Zinc-Mediated Defenses Against Toxic Heavy Metals and Metalloids: Mechanisms, Immunomodulation, and Therapeutic Relevance
Abstract
1. Introduction
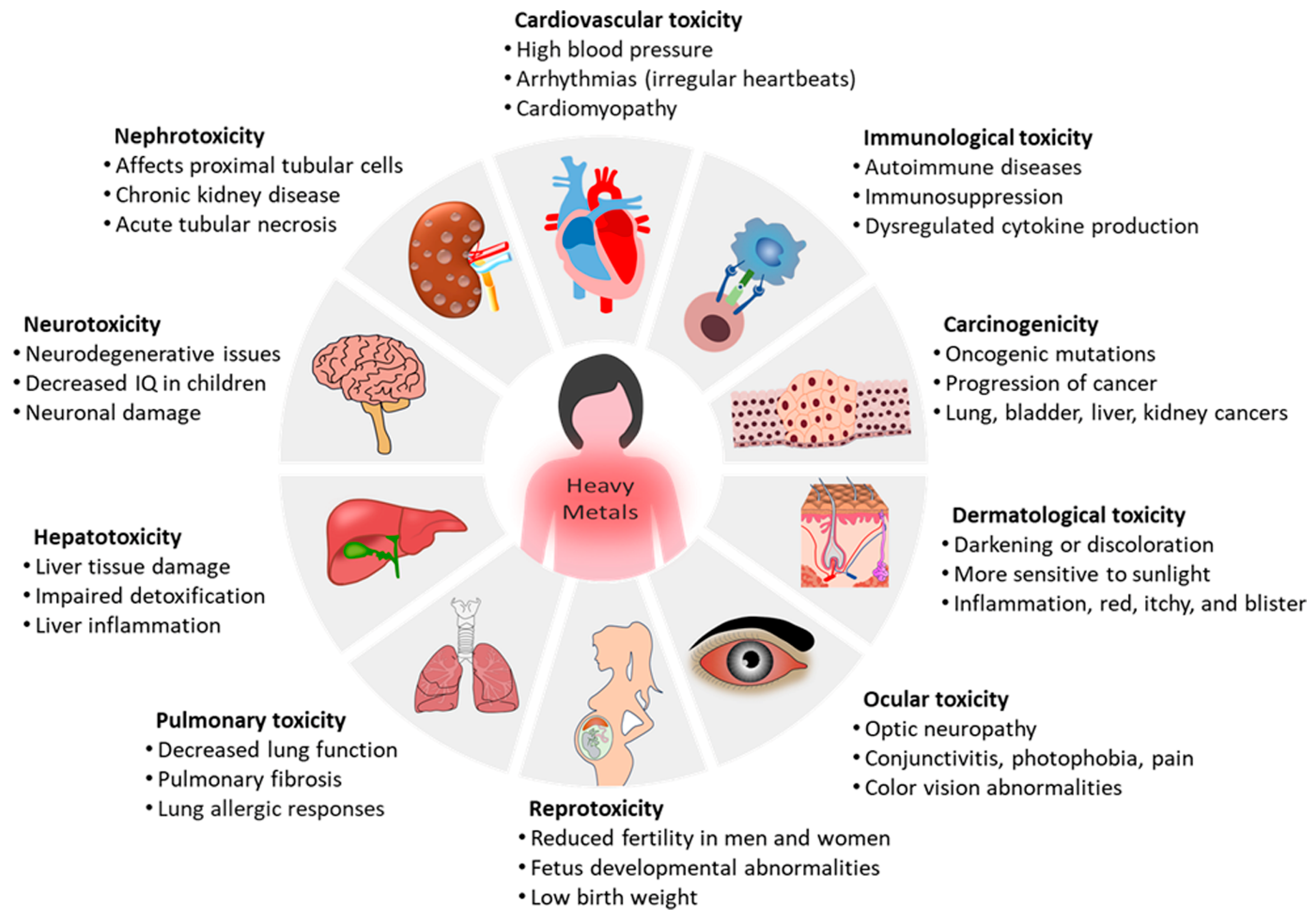
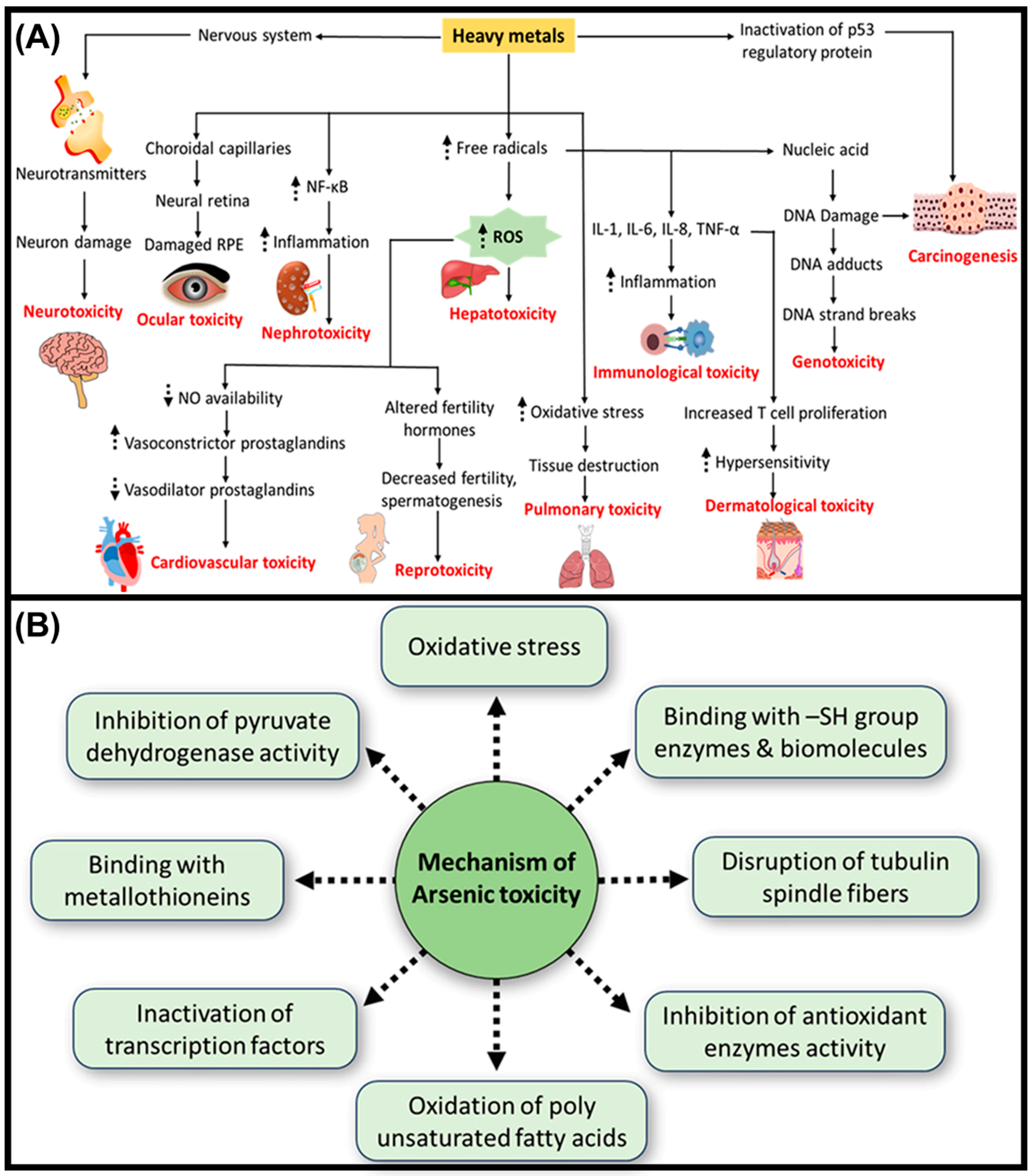
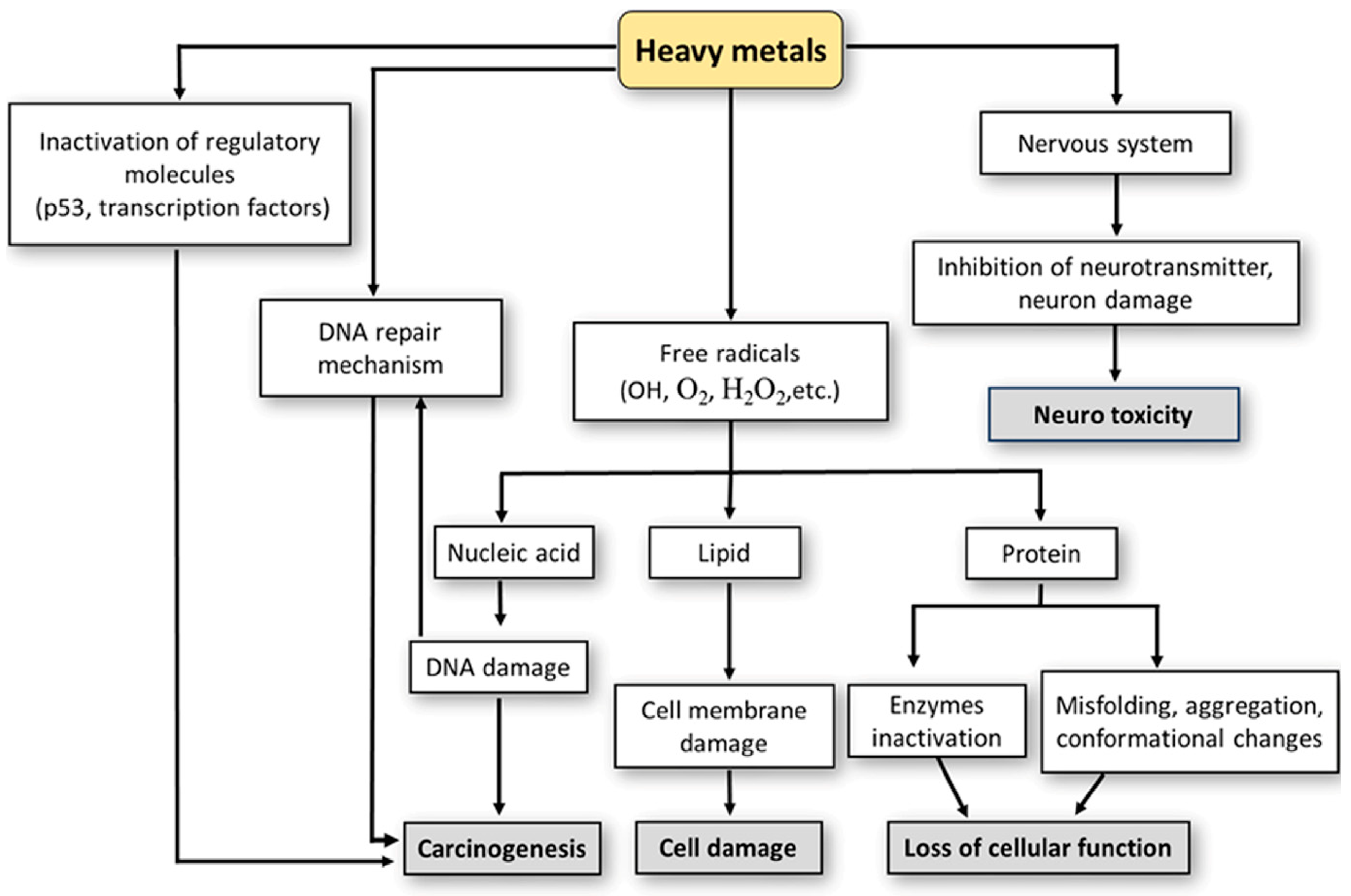
2. Suppressive Effects of Heavy Metals on the Immune System
3. Importance of Zinc in Biological Functions
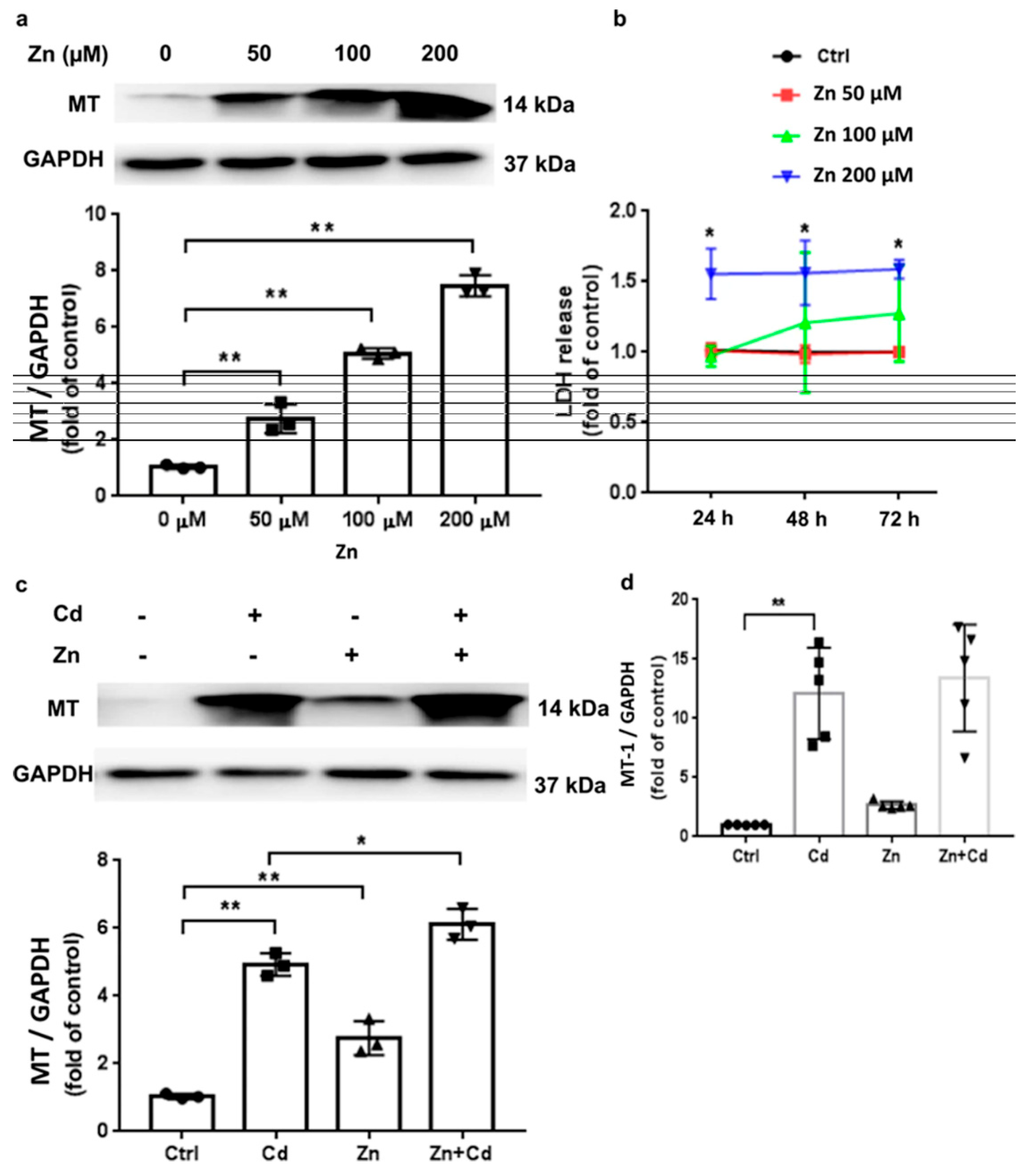
4. Zinc Deficiency and Its Adverse Effects: Significance of Zinc Supplementation in Human Health
- -
- Enhancing immune function to combat infections and diseases effectively, and thereby reducing respiratory tract infections (RTIs); cold, flu, sinusitis, pneumonia, and COVID-19.
- -
4.1. Zinc Supplementation in Cardiovascular Diseases
4.2. Zinc Supplementation in Neurodegenerative Disorders and Aging Process
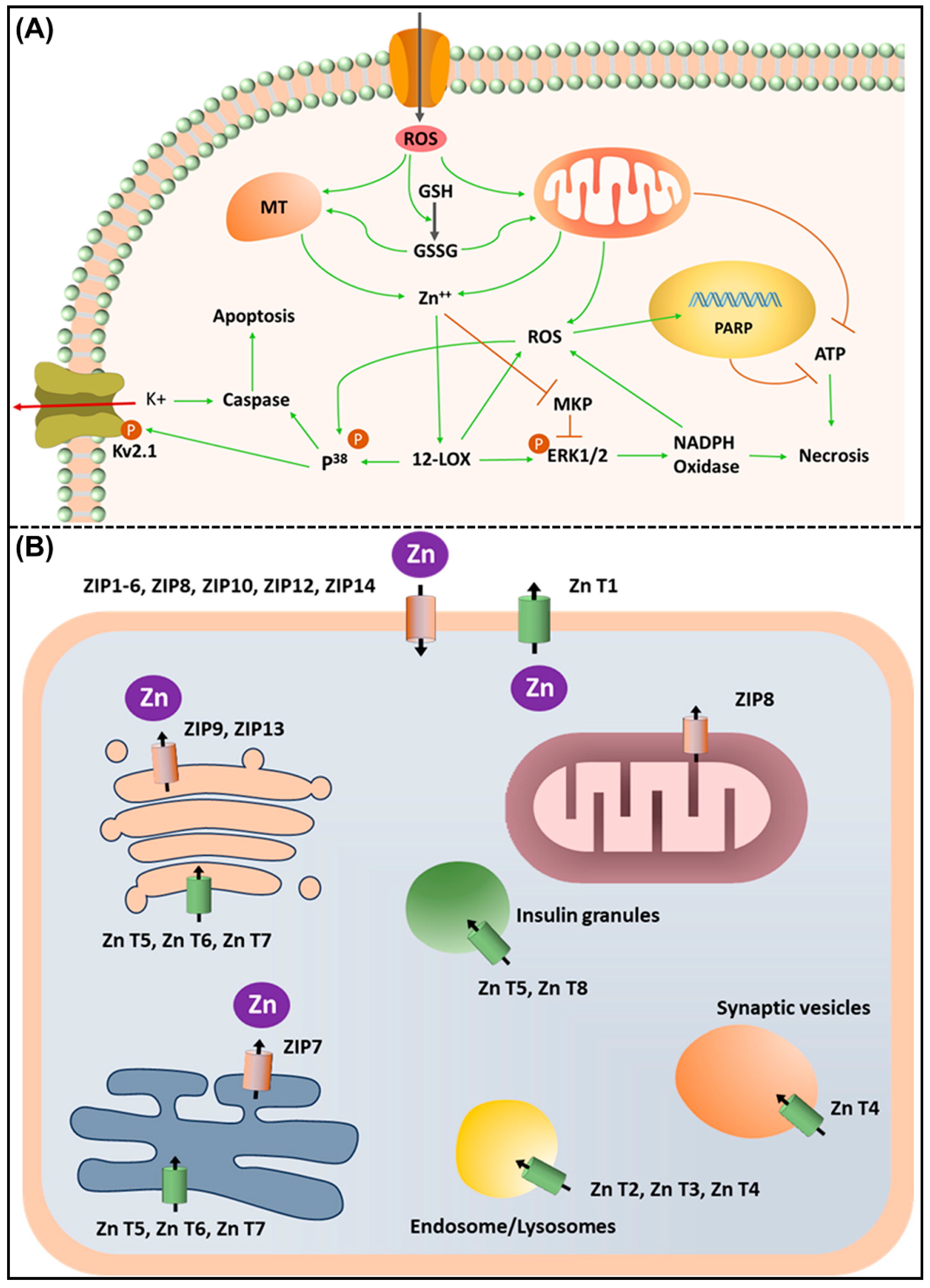
5. Role of Zinc, Zinc Importers, and Transporters in the Immune System and Associated Disorders
6. Dysregulation of ZIP Transporters in Cancer
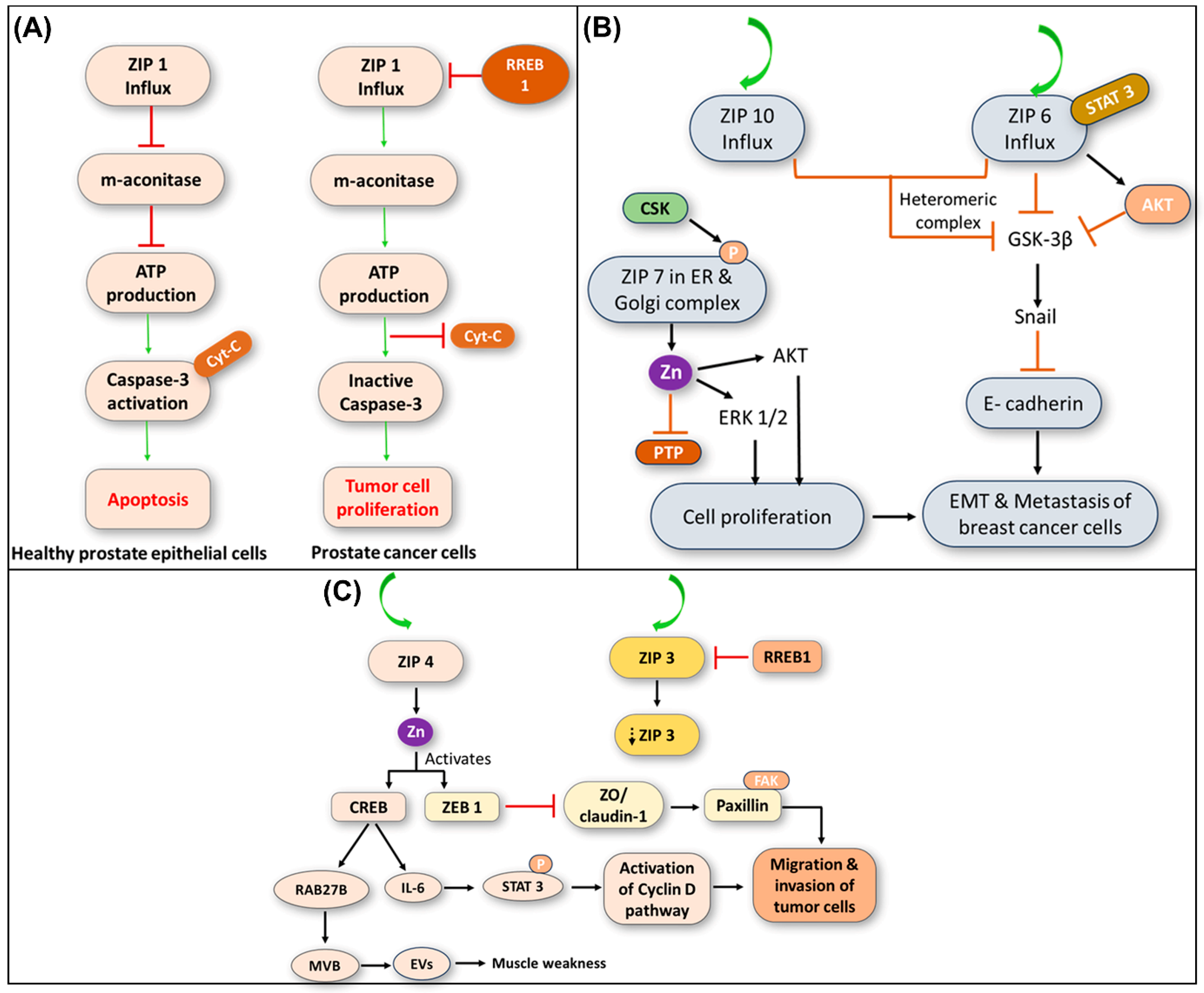
6.1. Role of ZIP1 in Prostate Cancer Development
6.2. Role of ZIP6, ZIP7, and ZIP10 in Breast Cancer Development
6.3. Role of ZIP4 in Pancreatic Cancer Development
- -
- Excess Zn accumulation mediated by ZIP4 overexpression may also affect the tumor microenvironment, consequently leading to induced inflammation and suppressed immunity, and finally, facilitating a conducive milieu for substantial cancer cell proliferation.
- -
- -
- The ZIP4-mediated Zn influx activates ZF-transcription factors, facilitating the release of cancer-associated mutant genes from extracellular vesicles (EVs) through Ras-related protein Rab-27B (RAB27B)-mediated activation. Additionally, ZIP4 inhibits tight junction proteins such as zona occludens-1 (ZO-1) and claudin-1 via ZF E-box binding homeobox 1 (ZEB1), leading to increased tumor cell movement and motility through EMT (Figure 5C) [124,125].
- -
- Targeting ZIP4 expression or its downstream signaling pathways presents a promising strategy for developing novel therapeutic interventions for pancreatic cancer. By disrupting Zn dysregulation mediated by ZIP4 overexpression, it may be possible to impede cancer progression and improve patient outcomes [126].
| ZIP Transporters (Importers) | Tissue Specificity | Functions | Mutation or Deficiency | Disorders | Ref |
|---|---|---|---|---|---|
| ZIP1 | Adults and fetal tissues | Rapid uptake and accumulation of Zn in prostate cells | Single or double ablation of ZIP1 | Prostate cancer; Abnormal embryonic development | [118] |
| ZIP2 | Prostate and uterine epithelial cells | Uptake of Zn; Contact inhibition of normal epithelial cells | Single or double ablation of ZIP2 | Abnormal embryonic development; Tumor genesis | [119] |
| ZIP3 | Bone marrow, spleen, small intestine, and liver | Responsible for Zn influx transporter | Single or double ablation of ZIP3 | Abnormal embryonic development | [122] |
| ZIP4 | Kidney, small intestine, stomach, colon, jejunum and duodenum | Regulates Zn homeostasis | Loss of function and targeted disruption of the ZIP4 genes | AE, TEWL, IgE, and Th1/Th2 balance | [124] |
| ZIP5 | Intestine, pancreas, liver, and kidney cells | Dietary uptake and homeostasis of Zn | Loss-of-function of ZIP5 gene | adHM | [125] |
| ZIP6 | Prostate, placenta, and mammary glands | Act as a metalloproteinase | Loss-of-function of ZIP6 gene | Placenta cancer and metastasis | [121] |
| ZIP7 | Mammary gland cells | Helps in Zn uptake | Loss-of-function of ZIP7 gene | Breast Cancer | [127] |
| ZIP8 | Fibroblasts and chondrocytes | Causes Cd transport and toxicity in fibroblasts and chondrocytes | Hypomorphic mutation of ZIP8,Zn influx into cartilage chondrocytes lead to MMPs, Non-synonymous variant in ZIP8 | Organ morphogenesis and hematopoiesis; Osteoarthritis due to IL-1β, IL-8 and TNF-α; MMPs and ROS; TH1 cytokines (IFN-γ, IL-2), and Schizophrenia | [128] |
| ZIP9 | Human lymphocytes | Activation of AKT in response to BCR activation | Lack of ZIP9 | Fecundity; Egg viability; Retardations in the offspring growth | [129] |
| ZIP10 | Mammary glands | Aid in the Zn influx | Lack of ZIP10 | Impaired B-cell development; HIR | [121] |
| ZIP11 | Testes, stomach, ileum, and cecum | For Zn transport | Lack of ZIP11 | Zn deficiency | [129] |
| ZIP12 | Brain and eye | For cellular Zn uptake | Targeted ZIP12 disruption | PH in hypoxic conditions; Possible schizophrenia | [121] |
| ZIP13 | Bone, teeth, and connective tissue | Maturation: osteoblasts/chondrocytes/fibroblasts | ZIP13 deficiency | EDS; SCD-EDS | [129] |
| ZIP14 | Mammalian cells | Cd transport and toxicity | ZIP14-deficiency homozygous loss-of-function mutations | Growth, bone metabolism, and gluconeogenesis; Dystonia-parkinsonism; Neurodegeneration with hypermanganesemia in childhood. | [129] |
| ZnT Transporters (Exporters) | Tissue Specificity | Functions | Mutations | Disorders | Ref |
|---|---|---|---|---|---|
| ZnT1 | Expressed in all tissues | Exporting metals from cytoplasm to extracellular medium | Targeted disruption of ZnT1 | Embryonic lethality and abnormal vulva formation | [68] |
| ZnT2 | Mammary gland, prostate, retina, pancreas, small intestine, and kidney | Accumulation of metals in organelles | Targeted disruption or mutation of ZnT2 | Extremely low Zn content of breast milk | [70] |
| ZnT3 | Brain, testes, and pancreas | Promotes the absorption of metal ions | Targeted ZnT3 disruption | Memory deficits with AD due to failure of Zn homeostasis proteins in neurons (MTIII- ZnT1-3) | [79] |
| ZnT4 | Expressed in all cells | Loss-of-function mutation in ZnT4 (lethal milk mutant) | Post-natal lethality | [80] | |
| ZnT5 and ZnT6 | PM/Golgi/vesicular membranes | Accumulation of metals in vesicles for transportation | ZnT5 and 6 -deficiency | Severe osteopenia with impaired DTH | [82] |
| ZnT7 | PM/Golgi/vesicular membranes | Accumulation of metals in vesicles, for transportation | ZnT7-deficiency | HFD-IGT | [86] |
| ZnT8 | Pancreatic β cells | Maintain the concentration of blood glucose | ZnT8-deficiency | Impaired insulin secretion and crystal formation in DM | [87] |
| ZnT10 | PM/Golgi/vesicular membranes | Accumulation of metals in vesicles, for transportation | ZnT10 mutation | Dystonia-parkinsonism with hyper manganesemia, polycythemia, and CLD | [87] |
| Type of Caner | Serum Zn Levels | Tissue Zn Levels | Abnormal Transporters | Ref |
|---|---|---|---|---|
| Breast | ZIP6 (), ZIP7 (), ZIP9 (), ZIP10 (), ZnT2 () | [70,121,129] | ||
| Lung (NSCLC) | ZIP4 () | [124] | ||
| Nasopharynx (NPC) | ZIP4 () | [124] | ||
| ESCC | ZIP5 (), ZIP6 () | [121,125] | ||
| Ovarian cancer | ZIP4 () | [124] | ||
| Cervical cancer | Uncertain | ZIP7 () | [121] | |
| Prostate cancer | ZIP1 , ZIP2 , ZIP3 , ZIP4 , ZIP9 (), ZnT4 | [80,118,119,122,124,129] |
7. Molecular Mechanisms, In Vitro, and In Vivo Studies
8. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ZFP | Zinc Finger Proteins |
| ZIP | Zrt- and Irt-like protein |
| ZnT | Zinc Transporter |
| ZEB1 | Zinc finger E-box binding homeobox 1 |
| MT | Metallothionein |
| ECM | Extracellular matrix |
| NK cells | Natural killer cells |
| TNF | Tumor Necrosis Factor |
| GPx | Glutathione peroxidase |
| ILs | Interleukins |
| IFNs | Interferons |
| NOX | NADPH Oxidase |
| PARP | Poly ADP-ribose polymerase |
| ROS | Reactive oxygen species |
| RREB1 | Ras-responsive element binding protein 1 |
| RAB27B | Ras-related protein Rab-27B |
| Aco2 | Mitochondrial aconitase |
| SOD | Superoxide Dismutase |
| MMPs | Matrix metalloproteinases |
| AMPs | Antimicrobial peptides |
| ATP | Adenosine triphosphate |
| NAD | Nicotinamide Adenine Dinucleotide |
| E-cad | Epithelial cadherin |
| GSK-3β | Glycogen Synthase Kinase-3 beta |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
References
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Knopf, B.; Konig, H. Biomethylation of heavy metals in soil and terrestrial invertebrates. In Soil Heavy Metals; Springer: Berlin/Heidelberg, Germany, 2010; pp. 315–328. [Google Scholar]
- Wuana, R.A.; Okieimen, F.E.; Imborvungu, J.A. Removal of heavy metals from a contaminated soil using organic chelating acids. Int. J. Environ. Sci. Technol. 2010, 7, 485–496. [Google Scholar] [CrossRef]
- Pueyo, M.; Rauret, G.; Lück, D.; Yli-Halla, M.; Muntau, H.; Quevauviller, P.; López-Sánchez, J.F. Certification of the extractable contents of Cd, Cr, Cu, Ni, Pb and Zn in a freshwater sediment following a collaboratively tested and optimised three-step sequential extraction procedure. J. Environ. Monit. 2001, 3, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Ghuge, S.A.; Nikalje, G.C.; Kadam, U.S.; Suprasanna, P.; Hong, J.C. Comprehensive mechanisms of heavy metal toxicity in plants, detoxification, and remediation. J. Hazard. Mater. 2023, 450, 131039. [Google Scholar] [CrossRef] [PubMed]
- Vasilachi, I.C.; Stoleru, V.; Gavrilescu, M. Analysis of heavy metal impacts on cereal crop growth and development in contaminated soils. Agriculture 2023, 13, 1983. [Google Scholar] [CrossRef]
- Li, Z.; Liang, Y.; Hu, H.; Shaheen, S.M.; Zhong, H.; Tack, F.M.G.; Wu, M.; Li, Y.-F.; Gao, Y.; Rinklebe, J.; et al. Speciation, transportation, and pathways of cadmium in soil-rice systems: A review on the environmental implications and remediation approaches for food safety. Environ. Int. 2021, 156, 106749. [Google Scholar] [CrossRef]
- Xia, W.; Ghouri, F.; Zhong, M.; Bukhari, S.A.H.; Ali, S.; Shahid, M.Q. Rice and heavy metals: A review of cadmium impact and potential remediation techniques. Sci. Total Environ. 2024, 957, 177403. [Google Scholar] [CrossRef]
- Rai, P.K.; Sonne, C.; Kim, K.H. Heavy metals and arsenic stress in food crops: Elucidating antioxidative defense mechanisms in hyperaccumulators for food security, agricultural sustainability, and human health. Sci. Total Environ. 2023, 874, 162327. [Google Scholar] [CrossRef]
- Eid, E.M.; El-Bebany, A.F.; Taher, M.A.; Alrumman, S.A.; Galal, T.M.; Shaltout, K.H.; Sewelam, N.A.; Ahmed, M.T. Heavy metal bioaccumulation, growth characteristics, and yield of Pisum sativum L. Grown in agricultural soil-sewage sludge mixtures. Plants 2020, 9, 1300. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, B.; Wu, Y.; Tan, M.; Shen, L.; Feng, G.; Yang, X.; Chen, S.; Xiong, Y.; Zhang, E.; et al. The lead and cadmium content in rice and risk to human health in China: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0278686. [Google Scholar] [CrossRef]
- Li, K.-Y.; Xiong, Y.-J.; Fu, J.-C.; Tian, X.-S.; Lu, C. Attenuated cadmium and arsenic enrichment in rice by co-application of organic composting and chemical fertilization. Sci. Rep. 2024, 14, 31942. [Google Scholar] [CrossRef]
- Mng’ong’o, M.E.; Mabagala, F.S. Arsenic and cadmium availability and its removal in paddy farming areas. J. Environ. Manag. 2024, 360, 121190. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, M.; Yang, H.; Pian, R.; Wang, J.; Wu, A.M. The Uptake, Transfer, and Detoxification of Cadmium in Plants and Its Exogenous Effects. Cells 2024, 13, 907. [Google Scholar] [CrossRef] [PubMed]
- Scutarasu, E.C.; Trinca, L.C. Heavy metals in foods and beverages: Global situation, health risks and reduction methods. Foods 2023, 12, 3340. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhao, X.; Li, Y.; Ling, J.; Ren, J.; Liao, Q.; Zhou, D.; Gu, X. Cadmium accumulation in wheat grain: Accumulation models and soil thresholds for safe production. Eco-Environ. Health 2025, 4, 100154. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, F.; Sun, S.; Li, Y.; Li, Y.; Mo, H.; Li, Z.; Zhuang, P. Effect of polishing on lead and cadmium bioavailability in rice and its health implications. Foods 2022, 11, 2718. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry. CERCLA Priority List of Hazardous Substances. 2019. Available online: https://www.atsdr.cdc.gov/programs/substance-priority-list.html (accessed on 30 August 2025).
- Honrado, A.; Miguel, M.; Ardila, P.; Beltrán, J.A.; Calanche, J.B. From waste to value: Fish protein hydrolysates as a technological and functional ingredient in human nutrition. Foods 2024, 13, 3120. [Google Scholar] [CrossRef]
- Shan, F.; Liu, L.; Li, L.; Wang, W.; Bi, Y.; Li, M. Management, safety, and efficacy evaluation of nutraceutical and functional food: A global perspective. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70222. [Google Scholar] [CrossRef]
- Zavrtnik, S.; Loborec, J.; Kapelj, S.; Grčić, I. Environmental biomonitoring of heavy and toxic metals using honeybees and their products—An overview of previous research. Sustainability 2024, 16, 8526. [Google Scholar] [CrossRef]
- Linquist, B.; Perry, H. Greenhouse gas emissions and grain arsenic and cadmium concentrations as affected by a weed control drainage in organic rice systems. Agrosyst, Geosci. Environ. 2023, 6, e20417. [Google Scholar] [CrossRef]
- Pinson, S.R.M.; Heuschele, D.J.; Edwards, J.D.; Jackson, A.K.; Sharma, S.; Barnaby, J.Y. Relationships Among Arsenic-Related Traits, Including Rice Grain Arsenic Concentration and Straighthead Resistance, as Revealed by Genome-Wide Association. Front. Genet. 2022, 12, 787767. [Google Scholar] [CrossRef]
- Banerjee, S.; Islam, J.; Mondal, S.; Saha, A.; Saha, B.; Sen, A. Proactive attenuation of arsenic-stress by nano-priming: Zinc oxide nanoparticles in Vigna mungo (L.) Hepper trigger antioxidant defense response and reduce root-shoot arsenic translocation. J. Hazard. Mater. 2023, 446, 130735. [Google Scholar] [CrossRef]
- Banerjee, S.; Mondal, S.; Islam, J.; Sarkar, R.; Saha, B.; Sen, A. Rhizospheric nano-remediation salvages arsenic genotoxicity: Zinc-oxide nanoparticles articulate better oxidative stress management, reduce arsenic uptake, and increase yield in Pisum sativum (L.). Sci. Total Environ. 2024, 913, 169493. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Koyama, H.; Kamogashira, T.; Yamasoba, T. Heavy metal exposure: Molecular pathways, clinical implications, and protective strategies. Antioxidants 2024, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Alomar, S.Y.; Nepovimova, E.; Kuca, K.; Valko, M. Heavy metals: Toxicity and human health effects. Arch. Toxicol. 2025, 99, 153–209. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, B.F.; Furieri, L.B.; Peçanha, F.M.; Wiggers, G.A.; Vassallo, P.F.; Simões, M.R.; Fiorim, J.; de Batista, P.R.; Fioresi, M.; Rossoni, L.; et al. Toxic effects of mercury on the cardiovascular and central nervous systems. J. Biomed. Biotechnol. 2012, 2012, 949048. [Google Scholar] [CrossRef] [PubMed]
- Cobbina, S.J.; Chen, Y.; Zhou, Z.; Wu, X.; Zhao, T.; Zhang, Z.; Feng, W.; Wang, W.; Li, Q.; Wu, X.; et al. Toxicity assessment due to sub-chronic exposure to individual and mixtures of four toxic heavy metals. J. Hazard. Mater. 2015, 294, 109–120. [Google Scholar] [CrossRef]
- Shin, D.Y.; Lee, S.M.; Jang, Y.; Lee, J.; Lee, C.M.; Cho, E.-M.; Seo, Y.R. Adverse human health effects of chromium by exposure route: A comprehensive review based on toxicogenomic approach. Int. J. Mol. Sci. 2023, 24, 3410. [Google Scholar] [CrossRef]
- Gazwi, H.S.; Yassien, E.E.; Hassan, H.M. Mitigation of lead neurotoxicity by the ethanolic extract of Laurus leaf in rats. Ecotoxicol. Environ. Saf. 2020, 192, 110297. [Google Scholar] [CrossRef]
- Bernhoft, R.A. Mercury toxicity and treatment: A review of the literature. J. Environ. Public Health 2012, 2012, 460508. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.G.; Bellinger, D.C.; Valeri, L.; Hasan, O.S.I.; Quamruzzaman, Q.; Golam, M.; Kile, M.L.; Christiani, D.C.; Wright, R.O.; Mazumdar, M. Neurodevelopmental outcomes among 2-to 3-year-old children in Bangladesh with elevated blood lead and exposure to arsenic and manganese in drinking water. Environ. Health 2016, 15, 44. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [PubMed]
- King, J.C.; Cousins, R.J.; Shils, M.; Shike, M.; Ross, A.C.; Caballero, B. Modern Nutrition in Health and Disease, 10th ed.; Lippincott Williams and Wilkins: Baltimore, MD, USA, 2006; pp. 271–285. [Google Scholar]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar]
- Bradl, H. Heavy Metals in the Environment: Origin, Interaction and Remediation, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 6, pp. 1–269. [Google Scholar]
- Anka, A.U.; Usman, A.B.; Kaoje, A.N.; Kabir, R.M.; Bala, A.; Arki, M.K.; Hossein-Khannazer, N.; Azizi, G. Potential mechanisms of some selected heavy metals in the induction of inflammation and autoimmunity. Eur. J. Inflamm. 2022, 20. [Google Scholar] [CrossRef]
- Hughes, J.P.; Polissar, L.; Van Belle, G. Evaluation and synthesis of health effects studies of communities surrounding arsenic producing industries. Int. J. Epidemiol. 1988, 17, 407–413. [Google Scholar] [CrossRef]
- Singh, N.; Kumar, D.; Sahu, A.P. Arsenic in the environment: Effects on human health and possible prevention. J. Environ. Biol. 2007, 28 (Suppl. S2), 359–365. [Google Scholar]
- Saha, J.C.; Dikshit, A.K.; Bandyopadhyay, M.; Saha, K.C. A review of arsenic poisoning and its effects on human health. Crit. Rev. Environ. Sci. Technol. 1999, 29, 281–313. [Google Scholar] [CrossRef]
- Gordon, J.J.; Quastel, G.H. Effect of organic arsenicals on enzyme system. Biochem. J. 1948, 42, 337–350. [Google Scholar] [CrossRef]
- Cirovic, A.; Cirovic, A.; Yimthiang, S.; Vesey, D.A.; Satarug, S. Modulation of adverse health effects of environmental cadmium exposure by zinc and its transporters. Biomolecules 2024, 14, 650, Correction in: J. Endocrinol. Investig. 2019, 42, 741. [Google Scholar] [CrossRef]
- Satarug, S.; Gobe, G.C.; Vesey, D.A. Multiple targets of toxicity in environmental exposure to low-dose cadmium. Toxics 2022, 10, 472. [Google Scholar] [CrossRef]
- JECFA. Summary and conclusions. In In Proceedings of the Joint FAO/WHO Expert Committee on Food Additives and Contaminants, Seventy-Third Meeting, JECFA/73/SC, Geneva, Switzerland, 8–17 June 2010; Food and Agriculture Organization of the United Nations/World Health Organization: Geneva, Switzerland, 2011. Available online: https://apps.who.int/iris/handle/10665/44521 (accessed on 30 August 2025).
- Engwa, G.A.; Ferdinand, P.U.; Nwalo, F.N.; Unachukwu, M.N. Mechanism and health effects of heavy metal toxicity in humans. In Poisoning in the Modern World-New Tricks for an Old Dog? Intechopen: Rijeka, Croatia, 2019; Volume 10, pp. 70–90. [Google Scholar]
- Germic, N.; Frangez, Z.; Yousefi, S.; Simon, H.U. Regulation of the innate immune system by autophagy: Neutrophils, eosinophils, mast cells, NK cells. Cell Death Differ. 2019, 26, 703–714. [Google Scholar] [CrossRef] [PubMed]
- IARC. Working group on the evaluation of carcinogenic risks to humans: Inorganic and organic lead compounds. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2006. [Google Scholar]
- Fittipaldi, S.; Bimonte, V.M.; Soricelli, A.; Aversa, A.; Lenzi, A.; Greco, E.A.; Migliaccio, S. Cadmium exposure alters steroid receptors and proinflammatory cytokine levels in endothelial cells in vitro: A potential mechanism of endocrine disruptor atherogenic effect. J. Endocrinol. Investig. 2019, 42, 727–739, Correction in: J. Endocrinol. Investig. 2019, 42, 741.. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gong, X.; Li, R.; Gao, W.; Hu, D.; Yi, X.; Liu, Y.; Fang, J.; Shao, J.; Ma, Y.; et al. Exposure to cadmium and lead is associated with diabetic kidney disease in diabetic patients. Environ. Health 2024, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Esteban, M.Á.; Cuesta, A.; Chaves-Pozo, E.; Meseguer, J. Phagocytosis in Teleosts: Implications of the new cells involved. Biology 2015, 4, 907–922. [Google Scholar] [CrossRef]
- Lin, J.; Knight, E.L.; Hogan, M.L.; Singh, A.K. A comparison of prediction equations for estimating glomerular filtration rate in adults without kidney disease. J. Am. Soc. Nephrol. 2003, 14, 2573–2580. [Google Scholar] [CrossRef]
- Kim, D.O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Shen, H.M.; Zhang, Q.F. Risk assessment of nickel carcinogenicity and occupational lung cancer. Environ. Health Perspect. 1994, 102, 275–282. [Google Scholar] [CrossRef]
- Fraker, P.J.; King, L.E. Reprogramming of the immune system during zinc deficiency. Annu. Rev. Nutr. 2004, 24, 277–298. [Google Scholar] [CrossRef]
- Shankar, A.H.; Prasad, A.S. Zinc and immune function: The biological basis of altered resistance to infection. Am. J. Clin. Nutr. 1998, 68, 447S–463S. [Google Scholar] [CrossRef]
- Fisher, E.B.; Strunk, R.C.; Sussman, L.K.; Sykes, R.K.; Walker, M.S. Community organization to reduce the need for acute care for asthma among african american children in low-income neighborhoods: The neighborhood asthma coalition. Pediatrics 2004, 114, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, M.; Olbert, M.; Wyszogrodzka, G.; Młyniec, K.; Librowski, T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology 2017, 25, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Schoofs, H.; Schmit, J.; Rink, L. Zinc Toxicity: Understanding the Limits. Molecules 2024, 29, 3130. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Prasad, A.S.; Bao, B. Molecular mechanisms of zinc as a pro-antioxidant mediator: Clinical therapeutic implications. Antioxidants 2019, 8, 164. [Google Scholar] [CrossRef]
- Kim, J.B.; Zhang, L. Accounting conservatism and stock price crash risk: Firm-level evidence. Contemp. Account. Res. 2016, 33, 412–441. [Google Scholar] [CrossRef]
- Agarwal, H.; Shanmugam, V. A review on anti-inflammatory activity of green synthesized zinc oxide nanoparticle: Mechanism-based approach. Bioorg. Chem. 2020, 94, 103423. [Google Scholar] [CrossRef]
- Mohammadi, F.; Marti, A.; Nayebzadeh, K.; Hosseini, S.M.; Tajdar-Oranj, B.; Jazaeri, S. Effect of washing, soaking and pH in combination with ultrasound on enzymatic rancidity, phytic acid, heavy metals and coliforms of rice bran. Food Chem. 2021, 334, 127583. [Google Scholar] [CrossRef]
- Rink, L.; Gabriel, P. Extracellular and immunological actions of zinc. Biometals 2001, 14, 367–383. [Google Scholar] [CrossRef]
- Stefanidou, M.; Maravelias, C.; Dona, A.; Spiliopoulou, C. Zinc: A multipurpose trace element. Arch. Toxicol. 2006, 80, 1–9. [Google Scholar] [CrossRef]
- Murakami, M.; Hirano, T. Intracellular zinc homeostasis and zinc signaling. Cancer Sci. 2008, 99, 1515–1522. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc: Role in immunity, oxidative stress and chronic inflammation. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [PubMed]
- Mengozzi, A.M.; Pugliese, N.R.M.; Chiriacò, M.M.; Masi, S.; Virdis, A.; Taddei, S. Microvascular ageing links metabolic disease to age-related disorders: The role of oxidative stress and inflammation in promoting microvascular dysfunction. J. Cardiovasc. Pharmacol. 2021, 78, S78–S87. [Google Scholar] [CrossRef] [PubMed]
- Tanislav, C.; Kostev, K. Fewer non-COVID-19 respiratory tract infections and gastrointestinal infections during the COVID-19 pandemic. J. Med. Virol. 2022, 94, 298–302. [Google Scholar] [CrossRef]
- Arshad, M.S.; Khan, U.; Sadiq, A.; Khalid, W.; Hussain, M.; Yasmeen, A.; Asghar, Z.; Rehana, H. Coronavirus Disease (COVID-19) and immunity booster green foods: A mini review. Food Sci. Nutr. 2020, 8, 3971–3976. [Google Scholar] [CrossRef]
- Bhowmik, D.; Chiranjib, K.; Kumar, S. A potential medicinal importance of zinc in human health and chronic diseases. Int. J. Pharm. 2010, 1, 5–11. [Google Scholar]
- Ho, E. Zinc deficiency, DNA damage and cancer risk. J. Nutr. Biochem. 2004, 15, 572–578. [Google Scholar] [CrossRef]
- Uriu-Adams, J.Y.; Keen, C.L. Zinc and reproduction: Effects of zinc deficiency on prenatal and early postnatal development. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2010, 89, 313–325. [Google Scholar] [CrossRef]
- Osredkar, J.; Sustar, N. Copper and zinc, biological role and significance of copper/zinc imbalance. J. Clin. Toxicol. 2011, S3, 0495. [Google Scholar] [CrossRef]
- Ali, M.; Shahzad, L.; Hayyat, M.U.; Sharif, F.; Ghafoor, G.Z.; Nasir, R. Determination of Heavy Metals and Their Associated Health Risk Assessment in the Commonly Available Sunblocks. Int. J. Environ. Anal. Chem. 2023, 104, 7408–7426. [Google Scholar] [CrossRef]
- Takagishi, T.; Hara, T.; Fukada, T. Recent advances in the role of SLC39A/ZIP zinc transporters in vivo. Int. J. Mol. Sci. 2017, 18, 2708. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Takeda, T.-A.; Takagishi, T.; Fukue, K.; Kambe, T.; Fukada, T. Physiological roles of zinc transporters: Molecular and genetic importance in zinc homeostasis. J. Physiol. Sci. 2017, 67, 283–301. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Jiang, W.; Wang, X.; Shahid, S.; Saba, N.; Ahmad, M.; Dar, A.; Masood, S.U.; Imran, M.; Mustafa, A. Mechanistic impact of zinc deficiency in human development. Front. Nutr. 2022, 9, 717064. [Google Scholar] [CrossRef]
- Harchegani, A.B.; Dahan, H.; Tahmasbpour, E.; Kaboutaraki, H.B.; Shahriary, A. Effects of zinc deficiency on impaired spermatogenesis and male infertility: The role of oxidative stress, inflammation and apoptosis. Hum. Fertil. 2018, 23, 5–16. [Google Scholar] [CrossRef]
- Huster, D. Wilson disease. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 531–539. [Google Scholar] [CrossRef]
- Watanabe, A.; Ito, H.; Chiba, M.; Ito, A.; Shimizu, H.; Fuji, S.-I.; Nakamura, S.-I.; Hattori, H.; Chino, M.; Satoh-Nagasawa, N.; et al. Isolation of novel types of Arabidopsis mutants with altered reactions to cadmium: Cadmium-gradient agar plates are an effective screen for the heavy metal-related mutants. Planta 2010, 232, 825–836. [Google Scholar] [CrossRef]
- Prasad, A.S. Effects of zinc deficiency on Th1 and Th2 cytokine shifts. J. Infect. Dis. 2000, 182, S62–S68. [Google Scholar] [CrossRef]
- Overbeck, S.; Rink, L.; Haase, H. Modulating the immune response by oral zinc supplementation: A single approach for multiple diseases. Arch. Immunol. Ther. Exp. 2008, 56, 15–30. [Google Scholar] [CrossRef]
- Choi, S.; Hu, Y.M.; Corkins, M.E.; Palmer, A.E.; Bird, A.J. Zinc transporters belonging to the cation diffusion facilitator (CDF) family have complementary roles in transporting zinc out of the cytosol. PLoS Genet. 2018, 14, e1007262. [Google Scholar] [CrossRef]
- Tranah, T.H.; Ballester, M.-P.; Carbonell-Asins, J.A.; Ampuero, J.; Alexandrino, G.; Caracostea, A.; Sánchez-Torrijos, Y.; Thomsen, K.L.; Kerbert, A.J.; Capilla-Lozano, M.; et al. Plasma ammonia levels predict hospitalisation with liver-related complications and mortality in clinically stable outpatients with cirrhosis. J. Hepatol. 2022, 77, 1554–1563. [Google Scholar] [CrossRef]
- Dardenne, M. Zinc and Immune Function. Eur. J. Clin. Nutr. 2002, 56, S20–S23. [Google Scholar] [CrossRef]
- Leem, A.Y.; Kim, S.K.; Chang, J.; Kang, Y.A.; Kim, Y.S.; Park, M.S.; Kim, S.Y.; Kim, E.Y.; Chung, K.S.; Jung, J.Y. Relationship between blood levels of heavy metals and lung function based on the Korean national health and nutrition examination survey IV–V. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 1559–1570. [Google Scholar] [CrossRef]
- Luo, X.; Wang, J.; Dooner, M.; Clarke, J. Overview of current development in electrical energy storage technologies and the application potential in power system operation. Appl. Energy 2015, 137, 511–536. [Google Scholar] [CrossRef]
- Jayawardena, R.; Ranasinghe, P.; Galappatthy, P.; Malkanthi, R.L.; Constantine, G.R.; Katulanda, P. Effects of zinc supplementation on diabetes mellitus: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2012, 4, 13. [Google Scholar] [CrossRef]
- Diglio, D.C.; Fernandes, S.A.; Stein, J.; Azeredo-da-Silva, A.; de Mattos, A.A.; Tovo, C.V. Role of zinc supplementation in the management of chronic liver diseases: A Systematic Review and Meta-Analysis. Ann. Hepatol. 2020, 19, 190–196. [Google Scholar] [CrossRef]
- Bloom, A.; Bloom, S.; Silva, H.; Nicoll, A.J.; Sawhney, R. Zinc supplementation and its benefits in the management of chronic liver disease: An in-depth literature review. Ann. Hepatol. 2021, 25, 100549. [Google Scholar] [CrossRef]
- Knez, M.; Glibetic, M. Zinc as a biomarker of cardiovascular health. Front. Nutr. 2021, 8, 686078. [Google Scholar] [CrossRef] [PubMed]
- Efeovbokhan, N.; Bhattacharya, S.K.; Ahokas, R.A.; Sun, Y.; Guntaka, R.V.; Gerling, I.C.; Weber, K.T. Zinc and the prooxidant heart failure phenotype. J. Cardiovasc. Pharmacol. 2014, 64, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Frustaci, A.; Neri, M.; Cesario, A.; Adams, J.B.; Domenici, E.; Dalla Bernardina, B.; Bonassi, S. Oxidative stress-related biomarkers in autism: Systematic review and meta-analyses. Free Radic. Biol. Med. 2012, 52, 2128–2141. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, D.; Gutkin, A.; Kedmi, R.; Ramishetti, S.; Veiga, N.; Jacobi, A.M.; Schubert, M.S.; Friedmann-Morvinski, D.; Cohen, Z.R.; Behlke, M.A.; et al. CRISPR-Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci. Adv. 2020, 6, eabc9450. [Google Scholar] [CrossRef]
- Aizenman, E. Zinc signaling in the life and death of neurons. In Zinc Signaling; Fukada, T., Kambe, T., Eds.; Springer: Singapore, 2019; pp. 165–185. [Google Scholar]
- Lee, S.H.; Lee, M.W.; Ko, D.G.; Choi, B.Y.; Suh, S.W. The role of NADPH oxidase in neuronal death and neurogenesis after acute neurological disorders. Antioxidants 2021, 10, 739. [Google Scholar] [CrossRef]
- Choe, J.Y.; Park, K.Y.; Kim, S.K. Oxidative stress by monosodium urate crystals promotes renal cell apoptosis through mitochondrial caspase-dependent pathway in human embryonic kidney 293 cells: Mechanism for urate-induced nephropathy. Apoptosis 2015, 20, 38–49. [Google Scholar] [CrossRef]
- Bredt, D.S. Endogenous nitric oxide synthesis: Biological Functions and Pathophysiology. Free Radic. Res. 1999, 31, 577–596. [Google Scholar] [CrossRef] [PubMed]
- Bitanihirwe, B.K.; Cunningham, M.G. Zinc: The Brain’s Dark Horse. Synapse 2009, 63, 1029–1049. [Google Scholar] [CrossRef] [PubMed]
- Frederickson, C.J.; Koh, J.Y.; Bush, A.I. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 2005, 6, 449–462. [Google Scholar] [CrossRef]
- Prasad, A.S.; Beck, F.W.; Snell, D.C.; Kucuk, O. Zinc in cancer prevention. Nutr. Cancer 2009, 61, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Reingold, A.L.; Menzies, D.; Pai, M. Tuberculosis among health-care workers in low-and middle-income countries: A systematic review. PLoS Med. 2006, 3, e494. [Google Scholar] [CrossRef]
- Mocchegiani, E.; Costarelli, L.; Giacconi, R.; Piacenza, F.; Basso, A.; Malavolta, M. Zinc, metallothioneins and immunosenescence: Effect of zinc supply as nutrigenomic approach. Biogerontology 2011, 12, 455–465. [Google Scholar] [CrossRef]
- Skrajnowska, D.; Bobrowska-Korczak, B. Role of zinc in immune system and anti-cancer defense mechanisms. Nutrients 2019, 11, 2273. [Google Scholar] [CrossRef]
- Fukada, T.; Kambe, T. Molecular and genetic features of zinc transporters in physiology and pathogenesis. Metallomics 2011, 3, 662–674. [Google Scholar] [CrossRef]
- Maywald, M.; Rink, L. Zinc homeostasis and immunosenescence. J. Trace Elem. Med. Biol. 2015, 29, 24–30. [Google Scholar] [CrossRef]
- Hojyo, S.; Fukada, T. Roles of zinc signaling in the immune system. J. Immunol. Res. 2016, 2016, 6762343. [Google Scholar] [CrossRef]
- Gropper, S.S.; Smith, J.L.; Groff, J.L. Advanced Nutrition and Human Metabolism; Wadsworth: Belmont, CA, USA, 2009. [Google Scholar]
- Song, Y.; Leonard, S.W.; Traber, M.G.; Ho, E. Zinc deficiency affects DNA damage, oxidative stress, antioxidant defenses, and DNA repair in rats. J. Nutr. 2009, 139, 1626–1631. [Google Scholar] [CrossRef]
- Alder, H.; Taccioli, C.; Chen, H.; Jiang, Y.; Smalley, K.J.; Fadda, P.; Ozer, H.G.; Huebner, K.; Farber, J.L.; Croce, C.M.; et al. Dysregulation of miR-31 and miR-21 induced by zinc deficiency promotes esophageal cancer. Carcinogenesis 2012, 33, 1736–1744. [Google Scholar] [CrossRef] [PubMed]
- Fong, L.Y.; Taccioli, C.; Palamarchuk, A.; Tagliazucchi, G.M.; Jing, R.; Smalley, K.J.; Fan, S.; Altemus, J.; Fiehn, O.; Huebner, K.; et al. Abrogation of esophageal carcinoma development in miR-31 Knockout Rats. Proc. Natl. Acad. Sci. USA 2020, 117, 6075–6085. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.; Muraina, I.A.; Brethour, D.; Schmitt-Ulms, G.; Nimmanon, T.; Ziliotto, S.; Kille, P.; Hogstrand, C. Zinc transporter ZIP10 forms a heteromer with ZIP6 which regulates embryonic development and cell migration. Biochem. J. 2016, 473, 2531–2544. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, H.; Nie, X.; Guo, M.; Jiang, G.; Xing, M. Zinc application alleviates the adverse renal effects of arsenic stress in a protein quality control way in common carp. Environ. Res. 2020, 191, 110063. [Google Scholar] [CrossRef]
- Gumulec, J.; Masarik, M.; Krizkova, S.; Adam, V.; Hubalek, J.; Hrabeta, J.; Eckschlager, T.; Stiborova, M.; Kizek, R. Insight to physiology and pathology of zinc (II) ions and their actions in breast and prostate carcinoma. Curr. Med. Chem. 2011, 18, 5041–5051. [Google Scholar] [CrossRef]
- Costello, L.C.; Zou, J.; Desouki, M.M.; Franklin, R.B. Evidence for changes in RREB-1, ZIP3, and zinc in the early development of pancreatic adenocarcinoma. J. Gastrointest. Cancer 2012, 43, 570–578. [Google Scholar] [CrossRef]
- Alam, S.; Kelleher, S.L. Cellular mechanisms of zinc dysregulation: A perspective on zinc homeostasis as an etiological factor in the development and progression of breast cancer. Nutrients 2012, 4, 875–903. [Google Scholar] [CrossRef]
- Hogstrand, C.; Kille, P.; Ackland, M.L.; Hiscox, S.; Taylor, K.M. A mechanism for epithelial-mesenchymal transition and anoikis resistance in breast cancer triggered by zinc channel ZIP6 and STAT3 (signal transducer and activator of transcription 3). Biochem. J. 2013, 455, 229–237. [Google Scholar] [CrossRef]
- Costello, L.C.; Levy, B.A.; Desouki, M.M.; Zou, J.; Bagasra, O.; Johnson, L.A.; Hanna, N.; Franklin, R.B. Decreased zinc and downregulation of ZIP3 zinc uptake transporter in the development of pancreatic adenocarcinoma. Cancer Biol. Ther. 2011, 12, 297–303. [Google Scholar] [CrossRef]
- Zou, J.; Milon, B.C.; Desouki, M.M.; Costello, L.C.; Franklin, R.B. hZIP1 zinc transporter down-regulation in prostate cancer involves the overexpression of Ras responsive element binding protein-1 (RREB-1). Prostate 2011, 71, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bharadwaj, U.; Logsdon, C.D.; Chen, C.; Yao, Q.; Li, M. ZIP4 regulates pancreatic cancer cell growth by activating IL-6/STAT3 pathway through zinc finger transcription factor CREB. Clin. Cancer Res. 2010, 16, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yang, J.; Zhang, Y.; Zhou, Z.; Cui, X.; Zhang, L.; Fung, K.-M.; Zheng, W.; Allard, F.D.; Yee, E.U.; et al. ZIP4 promotes pancreatic cancer progression by repressing ZO-1 and Claudin-1 through a ZEB1-dependent transcriptional mechanism. Clin. Cancer Res. 2018, 24, 3186–3196. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wallace, M.; Yang, J.; Jiang, L.; Zhai, Q.; Zhang, Y.; Hong, C.; Chen, Y.; Frank, T.; Stauffer, J.; et al. ZIP4 is a novel diagnostic and prognostic marker in human pancreatic cancer: A systemic comparison between EUS-FNA and surgical specimens. Curr. Mol. Med. 2014, 14, 309–315. [Google Scholar] [CrossRef]
- Grzywacz, A.; Argasińska, J.G.; Muszyńska, B.; Czochara, M.T.; Librowski, T.; Opoka, W. Metal responsive transcription factor 1 (MTF-1) regulates zinc dependent cellular processes at the molecular level. Acta Biochim. Pol. 2015, 62, 491–498. [Google Scholar] [CrossRef]
- Giménez, J.P.; Poirier, M.; Bühlmann, S.; Schuppisser, C.; Bhardwaj, R.; Awale, M.; Visini, R.; Javor, S.; Hediger, M.A.; Reymond, J.-L. Inhibitors of Human Divalent Metal Transporters DMT1 (SLC11A2) and ZIP8 (SLC39A8) from a GDB-17 Fragment Library. ChemMedChem 2021, 16, 3306–3314. [Google Scholar] [CrossRef]
- Jenkitkasemwong, S.; Wang, C.Y.; MacKenzie, B.; Knutson, M.D. Physiologic implications of metal-ion transport by ZIP14 and ZIP8. BioMetals 2012, 25, 643–655. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, B. The impact of zinc and zinc homeostasis on the intestinal mucosal barrier and intestinal diseases. Biomolecules 2022, 12, 900. [Google Scholar] [CrossRef]
- DiGuilio, K.M.; Rio, E.A.D.; Harty, R.N.; Mullin, J.M. Micronutrients at supplemental levels, tight junctions and epithelial barrier function: A narrative review. Int. J. Mol. Sci. 2024, 25, 3452. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a gatekeeper of immune function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
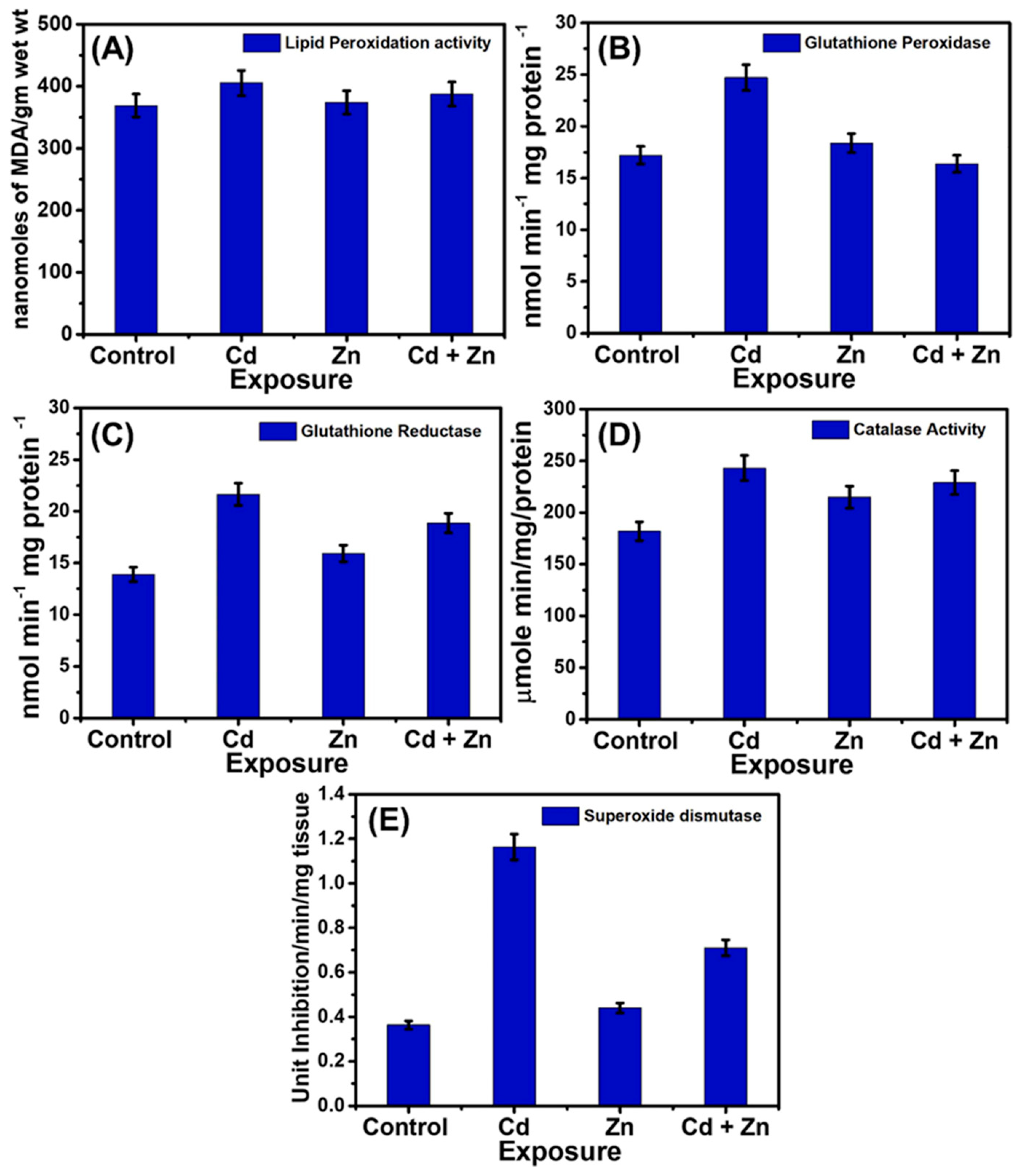
- Devarapogu, R.; Asupatri, U.R. Effects of zinc supplementation in mitigating the harmful effects of chronic cadmium exposure in a zebrafish model. Environ. Toxicol. Pharmacol. 2023, 100, 104158. [Google Scholar] [CrossRef] [PubMed]
- Gurer, H.; Ercal, N. Can antioxidants be beneficial in the treatment of lead poisoning? Free Radic. Biol. Med. 2000, 29, 927–945. [Google Scholar] [CrossRef]
- Kumar, A.; Malhotra, A.; Nair, P.; Garg, M.L.; Dhawan, D.K. Protective role of zinc in ameliorating arsenic-induced oxidative stress and histological changes in rat liver. J. Environ. Pathol. Toxicol. Oncol. 2010, 29, 91–100. [Google Scholar] [CrossRef]
- Ganger, R.; Garla, R.; Mohanty, B.P.; Bansal, M.P.; Garg, M.L. Protective effects of zinc against acute arsenic toxicity by regulating antioxidant defense system and cumulative metallothionein expression. Biol. Trace Elem. Res. 2016, 169, 218–229. [Google Scholar] [CrossRef]
- Adil, M.; Kandhare, A.; Visnagri, A.; Bodhankar, S. Naringin ameliorates sodium arsenite-induced renal and hepatic toxicity in rats: Decisive role of KIM-1, Caspase-3, TGF-β, and TNF-α. Ren. Fail. 2015, 37, 1396–1407. [Google Scholar] [CrossRef]
- Kharroubi, W.; Dhibi, M.; Mekni, M.; Haouas, Z.; Chreif, I.; Neffati, F.; Hammami, M.; Sakly, R. Sodium arsenate induce changes in fatty acids profiles and oxidative damage in kidney of rats. Environ. Sci. Pollut. Res. Int. 2014, 21, 12040–12049. [Google Scholar] [CrossRef]
- Cai, H.; Bao, Y.; Cheng, H.; Ge, X.; Zhang, M.; Feng, X.; Zheng, Y.; He, J.; Wei, Y.; Liu, C.; et al. Zinc homeostasis may reverse the synergistic neurotoxicity of heavy metal mixtures in Caenorhabditis elegans. Sci. Total Environ. 2023, 868, 161699. [Google Scholar] [CrossRef]
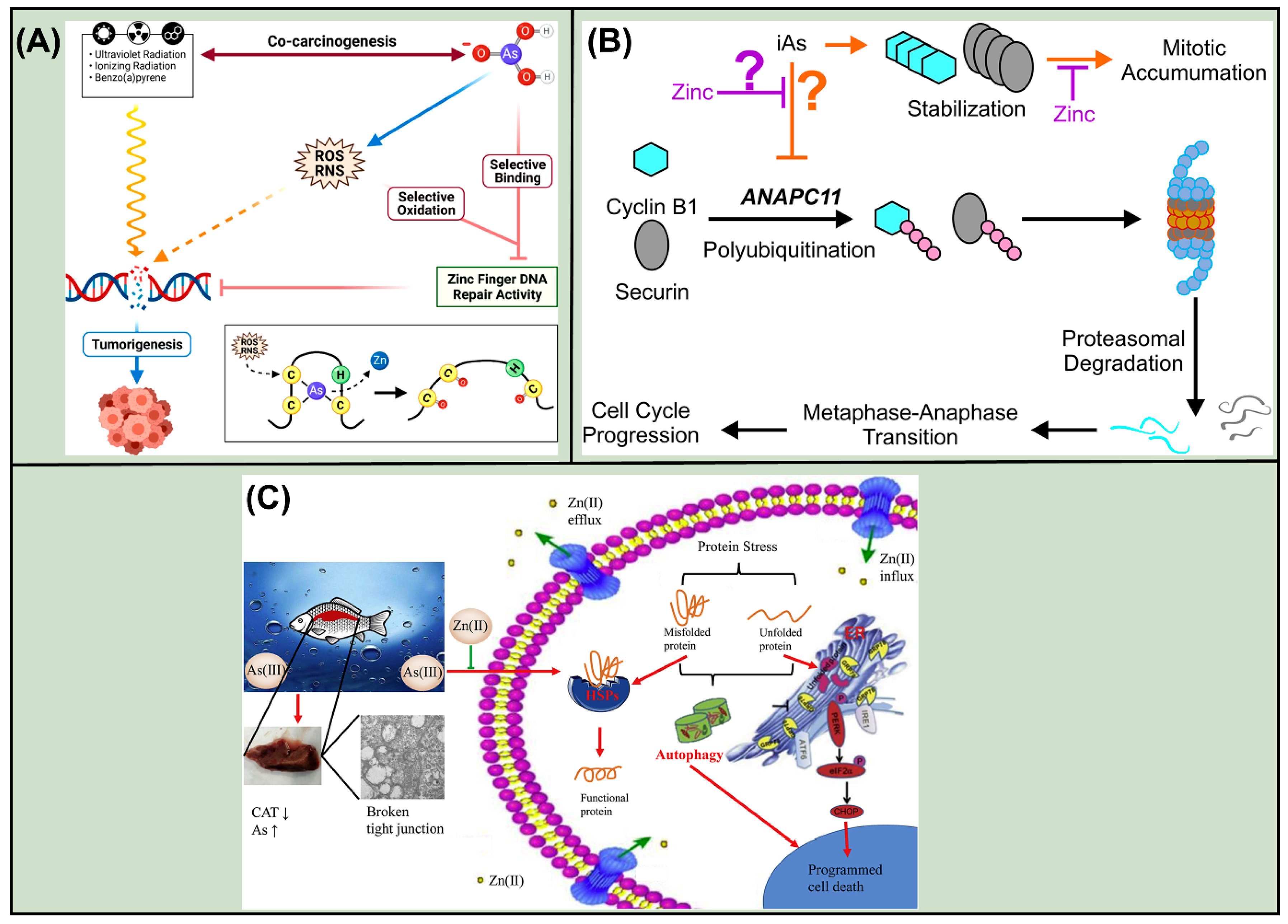
- Zhou, X.; Speer, R.M.; Volk, L.; Hudson, L.G.; Liu, K.J. Arsenic co-carcinogenesis: Inhibition of DNA repair and interaction with zinc finger proteins. Semin. Cancer Biol. 2021, 76, 86–98. [Google Scholar] [CrossRef]
- Banerjee, M.; Yaddanapudi, K.; States, J.C. Zinc supplementation prevents mitotic accumulation in human keratinocyte cell lines upon environmentally relevant arsenic exposure. Toxicol. Appl. Pharmacol. 2022, 454, 116255. [Google Scholar] [CrossRef]
- Knoell, D.L.; Smith, D.; Bao, S.; Sapkota, M.; Wyatt, T.A.; Zweier, J.L.; Flury, J.; Borchers, M.T.; Knutson, M. Imbalance in zinc homeostasis enhances lung tissue loss following cigarette smoke exposure. J. Trace Elem. Med. Biol. 2020, 60, 126483. [Google Scholar] [CrossRef]
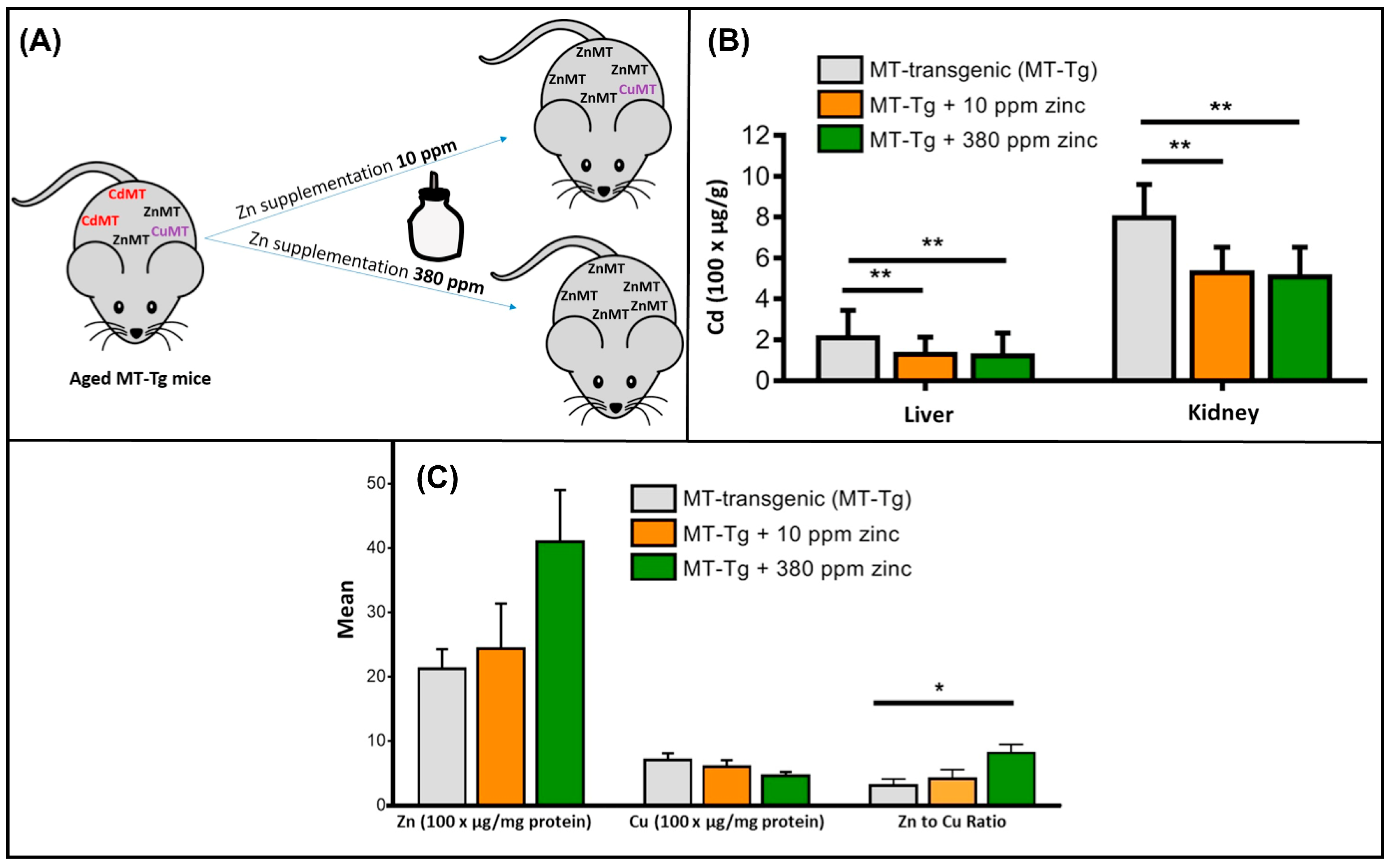
- Pabis, K.; Gundacker, C.; Giacconi, R.; Basso, A.; Costarelli, L.; Piacenza, F.; Strizzi, S.; Provinciali, M.; Malavolta, M. Zinc supplementation can reduce accumulation of cadmium in aged metallothionein transgenic mice. Chemosphere 2018, 211, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Dashner-Titus, E.J.; Schilz, J.R.; Alvarez, S.A.; Wong, C.P.; Simmons, K.; Ho, E.; Hudson, L.G. Zinc supplementation alters tissue distribution of Arsenic in Mus musculus. Toxicol. Appl. Pharmacol. 2023, 478, 116709. [Google Scholar] [CrossRef]
- Cooper, K.L.; King, B.S.; Sandoval, M.M.; Liu, K.J.; Hudson, L.G. Reduction of arsenite-enhanced ultraviolet radiation-induced DNA damage by supplemental zinc. Toxicol. Appl. Pharmacol. 2013, 269, 81–88. [Google Scholar] [CrossRef]
- Garla, R.; Sharma, N.; Shamli; Kaushal, N.; Garg, M.L. Effect of zinc on hepatic and renal tissues of chronically arsenic exposed rats: A biochemical and histopathological study. Biol. Trace Elem. Res. 2021, 199, 4237–4250. [Google Scholar] [CrossRef] [PubMed]
- Bastick, J.C.; Banerjee, M.; States, J.C. Zinc supplementation prevents Arsenic-Induced dysregulation of ZRANB2 splice function. Environ. Toxicol. Pharmacol. 2022, 94, 103921. [Google Scholar] [CrossRef] [PubMed]
- Kreppel, H.; Liu, J.; Liu, Y.; Reichl, F.X.; Klaassen, C.D. Zinc-induced arsenate tolerance in mice. Fundam. Appl. Toxicol. 1994, 23, 32–37. [Google Scholar] [CrossRef]
- Modi, M.; Kaul, R.K.; Kannan, G.M.; Flora, S.J.S. Co-administration of zinc and n-acetylcysteine prevents arsenic-induced tissue oxidative stress in male rats. J. Trace Elem. Med. Biol. 2006, 20, 197–204. [Google Scholar] [CrossRef]
- Ozoani, H.; Ezejiofor, A.N.; Okolo, K.O.; Orish, C.N.; Cirovic, A.; Cirovic, A.; Orisakwe, O.E. Ameliorative effects of Zn and Se supplementation on heavy metal mixture burden via increased renal metal excretion and restoration of redoxo-inflammatory alterations. Biol. Trace Elem. Res. 2024, 202, 643–658. [Google Scholar] [CrossRef] [PubMed]
- Skalny, A.A.; Tinkov, A.A.; Medvedeva, Y.S.; Alchinova, I.B.; Karganov, M.Y.; Ajsuvakova, O.P.; Skalny, A.V.; Nikonorov, A.A. Zinc asparaginate supplementation induces redistribution of toxic trace elements in rat tissues and organs. Interdiscip. Toxicol. 2015, 8, 131–138. [Google Scholar] [CrossRef]
- Yu, H.-T.; Zhen, J.; Xu, J.-X.; Cai, L.; Leng, J.-Y.; Ji, H.-L.; Keller, B.B. Zinc protects against cadmium-induced toxicity in neonatal murine engineered cardiac tissues via metallothionein-dependent and independent mechanisms. Acta Pharmacol. Sin. 2020, 41, 638–649. [Google Scholar] [CrossRef]
- Brzóska, M.M.; Kozłowska, M.; Rogalska, J.; Gałażyn-Sidorczuk, M.; Roszczenko, A.; Smereczański, N.M. Enhanced zinc intake protects against oxidative stress and its consequences in the brain: A study in an in vivo rat model of cadmium exposure. Nutrients 2021, 13, 478. [Google Scholar] [CrossRef]
- Brzóska, M.M.; Kozłowska, M.; Rogalska, J. The beneficial impact of zinc supplementation on the vascular tissue of the abdominal aorta under repeated intoxication with cadmium: A study in an in vivo experimental model. Nutrients 2022, 14, 4080. [Google Scholar] [CrossRef]
- Kodama, A.; Komori, K.; Koyama, A.; Sato, T.; Ikeda, S.; Tsuruoka, T.; Kawai, Y.; Niimi, K.; Sugimoto, M.; Banno, H.; et al. Impact of serum zinc level and oral zinc supplementation on clinical outcomes in patients undergoing infrainguinal bypass for chronic limb-threatening ischemia. Circ. J. 2022, 86, 995–1006. [Google Scholar] [CrossRef]
- El-Adl, M.; Rezk, S.; Ali, M.; Lashen, S.; Fouda, M.; El Sebaei, M.G.; Shukry, M.; Abdelkhalek, N.; Naiel, M.A. The efficiency of zinc sulfate immersion bath on improved wound healing via promoting antioxidant activity, gene expression biomarkers, and skin re-epithelization in a common carp-induced wound model. Appl. Water Sci. 2024, 14, 31. [Google Scholar] [CrossRef]
- Dadfar, R.; Khorsandi, L.; Goujani, R.; Mousavi, S.F.; Aslani, Z. Therapeutic utilization of zinc supplementation concurrent with ozone therapy ameliorates diabetic foot ulcer and attenuates serum level of C-reactive protein-A case report study. Adv. Biomed. Res. 2023, 12, 18. [Google Scholar] [CrossRef]
- Rembe, J.D.; Boehm, J.K.; Fromm-Dornieden, C.; Hauer, N.; Stuermer, E.K. Comprehensive analysis of zinc derivatives pro-proliferative, anti-apoptotic and antimicrobial effect on human fibroblasts and keratinocytes in a simulated, nutrient-deficient environment in vitro. Int. J. Mol. Cell. Med. 2020, 9, 165–178. [Google Scholar] [PubMed]
- Levin, N.; Gray, T.; Farsi, M.; Dorton, D.; Miller, R. Topical zinc may augment post-operative wound healing, including following Mohs micrographic surgery: A review of the literature. SKIN J. Cutan. Med. 2020, 4, 395–403. [Google Scholar] [CrossRef]
- Aryal, E.; Bhattarai, E.; Bhattarai, S. Zinc therapy in dermatology: A review and update. Nepal J. Dermatol. Venereol. Leprol. 2021, 19, 3–8. [Google Scholar] [CrossRef]
- Furihata, K.; Tsuchikawa, M.; Miwa, T.; Naito, Y.; Oba, K.; Sakagami, M. Efficacy and safety of polaprezinc (zinc compound) on zinc deficiency: A systematic review and dose–response meta-analysis of randomized clinical trials using individual patient data. Nutrients 2020, 12, 1128. [Google Scholar] [CrossRef]
- Jalil, S.; Nazir, M.M.; Ali, Q.; Zulfiqar, F.; Moosa, A.; Altaf, M.A.; Zaid, A.; Nafees, M.; Yong, J.W.H.; Jin, X. Zinc and nano zinc mediated alleviation of heavy metals and metalloids in plants: An overview. Funct. Plant Biol. 2023, 50, 870–888. [Google Scholar] [CrossRef] [PubMed]
- Bayram, E.N.; Al-Bakri, N.A.; Al-Shmgani, H.S. Zinc chloride can mitigate the alterations in metallothionein and some apoptotic proteins induced by cadmium chloride in mice hepatocytes: A histological and immunohistochemical study. J. Toxicol. 2023, 2023, 2200539. [Google Scholar] [CrossRef] [PubMed]
- Bagshaw, O.R.; Alva, R.; Goldman, J.; Drelich, J.W.; Stuart, J.A. Mitochondrial zinc toxicity. In Mitochondrial Intoxication; Academic Press: New York, NY, USA, 2023; pp. 723–744. [Google Scholar]
- Belyaeva, E.A. Impact of Zn2+ and Selenite on Rat Ascites Hepatoma AS-30D Cells and Rat Liver Mitochondria: A Comparison with Cd2+, Hg2+, Cu2+, and Ca2+. In Recent Developments in Chemistry and Biochemistry Research; BP International: London, UK, 2025; Volume 10, pp. 130–159. [Google Scholar]
- Seydi, E.; Soltani, M.; Ramazani, M.; Zarei, M.H.; Pourahmad, J. Occupational exposure in lead and zinc mines induces oxidative stress in miners lymphocytes: Role of mitochondrial/lysosomal damage. Main Group Met. Chem. 2020, 43, 154–163. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Zhang, L.; Huang, X. The paradoxical role of zinc on microglia. J. Trace Elem. Med. Biol. 2024, 83, 127380. [Google Scholar] [CrossRef]
- Ajitha, V.; Sreevidya, C.P.; Sarasan, M.; Park, J.C.; Mohandas, A.; Singh, I.S.B.; Puthumana, J.; Lee, J.S. Effects of zinc and mercury on ROS-mediated oxidative stress-induced physiological impairments and antioxidant responses in the microalga Chlorella vulgaris. Environ. Sci. Pollut. Res. 2021, 28, 32475–32492. [Google Scholar] [CrossRef]
- Ohiagu, F.O.; Chikezie, P.C.; Ahaneku, C.C.; Chikezie, C.M. Human exposure to heavy metals: Toxicity mechanisms and health implications. Mater. Sci. Eng. Int. J. 2022, 6, 78–87. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangubotla, R.; Syed, S.; Mastan, A.; Lakshmi, B.A.; Kim, J. Zinc-Mediated Defenses Against Toxic Heavy Metals and Metalloids: Mechanisms, Immunomodulation, and Therapeutic Relevance. Int. J. Mol. Sci. 2025, 26, 9797. https://doi.org/10.3390/ijms26199797
Sangubotla R, Syed S, Mastan A, Lakshmi BA, Kim J. Zinc-Mediated Defenses Against Toxic Heavy Metals and Metalloids: Mechanisms, Immunomodulation, and Therapeutic Relevance. International Journal of Molecular Sciences. 2025; 26(19):9797. https://doi.org/10.3390/ijms26199797
Chicago/Turabian StyleSangubotla, Roopkumar, Shameer Syed, Anthati Mastan, Buddolla Anantha Lakshmi, and Jongsung Kim. 2025. "Zinc-Mediated Defenses Against Toxic Heavy Metals and Metalloids: Mechanisms, Immunomodulation, and Therapeutic Relevance" International Journal of Molecular Sciences 26, no. 19: 9797. https://doi.org/10.3390/ijms26199797
APA StyleSangubotla, R., Syed, S., Mastan, A., Lakshmi, B. A., & Kim, J. (2025). Zinc-Mediated Defenses Against Toxic Heavy Metals and Metalloids: Mechanisms, Immunomodulation, and Therapeutic Relevance. International Journal of Molecular Sciences, 26(19), 9797. https://doi.org/10.3390/ijms26199797










