Ginkgo Biloba and Green Tea Polyphenols Captured into Collagen–Lipid Nanocarriers: A Promising Synergistically Approach for Apoptosis Activation and Tumoral Cell Cycle Arrest
Abstract
1. Introduction
2. Results and Discussion
2.1. Evaluation of the Size and Effectiveness of Individual and Mixed Capture of GBil and GTE + GBil in Various NLCs and Col-NLCs
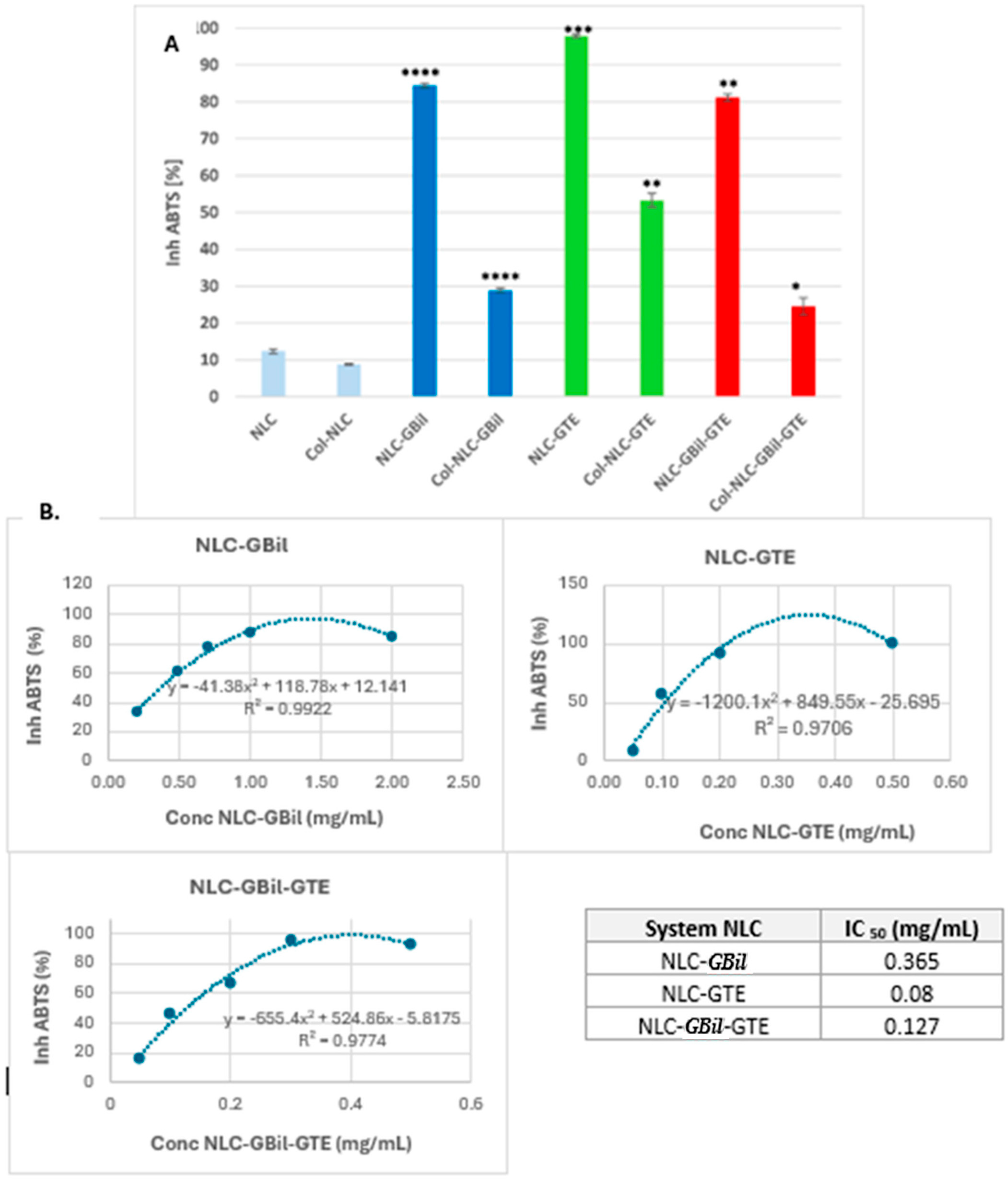
2.2. In Vitro Screening of Antioxidant Activity and Determination of IC50
2.3. Outcomes of In Vitro Antitumoral Effect of NLC- and Col-NLC-GBil-GTE
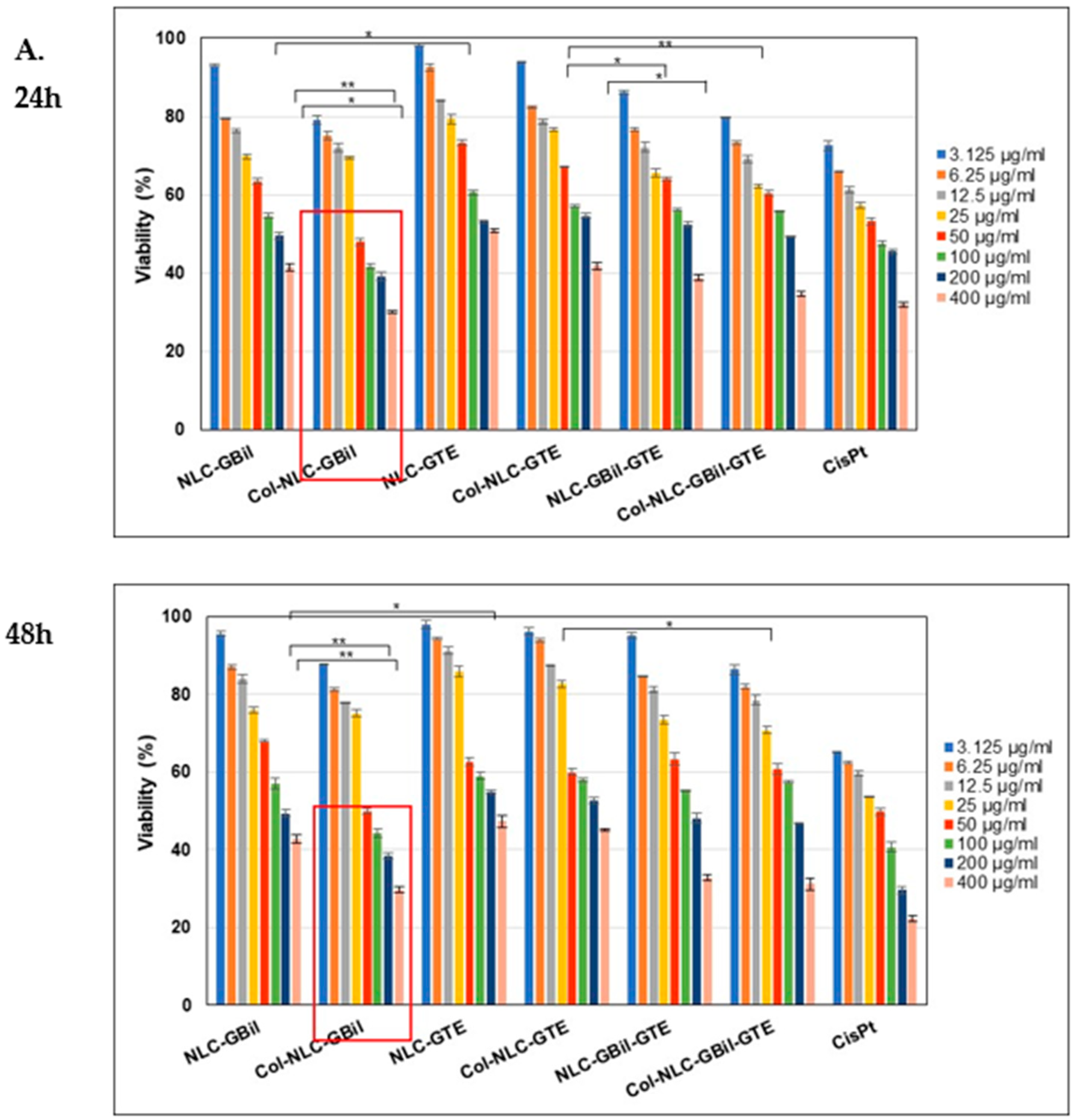
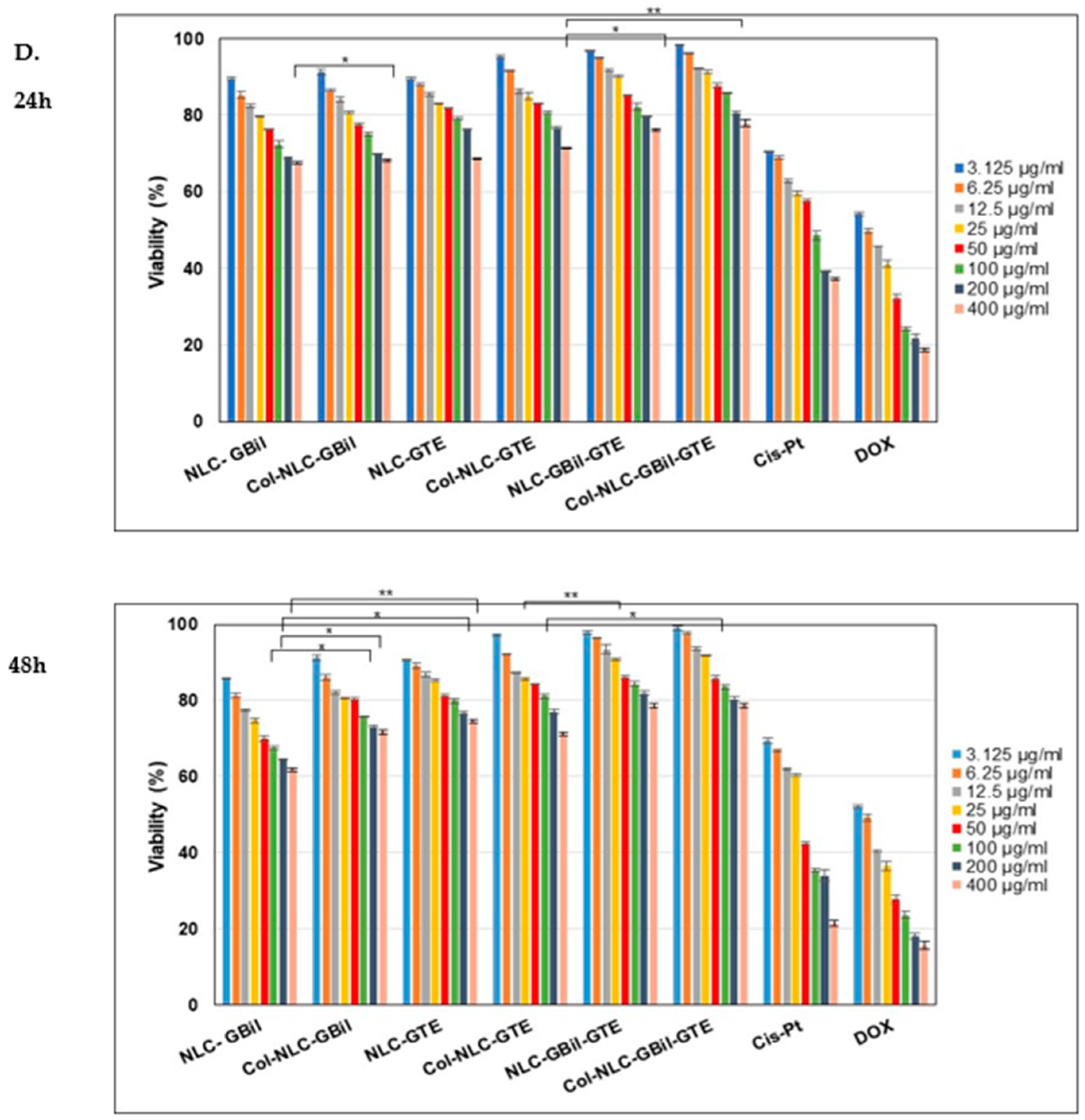
2.3.1. Cell Cytotoxicity Investigations for Different NLC and Col-NLC Phytochemicals Against Various Tumor Cells
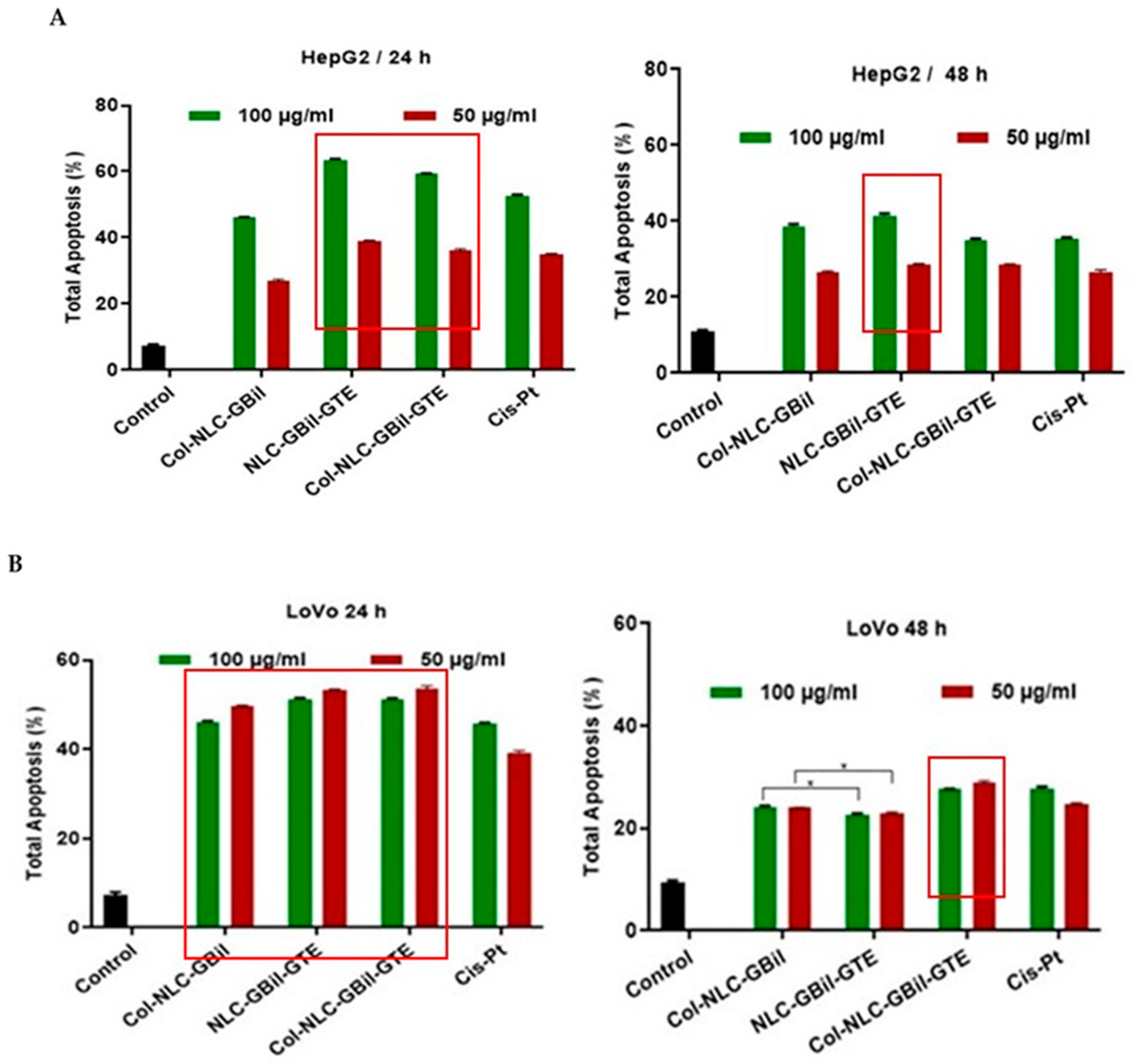
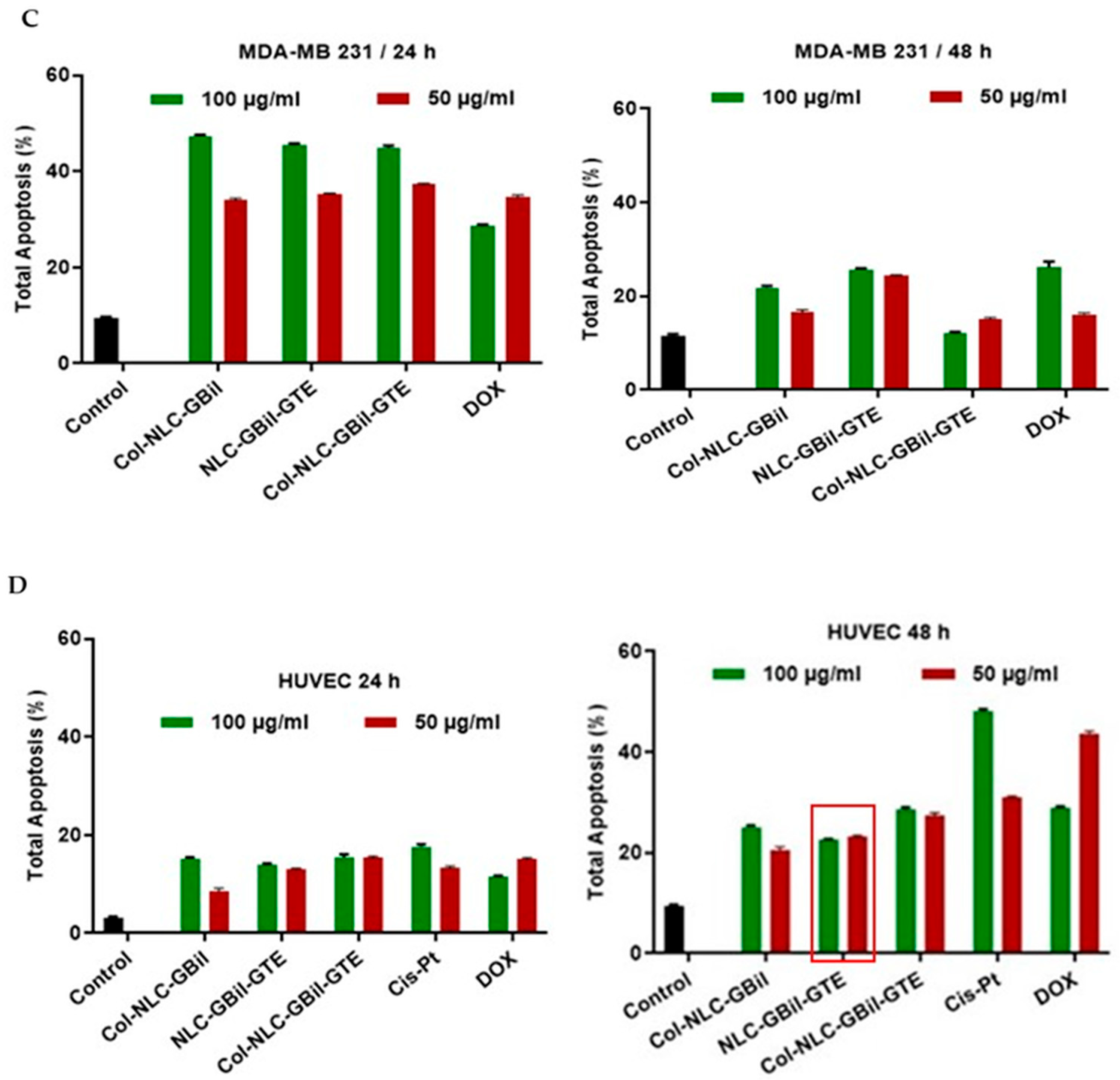
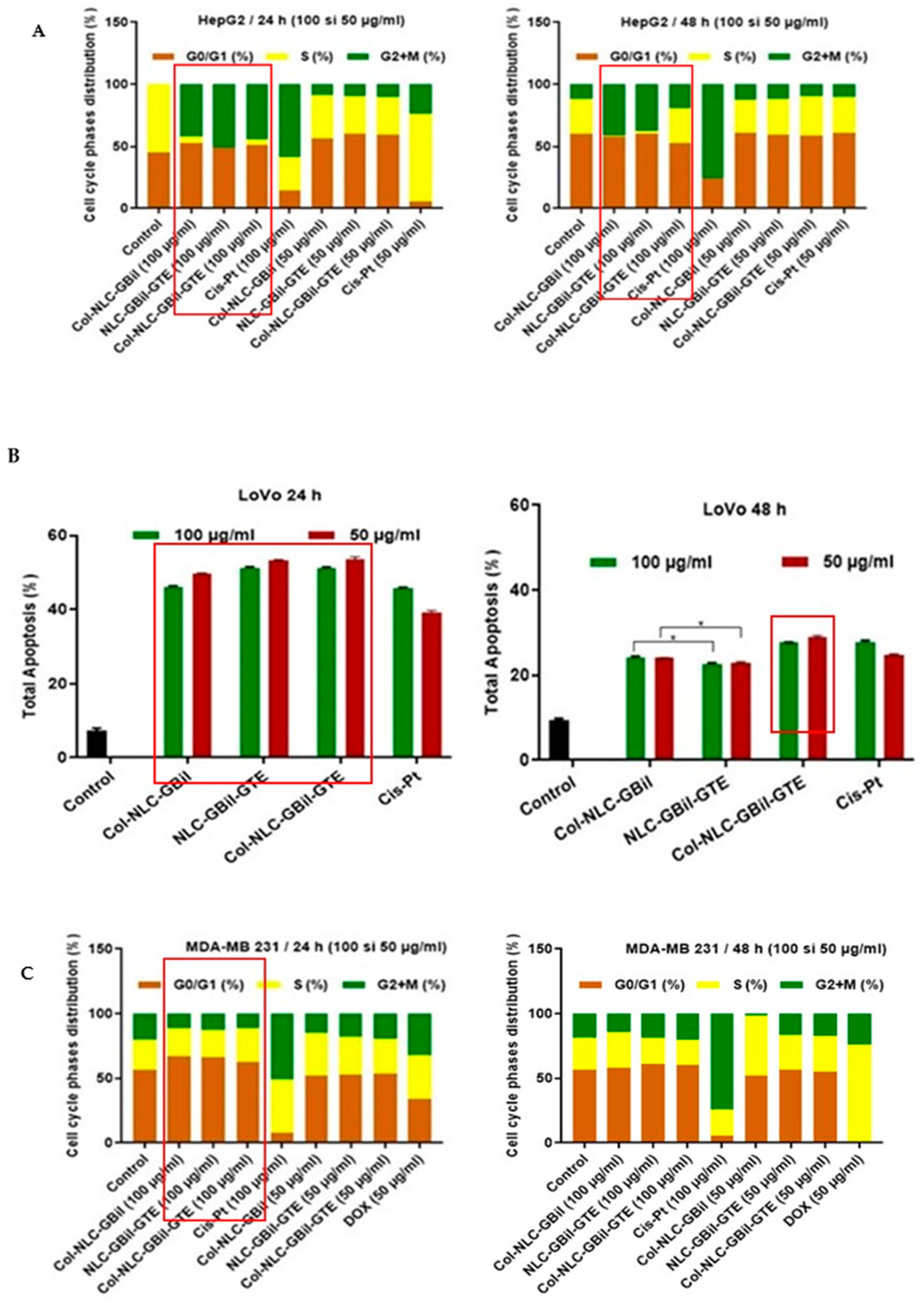
2.3.2. Apoptosis Induced by NLC- and Collagen-Decorated NLC-GBil-GTE
2.3.3. Cell Cycle Arrest by Col-NLC-GBil, NLC- and Col-NLC-GBil-GTE
3. Materials and Methods
3.1. Materials
3.2. Obtaining of NLC and Collagen-Decorated NLC Loaded with GBil and GTE
3.3. Characterization of Developed NLC and Collagen-NLC Formulations
3.3.1. Particle Size Evaluation
3.3.2. Determination of Zeta Potential
3.3.3. Entrapment Efficacy of GBil and Green Tea Extract
3.3.4. In Vitro Determination of Antioxidant Activity
3.3.5. Cell Cultures and Treatments
3.3.6. Assessment of Compounds’ Cytotoxicity by MTS Assay
3.3.7. Apoptosis Analysis by Flow Cytometry
3.3.8. Cell Cycle Evaluation Using Flow Cytometry
3.3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeinali, M.; Abbaspour-Ravasjani, S.; Ghorbani, M.; Babazadeh, A.; Soltanfam, T.; Santos, A.C.; Hamishehkar, H.; Hamblin, M.R. Nanovehicles for co-delivery of anticancer agents. Drug Discov. Today 2020, 25, 1416. [Google Scholar] [CrossRef] [PubMed]
- Alkhamach, D.; Khan, S.A.; Haider, M. Nanostructured lipid carriers in cancer therapy: Advances in passive and active targeting strategies. Int. J. Pharm. 2025, 678, 125736. [Google Scholar] [CrossRef]
- Paiva-Santos, A.C.; Silva, A.L.; Guerra, C.; Peixoto, D.; Pereira-Silva, M.; Zeinali, M.; Mascarenhas-Melo, F.; Castro, R.; Veiga, F. Ethosomes as Nanocarriers for the Development of Skin Delivery Formulations. Pharm. Res. 2021, 38, 947–970. [Google Scholar] [CrossRef]
- Zhu, Y.; Fan, Y.; Luo, S.; Zhang, L. Solid lipid nanoparticles as a promising delivery system for natural products: A review. Food Chem. 2022, 16, 100486. [Google Scholar]
- Lacatusu, I.; Iordache, T.A.; Mihaila, M.; Mihaiescu, D.E.; Pop, A.L.; Badea, N. Multifaceted role of dual herbal principles loaded-lipid nanocarriers in providing high therapeutic efficacy. Pharmaceutics 2021, 13, 1511. [Google Scholar] [CrossRef]
- Gunawan, M.; Boonkanokwong, V. Current applications of solid lipid nanoparticles and nanostructured lipid carriers as vehicles in oral delivery systems for antioxidant nutraceuticals: A review. Colloids Surf. B 2024, 233, 113608. [Google Scholar] [CrossRef]
- Shukla, S.; Mishra, A.K. Encapsulation of natural products in lipid-based nanocarriers for enhanced therapeutic efficacy: A review. Curr. Pharm. Des. 2020, 26, 2133–2150. [Google Scholar]
- Lacatusu, I.; Istrati, D.; Bordei, N.; Popescu, M.; Seciu, A.M.; Panteli, L.M.; Badea, N. Synergism of plant extract and vegetable oils-based lipid nanocarriers: Emerging trends in development of advanced cosmetic prototype products. Mater. Sci. Eng. C 2020, 108, 110412. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.S.; Silva, J.O.; Mussi, S.V.; Lopes, S.C.A.; Leite, E.A.; Cassali, G.D.; Cardoso, V.N.; Townsend, D.M.; Colletti, P.M.; Ferreira, L.A.M.; et al. Nanostructured Lipid Carrier Co-loaded with Doxorubicin and Docosahexaenoic Acid as a Theranostic Agent: Evaluation of Biodistribution and Antitumor Activity in Experimental Model. Mol. Imaging Biol. 2018, 20, 437–447. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Akbari, M. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Novel Drug Delivery Systems: A Review. J. Pharm. Sci. Res. 2017, 9, 2291–2299. [Google Scholar]
- Rajoriya, V.; Gupta, R.; Vengurlekar, S. Nanostructured lipid carriers (NLCs): A promising candidate for lung cancer targeting. Int. J. Pharm. 2024, 655, 123986. [Google Scholar] [CrossRef]
- Tincu, R.; Mihaila, M.; Bostan, M.; Istrati, D.; Badea, N.; Lacatusu, I. Hybrid Albumin Decorated Lipid-Nanocarrier Mediated Delivery of Polyphenol Rich Sambucus nigra L. in a Potential Multiple Antitumoral Therapy. Int. J. Mol. Sci. 2024, 25, 11206. [Google Scholar] [CrossRef]
- Boateng, I.D. Polyprenol, in Ginkgo biloba: A review of their chemistry (synthesis of polyprenols and their derivatives), extraction, purification, and bioactivities. Food Chem. 2023, 418, 136006. [Google Scholar] [CrossRef]
- Tabassum, N.-E.; Das, R.; Lami, M.S.; Chakraborty, A.J.; Mitra, S.; Tallei, T.E.; Idroes, R.; Mohamed, A.A.; Hossain, M.J.; Dhama, K.; et al. Ginkgo biloba: A Treasure of Functional Phytochemicals with Multimedicinal Applications. Evid.-Based Complement. Altern. Med. 2022, 2022, 8288818. [Google Scholar]
- Sharma, M.; Kumar, R. Ginkgo biloba: A comprehensive review of its phytochemistry, pharmacology, and clinical applications. J. Ethnopharm. 2020, 260, 112959. [Google Scholar]
- Akaberi, M.; Baharara, H.; Emami, S.A. Ginkgo biloba: An updated review on pharmacological, ethnobotanical, and phytochemical studies. Pharmacol. Res.-Mod. Chin. Med. 2023, 9, 100331. [Google Scholar] [CrossRef]
- Biernacka, P.; Adamska, I.; Felisiak, K. The Potential of Ginkgo biloba as a source of biologically active compounds—A Review of the Recent Literature and Patents. Molecules 2023, 28, 3993. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, Y.M.; Kim, D.S. Ginkgo biloba extract EGb 761 induces apoptosis in human leukaemia cells via activation of caspase-3 and down-regulation of Bcl-2. Biol. Pharm. Bull. 2004, 27, 1904–1907. [Google Scholar]
- Xu, Y.; Ma, Q.; Chen, D.; Yin, Q.; Zhang, W. Effects of Ginkgolide B on the Proliferation and Apoptosis of Cervical Cancer Cells. Curr. Top. Nutraceutical Res. 2020, 18, 227. [Google Scholar] [CrossRef]
- Huang, C.; Fang, G.; Lu, Y.; Wang, P.; Li, G.; Zhu, X. Ginkgo biloba extract EGb 761 inhibits colorectal cancer cell growth and induces apoptosis via the Wnt/β-catenin signaling pathway. J. Ethnopharm. 2018, 218, 178–185. [Google Scholar]
- Zhou, Y.; Yang, J.; Duan, Y.; Wang, J. Ginkgo biloba extract inhibits the proliferation of human ovarian cancer cells and induces apoptosis. J. Ethnopharm. 2011, 136, 268–275. [Google Scholar]
- Engel, S.; Dengler, J.; Graf, C. Effect of Ginkgo biloba extract EGb 761 on glioblastoma multiforme cell lines: An in vitro study. J. Neuro Oncol. 2011, 103, 1–9. [Google Scholar]
- Wang, Y.-Y.; Li, R.-H.; Sun, X.; Xia, Z.-M.; Zhang, G.-J.; Li, B.; Tian, Y.; Li, M.; Liu, S.-C. Comparative analysis of phytochemical constituents of Ginkgo biloba flowers and leaves and evaluation of their biological activities. Fitoterapia 2025, 185, 106673. [Google Scholar] [CrossRef]
- Menezes, J.C.J.M.D.S.; Diederich, M.F. Bioactivity of natural bioflavonoids in metabolism-related disease and cancer therapies. Pharmacol. Res. 2021, 167, 105525. [Google Scholar] [PubMed]
- Silva-Pinto, P.A.; Costa de Pontes, J.T.; Aguilar-Morón, B.; Carnero Canales, C.S.; Pavan, F.R.; Roque-Borda, C.A. Phytochemical insights into flavonoids in cancer: Mechanisms, therapeutic potential, and the case of quercetin. Helyon 2025, 11, e42682. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kang, S.; Go, G.-W. Exploring the multifaceted role of ginkgolides and bilobalide from Ginkgo biloba in mitigating metabolic disorders. Food Sci. Biotechnol. 2024, 33, 2903–2917. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Wang, Y.; Liu, X. Preparation and characterization of Ginkgo biloba extract-loaded solid lipid nanoparticles and their in vitro antioxidant activity. J. Drug Deliv. Sci. Technol. 2023, 85, 104592. [Google Scholar]
- Zhao, H.; Chen, J.; Li, Y.; Zhang, Q. Optimization of Ginkgo biloba extract-loaded nanostructured lipid carriers: Preparation, characterization, and in vitro antioxidant evaluation. J. Pharm. Sci. 2023, 112, 1543–1552. [Google Scholar]
- Wang, L.; Sun, P.; Li, X.; Gao, Y. Development and characterization of Ginkgo biloba extract-loaded solid lipid nanoparticles for improved oral bioavailability and antioxidant efficacy. RSC Adv. 2021, 11, 12247–12256. [Google Scholar]
- Jammanesh, A.; Bidgoli, S.A.; Ghaffari, S.; Avadi, M.R. Formulation, characterization and toxicity assessment of Ginkgo biloba extract solid lipid nanoparticle in female mice. Nanomed. Res. J. 2021, 6, 28–40. [Google Scholar]
- Liu, M.; Zhang, R.; Yu, H.; Wang, H. Enhanced antioxidant and anti-inflammatory activities of Ginkgo biloba flavonoids co-encapsulated in nanostructured lipid carriers. Int. J. Nanomed. 2022, 17, 4567–4580. [Google Scholar]
- Zhang, L.; Wang, Q.; Li, H.; Liu, P. In vitro antioxidant and anti-apoptotic effects of Ginkgo biloba extract encapsulated in novel lipid nanoparticles on oxidative stress-induced cells. Eur. J. Pharm. Sci. 2024, 196, 106720. [Google Scholar]
- Park, S.H.; Kim, J.H.; Lee, S.J. Antioxidant activity and neuroprotective effects of Ginkgo biloba extract-loaded nanostructured lipid carriers against oxidative stress in PC12 cells. J. Nanosci. Nanotechnol. 2020, 20, 6296–6304. [Google Scholar]
- Wang, Y.; Chen, Y.; Liu, D.; Zhang, S.; Zhang, Y. Solid lipid nanoparticles loaded with Ginkgo biloba extract (EGb 761) for breast cancer therapy. Int. J. Pharm. 2016, 506, 173–181. [Google Scholar]
- Yang, C.S.; Landau, J.M. Effects of tea consumption on nutrition and health. J. Nutr. 2000, 130, 2409S–2412S. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Tea polyphenols for health promotion. Life Sci. 2018, 194, 18–22. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, Z.; Wu, Z. A review on anti-cancer effect of green tea catechins. J. Funct. Foods 2020, 74, 104172. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.-Q.; Zhang, Q.; Zhu, J.-Y.; Li, Y.; Xie, C.-F.; Li, X.-T.; Wu, J.-S.; Geng, S.-S.; Zhong, C.-Y.; et al. (−)-Epigallocatechin-3-Gallate Inhibits Colorectal Cancer Stem Cells by Suppressing Wnt/β-Catenin Pathway. Nutrients 2017, 9, 572. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, X.; Lu, G.; Picinich, S.C. Cancer prevention by tea: Animal studies, molecular mechanisms and human efforts. J. Natl. Cancer Inst. 2009, 101, 1709–1719. [Google Scholar]
- Sehgal, A.; Bhat, M.A.; Dogra, D.; Rawat, S.; Dhatwalia, S.K. EGCG: The antioxidant powerhouse in lung cancer management and chemotherapy enhancement. Adv. Redox Res. 2023, 9, 100085. [Google Scholar] [CrossRef]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.J.; Bailey, H.H.; Mukhtar, H. Green tea polyphenols for prostate cancer chemoprevention: A translational perspective. Phytomedicine 2010, 17, 3–13. [Google Scholar] [CrossRef]
- Yin, Z.; Zheng, T.; Zhang, M. Improving the stability and bioavailability of tea polyphenols by encapsulations: A review. Food Sci. Hum. Well. 2022, 11, 537–556. [Google Scholar] [CrossRef]
- Shtay, R.; Keppler, J.K.; Schwarz, K. Encapsulation of (─)-epigallocatechin-3-gallate (EGCG) in solid lipid nanoparticles for food applications. J. Food Eng. 2019, 244, 91–100. [Google Scholar] [CrossRef]
- Prathumwon, C.; Anuchapreeda, S.; Ampasavate, C. Curcumin and EGCG combined formulation in nanostructured lipid carriers for anti-aging applications. Int. J. Pharm. 2025, 9, 100323. [Google Scholar]
- Sartaj, A.; Alam, M.; Biswas, L.; Yar, M.S.; Mir, S.R.; Verma, A.K.; Baboota, S.; Ali, J. Combinatorial delivery of Ribociclib and green tea extract mediated nanostructured lipid carrier for oral delivery for the treatment of breast cancer synchronising in silico, in vitro, and in vivo studies. J. Drug Target. 2022, 30, 1113–1134. [Google Scholar] [CrossRef]
- Verma, A.K. An Insight into Collagen-Based Nano Biomaterials for Drug Delivery Applications. In Engineered Biomaterials. Engineering Materials; Malviya, R., Sundram, S., Eds.; Springer: Singapore, 2023; pp. 397–428. [Google Scholar] [CrossRef]
- Tezgel, Ö.; Di Stasio, N.; Laghezza-Masci, V.; Taddei, A.-R.; Szarpak-Jankowska, A.; Auzély Velty, R.; Navarro, F.P.; Texier, I. Collagen scaffold-mediated delivery of NLC/siRNA as wound healing materials. J. Drug Deliv. Sci. Technol. 2020, 55, 101421. [Google Scholar]
- Laghezza Masci, V.; Taddei, A.-R.; Courant, T.; Tezgel, O.; Navarro, F.; Giorgi, F.; Mariolle, D.; Fausto, A.-M.; Texier, I. Characterization of collagen/lipid nanoparticle-curcumin cryostructurates for wound healing applications. Macromol. Biosci. 2019, 19, 1800446. [Google Scholar]
- Krishnamoorthy, G.; Stephen, P.; Prabhu, M.; Sehgal, P.K.; Sadulla, S. Collagen-coated nanoliposome as a targeted and controlled drug delivery system. AIP Conf. Proc. 2010, 1276, 163–168. [Google Scholar]
- Kusnadi, K.; Muchtaridi, Y.H. Collagen-based nanoparticles as a drug delivery system in wound healing applications. Int. J. Nanomed. 2024, 6, 11321–11341. [Google Scholar] [CrossRef]
- Oboulbiga, E.B.; Douamba, Z.; Compaoré-Sérémé, D.; Semporé, J.N.; Dabo, R.; Semde, Z.; Tapsoba, F.W.-B.; Hama-Ba, F.; Songré-Ouattara, L.T.; Parkouda, C.; et al. Physicochemical, potential nutritional, antioxidant and health properties of sesame seed oil: A review. Front. Nutr. 2023, 10, 1127926. [Google Scholar] [CrossRef] [PubMed]
- Ellinger, S.; Stehle, P. Impact of Cocoa Consumption on Inflammation Processes—A Critical Review of Randomized Controlled Trials. Nutrients 2016, 8, 321. [Google Scholar] [CrossRef]
- Lacatusu, I.; Balanuca, B.; Serafim, A.; Ott, C.; Prodana, M.; Badea, N. Salicin and Hederacoside C-Based Extracts and UV-Absorbers Co-Loaded into Bioactive Lipid Nanocarriers with Promoted Skin Antiaging and Hydrating Efficacy. Nanomaterials 2022, 12, 2362. [Google Scholar]
- Hu, F.Q.; Jiang, S.-P.; Du, Y.-Z.; Yuan, H.; Ye, Y.-Q.; Zeng, S. Preparation and characterization of stearic acid nanostructured lipid carriers by solvent diffusion method in an aqueous system. Colloids Surf. B Biointerfaces 2005, 45, 167–173. [Google Scholar] [CrossRef]
- Sarma, A.; Bania, R.; Das, M.K. Green tea: Current trends and prospects in nutraceutical and pharmaceutical aspects. J. Herbal. Med. 2023, 41, 100694. [Google Scholar]
- Yi, H.-Y.; Chun, J.-Y. Sargassum horneri extracts loaded nanostructured lipid carriers: Preparation, physicochemical properties, and in vitro antioxidant activity studies. Food Biosci. 2023, 53, 102804. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Total phenolic and flavonoid content, antioxidant activities and HPLC analysis of Garcinia mangostana Linn. extracts and their interaction with bovine serum albumin. Food Chem. 2014, 142, 337–345. [Google Scholar]
- De Rijke, E.; Zandtra, A.G.; Beekmann, C.J. Protein-polyphenol interactions: A review of the state of the art and future directions. Food Hydrocoll. 2006, 20, 297–308. [Google Scholar]
- Rana, J.N.; Gul, K.; Mumtaz, S. Isorhamnetin: Reviewing Recent Developments in Anticancer Mechanisms and Nanoformulation-Driven Delivery. Int. J. Mol. Sci. 2025, 26, 7381. [Google Scholar] [CrossRef]
- Butler, L.M.; Ferraldeschi, R.; Armstrong, H.K.; Centenera, M.M.; Workman, P. Maximizing the therapeutic potential of HSP90 inhibitors. Mol. Cancer Res. 2015, 13, 1445–1451. [Google Scholar] [CrossRef]
- Menezes, J.C.J.M.D.S.; Orlikova, B.; Morceau, F.; Diederich, M. Natural and synthetic flavonoids: Structure–activity relationship and chemotherapeutic potential for the treatment of leukemia. Crit. Rev. Food Sci. Nutr. 2016, 56, S4–S28. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Han, Y.; He, W.; Liang, F. Amentoflavone protects against high fat-induced metabolic dysfunction: Possible role of the regulation of adipogenic differentiation. Int. J. Mol. Med. 2016, 38, 1759–1767. [Google Scholar] [CrossRef]
- Xiong, B.; Lu, J.-J.; Guo, H.; Huang, M.; Li, T. Ginkgetin from Ginkgo biloba: Mechanistic insights into anticancer efficacy. Nat. Prod. Bioprospect. 2025, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, J.; Yang, J.; Ouyang, T.; Gao, H.; Kan, H.; Yang, Y. New insight into the mechanisms of Ginkgo biloba leaves in the treatment of cancer. Phytomedicine 2024, 122, 155088. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wang, Z.; Huang, W.; Zhao, X.; Xu, L.; Teng, C.; Li, Y. Hyaluronic acid-decorated lipid nanocarriers as novel vehicles for curcumin: Improved stability, cellular absorption, and anti-inflammatory effects. Food Chem. 2025, 463, 141420. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, H.; Zhang, S.; Zhang, Y.; Wei, R. Liposomes containing active constituents from Ginkgo biloba for liver cancer therapy: Preparation, characterization, and in vitro/in vivo evaluation. J. Liposome Res. 2019, 29, 74–84. [Google Scholar]
- Li, Y.; Feng, Z.; Shim, J.S. Green tea catechins as anticancer agents: Molecular targets and mechanisms. Mini Rev. Med. Chem. 2013, 13, 173–191. [Google Scholar]
- Zeng, L.; Yan, J.; Luo, L.; Ma, M.; Zhu, H. Preparation and characterization of (-)-epigallocatechin-3-gallate (EGCG)-loaded nanoparticles and their inhibitory effects on Human breast cancer MCF-7 cells. Sci. Rep. 2017, 7, 45521. [Google Scholar] [CrossRef]
- Nishikawa, T.; Nakajima, T.; ∙Moriguchi, M.; Jo, M.; Sekoguchi, S.; Ishii, M.; Takashima, H.; ∙Katagishi, T.; ∙Kimura, H.; Minami, M.; et al. A green tea polyphenol, epigalocatechin-3-gallate, induces apoptosis of human hepatocellular carcinoma, possibly through inhibition of Bcl-2 family proteins. J. Hepatol. 2006, 44, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Karavelioglu, Z.; Cakir-Koc, R. Preparation of chitosan nanoparticles as Ginkgo biloba extract carrier: In vitro neuroprotective effect on oxidative stress-induced human neuroblastoma cells (SH-SY5Y). Int. J. Biol. Macromol. 2021, 192, 675–683. [Google Scholar] [CrossRef]
- Chen, D.; Sun, S.; Cai, D.; Kong, G. Induction of mitochondrial-dependent apoptosis in T24 cells by a selenium (Se)-containing polysaccharide from Ginkgo biloba L. leaves. Int. J. Biol. Macromol. 2017, 101, 126–130. [Google Scholar] [CrossRef]
- Dong, C.; Wang, Z.; Shen, P.; Chen, Y.; Wang, J.; Wang, H. Epigallocatechin-3-gallate suppresses the growth of human osteosarcoma by inhibiting the Wnt/β-catenin signalling pathway. Bioengineered 2022, 13, 8490–8502. [Google Scholar] [CrossRef] [PubMed]
- Thipe, V.C.; Hal, L.N.; Pandurangi, A.; Ajayi, S.; Emeh, P.; Gauttam, I.; Ghamgui, R.; Hameedat, F.; Khelil, S.; Ly, N.K.; et al. Nano-ayurvedic medicine approaches using ginkgo biloba-phytochemicals functionalized gold nanoparticles against breast cancer. Nanotechnol. Sci. Appl. 2024, 17, 189–210. [Google Scholar]
- Mayr, C.; Wagner, A.; Neureiter, D.; Pichler, M.; Jakab, M.; Illig, R.; Berr, F.; Kiesslich, T. The green tea catechin epigallocatechin gallate induces cell cycle arrest and shows potential synergism with cisplatin in biliary tract cancer cells. BMC Complement. Altern. Med. 2015, 15, 194. [Google Scholar] [CrossRef]
- Nesran, Z.N.M.; Shafie, N.R.H.; Ishak, A.H.; Esa, N.M.; Ismail, A.; Tohid, S.F.M. Induction of Endoplasmic Reticulum Stress Pathway by Green Tea Epigallocatechin-3-Gallate (EGCG) in Colorectal Cancer Cells: Activation of PERK/p-eIF2α/ATF4 and IRE1α. BioMed Res. Int. 2019, 2019, 3480569. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, L.; Yin, W.; Nie, Y.; Zeng, P.; Yang, X. Anti-tumor effect of ginkgetin on human hepatocellular carcinoma cell lines by inducing cell cycle arrest and promoting cell apoptosis. Cell Cycle. 2022, 21, 74–85. [Google Scholar]
- Yu Bai, Feng Zhao, Yan Li, Lei Wang, Xiang-Jie Fang, Chen-Yu Wang, Ginkgo biloba extract induce cell apoptosis and G0/G1 cycle arrest in gastric cancer cells. Int. J. Clin. Exp. Med. 2015, 8, 20977–20982.
- Meo, F.D.; Cuciniello, R.; Margarucci, S.; Bergamo, P.; Petillo, O.; Peluso, G.; Filosa, S.; Crispi, S. Ginkgo biloba Prevents Oxidative Stress-Induced Apoptosis Blocking p53 Activation in Neuroblastoma Cells. Antioxidants 2020, 9, 279. [Google Scholar] [CrossRef]
- Li, X.; Zhou, M.; Zhu, Z.; Wang, Z.; Zhang, X.; Lu, L.; Xie, Z.; Wang, B.; Pan, Y.; Zhang, J.; et al. Kaempferol from Alpinia officinarum hance induces G2/M cell cycle arrest in hepatocellular carcinoma cells by regulating the ATM/CHEK2/KNL1 pathway. J. Ethnopharm. 2024, 333, 118430. [Google Scholar]
- Shen, X.; Zhang, Y.; Feng, Y.; Zhang, L.; Li, J.; Xie, Y.-A.; Luo, X. Epigallocatechin-3-gallate inhibits cell growth, induces apoptosis and causes S phase arrest in hepatocellular carcinoma by suppressing the AKT pathway. Int. J. Oncol. 2014, 44, 791–796. [Google Scholar] [CrossRef]
- Lacatusu, I.; Arsenie, L.V.; Badea, G.; Popa, O.; Oprea, O.; Badea, N. New cosmetic formulations with broad photoprotective 884 and antioxidative activities designed by amaranth and pumpkin seed oils nanocarriers. Ind. Crops Prod. 2018, 123, 424–433. [Google Scholar] [CrossRef]
- Niculae, G.; Lacatusu, I.; Badea, N.; Oprea, O.; Meghea, A. Optimization of lipid nanoparticles composition for sunscreen encapsulation. U.P.B. Sci. Bull. Ser. B 2013, 75, 79–92. [Google Scholar]
- Ivan, I.M.; Olaru, O.T.; Popovici, V.; Chițescu, C.L.; Popescu, L.; Luță, E.A.; Ilie, E.I.; Brașoveanu, L.I.; Hotnog, C.M.; Nițulescu, G.M.; et al. Antioxidant and Cytotoxic Properties of Berberis vulgaris (L.) Stem Bark Dry Extract. Molecules 2024, 29, 2053. [Google Scholar] [CrossRef]
- Tincu, R.; Mihaila, M.; Bostan, M.; Teodorescu, F.; Istrati, D.; Badea, N.; Lacatusu, I. Novel bovine serum albumin decorated–nanostructured lipid carriers able to modulate apoptosis and cell cycle response in ovarian, breast, and colon tumoral cells. Pharmaceutics 2023, 15, 1125. [Google Scholar] [CrossRef]
- Stecoza, C.E.; Nitulescu, G.M.; Draghici, C.; Caproiu, M.T.; Hanganu, A.; Olaru, O.T.; Mihai, D.P.; Bostan, M.; Mihaila, M. Synthesis of 1,3,4-Thiadiazole Derivatives and Their Anticancer Evaluation. Int. J. Mol. Sci. 2023, 24, 17476. [Google Scholar] [CrossRef]



| NLC and Collagen-Decorated NLC | EE% |
|---|---|
| NLC-GBil | 72.30 ± 0.74 |
| Col-NLC-GBil | 71.39 ± 1.76 |
| NLC-GTE | 86.75 ± 1.21 |
| Col-NLC-GTE | 83.59 ± 0.62 |
| NLC-GBil-GTE | 85.17 ± 0.67 |
| Col-NLC-GBil-GTE | 81.91 ± 1.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihaila, M.; Badea, N.; Birliga, M.; Bostan, M.; Albu Kaya, M.G.; Lacatusu, I. Ginkgo Biloba and Green Tea Polyphenols Captured into Collagen–Lipid Nanocarriers: A Promising Synergistically Approach for Apoptosis Activation and Tumoral Cell Cycle Arrest. Int. J. Mol. Sci. 2025, 26, 9648. https://doi.org/10.3390/ijms26199648
Mihaila M, Badea N, Birliga M, Bostan M, Albu Kaya MG, Lacatusu I. Ginkgo Biloba and Green Tea Polyphenols Captured into Collagen–Lipid Nanocarriers: A Promising Synergistically Approach for Apoptosis Activation and Tumoral Cell Cycle Arrest. International Journal of Molecular Sciences. 2025; 26(19):9648. https://doi.org/10.3390/ijms26199648
Chicago/Turabian StyleMihaila, Mirela, Nicoleta Badea, Marionela Birliga, Marinela Bostan, Madalina Georgiana Albu Kaya, and Ioana Lacatusu. 2025. "Ginkgo Biloba and Green Tea Polyphenols Captured into Collagen–Lipid Nanocarriers: A Promising Synergistically Approach for Apoptosis Activation and Tumoral Cell Cycle Arrest" International Journal of Molecular Sciences 26, no. 19: 9648. https://doi.org/10.3390/ijms26199648
APA StyleMihaila, M., Badea, N., Birliga, M., Bostan, M., Albu Kaya, M. G., & Lacatusu, I. (2025). Ginkgo Biloba and Green Tea Polyphenols Captured into Collagen–Lipid Nanocarriers: A Promising Synergistically Approach for Apoptosis Activation and Tumoral Cell Cycle Arrest. International Journal of Molecular Sciences, 26(19), 9648. https://doi.org/10.3390/ijms26199648







