Macrophage Migration Inhibitory Factor and Post-Discharge Inflammatory Profiles in Severe COVID-19: A Prospective Observational Study from Romania
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
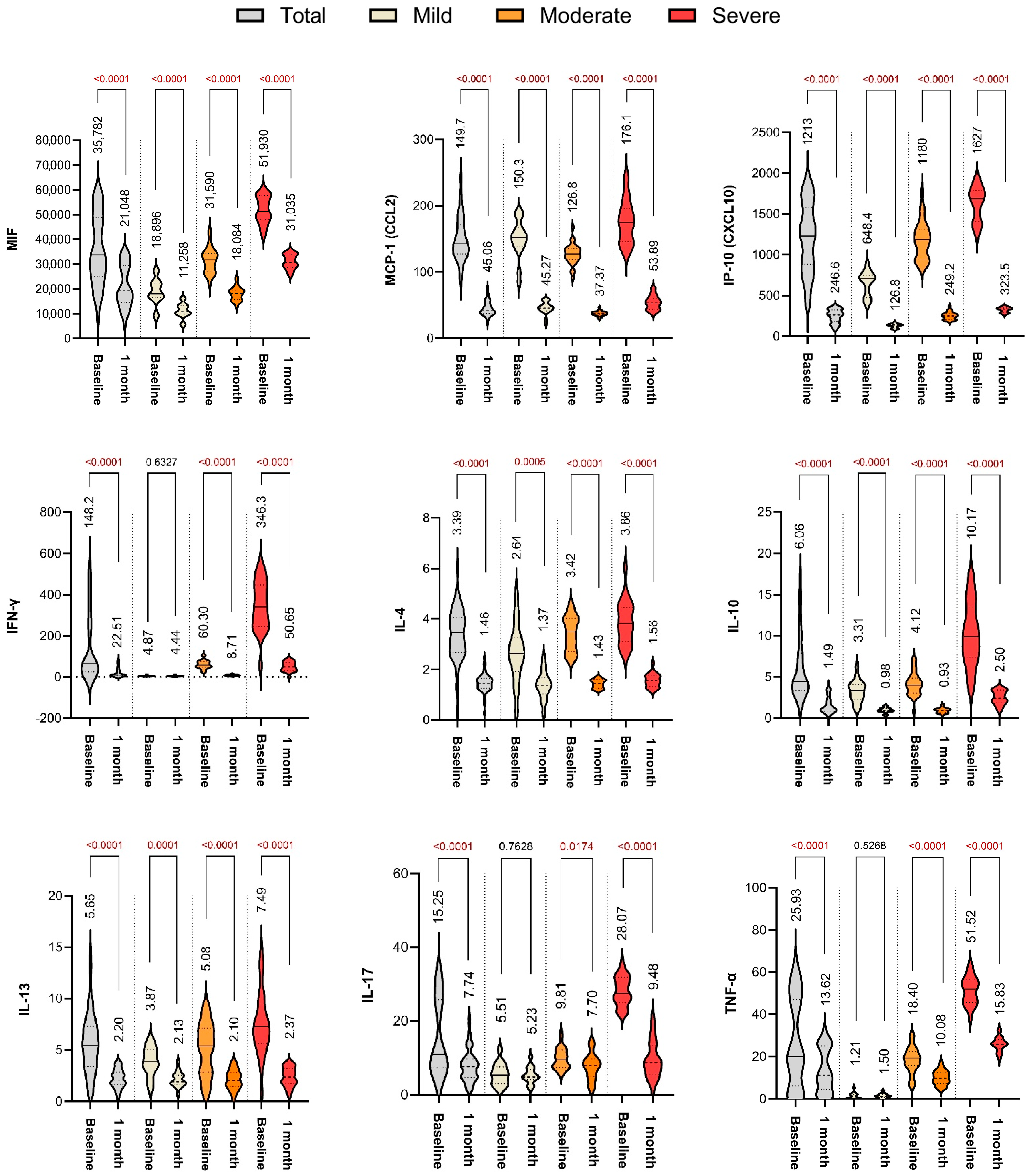
2.2. Baseline Cytokine Profiles
2.3. Cytokine Levels at 1-Month Follow-Up
2.4. Summary of Immune Resolution Trends
3. Discussion
3.1. MIF: A Key Mediator of COVID-19 Severity and Persistence
3.2. Persistent Cytokine Activation After Severe COVID-19
3.3. Comparison with Regional and Global Data
3.4. Clinical Implications
3.5. Future Directions
4. Materials and Methods
4.1. Study Design and Setting
4.2. Patient Population
4.3. Cytokine Quantification
4.4. Clinical and Demographic Data
4.5. Statistical Analysis
5. Conclusions
Study Limitations
- Single-country, single-region population: All patients were recruited from Mureș County, Romania, which might limit how well the results apply to populations with different genetic backgrounds, healthcare systems, or treatment protocols.
- Small cohort size and survival bias: The total cohort of 68 patients enabled comparisons across severity groups, but subgroup sizes were modest, particularly for mild cases (n = 16). Furthermore, patients who died before the 1-month follow-up were excluded, introducing survival bias and potentially underestimating persistent inflammation in the most severe cases.
- Lack of viral load and variant data: SARS-CoV-2 viral RNA was not quantified, nor were circulating variants sequenced. These details could have affected cytokine responses, especially considering the changing variant landscape during early 2022.
- Potential residual confounding: Although patients receiving immunosuppressive therapy or with active malignancy were excluded, common comorbidities such as obesity, diabetes, and COPD may still have affected cytokine levels during both the acute and recovery phases.
- Short clinical follow-up: Persistent cytokine elevations were documented at 1 month, but longer-term outcomes such as post-acute sequelae (long COVID), pulmonary fibrosis, or quality of life were not assessed.
- Single follow-up time point: The 1-month evaluation provides only a snapshot of immunological recovery, potentially missing earlier post-discharge dynamics or later convalescent changes.
- Lack of a healthy control group: Without a non-infected comparator population, it remains uncertain whether cytokine levels at 1 month represented a return to baseline or ongoing immune dysregulation.
- Restricted respiratory support capacity: The study centers were limited to conventional oxygen delivery (nasal cannula or Hudson-type mask). Non-invasive and invasive mechanical ventilation were not available, restricting direct comparison with cohorts treated in centers offering broader respiratory support.
- No cytokine–symptom correlation: Although 18% of patients reported persistent symptoms at 1 month, these did not significantly correlate with acute disease severity or cytokine levels. This limits interpretation of the link between immune activation and clinical expression of post-acute sequelae. Larger studies integrating both biological and clinical outcomes will be required to clarify these associations.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COVID-19 | Coronavirus Disease 2019 |
| COPD | Chronic Obstructive Pulmonary Disease |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| MIF | Macrophage Migration Inhibitory Factor |
| MCP-1 (CCL2) | Monocyte Chemoattractant Protein-1 (Chemokine (C-C motif) ligand 2) |
| IP-10 (CXCL10) | Interferon Gamma-Induced Protein 10 (C-X-C motif chemokine 10) |
| IFN-γ | Interferon Gamma |
| IL-4 | Interleukin-4 |
| IL-10 | Interleukin-10 |
| IL-13 | Interleukin-13 |
| IL-17 | Interleukin-17 |
| TNF-α | Tumor Necrosis Factor Alpha |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| ARDS | Acute Respiratory Distress Syndrome |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| ICU | Intensive Care Unit |
| PASC | Post-Acute Sequelae of COVID-19 |
| BMI | Body Mass Index |
| SpO2 | Peripheral Capillary Oxygen Saturation |
| CD74 | Cluster of Differentiation 74 (MIF receptor) |
| xMAP® | Multi-Analyte Profiling (Luminex technology) |
| SD | Standard Deviation |
References
- Matyas, B.; Polexa, S.; Benedek, I.; Buicu, A.; Benedek, T. Biomarkers of Systemic Versus Local Inflammation During the Acute Phase of Myocardial Infarction, as Predictors of Post-infarction Heart Failure. J. Cardiovasc. Emerg. 2021, 7, 70–76. [Google Scholar] [CrossRef]
- Han, H.; Ma, Q.; Li, C.; Liu, R.; Zhao, L.; Wang, W.; Zhang, P.; Liu, X.; Gao, G.; Liu, F.; et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes Infect. 2020, 9, 1123–1130. [Google Scholar] [CrossRef]
- Laing, A.G.; Lorenc, A.; Del Molino Del Barrio, I.; Das, A.; Fish, M.; Monin, L.; Munoz-Ruiz, M.; McKenzie, D.R.; Hayday, T.S.; Francos-Quijorna, I.; et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat. Med. 2020, 26, 1623–1635, Erratum in Nat. Med. 2020, 26, 1951. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Hou, H.; Luo, Y.; Tang, G.; Wu, S.; Huang, M.; Liu, W.; Zhu, Y.; Lin, Q.; Mao, L.; et al. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight 2020, 5, e137799. [Google Scholar] [CrossRef]
- Arunachalam, P.S.; Wimmers, F.; Mok, C.K.P.; Perera, R.A.P.M.; Scott, M.; Hagan, T.; Sigal, N.; Feng, Y.; Bristow, L.; Tsang, O.T.-Y.; et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science 2020, 369, 1210–1220. [Google Scholar] [CrossRef] [PubMed]
- Petrakis, D.; Margină, D.; Tsarouhas, K.; Tekos, F.; Stan, M.; Nikitovic, D.; Kouretas, D.; Spandidos, D.A.; Tsatsakis, A. Obesity a risk factor for increased COVID-19 prevalence, severity and lethality. Mol. Med. Rep. 2020, 22, 9–19. [Google Scholar] [CrossRef]
- Capraru, I.D.; Marian, C.; Vulcanescu, D.D.; Tanasescu, S.; Dragomir, T.L.; Marti, T.D.; Boru, C.; Avram, C.R.; Susan, M.; Vlad, C.S. Understanding the impact of COVID-19 on Roma vulnerable communities in Western Romania: Insights and predictive factors from a retrospective study. Viruses 2024, 16, 435. [Google Scholar] [CrossRef]
- Mátyás, B.; Benedek, I.; Opincariu, D.; Blîndu, E.; Rosea, A.; Benedek, B.; Benedek, T. Impact of COVID-19 Infection on Regional Periocoronary Inflammation: An Angio-CT Study of Epicardial Fat Attenuation. Rom. J. Cardiol. 2023, 33, 47–53. [Google Scholar] [CrossRef]
- Mărcău, F.C.; Purec, S.; Niculescu, G. Study on the Refusal of Vaccination against COVID-19 in Romania. Vaccines 2022, 10, 261. [Google Scholar] [CrossRef]
- Manole, C.; Baroiu, L.; Nechita, A.; Voinescu, D.C.; Ciubara, A.; Debita, M.; Tatu, A.L.; Ciubara, A.B.; Stefanopol, I.A.; Anghel, L.; et al. Comparative evaluation of the clinical severity of COVID-19 of vaccinated and unvaccinated patients in Southeastern Romania in the first 6 months of 2022, during the Omicron wave. Healthcare 2023, 11, 2184. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.B.; June, C.H. Cytokine release syndrome in severe COVID-19. Science 2020, 368, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef]
- Calandra, T.; Roger, T. Macrophage migration inhibitory factor: A regulator of innate immunity. Nat. Rev. Immunol. 2003, 3, 791–800. [Google Scholar] [CrossRef]
- Aharon, A.; Avni, B.; Louzoun, D.; Elias, S.; Stepensky, P.; Ram, R.; Zuckerman, T.; Meza-Romera, R.; Vandenbark, A.A.; Grisariu, S.; et al. MIF functional polymorphisms are associated with acute GVHD progression and steroid-refractoriness. Front. Immunol. 2025, 16, 1504976. [Google Scholar] [CrossRef]
- Bilsborrow, J.B.; Doherty, E.; Tilstam, P.V.; Bucala, R. Macrophage migration inhibitory factor (MIF) as a therapeutic target for rheumatoid arthritis and systemic lupus erythematosus. Expert Opin. Ther. Targets 2019, 23, 733–744. [Google Scholar] [CrossRef]
- Donnelly, S.C.; Haslett, C.; Reid, P.T.; Grant, I.S.; Wallace, W.A.H.; Metz, C.N.; Bruce, L.J.; Bucala, R. Regulatory role for macrophage migration inhibitory factor in acute respiratory distress syndrome. Nat. Med. 1997, 3, 320–323. [Google Scholar] [CrossRef]
- Bange, E.M.; Han, N.A.; Wileyto, P.; Kim, J.Y.; Gouma, S.; Robinson, J.; Greenplate, A.R.; Hwee, M.A.; Porterfield, F.; Owoyemi, O.; et al. CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat. Med. 2021, 27, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Grieb, G.; Merk, M.; Bernhagen, J.; Bucala, R. Macrophage migration inhibitory factor (MIF): A promising biomarker. Drug News Perspect. 2010, 23, 257–264. [Google Scholar] [CrossRef]
- Clinical Management of COVID-19: Living Guideline; World Health Organization: Geneva, Switzerland, 23 June 2022.
- Lucijanić, M.; Piskač Živković, N.; Režić, T.; Durlen, I.; Stojić, J.; Jurin, I.; Šakota, S.; Filipović, D.; Kurjaković, I.; Jordan, A.; et al. The performance of the WHO COVID-19 severity classification, COVID-GRAM, VACO Index, 4C Mortality, and CURB-65 prognostic scores in hospitalized COVID-19 patients: Data on 4014 patients from a tertiary center registry. Croat. Med. J. 2023, 64, 13–20. [Google Scholar] [CrossRef]
- Pan, Y.; Jiang, X.; Yang, L.; Chen, L.; Zeng, X.; Liu, G.; Tang, Y.; Qian, C.; Wang, X.; Cheng, F.; et al. SARS-CoV-2-specific immune response in COVID-19 convalescent individuals. Signal Transduct. Target. Ther. 2021, 6, 256. [Google Scholar] [CrossRef]
- Phetsouphanh, C.; Darley, D.R.; Wilson, D.B.; Howe, A.; Munier, C.M.L.; Patel, S.K.; Juno, J.A.; Burrell, L.M.; Kent, S.J.; Dore, G.J.; et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 210–216. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, B.; Zheng, X.; Wang, D.; Zhao, C.; Qi, Y.; Sun, R.; Tian, Z.; Xu, X.; Wei, H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl. Sci. Rev. 2020, 7, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022, 185, 881–895.e20. [Google Scholar] [CrossRef] [PubMed]
- Darcis, G.; Bouquegneau, A.; Maes, N.; Thys, M.; Hneket, M.; Labye, F.; Rousseau, A.-F.; Canivet, P.; Desir, C.; Calmes, D.; et al. Long-term clinical follow-up of patients suffering from moderate-to-severe COVID-19 infection: A monocentric prospective observational cohort study. Int. J. Infect. Dis. 2021, 109, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Gan, G.; Xu, J.; Hu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506, Erratum in Lancet 2020, 395, 496. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hiu, D.S.C.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]

 | |||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Total Study Cohort (n = 68) | Group 1 (Mild) (n = 16) | Group 2 (Moderate) (n = 28) | Group 3 (Severe) (n = 24) | p Value 1 | p Value 2 | p Value 3 |
| Age, (years), mean ± SD | 69.9 ± 12.4 | 64.4 ± 9.1 | 66.2 ± 10.2 | 69.4 ± 11.3 | 0.2411 | 0.1833 | 0.2134 |
| Male gender, n (%) | 40 (58.82%) | 10 (62.50%) | 16 (57.14%) | 14 (58.33%) | 0.9768 | 0.9891 | 0.9391 |
| BMI 4, (kg/m2), mean ± SD | 29.87 ± 3.9 | 29.05 ± 3.5 | 29.93 ± 3.9 | 29.07 ± 3.4 | 0.8723 | 0.7657 | 0.9133 |
| Hospital stay, (days), mean ± SD | 6.7 ± 2.8 | 4.5 ± 1.9 | 6.1 ± 2.4 | 7.9 ± 3.1 | <0.05 | <0.01 | <0.001 |
| Comorbidities: | |||||||
| Hypertension, n (%) | 11 (16.18%) | 2 (12.50%) | 5 (17.86%) | 4 (16.67%) | 0.6402 | 0.9099 | 0.8949 |
| Dyslipidemia, n (%) | 14 (20.59%) | 3 (18.75%) | 6 (21.43%) | 5 (20.83%) | 0.8322 | 0.9582 | 0.9772 |
| Cardiovascular disease, n (%) | 18 (26.47%) | 4 (25.00%) | 8 (28.57%) | 6 (25.00%) | 0.7983 | 0.7722 | 0.9475 |
| Chronic kidney failure, n (%) | 11 (16.18%) | 3 (18.75%) | 4 (14.29%) | 4 (16.67%) | 0.6969 | 0.8125 | 0.9249 |
| Obesity, n (%) | 13 (19.12%) | 3 (18.75%) | 5 (17.86%) | 5 (20.83%) | 0.9411 | 0.7861 | 0.9628 |
| Diabetes mellitus, n (%) | 7 (10.29%) | 2 (12.50%) | 3 (10.71%) | 2 (8.33%) | 0.8575 | 0.7716 | 0.9096 |
| COPD 5, n (%) | 15 (22.06%) | 4 (25.00%) | 6 (21.43%) | 5 (20.83%) | 0.7857 | 0.9582 | 0.9475 |
| Asthma, n (%) | 12 (17.65%) | 3 (18.75%) | 5 (17.86%) | 4 (16.67%) | 0.9411 | 0.9099 | 0.9851 |
| Presenting symptoms: | |||||||
| Fever, n (%) | 58 (85.29%) | 12 (75.00%) | 25 (89.29%) | 21 (87.50%) | 0.2127 | 0.8408 | 0.4065 |
| Cough, n (%) | 24 (35.29%) | 5 (31.25%) | 12 (42.86%) | 7 (29.17%) | 0.4469 | 0.3068 | 0.5467 |
| Expectoration, n (%) | 20 (29.41%) | 4 (25.00%) | 7 (25.00%) | 9 (37.50%) | >0.9999 | 0.3303 | 0.5575 |
| Myalgia, n (%) | 13 (19.12%) | 1 (6.25%) | 6 (21.43%) | 6 (25.00%) | 0.1854 | 0.7606 | 0.3093 |
| Diarrhea, n (%) | 6 (8.82%) | 0 (0.00%) | 5 (17.86%) | 1 (4.17%) | 0.0726 | 0.1234 | 0.0806 |
| Physiological variables: | |||||||
| Respiratory rate, mean ± SD | 23.3 ± 2.6 | 20.1 ± 2.1 | 22.4 ± 2.5 | 25.1 ± 3.1 | 0.0132 | 0.0090 | <0.0001 |
| O2 saturation, mean ± SD | 92.3 ± 3.9 | 96.3 ± 2.4 | 90.1 ± 4.1 | 82.2 ± 6.4 | <0.0001 | 0.0010 | <0.0001 |
 | |||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Total Study Cohort (n = 68) | Group 1 (Mild) (n = 16) | Group 2 (Moderate) (n = 28) | Group 3 (Severe) (n = 24) | p Value 1 | p Value 2 | p Value 3 |
| MIF 4, pg/mL, mean ± SD | 35,782 ± 14,005 | 18,896 ± 5202 | 31,590 ± 5179 | 51,930 ± 5511 | <0.0001 | <0.0001 | <0.0001 |
| MCP-1 5 (CCL2), pg/mL, mean ± SD | 149.7 ± 33.53 | 150.3 ± 28.69 | 126.8 ± 15.66 | 176.1 ± 32.95 | 0.0010 | <0.0001 | <0.0001 |
| IP-10 6 (CXCL10), pg/mL, mean ± SD | 1213 ± 425.1 | 648.4 ± 151.7 | 1180 ± 235.4 | 1627 ± 209.6 | <0.0001 | <0.0001 | <0.0001 |
| IFN-γ 7, pg/mL, mean ± SD | 148.2 ± 165.5 | 4.87 ± 2.58 | 60.30 ± 20.32 | 346.3 ± 121.1 | <0.0001 | <0.0001 | <0.0001 |
| IL-4 8, pg/mL, mean ± SD | 3.39 ± 0.98 | 2.64 ± 1.17 | 3.42 ± 0.68 | 3.86 ± 0.85 | 0.0081 | 0.0414 | 0.0003 |
| IL-10 9, pg/mL, mean ± SD | 6.06 ± 3.97 | 3.31 ± 1.36 | 4.12 ± 1.41 | 10.17 ± 3.86 | 0.0707 | <0.0001 | <0.0001 |
| IL-13 10, pg/mL, mean ± SD | 5.65 ± 2.91 | 3.87 ± 1.40 | 5.08 ± 2.54 | 7.49 ± 3.13 | 0.0873 | 0.0036 | 0.0001 |
| IL-17 11, pg/mL, mean ± SD | 15.25 ± 10.22 | 5.51 ± 2.91 | 9.81 ± 2.83 | 28.07 ± 4.01 | <0.0001 | <0.0001 | <0.0001 |
| TNF-α 12, pg/mL, mean ± SD | 25.93 ± 20.74 | 1.21 ± 1.57 | 18.40 ± 6.26 | 51.52 ± 6.62 | <0.0001 | <0.0001 | <0.0001 |
 | |||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Total Study Cohort (n = 68) | Group 1 (Mild) (n = 16) | Group 2 (Moderate) (n = 28) | Group 3 (Severe) (n = 24) | p Value 1 | p Value 2 | p Value 3 |
| MIF 4, pg/mL, mean ± SD | 21,048 ± 8396 | 11,258 ± 3031 | 18,084 ± 2782 | 31,035 ± 2968 | <0.0001 | <0.0001 | <0.0001 |
| MCP-1 5 (CCL2), pg/mL, mean ± SD | 45.06 ± 11.01 | 45.27 ± 9.86 | 37.37 ± 3.65 | 53.89 ± 10.96 | 0.0004 | <0.0001 | <0.0001 |
| IP-10 6 (CXCL10), pg/mL, mean ± SD | 246.6 ± 83.23 | 126.8 ± 26.71 | 249.2 ± 46.22 | 323.5 ± 32.48 | <0.0001 | <0.0001 | <0.0001 |
| IFN-γ 7, pg/mL, mean ± SD | 22.51 ± 25.12 | 4.44 ± 2.50 | 8.71 ± 3.23 | 50.65 ± 23.16 | <0.0001 | <0.0001 | <0.0001 |
| IL-4 8, pg/mL, mean ± SD | 1.46 ± 0.33 | 1.37 ± 0.55 | 1.43 ± 0.17 | 1.56 ± 0.27 | 0.6373 | 0.0441 | 0.1946 |
| IL-10 9, pg/mL, mean ± SD | 1.49 ± 0.93 | 0.98 ± 0.31 | 0.93 ± 0.31 | 2.50 ± 0.86 | 0.6264 | <0.0001 | <0.0001 |
| IL-13 10, pg/mL, mean ± SD | 2.20 ± 0.90 | 2.13 ± 0.77 | 2.10 ± 0.96 | 2.37 ± 0.91 | 0.8958 | 0.2957 | 0.5178 |
| IL-17 11, pg/mL, mean ± SD | 7.74 ± 4.20 | 5.23 ± 2.19 | 7.70 ± 3.57 | 9.48 ± 5.08 | 0.0165 | 0.1464 | 0.0059 |
| TNF-α 12, pg/mL, mean ± SD | 13.62 ± 10.13 | 1.50 ± 1.00 | 10.08 ± 3.41 | 25.83 ± 3.42 | <0.0001 | <0.0001 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
László, N.; Mărginean, C.; Mátyás, B.B.; Man, C.A.; Nagy, E.E.; Jimborean, G. Macrophage Migration Inhibitory Factor and Post-Discharge Inflammatory Profiles in Severe COVID-19: A Prospective Observational Study from Romania. Int. J. Mol. Sci. 2025, 26, 9697. https://doi.org/10.3390/ijms26199697
László N, Mărginean C, Mátyás BB, Man CA, Nagy EE, Jimborean G. Macrophage Migration Inhibitory Factor and Post-Discharge Inflammatory Profiles in Severe COVID-19: A Prospective Observational Study from Romania. International Journal of Molecular Sciences. 2025; 26(19):9697. https://doi.org/10.3390/ijms26199697
Chicago/Turabian StyleLászló, Nimród, Corina Mărginean, Botond Barna Mátyás, Cristina Alexandra Man, Előd Ernő Nagy, and Gabriela Jimborean. 2025. "Macrophage Migration Inhibitory Factor and Post-Discharge Inflammatory Profiles in Severe COVID-19: A Prospective Observational Study from Romania" International Journal of Molecular Sciences 26, no. 19: 9697. https://doi.org/10.3390/ijms26199697
APA StyleLászló, N., Mărginean, C., Mátyás, B. B., Man, C. A., Nagy, E. E., & Jimborean, G. (2025). Macrophage Migration Inhibitory Factor and Post-Discharge Inflammatory Profiles in Severe COVID-19: A Prospective Observational Study from Romania. International Journal of Molecular Sciences, 26(19), 9697. https://doi.org/10.3390/ijms26199697









