The Prognostic Power of miR-21 in Breast Cancer: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources and Search Strategy
2.4. Selection Process, Data Collection Process and Data Items
2.5. Quality Assessment
2.6. Statistical Methods
3. Results
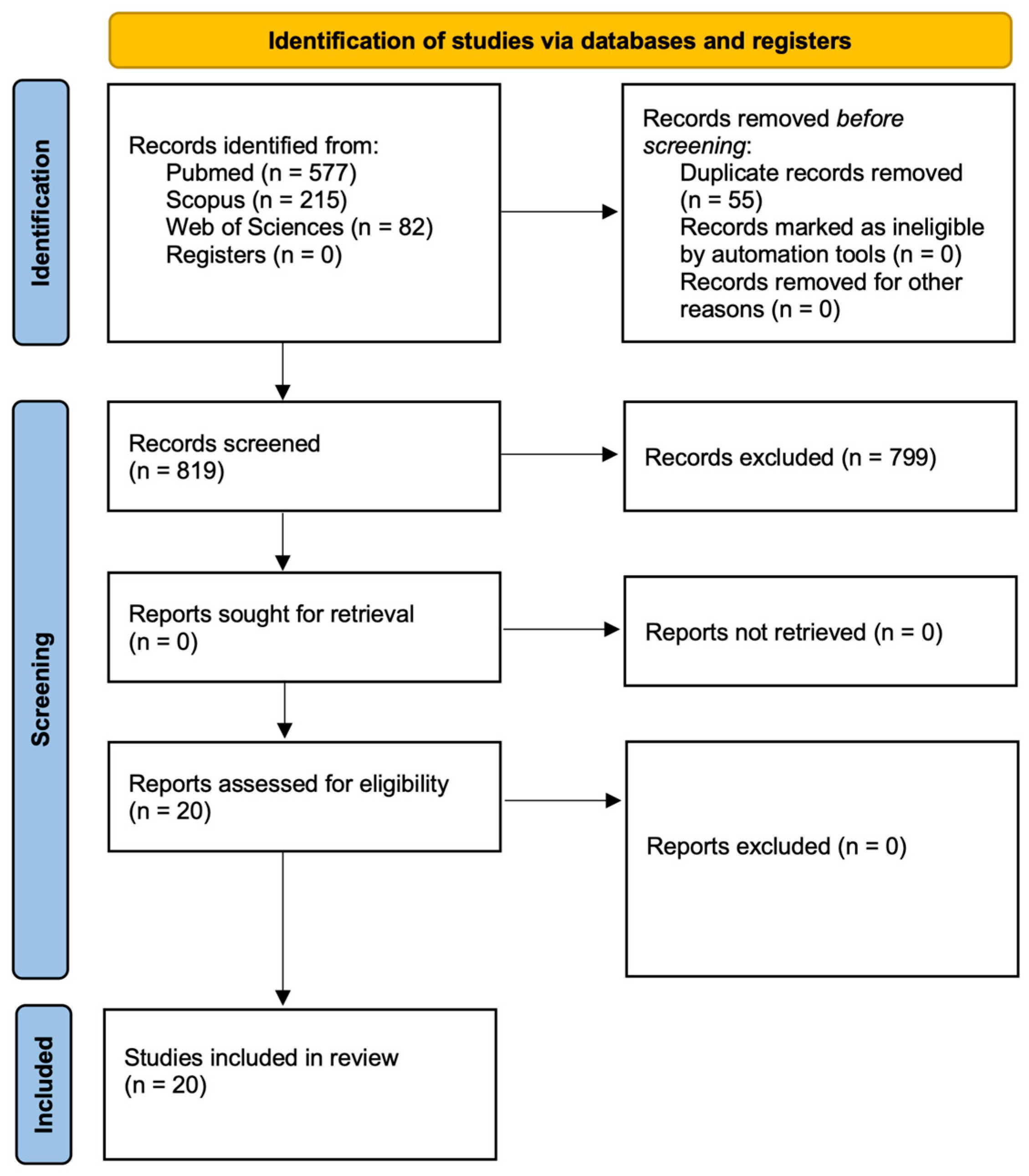
3.1. Literature Search
3.2. Summary of Included Studies
| First Author, Year, References | Study Design | Method | N Case Group | Sample Source | Stage | Ethnicity | Country | Cut-Off | Survival Analysis | HR (95%CI) for OS | HR (95%CI) for DFS/RFS/DFI | Follow-Up, Months (Range) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yan, 2008 [29] | Retrospective | RT-qPCR | 113 | Tumor Tissue | I-III | Asian | China | Mean | OS | 4.13 (1.80–9.50) | - | 66.2 (10.4–81.0) |
| Qian, 2009 [46] | Retrospective | RT-qPCR | 344 | Tumor Tissue | I-IV | Caucasian | USA | Median | OS | 0.99 (0.56–1.73) | 1.15 (0.69–1.93) | 86.2 (8.0–108) |
| Lee, 2011 [43] | Retrospective | RT-qPCR | 109 | Tumor Tissue | I-III | Asian | Korea | Mean | OS, DFS | 14.21 (1.34–15.10) | 0.88 (0.09–8.41) | Median 54 |
| Ota, 2011 [44] | Retrospective | RT-qPCR | 291 | BM | - | Asian | Japan | 5.84 | OS, DFS | 3.40 (1.26–9.18) | 1.04 (0.71–1.48) | 61 (2–90) |
| Walter, 2011 [47] | Retrospective | RT-qPCR | 25 | Tumor Tissue | I-IV | Caucasian | USA | Median | OS | 0.49 (0.06–3.71) | - | Median .5 |
| Radojicic, 2011 [35] | Retrospective | RT-qPCR | 49 TNBC | Tumor Tissue | I-IV | Caucasian | Greece | Median | OS, DFS | 0.85 (0.09–8.29) | 2.49 (0.72–8.58) | 120 |
| Markou, 2014 [45] | Retrospective | RT-qPCR | 112 | Tumor Tissue | I-IV | Caucasian | Greece | Median | OS, DFI | 1.48 (0.73–2.98) | 1.762 (1.010–3.074) | 84 (10–149) |
| Dong, 2014 [36] | Retrospective | RT-qPCR | 72 TNBC | Tumor Tissue | I-IV | Asian | China | 1.5 | RFS | - | 2.32 (1.24–4.12) | 95 |
| Muller, 2014 [40] | Retrospective | RT-qPCR | 127 HPBC | Serum | I-IV | Caucasian | Germany | Median | OS | 5.24 (1.58–17.35) | - | 62.15 (5.56–66.28) |
| Wang, 2015 [41] | Retrospective | RT-qPCR | 326 HPBC | Serum | I-III | Asian | China | - | RFS, DFS | - | RFS: 2.942 (1.420–8.325) DFS: 2.732 (1.038–7.273) | - |
| Yan, 2016 [48] | Retrospective | RT-qPCR | 320 | Tumor Tissue | I-III | Asian | China | Mean | OS | 2.47 (1.08–5.65) | - | - |
| Liu, 2017 [51] | Prospective | RT-qPCR | 118 | Serum | II-III | Asian | China | - | DFS | - | 51.579 (13.942–190.820) | 25 (10–36) |
| Papadaki, 2018 [52] | Prospective | RT-qPCR | 133 | Plasma | I-II | Caucasian | Greece | Median | DFS | - | 4.557 (1.685–12.869) | 94.3 (14.33–159.30) |
| Papadaki, 2019 [49] | Prospective | RT-qPCR | 70 metastatic BC 45 de novo metastatic | Plasma | I-III | Caucasian | Greece | Median | OS | 1.589 (0.916–2.756) | - | 27.33 (20.97–33.69) |
| Liu, 2019 [42] | Prospective | RT-qPCR | 83 HPBC | Serum | II-III | Asian | China | - | OS, DFS | 0.49 (0.21–1.11) | 0.51 (0.24–1.08) | Mean 23.6 (13–36) |
| Wu, 2020 [37] | Retrospective | Sequencing | 151 TNBC | Tumor Tissue | II-IV | Asian | China | Median | OS | 7.396 (1.590–34.411) | - | 60 |
| Romadhon, 2021 [50] | Retrospective | RT-qPCR | 49 | Plasma | I-II | Asian | Indonesia | Mean | OS | 5.5 (3.2–9.46) | - | 12 |
| Kujala, 2024 [38] | Prospective | Sequencing | 14 recurrent TNBC 19 non recurrent | Serum | II-III | Caucasian | Finland | - | RFS | - | 1.87 (1.06–3.30) | 60 |
| MacKenzie 2014 [39] | Retrospective | in situ hybridization | 901 TNBC | Tumor Tissue | I-II | Caucasian | USA | - | RFS | - | 1.71 (1.265–2.319) | median, 10.33 years |
| Amirfallah 2021 [53] | Retrospective | RT-qPCR | 139 | Tumor Tissue | I-III | Caucasian | Iceland | Median | DFS | - | 1.89 (1.18–3.04) | - |
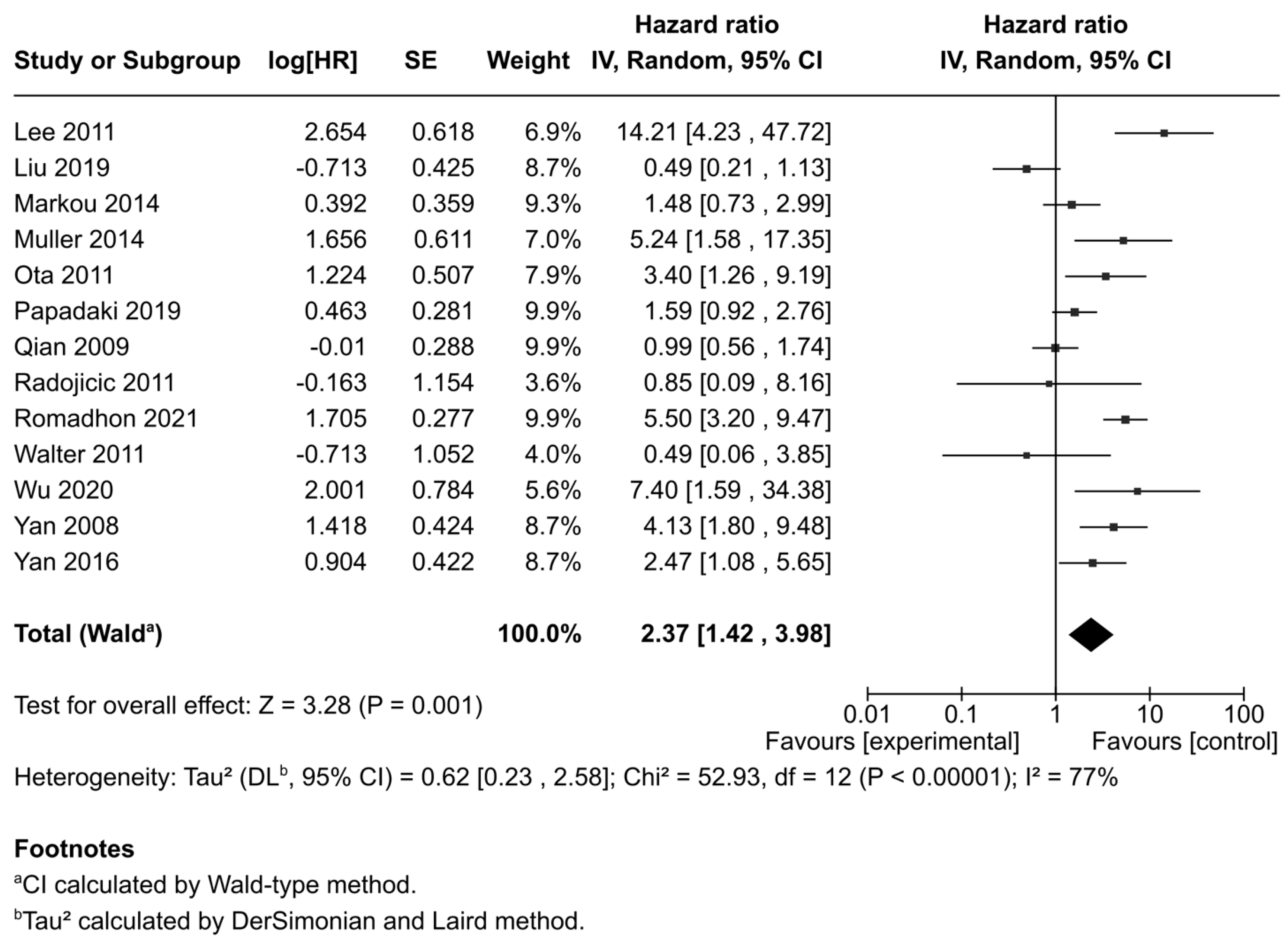
3.3. Correlation Between miR-21 Expression and Overall Survival (OS)
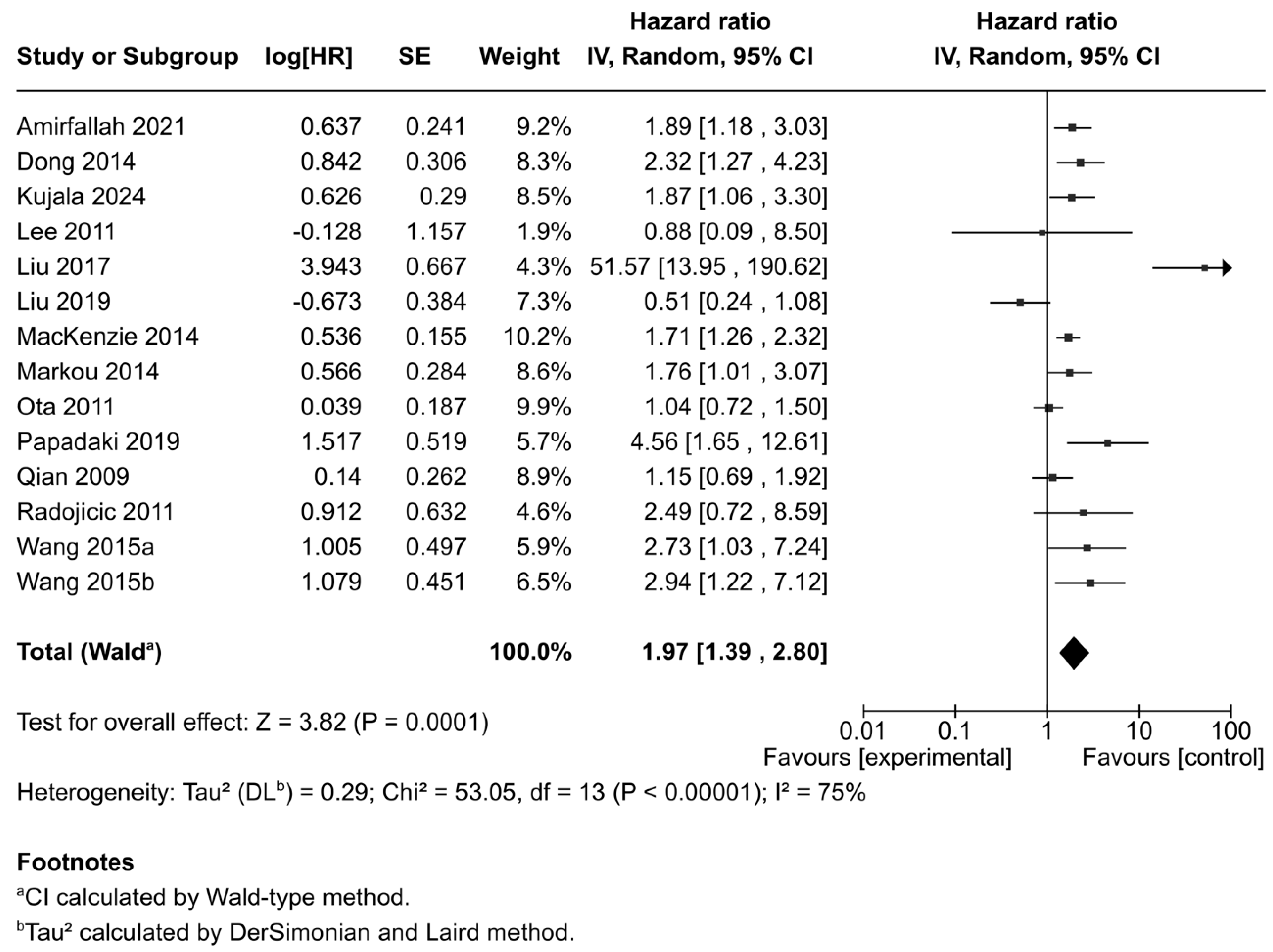
3.4. Correlation Between miR-21 Expression and Disease-Free Survival/Regression Free Survival
3.5. Subgroup Analysis
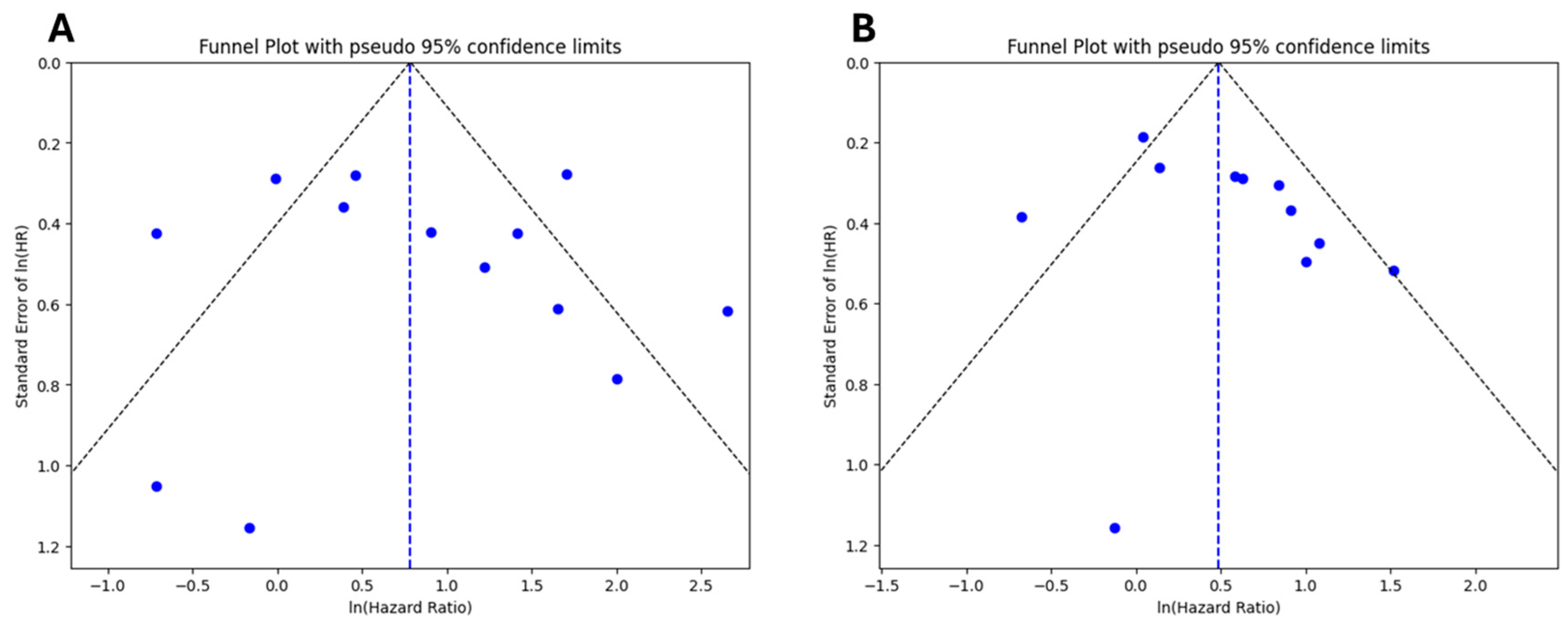
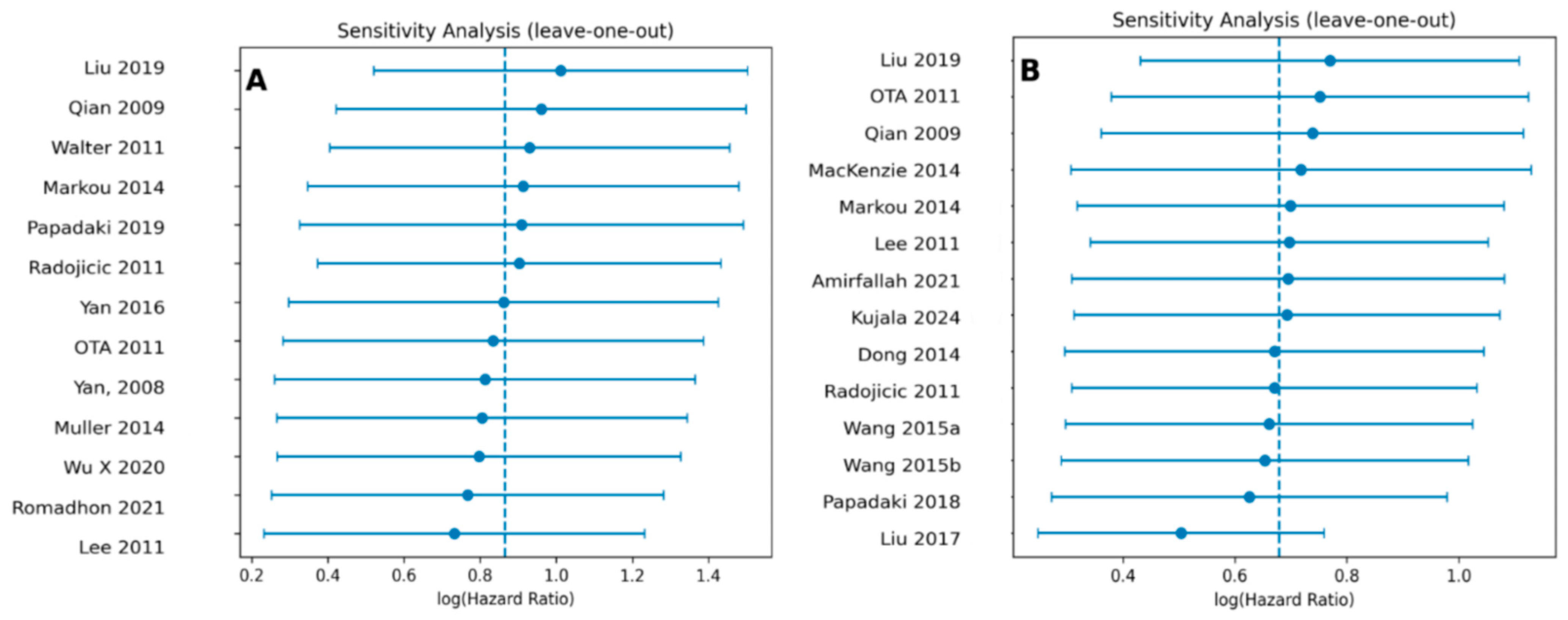
3.6. Publication Bias and Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- WHO Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 9 July 2025).
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and Future Burden of Breast Cancer: Global Statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Conte, L.; Tafuri, B.; Portaluri, M.; Galiano, A.; Maggiulli, E.; De Nunzio, G. Breast Cancer Mass Detection in DCE–MRI Using Deep-Learning Features Followed by Discrimination of Infiltrative vs. In Situ Carcinoma through a Machine-Learning Approach. Appl. Sci. 2020, 10, 6109. [Google Scholar] [CrossRef]
- Weigelt, B.; Reis-Filho, J.S. Histological and Molecular Types of Breast Cancer: Is There a Unifying Taxonomy? Nat. Rev. Clin. Oncol. 2009, 6, 718–730. [Google Scholar] [CrossRef]
- Conte, L.; De Nunzio, G.; Lupo, R.; Mieli, M.; Lezzi, A.; Vitale, E.; Carriero, M.C.; Calabrò, A.; Carvello, M.; Rubbi, I.; et al. Breast Cancer Prevention: The Key Role of Population Screening, Breast Self-Examination (BSE) and Technological Tools. Survey of Italian Women. J. Cancer Educ. 2023, 38, 1728–1742. [Google Scholar] [CrossRef] [PubMed]
- Thuc Nguyen, T.M.; Dinh Le, R.; Nguyen, C. Van Breast Cancer Molecular Subtype and Relationship with Clinicopathological Profiles among Vietnamese Women: A Retrospective Study. Pathol. Res. Pract. 2023, 250, 154819. [Google Scholar] [CrossRef]
- Cortesi, L.; Galli, G.R.; Domati, F.; Conte, L.; Manca, L.; Berio, M.A.; Toss, A.; Iannone, A.; Federico, M. Obesity in Postmenopausal Breast Cancer Patients: It Is Time to Improve Actions for a Healthier Lifestyle. The Results of a Comparison Between Two Italian Regions with Different “Presumed” Lifestyles. Front. Oncol. 2021, 11, 769683. [Google Scholar] [CrossRef] [PubMed]
- Conte, L.; Lupo, R.; Lezzi, A.; Sciolti, S.; Rubbi, I.; Carvello, M.; Calabrò, A.; Botti, S.; Fanizzi, A.; Massafra, R.; et al. Breast Cancer Prevention Practices and Knowledge in Italian and Chinese Women in Italy: Clinical Checkups, Free NHS Screening Adherence, and Breast Self-Examination (BSE). J. Cancer Educ. 2025, 40, 30–43. [Google Scholar] [CrossRef]
- Survival Rates for Breast Cancer | American Cancer Society. Available online: https://www.cancer.org/cancer/types/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html (accessed on 10 June 2025).
- Conte, L.; Lupo, R.; Sciolti, S.; Lezzi, A.; Rubbi, I.; Botti, S.; Carvello, M.; Fanizzi, A.; Massafra, R.; Vitale, E.; et al. Exploring the Landscape of Breast Cancer Prevention among Chinese Residents in Italy: An In-Depth Analysis of Screening Adherence, Breast Self-Examination (BSE) Practices, the Role of Technological Tools, and Misconceptions Surrounding Risk Factors and Symptoms. Int. J. Environ. Res. Public Health 2024, 21, 308. [Google Scholar] [CrossRef]
- Li, C.; Yuan, Q.; Deng, T.; Xu, G.; Hou, J.; Zheng, L.; Wu, G. Prognosis Difference between HER2-Low and HER2-Zero Breast Cancer Patients: A Systematic Review and Meta-Analysis. Breast Cancer 2023, 30, 965–975. [Google Scholar] [CrossRef]
- Rubbi, I.; Lupo, R.; Lezzi, A.; Cremonini, V.; Carvello, M.; Caricato, M.; Conte, L.; Antonazzo, M.; Caldararo, C.; Botti, S.; et al. The Social and Professional Image of the Nurse: Results of an Online Snowball Sampling Survey among the General Population in the Post-Pandemic Period. Nurs. Rep. 2023, 13, 1291–1303. [Google Scholar] [CrossRef]
- Conte, L.; Lupo, R.; Lezzi, A.; Paolo, V.; Rubbi, I.; Rizzo, E.; Carvello, M.; Calabrò, A.; Botti, S.; De Matteis, E.; et al. A Nationwide Cross-Sectional Study Investigating Adherence to the Mediterranean Diet, Smoking, Alcohol and Work Habits, Hormonal Dynamics between Breast Cancer Cases and Healthy Subjects. Clin. Nutr. Open Sci. 2024, 55, 1–19. [Google Scholar] [CrossRef]
- Conte, L.; Rizzo, E.; Grassi, T.; Bagordo, F.; De Matteis, E.; De Nunzio, G. Artificial Intelligence Techniques and Pedigree Charts in Oncogenetics: Towards an Experimental Multioutput Software System for Digitization and Risk Prediction. Computation 2024, 12, 47. [Google Scholar] [CrossRef]
- Conte, L.; Rizzo, E.; Civino, E.; Tarantino, P.; De Nunzio, G.; De Matteis, E. Enhancing Breast Cancer Risk Prediction with Machine Learning: Integrating BMI, Smoking Habits, Hormonal Dynamics, and BRCA Gene Mutations—A Game-Changer Compared to Traditional Statistical Models? Appl. Sci. 2024, 14, 8474. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, C.; Zhao, Y.; Wang, Q.; Guo, J.; Ye, B.; Yu, G. Overview of MicroRNAs as Diagnostic and Prognostic Biomarkers for High-Incidence Cancers in 2021. Int. J. Mol. Sci. 2022, 23, 11389. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating MicroRNAs as Stable Blood-Based Markers for Cancer Detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef] [PubMed]
- Treiber, T.; Treiber, N.; Meister, G. Regulation of MicroRNA Biogenesis and Its Crosstalk with Other Cellular Pathways. Nat. Rev. Mol. Cell Biol. 2019, 20, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Otmani, K.; Rouas, R.; Berehab, M.; Lewalle, P. The Regulatory Mechanisms of OncomiRs in Cancer. Biomed. Pharmacother. 2024, 171, 116165. [Google Scholar] [CrossRef]
- Kontomanolis, E.N.; Koutras, A.; Syllaios, A.; Schizas, D.; Mastoraki, A.; Garmpis, N.; Diakosavvas, M.; Angelou, K.; Tsatsaris, G.; Pagkalos, A.; et al. Role of Oncogenes and Tumor-Suppressor Genes in Carcinogenesis: A Review. Anticancer Res. 2020, 40, 6009–6015. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X. MicroRNA-21 in Breast Cancer: Diagnostic and Prognostic Potential. Clin. Transl. Oncol. 2014, 16, 225–233. [Google Scholar] [CrossRef]
- Sukhija, S.; Purohit, P.; Pareek, P.; Garg, P.K.; Vishnoi, J.R.; Elhence, P.A.; Varthya, S.B.; Sharma, P.; Ambwani, S.; Charan, J. Circulating MiRNA-21 Levels in Breast Cancer Patients Before and After Chemotherapy and Its Association with Clinical Improvement. Indian J. Clin. Biochem. 2024, 39, 214–220. [Google Scholar] [CrossRef]
- Zhu, S.; Si, M.-L.; Wu, H.; Mo, Y.-Y. MicroRNA-21 Targets the Tumor Suppressor Gene Tropomyosin 1 (TPM1). J. Biol. Chem. 2007, 282, 14328–14336. [Google Scholar] [CrossRef]
- Frankel, L.B.; Christoffersen, N.R.; Jacobsen, A.; Lindow, M.; Krogh, A.; Lund, A.H. Programmed Cell Death 4 (PDCD4) Is an Important Functional Target of the MicroRNA MiR-21 in Breast Cancer Cells. J. Biol. Chem. 2008, 283, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Henson, R.; Wehbe–Janek, H.; Ghoshal, K.; Jacob, S.T.; Patel, T. MicroRNA-21 Regulates Expression of the PTEN Tumor Suppressor Gene in Human Hepatocellular Cancer. Gastroenterology 2007, 133, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Y.; Tian, X.; Wang, Q.; Huang, H.; Lu, X.; Qi, M.; Cao, X.; Lei, J. Diagnostic and Predictive Value of Liquid Biopsy-Derived Exosome MiR-21 for Breast Cancer: A Systematic Review and Meta-Analysis. Expert Rev. Mol. Diagn. 2023, 23, 315–324. [Google Scholar] [CrossRef]
- Kim, K.; Jung, K.O.; Oh, S.; Kim, Y.-H.; Lee, S.-Y.; Hong, S.; Cho, S.H.; Kim, H.; Rhee, S.; Cheon, G.J.; et al. Radiation-Induced Exosomal MiR-21 Enhances Tumor Proliferation and Invasiveness in Breast Cancer: Implications for Poor Prognosis in Radiotherapy Patients. Exp. Hematol. Oncol. 2024, 13, 120. [Google Scholar] [CrossRef]
- Pan, F.; Mao, H.; Deng, L.; Li, G.; Geng, P. Prognostic and Clinicopathological Significance of MicroRNA-21 Overexpression in Breast Cancer: A Meta-Analysis. Int. J. Clin. Exp. Pathol. 2014, 7, 5622–5633. [Google Scholar] [PubMed]
- Yan, L.-X.; Huang, X.-F.; Shao, Q.; Huang, M.-Y.; Deng, L.; Wu, Q.-L.; Zeng, Y.-X.; Shao, J.-Y. MicroRNA MiR-21 Overexpression in Human Breast Cancer Is Associated with Advanced Clinical Stage, Lymph Node Metastasis and Patient Poor Prognosis. RNA 2008, 14, 2348–2360. [Google Scholar] [CrossRef]
- Santana, T.A.B.d.S.; de Oliveira Passamai, L.; de Miranda, F.S.; Borin, T.F.; Borges, G.F.; Luiz, W.B.; Campos, L.C.G. The Role of MiRNAs in the Prognosis of Triple-Negative Breast Cancer: A Systematic Review and Meta-Analysis. Diagnostics 2022, 13, 127. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA 2000, 283, 2008. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Mantel, N. Statistical Aspects of the Analysis of Data from Retrospective Studies of Disease. JNCI J. Natl. Cancer Inst. 1959, 22, 719–748. [Google Scholar] [CrossRef]
- Radojicic, J.; Zaravinos, A.; Vrekoussis, T.; Kafousi, M.; Spandidos, D.A.; Stathopoulos, E.N. MicroRNA Expression Analysis in Triple-Negative (ER, PR and Her2/Neu) Breast Cancer. Cell Cycle 2011, 10, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Liang, X.; Wang, D.; Gao, H.; Wang, L.; Wang, L.; Liu, J.; Du, Z. High Expression of MiR-21 in Triple-Negative Breast Cancers Was Correlated with a Poor Prognosis and Promoted Tumor Cell in Vitro Proliferation. Med. Oncol. 2014, 31, 57. [Google Scholar] [CrossRef]
- Wu, X.; Ding, M.; Lin, J. Three-microRNA Expression Signature Predicts Survival in Triple-negative Breast Cancer. Oncol. Lett. 2020, 19, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Kujala, J.; Tengström, M.; Heikkinen, S.; Taipale, M.; Kosma, V.-M.; Hartikainen, J.M.; Mannermaa, A. Circulating Micro-RNAs Predict the Risk of Recurrence in Triple-Negative Breast Cancer. Cells 2024, 13, 1884. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, T.A.; Schwartz, G.N.; Calderone, H.M.; Graveel, C.R.; Winn, M.E.; Hostetter, G.; Wells, W.A.; Sempere, L.F. Stromal expression of miR-21 identifies high-risk group in triple-negative breast cancer. Am. J. Pathol. 2014, 184, 3217–3225. [Google Scholar] [CrossRef]
- Müller, V.; Gade, S.; Steinbach, B.; Loibl, S.; von Minckwitz, G.; Untch, M.; Schwedler, K.; Lübbe, K.; Schem, C.; Fasching, P.A.; et al. Changes in Serum Levels of MiR-21, MiR-210, and MiR-373 in HER2-Positive Breast Cancer Patients Undergoing Neoadjuvant Therapy: A Translational Research Project within the Geparquinto Trial. Breast Cancer Res. Treat. 2014, 147, 61–68. [Google Scholar] [CrossRef]
- Wang, G.; Wang, L.; Sun, S.; Wu, J.; Wang, Q. Quantitative Measurement of Serum MicroRNA-21 Expression in Relation to Breast Cancer Metastasis in Chinese Females. Ann. Lab. Med. 2015, 35, 226–232. [Google Scholar] [CrossRef]
- Liu, B.; Su, F.; Lv, X.; Zhang, W.; Shang, X.; Zhang, Y.; Zhang, J. Serum MicroRNA-21 Predicted Treatment Outcome and Survival in HER2-Positive Breast Cancer Patients Receiving Neoadjuvant Chemotherapy Combined with Trastuzumab. Cancer Chemother. Pharmacol. 2019, 84, 1039–1049. [Google Scholar] [CrossRef]
- Lee, J.A.; Lee, H.Y.; Lee, E.S.; Kim, I.; Bae, J.W. Prognostic Implications of MicroRNA-21 Overexpression in Invasive Ductal Carcinomas of the Breast. J. Breast Cancer 2011, 14, 269. [Google Scholar] [CrossRef] [PubMed]
- Ota, D.; Mimori, K.; Yokobori, T.; Iwatsuki, M.; Kataoka, A.; Masuda, N.; Ishii, H.; Ohno, S.; Mori, M. Identification of recurrence-related microRNAs in the bone marrow of breast cancer patients. Int. J. Oncol. 2011, 38, 955–962. [Google Scholar] [CrossRef]
- Markou, A.; Yousef, G.M.; Stathopoulos, E.; Georgoulias, V.; Lianidou, E. Prognostic Significance of Metastasis-Related MicroRNAs in Early Breast Cancer Patients with a Long Follow-Up. Clin. Chem. 2014, 60, 197–205. [Google Scholar] [CrossRef]
- Qian, B.; Katsaros, D.; Lu, L.; Preti, M.; Durando, A.; Arisio, R.; Mu, L.; Yu, H. High MiR-21 Expression in Breast Cancer Associated with Poor Disease-Free Survival in Early Stage Disease and High TGF-Β1. Breast Cancer Res. Treat. 2009, 117, 131–140. [Google Scholar] [CrossRef]
- Walter, B.A.; Gómez-Macias, G.; Valera, V.A.; Sobel, M.; Merino, M.J. MiR-21 Expression in Pregnancy-Associated Breast Cancer: A Possible Marker of Poor Prognosis. J. Cancer 2011, 2, 67–75. [Google Scholar] [CrossRef]
- Yan, L.-X.; Liu, Y.-H.; Xiang, J.-W.; Wu, Q.-N.; Xu, L.-B.; Luo, X.-L.; Zhu, X.-L.; Liu, C.; Xu, F.-P.; Luo, D.-L.; et al. PIK3R1 Targeting by MiR-21 Suppresses Tumor Cell Migration and Invasion by Reducing PI3K/AKT Signaling and Reversing EMT, and Predicts Clinical Outcome of Breast Cancer. Int. J. Oncol. 2016, 48, 471–484. [Google Scholar] [CrossRef]
- Papadaki, C.; Stoupis, G.; Tsalikis, L.; Monastirioti, A.; Papadaki, M.; Maliotis, N.; Stratigos, M.; Mastrostamatis, G.; Mavroudis, D.; Agelaki, S. Circulating MiRNAs as a Marker of Metastatic Disease and Prognostic Factor in Metastatic Breast Cancer. Oncotarget 2019, 10, 966–981. [Google Scholar] [CrossRef]
- Romadhon, P.Z.; Prayoga, A.A.; Bintoro, S.U.Y.; Diansyah, M.N.; Amrita, P.N.; Savitri, M.; Windradi, C. The Association between Plasma MiRNA-21 Levels with Overall 1-Year Survival Rate of Breast Cancer Patients at Various Stages. Acta Med. Indones. 2021, 53, 432–441. [Google Scholar]
- Liu, B.; Su, F.; Chen, M.; Li, Y.; Qi, X.; Xiao, J.; Li, X.; Liu, X.; Liang, W.; Zhang, Y.; et al. Serum MiR-21 and MiR-125b as Markers Predicting Neoadjuvant Chemotherapy Response and Prognosis in Stage II/III Breast Cancer. Hum. Pathol. 2017, 64, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, C.; Stratigos, M.; Markakis, G.; Spiliotaki, M.; Mastrostamatis, G.; Nikolaou, C.; Mavroudis, D.; Agelaki, S. Circulating MicroRNAs in the Early Prediction of Disease Recurrence in Primary Breast Cancer. Breast Cancer Res. 2018, 20, 72. [Google Scholar] [CrossRef] [PubMed]
- Amirfallah, A.; Knutsdottir, H.; Arason, A.; Hilmarsdottir, B.; Johannsson, O.T.; Agnarsson, B.A.; Barkardottir, R.B.; Reynisdottir, I. Hsa-MiR-21-3p Associates with Breast Cancer Patient Survival and Targets Genes in Tumor Suppressive Pathways. PLoS ONE 2021, 16, e0260327. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Sánchez, D.; Arriaga-Canon, C.; Pedroza-Torres, A.; De La Rosa-Velázquez, I.A.; González-Barrios, R.; Contreras-Espinosa, L.; Montiel-Manríquez, R.; Castro-Hernández, C.; Fragoso-Ontiveros, V.; Álvarez-Gómez, R.M.; et al. The Promising Role of MiR-21 as a Cancer Biomarker and Its Importance in RNA-Based Therapeutics. Mol. Ther. Nucleic Acids 2020, 20, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, S.R.; Yang, C.H.; Pfeffer, L.M. The Role of MiR-21 in Cancer. Drug Dev. Res. 2015, 76, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Maghsoudloo, M.; Kaboli, P.J.; Babaeizad, A.; Cui, Y.; Fu, J.; Wang, Q.; Imani, S. Decoding the Promise and Challenges of MiRNA-Based Cancer Therapies: An Essential Update on MiR-21, MiR-34, and MiR-155. Int. J. Med. Sci. 2024, 21, 2781–2798. [Google Scholar] [CrossRef]
- Fang, H.; Xie, J.; Zhang, M.; Zhao, Z.; Wan, Y.; Yao, Y. MiRNA-21 Promotes Proliferation and Invasion of Triple-Negative Breast Cancer Cells through Targeting PTEN. Am. J. Transl. Res. 2017, 9, 953–961. [Google Scholar]
- Giordo, R.; Ahmadi, F.A.M.; Al Husaini, N.; Al-Nuaimi, N.R.A.M.; Ahmad, S.M.S.; Pintus, G.; Zayed, H. MicroRNA 21 and Long Non-Coding RNAs Interplays Underlie Cancer Pathophysiology: A Narrative Review. Noncoding RNA Res. 2024, 9, 831–852. [Google Scholar] [CrossRef]
| Population (P) | Prognostic Factors (F) | Outcome (O) |
|---|---|---|
| Patients diagnosed with breast cancer | Expression of miR-21, measured in tissue, serum, plasma, or exosomes | Survival outcomes (OS, DFS, RFS, DFI) |
| Subgroup Analysis | Number of Studies | Model | HR (95% CI) | p Value | Heterogeneity (I2, p-Value) |
|---|---|---|---|---|---|
| All | 13 | Random | 2.37 (1.42–3.98) | 0.001 | 77%, <0.001 |
| Cut-off | |||||
| Mean | 4 | Fixed | 4.81 (3.29–7.02) | <0.001 | 49%, 0.11 |
| Median | 7 | Fixed | 1.50 (1.10–2.06) | 0.01 | 49%, 0.07 |
| Type of Breast Cancer | |||||
| Mixed | 9 | Random | 2.55 (1.47–4.40) | <0.001 | 77%, <0.001 |
| Her2+ | 2 | Fixed | 1.06 (0.54–2.10) | 0.86 | 90%, 0.86 |
| Triple-negative | 2 | Fixed | 5.69 (3.41–9.49) | <0.001 | 0%, 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conte, L.; Tumolo, M.R.; De Nunzio, G.; De Giorgi, U.; Guarino, R.; Cascio, D.; Cucci, F. The Prognostic Power of miR-21 in Breast Cancer: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 9713. https://doi.org/10.3390/ijms26199713
Conte L, Tumolo MR, De Nunzio G, De Giorgi U, Guarino R, Cascio D, Cucci F. The Prognostic Power of miR-21 in Breast Cancer: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2025; 26(19):9713. https://doi.org/10.3390/ijms26199713
Chicago/Turabian StyleConte, Luana, Maria Rosaria Tumolo, Giorgio De Nunzio, Ugo De Giorgi, Roberto Guarino, Donato Cascio, and Federico Cucci. 2025. "The Prognostic Power of miR-21 in Breast Cancer: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 26, no. 19: 9713. https://doi.org/10.3390/ijms26199713
APA StyleConte, L., Tumolo, M. R., De Nunzio, G., De Giorgi, U., Guarino, R., Cascio, D., & Cucci, F. (2025). The Prognostic Power of miR-21 in Breast Cancer: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 26(19), 9713. https://doi.org/10.3390/ijms26199713








