Lipid Peroxidation in Diabetic Kidney Disease: Mechanism and Natural Solution
Abstract
1. Introduction
2. Mechanisms of Lipid Peroxidation in DKD
2.1. Lipid Metabolism Disorder
2.2. Oxidative Stress and Lipid Peroxidation
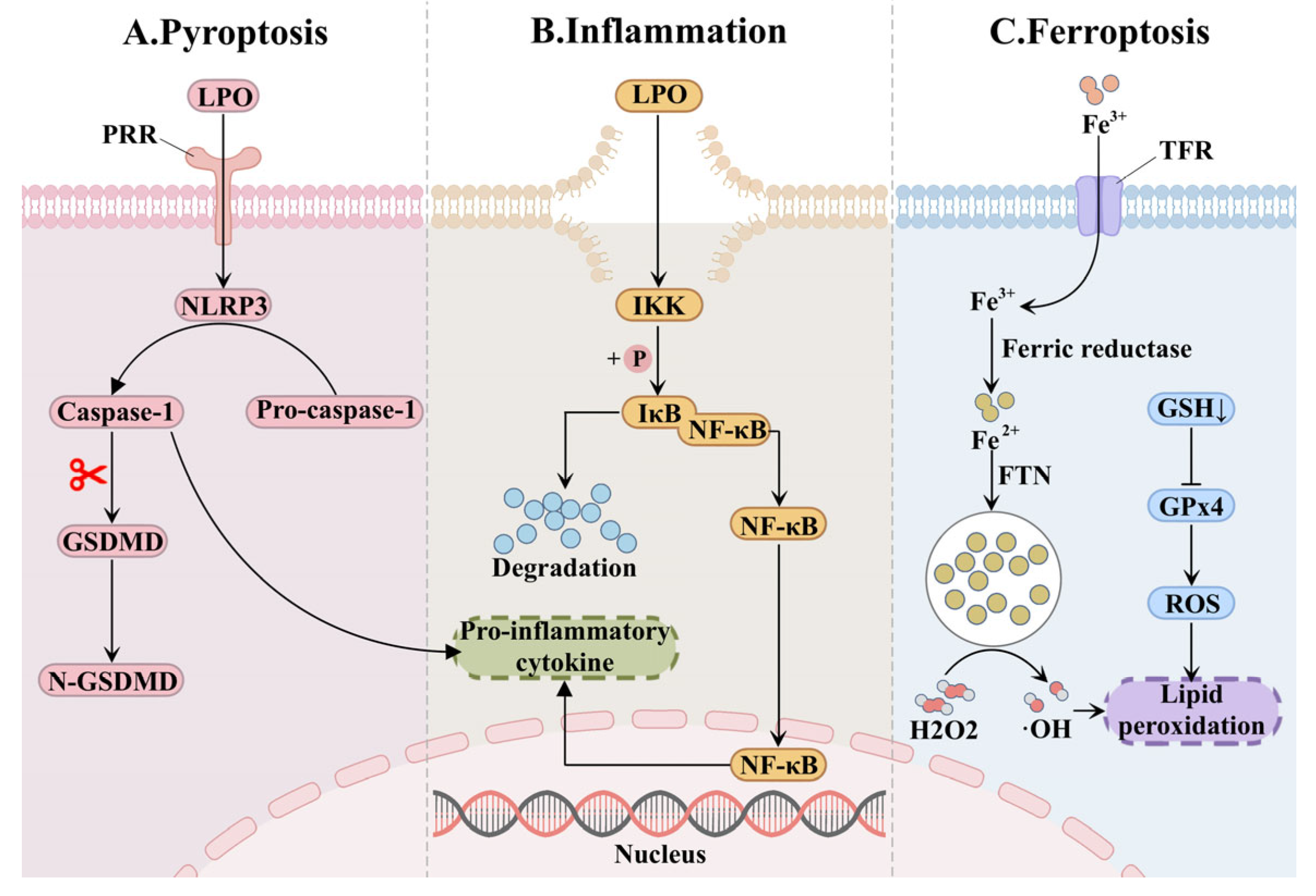
2.3. Lipid Peroxide-Mediated Renal Injury
3. Natural Intervention Strategies for Lipid Peroxidation in Diabetic Kidney Disease
3.1. Cutting Off the Substrate Supply: Restoring Lipid Metabolic Homeostasis
3.2. Reducing Triggers: Reinforcing the Antioxidant Barrier
3.3. Ameliorating Renal Damage: Delaying the Progression of Kidney Pathology
| Natural Product | Source | Application Model | Mechanism | Refs. |
|---|---|---|---|---|
| Resveratrol | Reynoutria japonica Houtt | HFD induced C57BL/6J mice C57BL/KsJ db/db mice HG induced NMS2 cells STZ injected male Wistar rats | Activate SIRT1/PGC1, upregulate PPARa/CPT1, suppress SREBP-1 and ChREBP, suppress AGE/RAGE, reduce 4-HNE | [74,75,76] |
| Curcumin | Curcuma longa L. | OLETF rats | Enhance AMPK phosphorylation, downregulate SREBP-1 and promote ACC phosphorylation | [78] |
| Apigenin | Apium graveolens L. | HFD induced male C57BL/6J mice HF induced SD rats | Suppress SREBP-1c and SREBP-2, downregulate FAS and GLUT1 | [80,81] |
| Soy isoflavones | Glycine max (L.) Merr | obese Zucker rats HFD + STZ injected male SD rats | Active PPARa and suppress SREBP-1c | [82,83] |
| Anthocyanin | Vaccinium uliginosum L. | HFSD + STZ injected male C57BL/6J mice | Scavenge free radicals, activate AMPK, suppress SREBP-1c and ACC | [85] |
| Myricetin | Morella rubra Lour | STZ-Cd induced male albino Wistar rats | Downregulate SREBP-1c and SREBP-2, upregulate PPARa, suppress TGF-β | [86] |
| Hesperidin | Citrus reticulata Blanco | STZ injected male SD rats HFD + STZ injected Wistar rats | Scavenge free radicals, activate Nrf2/ARE signaling pathway, promote Glo-1 and suppress AGE/RAGE, downregulate NF-κB and NLRP3, reduces IL-6 and IL-1β | [88,89] |
| Quercetin | Various herbal plants | HG induced HK-2 cells Male C57BL/KsJ db/db mice | Scavenge ROS, activate Nrf2, downregulate TFR1 and upregulate GPx4, enhance CAT, SOD and GST | [91] |
| Astragaloside IV | Astragalus membranaceus (Fisch.) Bunge | STZ injected male C57BL/6J rats HG induced MPC cells HFD + STZ injected Sprague Dawley rats PA induced HMC cells BKS-db/db mice HG induced MPC cells | Promote Nrf2, downregulate CD36, suppress NOX, inhibit NLRP3 inflammasome, reduce IL-1β, TNF-α, and MCP-1 | [93,94,96] |
| Sinapic acid | Various herbal plants | STZ injected male Wistar rats HFD induced male Syrian hamsters | Scavenge ROS, activate Nrf2, upregulate SOD and GPx4, downregulate NF-κB signaling pathway, promote PPAR and CPT1, suppress SREBP-1, ACC, and FAS | [98,99] |
| Chlorogenic acid | Lonicera japonica Thunb. | STZ injected HBZY-1 cells HFD + STZ injected Wistar rats HG induced HK-2 cells | Upregulate Nrf2/HO-1, downregulate NF-κB and NLRP3 inflammasome signaling pathway | [100,101] |
| Proanthocyanidin | Vitis vinifera L. | Cd + HFSD induced male KM mice | Scavenge ROS, activate Keap1/Nrf2 signaling pathway, upregulate GSH and SOD, suppress NOX via the AGE/RAGE axis | [102] |
| Geniposide | Gardenia jasminoides J. Ellis | HFD + UNx + STZ injected C57BL/6 male mice HFD + STZ injected C57BL/6 mice | Activate PKA, enhance AMPK and autophagy, activate SIRT1 and suppress NF-κB | [104,105] |
| Lycopene | Solanum lycopersicum L. | HFD induced male Wistar rats STZ injected male Wistar rats | upregulate Nrf2, suppress NF-κB, reduce IL-1β and TNF-α, suppress AGEs, upregulate SOD and CAT, promotes GPx | [106,107] |
| Coumarin derivatives | Dipteryx odorata (Aubl.) Willd. | STZ injected male Wistar rats HG induced HBZY-1 cells HFD + STZ injected male C57BL/6 mice AGEs induced HK2 cells | Activate Nrf2, scavenge ROS, activate SIRT3, promote FOXO3a, enhance MnSOD, inhibit Smad2/3, reduce fibronectin, downregulate SREBP | [108,109] |
| Silymarin | Silybum marianum (L.) Gaertn | HG induced MPC5 cells STZ injected male SD rats | Inhibit TGF-β/Smad signaling pathway, upregulate CAT and GPx4 | [110,112] |
| Epigallocatechin gallate | Camellia sinensis (L.) Kuntze | HG induced HPC cells Male C57BL/KsJ db/db mice | Inhibit DNA methyltransferase, reverse ACTN4 hypermethylation, suppress NF-κB and IκBα, reduce α-SMA and inhibit NLRP3 | [115,116] |
| Isoliquiritigenin | Glycyrrhiza uralensis Fisch | HFD + STZ injected Wistar rats HG induced HK-2 cells HFD + STZ injected male SD rats | Scavenge free radicals, suppress NLRP3, NF-κB and JAK/STAT signaling pathways, downregulate TGF-β, fibronectin, and collagen | [89,120] |
| Andrographolide | Andrographis paniculata (Burm. f.) Nees | HFD + STZ induced SD rats AGEs induced MPC5 cells HFD + STZ injected C57BL/6 mice | Suppress STAT3/PI3K/Akt signaling pathway, inhibit NF-κB | [121,122] |
| Berberine | Coptis chinensis Franch | HFD induced C57BL/6J mice and AopE−/− mice | Upregulate GPx4 and Nrf2 | [124] |
| Ginkgolide B | Ginkgo biloba L. | Male C57BL/KsJ db/db mice PA-G induced MPC5 cells HFD induced SD rats | Inhibit TFR1, enhance FTH1, reduce intracellular free iron, suppress the ubiquitination of GPx4, activate PPARa and upregulate Nrf2 | [125,126] |
| Tanshinone IIA | Salvia miltiorrhiza Bunge | db/db mice HG induced HRGEC cells HG induced MPC5 cells | Inhibit NLRP3/caspase-1/GSDMD mediated pyroptosis, downregulate ACSL4, upregulate GSH and scavenge free radicals | [127,128] |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4-HNE | 4-Hydroxy-nonenal |
| ACC | Acetyl-CoA carboxylase |
| ACSL4 | Acyl-CoA synthetase long chain family member 4 |
| AMPK | Adenosine 5′-monophosphate (AMP)-activated protein kinase |
| AGE | Advanced glycation end product |
| ARE | Antioxidant response element |
| ChREBP | Carbohydrate response element binding protein |
| CPT1 | Carnitine palmitoyl transferase 1 |
| CAT | Catalase |
| CD36 | Cluster of differentiation 36 |
| DKD | Diabetic kidney disease |
| ETC | Electron transport chain |
| ESRD | End-stage renal disease |
| EMT | Epithelial–mesenchymal transition |
| FAO | Fatty acid oxidation |
| FAS | Fatty acid synthase |
| FTN | Ferritin |
| GSDMD | Gasdermin D |
| GLUT | Glucose transporter |
| GSH | Glutathione |
| GPx | Glutathione peroxidase |
| GST | Glutathione S-transferase |
| HO-1 | Heme oxygenase-1 |
| IKK | Inhibitor of kappa B kinase |
| IκB | Inhibitor of NF-κB |
| JAK | Janus kinase |
| KeaP1 | Kelch-like ECH-associated protein 1 |
| LOOH | Lipid hydroperoxides |
| MDA | Malondialdehyde |
| MPC | Mitochondrial pyruvate carrier |
| NOX | NADPH oxidase |
| NLRP3 | NOD-like receptor thermal protein domain associated protein 3 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NF-κB | Nuclear factor kappa-B |
| PRR | Pattern recognition receptors |
| PPARa | Perixisome proliferator-activated receptor alpha |
| PI3K | Phosphoinositide 3-kinase |
| PUFA-PE | Polyunsaturated fatty acid-phosphatidyl ethanolamine |
| PUFA | Polyunsaturated fatty acid |
| PKA | Protein kinase A |
| Akt | Protein kinase B |
| ROS | Reactive oxygen species |
| RAGE | Receptor for AGE |
| RAASi | Renin–angiotensin–aldosterone system inhibitor |
| STAT | Signal transducer and activator of transcription |
| SIRT1 | Sirtuin 1 |
| SGLT2i | Sodium-dependent glucose transporters 2 inhibitor |
| SREBP-1c | Sterol regulatory element binding protein-1c |
| SOD | Superoxide dismutase |
| TFR | Transferrin receptor |
| TG | Triglycerides |
| ACTN4 | α-actinin-4 |
| α-SMA | α-smooth muscle actin |
References
- Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2022, 102, S1–S127. [Google Scholar] [CrossRef]
- van Raalte, D.H.; Bjornstad, P.; Cherney, D.Z.I.; de Boer, I.H.; Fioretto, P.; Gordin, D.; Persson, F.; Rosas, S.E.; Rossing, P.; Schaub, J.A.; et al. Combination therapy for kidney disease in people with diabetes mellitus. Nat. Rev. Nephrol. 2024, 20, 433–446. [Google Scholar] [CrossRef]
- Danta, C.C.; Boa, A.N.; Bhandari, S.; Sathyapalan, T.; Xu, S.Z. Recent advances in drug discovery for diabetic kidney disease. Expert Opin. Drug Discov. 2021, 16, 447–461. [Google Scholar] [CrossRef]
- Njeim, R.; Alkhansa, S.; Fornoni, A. Unraveling the crosstalk between lipids and NADPH oxidases in diabetic kidney disease. Pharmaceutics 2023, 15, 1360. [Google Scholar] [CrossRef]
- Hou, Y.; Tan, E.; Shi, H.; Ren, X.; Wan, X.; Wu, W.; Chen, Y.; Niu, H.; Zhu, G.; Li, J.; et al. Mitochondrial oxidative damage reprograms lipid metabolism of renal tubular epithelial cells in the diabetic kidney. Cell. Mol. Life Sci. 2024, 81, 23. [Google Scholar] [CrossRef] [PubMed]
- Raz, I.; Eldor, R.; Cernea, S.; Shafrir, E. Diabetes: Insulin resistance and derangements in lipid metabolism. Cure through intervention in fat transport and storage. Diabetes Metab. Res. Rev. 2005, 21, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Gai, Z.; Wang, T.; Visentin, M.; Kullak-Ublick, G.A.; Fu, X.; Wang, Z. Lipid accumulation and chronic kidney disease. Nutrients 2019, 11, 722. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Zi, X.; Song, J.; Zhao, Q.; Liu, J.; Bao, H.; Li, L. Molecular mechanistic pathways targeted by natural compounds in the prevention and treatment of diabetic kidney disease. Molecules 2022, 27, 6221. [Google Scholar] [CrossRef]
- Zhao, X.; An, X.; Yang, C.; Sun, W.; Ji, H.; Lian, F. The crucial role and mechanism of insulin resistance in metabolic disease. Front. Endocrinol. 2023, 14, 1149239. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Lu, X.; Xie, Q.; Pan, X.; Zhang, R.; Zhang, X.; Peng, G.; Zhang, Y.; Shen, S.; Tong, N. Type 2 diabetes mellitus in adults: Pathogenesis, prevention and therapy. Signal Transduct. Target. Ther. 2024, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Puchałowicz, K.; Rać, M.E. The multifunctionality of CD36 in diabetes mellitus and its complications-update in pathogenesis, treatment and monitoring. Cells 2020, 9, 1877. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D. Fatty acid oxidation and its relation with insulin resistance and associated disorders. Ann. Nutr. Metab. 2016, 68 (Suppl. 3), 15–20. [Google Scholar] [CrossRef] [PubMed]
- Schlaepfer, I.R.; Joshi, M. CPT1A-mediated fat oxidation, mechanisms, and therapeutic potential. Endocrinology 2020, 161, bqz046. [Google Scholar] [CrossRef]
- Jang, H.S.; Noh, M.R.; Kim, J.; Padanilam, B.J. Defective mitochondrial fatty acid oxidation and lipotoxicity in kidney diseases. Front. Med. 2020, 7, 65. [Google Scholar] [CrossRef]
- Bjørndal, B.; Alterås, E.K.; Lindquist, C.; Svardal, A.; Skorve, J.; Berge, R.K. Associations between fatty acid oxidation, hepatic mitochondrial function, and plasma acylcarnitine levels in mice. Nutr. Metab. 2018, 15, 10. [Google Scholar] [CrossRef]
- Herman-Edelstein, M.; Scherzer, P.; Tobar, A.; Levi, M.; Gafter, U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J. Lipid Res. 2014, 55, 561–572. [Google Scholar] [CrossRef]
- Miguel, V.; Tituaña, J.; Herrero, J.I.; Herrero, L.; Serra, D.; Cuevas, P.; Barbas, C.; Puyol, D.R.; Márquez-Expósito, L.; Ruiz-Ortega, M.; et al. Renal tubule CPT1A overexpression protects from kidney fibrosis by restoring mitochondrial homeostasis. J. Clin. Invest. 2021, 131, e140695. [Google Scholar] [CrossRef]
- Nakamura, M.T.; Nara, T.Y. Essential fatty acid synthesis and its regulation in mammals. Prostaglandins Leukot. Essent. Fat. Acids 2003, 68, 145–150. [Google Scholar] [CrossRef]
- Li, L.; Long, J.; Mise, K.; Poungavrin, N.; Lorenzi, P.L.; Mahmud, I.; Tan, L.; Saha, P.K.; Kanwar, Y.S.; Chang, B.H.; et al. The transcription factor ChREBP links mitochondrial lipidomes to mitochondrial morphology and progression of diabetic kidney disease. J. Biol. Chem. 2023, 299, 105185. [Google Scholar] [CrossRef]
- Wedan, R.J.; Longenecker, J.Z.; Nowinski, S.M. Mitochondrial fatty acid synthesis is an emergent central regulator of mammalian oxidative metabolism. Cell Metab. 2024, 36, 36–47. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, L.; Hao, J.; Duan, H.; Liu, S.; Zhao, S.; Liu, Q.; Liu, W. Co-regulation of SREBP-1 and mTOR ameliorates lipid accumulation in kidney of diabetic mice. Exp. Cell Res. 2015, 336, 76–84. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, X.Y.; Tan, H.B.; Huang, M.Y.; Yang, Y.Q.; Guo, J. FTZ alleviates lipid deposition in diabetic kidney disease by AMPK/ACC/SREBP signaling pathway. Acta Diabetol. 2025, 62, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mitrofanova, A.; Merscher, S.; Fornoni, A. Kidney lipid dysmetabolism and lipid droplet accumulation in chronic kidney disease. Nat. Rev. Nephrol. 2023, 19, 629–645. [Google Scholar] [CrossRef] [PubMed]
- Ducasa, G.M.; Mitrofanova, A.; Fornoni, A. Crosstalk between lipids and mitochondria in diabetic kidney disease. Curr. Diab. Rep. 2019, 19, 144. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.T.; Yudell, B.E.; Loor, J.J. Regulation of energy metabolism by long-chain fatty acids. Prog. Lipid Res. 2014, 53, 124–144. [Google Scholar] [CrossRef]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef]
- Yao, L.; Liang, X.; Qiao, Y.; Chen, B.; Wang, P.; Liu, Z. Mitochondrial dysfunction in diabetic tubulopathy. Metabolism 2022, 131, 155195. [Google Scholar] [CrossRef]
- Ito, M.; Gurumani, M.Z.; Merscher, S.; Fornoni, A. Glucose- and non-glucose-induced mitochondrial dysfunction in diabetic kidney disease. Biomolecules 2022, 12, 351. [Google Scholar] [CrossRef]
- Braga, P.C.; Alves, M.G.; Rodrigues, A.S.; Oliveira, P.F. Mitochondrial pathophysiology on chronic kidney disease. Int. J. Mol. Sci. 2022, 23, 1776. [Google Scholar] [CrossRef]
- Efiong, E.E.; Bazireh, H.; Fuchs, M.; Amadi, P.U.; Effa, E.; Sharma, S.; Schmaderer, C. Crosstalk of hyperglycaemia and cellular mechanisms in the pathogenesis of diabetic kidney disease. Int. J. Mol. Sci. 2024, 25, 10882. [Google Scholar] [CrossRef]
- Sanajou, D.; Ghorbani Haghjo, A.; Argani, H.; Aslani, S. AGE-RAGE axis blockade in diabetic nephropathy: Current status and future directions. Eur. J. Pharmacol. 2018, 833, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Pathomthongtaweechai, N.; Chutipongtanate, S. AGE/RAGE signaling-mediated endoplasmic reticulum stress and future prospects in non-coding RNA therapeutics for diabetic nephropathy. Biomed. Pharmacother. 2020, 131, 110655. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ananthakrishnan, R.; Qu, W.; Lu, Y.; Reiniger, N.; Zeng, S.; Ma, W.; Rosario, R.; Yan, S.F.; Ramasamy, R.; et al. RAGE mediates podocyte injury in adriamycin-induced glomerulosclerosis. J. Am. Soc. Nephrol. 2008, 19, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Yiu, W.H.; Wong, D.W.; Wu, H.J.; Li, R.X.; Yam, I.; Chan, L.Y.; Leung, J.C.; Lan, H.Y.; Lai, K.N.; Tang, S.C. Kallistatin protects against diabetic nephropathy in db/db mice by suppressing AGE-RAGE-induced oxidative stress. Kidney Int. 2016, 89, 386–398. [Google Scholar] [CrossRef]
- Kaseda, K.; Kai, Y.; Tajima, M.; Suematsu, M.; Iwata, S.; Miyata, M.; Mifude, C.K.; Yamashita, N.; Seiryu, W.A.; Fukada, M.; et al. Oral administration of spa-derived green alga improves insulin resistance in overweight subjects: Mechanistic insights from fructose-fed rats. Pharmacol. Res. 2020, 152, 104633. [Google Scholar] [CrossRef]
- Eid, A.A.; Gorin, Y.; Fagg, B.M.; Maalouf, R.; Barnes, J.L.; Block, K.; Abboud, H.E. Mechanisms of podocyte injury in diabetes: Role of cytochrome P450 and NADPH oxidases. Diabetes 2009, 58, 1201–1211. [Google Scholar] [CrossRef]
- Kawamura, M.; Kobashi, Y.; Tanaka, H.; Bohno-Mikami, A.; Hamada, M.; Ito, Y.; Hirata, T.; Ohara, H.; Kojima, N.; Koretsune, H.; et al. Discovery of novel pyrazolylpyridine derivatives for 20-hydroxyeicosatetraenoic acid synthase inhibitors with selective CYP4A11/4F2 inhibition. J. Med. Chem. 2022, 65, 14599–14613. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Liu, Y.; Uruno, A.; Saito, R.; Matsukawa, N.; Hishinuma, E.; Saigusa, D.; Liu, H.; Yamamoto, M. Nrf2 deficiency deteriorates diabetic kidney disease in Akita model mice. Redox Biol. 2022, 58, 102525. [Google Scholar] [CrossRef]
- Hashemi, M.; Zandieh, M.A.; Ziaolhagh, S.; Mojtabavi, S.; Sadi, F.H.; Koohpar, Z.K.; Ghanbarirad, M.; Haghighatfard, A.; Behroozaghdam, M.; Khorrami, R.; et al. Nrf2 signaling in diabetic nephropathy, cardiomyopathy and neuropathy: Therapeutic targeting, challenges and future prospective. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166714. [Google Scholar] [CrossRef] [PubMed]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress-A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Niedowicz, D.M.; Daleke, D.L. The role of oxidative stress in diabetic complications. Cell Biochem. Biophys. 2005, 43, 289–330. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Medina-Navarro, R.; Nieto-Aguilar, R.; Alvares-Aguilar, C. Protein conjugated with aldehydes derived from lipid peroxidation as an independent parameter of the carbonyl stress in the kidney damage. Lipids Health Dis. 2011, 10, 201. [Google Scholar] [CrossRef]
- Vazdar, K.; Škulj, S.; Bakarić, D.; Margetić, D.; Vazdar, M. Chemistry and reactivity of 4-hydroxy-2-nonenal (HNE) in model biological systems. Mini Rev. Med. Chem. 2021, 21, 1394–1405. [Google Scholar] [CrossRef]
- Epizzimenti, S.; Ciamporcero, E.S.; Edaga, M.; Epettazzoni, P.; Earcaro, A.; Ecetrangolo, G.; Eminelli, R.; Edianzani, C.; Elepore, A.; Egentile, F.; et al. Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front. Physiol. 2013, 4, 242. [Google Scholar] [CrossRef]
- Schaur, R.J.; Siems, W.; Bresgen, N.; Eckl, P.M. 4-Hydroxy-nonenal-a bioactive lipid peroxidation product. Biomolecules 2015, 5, 2247–2337. [Google Scholar] [CrossRef]
- Soulage, C.O.; Pelletier, C.C.; Florens, N.; Lemoine, S.; Dubourg, L.; Juillard, L.; Guebre-Egziabher, F. Two toxic lipid aldehydes, 4-hydroxy-2-hexenal (4-HHE) and 4-hydroxy-2-nonenal (4-HNE), accumulate in patients with chronic kidney disease. Toxins 2020, 12, 567. [Google Scholar] [CrossRef]
- Mu, F.; Luo, P.; Zhu, Y.; Nie, P.; Li, B.; Bai, X. Iron metabolism and ferroptosis in diabetic kidney disease. Cell Biochem. Funct. 2025, 43, e70067. [Google Scholar] [CrossRef] [PubMed]
- Barnabei, L.; Laplantine, E.; Mbongo, W.; Rieux-Laucat, F.; Weil, R. NF-κB: At the borders of autoimmunity and inflammation. Front. Immunol. 2021, 12, 716469. [Google Scholar] [CrossRef] [PubMed]
- White, S.; Lin, L.; Hu, K. NF-κB and tPA signaling in kidney and other diseases. Cells 2020, 9, 1348. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, I.; Liu, W.; Akhand, A.A.; Takeda, K.; Kawamoto, Y.; Kato, M.; Suzuki, H. 4-hydroxynonenal triggers multistep signal transduction cascades for suppression of cellular functions. Mol. Asp. Med. 2003, 24, 231–238. [Google Scholar] [CrossRef]
- Ioannidis, M.; Tjepkema, J.; Uitbeijerse, M.R.P.; van den Bogaart, G. Immunomodulatory effects of 4-hydroxynonenal. Redox Biol. 2025, 85, 103719. [Google Scholar] [CrossRef]
- Uchida, K. Redox-derived damage-associated molecular patterns: Ligand function of lipid peroxidation adducts. Redox Biol. 2013, 1, 94–96. [Google Scholar] [CrossRef]
- Tang, S.C.W.; Yiu, W.H. Innate immunity in diabetic kidney disease. Nat. Rev. Nephrol. 2020, 16, 206–222. [Google Scholar] [CrossRef]
- Fu, J.; Wu, H. Structural mechanisms of NLRP3 inflammasome assembly and activation. Annu. Rev. Immunol. 2023, 41, 301–316. [Google Scholar] [CrossRef]
- Henedak, N.T.; El-Abhar, H.S.; Soubh, A.A.; Abdallah, D.M. NLRP3 inflammasome: A central player in renal pathologies and nephropathy. Life Sci. 2024, 351, 122813. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Z.; Li, Y. Relevance of the pyroptosis-related inflammasome pathway in the pathogenesis of diabetic kidney disease. Front. Immunol. 2021, 12, 603416. [Google Scholar] [CrossRef]
- Foresto-Neto, O.; Albino, A.H.; Arias, S.C.A.; Faustino, V.D.; Zambom, F.F.F.; Cenedeze, M.A.; Elias, R.M.; Malheiros, D.M.A.C.; Camara, N.O.S.; Fujihara, C.K.; et al. NF-κB system is chronically activated and promotes glomerular injury in experimental type 1 diabetic kidney disease. Front. Physiol. 2020, 11, 84. [Google Scholar] [CrossRef]
- Wu, K.; Zha, H.; Wu, T.; Liu, H.; Peng, R.; Lin, Z.; Lv, D.; Liao, X.; Sun, Y.; Zhang, Z. Cytosolic Hmgb1 accumulation in mesangial cells aggravates diabetic kidney disease progression via NFκB signaling pathway. Cell. Mol. Life Sci. 2024, 81, 408. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, P.; Bailey, T.; Bhattarai, S.; Subedi, U.; Miller, C.; Ara, H.; Kidambi, S.; Sun, H.; Panchatcharam, M.; et al. Electrophilic aldehyde 4-hydroxy-2-nonenal mediated signaling and mitochondrial dysfunction. Biomolecules 2022, 12, 1555. [Google Scholar] [CrossRef] [PubMed]
- Maiorino, M.; Conrad, M.; Ursini, F. GPx4, lipid oeroxidation, and cell death: Discoveries, rediscoveries, and open issues. Antioxid. Redox Signal 2018, 29, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Takahashi, K.; Hatakawa, Y.; Oe, T. Lipid peroxidation-derived modification and its effect on the activity of glutathione peroxidase 1. Free Radic. Biol. Med. 2023, 208, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Morell, F.; Flohé, L.; Marín, N.; Romero, F.J. 4-Hydroxynonenal inhibits glutathione peroxidase: Protection by glutathione. Free Radic. Biol. Med. 1999, 26, 1383–1387. [Google Scholar] [CrossRef]
- Gao, G.; Li, J.; Zhang, Y.; Chang, Y.Z. Cellular iron metabolism and regulation. Adv. Exp. Med. Biol. 2019, 1173, 21–32. [Google Scholar] [CrossRef]
- Zhong, S.; Wang, N.; Zhang, C. Podocyte death in diabetic kidney disease: Potential molecular mechanisms and therapeutic targets. Int. J. Mol. Sci. 2024, 25, 9035. [Google Scholar] [CrossRef]
- Wang, H.; Liu, D.; Zheng, B.; Yang, Y.; Qiao, Y.; Li, S.; Pan, S.; Liu, Y.; Feng, Q.; Liu, Z. Emerging role of ferroptosis in diabetic kidney disease: Molecular mechanisms and therapeutic opportunities. Int. J. Biol. Sci. 2023, 19, 2678–2694. [Google Scholar] [CrossRef]
- Wu, K.; Zhu, E.; Chen, J.; Kuang, Q.; Lin, J.; Zhao, S.; Xu, X.; Li, S.; Sui, Y.; Huang, M.; et al. Overexpression of GPX4 in diabetic rat kidney alleviates renal injury induced by ferroptosis. Biometals 2025, 38, 1281–1297. [Google Scholar] [CrossRef]
- Khamis, M.M.; Moselhy, S.S.; Rihan, S. Role of trans-resveratrol in ameliorating biochemical and molecular alterations in obese rats induced by a high fructose/fat diet. Sci. Rep. 2025, 15, 7879. [Google Scholar] [CrossRef]
- Sikur, N.; Böröczky, C.; Paszternák, A.; Gyöngyössy, R.; Szökő, É.; Varga, K.; Tábi, T. Resveratrol and its derivatives diminish lipid accumulation in adipocytes in vitro-mechanism of action and structure-activity relationship. Nutrients 2024, 16. [Google Scholar] [CrossRef]
- Gu, W.; Wang, X.; Zhao, H.; Geng, J.; Li, X.; Zheng, K.; Guan, Y.; Hou, X.; Wang, C.; Song, G. Resveratrol ameliorates diabetic kidney injury by reducing lipotoxicity and modulates expression of components of the junctional adhesion molecule-like/sirtuin 1 lipid metabolism pathway. Eur. J. Pharmacol. 2022, 918, 174776. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Lim, J.H.; Youn, H.H.; Hong, Y.A.; Yang, K.S.; Park, H.S.; Chung, S.; Ko, S.H.; Shin, S.J.; Choi, B.S.; et al. Resveratrol prevents renal lipotoxicity and inhibits mesangial cell glucotoxicity in a manner dependent on the AMPK-SIRT1-PGC1α axis in db/db mice. Diabetologia 2013, 56, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Al-Hussaini, H.; Kilarkaje, N. Trans-resveratrol mitigates type 1 diabetes-induced oxidative DNA damage and accumulation of advanced glycation end products in glomeruli and tubules of rat kidneys. Toxicol. Appl. Pharmacol. 2018, 339, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Witkin, J.M.; Li, X. Curcumin, an active constiuent of the ancient medicinal herb Curcuma longa L.: Some uses and the establishment and biological basis of medical efficacy. CNS Neurol. Disord. Drug Targets 2013, 12, 487–497. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, E.S.; Choi, R.; Nawaboot, J.; Lee, M.Y.; Lee, E.Y.; Kim, H.S.; Chung, C.H. Protective effects of curcumin on renal oxidative stress and lipid metabolism in a rat model of type 2 diabetic nephropathy. Yonsei Med. J. 2016, 57, 664–673. [Google Scholar] [CrossRef]
- Allemailem, K.S.; Almatroudi, A.; Alharbi, H.O.A.; AlSuhaymi, N.; Alsugoor, M.H.; Aldakheel, F.M.; Khan, A.A.; Rahmani, A.H. Apigenin: A bioflavonoid with a promising role in disease prevention and treatment. Biomedicines 2024, 12, 1353. [Google Scholar] [CrossRef]
- Wu, L.; Guo, T.; Deng, R.; Liu, L.; Yu, Y. Apigenin ameliorates insulin resistance and lipid accumulation by endoplasmic reticulum stress and SREBP-1c/SREBP-2 pathway in palmitate-induced HepG2 cells and high-fat diet-fed mice. J. Pharmacol. Exp. Ther. 2021, 377, 146–156. [Google Scholar] [CrossRef]
- Chen, X.; Tan, J.; Zhang, L.; Liu, Y.; Cheng, Y.; Zhang, Q.; Ding, H. Apigenin ameliorates vascular injury in rats with high fructose-induced metabolic disturbance by inhibiting PI3K/AKT/GLUT1. RSC Adv. 2018, 8, 24470–24476. [Google Scholar] [CrossRef] [PubMed]
- Mezei, O.; Banz, W.J.; Steger, R.W.; Peluso, M.R.; Winters, T.A.; Shay, N. Soy isoflavones exert antidiabetic and hypolipidemic effects through the PPAR pathways in obese Zucker rats and murine RAW 264.7 cells. J. Nutr. 2003, 133, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Misiakiewicz-Has, K.; Maciejewska-Markiewicz, D.; Szypulska-Koziarska, D.; Kolasa, A.; Wiszniewska, B. The influence of soy isoflavones and soy isoflavones with inulin on kidney morphology, fatty acids, and associated parameters in rats with and without induced diabetes type 2. Int. J. Mol. Sci. 2024, 25, 5418. [Google Scholar] [CrossRef] [PubMed]
- Danielewski, M.; Matuszewska, A.; Szeląg, A.; Sozański, T. The impact of anthocyanins and iridoids on transcription factors crucial for lipid and cholesterol homeostasis. Int. J. Mol. Sci. 2021, 22, 6074. [Google Scholar] [CrossRef]
- Herrera-Balandrano, D.D.; Chai, Z.; Hutabarat, R.P.; Beta, T.; Feng, J.; Ma, K.; Li, D.; Huang, W. Hypoglycemic and hypolipidemic effects of blueberry anthocyanins by AMPK activation: In vitro and in vivo studies. Redox Biol. 2021, 46, 102100. [Google Scholar] [CrossRef]
- Kandasamy, N.; Ashokkumar, N. Renoprotective effect of myricetin restrains dyslipidemia and renal mesangial cell proliferation by the suppression of sterol regulatory element binding proteins in an experimental model of diabetic nephropathy. Eur. J. Pharmacol. 2014, 743, 53–62. [Google Scholar] [CrossRef]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef]
- Chen, Y.J.; Kong, L.; Tang, Z.Z.; Zhang, Y.M.; Liu, Y.; Wang, T.Y.; Liu, Y.W. Hesperetin ameliorates diabetic nephropathy in rats by activating Nrf2/ARE/glyoxalase 1 pathway. Biomed. Pharmacother. 2019, 111, 1166–1175. [Google Scholar] [CrossRef]
- Meenu; Bano, U.; Mujeeb, M.; Akhtar, M.; Aqil, M.; Haque, S.E.U.; Najmi, A.K. Hesperetin and isoliquiritigenin attenuate the progression of diabetic nephropathy via inhibition of NF-κB/NLRP3 pathways in type 2 diabetic rats. ACS Omega 2025, 10, 34342–34351. [Google Scholar] [CrossRef]
- Muñoz-Reyes, D.; Morales, A.I.; Prieto, M. Transit and metabolic pathways of quercetin in tubular cells: Involvement of its antioxidant properties in the kidney. Antioxidants 2021, 10, 909. [Google Scholar] [CrossRef]
- Feng, Q.; Yang, Y.; Qiao, Y.; Zheng, Y.; Yu, X.; Liu, F.; Wang, H.; Zheng, B.; Pan, S.; Ren, K.; et al. Quercetin ameliorates diabetic kidney injury by inhibiting ferroptosis via activating Nrf2/HO-1 signaling pathway. Am. J. Chin. Med. 2023, 51, 997–1018. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.H.; Alsahli, M.A.; Khan, A.A.; Almatroodi, S.A. Quercetin, a plant flavonol attenuates diabetic complications, renal tissue damage, renal oxidative stress and inflammation in streptozotocin-induced diabetic rats. Metabolites 2023, 13, 130. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Fang, J.; Guo, H.; Su, X.; Zhu, B.; Yao, X.; Wang, Y.; Cao, A.; Wang, H.; Wang, L. Astragaloside IV attenuates podocyte apoptosis through ameliorating mitochondrial dysfunction by up-regulated Nrf2-ARE/TFAM signaling in diabetic kidney disease. Free Radic. Biol. Med. 2023, 203, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Chen, Q.; Ma, K.; Ju, Y.; Ji, T.; Wang, Z.; Li, W.; Li, W. Astragaloside IV inhibits palmitate-mediated oxidative stress and fibrosis in human glomerular mesangial cells via downregulation of CD36 expression. Pharmacol. Rep. 2019, 71, 319–329. [Google Scholar] [CrossRef]
- Zang, J.; Peters, F.; Cambet, Y.; Cifuentes-Pagano, E.; Hissabu, M.M.S.; Dustin, C.M.; Svensson, L.H.; Olesen, M.M.; Poulsen, M.F.L.; Jacobsen, S.; et al. Targeting NOX2 with bivalent small-molecule p47phox-p22phox inhibitors. J. Med. Chem. 2023, 66, 14963–15005. [Google Scholar] [CrossRef]
- Feng, H.; Zhu, X.; Tang, Y.; Fu, S.; Kong, B.; Liu, X. Astragaloside IV ameliorates diabetic nephropathy in db/db mice by inhibiting NLRP3 inflammasome-mediated inflammation. Int. J. Mol. Med. 2021, 48, 164. [Google Scholar] [CrossRef]
- Chen, C. Sinapic acid and its derivatives as medicine in oxidative stress-induced diseases and aging. Oxid. Med. Cell Longev. 2016, 2016, 3571614. [Google Scholar] [CrossRef]
- Alaofi, A.L. Sinapic acid ameliorates the progression of streptozotocin (STZ)-induced diabetic nephropathy in rats via NRF2/HO-1 mediated pathways. Front. Pharmacol. 2020, 11, 1119. [Google Scholar] [CrossRef]
- Wang, K.; Liang, C.; Cao, W.; Luo, G.; Zhong, S.; Zeng, Z.; Dai, L.; Song, J.L. Dietary sinapic acid attenuated high-fat diet-induced lipid metabolism and oxidative stress in male Syrian hamsters. J. Food Biochem. 2022, 46, e14203. [Google Scholar] [CrossRef]
- Bao, L.; Li, J.; Zha, D.; Zhang, L.; Gao, P.; Yao, T.; Wu, X. Chlorogenic acid prevents diabetic nephropathy by inhibiting oxidative stress and inflammation through modulation of the Nrf2/HO-1 and NF-ĸB pathways. Int. Immunopharmacol. 2018, 54, 245–253. [Google Scholar] [CrossRef]
- Bao, L.; Gong, Y.; Xu, W.; Dao, J.; Rao, J.; Yang, H. Chlorogenic acid inhibits NLRP3 inflammasome activation through Nrf2 activation in diabetic nephropathy. PLoS ONE 2025, 20, e0316615. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Wang, P.; Pi, S.; Guo, Y.; Pei, S.; Yang, W.; Chang, X.; Wang, L.; Chen, F. Proanthocyanidins protect against cadmium-induced diabetic nephropathy through p38 MAPK and Keap1/Nrf2 signaling pathways. Front. Pharmacol. 2021, 12, 801048. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Cheng, K.W.; Ma, J.; Chen, B.; Ho, C.T.; Lo, C.; Chen, F.; Wang, M. Cinnamon bark proanthocyanidins as reactive carbonyl scavengers to prevent the formation of advanced glycation endproducts. J. Agric. Food Chem. 2008, 56, 1907–1911. [Google Scholar] [CrossRef] [PubMed]
- Dusabimana, T.; Park, E.J.; Je, J.; Jeong, K.; Yun, S.P.; Kim, H.J.; Kim, H.; Park, S.W. Geniposide improves diabetic nephropathy by enhancing ULK1-mediated autophagy and reducing oxidative stress through AMPK activation. Int. J. Mol. Sci. 2021, 22, 1651. [Google Scholar] [CrossRef]
- Li, F.; Chen, Y.; Li, Y.; Huang, M.; Zhao, W. Geniposide alleviates diabetic nephropathy of mice through AMPK/SIRT1/NF-κB pathway. Eur. J. Pharmacol. 2020, 886, 173449. [Google Scholar] [CrossRef]
- Albrahim, T.; Robert, A.A. Lycopene effects on metabolic syndrome and kidney injury in rats fed a high-fat diet: An experimental study. ACS Omega 2022, 7, 30930–30938. [Google Scholar] [CrossRef]
- Figueiredo, I.D.; Lima, T.F.O.; Inácio, M.D.; Costa, M.C.; Assis, R.P.; Brunetti, I.L.; Baviera, A.M. Lycopene improves the metformin effects on glycemic control and decreases biomarkers of glycoxidative stress in diabetic rats. Diabetes Metab. Syndr. Obes. 2020, 13, 3117–3135. [Google Scholar] [CrossRef]
- Sen, Z.; Weida, W.; Jie, M.; Li, S.; Dongming, Z.; Xiaoguang, C. Coumarin glycosides from Hydrangea paniculata slow down the progression of diabetic nephropathy by targeting Nrf2 anti-oxidation and smad2/3-mediated profibrosis. Phytomedicine 2019, 57, 385–395. [Google Scholar] [CrossRef]
- Liu, M.; Xuan, A.; Zheng, L.; Li, D.; Chen, C.; Liu, H.; Lu, G.; Cheng, Z.; Zou, Y.; Zhi, S.; et al. Novel coumarin derivative SZC-6 as an allosteric activator of SIRT3 alleviates diabetic kidney disease via the SIRT3-Foxo3a signaling axis. Free Radic. Biol. Med. 2025, 240, 29–45. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Yang, T. Silymarin nanoliposomes attenuate renal injury on diabetic nephropathy rats via co-suppressing TGF-β/Smad and JAK2/STAT3/SOCS1 pathway. Life Sci. 2021, 271, 119197. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, D.; Kang, X.; Zhou, R.; Sun, Y.; Lian, F.; Tong, X. Signaling pathways involved in diabetic renal fibrosis. Front. Cell Dev. Biol. 2021, 9, 696542. [Google Scholar] [CrossRef] [PubMed]
- Vessal, G.; Akmali, M.; Najafi, P.; Moein, M.R.; Sagheb, M.M. Silymarin and milk thistle extract may prevent the progression of diabetic nephropathy in streptozotocin-induced diabetic rats. Ren. Fail. 2010, 32, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Kumar, M.; Muntel, J.; Gurley, S.B.; Birrane, G.; Stillman, I.E.; Ding, L.; Wang, M.; Ahmed, S.; Schlondorff, J.; et al. Phosphorylation of ACTN4 leads to podocyte vulnerability and proteinuric glomerulosclerosis. J. Am. Soc. Nephrol. 2020, 31, 1479–1495. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Natarajan, R. Epigenetics and epigenomics in diabetic kidney disease and metabolic memory. Nat. Rev. Nephrol. 2019, 15, 327–345. [Google Scholar] [CrossRef]
- He, C.; Wang, D.; Wang, R.; Huang, Y.; Huang, X.; Shen, S.; Lv, J.; Wu, M. Epigallocatechin gallate induces the demethylation of actinin alpha 4 to inhibit diabetic nephropathy renal fibrosis via the NF-KB signaling pathway in vitro. Dose Response 2022, 20, 15593258221105704. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Wang, M.; Wang, X.; Liu, Q.; Lv, S.; Nie, H.; Liu, G. Epigallocatechin-3-gallate ameliorates diabetic kidney disease by inhibiting the TXNIP/NLRP3/IL-1β signaling pathway. Food Sci. Nutr. 2024, 12, 10800–10815. [Google Scholar] [CrossRef]
- Skorokhod, O.; Barrera, V.; Mandili, G.; Costanza, F.; Valente, E.; Ulliers, D.; Schwarzer, E. Malaria pigment hemozoin impairs GM-CSF receptor expression and function by 4-hydroxynonenal. Antioxidants 2021, 10, 1259. [Google Scholar] [CrossRef]
- Achuthan, A.A.; Lee, K.M.C.; Hamilton, J.A. Targeting GM-CSF in inflammatory and autoimmune disorders. Semin. Immunol. 2021, 54, 101523. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Zhang, J.; Gao, S.; Xu, T.; Yin, Y. JAK/STAT signaling in diabetic kidney disease. Front. Cell Dev. Biol. 2023, 11, 1233259. [Google Scholar] [CrossRef]
- Sun, L.; Yang, Z.; Zhang, J.; Wang, J. Isoliquiritigenin attenuates acute renal injury through suppressing oxidative stress, fibrosis and JAK2/STAT3 pathway in streptozotocin-induced diabetic rats. Bioengineered 2021, 12, 11188–11200. [Google Scholar] [CrossRef]
- Yin, Y.; He, J.; Fang, Y.; Wei, M.; Zhang, W. Andrographolide as a multi-target therapeutic agent in diabetic nephropathy: Insights into STAT3/PI3K/Akt pathway modulation. Biomol. Ther. 2025, 33, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Li, C.; Ou, Y.; Li, N.; Yuan, K.; Yang, G.; Chen, X.; Yang, Z.; Liu, B.; Cheung, W.W.; et al. Andrographolide ameliorates diabetic nephropathy by attenuating hyperglycemia-mediated renal oxidative stress and inflammation via Akt/NF-κB pathway. Mol. Cell. Endocrinol. 2016, 437, 268–279. [Google Scholar] [CrossRef]
- Ma, X.; Chen, Z.; Wang, L.; Wang, G.; Wang, Z.; Dong, X.; Wen, B.; Zhang, Z. The pathogenesis of diabetes mellitus by oxidative stress and inflammation: Its inhibition by berberine. Front. Pharmacol. 2018, 9, 782. [Google Scholar] [CrossRef]
- Wang, T.T.; Yu, L.L.; Zheng, J.M.; Han, X.Y.; Jin, B.Y.; Hua, C.J.; Chen, Y.S.; Shang, S.S.; Liang, Y.Z.; Wang, J.R. Berberine inhibits ferroptosis and stabilizes atherosclerotic plaque through NRF2/SLC7A11/GPX4 pathway. Chin. J. Integr. Med. 2024, 30, 906–916. [Google Scholar] [CrossRef]
- Chen, J.; Ou, Z.; Gao, T.; Yang, Y.; Shu, A.; Xu, H.; Chen, Y.; Lv, Z. Ginkgolide B alleviates oxidative stress and ferroptosis by inhibiting GPX4 ubiquitination to improve diabetic nephropathy. Biomed. Pharmacother. 2022, 156, 113953. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, F.; Xu, H.; Wang, H.; Lu, M.; Cheng, L. Ginkgolide B attenuates hyperlipidemia by restoring sphingolipid homeostasis and activating PPARα and Nrf2 pathways. Sci. Rep. 2025, 15, 28774. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Guan, Y.B.; Zhang, K.J.; Li, L.; Zhou, Y. Tanshinone IIA mediates protection from diabetes kidney disease by inhibiting oxidative stress induced pyroptosis. J. Ethnopharmacol. 2023, 316, 116667. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Kang, Z.; Zhang, F. Tanshinone IIA suppresses ferroptosis to attenuate renal podocyte injury in diabetic nephropathy through the embryonic lethal abnormal visual-like protein 1 and acyl-coenzyme A synthetase long-chain family member 4 signaling pathway. J. Diabetes Investig. 2024, 15, 1003–1016. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Tong, Y. Lipid Peroxidation in Diabetic Kidney Disease: Mechanism and Natural Solution. Int. J. Mol. Sci. 2025, 26, 9764. https://doi.org/10.3390/ijms26199764
Dong Y, Tong Y. Lipid Peroxidation in Diabetic Kidney Disease: Mechanism and Natural Solution. International Journal of Molecular Sciences. 2025; 26(19):9764. https://doi.org/10.3390/ijms26199764
Chicago/Turabian StyleDong, Yuxin, and Yanqing Tong. 2025. "Lipid Peroxidation in Diabetic Kidney Disease: Mechanism and Natural Solution" International Journal of Molecular Sciences 26, no. 19: 9764. https://doi.org/10.3390/ijms26199764
APA StyleDong, Y., & Tong, Y. (2025). Lipid Peroxidation in Diabetic Kidney Disease: Mechanism and Natural Solution. International Journal of Molecular Sciences, 26(19), 9764. https://doi.org/10.3390/ijms26199764






