Mutation of the GDP-Fucose Biosynthesis Gene gmds Increases Hair Cell Number and Neuromast Regenerative Capacity in Zebrafish
Abstract
1. Introduction
2. Results
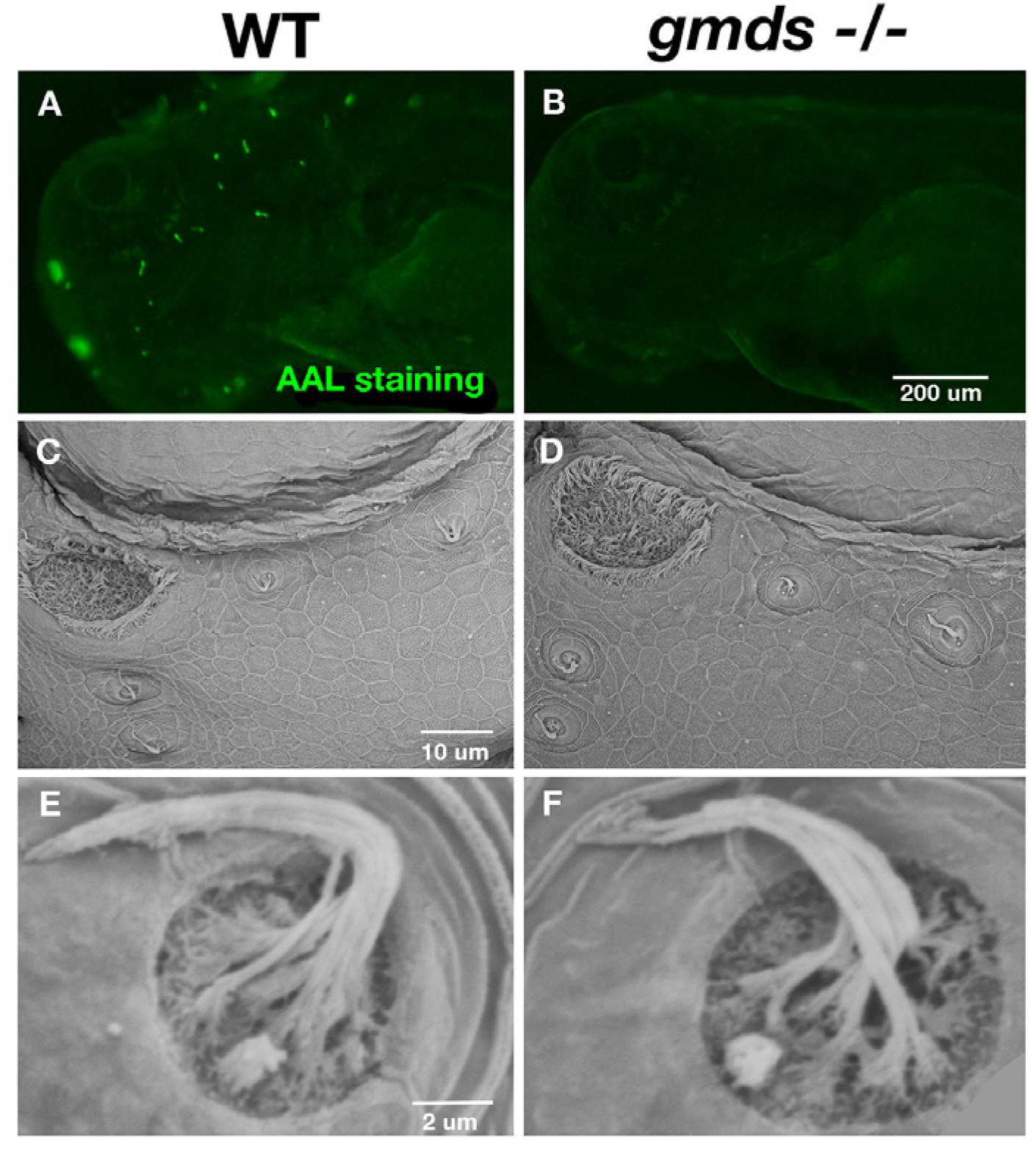
2.1. Abnormal Fucosylation but Normal Neuromast Morphology in gmds−/− Mutants
2.2. Circular Swimming and Increased Hair Cell Number in gmds Mutant Neuromasts Under Homeostatic Conditions
2.3. Neuromast Markers Are Not Altered in gmds Mutants Under Homeostatic Conditions
2.4. Increased Neuromast Regeneration Capacity of gmds−/− Mutants with DASPEI Staining
2.5. Scanning Electron Microscopy Imaging of Neuromasts
2.6. Quantitative Assessment of Increased Hair Cell Regeneration Capacity of gmds−/− Mutants with YO-PRO-1 Staining
2.7. Inhibition of Notch Signalling Affects Hair Cell Regeneration in gmds−/− Mutants
3. Discussion
4. Materials and Methods
4.1. Zebrafish Husbandry
4.2. In Situ Hybridization Gene Expression
4.3. AAL Staining for Fucose Detection and Immunohistochemistry
4.4. Hair Cell Damage Induction
4.5. Neuromast Regeneration Time-Lapse Imaging with DASPEI
4.6. YO-PRO-1 Hair Cell Quantification Under Homeostatic Conditions and After Ablation
4.7. Scanning Electron Microscopy (SEM) of Hair Cell Regeneration
4.8. Inhibition of Notch Signalling During Regeneration
4.9. Data Preparation and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss (accessed on 1 May 2025).
- Kros, C.J.; Steyger, P.S. Aminoglycoside- and Cisplatin-Induced Ototoxicity: Mechanisms and Otoprotective Strategies. Cold Spring Harb. Perspect. Med. 2019, 9, a033548. [Google Scholar] [CrossRef]
- Rubel, E.W.; Furrer, S.A.; Stone, J.S. A brief history of hair cell regeneration research and speculations on the future. Hear. Res. 2013, 297, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Kniss, J.S.; Jiang, L.; Piotrowski, T. Insights into sensory hair cell regeneration from the zebrafish lateral line. Curr. Opin. Genet. Dev. 2016, 40, 32–40. [Google Scholar] [CrossRef]
- Lush, M.E.; Piotrowski, T. Sensory hair cell regeneration in the zebrafish lateral line. Dev. Dyn. 2014, 243, 1187–1202. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, T.T. Zebrafish as a model for hearing and deafness. J. Neurobiol. 2002, 53, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, T. The genetics of hearing and balance in zebrafish. Annu. Rev. Genet. 2005, 39, 9–22. [Google Scholar] [CrossRef]
- Kindig, K.; Stepanyan, R.; Kindt, K.S.; McDermott, B.M., Jr. Asymmetric mechanotransduction by hair cells of the zebrafish lateral line. Curr. Biol. 2023, 33, 1295–1307 e3. [Google Scholar] [CrossRef]
- Pickett, S.B.; Raible, D.W. Water Waves to Sound Waves: Using Zebrafish to Explore Hair Cell Biology. J. Assoc. Res. Otolaryngol. 2019, 20, 1–19. [Google Scholar] [CrossRef]
- Lush, M.E.; Diaz, D.C.; Koenecke, N.; Baek, S.; Boldt, H.; St Peter, M.K.; Gaitan-Escudero, T.; Romero-Carvajal, A.; Busch-Nentwich, E.M.; Perera, A.G.; et al. scRNA-Seq reveals distinct stem cell populations that drive hair cell regeneration after loss of Fgf and Notch signaling. Elife 2019, 8. [Google Scholar] [CrossRef]
- Bai, H.; Jiang, L.; Wang, X.; Gao, X.; Bing, J.; Xi, C.; Wang, W.; Zhang, M.; Zhang, X.; Han, Z.; et al. Transcriptomic analysis of mouse cochleae suffering from gentamicin damage reveals the signalling pathways involved in hair cell regeneration. Sci. Rep. 2019, 9, 10494. [Google Scholar] [CrossRef]
- Hu, Z.; Singh, A.; Bojrab, D., 2nd; Sim, N. Insights into the molecular mechanisms regulating mammalian hair cell regeneration. Curr. Opin. Otolaryngol. Head. Neck Surg. 2021, 29, 400–406. [Google Scholar] [CrossRef]
- Head, J.R.; Gacioch, L.; Pennisi, M.; Meyers, J.R. Activation of canonical Wnt/beta-catenin signaling stimulates proliferation in neuromasts in the zebrafish posterior lateral line. Dev. Dyn. 2013, 242, 832–846. [Google Scholar] [CrossRef]
- Tang, D.; He, Y.; Li, W.; Li, H. Wnt/beta-catenin interacts with the FGF pathway to promote proliferation and regenerative cell proliferation in the zebrafish lateral line neuromast. Exp. Mol. Med. 2019, 51, 1–16. [Google Scholar] [CrossRef]
- Romero-Carvajal, A.; Navajas Acedo, J.; Jiang, L.; Kozlovskaja-Gumbriene, A.; Alexander, R.; Li, H.; Piotrowski, T. Regeneration of Sensory Hair Cells Requires Localized Interactions between the Notch and Wnt Pathways. Dev. Cell 2015, 34, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.Y.; Rubel, E.W.; Raible, D.W. Notch signaling regulates the extent of hair cell regeneration in the zebrafish lateral line. J. Neurosci. 2008, 28, 2261–2273. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Romero-Carvajal, A.; Haug, J.S.; Seidel, C.W.; Piotrowski, T. Gene-expression analysis of hair cell regeneration in the zebrafish lateral line. Proc. Natl. Acad. Sci. USA 2014, 111, E1383–E1392, Erratum in Proc. Natl. Acad. Sci. USA 2015, 112, E6080. [Google Scholar] [CrossRef]
- Skurska, E.; Olczak, M. Interplay between de novo and salvage pathways of GDP-fucose synthesis. PLoS ONE 2024, 19, e0309450. [Google Scholar] [CrossRef]
- Becker, D.J.; Lowe, J.B. Fucose: Biosynthesis and biological function in mammals. Glycobiology 2003, 13, 41R–53R. [Google Scholar] [CrossRef]
- Fowler, G.; French, D.V.; Rose, A.; Squires, P.; Aniceto da Silva, C.; Ohata, S.; Okamoto, H.; French, C.R. Protein fucosylation is required for Notch dependent vascular integrity in zebrafish. Dev. Biol. 2021, 480, 62–68. [Google Scholar] [CrossRef]
- Stanley, P. Regulation of Notch signaling by glycosylation. Curr. Opin. Struct. Biol. 2007, 17, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Holdener, B.C.; Haltiwanger, R.S. Protein O-fucosylation: Structure and function. Curr. Opin. Struct. Biol. 2019, 56, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Rampal, R.; Arboleda-Velasquez, J.F.; Nita-Lazar, A.; Kosik, K.S.; Haltiwanger, R.S. Highly conserved O-fucose sites have distinct effects on Notch1 function. J. Biol. Chem. 2005, 280, 32133–32140. [Google Scholar] [CrossRef]
- Sasamura, T.; Sasaki, N.; Miyashita, F.; Nakao, S.; Ishikawa, H.O.; Ito, M.; Kitagawa, M.; Harigaya, K.; Spana, E.; Bilder, D.; et al. neurotic, a novel maternal neurogenic gene, encodes an O-fucosyltransferase that is essential for Notch-Delta interactions. Development 2003, 130, 4785–4795. [Google Scholar] [CrossRef] [PubMed]
- Koleilat, A.; Dugdale, J.A.; Christenson, T.A.; Bellah, J.L.; Lambert, A.M.; Masino, M.A.; Ekker, S.C.; Schimmenti, L.A. L-type voltage-gated calcium channel agonists mitigate hearing loss and modify ribbon synapse morphology in the zebrafish model of Usher syndrome type 1. Dis. Model. Mech. 2020, 13, dmm043885. [Google Scholar] [CrossRef]
- Nicolson, T.; Rusch, A.; Friedrich, R.W.; Granato, M.; Ruppersberg, J.P.; Nusslein-Volhard, C. Genetic analysis of vertebrate sensory hair cell mechanosensation: The zebrafish circler mutants. Neuron 1998, 20, 271–283. [Google Scholar] [CrossRef]
- Whitfield, T.T.; Granato, M.; van Eeden, F.J.; Schach, U.; Brand, M.; Furutani-Seiki, M.; Haffter, P.; Hammerschmidt, M.; Heisenberg, C.P.; Jiang, Y.J.; et al. Mutations affecting development of the zebrafish inner ear and lateral line. Development 1996, 123, 241–254. [Google Scholar] [CrossRef]
- Song, Y.; Willer, J.R.; Scherer, P.C.; Panzer, J.A.; Kugath, A.; Skordalakes, E.; Gregg, R.G.; Willer, G.B.; Balice-Gordon, R.J. Neural and synaptic defects in slytherin, a zebrafish model for human congenital disorders of glycosylation. PLoS ONE 2010, 5, e13743. [Google Scholar] [CrossRef]
- Ameen, M.T.; Alloway, H.; Longjohn, M.N.; Gendron, R.L.; Paradis, H.; Benoukraf, T.; French, C.R. Genomic analysis of glaucoma pathogenesis due to gmds mutation in zebrafish. Exp. Eye Res. 2025, 258, 110497. [Google Scholar] [CrossRef]
- Ryu, N.; Lee, S.; Park, H.J.; Lee, B.; Kwon, T.J.; Bok, J.; Park, C.I.; Lee, K.Y.; Baek, J.I.; Kim, U.K. Identification of a novel splicing mutation within SLC17A8 in a Korean family with hearing loss by whole-exome sequencing. Gene 2017, 627, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Ruel, J.; Emery, S.; Nouvian, R.; Bersot, T.; Amilhon, B.; Van Rybroek, J.M.; Rebillard, G.; Lenoir, M.; Eybalin, M.; Delprat, B.; et al. Impairment of SLC17A8 encoding vesicular glutamate transporter-3, VGLUT3, underlies nonsyndromic deafness DFNA25 and inner hair cell dysfunction in null mice. Am. J. Hum. Genet. 2008, 83, 278–292. [Google Scholar] [CrossRef]
- Hagstrom, S.A.; Pauer, G.J.; Reid, J.; Simpson, E.; Crowe, S.; Maumenee, I.H.; Traboulsi, E.I. SOX2 mutation causes anophthalmia, hearing loss, and brain anomalies. Am. J. Med. Genet. A 2005, 138A, 95–98. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Long, R.; Yu, L. A novel deletion mutation of the SOX2 gene in a child of Chinese origin with congenital bilateral anophthalmia and sensorineural hearing loss. J. Genet. 2018, 97, 1007–1011. [Google Scholar] [CrossRef]
- Panzer, J.A.; Gibbs, S.M.; Dosch, R.; Wagner, D.; Mullins, M.C.; Granato, M.; Balice-Gordon, R.J. Neuromuscular synaptogenesis in wild-type and mutant zebrafish. Dev. Biol. 2005, 285, 340–357. [Google Scholar] [CrossRef]
- Thomas, E.D.; Cruz, I.A.; Hailey, D.W.; Raible, D.W. There and back again: Development and regeneration of the zebrafish lateral line system. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 1–16. [Google Scholar] [CrossRef]
- Patterson, A.S.; Dugdale, J.; Koleilat, A.; Krauss, A.; Hernandez-Herrera, G.A.; Wallace, J.G.; Petree, C.; Varshney, G.K.; Schimmenti, L.A. Vital Dye Uptake of YO-PRO-1 and DASPEI Depends Upon Mechanoelectrical Transduction Function in Zebrafish Hair Cells. J. Assoc. Res. Otolaryngol. 2024, 25, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Okajima, T.; Irvine, K.D. Regulation of notch signaling by o-linked fucose. Cell 2002, 111, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Feng, L.; Yang, Y.; Jiang, H.; Hou, X.; Guo, P.; Marlow, F.L.; Stanley, P.; Wu, P. In Situ Fucosylation of the Wnt Co-receptor LRP6 Increases Its Endocytosis and Reduces Wnt/beta-Catenin Signaling. Cell Chem. Biol. 2020, 27, 1140–1150 e4. [Google Scholar] [CrossRef]
- Pei, W.; Huang, S.C.; Xu, L.; Pettie, K.; Ceci, M.L.; Sanchez, M.; Allende, M.L.; Burgess, S.M. Loss of Mgat5a-mediated N-glycosylation stimulates regeneration in zebrafish. Cell Regen. 2016, 5, 3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shen, N.; Lin, H.; Wu, T.; Wang, D.; Wang, W.; Xie, H.; Zhang, J.; Feng, Z. Inhibition of TGF-beta1-receptor posttranslational core fucosylation attenuates rat renal interstitial fibrosis. Kidney Int. 2013, 84, 64–77. [Google Scholar] [CrossRef]
- Wang, X.; Inoue, S.; Gu, J.; Miyoshi, E.; Noda, K.; Li, W.; Mizuno-Horikawa, Y.; Nakano, M.; Asahi, M.; Takahashi, M.; et al. Dysregulation of TGF-beta1 receptor activation leads to abnormal lung development and emphysema-like phenotype in core fucose-deficient mice. Proc. Natl. Acad. Sci. USA 2005, 102, 15791–15796. [Google Scholar] [CrossRef]
- Blokzijl, A.; Dahlqvist, C.; Reissmann, E.; Falk, A.; Moliner, A.; Lendahl, U.; Ibáñez, C.F. Cross-talk between the Notch and TGF-beta signaling pathways mediated by interaction of the Notch intracellular do-main with Smad3. J. Cell Biol. 2003, 163, 723–728. [Google Scholar] [CrossRef]
- Moon, I.S.; So, J.H.; Jung, Y.M.; Lee, W.S.; Kim, E.Y.; Choi, J.H.; Kim, C.H.; Choi, J.Y. Fucoidan promotes mechanosensory hair cell regeneration following amino glycoside-induced cell death. Hear. Res. 2011, 282, 236–242. [Google Scholar] [CrossRef]
- Liu, Y.; Sweet, I.R.; Boons, G.J. 2,2-Difluoro Derivatives of Fucose Can Inhibit Cell Surface Fucosylation without Causing Slow Transfer to Acceptors. JACS Au 2024, 4, 3953–3963. [Google Scholar] [CrossRef] [PubMed]
- Gilormini, P.A.; Thota, V.N.; Fers-Lidou, A.; Ashmus, R.A.; Nodwell, M.; Brockerman, J.; Kuo, C.W.; Wang, Y.; Gray, T.E.; Nitin; et al. A metabolic inhibitor blocks cellular fucosylation and enables production of afucosylated antibodies. Proc. Natl. Acad. Sci. USA 2024, 121, e2314026121. [Google Scholar] [CrossRef] [PubMed]
- Thisse, C.; Thisse, B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 2008, 3, 59–69. [Google Scholar] [CrossRef]
- Harris, J.A.; Cheng, A.G.; Cunningham, L.L.; MacDonald, G.; Raible, D.W.; Rubel, E.W. Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio). J. Assoc. Res. Otolaryngol. 2003, 4, 219–234. [Google Scholar] [CrossRef]
- Jensen, K.H.; Rekling, J.C. Development of a no-wash assay for mitochondrial membrane potential using the styryl dye DASPEI. J. Biomol. Screen. 2010, 15, 1071–1081. [Google Scholar] [CrossRef] [PubMed][Green Version]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ameen, M.T.; Fowler, G.; French, C.R. Mutation of the GDP-Fucose Biosynthesis Gene gmds Increases Hair Cell Number and Neuromast Regenerative Capacity in Zebrafish. Int. J. Mol. Sci. 2025, 26, 9737. https://doi.org/10.3390/ijms26199737
Ameen MT, Fowler G, French CR. Mutation of the GDP-Fucose Biosynthesis Gene gmds Increases Hair Cell Number and Neuromast Regenerative Capacity in Zebrafish. International Journal of Molecular Sciences. 2025; 26(19):9737. https://doi.org/10.3390/ijms26199737
Chicago/Turabian StyleAmeen, Muhammad T., Gerissa Fowler, and Curtis R. French. 2025. "Mutation of the GDP-Fucose Biosynthesis Gene gmds Increases Hair Cell Number and Neuromast Regenerative Capacity in Zebrafish" International Journal of Molecular Sciences 26, no. 19: 9737. https://doi.org/10.3390/ijms26199737
APA StyleAmeen, M. T., Fowler, G., & French, C. R. (2025). Mutation of the GDP-Fucose Biosynthesis Gene gmds Increases Hair Cell Number and Neuromast Regenerative Capacity in Zebrafish. International Journal of Molecular Sciences, 26(19), 9737. https://doi.org/10.3390/ijms26199737





