The Role of Hypoxia-Sensitive miRNA181a, miRNA199a, SIRT1, and Adiponectin in Diabetes Mellitus Type 2 Development in Obstructive Sleep Apnea Patients †
Abstract
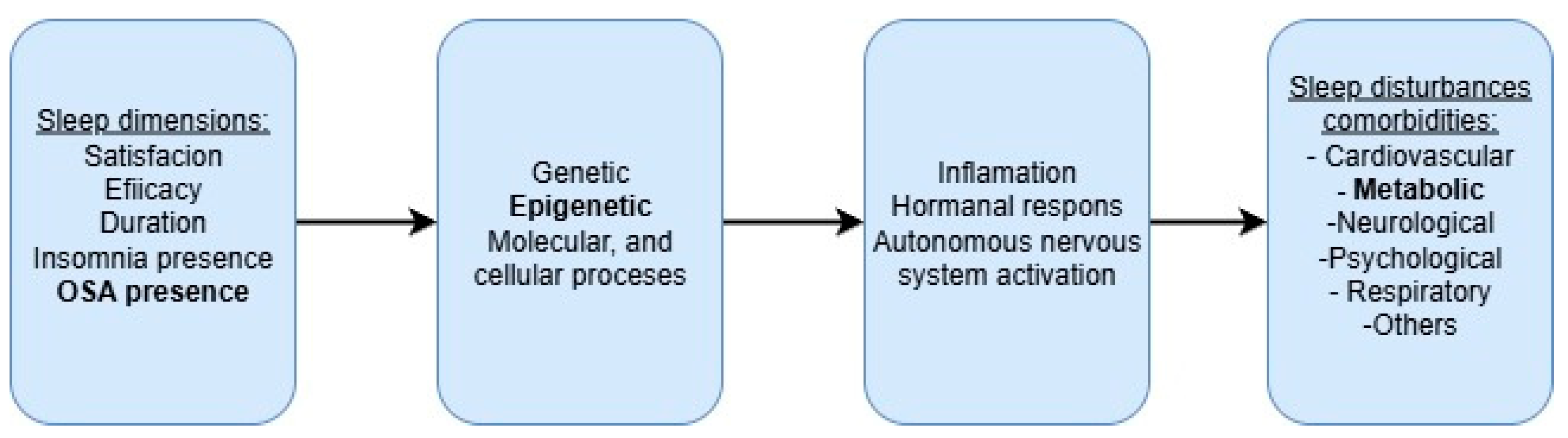
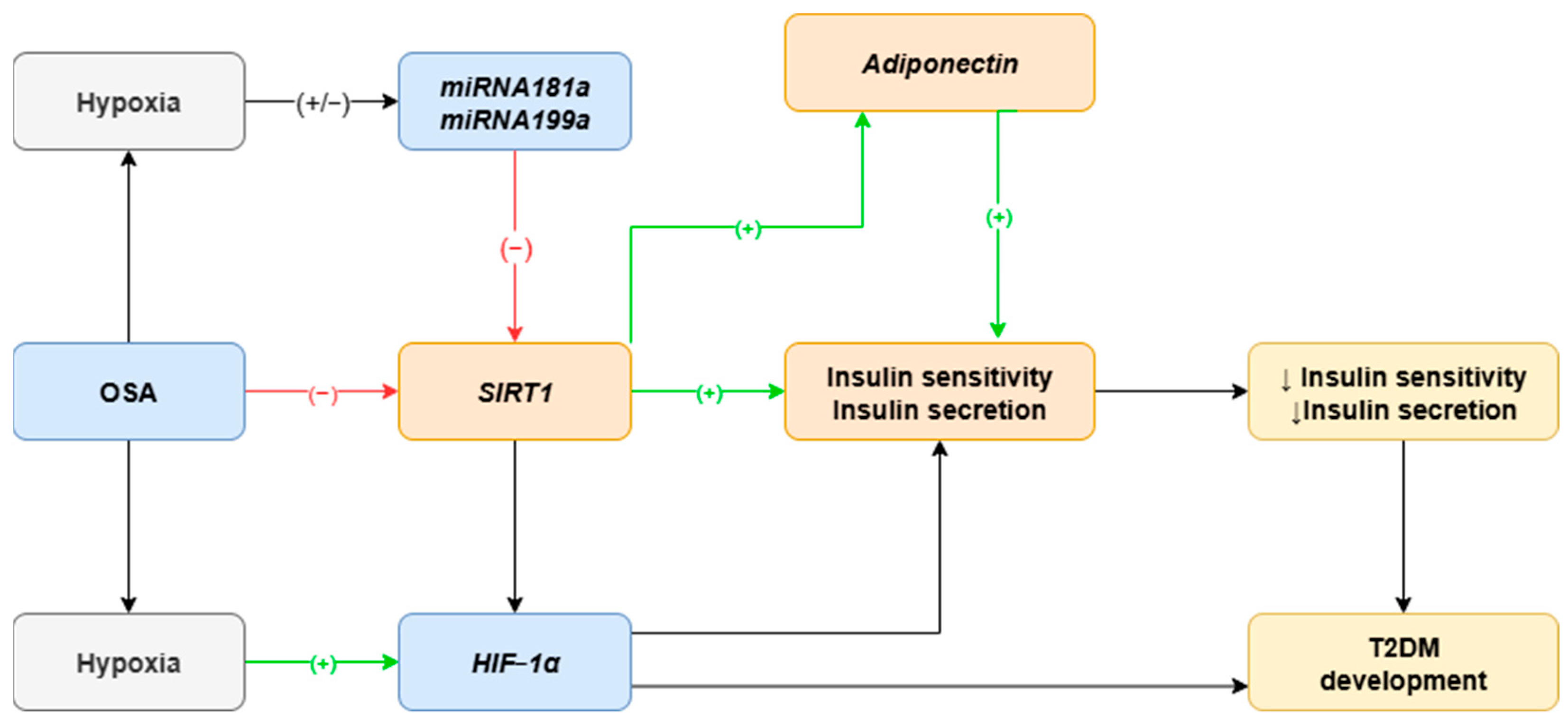
1. Introduction
2. Results
2.1. Study Groups
2.2. Hypoxia-Sensitive miRNAs: microRNA-181a and miRNA-199a
2.3. Metabolic Guardians: SIRT1 and Adiponectin
2.4. Results After One-Night CPAP Trial and at Least 3 Month CPAP Treatment
3. Discussion
Study Limitations
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, C.M.; Davidson, T.M.; Ancoli-Israel, S. Gender Differences in Obstructive Sleep Apnea and Treatment Implications. Sleep Med. Rev. 2008, 12, 481. [Google Scholar] [CrossRef] [PubMed]
- Gabryelska, A.; Karuga, F.F.; Szmyd, B.; Białasiewicz, P. HIF-1α as a Mediator of Insulin Resistance, T2DM, and Its Complications: Potential Links With Obstructive Sleep Apnea. Front. Physiol. 2020, 11, 540381. [Google Scholar] [CrossRef]
- Labarca, G.; Reyes, T.; Jorquera, J.; Dreyse, J.; Drake, L. CPAP in Patients with Obstructive Sleep Apnea and Type 2 Diabetes Mellitus: Systematic Review and Meta-Analysis. Clin. Respir. J. 2018, 12, 2361–2368. [Google Scholar] [CrossRef]
- Bonsignore, M.R.; Baiamonte, P.; Mazzuca, E.; Castrogiovanni, A.; Marrone, O. Obstructive Sleep Apnea and Comorbidities: A Dangerous Liaison. Multidiscip. Respir. Med. 2019, 14, 8. [Google Scholar] [CrossRef]
- Karuga, F.F.; Jaromirska, J.; Sochal, M.; Bialasiewicz, P.; Gabryelska, A. Association between Glucose Metabolism, the Circadian Cycle and Hypoxia: Evaluation of the NPAS2 and Rev-Erb-α Protein Serum Levels in Obstructive Sleep Apnea Patients—A Pilot Study. Dent. Med. Probl. 2024, 61, 463–467. [Google Scholar] [CrossRef]
- Mahmood, K.; Akhter, N.; Eldeirawi, K.; Önal, E.; Christman, J.W.; Carley, D.W.; Herdegen, J.J. Prevalence of Type 2 Diabetes in Patients with Obstructive Sleep Apnea in a Multi-Ethnic Sample. J. Clin. Sleep Med. 2009, 5, 215–221. [Google Scholar] [CrossRef]
- Reutrakul, S.; Mokhlesi, B. Obstructive Sleep Apnea and Diabetes: A State of the Art Review. Chest 2017, 152, 1070–1086. [Google Scholar] [CrossRef]
- Gabryelska, A.; Szmyd, B.; Panek, M.; Szemraj, J.; Kuna, P.; Białasiewicz, P. Serum Hypoxia–Inducible Factor–1α Protein Level as a Diagnostic Marker of Obstructive Sleep Apnea. Polish Arch. Intern. Med. 2020, 130, 158–160. [Google Scholar] [CrossRef]
- Bielicki, P.; Pływaczewski, R.; Brzóska, K.; Kumor, M.; Barnas, M.; Jonczak, L.; Stȩpkowski, T.M.; Piechuta, A.; Chazan, R.; Sliwiński, P.; et al. Impact of Polymorphism of Selected Genes on the Diagnosis of Type 2 Diabetes in Patients with Obstructive Sleep Apnea. Polish Arch. Intern. Med. 2019, 129, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Morris, B.J. Seven Sirtuins for Seven Deadly Diseases Ofaging. Free Radic. Biol. Med. 2013, 56, 133–171. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.-Y.; Yun, M.; Jeong, J.; Park, E.-R.; Shin, H.-J.; Woo, S.R.; Jung, J.K.; Kim, Y.-M.; Park, J.-J.; Kim, J.; et al. SIRT1 Deacetylates and Stabilizes Hypoxia-Inducible Factor-1α (HIF-1α) via Direct Interactions during Hypoxia. Biochem. Biophys. Res. Commun. 2015, 462, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Dioum, E.M.; Chen, R.; Alexander, M.S.; Zhang, Q.; Hogg, R.T.; Gerard, R.D.; Garcia, J.A. Regulation of Hypoxia-Inducible Factor 2α Signaling by the Stress-Responsive Deacetylase Sirtuin 1. Science 2009, 324, 1289–1293. [Google Scholar] [CrossRef]
- Lim, J.H.; Lee, Y.M.; Chun, Y.S.; Chen, J.; Kim, J.E.; Park, J.W. Sirtuin 1 Modulates Cellular Responses to Hypoxia by Deacetylating Hypoxia-Inducible Factor 1alpha. Mol. Cell 2010, 38, 864–878. [Google Scholar] [CrossRef]
- Laemmle, A.; Lechleiter, A.; Roh, V.; Schwarz, C.; Portmann, S.; Furer, C.; Keogh, A.; Tschan, M.P.; Candinas, D.; Vorburger, S.A.; et al. Inhibition of SIRT1 Impairs the Accumulation and Transcriptional Activity of HIF-1α Protein under Hypoxic Conditions. PLoS ONE 2012, 7, e33433. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Koya, D. SIRT1 in Type 2 Diabetes: Mechanisms and Therapeutic Potential. Diabetes Metab. J. 2013, 37, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Ogura, Y.; Monno, I.; Koya, D. Sirtuins and Type 2 Diabetes: Role in Inflammation, Oxidative Stress, and Mitochondrial Function. Front. Endocrinol. 2019, 10, 187. [Google Scholar] [CrossRef]
- Song, J.; Yang, B.; Jia, X.; Li, M.; Tan, W.; Ma, S.; Shi, X.; Feng, L. Distinctive Roles of Sirtuins on Diabetes, Protective or Detrimental? Front. Endocrinol. 2018, 9, 724. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, F.; Ge, X.; Yan, T.; Chen, X.; Shi, X.; Zhai, Q. SIRT1 Improves Insulin Sensitivity under Insulin-Resistant Conditions by Repressing PTP1B. Cell Metab. 2007, 6, 307–319. [Google Scholar] [CrossRef]
- Celikhisar, H.; Ilkhan, G.D. Alterations in Serum Adropin, Adiponectin, and Proinflammatory Cytokine Levels in OSAS. Can. Respir. J. 2020, 2020, 2571283. [Google Scholar] [CrossRef]
- Hotta, K.; Funahashi, T.; Arita, Y.; Takahashi, M.; Matsuda, M.; Okamoto, Y.; Iwahashi, H.; Kuriyama, H.; Ouchi, N.; Maeda, K.; et al. Plasma Concentrations of a Novel, Adipose-Specific Protein, Adiponectin, in Type 2 Diabetic Patients. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1595–1599. [Google Scholar] [CrossRef]
- Qiao, L.; Shao, J. SIRT1 Regulates Adiponectin Gene Expression through Foxo1-C/Enhancer- Binding Protein α Transcriptional Complex. J. Biol. Chem. 2006, 281, 39915–39924. [Google Scholar] [CrossRef]
- Qiang, L.; Wang, H.; Farmer, S.R. Adiponectin Secretion Is Regulated by SIRT1 and the Endoplasmic Reticulum Oxidoreductase Ero1-L α. Mol. Cell. Biol. 2007, 27, 4698–4707. [Google Scholar] [CrossRef]
- Kulshreshtha, R.; Ferracin, M.; Wojcik, S.E.; Garzon, R.; Alder, H.; Agosto-Perez, F.J.; Davuluri, R.; Liu, C.-G.; Croce, C.M.; Negrini, M.; et al. A MicroRNA Signature of Hypoxia. Mol. Cell. Biol. 2007, 27, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Li, X.; Jia, Y.F.; Piazza, G.A.; Xi, Y. Hypoxia-Regulated MicroRNAs in Human Cancer. Acta Pharmacol. Sin. 2013, 34, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Santamaria-Martos, F.; Benítez, I.; Ortega, F.; Zapater, A.; Giron, C.; Pinilla, L.; Pascual, L.; Cortijo, A.; Dalmases, M.; Fernandez-Real, J.M.; et al. Circulating MicroRNA Profile as a Potential Biomarker for Obstructive Sleep Apnea Diagnosis. Sci. Rep. 2019, 9, 13456. [Google Scholar] [CrossRef] [PubMed]
- Pinilla, L.; Barbé, F.; de Gonzalo-Calvo, D. MicroRNAs to Guide Medical Decision-Making in Obstructive Sleep Apnea: A Review. Sleep Med. Rev. 2021, 59, 101458. [Google Scholar] [CrossRef]
- Li, K.; Wei, P.; Qin, Y.; Wei, Y. MicroRNA Expression Profiling and Bioinformatics Analysis of Dysregulated MicroRNAs in Obstructive Sleep Apnea Patients. Medicine 2017, 96, e7917. [Google Scholar] [CrossRef]
- Lacedonia, D.; Scioscia, G.; Palladino, G.P.; Gallo, C.; Carpagnano, G.E.; Sabato, R.; Foschino Barbaro, M.P. MicroRNA Expression Profile during Different Conditions of Hypoxia. Oncotarget 2018, 9, 35114–35122. [Google Scholar] [CrossRef]
- Santamaria-Martos, F.; Benítez, I.; Zapater, A.; Girón, C.; Pinilla, L.; Fernandez-Real, J.M.; Barbé, F.; Ortega, F.J.; Sánchez-de-la-Torre, M. Identification and Validation of Circulating MiRNAs as Endogenous Controls in Obstructive Sleep Apnea. PLoS ONE 2019, 14, e0213622. [Google Scholar] [CrossRef]
- Rane, S.; He, M.; Sayed, D.; Vashistha, H.; Malhotra, A.; Sadoshima, J.; Vatner, D.E.; Vatner, S.F.; Abdellatif, M. Downregulation of MiR-199a Derepresses Hypoxia-Inducible Factor-1α and Sirtuin 1 and Recapitulates Hypoxia Preconditioning in Cardiac Myocytes. Circ. Res. 2009, 104, 879–886. [Google Scholar] [CrossRef]
- Zhou, B.; Li, C.; Qi, W.; Zhang, Y.; Zhang, F.; Wu, J.X.; Hu, Y.N.; Wu, D.M.; Liu, Y.; Yan, T.T.; et al. Downregulation of MiR-181a Upregulates Sirtuin-1 (SIRT1) and Improves Hepatic Insulin Sensitivity. Diabetologia 2012, 55, 2032–2043. [Google Scholar] [CrossRef]
- Cuyàs, E.; Verdura, S.; Llorach-Parés, L.; Fernández-Arroyo, S.; Joven, J.; Martin-Castillo, B.; Bosch-Barrera, J.; Brunet, J.; Nonell-Canals, A.; Sanchez-Martinez, M.; et al. Metformin Is a Direct SIRT1-Activating Compound: Computational Modeling and Experimental Validation. Front. Endocrinol. 2018, 9, 657. [Google Scholar] [CrossRef]
- Khowailed, E.A.; Seddiek, H.A.; Mahmoud, M.M.; Rashed, L.A.; Ibrahim, F.E. Effect of Metformin on Sirtuin-1 Disorders Associated with Diabetes in Male Rats. Alexandria J. Med. 2018, 54, 373–381. [Google Scholar] [CrossRef]
- Oliveras-Ferraros, C.; Cufí, S.; Vazquez-Martin, A.; Torres-Garcia, V.Z.; Del Barco, S.; Martin-Castillo, B.; Menendez, J.A. Micro(Mi)RNA Expression Profile of Breast Cancer Epithelial Cells Treated with the Anti-Diabetic Drug Metformin: Induction of the Tumor Suppressor MiRNA Let-7a and Suppression of the TGFβ-Induced OncomiR MiRNA-181a. Cell Cycle 2011, 10, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Zunica, E.R.M.; Heintz, E.C.; Dantas, W.S.; Hebert, R.C.; Tanksley, M.K.; Beyl, R.A.; Mader, E.C.; Kirwan, J.P.; Axelrod, C.L.; Singh, P. Effects of Metformin on Glucose Metabolism and Mitochondrial Function in Patients with Obstructive Sleep Apnea: A Pilot Randomized Trial. Physiol. Rep. 2024, 12, e15948. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Tan, X.; Benedict, C. Association of Poor Sleep and HbA1c in Metformin-Treated Patients with Type 2 Diabetes: Findings from the UK Biobank Cohort Study. J. Sleep Res. 2023, 32, e13917. [Google Scholar] [CrossRef] [PubMed]
- Mcclelland, A.D.; Kantharidis, P. MicroRNA in the Development of Diabetic Complications. Clin. Sci. 2014, 126, 95–110. [Google Scholar] [CrossRef]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef]
- Subramanian, A.; Adderley, N.J.; Tracy, A.; Taverner, T.; Hanif, W.; Toulis, K.A.; Neil Thomas, G.; Tahrani, A.A.; Nirantharakumar, K. Risk of Incident Obstructive Sleep Apnea Among Patients With Type 2 Diabetes. Diabetes Care 2019, 42, 954–963. [Google Scholar] [CrossRef]
- Karuga, F.F.; Kaczmarski, P.; Sochal, M.; Szmyd, B.; Białasiewicz, P.; Gabryelska, A. Cross-Sectional Analysis of Hypoxia-Regulated MiRNA-181a, MiRNA-199a, HIF-1α, and SIRT1 in the Development of Type 2 Diabetes in Patients with Obstructive Sleep Apnea—Preliminary Study. J. Clin. Med. 2024, 13, 7644. [Google Scholar] [CrossRef]
- Araszkiewicz, A.; Bandurska-Stankiewicz, E.; Borys, S.; Budzyński, A.; Cyganek, K.; Cypryk, K.; Czech, A.; Czupryniak, L.; Drzewoski, J.; Dzida, G.; et al. Zalecenia Kliniczne Dotyczące Postępowania u Chorych z Cukrzycą 2021. Stanowisko Polskiego Towarzystwa Diabetologicznego. Diabetol. Prakt. 2021, 7, 1–121. [Google Scholar]
| OSA | OSA + T2DM | Control Group | p-Value | |
|---|---|---|---|---|
| N | 26 (29.9%) | 29 (33.3%) | 32 (36.8%) | N.A. |
| Female sex | 4 (15.4%) | 5 (17.2%) | 7 (21.9%) | 0.441 |
| Age [years old] | 50.5 (IQR: 45.25–62) | 58 (IQR: 49–67) | 47 (IQR: 42–54) | 0.004 |
| BMI [kg/m2] | 31.794 (IQR: 28.091–36.499) | 35.891 (IQR: 32.084–39.246) | 28.532 (IQR: 26.967–31.868) | <0.001 |
| AHI [incidents/h] | 40.35 (IQR: 36.25–59.375) | 55.1 (IQR: 28.4–80.3) | 2.1 (IQR: 1.175–4.2) | <0.001 |
| Desaturation index | 43 (IQR: 24–56.3) | 62 (IQR: 33.3–80) | 2 (IQR: 1–3.55) | <0.001 |
| Total sleep time [h] | 6.3 (IQR: 5.2–7) | 5.88 (IQR: 5.201–7) | 6.2 (IQR: 5.4–6.5) | 0.771 |
| Glucose [mmol/L] | 5.755 IQR: 5.32–5.957 | 6.89 IQR: 6.35–7.46 | 5.31 IQR: 5.177–5.565 | <0.001 |
| Insulin [pmol/L] | 137 IQR: 102.375–168.175 | 151.7 IQR: 110.2–212.6 | 68.05 IQR: 59.7–92.575 | <0.001 |
| OSA n = 26 | OSA + T2DM n = 29 | Control n = 32 | p-Value | |
|---|---|---|---|---|
| miRNA-181a evening | 36.746 IQR: 18.11–108.35 | 16.78 IQR: 13.46–27.546 | 25.619 IQR: 7.002–114.02 | 0.041 |
| miRNA-199a evening | 2.988 IQR: 1.01–8.23 | 0.752 IQR: 0.398–2.264 | 1.237 IQR: 0.481–5.27 | 0.047 |
| miRNA-181a morning | 34.301 IQR: 10.96–81.19 | 18.279 IQR: 11.335–24.91 | 15.643 IQR: 9.31–144.19 | 0.345 |
| miRNA-199a morning | 2.788 IQR: 0.741–7.886 | 0.591 IQR: 0.301–1.587 | 1.137 IQR: 0.468–7.342 | 0.034 |
| OSA n = 26 | OSA + T2DM n = 29 | Control n = 32 | p-Value | |
|---|---|---|---|---|
| SIRT1 [* 10−6] evening | 44.12 IQR: 27.88–267.96 | 506.60 IQR: 165.5–1176.7 | 323.08 IQR: 32.50–1136.35 | 0.012 |
| SIRT1 [* 10−6] morning | 84.44 IQR: 48.49–156.49 | 553.75 IQR: 206.18–1254.58 | 214.86 IQR: 46.02–1055.07 | 0.008 |
| Adiponectin evening [ng/mL] | 3849.005 IQR: 2812.635–6048 | 3170.35 IQR: 2658.19–4366.99 | 5907.54 IQR: 4142.64–8347.63 | 0.001 |
| Adiponectin morning [ng/mL] | 3327.035 IQR: 2678.85–5013.067 | 2953.49 IQR: 2296.637–3915.38 | 5207.775 IQR: 3604.97–7138.832 | 0.001 |
| Biomolecule | Sample Type | Expression |
|---|---|---|
| miRNA-181a | buffy coat | yes |
| miRNA199a | buffy coat | yes |
| SIRT1 mRNA | buffy coat | yes, but low |
| SIRT1 protein | buffy coat | no |
| Adiponectin | serum | yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karuga, F.F.; Kaczmarski, P.; Sochal, M.; Szmyd, B.; Urbonaitė, G.V.; Turkiewicz, S.; Białasiewicz, P.; Gabryelska, A. The Role of Hypoxia-Sensitive miRNA181a, miRNA199a, SIRT1, and Adiponectin in Diabetes Mellitus Type 2 Development in Obstructive Sleep Apnea Patients. Int. J. Mol. Sci. 2025, 26, 9699. https://doi.org/10.3390/ijms26199699
Karuga FF, Kaczmarski P, Sochal M, Szmyd B, Urbonaitė GV, Turkiewicz S, Białasiewicz P, Gabryelska A. The Role of Hypoxia-Sensitive miRNA181a, miRNA199a, SIRT1, and Adiponectin in Diabetes Mellitus Type 2 Development in Obstructive Sleep Apnea Patients. International Journal of Molecular Sciences. 2025; 26(19):9699. https://doi.org/10.3390/ijms26199699
Chicago/Turabian StyleKaruga, Filip Franciszek, Piotr Kaczmarski, Marcin Sochal, Bartosz Szmyd, Greta Veronika Urbonaitė, Szymon Turkiewicz, Piotr Białasiewicz, and Agata Gabryelska. 2025. "The Role of Hypoxia-Sensitive miRNA181a, miRNA199a, SIRT1, and Adiponectin in Diabetes Mellitus Type 2 Development in Obstructive Sleep Apnea Patients" International Journal of Molecular Sciences 26, no. 19: 9699. https://doi.org/10.3390/ijms26199699
APA StyleKaruga, F. F., Kaczmarski, P., Sochal, M., Szmyd, B., Urbonaitė, G. V., Turkiewicz, S., Białasiewicz, P., & Gabryelska, A. (2025). The Role of Hypoxia-Sensitive miRNA181a, miRNA199a, SIRT1, and Adiponectin in Diabetes Mellitus Type 2 Development in Obstructive Sleep Apnea Patients. International Journal of Molecular Sciences, 26(19), 9699. https://doi.org/10.3390/ijms26199699







