,3-b]pyridine-2-carboxamides with ninhydrin. Russ. J. Gen. Chem. 2020, 90, 948–960. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Lukina, D.Y.; Buryi, D.S.; Strelkov, V.D.; Aksenov, N.A.; Aksenova, I.V. Synthesis of new polycyclic compounds containing thieno[2′,3′:5,6]pyrimido[2,1-a]isoindole fragment. Russ. J. Gen. Chem. 2021, 91, 1292–1296. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Rudenko, S.V.; Lukina, D.Y.; Smirnova, A.K.; Krivokolysko, S.G.; Temerdashev, A.Z.; Harutyunyan, A.S.; Paronikyan, E.G.; Aksenov, N.A.; Aksenova, I.V. Synthesis of 2,2-dimethyl-2,3-dihydropyrido[3′,2′: 4,5]thieno[3,2-d]pyrimidin-4(1H)-ones by reaction of 3-aminothieno[2,3-b]pyridine- 2-carboxamides with acetone. Russ. J. Gen. Chem. 2025, 95, 1236–1247. [Google Scholar] [CrossRef]
- Pakholka, N.A.; Dotsenko, V.V.; Churakov, A.V.; Krivokolysko, S.G. Synthesis, Structure, and bromination of 3-(arylamino)-2-(4-arylthiazol-2-yl)acrylonitriles. Russ. J. Gen. Chem. 2025, 95, 1210–1224. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Bespalov, A.V.; Sinotsko, A.E.; Temerdashev, A.Z.; Vasilin, V.K.; Varzieva, E.A.; Strelkov, V.D.; Aksenov, N.A.; Aksenova, I.V. 6-Amino-4-aryl-7-phenyl-3-(phenylimino)-4,7-dihydro-3H-[1,2]dithiolo[3,4-b]pyridine-5-carboxamides: Synthesis, biological activity, quantum chemical studies and in silico docking studies. Int. J. Mol. Sci. 2024, 25, 769. [Google Scholar] [CrossRef] [PubMed]
- Mosnaim, A.D.; Ranade, V.V.; Wolf, M.E.; Puente, J.; Antonieta Valenzuela, M. Phenothiazine molecule provides the basic chemical structure for various classes of pharmacotherapeutic agents. Am. J. Ther. 2006, 13, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Ohlow, M.J.; Moosmann, B. Phenothiazine: The seven lives of pharmacology’s first lead structure. Drug Discov. Today 2011, 16, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Zaharia, C.A. Phenothiazine-based dopamine D2 antagonists for the treatment of schizophrenia (Chapter 5). In Bioactive Heterocyclic Compound Classes: Pharmaceuticals, 1st ed.; Dinges, J., Lamberth, C., Eds.; Wiley: Hoboken, NJ, USA, 2012; pp. 65–79. [Google Scholar]
- Wainwright, M. The development of phenothiazinium photosensitisers. Photodiagnosis Photodyn. Ther. 2005, 2, 263–272. [Google Scholar] [CrossRef]
- Medina, D.X.; Caccamo, A.; Oddo, S. Methylene Blue reduces aβ levels and rescues early cognitive deficit by increasing proteasome activity. Brain Pathol. 2011, 21, 140–149. [Google Scholar] [CrossRef]
- Padnya, P.L.; Khadieva, A.I.; Stoikov, I.I. Current achievements and perspectives in synthesis and applications of 3,7-disubstituted phenothiazines as Methylene Blue analogues. Dye. Pigment. 2023, 208, 110806. [Google Scholar] [CrossRef]
- Oz, M.; Lorke, D.E.; Petroianu, G.A. Methylene Blue and Alzheimer’s Disease. Biochem. Pharmacol. 2009, 78, 927–932. [Google Scholar] [CrossRef]
- Seitkazina, A.; Yang, J.K.; Kim, S. Clinical effectiveness and prospects of Methylene Blue: A Systematic Review. Precis. Future Med. 2022, 6, 193–208. [Google Scholar] [CrossRef]
- Taldaev, A.; Terekhov, R.; Nikitin, I.; Melnik, E.; Kuzina, V.; Klochko, M.; Reshetov, I.; Shiryaev, A.; Loschenov, V.; Ramenskaya, G. Methylene Blue in anticancer photodynamic therapy: Systematic review of preclinical studies. Front. Pharmacol. 2023, 14, 1264961. [Google Scholar] [CrossRef]
- Kumar, A.; Vigato, C.; Boschi, D.; Lolli, M.L.; Kumar, D. Phenothiazines as anti-cancer agents: SAR Overview and Synthetic Strategies. Eur. J. Med. Chem. 2023, 254, 115337. [Google Scholar] [CrossRef]
- González-González, A.; Vazquez-Jimenez, L.K.; Paz-González, A.D.; Bolognesi, M.L.; Rivera, G. Recent advances in the medicinal chemistry of phenothiazines, new anticancer and antiprotozoal agents. Curr. Med. Chem. 2021, 28, 7910–7936. [Google Scholar] [CrossRef] [PubMed]
- Babalola, B.A.; Malik, M.; Sharma, L.; Olowokere, O.; Folajimi, O. Exploring the therapeutic potential of phenothiazine derivatives in medicinal chemistry. Results Chem. 2024, 8, 101565. [Google Scholar] [CrossRef]
- Voronova, O.; Zhuravkov, S.; Korotkova, E.; Artamonov, A.; Plotnikov, E. Antioxidant properties of new phenothiazine derivatives. Antioxidants 2022, 11, 1371. [Google Scholar] [CrossRef] [PubMed]
- El-Sedik, M.S.; Mohamed, M.B.I.; Abdel-Aziz, M.S.; Aysha, T.S. Synthesis of New D–Π–A phenothiazine-based fluorescent dyes: Aggregation induced emission and antibacterial activity. J. Fluoresc. 2024, 35, 3119–3130. [Google Scholar] [CrossRef]
- Khan, F.; Misra, R. Recent advances in the development of phenothiazine and its fluorescent derivatives for optoelectronic applications. J. Mater. Chem. C 2023, 11, 2786–2825. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, Y.; Liu, J.; Zhang, Y.; Zhang, H.; Zhang, M.X. Asymmetric Phenothiazine derivatives modified with diphenylamine and carbazole: Photophysical properties and hypochlorite sensing. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 340, 126346. [Google Scholar] [CrossRef]
- Ilakiyalakshmi, M.; Dhanasekaran, K.; Napoleon, A.A. A Review on recent development of phenothiazine-based chromogenic and fluorogenic sensors for the detection of cations, anions, and neutral analytes. Top. Curr. Chem. 2024, 382, 29. [Google Scholar] [CrossRef]
- Ilakiyalakshmi, M.; Arumugam Napoleon, A. Phenothiazine-derived fluorescent chemosensor: A versatile platform enabling swift cyanide ion detection and its multifaceted utility in strips, environmental water, food samples and living cells. J. Photochem. Photobiol. A Chem. 2024, 447, 115213. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, C.; Li, J.; Sun, J. A New phenothiazine-based fluorescent sensor for detection of cyanide. Biosensors 2024, 14, 51. [Google Scholar] [CrossRef]
- Serkov, I.V.; Proshin, A.N.; Ustinov, A.K.; Bachurin, S.O. Phenothiazine derivatives containing a NO-generating fragment. Russ. Chem. Bull. 2022, 71, 2757–2760. [Google Scholar] [CrossRef]
- Malanina, A.N.; Kuzin, Y.I.; Padnya, P.L.; Ivanov, A.N.; Stoikov, I.I.; Evtugyn, G.A. Cationic and anionic phenothiazine derivatives: Electrochemical behavior and application in DNA sensor development. Analyst 2025, 150, 2087–2100. [Google Scholar] [CrossRef]
- Kononov, A.I.; Strekalova, S.O.; Budnikova, Y.H. Electrochemical and photochemical functionalization of phenothiazines towards the synthesis of N-Aryl phenothiazines: Recent updates and prospects. Eur. J. Org. Chem. 2025, 28, e202401472. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances on visible light phenothiazine-based photoinitiators of polymerization. Eur. Polym. J. 2022, 165, 110999. [Google Scholar] [CrossRef]
- Hölter, N.; Rendel, N.H.; Spierling, L.; Kwiatkowski, A.; Kleinmans, R.; Daniliuc, C.G.; Wenger, O.S.; Glorius, F. Phenothiazine sulfoxides as active photocatalysts for the synthesis of γ-lactones. J. Am. Chem. Soc. 2025, 147, 12908–12916. [Google Scholar] [CrossRef] [PubMed]
- Posso, M.C.; Domingues, F.C.; Ferreira, S.; Silvestre, S. Development of phenothiazine hybrids with potential medicinal interest: A Review. Molecules 2022, 27, 276. [Google Scholar] [CrossRef] [PubMed]
- Bachurin, S.O.; Shevtsova, E.F.; Makhaeva, G.F.; Aksinenko, A.Y.; Grigoriev, V.V.; Goreva, T.V.; Epishina, T.A.; Kovaleva, N.V.; Boltneva, N.P.; Lushchekina, S.V.; et al. Conjugates of Methylene Blue With cycloalkaneindoles as new multifunctional agents for potential treatment of neurodegenerative disease. Int. J. Mol. Sci. 2022, 23, 13925. [Google Scholar] [CrossRef]
- Kisla, M.M.; Yaman, M.; Zengin-Karadayi, F.; Korkmaz, B.; Bayazeid, O.; Kumar, A.; Peravali, R.; Gunes, D.; Tiryaki, R.S.; Gelinci, E.; et al. Synthesis and structure of novel phenothiazine derivatives, and compound prioritization via in silico target search and screening for cytotoxic and cholinesterase modulatory activities in liver cancer cells and in Vivo in Zebrafish. ACS Omega 2024, 9, 30594–30614. [Google Scholar] [CrossRef]
- Gorecki, L.; Uliassi, E.; Bartolini, M.; Janockova, J.; Hrabinova, M.; Hepnarova, V.; Prchal, L.; Muckova, L.; Pejchal, J.; Karasova, J.Z.; et al. Phenothiazine-tacrine heterodimers: Pursuing multitarget directed approach in Alzheimer’s disease. ACS Chem. Neurosci. 2021, 12, 1698–1715. [Google Scholar] [CrossRef]
- Carocci, A.; Barbarossa, A.; Leuci, R.; Carrieri, A.; Brunetti, L.; Laghezza, A.; Catto, M.; Limongelli, F.; Chaves, S.; Tortorella, P.; et al. Novel phenothiazine/donepezil-like hybrids endowed with antioxidant activity for a multi-target approach to the therapy of Alzheimer’s disease. Antioxidants 2022, 11, 1631. [Google Scholar] [CrossRef]
- Spivak, A.Y.; Nedopekina, D.A.; Davletshin, E.V.; Khalitova, R.R. Efficient and practical synthesis of a novel lipophilic phenothiazine derivative with an α-tocopherol isoprenoid side chain. Russ. J. Gen. Chem. 2025, 95, 1494–1500. [Google Scholar] [CrossRef]
- Cibotaru, S.; Sandu, A.I.; Nicolescu, A.; Marin, L. Antitumor activity of PEGylated and TEGylated phenothiazine derivatives: Structure–Activity Relationship. Int. J. Mol. Sci. 2023, 24, 5449. [Google Scholar] [CrossRef] [PubMed]
- Iniyaval, S.; Saravanan, V.; Mai, C.W.; Ramalingan, C. Tetrazolopyrimidine-tethered phenothiazine molecular hybrids: Synthesis, biological and molecular docking studies. New J. Chem. 2024, 48, 13384–13396. [Google Scholar] [CrossRef]
- Doddagaddavalli, M.A.; Bhat, S.S.; Seetharamappa, J. Characterization, crystal structure, anticancer and antioxidant activity of novel N-(2-oxo-2-(10H-phenothiazin-10-yl) ethyl)piperidine-1-carboxamide. J. Struct. Chem. 2023, 64, 131–141. [Google Scholar] [CrossRef]
- Sarhan, M.O.; Haffez, H.; Elsayed, N.A.; El-Haggar, R.S.; Zaghary, W.A. New phenothiazine conjugates as apoptosis inducing agents: Design, synthesis, in-vitro anti-cancer screening and 131I-radiolabeling for in-vivo evaluation. Bioorg. Chem. 2023, 141, 106924. [Google Scholar] [CrossRef]
- Litvinov, V.P. The chemistry of 3-cyanopyridine-2(1H)-chalcogenones. Russ. Chem. Rev. 2006, 75, 577–599. [Google Scholar] [CrossRef]
- Litvinov, V.P. Partially hydrogenated pyridinechalcogenones. Russ. Chem. Bull. 1998, 47, 2053–2073. [Google Scholar] [CrossRef]
- Litvinov, V.P.; Krivokolysko, S.G.; Dyachenko, V.D. Synthesis and properties of 3-cyanopyridine-2(1H)-chalcogenones. Review. Chem. Heterocycl. Compd. 1999, 35, 509–540. [Google Scholar] [CrossRef]
- Gouda, M.A.; Berghot, M.A.; Abd El Ghani, G.E.; Khalil, A.E.G.M. Chemistry of 2-amino-3-cyanopyridines. Synth. Commun. 2014, 44, 297–330. [Google Scholar] [CrossRef]
- Salem, M.A.; Helel, M.H.; Gouda, M.A.; Ammar, Y.A.; El-Gaby, M.S.A. Overview on the synthetic routes to nicotine nitriles. Synth. Commun. 2018, 48, 345–374. [Google Scholar] [CrossRef]
- Gouda, M.A.; Hussein, B.H.; Helal, M.H.; Salem, M.A. A Review: Synthesis and medicinal importance of nicotinonitriles and their analogous. J. Heterocycl. Chem. 2018, 55, 1524–1553. [Google Scholar] [CrossRef]
- Gouda, M.A.; Attia, E.; Helal, M.H.; Salem, M.A. Recent progress on nicotinonitrile scaffold-based anticancer, antitumor, and antimicrobial agents: A literature review. J. Heterocycl. Chem. 2018, 55, 2224–2250. [Google Scholar] [CrossRef]
- Hassan, H.; Hisham, M.; Osman, M.; Hayallah, A. Nicotinonitrile as an essential scaffold in medicinal chemistry: An updated review. J. Adv. Biomed. Pharm. Sci. 2023, 6, 1–11. [Google Scholar] [CrossRef]
- Anwer, K.E.; Sayed, G.H. Synthesis and reactions of 2-amino-3-cyanopyridine derivatives (A Review). Russ. J. Org. Chem. 2024, 60, 2170–2227. [Google Scholar] [CrossRef]
- Khlus, A.V.; Egorov, D.M. Synthetic approaches to 2-aminopyridine-3-carbonitriles (A Review). Russ. J. Org. Chem. 2025, 61, 195–211. [Google Scholar] [CrossRef]
- Mekky, A.E.M.; Sanad, S.M.H. [3+2] Cycloaddition Synthesis of new (nicotinonitrile-chromene)-based bis(pyrazole) hybrids as potential acetylcholinesterase inhibitors. J. Heterocycl. Chem. 2023, 60, 156–160. [Google Scholar] [CrossRef]
- Ashmawy, F.O.; Gomha, S.M.; Abdallah, M.A.; Zaki, M.E.A.; Al-Hussain, S.A.; El-desouky, M.A. Synthesis, in vitro evaluation and molecular docking studies of novel thiophenyl thiazolyl-pyridine hybrids as potential anticancer agents. Molecules 2023, 28, 4270. [Google Scholar] [CrossRef]
- Ali, S.S.; Nafie, M.S.; Farag, H.A.; Amer, A.M. Anticancer potential of nicotinonitrile derivatives as PIM-1 kinase inhibitors through apoptosis: In vitro and in vivo studies. Med. Chem. Res. 2025, 34, 1074–1088. [Google Scholar] [CrossRef]
- Bardasov, I.N.; Ievlev, M.Y.; Chunikhin, S.S.; Alekseeva, A.U.; Ershov, O.V. Synthesis and photophysical properties of novel nicotinonitrile-based chromophores of 1,4-diarylbuta-1,3-diene series. Dye. Pigment. 2023, 217, 111432. [Google Scholar] [CrossRef]
- Ketova, E.S.; Myazina, A.V.; Bibik, E.Y.; Krivokolysko, S.G. New compounds with a dihydropyridine framework as promising hypolipidemic and hepatoprotective agents. Res. Results Pharmacol. 2024, 10, 61–71. [Google Scholar] [CrossRef]
- Tilchenko, D.A.; Bibik, E.Y.; Dotsenko, V.V.; Krivokolysko, S.G.; Frolov, K.A.; Aksenov, N.A.; Aksenova, I.V. Synthesis and hypoglycemic activity of new nicotinonitrile-furan molecular hybrids. Russ. J. Bioorg. Chem. 2024, 50, 554–570. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Krivokolysko, B.S.; Bibik, E.Y.; Frolov, K.A.; Aksenov, N.A.; Aksenova, I.V.; Krivokolysko, S.G. Synthesis and in vivo evaluation of hepatoprotective effects of novel sulfur-containing 1,4-dihydropyridines and 1,2,3,4-tetrahydropyridines. Curr. Bioact. Compd. 2023, 19, e171022210054. [Google Scholar] [CrossRef]
- Krivokolysko, D.S.; Dotsenko, V.V.; Bibik, E.Y.; Samokish, A.A.; Venidiktova, Y.S.; Frolov, K.A.; Krivokolysko, S.G.; Vasilin, V.K.; Pankov, A.A.; Aksenov, N.A.; et al. New 4-(2-furyl)-1,4-dihydronicotinonitriles and 1,4,5,6-tetrahydronicotinonitriles: Synthesis, structure, and analgesic activity. Russ. J. Gen. Chem. 2021, 91, 1646–1660. [Google Scholar] [CrossRef]
- Krivokolysko, D.S.; Dotsenko, V.V.; Bibik, E.Y.; Myazina, A.V.; Krivokolysko, S.G.; Vasilin, V.K.; Pankov, A.A.; Aksenov, N.A.; Aksenova, I.V. Synthesis, structure, and analgesic activity of 4-(5-cyano-{4-(fur-2-yl)-1,4- dihydropyridin-3-yl}carboxamido)benzoic acids ethyl esters. Russ. J. Gen. Chem. 2021, 91, 2588–2605. [Google Scholar] [CrossRef]
- Krivokolysko, D.S.; Dotsenko, V.V.; Bibik, E.Y.; Samokish, A.A.; Venidiktova, Y.S.; Frolov, K.A.; Krivokolysko, S.G.; Pankov, A.A.; Aksenov, N.A.; Aksenova, I.V. New hybrid molecules based on sulfur-containing nicotinonitriles: Synthesis, analgesic activity in acetic acid-induced writhing test, and molecular docking studies. Russ. J. Bioorg. Chem. 2022, 48, 628–635. [Google Scholar] [CrossRef]
- Bibik, I.V.; Bibik, E.Y.; Pankov, A.A.; Frolov, K.A.; Dotsenko, V.V.; Krivokolysko, S.G. Study of anti-inflammatory and antinociceptive properties of new derivatives of condensed 3-aminothieno[2,3-b]pyridines and 1,4-dihydropyridines. Acta Biomed. Sci. 2023, 8, 220–233. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Jassim, N.T.; Temerdashev, A.Z.; Abdul-Hussein, Z.R.; Aksenov, N.A.; Aksenova, I.V. New 6′-amino-5′-cyano-2-oxo-1,2-dihydro-1′H-spiro[indole-3,4′-pyridine]-3′-carboxamides: Synthesis, reactions, molecular docking studies and biological activity. Molecules 2023, 28, 3161. [Google Scholar] [CrossRef]
- Dyadyuchenko, L.V.; Dmitrieva, I.G.; Aksenov, N.A.; Dotsenko, V.V. Synthesis, structure, and biological activity of 2,6-diazido-4-methylnicotinonitrile derivatives. Chem. Heterocycl. Compd. 2018, 54, 964–970. [Google Scholar] [CrossRef]
- Al-Wahaibi, L.H.; Abou-Zied, H.A.; Hisham, M.; Beshr, E.A.M.; Youssif, B.G.M.; Bräse, S.; Hayallah, A.M.; Abdel-Aziz, M. Design, synthesis, and biological evaluation of novel 3-cyanopyridone/pyrazoline hybrids as potential apoptotic antiproliferative agents targeting EGFR/BRAFV600E inhibitory pathways. Molecules 2023, 28, 6586. [Google Scholar] [CrossRef]
- Litvinov, V.P.; Dotsenko, V.V.; Krivokolysko, S.G. Thienopyridines: Synthesis, properties, and biological activity. Russ. Chem. Bull. 2005, 54, 864–904. [Google Scholar] [CrossRef]
- Litvinov, V.P.; Dotsenko, V.V.; Krivokolysko, S.G. The chemistry of thienopyridines. Adv. Heterocycl. Chem. 2007, 93, 117–178. [Google Scholar]
- El-Sayed, H.A. Heterocyclization of ethyl 3-amino-4,6-dimethylthieno[2,3-b]pyridine-2-carboxylate (Review). J. Iran. Chem. Soc. 2014, 11, 131–145. [Google Scholar] [CrossRef]
- Salem, M.A.; Abu-Hashem, A.A.; Abdelgawad, A.A.M.; Gouda, M.A. Synthesis and reactivity of thieno[2,3-b]quinoline derivatives (part II). J. Heterocycl. Chem. 2021, 58, 1705–1740. [Google Scholar] [CrossRef]
- Shaw, R.; Tewari, R.; Yadav, M.; Pandey, E.; Tripathi, K.; Rani, J.; Althagafi, I.; Pratap, R. Recent advancements in the synthesis of fused thienopyridines and their therapeutic applications. Eur. J. Med. Chem. Rep. 2024, 12, 100185. [Google Scholar] [CrossRef]
- Anighoro, A.; Pinzi, L.; Marverti, G.; Bajorath, J.; Rastelli, G. Heat Shock Protein 90 and Serine/Threonine Kinase B-Raf Inhibitors have overlapping chemical space. RSC Adv. 2017, 7, 31069–31074. [Google Scholar] [CrossRef]
- Masch, A.; Kunick, C. Selective Inhibitors of Plasmodium falciparum Glycogen Synthase-3 (PfGSK-3): New antimalarial agents? Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2015, 1854, 1644–1649. [Google Scholar] [CrossRef]
- Schweda, S.I.; Alder, A.; Gilberger, T.; Kunick, C. 4-Arylthieno[2,3-b]pyridine-2-carboxamides are a new class of antiplasmodial agents. Molecules 2020, 25, 3187. [Google Scholar] [CrossRef]
- Masch, A.; Nasereddin, A.; Alder, A.; Bird, M.J.; Schweda, S.I.; Preu, L.; Doerig, C.; Dzikowski, R.; Gilberger, T.W.; Kunick, C. Structure–Activity Relationships in a series of antiplasmodial thieno[2,3-b]pyridines. Malar. J. 2019, 18, 89. [Google Scholar] [CrossRef]
- Fugel, W.; Oberholzer, A.E.; Gschloessl, B.; Dzikowski, R.; Pressburger, N.; Preu, L.; Pearl, L.H.; Baratte, B.; Ratin, M.; Okun, I.; et al. 3,6-Diamino-4-(2-halophenyl)-2-benzoylthieno[2,3-b]pyridine-5-carbonitriles are selective inhibitors of Plasmodium falciparum glycogen synthase kinase-3. J. Med. Chem. 2013, 56, 264–275. [Google Scholar] [CrossRef]
- Nkomba, G.; Terre’Blanche, G.; Janse van Rensburg, H.D.; Legoabe, L.J. Design, synthesis and evaluation of amino-3,5-dicyanopyridines and thieno[2,3-b]pyridines as ligands of adenosine A1 Receptors for the potential treatment of epilepsy. Med. Chem. Res. 2022, 31, 1277–1297. [Google Scholar] [CrossRef]
- Betti, M.; Catarzi, D.; Varano, F.; Falsini, M.; Varani, K.; Vincenzi, F.; Dal Ben, D.; Lambertucci, C.; Colotta, V. The aminopyridine-3,5-dicarbonitrile core for the design of new non-nucleoside-like agonists of the human adenosine A2B Receptor. Eur. J. Med. Chem. 2018, 150, 127–139. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Krivokolysko, S.G.; Chernega, A.N.; Litvinov, V.P. Anilinomethylidene derivatives of cyclic 1,3-dicarbonyl compounds in the synthesis of new sulfur-containing pyridines and quinolines. Russ. Chem. Bull. 2002, 51, 1556–1561. [Google Scholar] [CrossRef]
- Dayam, R.; Al-Mawsawi, L.Q.; Zawahir, Z.; Witvrouw, M.; Debyser, Z.; Neamati, N. Quinolone 3-Carboxylic Acid Pharmacophore: Design of Second Generation HIV-1 Integrase Inhibitors. J. Med. Chem. 2008, 51, 1136–1144. [Google Scholar] [CrossRef]
- Nagarajan, S.; Doddareddy, M.; Choo, H.; Cho, Y.S.; Oh, K.S.; Lee, B.H.; Pae, A.N. IKKβ Inhibitors Identification Part I: Homology Model Assisted Structure Based Virtual Screening. Bioorg. Med. Chem. 2009, 17, 2759–2766. [Google Scholar] [CrossRef] [PubMed]
- Mermerian, A.H.; Case, A.; Stein, R.L.; Cuny, G.D. Structure–Activity relationship, kinetic mechanism, and selectivity for a new class of ubiquitin c-terminal hydrolase-L1 (UCH-L1) inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 3729–3732. [Google Scholar] [CrossRef] [PubMed]
- Sorci, L.; Pan, Y.; Eyobo, Y.; Rodionova, I.; Huang, N.; Kurnasov, O.; Zhong, S.; MacKerell, A.D.; Zhang, H.; Osterman, A.L. Targeting NAD biosynthesis in bacterial pathogens: Structure-based development of inhibitors of nicotinate mononucleotide adenylyltransferase NadD. Chem. Biol. 2009, 16, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zheng, R.; Zhou, Y.; Peng, F.; Lin, H.; Bu, Q.; Mao, Y.; Yu, L.; Yang, L.; Yang, S.; et al. Small molecular anticancer agent SKLB703 induces apoptosis in human hepatocellular carcinoma cells via the mitochondrial apoptotic pathway in vitro and inhibits tumor growth in vivo. Cancer Lett. 2011, 313, 44–53. [Google Scholar] [CrossRef]
- Nikkhoo, A.R.; Miri, R.; Arianpour, N.; Firuzi, O.; Ebadi, A.; Salarian, A.A. Cytotoxic activity assessment and C-Src Tyrosine kinase docking simulation of thieno[2,3-b]pyridine-based derivatives. Med. Chem. Res. 2014, 23, 1225–1233. [Google Scholar] [CrossRef]
- Leung, E.; Hung, J.M.; Barker, D.; Reynisson, J. The effect of a thieno[2,3-b]pyridine PLC-γ inhibitor on the proliferation, morphology, migration and cell cycle of breast cancer cells. Med. Chem. Commun. 2013, 5, 99–106. [Google Scholar] [CrossRef]
- Arabshahi, H.J.; Leung, E.; Barker, D.; Reynisson, J. The development of thieno[2,3-b]pyridine analogues as anticancer agents applying in silico methods. MedChemComm 2014, 5, 186. [Google Scholar] [CrossRef]
- Zafar, A.; Pilkington, L.; Haverkate, N.; van Rensburg, M.; Leung, E.; Kumara, S.; Denny, W.; Barker, D.; Alsuraifi, A.; Hoskins, C.; et al. Investigation into improving the aqueous solubility of the thieno[2,3-b]pyridine anti-proliferative agents. Molecules 2018, 23, 145. [Google Scholar] [CrossRef] [PubMed]
- Arabshahi, H.J.; van Rensburg, M.; Pilkington, L.I.; Jeon, C.Y.; Song, M.; Gridel, L.M.; Leung, E.; Barker, D.; Vuica-Ross, M.; Volcho, K.P.; et al. A Synthesis, in silico, in vitro and in vivo study of thieno[2,3-b]pyridine anticancer analogues. MedChemComm 2015, 6, 1987–1997. [Google Scholar] [CrossRef]
- Binsaleh, N.K.; Wigley, C.A.; Whitehead, K.A.; van Rensburg, M.; Reynisson, J.; Pilkington, L.I.; Barker, D.; Jones, S.; Dempsey-Hibbert, N.C. Thieno[2,3-b]pyridine derivatives are potent anti-platelet drugs, inhibiting platelet activation, aggregation and showing synergy with aspirin. Eur. J. Med. Chem. 2018, 143, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.P.; Fleck, R.; Brickwood, J.; Capolino, A.; Catron, K.; Chen, Z.; Cywin, C.; Emeigh, J.; Foerst, M.; Ginn, J.; et al. The discovery of thienopyridine analogues as potent IκB kinase β inhibitors. Part II. Bioorg. Med. Chem. Lett. 2009, 19, 5547–5551. [Google Scholar] [CrossRef]
- Mekky, A.E.M.; Sanad, S.M.H.; Said, A.Y.; Elneairy, M.A.A. Synthesis, cytotoxicity, in-vitro antibacterial screening and in-silico study of novel thieno[2,3-b]pyridines as potential pim-1 inhibitors. Synth. Commun. 2020, 50, 2376–2389. [Google Scholar] [CrossRef]
- Laudette, M.; Coluccia, A.; Sainte-Marie, Y.; Solari, A.; Fazal, L.; Sicard, P.; Silvestri, R.; Mialet-Perez, J.; Pons, S.; Ghaleh, B.; et al. Identification of a pharmacological inhibitor of Epac1 that protects the heart against acute and chronic models of cardiac stress. Cardiovasc. Res. 2019, 115, 1766–1777. [Google Scholar] [CrossRef]
- Li, X.D.; Liu, L.; Cheng, L. Identification of thienopyridine carboxamides as selective binders of HIV-1 trans activation response (TAR) and Rev Response Element (RRE) RNAs. Org. Biomol. Chem. 2018, 16, 9191–9196. [Google Scholar] [CrossRef]
- Bakhite, E.A.; Gad, M.A.; Khamies, E.; Thagfan, F.A.; Mohamed, R.A.E.H.; Bakry, M.M.S. Exploration of some thieno[2,3-b]pyridines, thieno[3,2-d]pyrimidinones, and thieno[3,2-d][1,2,3]triazinones as insecticidal agents against Aonidiella aurantii. Russ. J. Bioorg. Chem. 2025, 51, 816–826. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Buryi, D.S.; Lukina, D.Y.; Stolyarova, A.N.; Aksenov, N.A.; Aksenova, I.V.; Strelkov, V.D.; Dyadyuchenko, L.V. Substituted N-(thieno[2,3-b]pyridine-3-yl)acetamides: Synthesis, reactions, and biological activity. Monatsh. Chem. 2019, 150, 1973–1985. [Google Scholar] [CrossRef]
- Sanad, S.M.H.; Mekky, A.E.M. Ultrasound-mediated synthesis of new (piperazine-chromene)-linked bis(thieno[2,3-b]pyridine) hybrids as potential anti-acetylcholinesterase. ChemistrySelect 2022, 7, e202203020. [Google Scholar] [CrossRef]
- Alenazi, N.A.; Alharbi, H.; Fawzi Qarah, A.; Alsoliemy, A.; Abualnaja, M.M.; Karkashan, A.; Abbas, B.; El-Metwaly, N.M. New thieno[2,3-b]pyridine-based compounds: Synthesis, molecular modelling, antibacterial and antifungal activities. Arab. J. Chem. 2023, 16, 105226. [Google Scholar] [CrossRef]
- Xu, L.; Mu, X.; Liu, M.; Wang, Z.; Shen, C.; Mu, Q.; Feng, B.; Xu, Y.; Hou, T.; Gao, L.; et al. Novel Thieno[2,3-b]quinoline-procaine hybrid molecules: A new class of allosteric SHP-1 activators evolved from PTP1B Inhibitors. Chin. Chem. Lett. 2023, 34, 108063. [Google Scholar] [CrossRef]
- Dyachenko, V.D.; Dyachenko, I.V.; Nenajdenko, V.G. Cyanothioacetamide: A polyfunctional reagent with broad synthetic utility. Russ. Chem. Rev. 2018, 87, 1. [Google Scholar] [CrossRef]
- Sharanin, Y.A.; Rodinovskaya, L.A.; Litvinov, V.P.; Promonenkov, V.K.; Mortikov, V.Y.; Shestopalov, A.M. Reactions of arylidenecyanothioacetamides with carbonyl compounds and their enamines. J. Org. Chem. USSR 1985, 21, 619–620. [Google Scholar]
- Sharanin, Y.A.; Litvinov, V.P.; Shestopalov, A.M.; Nesterov, V.N.; Struchkov, Y.T.; Shklover, V.E.; Promonenkov, V.K.; Mortikov, V.Y. Structure of 4-amino-6-phenyl-5-cyano-2-cyclohexanespiro-1,3-dithia-4-cyclohexene and its recyclization to 5,6-tetramethylene-4-phenyl-3-cyano-2[1H]Pyridinethione. Bull. Acad. Sci. USSR Div. Chem. Sci. 1985, 34, 1619–1625. [Google Scholar] [CrossRef]
- Litvinov, V.P.; Promonenkov, V.K.; Sharanin, Y.A.; Shestopalov, A.M.; Rodinovskaya, L.A.; Mortikov, V.Y.; Bogdanov, V.S. Condensed pyridines communication 3. Arylidenethio(seleno)acetamides in the synthesis of 4-aryl-3-cyano- 2[1H]pyridinethiones and 4-aryl-3-cyano-2[1H]pyridineselenones. Bull. Acad. Sci. USSR Div. Chem. Sci. 1985, 34, 1940–1947. [Google Scholar] [CrossRef]
- Kindop, V.K.; Bespalov, A.V.; Dotsenko, V.V.; Strelkov, V.D.; Lukina, D.Y.; Baichurin, R.I.; Paronikyan, E.G.; Harutyunyan, A.S.; Ovcharov, S.N.; Aksenov, N.A.; et al. Synthesis and structural characterization of 2-iminothiazoline/quinoline and 2-iminothiazoline/thieno[2,3-b]quinoline molecular hybrids with herbicide safening properties. Tetrahedron 2025, 186, 134889. [Google Scholar] [CrossRef]
- Narushyavichus, É.V.; Garalene, V.N.; Krauze, A.A.; Dubur, G.Y. Cardiotropic activity of pyridin-2(1H)-ones. Pharm. Chem. J. 1989, 23, 983–986. [Google Scholar] [CrossRef]
- Frolova, N.G.; Zav’yalova, V.K.; Litvinov, V.P. Synthesis of 4,5,6-trisubstituted 3-cyanopyridine-2(1H)-thiones based on α-substituted β-diketones. Russ. Chem. Bull. 1996, 45, 2578–2580. [Google Scholar] [CrossRef]
- Hussain, B.A.; Attia, A.M.; Elgemeie, G.E.H. Synthesis of N-glycosylated pyridines as new antimetabolite agents. Nucleosides Nucleotides 1999, 18, 2335–2343. [Google Scholar] [CrossRef]
- Rodinovskaya, L.A.; Belukhina, E.V.; Shestopalov, A.M.; Litvinov, V.P. Regioselective synthesis of 5,6-polymethylene-3-cyanopyridine-2(1H)-thiones and fused heterocycles based on them. Russ. Chem. Bull. 1994, 43, 449–457. [Google Scholar] [CrossRef]
- Marcu, A.; Schurigt, U.; Müller, K.; Moll, H.; Krauth-Siegel, R.L.; Prinz, H. Inhibitory effect of phenothiazine- and phenoxazine-derived chloroacetamides on Leishmania major growth and Trypanosoma brucei trypanothione reductase. Eur. J. Med. Chem. 2016, 108, 436–443. [Google Scholar] [CrossRef]
- Khelwati, H.; van Geelen, L.; Kalscheuer, R.; Müller, T.J.J. Synthesis, electronic, and antibacterial properties of 3,7-di(hetero)aryl-substituted phenothiazinyl N-propyl trimethylammonium salts. Molecules 2024, 29, 2126. [Google Scholar] [CrossRef]
- Nizi, M.G.; Desantis, J.; Nakatani, Y.; Massari, S.; Mazzarella, M.A.; Shetye, G.; Sabatini, S.; Barreca, M.L.; Manfroni, G.; Felicetti, T.; et al. Antitubercular polyhalogenated phenothiazines and phenoselenazine with reduced binding to CNS Receptors. Eur. J. Med. Chem. 2020, 201, 112420. [Google Scholar] [CrossRef]
- Kindop, V.K.; Kindop, V.K.; Dotsenko, V.V.; Lukina, D.Y.; Aksenov, N.A.; Aksenova, I.V. The unexpected result of the Thorpe-Ziegler heterocyclization of N-[(3-cyanoquinolin-2-yl)thio]acetylphenothiazines promoted by KOH–CH3OH. Russ. Chem. Bull. 2025; accepted. [Google Scholar]
- Gruner, M.; Rehwald, M.; Eckert, K.; Gewald, K. New syntheses of 2-alkylthio-4-oxo-3,4-dihydroquinazolines, 2-alkylthio- quinazolines, as well as their hetero analogues. Heterocycles 2000, 53, 2363–2377. [Google Scholar] [CrossRef]
- Zaki, R.M.; Kamal El-Dean, A.M.; Radwan, S.M.; Ammar, M.A. Efficient synthesis, reactions and anti-inflammatory evaluation of novel cyclopenta[d]thieno[2,3-b]pyridines and their related heterocycles. Russ. J. Bioorg. Chem. 2022, 48, S121–S135. [Google Scholar] [CrossRef]
- Zaki, R.M.; Radwan, S.M.; El-Dean, A.M.K. Synthesis and reactions of 1-amino-5-morpholin-4-yl- 6,7,8,9-tetrahydrothieno[2,3-c]isoquinoline. J. Chin. Chem. Soc. 2011, 58, 544–554. [Google Scholar] [CrossRef]
- El-Mariah, F. Thieno[2,3-c]pyridazine derivatives: Synthesis and antimicrobial activity. Phosphorus Sulfur Silicon Relat. Elem. 2008, 183, 2795–2806. [Google Scholar] [CrossRef]
- Regal, M.K.A.; Rafat, E.H.; El-Sattar, N.E.A.A. Synthesis, characterization, and dyeing performance of some azo thienopyridine and thienopyrimidine dyes based on wool and nylon. J. Heterocycl. Chem. 2020, 57, 1173–1182. [Google Scholar] [CrossRef]
- Abu El-Azm, F.S.M.; Ali, A.T.; Hekal, M.H. Facile synthesis and anticancer activity of novel 4-aminothieno[2,3-d]pyrimidines and triazolothienopyrimidines. Org. Prep. Proced. Int. 2019, 51, 507–520. [Google Scholar] [CrossRef]
- Saravanan, G.; Selvaraju, R.; Nagarajan, S. Synthesis of novel 2-iminothiazolidin-4-ones. Synth. Commun. 2012, 42, 3361–3367. [Google Scholar] [CrossRef]
- Tikhonov, D.S.; Gordiy, I.; Iakovlev, D.A.; Gorislav, A.A.; Kalinin, M.A.; Nikolenko, S.A.; Malaskeevich, K.M.; Yureva, K.; Matsokin, N.A.; Schnell, M. Harmonic scale factors of fundamental transitions for dispersion-corrected quantum chemical methods. ChemPhysChem 2024, 25, e202400547. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Sander, T. OSIRIS Property Explorer; Idorsia Pharmaceuticals Ltd.: Basel-Landschaft, Switzerland. Available online: http://www.organic-chemistry.org/prog/peo/ (accessed on 15 September 2025).
- Gu, Y.; Yu, Z.; Wang, Y.; Chen, L.; Lou, C.; Yang, C.; Li, W.; Liu, G.; Tang, Y. AdmetSAR3.0: A comprehensive platform for exploration, prediction and optimization of chemical ADMET properties. Nucl. Acids Res. 2024, 52, W432–W438. [Google Scholar] [CrossRef]
- GalaxyWEB. A Web Server for Protein Structure Prediction, Refinement, and Related Methods. Computational Biology Lab, Department of Chemistry, Seoul National University, S. Korea. Available online: http://galaxy.seoklab.org/index.html (accessed on 15 September 2025).
- Yang, J.; Kwon, S.; Bae, S.H.; Park, K.M.; Yoon, C.; Lee, J.H.; Seok, C. GalaxySagittarius: Structure- and similarity-based prediction of protein targets for druglike compounds. J. Chem. Inf. Model. 2020, 60, 3246–3254. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- UCSF Chimera. Visualization System for Exploratory Research and Analysis Developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, US. Available online: https://www.rbvi.ucsf.edu/chimera/ (accessed on 15 September 2025).
- Dotsenko, V.V.; Krivokolysko, S.G.; Polovinko, V.V.; Litvinov, V.P. On the regioselectivity of the reaction of cyanothioacetamide with 2-acetylcyclohexanone, 2-acetylcyclopentanone, and 2-acetyl-1-(morpholin-4-yl)-1-cycloalkenes. Chem. Heterocycl. Compd. 2012, 48, 309–319. [Google Scholar] [CrossRef]
- Sharanin, Y.A.; Shestopalov, A.M.; Promonenkov, V.K.; Rodinovskaya, L.A. Cyclization of nitriles. X. Enamino nitriles of the 1,3-dithia-4-cyclohexene series and their recyclization to derivatives of pyridine and thiazole. J. Org. Chem. USSR 1984, 20, 1402–1415. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. The ORCA Program System. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software Update: The ORCA Program System—Version 6.0. WIREs Comput. Mol. Sci. 2025, 15, e70019. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 2002, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 2002, 37, 785–789. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar] [CrossRef]
- de Souza, B. GOAT: A Global Optimization Algorithm for molecules and atomic clusters. Angew. Chem. Int. Ed. 2025, 64, e202500393. [Google Scholar] [CrossRef]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-XTB—An Accurate and broadly parametrized self-consistent tight-binding quantum chemical method with multipole electrostatics and density-dependent dispersion contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef]

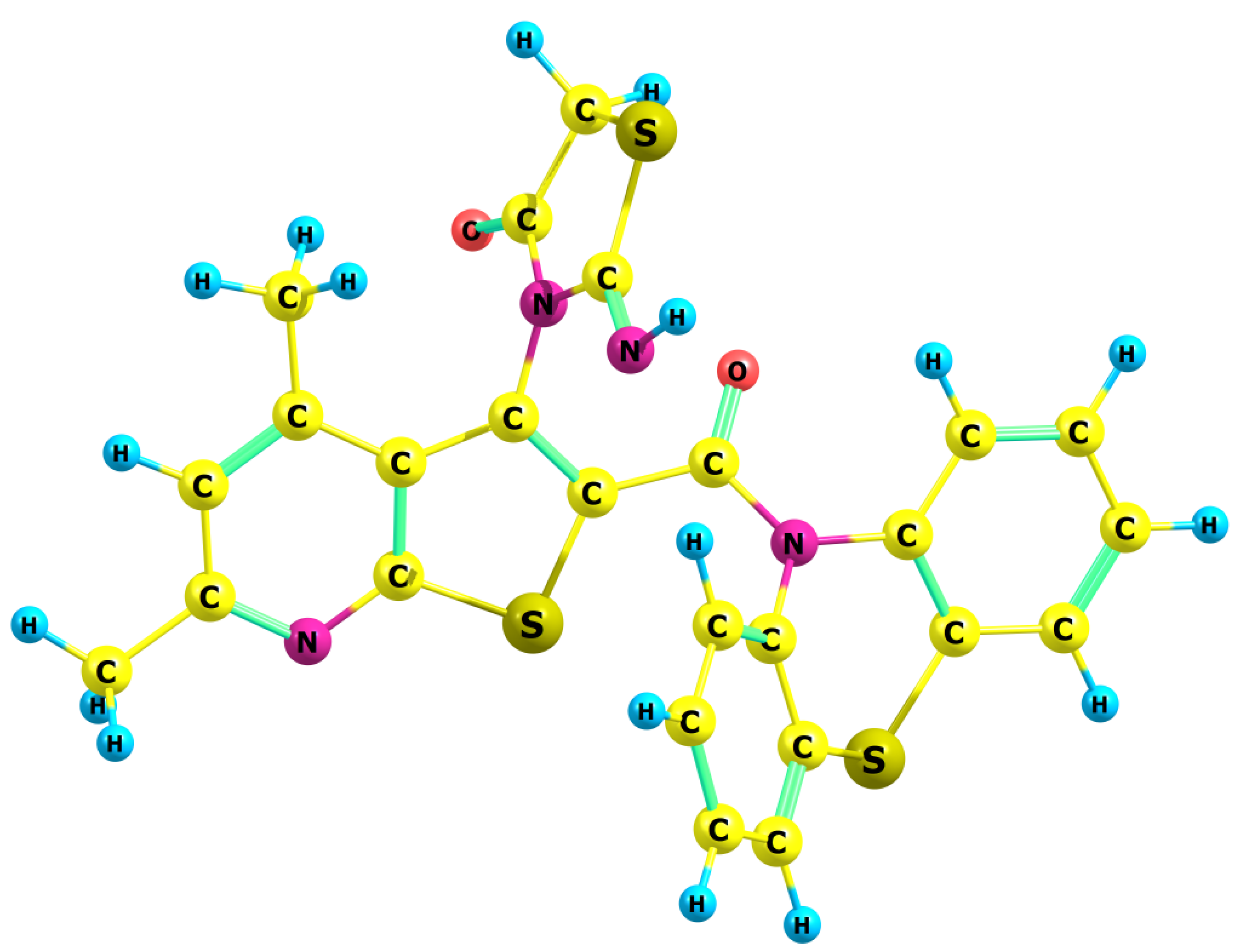

| Vibrations | Experimental Bands, cm−1 | Calculated Vibrational Frequencies, cm−1 | |
|---|---|---|---|
| No Correction Factors | With Correction Factors * | ||
| ν N-H | 3294 | 3503 | 3388 |
| ν C–H(Ar) | 3063 | 3196 | 3091 |
| νas CH3 | 2978 | 3113 | 3011 |
| νs CH3 | 2935 | 3037 | 2938 |
| ν C=O thiazolidinone | 1720 | 1805 | 1746 |
| ν C=O (amide I) | 1659 | 1701 | 1665 |
| ν C=N | 1624 | 1672 | 1637 |
| ν C–C(Ar) ThPy | 1585 | 1622 | 1588 |
| ν C–C(Ar) ThPy | 1555 | 1596 | 1562 |
| ν C–C(Ar) ThPy | 1524 | 1558 | 1525 |
| ν C–C(Ar) + δ C–H(Ar) PhTz | 1458 | 1502 | 1470 |
| ν C-N + δ CH3 | 1396 | 1423 | 1393 |
| Skeletal | 1331 | 1367 | 1338 |
| Skeletal | 1261 | 1299 | 1272 |
| Skeletal | 1211 | 1233 | 1207 |
| δ N-H + δ C–H(Ar) | 1126 | 1169 | 1144 |
| δ C–H(Ar) | 1030 | 1060 | 1038 |
| Skeletal | 918 | 938 | 918 |
| Skeletal | 895 | 907 | 888 |
| ν C-S PhTz | 868 | 878 | 860 |
| δ N-H | 787 | 795 | 778 |
| δ C–H(Ar) | 764 | 777 | 761 |
| Skeletal | 725 | 738 | 723 |
| Skeletal | 613 | 633 | 620 |
| MAPE **, % | - | 2.83 | 0.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dotsenko, V.V.; Kindop, V.K.; Kindop, V.K.; Daus, E.S.; Yudaev, I.V.; Daus, Y.V.; Bespalov, A.V.; Buryi, D.S.; Lukina, D.Y.; Aksenov, N.A.; et al. Synthesis of New Phenothiazine/3-cyanoquinoline and Phenothiazine/3-aminothieno[2,3-b]pyridine(-quinoline) Heterodimers. Int. J. Mol. Sci. 2025, 26, 9798. https://doi.org/10.3390/ijms26199798
Dotsenko VV, Kindop VK, Kindop VK, Daus ES, Yudaev IV, Daus YV, Bespalov AV, Buryi DS, Lukina DY, Aksenov NA, et al. Synthesis of New Phenothiazine/3-cyanoquinoline and Phenothiazine/3-aminothieno[2,3-b]pyridine(-quinoline) Heterodimers. International Journal of Molecular Sciences. 2025; 26(19):9798. https://doi.org/10.3390/ijms26199798
Chicago/Turabian StyleDotsenko, Victor V., Vladislav K. Kindop, Vyacheslav K. Kindop, Eva S. Daus, Igor V. Yudaev, Yuliia V. Daus, Alexander V. Bespalov, Dmitrii S. Buryi, Darya Yu. Lukina, Nicolai A. Aksenov, and et al. 2025. "Synthesis of New Phenothiazine/3-cyanoquinoline and Phenothiazine/3-aminothieno[2,3-b]pyridine(-quinoline) Heterodimers" International Journal of Molecular Sciences 26, no. 19: 9798. https://doi.org/10.3390/ijms26199798
APA StyleDotsenko, V. V., Kindop, V. K., Kindop, V. K., Daus, E. S., Yudaev, I. V., Daus, Y. V., Bespalov, A. V., Buryi, D. S., Lukina, D. Y., Aksenov, N. A., & Aksenova, I. V. (2025). Synthesis of New Phenothiazine/3-cyanoquinoline and Phenothiazine/3-aminothieno[2,3-b]pyridine(-quinoline) Heterodimers. International Journal of Molecular Sciences, 26(19), 9798. https://doi.org/10.3390/ijms26199798







