Obesity-Driven Metabolic Disorders: The Interplay of Inflammation and Mitochondrial Dysfunction
Abstract
1. Introduction
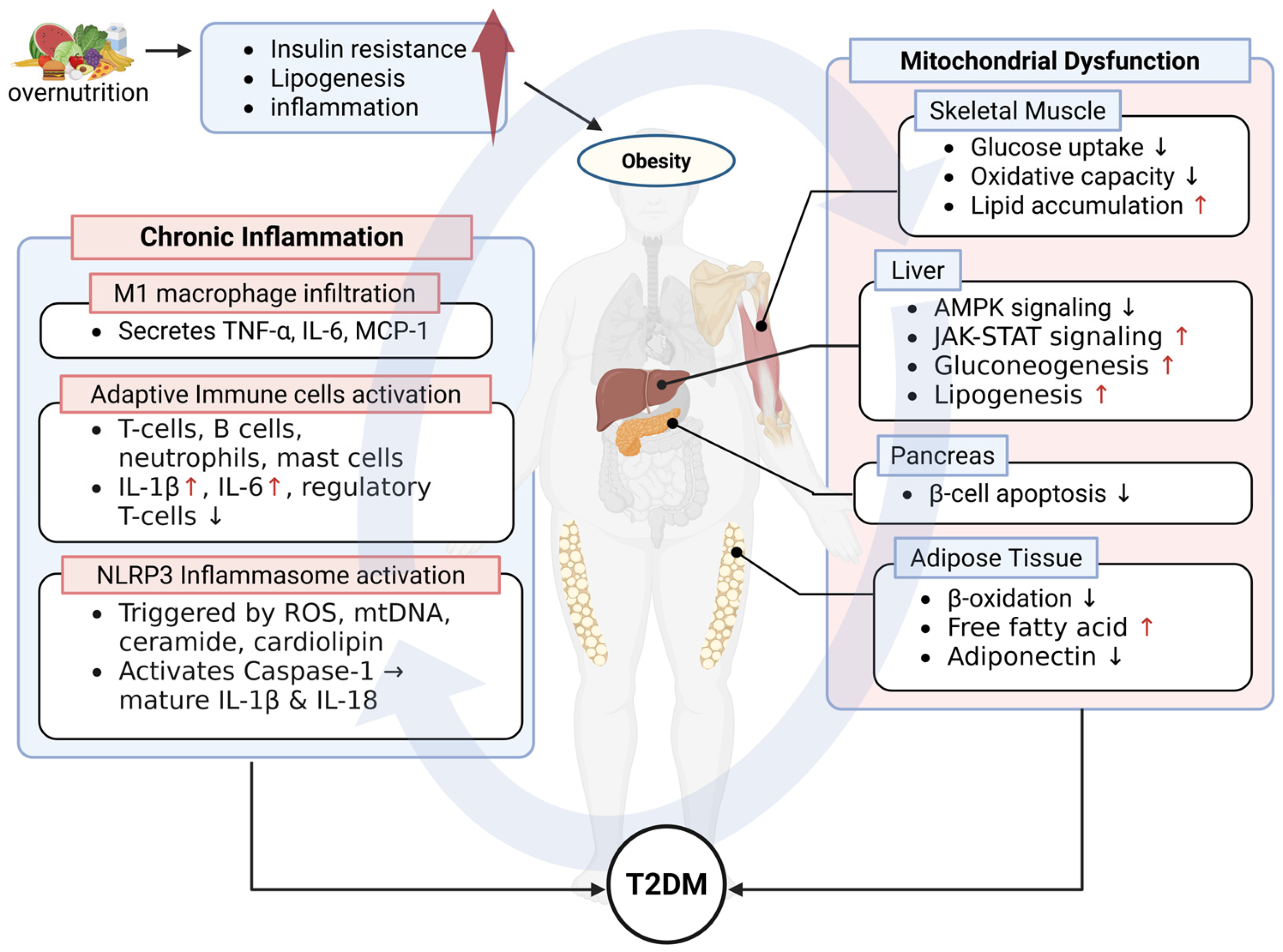
2. Pathophysiological Insights into Obesity-Driven Metabolic Disorder
2.1. Type II Diabetes Mellitus (T2DM)
2.1.1. Overview of T2DM
2.1.2. Mitochondrial Dysfunction in T2DM Pathogenesis
2.1.3. The Role of Chronic Inflammation in T2DM
2.1.4. Therapeutic Implications
| Drug | Mechanism & Molecular Target | Developer | ClinicalTrials.gov Identifier | Phase | References |
|---|---|---|---|---|---|
| Imeglimin | Oral Glimin class; enhances mitochondrial function | Poxel & Sumitomo Dainippon; approved by Japan’s PMDA in 2021 as Twymeeg®. Approval was based on Phase 3 trials in Japan. In U.S., only Phase II trials has been completed. | N/A | 2 (FDA) | [99,100] |
| MSDC-0602K | Azemiglita zone; MPC inhibitor; PPARγ-sparing insulin sensitizer targeting mitochondrial pyruvate carrier (mTOT) | Cirius Therapeutics (Metabolic Solutions) | NCT02784444 (EMMINECE), NCT03970031 (planned) | 3 | [101] |
| BGP-15 | HSP modulator; orally active co-inducer of heat shock proteins (HSP72); reduces oxidative and ER stress | N-Gene/Mitochon (HU) | NCT01069965 | 2a | [102] |
| HU6 | Mitochondrial uncoupler | Rivus Pharmaceuticals (Phase 2b in obesity+T2DM ongoing) | NCT04874233 | 2b | [103] |
| Pegbelfermin | FGF21 analog; enhances lipid oxidation, weight loss, and insulin-independent glucose uptake | Bristol Myers Squibb (BMS-986036) | NCT02071509 | 2a | [104] |
| SS-31 (Elamipretide) | Selectively binds to Cardiolipin in inner mitochondrial membrane, stabilizing mitochondrial structure and function | Stealth Bio Therapeutics (developing for Barth syndrome) | N/A (Preclinical Studies Only) | N/A | [94] |
2.2. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)
2.2.1. Overview of MASLD
2.2.2. Mitochondrial Dysfunction in MASLD Progression
2.2.3. Hepatic Inflammation in MASLD
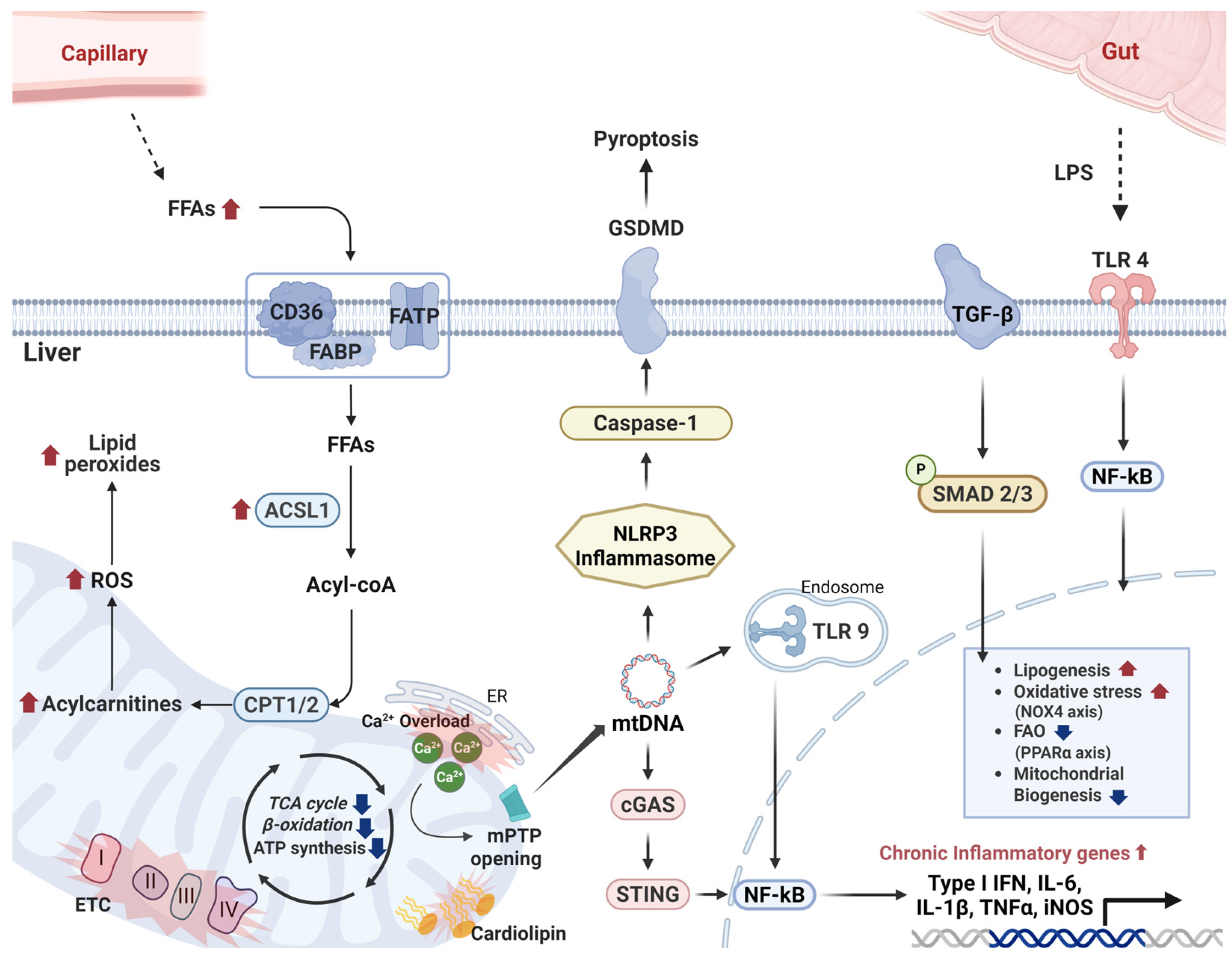
2.2.4. Therapeutic Implications
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACOX1 | Acyl-CoA oxidase 1 |
| ACSL1 | Acyl-CoA synthetase long-chain family member 1 |
| ACSL5 | Acyl-CoA synthetase long-chain family member 5 |
| AMPK | AMP-activated protein kinase |
| BAT | Brown adipose tissue |
| BAX | BCL2-associated X protein |
| BGP-15 | Hydroximic acid derivative BGP-15 |
| BMI | Body mass index |
| CACT | Carnitine-acylcarnitine translocase |
| CARD | Caspase recruitment domain |
| CCL2 | C-C motif chemokine ligand 2 |
| cf-mtDNA | Cell-free mitochondrial DNA |
| CMPK2 | Cytidine/uridine monophosphate kinase 2 |
| CPT1A | Carnitine palmitoyltransferase 1A |
| CPT2 | Carnitine palmitoyltransferase 2 |
| CRID3 | Cytokine release inhibitory drug 3 |
| CRP | C-reactive protein |
| CS | Citrate synthase |
| CVOT | Cardiovascular outcome trial |
| DAG | Diacylglycerol |
| DAMPs | Damage-associated molecular patterns |
| Δψm | Mitochondrial membrane potential |
| ETC | Electron transport chain |
| ETF | Electron transfer flavoprotein |
| ETFDH | Electron transfer flavoprotein dehydrogenase |
| FABP1 | Fatty acid-binding protein 1 |
| FAO | Fatty acid β-oxidation |
| FFAs | Free fatty acids |
| FGF21 | Fibroblast growth factor 21 |
| GAS | Gastrocnemius |
| GCN2 | General control nonderepressible 2 |
| GSIS | Glucose-stimulated insulin secretion |
| GSDMD | Gasdermin D |
| HCC | Hepatocellular carcinoma |
| HFD | High-fat diet |
| HIF | Hypoxia-inducible factor |
| HOMA-β | Homeostatic model assessment of β-cell function |
| HSP | Heat shock protein |
| IL | Interleukin |
| IP3R | Inositol 1,4,5-trisphosphate receptor |
| IRS | Insulin receptor substrate |
| JAK | Janus kinase |
| JNK | c-Jun N-terminal kinase |
| LPS | Lipopolysaccharide |
| MAMs | Mitochondria-associated membranes |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| MASH | Metabolic dysfunction-associated steatohepatitis |
| MCC950 | NLRP3 inflammasome inhibitor MCC950 |
| MCL-1 | Myeloid cell leukemia 1 |
| MetALD | Metabolic dysfunction-associated alcohol-related liver disease |
| MLKL | Mixed lineage kinase domain-like protein |
| MPC | Mitochondrial pyruvate carrier |
| mPTP | Mitochondrial permeability transition pore |
| mtDNA | Mitochondrial DNA |
| mtDAMPs | Mitochondrial damage-associated molecular patterns |
| mTOT | Mitochondrial target of thiazolidinediones |
| NAFLD | Non-alcoholic fatty liver disease |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NK | Natural killer |
| NLRP3 | NOD-, LRR- and pyrin domain-containing protein 3 |
| NOX4 | NADPH oxidase 4 |
| OXPHOS | Oxidative phosphorylation |
| PAMPs | Pathogen-associated molecular patterns |
| PAX4 | Paired box gene 4 |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PKC | Protein kinase C |
| PPARα | Peroxisome proliferator-activated receptor alpha |
| PRRs | Pattern recognition receptors |
| RIPK3 | Receptor-interacting serine/threonine-protein kinase 3 |
| ROS | Reactive oxygen species |
| SIRT1 | Sirtuin 1 |
| SLD | Steatotic liver disease |
| SREBP-1c | Sterol regulatory element-binding protein 1c |
| STING | Stimulator of interferon genes |
| T2DM | Type 2 diabetes mellitus |
| TBK1 | TANK-binding kinase 1 |
| TFAM | Mitochondrial transcription factor A |
| TGF-β | Transforming growth factor beta |
| TLR4 | Toll-like receptor 4 |
| TLR9 | Toll-like receptor 9 |
| TNF-α | Tumor necrosis factor alpha |
| UCP1 | Uncoupling protein 1 |
| USP29 | Ubiquitin-specific protease 29 |
| VDAC | Voltage-dependent anion channel |
References
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Milano, W.; Carizzone, F.; Foia, M.; Marchese, M.; Milano, M.; Saetta, B.; Capasso, A. Obesity and Its Multiple Clinical Implications between Inflammatory States and Gut Microbiotic Alterations. Diseases 2022, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Mili, N.; Paschou, S.A.; Goulis, D.G.; Dimopoulos, M.A.; Lambrinoudaki, I.; Psaltopoulou, T. Obesity, metabolic syndrome, and cancer: Pathophysiological and therapeutic associations. Endocrine 2021, 74, 478–497. [Google Scholar] [CrossRef]
- Ruze, R.; Liu, T.; Zou, X.; Song, J.; Chen, Y.; Xu, R.; Yin, X.; Xu, Q. Obesity and type 2 diabetes mellitus: Connections in epidemiology, pathogenesis, and treatments. Front. Endocrinol. 2023, 14, 1161521. [Google Scholar] [CrossRef]
- De Mello, A.H.; Costa, A.B.; Engel, J.D.G.; Rezin, G.T. Mitochondrial dysfunction in obesity. Life Sci. 2018, 192, 26–32. [Google Scholar] [CrossRef]
- Prasun, P. Mitochondrial dysfunction in metabolic syndrome. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165838. [Google Scholar] [CrossRef]
- Russo, S.; Kwiatkowski, M.; Govorukhina, N.; Bischoff, R.; Melgert, B.N. Meta-Inflammation and Metabolic Reprogramming of Macrophages in Diabetes and Obesity: The Importance of Metabolites. Front. Immunol. 2021, 12, 746151. [Google Scholar] [CrossRef]
- Staal, J.; Blanco, L.P.; Perl, A. Editorial: Mitochondrial dysfunction in inflammation and autoimmunity. Front. Immunol. 2023, 14, 1304315. [Google Scholar] [CrossRef]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef] [PubMed]
- Marchi, S.; Guilbaud, E.; Tait, S.W.G.; Yamazaki, T.; Galluzzi, L. Mitochondrial control of inflammation. Nat. Rev. Immunol. 2023, 23, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Flamment, M.; Hajduch, E.; Ferre, P.; Foufelle, F. New insights into ER stress-induced insulin resistance. Trends Endocrinol. Metab. 2012, 23, 381–390. [Google Scholar] [CrossRef]
- Namkoong, S.; Cho, C.S.; Semple, I.; Lee, J.H. Autophagy Dysregulation and Obesity-Associated Pathologies. Mol. Cells 2018, 41, 3–10. [Google Scholar] [CrossRef]
- Jobe, M.; Agbla, S.C.; Todorcevic, M.; Darboe, B.; Danso, E.; de Barros, J.-P.P.; Lagrost, L.; Karpe, F.; Prentice, A.M. Possible mediators of metabolic endotoxemia in women with obesity and women with obesity-diabetes in The Gambia. Int. J. Obes. 2022, 46, 1892–1900. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Das, S.R.; Hilliard, M.E.; Isaacs, D.; et al. Erratum. 10. Cardiovascular disease and risk management: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. S1), S158–S190, Erratum in Diabetes Care 2023, 46, 898. [Google Scholar] [CrossRef]
- Collaborators, G.B.D.D. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Department of Error. Lancet 2025, 405, 202. [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Ritz, E.; Orth, S.R. Nephropathy in patients with type 2 diabetes mellitus. N. Engl. J. Med. 1999, 341, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Nørregaard, R.; Mutsaers, H.A.M.; Frøkiær, J.; Kwon, T.H. Obstructive nephropathy and molecular pathophysiology of renal interstitial fibrosis. Physiol. Rev. 2023, 103, 2827–2872. [Google Scholar] [CrossRef] [PubMed]
- Fioretto, P.; Bruseghin, M.; Berto, I.; Gallina, P.; Manzato, E.; Mussap, M. Renal protection in diabetes: Role of glycemic control. J. Am. Soc. Nephrol. 2006, 17, S86–S89. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. Mechanisms for insulin resistance: Common threads and missing links. Cell 2012, 148, 852–871. [Google Scholar] [CrossRef]
- Olefsky, J.M.; Glass, C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef]
- Klein, S.; Gastaldelli, A.; Yki-Jarvinen, H.; Scherer, P.E. Why does obesity cause diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Unoki, H.; Takahashi, A.; Kawaguchi, T.; Hara, K.; Horikoshi, M.; Andersen, G.; Ng, D.P.; Holmkvist, J.; Borch-Johnsen, K.; Jørgensen, T.; et al. SNPs in KCNQ1 are associated with susceptibility to type 2 diabetes in East Asian and European populations. Nat. Genet. 2008, 40, 1098–1102. [Google Scholar] [CrossRef]
- Lau, H.H.; Krentz, N.A.J.; Abaitua, F.; Perez-Alcantara, M.; Chan, J.W.; Ajeian, J.; Ghosh, S.; Lee, Y.; Yang, J.; Thaman, S.; et al. PAX4 loss of function increases diabetes risk by altering human pancreatic endocrine cell development. Nat. Commun. 2023, 14, 6119. [Google Scholar] [CrossRef]
- Ling, C.; Rönn, T. Epigenetics in Human Obesity and Type 2 Diabetes. Cell Metab. 2019, 29, 1028–1044. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.; Martin, S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Xie, Q.; Pan, X.; Zhang, R.; Zhang, X.; Peng, G.; Zhang, Y.; Shen, S.; Tong, N. Type 2 diabetes mellitus in adults: Pathogenesis, prevention and therapy. Signal Transduct. Target. Ther. 2024, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Gong, M.; Pande, A.; Margineanu, A.; Lisewski, U.; Purfürst, B.; Zhu, H.; Liang, L.; Jia, S.; Froehler, S.; et al. Atypical KCNQ1/Kv7 channel function in a neonatal diabetes patient: Hypersecretion preceded the failure of pancreatic β-cells. iScience 2024, 27, 110291. [Google Scholar] [CrossRef]
- Asahara, S.-i.; Etoh, H.; Inoue, H.; Teruyama, K.; Shibutani, Y.; Ihara, Y.; Kawada, Y.; Bartolome, A.; Hashimoto, N.; Matsuda, T.; et al. Paternal allelic mutation at the Kcnq1 locus reduces pancreatic β-cell mass by epigenetic modification of Cdkn1c. Proc. Natl. Acad. Sci. USA 2015, 112, 8332–8337. [Google Scholar] [CrossRef]
- Mellado-Gil, J.M.; Jiménez-Moreno, C.M.; Martin-Montalvo, A.; Alvarez-Mercado, A.I.; Fuente-Martin, E.; Cobo-Vuilleumier, N.; Lorenzo, P.I.; Bru-Tari, E.; Herrera-Gómez Ide, G.; López-Noriega, L.; et al. PAX4 preserves endoplasmic reticulum integrity preventing beta cell degeneration in a mouse model of type 1 diabetes mellitus. Diabetologia 2016, 59, 755–765. [Google Scholar] [CrossRef]
- Montgomery, M.K.; Turner, N. Mitochondrial dysfunction and insulin resistance: An update. Endocr. Connect. 2015, 4, R1–R15. [Google Scholar] [CrossRef]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef]
- Fiorenza, M.; Onslev, J.; Henríquez-Olguín, C.; Persson, K.W.; Hesselager, S.A.; Jensen, T.E.; Wojtaszewski, J.F.P.; Hostrup, M.; Bangsbo, J. Reducing the mitochondrial oxidative burden alleviates lipid-induced muscle insulin resistance in humans. Sci. Adv. 2024, 10, eadq4461. [Google Scholar] [CrossRef]
- Whytock, K.L.; Pino, M.F.; Sun, Y.; Yu, G.; De Carvalho, F.G.; Yeo, R.X.; Vega, R.B.; Parmar, G.; Divoux, A.; Kapoor, N.; et al. Comprehensive interrogation of human skeletal muscle reveals a dissociation between insulin resistance and mitochondrial capacity. Am. J. Physiol. Endocrinol. Metab. 2023, 325, E291–E302. [Google Scholar] [CrossRef]
- Mezincescu, A.M.; Rudd, A.; Cheyne, L.; Horgan, G.; Philip, S.; Cameron, D.; van Loon, L.; Whitfield, P.; Gribbin, R.; Hu, M.K.; et al. Comparison of intramyocellular lipid metabolism in patients with diabetes and male athletes. Nat. Commun. 2024, 15, 3690. [Google Scholar] [CrossRef]
- Anello, M.; Lupi, R.; Spampinato, D.; Piro, S.; Masini, M.; Boggi, U.; Del Prato, S.; Rabuazzo, A.M.; Purrello, F.; Marchetti, P. Functional and morphological alterations of mitochondria in pancreatic beta cells from type 2 diabetic patients. Diabetologia 2005, 48, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Genders, A.J.; Holloway, G.P.; Bishop, D.J. Are Alterations in Skeletal Muscle Mitochondria a Cause or Consequence of Insulin Resistance? Int. J. Mol. Sci. 2020, 21, 6948. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, S.K.; Teede, H.J.; Rachon, D.; Harrison, C.L.; Strauss, B.J.; Stepto, N.K. Effect of exercise training on insulin sensitivity, mitochondria and computed tomography muscle attenuation in overweight women with and without polycystic ovary syndrome. Diabetologia 2012, 55, 1424–1434. [Google Scholar] [CrossRef] [PubMed]
- Morino, K.; Petersen, K.F.; Dufour, S.; Befroy, D.; Frattini, J.; Shatzkes, N.; Neschen, S.; White, M.F.; Bilz, S.; Sono, S.; et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J. Clin. Investig. 2005, 115, 3587–3593. [Google Scholar] [CrossRef]
- Lowell, B.B.; Shulman, G.I. Mitochondrial dysfunction and type 2 diabetes. Science 2005, 307, 384–387. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Invest. 2006, 116, 1793–1801. [Google Scholar] [CrossRef]
- Lee, H.K. Mitochondrial dysfunction and insulin resistance: The contribution of dioxin-like substances. Diabetes Metab. J. 2011, 35, 207–215. [Google Scholar] [CrossRef]
- Heinonen, S.; Buzkova, J.; Muniandy, M.; Kaksonen, R.; Ollikainen, M.; Ismail, K.; Hakkarainen, A.; Lundbom, J.; Lundbom, N.; Vuolteenaho, K.; et al. Impaired Mitochondrial Biogenesis in Adipose Tissue in Acquired Obesity. Diabetes 2015, 64, 3135–3145. [Google Scholar] [CrossRef]
- Mantena, S.K.; King, A.L.; Andringa, K.K.; Eccleston, H.B.; Bailey, S.M. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol- and obesity-induced fatty liver diseases. Free Radic. Biol. Med. 2008, 44, 1259–1272. [Google Scholar] [CrossRef]
- Yuzefovych, L.V.; Musiyenko, S.I.; Wilson, G.L.; Rachek, L.I. Mitochondrial DNA Damage and Dysfunction, and Oxidative Stress Are Associated with Endoplasmic Reticulum Stress, Protein Degradation and Apoptosis in High Fat Diet-Induced Insulin Resistance Mice. PLoS ONE 2013, 8, e54059. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Xu, S.; Huang, L.; Chen, C. Obesity and insulin resistance: Pathophysiology and treatment. Drug Discov. Today 2022, 27, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Kojta, I.; Chacinska, M.; Blachnio-Zabielska, A. Obesity, Bioactive Lipids, and Adipose Tissue Inflammation in Insulin Resistance. Nutrients 2020, 12, 1305. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tang, Y.; Luo, Y.; Gao, Y.; He, L. Role and mechanism of specialized pro-resolving mediators in obesity-associated insulin resistance. Lipids Health Dis. 2024, 23, 234. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef]
- Holland, W.L.; Brozinick, J.T.; Wang, L.P.; Hawkins, E.D.; Sargent, K.M.; Liu, Y.; Narra, K.; Hoehn, K.L.; Knotts, T.A.; Siesky, A.; et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007, 5, 167–179. [Google Scholar] [CrossRef]
- Donath, M.Y.; Ehses, J.A.; Maedler, K.; Schumann, D.M.; Ellingsgaard, H.; Eppler, E.; Reinecke, M. Mechanisms of beta-cell death in type 2 diabetes. Diabetes 2005, 54 (Suppl. S2), S108–S113. [Google Scholar] [CrossRef]
- De Gaetano, A.; Solodka, K.; Zanini, G.; Selleri, V.; Mattioli, A.V.; Nasi, M.; Pinti, M. Molecular Mechanisms of mtDNA-Mediated Inflammation. Cells 2021, 10, 2898. [Google Scholar] [CrossRef]
- Ajaz, S.; McPhail, M.J.; Gnudi, L.; Trovato, F.M.; Mujib, S.; Napoli, S.; Carey, I.; Agarwal, K. Mitochondrial dysfunction as a mechanistic biomarker in patients with non-alcoholic fatty liver disease (NAFLD). Mitochondrion 2021, 57, 119–130. [Google Scholar] [CrossRef]
- Ramanathan, R.; Ali, A.H.; Ibdah, J.A. Mitochondrial Dysfunction Plays Central Role in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 7280. [Google Scholar] [CrossRef]
- Sakuma, T.; Nakamura, M.; Chiba, T.; Iwanaga, T.; Kan, M.; Kojima, R.; Ao, J.; Ma, Y.; Unozawa, H.; Fujita, N.; et al. A diet-induced murine model for non-alcoholic fatty liver disease with obesity and insulin resistance that rapidly develops steatohepatitis and fibrosis. Lab. Invest. 2022, 102, 1150–1157. [Google Scholar] [CrossRef]
- Vincent, A.M.; Callaghan, B.C.; Smith, A.L.; Feldman, E.L. Diabetic neuropathy: Cellular mechanisms as therapeutic targets. Nat. Rev. Neurol. 2011, 7, 573–583. [Google Scholar] [CrossRef]
- Lee, M.W.; Lee, M.; Oh, K.J. Adipose Tissue-Derived Signatures for Obesity and Type 2 Diabetes: Adipokines, Batokines and MicroRNAs. J. Clin. Med. 2019, 8, 854. [Google Scholar] [CrossRef] [PubMed]
- Valaiyapathi, B.; Gower, B.; Ashraf, A.P. Pathophysiology of Type 2 Diabetes in Children and Adolescents. Curr. Diabetes Rev. 2020, 16, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Barshad, G.; Marom, S.; Cohen, T.; Mishmar, D. Mitochondrial DNA Transcription and Its Regulation: An Evolutionary Perspective. Trends Genet. 2018, 34, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ren, J.; Li, Y.; Wu, Q.; Wei, J. Oxidative stress: The nexus of obesity and cognitive dysfunction in diabetes. Front. Endocrinol. 2023, 14, 1134025. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, Y.; Lang, X.; Zhang, Y. Role of mitochondrial metabolic disorder and immune infiltration in diabetic cardiomyopathy: New insights from bioinformatics analysis. J. Transl. Med. 2023, 21, 66. [Google Scholar] [CrossRef]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef]
- West, A.P. Mitochondrial dysfunction as a trigger of innate immune responses and inflammation. Toxicology 2017, 391, 54–63. [Google Scholar] [CrossRef]
- Lopez-Armada, M.J.; Riveiro-Naveira, R.R.; Vaamonde-Garcia, C.; Valcarcel-Ares, M.N. Mitochondrial dysfunction and the inflammatory response. Mitochondrion 2013, 13, 106–118. [Google Scholar] [CrossRef]
- West, A.P.; Shadel, G.S. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat. Rev. Immunol. 2017, 17, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Barja, G. Free radicals and aging. Trends Neurosci. 2004, 27, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstråle, M.; Laurila, E.; et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Microbiota and diabetes: An evolving relationship. Gut 2014, 63, 1513–1521. [Google Scholar] [CrossRef]
- Scheithauer, T.P.M.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; van Raalte, D.H.; Herrema, H. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front. Immunol. 2020, 11, 571731. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Thomou, T.; Mori, M.A.; Dreyfuss, J.M.; Konishi, M.; Sakaguchi, M.; Wolfrum, C.; Rao, T.N.; Winnay, J.N.; Garcia-Martin, R.; Grinspoon, S.K.; et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 2017, 542, 450–455. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Invest. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Larsen, C.M.; Faulenbach, M.; Vaag, A.; Vølund, A.; Ehses, J.A.; Seifert, B.; Mandrup-Poulsen, T.; Donath, M.Y. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 2007, 356, 1517–1526. [Google Scholar] [CrossRef]
- Kataria, Y.; Ellervik, C.; Mandrup-Poulsen, T. Treatment of type 2 diabetes by targeting interleukin-1: A meta-analysis of 2921 patients. Semin Immunopathol 2019, 41, 413–425. [Google Scholar] [CrossRef]
- Vandanmagsar, B.; Youm, Y.H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef]
- Ruscitti, P.; Cipriani, P.; Di Benedetto, P.; Liakouli, V.; Berardicurti, O.; Carubbi, F.; Ciccia, F.; Alvaro, S.; Triolo, G.; Giacomelli, R. Monocytes from patients with rheumatoid arthritis and type 2 diabetes mellitus display an increased production of interleukin (IL)-1β via the nucleotide-binding domain and leucine-rich repeat containing family pyrin 3(NLRP3)-inflammasome activation: A possible implication for therapeutic decision in these patients. Clin. Exp. Immunol. 2015, 182, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Coll, R.C.; Hill, J.R.; Day, C.J.; Zamoshnikova, A.; Boucher, D.; Massey, N.L.; Chitty, J.L.; Fraser, J.A.; Jennings, M.P.; Robertson, A.A.B.; et al. MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat. Chem. Biol. 2019, 15, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Everett, B.M.; Donath, M.Y.; Pradhan, A.D.; Thuren, T.; Pais, P.; Nicolau, J.C.; Glynn, R.J.; Libby, P.; Ridker, P.M. Anti-Inflammatory Therapy With Canakinumab for the Prevention and Management of Diabetes. J. Am. Coll. Cardiol. 2018, 71, 2392–2401. [Google Scholar] [CrossRef] [PubMed]
- Sloan-Lancaster, J.; Abu-Raddad, E.; Polzer, J.; Miller, J.W.; Scherer, J.C.; De Gaetano, A.; Berg, J.K.; Landschulz, W.H. Double-blind, randomized study evaluating the glycemic and anti-inflammatory effects of subcutaneous LY2189102, a neutralizing IL-1β antibody, in patients with type 2 diabetes. Diabetes Care 2013, 36, 2239–2246. [Google Scholar] [CrossRef]
- Goldfine, A.B.; Fonseca, V.; Jablonski, K.A.; Pyle, L.; Staten, M.A.; Shoelson, S.E. The effects of salsalate on glycemic control in patients with type 2 diabetes: A randomized trial. Ann. Intern. Med. 2010, 152, 346–357. [Google Scholar] [CrossRef]
- Demidowich, A.P.; Levine, J.A.; Apps, R.; Cheung, F.K.; Chen, J.; Fantoni, G.; Zhou, H.; Shi, R.; Subramanian, P.; Tsang, J.; et al. Colchicine’s effects on metabolic and inflammatory molecules in adults with obesity and metabolic syndrome: Results from a pilot randomized controlled trial. Int. J. Obes. 2020, 44, 1793–1799. [Google Scholar] [CrossRef]
- Tunc, S.E.; Delibasi, B.T.; Delibasi, T. Targeting Inflammation in Type 2 Diabetes: The Role of Colchicine. Curr. Diabetes Rev. 2025. [Google Scholar] [CrossRef]
- Zhu, D.; Li, X.; Ma, J.; Zeng, J.e.; Gan, S.; Dong, X.; Yang, J.; Lin, X.; Cai, H.; Song, W.; et al. Dorzagliatin in drug-naïve patients with type 2 diabetes: A randomized, double-blind, placebo-controlled phase 3 trial. Nat. Med. 2022, 28, 965–973. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Dhokte, S.; Czaja, K. Visceral Adipose Tissue: The Hidden Culprit for Type 2 Diabetes. Nutrients 2024, 16, 1015. [Google Scholar] [CrossRef]
- Sun, K.; Tordjman, J.; Clement, K.; Scherer, P.E. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013, 18, 470–477. [Google Scholar] [CrossRef]
- Zhu, Y.; Luo, M.; Bai, X.; Li, J.; Nie, P.; Li, B.; Luo, P. SS-31, a Mitochondria-Targeting Peptide, Ameliorates Kidney Disease. Oxid. Med. Cell Longev. 2022, 2022, 1295509. [Google Scholar] [CrossRef]
- Escribano-Lopez, I.; Diaz-Morales, N.; Iannantuoni, F.; Lopez-Domenech, S.; de Marañon, A.M.; Abad-Jimenez, Z.; Bañuls, C.; Rovira-Llopis, S.; Herance, J.R.; Rocha, M.; et al. The mitochondrial antioxidant SS-31 increases SIRT1 levels and ameliorates inflammation, oxidative stress and leukocyte-endothelium interactions in type 2 diabetes. Sci. Rep. 2018, 8, 15862. [Google Scholar] [CrossRef] [PubMed]
- Sweetwyne, M.T.; Pippin, J.W.; Eng, D.G.; Hudkins, K.L.; Chiao, Y.A.; Campbell, M.D.; Marcinek, D.J.; Alpers, C.E.; Szeto, H.H.; Rabinovitch, P.S.; et al. The mitochondrial-targeted peptide, SS-31, improves glomerular architecture in mice of advanced age. Kidney Int. 2017, 91, 1126–1145. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, V.; La Grotta, R.; Carreras, F.; Giuliani, A.; Sabbatinelli, J.; Olivieri, F.; Berra, C.C.; Ceriello, A.; Prattichizzo, F. Inflammatory Trajectory of Type 2 Diabetes: Novel Opportunities for Early and Late Treatment. Cells 2024, 13, 1662. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, X.; Wang, C.; He, H. Effects of Exercise on Inflammatory Cytokines in Patients with Type 2 Diabetes: A Meta-analysis of Randomized Controlled Trials. Oxid. Med. Cell Longev. 2020, 2020, 6660557. [Google Scholar] [CrossRef]
- Vinayagam, P.; Senathipathi, V.; Shivam, V.; Velraju, N. The role of Imeglimin in glycemic control, beta cell function and safety outcomes in patients with type 2 diabetes mellitus: A comprehensive meta-analysis. Diabetes Epidemiol. Manag. 2023, 12, 100164. [Google Scholar] [CrossRef]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Katsuyama, H. Glucose-Lowering Effects of Imeglimin and Its Possible Beneficial Effects on Diabetic Complications. Biology 2023, 12, 726. [Google Scholar] [CrossRef]
- Kamm, D.R.; Pyles, K.D.; Sharpe, M.C.; Healy, L.N.; Colca, J.R.; McCommis, K.S. Novel insulin sensitizer MSDC-0602K improves insulinemia and fatty liver disease in mice, alone and in combination with liraglutide. J. Biol. Chem. 2021, 296, 100807. [Google Scholar] [CrossRef]
- Literáti-Nagy, B.; Kulcsár, E.; Literáti-Nagy, Z.; Buday, B.; Péterfai, E.; Horváth, T.; Tory, K.; Kolonics, A.; Fleming, A.; Mandl, J.; et al. Improvement of insulin sensitivity by a novel drug, BGP-15, in insulin-resistant patients: A proof of concept randomized double-blind clinical trial. Horm. Metab. Res. 2009, 41, 374–380. [Google Scholar] [CrossRef]
- Noureddin, M.; Khan, S.; Portell, F.; Jorkasky, D.; Dennis, J.; Khan, O.; Johansson, L.; Johansson, E.; Sanyal, A.J. Safety and efficacy of once-daily HU6 versus placebo in people with non-alcoholic fatty liver disease and high BMI: A randomised, double-blind, placebo-controlled, phase 2a trial. Lancet Gastroenterol. Hepatol. 2023, 8, 1094–1105. [Google Scholar] [CrossRef] [PubMed]
- Charles, E.D.; Neuschwander-Tetri, B.A.; Pablo Frias, J.; Kundu, S.; Luo, Y.; Tirucherai, G.S.; Christian, R. Pegbelfermin (BMS-986036), PEGylated FGF21, in Patients with Obesity and Type 2 Diabetes: Results from a Randomized Phase 2 Study. Obesity 2019, 27, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Kalligeros, M.; Henry, L. Epidemiology of metabolic dysfunction-associated steatotic liver disease. Clin. Mol. Hepatol. 2025, 31, S32–S50. [Google Scholar] [CrossRef]
- Leyh, C.; Coombes, J.D.; Schmidt, H.H.; Canbay, A.; Manka, P.P.; Best, J. MASLD-Related HCC-Update on Pathogenesis and Current Treatment Options. J. Pers. Med. 2024, 14, 370. [Google Scholar] [CrossRef]
- Danpanichkul, P.; Suparan, K.; Prasitsumrit, V.; Ahmed, A.; Wijarnpreecha, K.; Kim, D. Long-term outcomes and risk modifiers of metabolic dysfunction-associated steatotic liver disease between lean and non-lean populations. Clin. Mol. Hepatol. 2025, 31, 74–89. [Google Scholar] [CrossRef]
- Meyer, M.; Schwärzler, J.; Jukic, A.; Tilg, H. Innate Immunity and MASLD. Biomolecules 2024, 14, 476. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Jia, Z.; Xiao, Y.; Shi, A.; Ma, X. Mitochondrial Dysfunction as a Pathogenesis and Therapeutic Strategy for Metabolic-Dysfunction-Associated Steatotic Liver Disease. Int. J. Mol. Sci. 2025, 26, 4256. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, T.; Zhao, S.; Yang, S.; Jiang, K.; Li, S.; Kang, Y.; Yang, Z.; Shen, J.; Shen, S.; et al. O-GlcNAcylation promotes the progression of nonalcoholic fatty liver disease by upregulating the expression and function of CD36. Metabolism 2024, 156, 155914. [Google Scholar] [CrossRef]
- Beyoğlu, D.; Popov, Y.V.; Idle, J.R. Metabolomic Hallmarks of Obesity and Metabolic Dysfunction-Associated Steatotic Liver Disease. Int. J. Mol. Sci. 2024, 25, 12809. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Liu, Y.; Xia, J.; Liu, J.; Wang, K.; Liang, H.; Xu, F.; Liu, D.; Nie, D.; Tang, X.; et al. Loss of SLC27A5 Activates Hepatic Stellate Cells and Promotes Liver Fibrosis via Unconjugated Cholic Acid. Adv. Sci. 2024, 11, e2304408. [Google Scholar] [CrossRef] [PubMed]
- Mukai, T.; Egawa, M.; Takeuchi, T.; Yamashita, H.; Kusudo, T. Silencing of FABP1 ameliorates hepatic steatosis, inflammation, and oxidative stress in mice with nonalcoholic fatty liver disease. FEBS Open Bio 2017, 7, 1009–1016. [Google Scholar] [CrossRef]
- Li, L.O.; Ellis, J.M.; Paich, H.A.; Wang, S.; Gong, N.; Altshuller, G.; Thresher, R.J.; Koves, T.R.; Watkins, S.M.; Muoio, D.M.; et al. Liver-specific loss of long chain acyl-CoA synthetase-1 decreases triacylglycerol synthesis and beta-oxidation and alters phospholipid fatty acid composition. J. Biol. Chem. 2009, 284, 27816–27826. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, Z.; Zhu, K.; Shi, H.; Qin, F.; Zhang, T.; Tian, S.; Ji, Y.; Zhang, J.; Qin, J.; et al. USP29 alleviates the progression of MASLD by stabilizing ACSL5 through K48 deubiquitination. Clin. Mol. Hepatol. 2025, 31, 147–165. [Google Scholar] [CrossRef]
- Şen, İ.; Dumlu, Ş. Liver Fatty Acid-binding Protein Is a More Reliable Biomarker for Liver Injury in Nonalcoholic Steatohepatitis than Other Etiologies of Hepatitis. Turk. J. Gastroenterol. 2024, 35, 568–576. [Google Scholar] [CrossRef]
- Choi, M.G.; Lee, N.Y.; Koo, J.H. Stabilizing hepatic fatty acid oxidation: Editorial on “USP29 alleviates the progression of MASLD by stabilizing ACSL5 through K48 deubiquitination”. Clin. Mol. Hepatol. 2025, 31, 592–595. [Google Scholar] [CrossRef]
- Lu, D.; He, A.; Tan, M.; Mrad, M.; El Daibani, A.; Hu, D.; Liu, X.; Kleiboeker, B.; Che, T.; Hsu, F.F.; et al. Liver ACOX1 regulates levels of circulating lipids that promote metabolic health through adipose remodeling. Nat. Commun. 2024, 15, 4214. [Google Scholar] [CrossRef]
- Carli, F.; Della Pepa, G.; Sabatini, S.; Vidal Puig, A.; Gastaldelli, A. Lipid metabolism in MASLD and MASH: From mechanism to the clinic. JHEP Rep. 2024, 6, 101185. [Google Scholar] [CrossRef]
- Jokinen, M.J.; Luukkonen, P.K. Hepatic mitochondrial reductive stress in the pathogenesis and treatment of steatotic liver disease. Trends Pharmacol. Sci. 2024, 45, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Gao, X.Q.; Shen, N.; He, J.; Fan, X.; Chen, K.; Lin, X.H.; Li, H.M.; Tian, F.S.; Li, H. A targeted metabolomic profiling of plasma acylcarnitines in nonalcoholic fatty liver disease. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7433–7441. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Jellinek, M.J.; Mehta, K.; Seok, S.M.; Kuo, S.H.; Lu, W.; Shi, R.; Lee, R.; Lau, G.W.; Kemper, J.K.; et al. Membrane phospholipid remodeling modulates nonalcoholic steatohepatitis progression by regulating mitochondrial homeostasis. Hepatology 2024, 79, 882–897. [Google Scholar] [CrossRef] [PubMed]
- Fromenty, B.; Roden, M. Mitochondrial alterations in fatty liver diseases. J. Hepatol. 2023, 78, 415–429. [Google Scholar] [CrossRef]
- Brothwell, M.J.; Cao, G.; Maschek, J.A.; Poss, A.M.; Peterlin, A.D.; Wang, L.; Baker, T.B.; Shahtout, J.L.; Siripoksup, P.; Pearce, Q.J.; et al. Cardiolipin deficiency disrupts electron transport chain to drive steatohepatitis. bioRxiv 2025. [Google Scholar] [CrossRef]
- Amorim, R.; Simões, I.C.M.; Veloso, C.; Carvalho, A.; Simões, R.F.; Pereira, F.B.; Thiel, T.; Normann, A.; Morais, C.; Jurado, A.S.; et al. Exploratory Data Analysis of Cell and Mitochondrial High-Fat, High-Sugar Toxicity on Human HepG2 Cells. Nutrients 2021, 13, 1723. [Google Scholar] [CrossRef]
- Yin, W.; Xu, H.; Bai, Z.; Wu, Y.; Zhang, Y.; Liu, R.; Wang, Z.; Zhang, B.; Shen, J.; Zhang, H.; et al. Inhibited peroxidase activity of peroxiredoxin 1 by palmitic acid exacerbates nonalcoholic steatohepatitis in male mice. Nat. Commun. 2025, 16, 598. [Google Scholar] [CrossRef]
- Abdelmalek, M.F.; Lazo, M.; Horska, A.; Bonekamp, S.; Lipkin, E.W.; Balasubramanyam, A.; Bantle, J.P.; Johnson, R.J.; Diehl, A.M.; Clark, J.M. Higher dietary fructose is associated with impaired hepatic adenosine triphosphate homeostasis in obese individuals with type 2 diabetes. Hepatology 2012, 56, 952–960. [Google Scholar] [CrossRef]
- Li, H.; Yao, L.; Xiao, Z.; Li, S. Detecting the Stage of Fibrosis in Non-alcoholic Fatty Liver Disease by 9.4T Phosphorus Magnetic Resonance Spectroscopy. Magn. Reson. Med. Sci. 2025. [Google Scholar] [CrossRef]
- Zhao, P.; Saltiel, A.R. From overnutrition to liver injury: AMP-activated protein kinase in nonalcoholic fatty liver diseases. J. Biol. Chem. 2020, 295, 12279–12289. [Google Scholar] [CrossRef]
- Boudaba, N.; Marion, A.; Huet, C.; Pierre, R.; Viollet, B.; Foretz, M. AMPK Re-Activation Suppresses Hepatic Steatosis but its Downregulation Does Not Promote Fatty Liver Development. EBioMedicine 2018, 28, 194–209. [Google Scholar] [CrossRef]
- Hendrikx, T.; Binder, C.J. Oxidation-Specific Epitopes in Non-Alcoholic Fatty Liver Disease. Front. Endocrinol. 2020, 11, 607011. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Ou, Z.; Cai, C.; Li, P.; Gong, J.; Ruan, X.Z.; He, K. Fatty acid activates NLRP3 inflammasomes in mouse Kupffer cells through mitochondrial DNA release. Cell Immunol. 2018, 332, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, R.; Cao, Y.; Li, Y.; Zhu, Y.; Zhang, Z.; Fleishman, J.S.; Chen, J.; Ding, M. cGAS-STING Targeting Offers Novel Therapeutic Opportunities in Liver Diseases. Drug Des. Devel Ther. 2025, 19, 5835–5853. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, K.; Gohil, V.; Stuart, R.A.; Hunte, C.; Brandt, U.; Greenberg, M.L.; Schägger, H. Cardiolipin stabilizes respiratory chain supercomplexes. J. Biol. Chem. 2003, 278, 52873–52880. [Google Scholar] [CrossRef]
- Schwall, C.T.; Greenwood, V.L.; Alder, N.N. The stability and activity of respiratory Complex II is cardiolipin-dependent. Biochim. Biophys. Acta 2012, 1817, 1588–1596. [Google Scholar] [CrossRef]
- Chi, Y.J.; Bai, Z.Y.; Feng, G.L.; Lai, X.H.; Song, Y.F. ER-mitochondria contact sites regulate hepatic lipogenesis via Ip3r-Grp75-Vdac complex recruiting Seipin. Cell Commun. Signal 2024, 22, 464. [Google Scholar] [CrossRef]
- Guo, M.; Liu, R.; Zhang, F.; Qu, J.; Yang, Y.; Li, X. A new perspective on liver diseases: Focusing on the mitochondria-associated endoplasmic reticulum membranes. Pharmacol. Res. 2024, 208, 107409. [Google Scholar] [CrossRef]
- Lai, X.H.; Feng, G.L.; Hu, N.J.; Zheng, F.F.; Song, Y.F. High-Fat Diet Impairs Translocation of Phosphatidylserine From Mitochondria-Associated Membranes (MAM) Into Mitochondria by the Conservative Mechanism From Fish to Tetrapod. FASEB J. 2025, 39, e70889. [Google Scholar] [CrossRef]
- Li, Y.E.; Sowers, J.R.; Hetz, C.; Ren, J. Cell death regulation by MAMs: From molecular mechanisms to therapeutic implications in cardiovascular diseases. Cell Death Dis. 2022, 13, 504. [Google Scholar] [CrossRef]
- Barazzuol, L.; Giamogante, F.; Calì, T. Mitochondria Associated Membranes (MAMs): Architecture and physiopathological role. Cell Calcium 2021, 94, 102343. [Google Scholar] [CrossRef]
- Endlicher, R.; Drahota, Z.; Štefková, K.; Červinková, Z.; Kučera, O. The Mitochondrial Permeability Transition Pore-Current Knowledge of Its Structure, Function, and Regulation, and Optimized Methods for Evaluating Its Functional State. Cells 2023, 12, 1273. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, M.; Sun, H.; Ferdous, M.R.U.; Gao, L.; Zhao, J.; Song, Y. Liver cyclophilin D deficiency inhibits the progression of early NASH by ameliorating steatosis and inflammation. Biochem. Biophys. Res. Commun. 2022, 594, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, W.T.; Bobardt, M.; Ure, D.R.; Foster, R.T.; Gallay, P. Cyclophilin D knockout significantly prevents HCC development in a streptozotocin-induced mouse model of diabetes-linked NASH. PLoS ONE 2024, 19, e0301711. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Du, H.; Shao, S.; Bo, T.; Yu, C.; Chen, W.; Zhao, L.; Li, Q.; Wang, L.; Liu, X.; et al. Cyclophilin D deficiency attenuates mitochondrial perturbation and ameliorates hepatic steatosis. Hepatology 2018, 68, 62–77. [Google Scholar] [CrossRef]
- Li, S.; Chen, F.; Liu, M.; Zhang, Y.; Xu, J.; Li, X.; Shang, Z.; Huang, S.; Song, S.; Tu, C. Knockdown of hepatic mitochondrial calcium uniporter mitigates MASH and fibrosis in mice. Cell Biosci. 2024, 14, 135. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, Z.; Yu, L.; Xiao, Y.; Liu, S.; Z, A.L.; Ma, Z.; Huang, L.; Xiao, L.; Jia, M.; et al. Ruthenium 360 and mitoxantrone inhibit mitochondrial calcium uniporter channel to prevent liver steatosis induced by high-fat diet. Br. J. Pharmacol. 2022, 179, 2678–2696. [Google Scholar] [CrossRef]
- Li, F.; Guan, Z.; Gao, Y.; Bai, Y.; Zhan, X.; Ji, X.; Xu, J.; Zhou, H.; Rao, Z. ER stress promotes mitochondrial calcium overload and activates the ROS/NLRP3 axis to mediate fatty liver ischemic injury. Hepatol. Commun. 2024, 8, e0399. [Google Scholar] [CrossRef]
- Chen, F.; Li, S.; Liu, M.; Qian, C.; Shang, Z.; Song, X.; Jiang, W.; Tu, C. Targeting BRD4 mitigates hepatocellular lipotoxicity by suppressing the NLRP3 inflammasome activation and GSDMD-mediated hepatocyte pyroptosis. Cell Mol. Life Sci. 2024, 81, 295. [Google Scholar] [CrossRef]
- Newman, L.E.; Shadel, G.S. Mitochondrial DNA Release in Innate Immune Signaling. Annu. Rev. Biochem. 2023, 92, 299–332. [Google Scholar] [CrossRef]
- Victorelli, S.; Salmonowicz, H.; Chapman, J.; Martini, H.; Vizioli, M.G.; Riley, J.S.; Cloix, C.; Hall-Younger, E.; Machado Espindola-Netto, J.; Jurk, D.; et al. Apoptotic stress causes mtDNA release during senescence and drives the SASP. Nature 2023, 622, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Riley, J.S.; Tait, S.W. Mitochondrial DNA in inflammation and immunity. EMBO Rep. 2020, 21, e49799. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martinez, I.; Santoro, N.; Chen, Y.; Hoque, R.; Ouyang, X.; Caprio, S.; Shlomchik, M.J.; Coffman, R.L.; Candia, A.; Mehal, W.Z. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J. Clin. Invest. 2016, 126, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, Y.; An, W.; Song, J.; Zhang, Y.; Zhao, X. STING-mediated inflammation in Kupffer cells contributes to progression of nonalcoholic steatohepatitis. J. Clin. Invest. 2019, 129, 546–555. [Google Scholar] [CrossRef]
- Xu, D.; Qu, X.; Yang, T.; Sheng, M.; Bian, X.; Zhan, Y.; Tian, Y.; Lin, Y.; Jin, Y.; Wang, X.; et al. The Foxo1-YAP-Notch1 axis reprograms STING-mediated innate immunity in NASH progression. Exp. Mol. Med. 2024, 56, 1843–1855. [Google Scholar] [CrossRef]
- Zhu, S.; Liao, L.; Zhong, Y.; Liu, Z.; Lu, J.; Yang, Z.; Xiao, Y.; Xu, X. Hepatocellular CMPK2 promotes the development of metabolic dysfunction-associated steatohepatitis. J. Hepatol. 2025, 83, 383–396. [Google Scholar] [CrossRef]
- Chen, S.; Cao, Y.; Fan, Z.; Xu, L.; Pan, Z.; Gao, Y.; Wei, L.; Wei, Q.; Tian, Y.; Zhang, X.; et al. Depressed TFAM promotes acetaminophen-induced hepatotoxicity regulated by DDX3X-PGC1α-NRF2 signaling pathway. Mol. Med. 2024, 30, 246. [Google Scholar] [CrossRef]
- Xu, H.L.; Wan, S.R.; An, Y.; Wu, Q.; Xing, Y.H.; Deng, C.H.; Zhang, P.P.; Long, Y.; Xu, B.T.; Jiang, Z.Z. Targeting cell death in NAFLD: Mechanisms and targeted therapies. Cell Death Discov. 2024, 10, 399. [Google Scholar] [CrossRef]
- Yu, L.; Hong, W.; Lu, S.; Li, Y.; Guan, Y.; Weng, X.; Feng, Z. The NLRP3 Inflammasome in Non-Alcoholic Fatty Liver Disease and Steatohepatitis: Therapeutic Targets and Treatment. Front. Pharmacol. 2022, 13, 780496. [Google Scholar] [CrossRef]
- Xian, H.; Watari, K.; Sanchez-Lopez, E.; Offenberger, J.; Onyuru, J.; Sampath, H.; Ying, W.; Hoffman, H.M.; Shadel, G.S.; Karin, M. Oxidized DNA fragments exit mitochondria via mPTP- and VDAC-dependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity 2022, 55, 1370–1385.e1378. [Google Scholar] [CrossRef]
- Wu, N.N.; Wang, L.; Wang, L.; Xu, X.; Lopaschuk, G.D.; Zhang, Y.; Ren, J. Site-specific ubiquitination of VDAC1 restricts its oligomerization and mitochondrial DNA release in liver fibrosis. Exp. Mol. Med. 2023, 55, 269–280. [Google Scholar] [CrossRef]
- Baik, S.H.; Ramanujan, V.K.; Becker, C.; Fett, S.; Underhill, D.M.; Wolf, A.J. Hexokinase dissociation from mitochondria promotes oligomerization of VDAC that facilitates NLRP3 inflammasome assembly and activation. Sci. Immunol. 2023, 8, eade7652. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Tang, C.; Song, X.; Liu, Z.; Zhou, J.; Shi, Q.; Yu, C.; Xu, C. High uric acid exacerbates nonalcoholic steatohepatitis through NLRP3 inflammasome and gasdermin D-mediated pyroptosis. J. Biol. Chem. 2025, 301, 110249. [Google Scholar] [CrossRef] [PubMed]
- Santa Cruz Garcia, A.B.; Schnur, K.P.; Malik, A.B.; Mo, G.C.H. Gasdermin D pores are dynamically regulated by local phosphoinositide circuitry. Nat. Commun. 2022, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cai, Z.; Schindler, F.; Afjehi-Sadat, L.; Montsch, B.; Heffeter, P.; Heiss, E.H.; Weckwerth, W. Elevated PINK1/Parkin-Dependent Mitophagy and Boosted Mitochondrial Function Mediate Protection of HepG2 Cells from Excess Palmitic Acid by Hesperetin. J. Agric. Food Chem. 2024, 72, 13039–13053. [Google Scholar] [CrossRef]
- Xu, Y.; Tang, Y.; Lu, J.; Zhang, W.; Zhu, Y.; Zhang, S.; Ma, G.; Jiang, P.; Zhang, W. PINK1-mediated mitophagy protects against hepatic ischemia/reperfusion injury by restraining NLRP3 inflammasome activation. Free Radic. Biol. Med. 2020, 160, 871–886. [Google Scholar] [CrossRef]
- Zhao, R.; Guo, Z.; Lu, K.; Chen, Q.; Riaz, F.; Zhou, Y.; Yang, L.; Cheng, X.; Wu, L.; Cheng, K.; et al. Hepatocyte-specific NR5A2 deficiency induces pyroptosis and exacerbates non-alcoholic steatohepatitis by downregulating ALDH1B1 expression. Cell Death Dis. 2024, 15, 770. [Google Scholar] [CrossRef]
- Lawlor, K.E.; Murphy, J.M.; Vince, J.E. Gasdermin and MLKL necrotic cell death effectors: Signaling and diseases. Immunity 2024, 57, 429–445. [Google Scholar] [CrossRef]
- Afonso, M.B.; Islam, T.; Magusto, J.; Amorim, R.; Lenoir, V.; Simões, R.F.; Teixeira, J.; Silva, L.C.; Wendum, D.; Jéru, I.; et al. RIPK3 dampens mitochondrial bioenergetics and lipid droplet dynamics in metabolic liver disease. Hepatology 2023, 77, 1319–1334. [Google Scholar] [CrossRef]
- Xu, J.; Wu, D.; Zhou, S.; Hu, H.; Li, F.; Guan, Z.; Zhan, X.; Gao, Y.; Wang, P.; Rao, Z. MLKL deficiency attenuated hepatocyte oxidative DNA damage by activating mitophagy to suppress macrophage cGAS-STING signaling during liver ischemia and reperfusion injury. Cell Death Discov. 2023, 9, 58. [Google Scholar] [CrossRef]
- Xu, G.X.; Wei, S.; Yu, C.; Zhao, S.Q.; Yang, W.J.; Feng, Y.H.; Pan, C.; Yang, K.X.; Ma, Y. Activation of Kupffer cells in NAFLD and NASH: Mechanisms and therapeutic interventions. Front. Cell Dev. Biol. 2023, 11, 1199519. [Google Scholar] [CrossRef]
- Jeelani, I.; Moon, J.S.; da Cunha, F.F.; Nasamran, C.A.; Jeon, S.; Zhang, X.; Bandyopadhyay, G.K.; Dobaczewska, K.; Mikulski, Z.; Hosseini, M.; et al. HIF-2α drives hepatic Kupffer cell death and proinflammatory recruited macrophage activation in nonalcoholic steatohepatitis. Sci. Transl. Med. 2024, 16, eadi0284. [Google Scholar] [CrossRef]
- Tran, S.; Baba, I.; Poupel, L.; Dussaud, S.; Moreau, M.; Gélineau, A.; Marcelin, G.; Magréau-Davy, E.; Ouhachi, M.; Lesnik, P.; et al. Impaired Kupffer Cell Self-Renewal Alters the Liver Response to Lipid Overload during Non-alcoholic Steatohepatitis. Immunity 2020, 53, 627–640.e625. [Google Scholar] [CrossRef]
- Kostallari, E.; Schwabe, R.F.; Guillot, A. Inflammation and immunity in liver homeostasis and disease: A nexus of hepatocytes, nonparenchymal cells and immune cells. Cell Mol. Immunol. 2025, 22, 1205–1225. [Google Scholar] [CrossRef] [PubMed]
- Czopik, A.K.; McNamee, E.N.; Vaughn, V.; Huang, X.; Bang, I.H.; Clark, T.; Wang, Y.; Ruan, W.; Nguyen, T.; Masterson, J.C.; et al. HIF-2α-dependent induction of miR-29a restrains T(H)1 activity during T cell dependent colitis. Nat. Commun. 2024, 15, 8042. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Khalil, M.; Angelis, M.; Calabrese, F.M.; D’Amato, M.; Wang, D.Q.; Di Ciaula, A. Intestinal Barrier and Permeability in Health, Obesity and NAFLD. Biomedicines 2021, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.; Zhao, N.; Mlcochova, P.; Ferreira, I.; Ortmann, B.M.; Davis, T.; Wit, N.; Rehwinkel, J.; Cook, S.; Maxwell, P.H.; et al. Hypoxia drives HIF2-dependent reversible macrophage cell cycle entry. Cell Rep. 2024, 43, 114471. [Google Scholar] [CrossRef]
- Huang, H.Y.; Chen, Y.Z.; Zhao, C.; Zheng, X.N.; Yu, K.; Yue, J.X.; Ju, H.Q.; Shi, Y.X.; Tian, L. Alternations in inflammatory macrophage niche drive phenotypic and functional plasticity of Kupffer cells. Nat. Commun. 2024, 15, 9337. [Google Scholar] [CrossRef]
- Xia, J.; Chen, H.; Wang, X.; Chen, W.; Lin, J.; Xu, F.; Nie, Q.; Ye, C.; Zhong, B.; Zhao, M.; et al. Sphingosine d18:1 promotes nonalcoholic steatohepatitis by inhibiting macrophage HIF-2α. Nat. Commun. 2024, 15, 4755. [Google Scholar] [CrossRef]
- Kang, J.; Postigo-Fernandez, J.; Kim, K.; Zhu, C.; Yu, J.; Meroni, M.; Mayfield, B.; Bartolomé, A.; Dapito, D.H.; Ferrante, A.W., Jr.; et al. Notch-mediated hepatocyte MCP-1 secretion causes liver fibrosis. JCI Insight 2023, 8, e165369. [Google Scholar] [CrossRef]
- Geervliet, E.; Karkdijk, E.; Bansal, R. Inhibition of intrahepatic monocyte recruitment by Cenicriviroc and extracellular matrix degradation by MMP1 synergistically attenuate liver inflammation and fibrogenesis in vivo. Sci. Rep. 2024, 14, 16897. [Google Scholar] [CrossRef]
- Reiter, F.P.; Wimmer, R.; Wottke, L.; Artmann, R.; Nagel, J.M.; Carranza, M.O.; Mayr, D.; Rust, C.; Fickert, P.; Trauner, M.; et al. Role of interleukin-1 and its antagonism of hepatic stellate cell proliferation and liver fibrosis in the Abcb4(-/-) mouse model. World J. Hepatol. 2016, 8, 401–410. [Google Scholar] [CrossRef]
- Dudek, M.; Pfister, D.; Donakonda, S.; Filpe, P.; Schneider, A.; Laschinger, M.; Hartmann, D.; Hüser, N.; Meiser, P.; Bayerl, F.; et al. Auto-aggressive CXCR6(+) CD8 T cells cause liver immune pathology in NASH. Nature 2021, 592, 444–449. [Google Scholar] [CrossRef]
- Adams, V.R.; Collins, L.B.; Williams, T.I.; Holmes, J.; Hess, P.; Atkins, H.M.; Scheidemantle, G.; Liu, X.; Lodge, M.; Johnson, A.J.; et al. Myeloid cell MHC I expression drives CD8(+) T cell activation in nonalcoholic steatohepatitis. Front. Immunol. 2023, 14, 1302006. [Google Scholar] [CrossRef] [PubMed]
- Tosello-Trampont, A.C.; Krueger, P.; Narayanan, S.; Landes, S.G.; Leitinger, N.; Hahn, Y.S. NKp46(+) natural killer cells attenuate metabolism-induced hepatic fibrosis by regulating macrophage activation in mice. Hepatology 2016, 63, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Saffarzadeh, M.; Juenemann, C.; Queisser, M.A.; Lochnit, G.; Barreto, G.; Galuska, S.P.; Lohmeyer, J.; Preissner, K.T. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: A predominant role of histones. PLoS ONE 2012, 7, e32366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, R.; Zhan, X.; Wang, X.Y.; Xiang, L.W.; Duan, Y.Q.; You, Y.; Zhang, J.B.; Wu, R.; Zhang, Y.Y.; et al. Neutrophil extracellular traps facilitate liver inflammation/fibrosis progression by entering macrophages and triggering AIM2 inflammasome-dependent pyroptosis. Cell Commun. Signal 2024, 22, 556. [Google Scholar] [CrossRef]
- Daemen, S.; Gainullina, A.; Kalugotla, G.; He, L.; Chan, M.M.; Beals, J.W.; Liss, K.H.; Klein, S.; Feldstein, A.E.; Finck, B.N.; et al. Dynamic Shifts in the Composition of Resident and Recruited Macrophages Influence Tissue Remodeling in NASH. Cell Rep. 2021, 34, 108626. [Google Scholar] [CrossRef]
- Vonderlin, J.; Chavakis, T.; Sieweke, M.; Tacke, F. The Multifaceted Roles of Macrophages in NAFLD Pathogenesis. Cell Mol. Gastroenterol. Hepatol. 2023, 15, 1311–1324. [Google Scholar] [CrossRef]
- Cherry, A.D.; Piantadosi, C.A. Regulation of mitochondrial biogenesis and its intersection with inflammatory responses. Antioxid. Redox Signal 2015, 22, 965–976. [Google Scholar] [CrossRef]
- Gehrke, N.; Hofmann, L.J.; Straub, B.K.; Rühle, F.; Waisman, A.; Galle, P.R.; Schattenberg, J.M. Hepatic interleukin-1 receptor type 1 signalling regulates insulin sensitivity in the early phases of nonalcoholic fatty liver disease. Clin. Transl. Med. 2022, 12, e1048. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.I.; Parra, E.R.; Jiao, J.; Solis Soto, L.M.; Ledesma, D.A.; Saldarriaga, O.A.; Stevenson, H.L.; Beretta, L. Cellular and Molecular Mechanisms of Liver Fibrosis in Patients with NAFLD. Cancers 2023, 15, 2871. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Zhong, W.; Dong, H.; Guo, W.; Sun, X.; Zhang, W.; Yue, R.; Li, T.; Griffiths, A.; Ahmadi, A.R.; et al. ATF4 activation promotes hepatic mitochondrial dysfunction by repressing NRF1-TFAM signalling in alcoholic steatohepatitis. Gut 2021, 70, 1933–1945. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Pang, J.; Tripathi, M.; Ho, J.P.; Widjaja, A.A.; Shekeran, S.G.; Cook, S.A.; Suzuki, A.; Diehl, A.M.; Petretto, E.; et al. Spermidine-mediated hypusination of translation factor EIF5A improves mitochondrial fatty acid oxidation and prevents non-alcoholic steatohepatitis progression. Nat. Commun. 2022, 13, 5202. [Google Scholar] [CrossRef]
- Cheng, D.; Zhang, M.; Zheng, Y.; Wang, M.; Gao, Y.; Wang, X.; Liu, X.; Lv, W.; Zeng, X.; Belosludtsev, K.N.; et al. α-Ketoglutarate prevents hyperlipidemia-induced fatty liver mitochondrial dysfunction and oxidative stress by activating the AMPK-pgc-1α/Nrf2 pathway. Redox Biol. 2024, 74, 103230. [Google Scholar] [CrossRef]
- Gehrke, N.; Hövelmeyer, N.; Waisman, A.; Straub, B.K.; Weinmann-Menke, J.; Wörns, M.A.; Galle, P.R.; Schattenberg, J.M. Hepatocyte-specific deletion of IL1-RI attenuates liver injury by blocking IL-1 driven autoinflammation. J. Hepatol. 2018, 68, 986–995. [Google Scholar] [CrossRef]
- Gehrke, N.; Hofmann, L.J.; Straub, B.K.; Ridder, D.A.; Waisman, A.; Kaps, L.; Galle, P.R.; Schattenberg, J.M. Blocking interleukin-1 receptor type 1 (IL-1R1) signaling in hepatocytes slows down diethylnitrosamine-induced liver tumor growth in obese mice. Hepatol. Commun. 2024, 8, e0568. [Google Scholar] [CrossRef]
- Undamatla, R.; Fagunloye, O.G.; Chen, J.; Edmunds, L.R.; Murali, A.; Mills, A.; Xie, B.; Pangburn, M.M.; Sipula, I.; Gibson, G.; et al. Reduced mitophagy is an early feature of NAFLD and liver-specific PARKIN knockout hastens the onset of steatosis, inflammation and fibrosis. Sci. Rep. 2023, 13, 7575. [Google Scholar] [CrossRef]
- Yao, J.; Yang, H.; Wang, H.; Shi, H.; Jiao, Y.; Zhang, Y.; Chen, D.; Shi, H. ASPP2 Coordinates ERS-Mediated Autophagy and Apoptosis Through mTORC1 Pathway in Hepatocyte Injury Induced by TNF-α. Front. Pharmacol. 2022, 13, 865389. [Google Scholar] [CrossRef]
- Jin, K.; Shi, Y.; Zhang, H.; Zhangyuan, G.; Wang, F.; Li, S.; Chen, C.; Zhang, J.; Wang, H.; Zhang, W.; et al. A TNFα/Miz1-positive feedback loop inhibits mitophagy in hepatocytes and propagates non-alcoholic steatohepatitis. J. Hepatol. 2023, 79, 403–416. [Google Scholar] [CrossRef]
- Rho, H.; Kim, S.; Kim, S.U.; Kim, J.W.; Lee, S.H.; Park, S.H.; Escorcia, F.E.; Chung, J.Y.; Song, J. CHIP ameliorates nonalcoholic fatty liver disease via promoting K63- and K27-linked STX17 ubiquitination to facilitate autophagosome-lysosome fusion. Nat. Commun. 2024, 15, 8519. [Google Scholar] [CrossRef]
- Goncalves, R.L.S.; Wang, Z.B.; Riveros, J.K.; Parlakgül, G.; Inouye, K.E.; Lee, G.Y.; Fu, X.; Saksi, J.; Rosique, C.; Hui, S.T.; et al. CoQ imbalance drives reverse electron transport to disrupt liver metabolism. Nature 2025, 643, 1057–1065. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, H.; Han, F.; Guo, P. Reactive Oxygen Species as Key Molecules in the Pathogenesis of Alcoholic Fatty Liver Disease and Nonalcoholic Fatty Liver Disease: Future Perspectives. Curr. Issues Mol. Biol. 2025, 47, 464. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Niu, M.; Ni, H.M.; Ding, W.X. Mitochondrial dynamics, quality control, and mtDNA in alcohol-associated liver disease and liver cancer. Hepatology 2024. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Wang, Y.; Zhang, C.; Sullivan, M.A.; Chen, W.; Jing, X.; Yu, H.; Li, F.; Wang, Q.; Zhou, Z.; et al. Quercetin ameliorates nonalcoholic fatty liver disease (NAFLD) via the promotion of AMPK-mediated hepatic mitophagy. J. Nutr. Biochem. 2023, 120, 109414. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ojogun, N.; Liu, Y.; Gan, L.; Xiao, Z.; Feng, J.; Jiang, W.; Chen, Y.; Zou, B.; Yu, C.; et al. A host enzyme reduces metabolic dysfunction-associated steatotic liver disease (MASLD) by inactivating intestinal lipopolysaccharide. Elife 2025, 13, RP100731. [Google Scholar] [CrossRef]
- Han, Y.H.; Onufer, E.J.; Huang, L.H.; Sprung, R.W.; Davidson, W.S.; Czepielewski, R.S.; Wohltmann, M.; Sorci-Thomas, M.G.; Warner, B.W.; Randolph, G.J. Enterically derived high-density lipoprotein restrains liver injury through the portal vein. Science 2021, 373. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D.; Tilg, H. MASLD: A systemic metabolic disorder with cardiovascular and malignant complications. Gut 2024, 73, 691–702. [Google Scholar] [CrossRef]
- Wu, K.K.; Xu, X.; Wu, M.; Li, X.; Hoque, M.; Li, G.H.Y.; Lian, Q.; Long, K.; Zhou, T.; Piao, H.; et al. MDM2 induces pro-inflammatory and glycolytic responses in M1 macrophages by integrating iNOS-nitric oxide and HIF-1α pathways in mice. Nat. Commun. 2024, 15, 8624. [Google Scholar] [CrossRef]
- Cyr, A.; Chambers, L.; Waltz, P.K.; Whelan, S.P.; Kohut, L.; Carchman, E.; Dyer, M.; Luciano, J.; Kautza, B.; Gomez, H.D.; et al. Endotoxin Engages Mitochondrial Quality Control via an iNOS-Reactive Oxygen Species Signaling Pathway in Hepatocytes. Oxid. Med. Cell Longev. 2019, 2019, 4745067. [Google Scholar] [CrossRef]
- Palmieri, E.M.; Gonzalez-Cotto, M.; Baseler, W.A.; Davies, L.C.; Ghesquière, B.; Maio, N.; Rice, C.M.; Rouault, T.A.; Cassel, T.; Higashi, R.M.; et al. Nitric oxide orchestrates metabolic rewiring in M1 macrophages by targeting aconitase 2 and pyruvate dehydrogenase. Nat. Commun. 2020, 11, 698. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Liu, Y.; Li, N.; Geng, Q. Lactate and lactylation in macrophage metabolic reprogramming: Current progress and outstanding issues. Front. Immunol. 2024, 15, 1395786. [Google Scholar] [CrossRef] [PubMed]
- Kuraji, R.; Ye, C.; Zhao, C.; Gao, L.; Martinez, A.; Miyashita, Y.; Radaic, A.; Kamarajan, P.; Le, C.; Zhan, L.; et al. Nisin lantibiotic prevents NAFLD liver steatosis and mitochondrial oxidative stress following periodontal disease by abrogating oral, gut and liver dysbiosis. NPJ Biofilm. Microbiomes 2024, 10, 3. [Google Scholar] [CrossRef]
- Kaliannan, K.; Hamarneh, S.R.; Economopoulos, K.P.; Nasrin Alam, S.; Moaven, O.; Patel, P.; Malo, N.S.; Ray, M.; Abtahi, S.M.; Muhammad, N.; et al. Intestinal alkaline phosphatase prevents metabolic syndrome in mice. Proc. Natl. Acad. Sci. USA 2013, 110, 7003–7008. [Google Scholar] [CrossRef]
- Zhang, Q.; Shen, X.; Yuan, X.; Huang, J.; Zhu, Y.; Zhu, T.; Zhang, T.; Wu, H.; Wu, Q.; Fan, Y.; et al. Lipopolysaccharide binding protein resists hepatic oxidative stress by regulating lipid droplet homeostasis. Nat. Commun. 2024, 15, 3213. [Google Scholar] [CrossRef]
- Barchetta, I.; Cimini, F.A.; Sentinelli, F.; Chiappetta, C.; Di Cristofano, C.; Silecchia, G.; Leonetti, F.; Baroni, M.G.; Cavallo, M.G. Reduced Lipopolysaccharide-Binding Protein (LBP) Levels Are Associated with Non-Alcoholic Fatty Liver Disease (NAFLD) and Adipose Inflammation in Human Obesity. Int. J. Mol. Sci. 2023, 24, 17174. [Google Scholar] [CrossRef]
- Schnabl, B.; Damman, C.J.; Carr, R.M. Metabolic dysfunction-associated steatotic liver disease and the gut microbiome: Pathogenic insights and therapeutic innovations. J. Clin. Invest. 2025, 135, 135. [Google Scholar] [CrossRef]
- Gao, L.; Zuo, X.L.; Dong, L.L.; Zhou, S.F.; Wang, Z.J.; Duan, Y.S.; Chen, M.Y.; Zhu, Q.X.; Zhang, J.X. Hepatocyte mitochondrial DNA mediates macrophage immune response in liver injury induced by trichloroethylene. Ecotoxicol. Environ. Saf. 2024, 276, 116317. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, J.; Liang, W.; Li, K.; Huang, Y.; Song, J.; Zhang, B.; Qiu, X.; Qiu, D.; Zhang, Q.; et al. Blockade of the mitochondrial DNA release ameliorates hepatic ischemia-reperfusion injury through avoiding the activation of cGAS-Sting pathway. J. Transl. Med. 2024, 22, 796. [Google Scholar] [CrossRef]
- Giordano, L.; Ware, S.A.; Lagranha, C.J.; Kaufman, B.A. Mitochondrial DNA signals driving immune responses: Why, How, Where? Cell Commun. Signal 2025, 23, 192. [Google Scholar] [CrossRef]
- Wu, X.Y.; Chen, Y.J.; Liu, C.A.; Gong, J.H.; Xu, X.S. STING Induces Liver Ischemia-Reperfusion Injury by Promoting Calcium-Dependent Caspase 1-GSDMD Processing in Macrophages. Oxid. Med. Cell Longev. 2022, 2022, 8123157. [Google Scholar] [CrossRef]
- Dewidar, B.; Meyer, C.; Dooley, S.; Meindl-Beinker, A.N. TGF-β in Hepatic Stellate Cell Activation and Liver Fibrogenesis-Updated 2019. Cells 2019, 8, 1419. [Google Scholar] [CrossRef]
- Hui, S.T.; Gong, L.; Swichkow, C.; Blencowe, M.; Kaminska, D.; Diamante, G.; Pan, C.; Dalsania, M.; French, S.W.; Magyar, C.E.; et al. Role of Matrix Gla Protein in Transforming Growth Factor-β Signaling and Nonalcoholic Steatohepatitis in Mice. Cell Mol. Gastroenterol. Hepatol. 2023, 16, 943–960. [Google Scholar] [CrossRef]
- Yang, L.; Roh, Y.S.; Song, J.; Zhang, B.; Liu, C.; Loomba, R.; Seki, E. Transforming growth factor beta signaling in hepatocytes participates in steatohepatitis through regulation of cell death and lipid metabolism in mice. Hepatology 2014, 59, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Hu, L.; Gui, W.; Xiao, L.; Wang, W.; Xia, J.; Fan, H.; Li, Z.; Zhu, Q.; Hou, X.; et al. Hepatocyte TGF-β Signaling Inhibiting WAT Browning to Promote NAFLD and Obesity Is Associated With Let-7b-5p. Hepatol. Commun. 2022, 6, 1301–1321. [Google Scholar] [CrossRef] [PubMed]
- Traussnigg, S.; Kienbacher, C.; Gajdošík, M.; Valkovič, L.; Halilbasic, E.; Stift, J.; Rechling, C.; Hofer, H.; Steindl-Munda, P.; Ferenci, P.; et al. Ultra-high-field magnetic resonance spectroscopy in non-alcoholic fatty liver disease: Novel mechanistic and diagnostic insights of energy metabolism in non-alcoholic steatohepatitis and advanced fibrosis. Liver Int. 2017, 37, 1544–1553. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Link, F.; Han, M.; Chaudhary, R.; Asimakopoulos, A.; Liebe, R.; Yao, Y.; Hammad, S.; Dropmann, A.; Krizanac, M.; et al. The Interplay of TGF-β1 and Cholesterol Orchestrating Hepatocyte Cell Fate, EMT, and Signals for HSC Activation. Cell Mol. Gastroenterol. Hepatol. 2024, 17, 567–587. [Google Scholar] [CrossRef]
- Satapati, S.; Kucejova, B.; Duarte, J.A.; Fletcher, J.A.; Reynolds, L.; Sunny, N.E.; He, T.; Nair, L.A.; Livingston, K.A.; Fu, X.; et al. Mitochondrial metabolism mediates oxidative stress and inflammation in fatty liver. J. Clin. Invest. 2015, 125, 4447–4462. [Google Scholar] [CrossRef]
- Matuz-Mares, D.; Vázquez-Meza, H.; Vilchis-Landeros, M.M. NOX as a Therapeutic Target in Liver Disease. Antioxidants 2022, 11, 2038. [Google Scholar] [CrossRef]
- Herranz-Itúrbide, M.; Peñuelas-Haro, I.; Espinosa-Sotelo, R.; Bertran, E.; Fabregat, I. The TGF-β/NADPH Oxidases Axis in the Regulation of Liver Cell Biology in Health and Disease. Cells 2021, 10, 2312. [Google Scholar] [CrossRef]
- Yang, W.; Yan, X.; Chen, R.; Xin, X.; Ge, S.; Zhao, Y.; Yan, X.; Zhang, J. Smad4 deficiency in hepatocytes attenuates NAFLD progression via inhibition of lipogenesis and macrophage polarization. Cell Death Dis. 2025, 16, 58. [Google Scholar] [CrossRef]
- Sasaki, Y.; Asahiyama, M.; Tanaka, T.; Yamamoto, S.; Murakami, K.; Kamiya, W.; Matsumura, Y.; Osawa, T.; Anai, M.; Fruchart, J.C.; et al. Pemafibrate, a selective PPARα modulator, prevents non-alcoholic steatohepatitis development without reducing the hepatic triglyceride content. Sci. Rep. 2020, 10, 7818. [Google Scholar] [CrossRef]
- Cooreman, M.P.; Butler, J.; Giugliano, R.P.; Zannad, F.; Dzen, L.; Huot-Marchand, P.; Baudin, M.; Beard, D.R.; Junien, J.L.; Broqua, P.; et al. The pan-PPAR agonist lanifibranor improves cardiometabolic health in patients with metabolic dysfunction-associated steatohepatitis. Nat. Commun. 2024, 15, 3962. [Google Scholar] [CrossRef]
- Le Roux, C.W.; Steen, O.; Lucas, K.J.; Startseva, E.; Unseld, A.; Hennige, A.M. Glucagon and GLP-1 receptor dual agonist survodutide for obesity: A randomised, double-blind, placebo-controlled, dose-finding phase 2 trial. Lancet Diabetes Endocrinol. 2024, 12, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef] [PubMed]
- EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [CrossRef] [PubMed]
- Food, U.S.; Drug, A. Approval Letter: Rezdiffra (Resmetirom) Tablets (NDA 217785); Center for Drug Evaluation and Research: Silver Spring, MD, USA, 2024. [Google Scholar]
- Food, U.S.; Drug, A. Approval Letter: Wegovy (Semaglutide) Solution (sNDA 215256/S-024)—Accelerated Approval for Noncirrhotic MASH with Moderate to Advanced Fibrosis; Center for Drug Evaluation and Research: Silver Spring, MD, USA, 2025. [Google Scholar]
- Sanyal, A.J.; Newsome, P.N.; Kliers, I.; Østergaard, L.H.; Long, M.T.; Kjær, M.S.; Cali, A.M.G.; Bugianesi, E.; Rinella, M.E.; Roden, M.; et al. Phase 3 Trial of Semaglutide in Metabolic Dysfunction-Associated Steatohepatitis. N. Engl. J. Med. 2025, 392, 2089–2099. [Google Scholar] [CrossRef]
- Francque, S.M.; Bedossa, P.; Ratziu, V.; Anstee, Q.M.; Bugianesi, E.; Sanyal, A.J.; Loomba, R.; Harrison, S.A.; Balabanska, R.; Mateva, L.; et al. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. N. Engl. J. Med. 2021, 385, 1547–1558. [Google Scholar] [CrossRef]
- Harrison, S.A.; Frias, J.P.; Neff, G.; Abrams, G.A.; Lucas, K.J.; Sanchez, W.; Gogia, S.; Sheikh, M.Y.; Behling, C.; Bedossa, P.; et al. Safety and efficacy of once-weekly efruxifermin versus placebo in non-alcoholic steatohepatitis (HARMONY): A multicentre, randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Gastroenterol. Hepatol. 2023, 8, 1080–1093. [Google Scholar] [CrossRef]
- Loomba, R.; Sanyal, A.J.; Kowdley, K.V.; Bhatt, D.L.; Alkhouri, N.; Frias, J.P.; Bedossa, P.; Harrison, S.A.; Lazas, D.; Barish, R.; et al. Randomized, Controlled Trial of the FGF21 Analogue Pegozafermin in NASH. N. Engl. J. Med. 2023, 389, 998–1008. [Google Scholar] [CrossRef]
- Harrison, S.A.; Rolph, T.; Knott, M.; Dubourg, J. FGF21 agonists: An emerging therapeutic for metabolic dysfunction-associated steatohepatitis and beyond. J. Hepatol. 2024, 81, 562–576. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Bedossa, P.; Fraessdorf, M.; Neff, G.W.; Lawitz, E.; Bugianesi, E.; Anstee, Q.M.; Hussain, S.A.; Newsome, P.N.; Ratziu, V.; et al. A Phase 2 Randomized Trial of Survodutide in MASH and Fibrosis. N. Engl. J. Med. 2024, 391, 311–319. [Google Scholar] [CrossRef]
- Petersen, K.F.; Dufour, S.; Mehal, W.Z.; Shulman, G.I. Glucagon promotes increased hepatic mitochondrial oxidation and pyruvate carboxylase flux in humans with fatty liver disease. Cell Metab. 2024, 36, 2359–2366.e2353. [Google Scholar] [CrossRef]
- Loomba, R.; Hartman, M.L.; Lawitz, E.J.; Vuppalanchi, R.; Boursier, J.; Bugianesi, E.; Yoneda, M.; Behling, C.; Cummings, O.W.; Tang, Y.; et al. Tirzepatide for Metabolic Dysfunction-Associated Steatohepatitis with Liver Fibrosis. N. Engl. J. Med. 2024, 391, 299–310. [Google Scholar] [CrossRef]
| Drug | Mechanism & Molecular Target | Developer | ClinicalTrials.gov Identifier | Phase | References |
|---|---|---|---|---|---|
| Anakinra | Recombinant IL-1 receptor antagonist; blocks IL-1α/β signaling | Swedish Orphan Biovitrum | NCT00303394 | 2 | [80] |
| Canakinumab | Human monoclonal antibody against IL-1β; reduces systemic inflammation | Novartis | NCT01327846 (CANTOS trial) | 3 CVOT | [85] |
| LY2189102 | Neutralizing IL-1β; inhibits IL-1β mediated inflammatory signaling | Eli Lilly | NCT00711556 | 2 | [86] |
| Salsalate | Non-acetylated salicylate; inhibits IKKβ/NF-κB activation | Generic; studied in T2DM | NCT00799643 (TINSAL trials by NIH) | 2/3 | [87] |
| Colchicine | Anti-inflammatory alkaloid; disrupts microtubules, inhibiting NLRP3 inflammasome activation | Generic; repurposed for T2DM inflammation (on going trials) | NCT04181996 (CADENCE trial) | 3 | [88,89] |
| Dorzagliatin | First-in-class glucokinase allosteric activator; restores glucose sensing in β-cells & liver | Approved by China’s NMPA in 2022 as HuaTangNing® for T2DM. Completed Phase 3 trials in China. No approval in the U.S. | NCT03173391 & NCT03141073 (DAWN) | N/A | [90] |
| MCC950 | Direct NLRP3 inflammasome inhibitor | Pfizer (CP-456773/CRID3); halted due to drug-induced hepatotoxicity | No T2DM clinical trial (Pre-clinical ONLY) | N/A | [84] |
| Drug | Target | Developer | ClinicalTrials.gov Identifier | Phase | References |
|---|---|---|---|---|---|
| Lanifibranor | pan-PPAR (α/δ/γ) agonist | Inventiva | NCT04849728 | Phase 3 (ongoing) | [240] |
| Efruxifermin (EFX) | FGF21 analogue (FGFR1c/2c/3c agonism) | Akero Therapeutics | NCT06215716 | Phase 3 (ongoing) | [241] |
| Pegozafermin | FGF21 analogue | 89bio | NCT06318169 | Phase 3 (ongoing) | [242,243] |
| Survodutide (BI-456906) | Dual GLP-1/Glucagon receptor agonist | Boehringer Ingelheim · Zealand Pharma | NCT06632444; NCT06632457 | Phase 3 (ongoing) | [244,245] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, W.; Woo, G.H.; Kwon, T.-H.; Jeon, J.-H. Obesity-Driven Metabolic Disorders: The Interplay of Inflammation and Mitochondrial Dysfunction. Int. J. Mol. Sci. 2025, 26, 9715. https://doi.org/10.3390/ijms26199715
Choi W, Woo GH, Kwon T-H, Jeon J-H. Obesity-Driven Metabolic Disorders: The Interplay of Inflammation and Mitochondrial Dysfunction. International Journal of Molecular Sciences. 2025; 26(19):9715. https://doi.org/10.3390/ijms26199715
Chicago/Turabian StyleChoi, Wooyoung, Gun Ha Woo, Tae-Hwan Kwon, and Jae-Han Jeon. 2025. "Obesity-Driven Metabolic Disorders: The Interplay of Inflammation and Mitochondrial Dysfunction" International Journal of Molecular Sciences 26, no. 19: 9715. https://doi.org/10.3390/ijms26199715
APA StyleChoi, W., Woo, G. H., Kwon, T.-H., & Jeon, J.-H. (2025). Obesity-Driven Metabolic Disorders: The Interplay of Inflammation and Mitochondrial Dysfunction. International Journal of Molecular Sciences, 26(19), 9715. https://doi.org/10.3390/ijms26199715



.jpg)




