The Establishment of a Sheep Embryo Genomic Selection System
Abstract
1. Introduction
2. Results
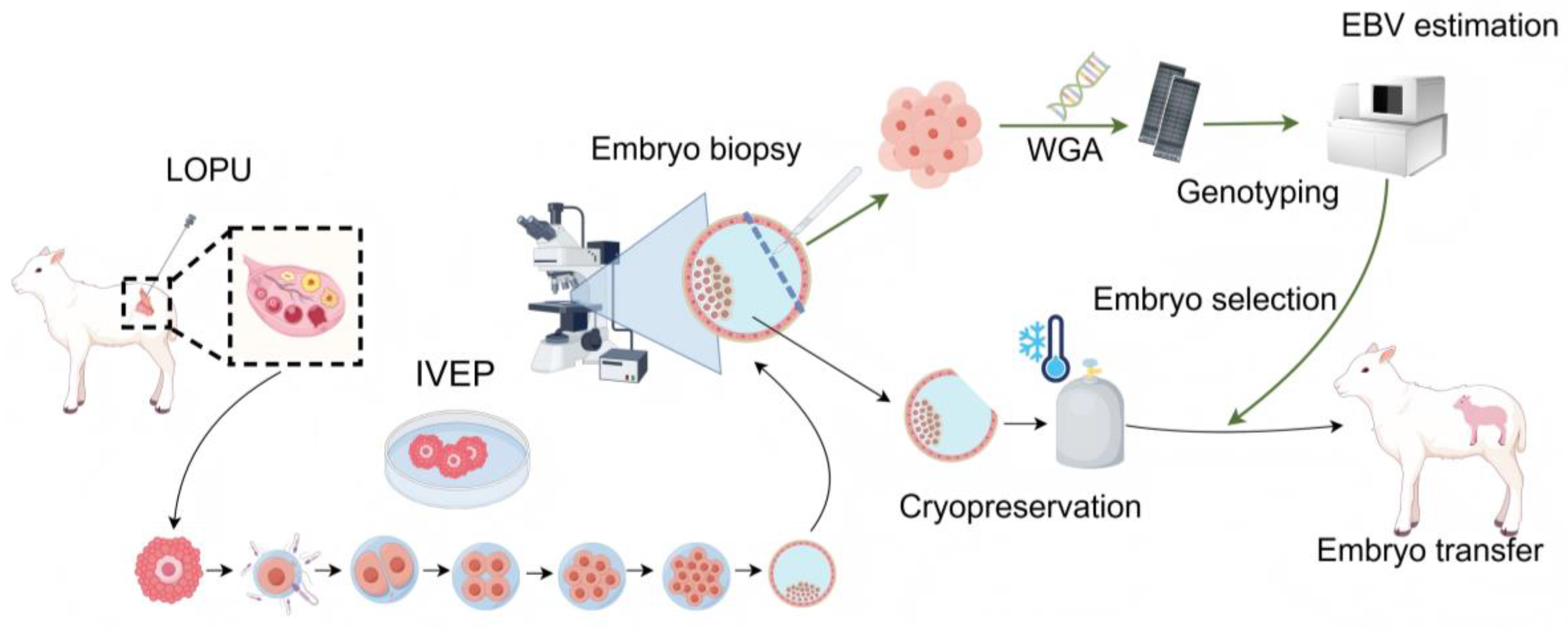
2.1. Sheep Embryo Genomic Selection Breeding System
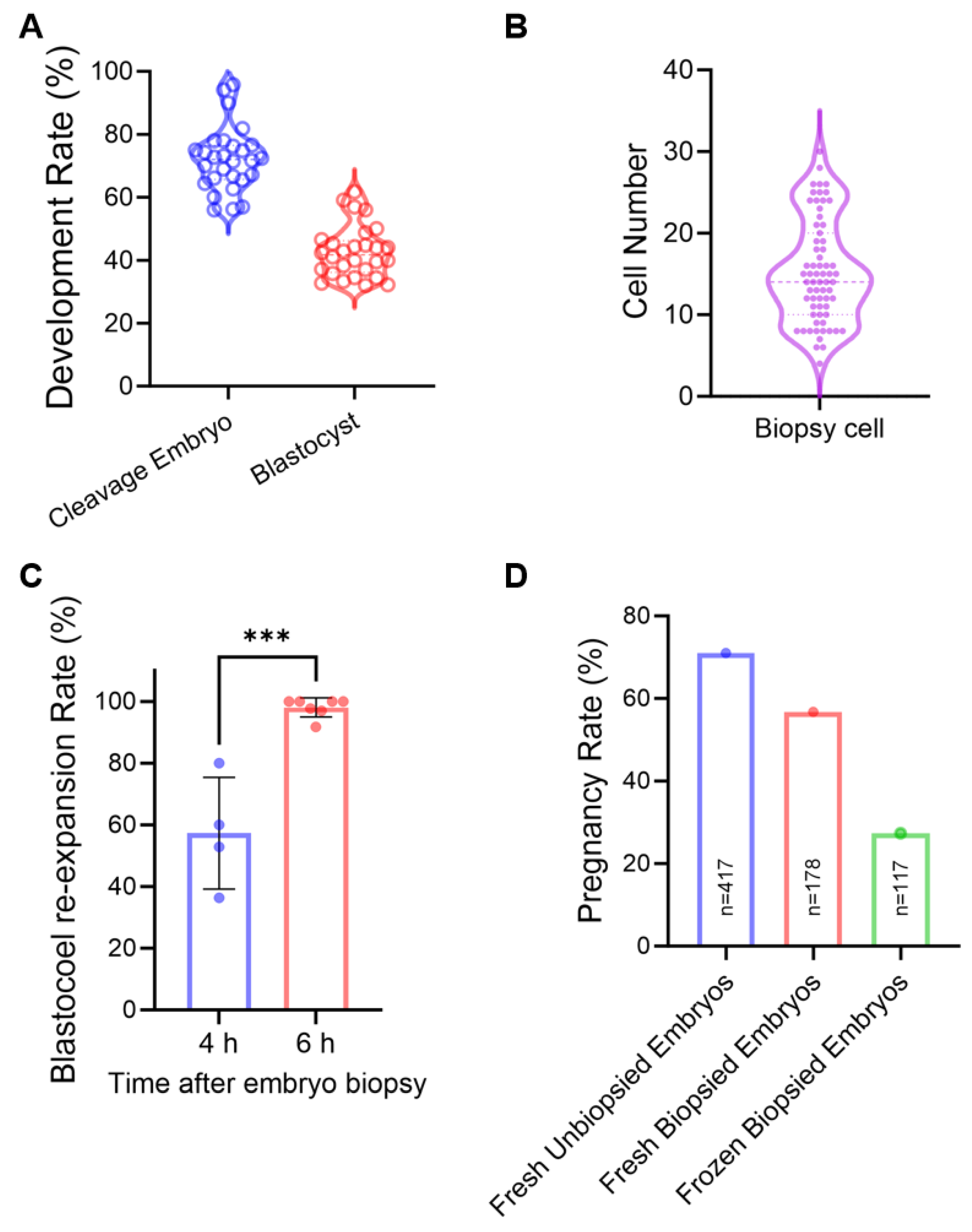
2.2. In Vitro Sheep Embryo Production and Embryo Biopsy
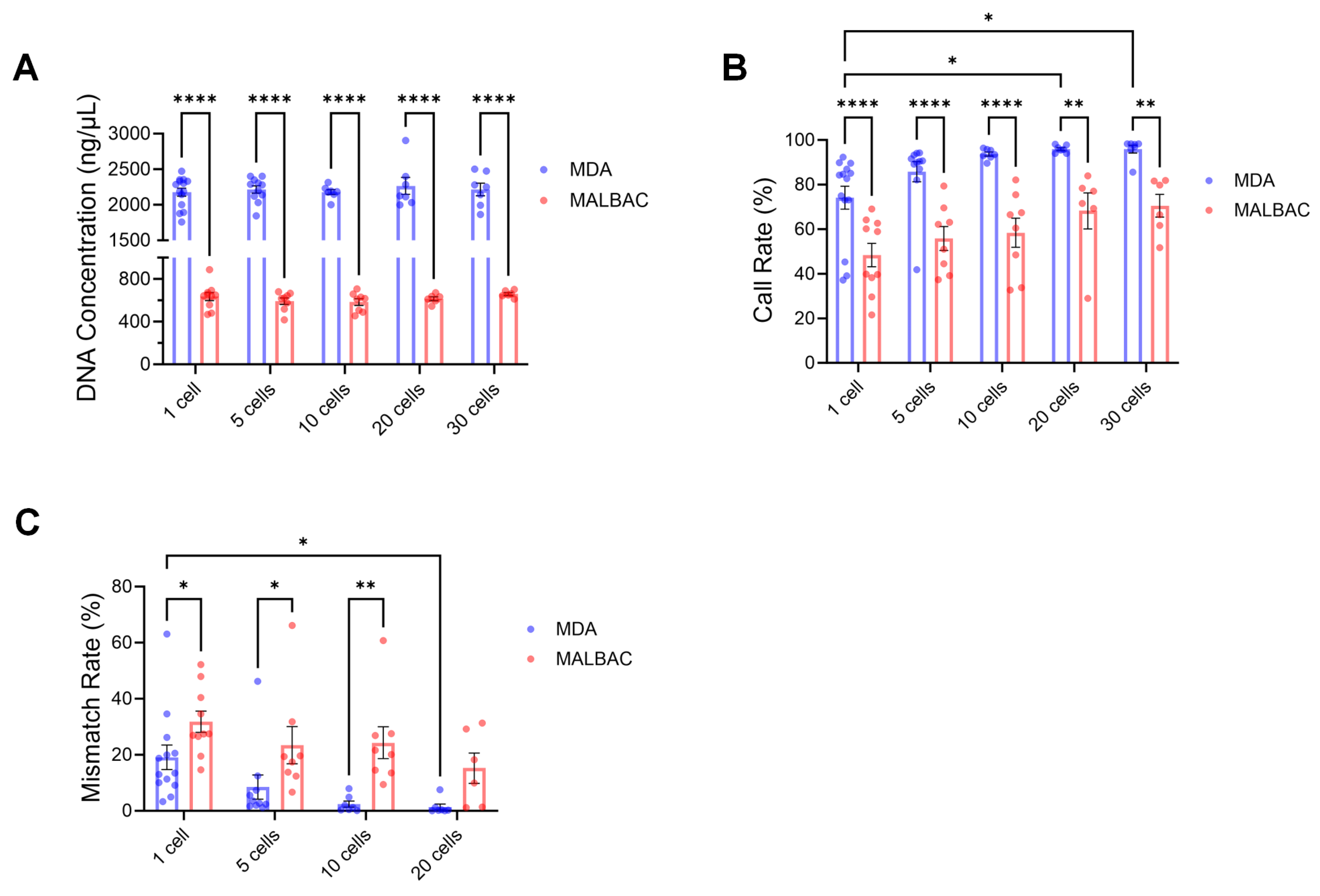
2.3. Effects of Biopsied Cell Numbers and Amplification Methods on SNP Genotyping
2.4. Optimization of Biopsy Cell Amplification System
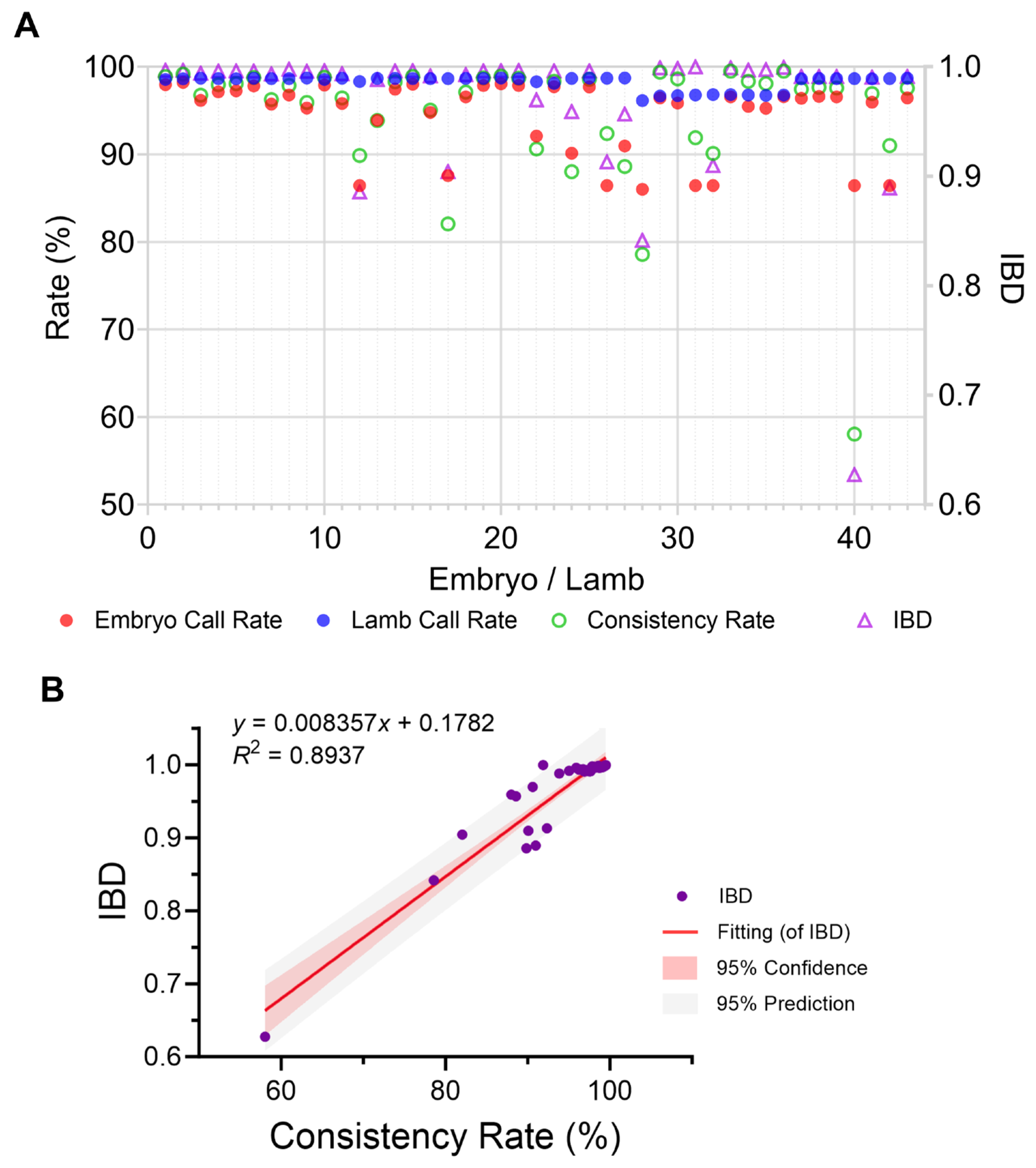
2.5. High Genotyping Quality and Consistency Between Embryo Biopsy and Offspring Samples
2.6. Improved Genotyping Quality Through 10× Whole Genome Resequencing Imputation
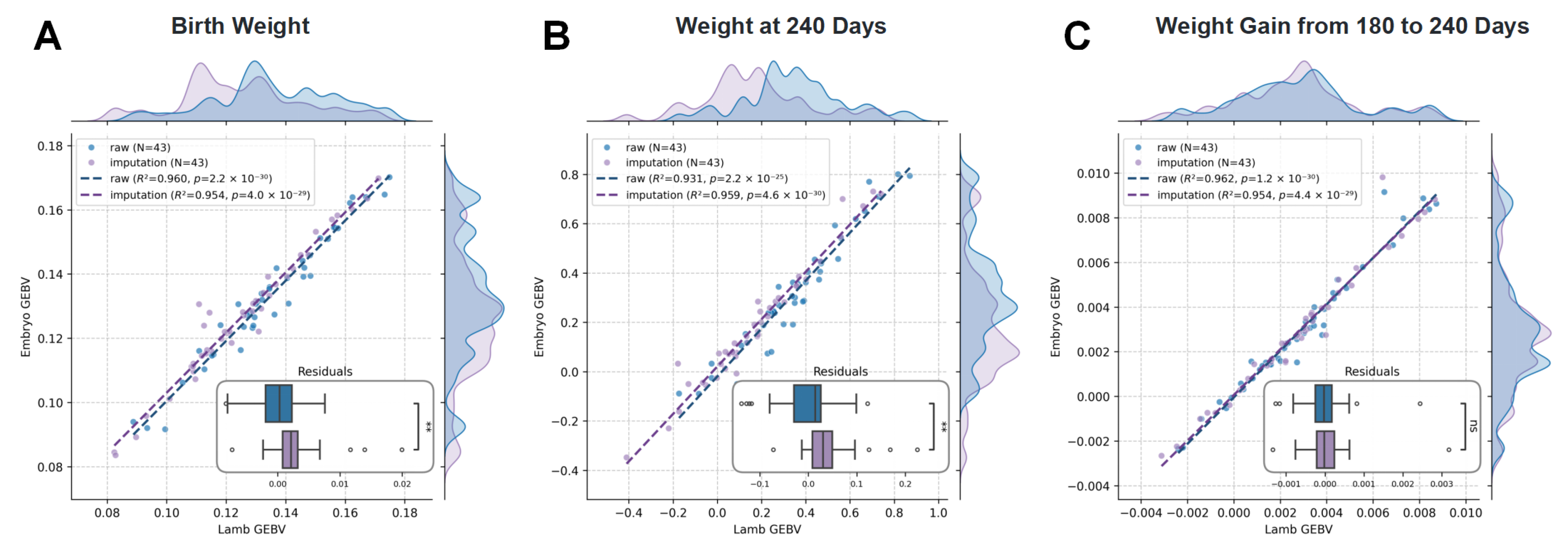
2.7. High Concordance in GEBVs Between Embryos and Offspring
3. Discussion
4. Materials and Methods
4.1. Animals and Sample Collection
4.2. Laparoscopic Ovum Pick-Up (LOPU)
4.3. Oocytes Collection and In Vitro Maturation (IVM)
4.4. In Vitro Fertilization and Embryo Culture
4.5. Embryo Transfer
4.6. Embryo Biopsy and Sample Preparation
4.7. Whole-Genome Amplification
4.8. DNA Quantification and Quality Control
4.9. SNP Chip-Based Genotyping
4.10. Whole-Genome Sequencing and Imputation
4.11. Genomic Prediction and GEBV Calculation
4.12. Technical Replicate and Batch Effect Management
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GS | Genomic selection |
| MDA | Multiple Displacement Amplification |
| MALBAC | Multiple Annealing and Looping Based Amplification Cycles |
| WGA | Whole-genome amplification |
| GEBV | Genomic estimated breeding value |
| LOPU | Laparoscopic Ovum Pick-up |
| IVM | In vitro maturation |
| IVF | In vitro fertilization |
| IBD | Identity-by-Descent |
| SNP | Single nucleotide polymorphism |
| IVEP | In vitro embryo production |
| OPU | Ovum pick-up |
| LIANTI | Linear amplification via transposon insertion |
| CIDR | Controlled internal drug release |
| COCs | Cumulus-oocyte complexes |
| GBLUP | Genomic best linear unbiased prediction |
References
- Meuwissen, T.H.; Hayes, B.J.; Goddard, M.E. Prediction of Total Genetic Value Using Genome-Wide Dense Marker Maps. Genetics 2001, 157, 1819–1829. [Google Scholar] [CrossRef]
- Santos, B.F.S.; Van Der Werf, J.H.J.; Gibson, J.P.; Byrne, T.J.; Amer, P.R. Genetic and Economic Benefits of Selection Based on Performance Recording and Genotyping in Lower Tiers of Multi-Tiered Sheep Breeding Schemes. Genet. Sel. Evol. 2017, 49, 10. [Google Scholar] [CrossRef]
- VanRaden, P.M. Efficient Methods to Compute Genomic Predictions. J. Dairy Sci. 2008, 91, 4414–4423. [Google Scholar] [CrossRef]
- Hayes, B.; Goddard, M.E. The Distribution of the Effects of Genes Affecting Quantitative Traits in Livestock. Genet. Sel. Evol. 2001, 33, 209. [Google Scholar] [CrossRef]
- Newton, J.E.; Brown, D.J.; Dominik, S.; Van Der Werf, J.H.J. Impact of Young Ewe Fertility Rate on Risk and Genetic Gain in Sheep-Breeding Programs Using Genomic Selection. Anim. Prod. Sci. 2017, 57, 1653. [Google Scholar] [CrossRef]
- Daetwyler, H.D.; Kemper, K.E.; van der Werf, J.H.J.; Hayes, B.J. Components of the Accuracy of Genomic Prediction in a Multi-Breed Sheep Population. J. Anim. Sci. 2012, 90, 3375–3384. [Google Scholar] [CrossRef]
- Dodds, K.; Auvray, B.; Lee, M.; Newman, S.-A.; McEwan, J. Genomic Selection in New Zealand Dual Purpose Sheep. In Proceedings of the 10th World Congress of Genetics Applied to Livestock, Vancouver, BC, Canada, 17–22 August 2014. [Google Scholar]
- McLaren, A.; Kaseja, K.; Yates, J.; Mucha, S.; Lambe, N.R.; Conington, J. New Mastitis Phenotypes Suitable for Genomic Selection in Meat Sheep and Their Genetic Relationships with Udder Conformation and Lamb Live Weights. Animal 2018, 12, 2470–2479. [Google Scholar] [CrossRef]
- Daetwyler, H.D.; Swan, A.A.; van der Werf, J.H.; Hayes, B.J. Accuracy of Pedigree and Genomic Predictions of Carcass and Novel Meat Quality Traits in Multi-Breed Sheep Data Assessed by Cross-Validation. Genet. Sel. Evol. 2012, 44, 33. [Google Scholar] [CrossRef] [PubMed]
- Hayes, B.J.; Bowman, P.J.; Chamberlain, A.J.; Goddard, M.E. Invited Review: Genomic Selection in Dairy Cattle: Progress and Challenges. J. Dairy Sci. 2009, 92, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, J.C.M. Prediction of Response to Marker-Assisted and Genomic Selection Using Selection Index Theory. J. Anim. Breed. Genet. 2007, 124, 331–341. [Google Scholar] [CrossRef]
- Kasinathan, P.; Wei, H.; Xiang, T.; Molina, J.A.; Metzger, J.; Broek, D.; Kasinathan, S.; Faber, D.C.; Allan, M.F. Acceleration of Genetic Gain in Cattle by Reduction of Generation Interval. Sci. Rep. 2015, 5, 8674. [Google Scholar] [CrossRef]
- Granleese, T.; Clark, S.A.; Swan, A.A.; van der Werf, J.H.J. Increased Genetic Gains in Sheep, Beef and Dairy Breeding Programs from Using Female Reproductive Technologies Combined with Optimal Contribution Selection and Genomic Breeding Values. Genet. Sel. Evol. 2015, 47, 70. [Google Scholar] [CrossRef]
- Mullaart, E.; Wells, D. Embryo Biopsies for Genomic Selection. In Animal Biotechnology 2; Niemann, H., Wrenzycki, C., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 81–94. ISBN 978-3-319-92347-5. [Google Scholar]
- Ramos-Ibeas, P.; Calle, A.; Pericuesta, E.; Laguna-Barraza, R.; Moros-Mora, R.; Lopera-Vásquez, R.; Maillo, V.; Yáñez-Mó, M.; Gutiérrez-Adán, A.; Rizos, D.; et al. An Efficient System to Establish Biopsy-Derived Trophoblastic Cell Lines from Bovine Embryos1. Biol. Reprod. 2014, 91, 15. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.S.; Camargo, L.S.A.; Silva, M.V.G.B.D.; Saraiva, N.Z.; Quintão, C.C.; Machado, M.A. Embryo Biopsies for Genomic Selection in Tropical Dairy Cattle. Anim. Reprod. 2023, 20, e20230064. [Google Scholar] [CrossRef]
- Kadarmideen, H.; Mazzoni, G.; Watanabe, Y.; Stroebech, L.; Baruselli, P.; Meirelles, F.; Callesen, H.; Hyttel, P.; Ferraz, J.; Nogueira, M. Genomic Selection of in Vitro Produced and Somatic Cell Nuclear Transfer Embryos for Rapid Genetic Improvement in Cattle Production. Anim. Reprod. 2015, 12, 389–396. [Google Scholar]
- Oliveira, C.S.; Silva, M.V.G.B.D.; Quintão, C.C.; Otto, P.I.; Alonso, R.V.; Feres, L.F.; Panetto, J.C.D.C.; Machado, M.A.; Camargo, L.S.D.A. Imputation Accuracy for Genomic Selection Using Embryo Biopsy Samples in Gir. Reprod. Biol. 2023, 23, 100765. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Naito, A.; Hirayama, H.; Kashima, M.; Yoshino, H.; Hanamure, T.; Domon, Y.; Hayakawa, H.; Watanabe, T.; Moriyasu, S.; et al. Potential of Preimplantation Genomic Selection for Carcass Traits in Japanese Black Cattle. J. Reprod. Dev. 2019, 65, 251–258. [Google Scholar] [CrossRef]
- Fujii, T.; Naito, A.; Moriyasu, S.; Kageyama, S. Potential of Preimplantation Genomic Selection Using the Blastomere Separation Technique in Bovine in Vitro Fertilized Embryos. J. Reprod. Dev. 2021, 67, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Campos-Chillon, F.; Mancino, J.; Barceló-Fimbres, M.; Altermatt, J.L. Progress in Genotyping in Vitro-Produced Embryos: Are We Close? In Proceedings of the Applied Reproductive Strategies in Beef Cattle, Davis, CA, USA, 17–18 September 2015. [Google Scholar]
- Shojaei Saadi, H.A.; Vigneault, C.; Sargolzaei, M.; Gagné, D.; Fournier, É.; De Montera, B.; Chesnais, J.; Blondin, P.; Robert, C. Impact of Whole-Genome Amplification on the Reliability of Pre-Transfer Cattle Embryo Breeding Value Estimates. BMC Genom. 2014, 15, 889. [Google Scholar] [CrossRef]
- Fisher, P.; Hyndman, D.; Bixley, M.; Oback, F.C.; Popovic, L.; McGowan, L.T.; Berg, M.C.; Wells, D. Potential for Genomic Selection of Bovine Embryos. Proc. New Zealand Soc. Anim. Prod. 2012, 72, 156–158. [Google Scholar]
- Bourhis, D.; Mullaart, E.; Schrooten, C.; Fritz, S.; Coppieters, W.; Ponsart, C. 135 Breeding Values Concordance between Embryos and Corresponding Calves. Reprod. Fertil. Dev. 2011, 24, 180. [Google Scholar] [CrossRef]
- Gualtieri, R.; De Gregorio, V.; Candela, A.; Travaglione, A.; Genovese, V.; Barbato, V.; Talevi, R. In Vitro Culture of Mammalian Embryos: Is There Room for Improvement? Cells 2024, 13, 996. [Google Scholar] [CrossRef]
- Holm, P.; Booth, P.J.; Callesen, H. Kinetics of Early in Vitro Development of Bovine in Vivo- and in Vitro-Derived Zygotes Produced and/or Cultured in Chemically Defined or Serum-Containing Media. Reproduction 2002, 123, 553–565. [Google Scholar] [CrossRef]
- Khaliq, A.; Hamza, M.A.; Ashraf, T.; Husnain, A.; Yaseen, M.; Rehman, A.; Binyameen, M.; Zahoor, M.Y.; Riaz, A. Effect of Supplementing Epinephrine in Maturation Media on in-Vitro Developmental Competence of Cattle and Buffalo Oocytes. Theriogenology 2024, 226, 219–227. [Google Scholar] [CrossRef]
- Rossant, J.; Tam, P.P.L. Early Human Embryonic Development: Blastocyst Formation to Gastrulation. Dev. Cell 2022, 57, 152–165. [Google Scholar] [CrossRef]
- Tutt, D.A.R.; Passaro, C.; Whitworth, D.J.; Holland, M.K. Laser Assisted Blastomere Extrusion Biopsy of in Vitro Produced Cattle Embryos—A Potential High Throughput, Minimally Invasive Approach for Sampling Pre-Morula and Morula Stage Embryos. Anim. Reprod. Sci. 2020, 219, 106546. [Google Scholar] [CrossRef]
- ESHRE Add-ons working group; Lundin, K.; Bentzen, J.G.; Bozdag, G.; Ebner, T.; Harper, J.; Le Clef, N.; Moffett, A.; Norcross, S.; Polyzos, N.P.; et al. Good Practice Recommendations on Add-Ons in Reproductive Medicine. Hum. Reprod. 2023, 38, 2062–2104. [Google Scholar] [CrossRef] [PubMed]
- Joris, H.; Van den Abbeel, E.; Vos, A.D.; Van Steirteghem, A. Reduced Survival after Human Embryo Biopsy and Subsequent Cryopreservation. Hum. Reprod. 1999, 14, 2833–2837. [Google Scholar] [CrossRef][Green Version]
- Aghajani, S.; Salehzadeh, A.; Ghasemian, F.; Mehrafza, M.; Hosseini, A. Effect of Single Embryo Blastomere Biopsy from Human Frozen Embryos on Assisted Reproductive Outcomes. Cell J. 2022, 24, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Bartolacci, A.; Vitiello, C.; de Girolamo, S.; Papaleo, E.; Pagliardini, L. Does Double Cryopreservation as Well as Double Biopsy Affect Embryo Viability and Clinical Outcomes? Evidence from a Systematic Review of the Literature. J. Assist. Reprod. Genet. 2025, 42, 1053–1066. [Google Scholar] [CrossRef] [PubMed]
- Cenariu, M.; Pall, E.; Cernea, C.; Groza, I. Evaluation of Bovine Embryo Biopsy Techniques According to Their Ability to Preserve Embryo Viability. J. Biomed. Biotechnol. 2012, 2012, 541384. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.L.; Said, A.-H.; Thompson, D.J.; Caperton, C.L. Large-Volume Vitrification of Human Biopsied and Non-Biopsied Blastocysts: A Simple, Robust Technique for Cryopreservation. J. Assist. Reprod. Genet. 2015, 32, 207–214. [Google Scholar] [CrossRef][Green Version]
- Magli, M.C.; Gianaroli, L.; Fortini, D.; Ferraretti, A.P.; Munné, S. Impact of Blastomere Biopsy and Cryopreservation Techniques on Human Embryo Viability. Hum. Reprod. 1999, 14, 770–773. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Liu, H.; Pan, W.; Ren, J.; Zheng, X.; Tan, Y.; Chen, Z.; Deng, Y.; He, N.; et al. Recent Advances and Application of Whole Genome Amplification in Molecular Diagnosis and Medicine. MedComm 2022, 3, e116. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, N.; Yue, Y.; Chen, D.; Chou, C.; An, L.; Cheng, L.; Zhang, J.; Tian, J. Field Outcomes of Laparoscopic Ovum Pick-up Combined with in Vitro Embryo Production in Sheep: Effects of Long-Acting Recombinant Ovine FSH Pre-Stimulation, Collection Frequency, and Donor Breed. Domest. Anim. Endocrinol. 2024, 87, 106826. [Google Scholar] [CrossRef]
- Dos Santos-Neto, P.C.; Cuadro, F.; Souza-Neves, M.; Crispo, M.; Menchaca, A. Refinements in Embryo Manipulation Applied to CRISPR Technology in Livestock. Theriogenology 2023, 208, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Bredbacka, P.; Kankaanpää, A.; Peippo, J. PCR-Sexing of Bovine Embryos: A Simplified Protocol. Theriogenology 1995, 44, 167–176. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Qin, H.; Li, K.; Hao, J.; Liu, X.; Chen, D.; Cheng, L.; He, H.; Wu, R.; Wu, Y.; et al. The Establishment of a Sheep Embryo Genomic Selection System. Int. J. Mol. Sci. 2025, 26, 9738. https://doi.org/10.3390/ijms26199738
Wang Y, Qin H, Li K, Hao J, Liu X, Chen D, Cheng L, He H, Wu R, Wu Y, et al. The Establishment of a Sheep Embryo Genomic Selection System. International Journal of Molecular Sciences. 2025; 26(19):9738. https://doi.org/10.3390/ijms26199738
Chicago/Turabian StyleWang, Yubing, Hao Qin, Ke Li, Jia Hao, Xingyuan Liu, Dayong Chen, Lei Cheng, Huijie He, Riga Wu, Yingjie Wu, and et al. 2025. "The Establishment of a Sheep Embryo Genomic Selection System" International Journal of Molecular Sciences 26, no. 19: 9738. https://doi.org/10.3390/ijms26199738
APA StyleWang, Y., Qin, H., Li, K., Hao, J., Liu, X., Chen, D., Cheng, L., He, H., Wu, R., Wu, Y., Wang, Y., Guo, M., Li, Q., An, L., Tian, J., Han, H., & Xi, G. (2025). The Establishment of a Sheep Embryo Genomic Selection System. International Journal of Molecular Sciences, 26(19), 9738. https://doi.org/10.3390/ijms26199738





