Dietary and Pharmacological Modulation of Aging-Related Metabolic Pathways: Molecular Insights, Clinical Evidence, and a Translational Model
Abstract
1. Introduction
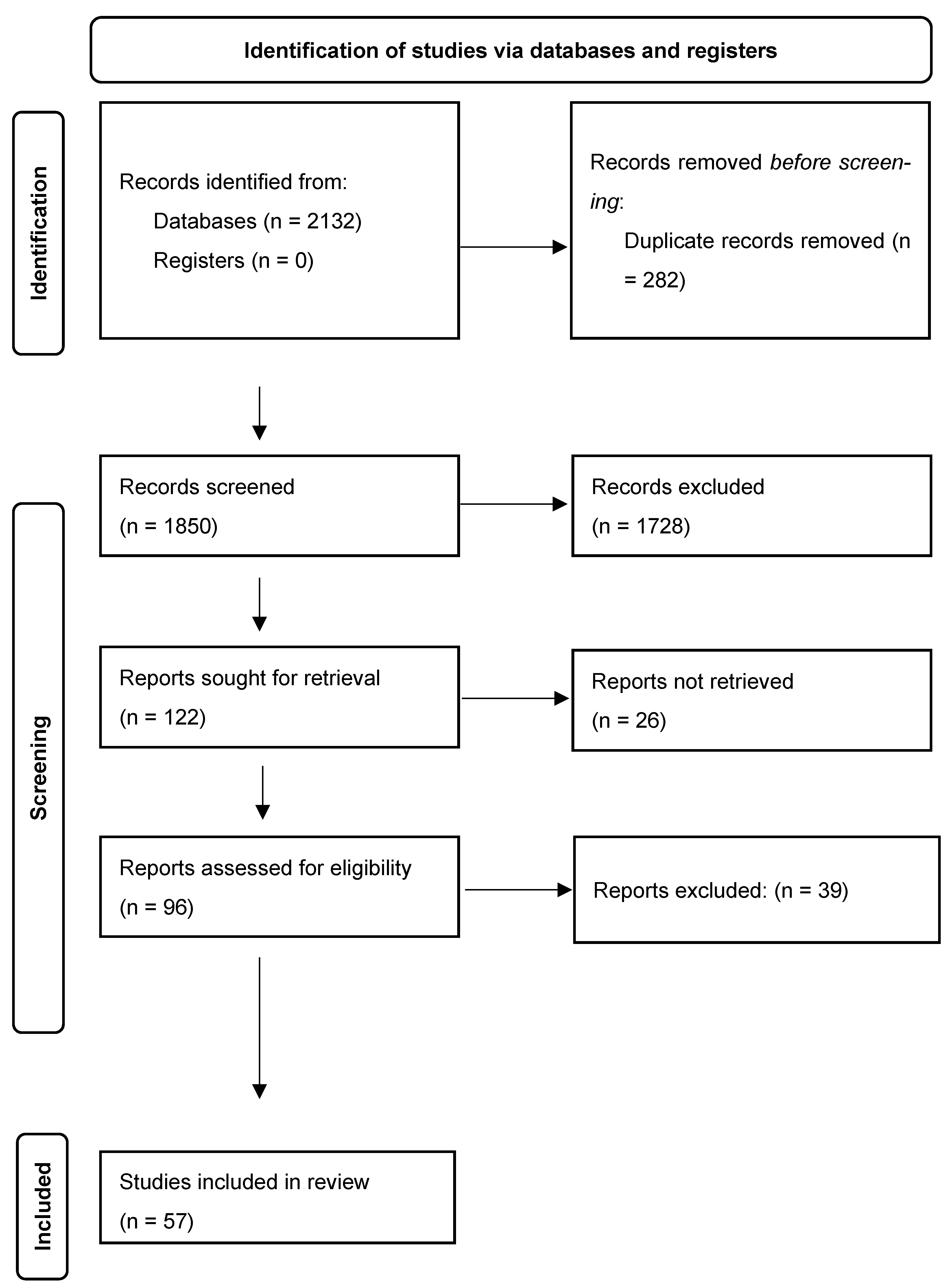
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
- Clinical studies in humans and systematic reviews with a comparative focus on caloric restriction (CR), intermittent fasting (IF), or CR mimetics (e.g., metformin, resveratrol, rapamycin, spermidine, FMD) with defined protocols.
- Preclinical studies only if they provide a straightforward pathophysiological extrapolation to humans.
- Reporting of molecular mechanisms (mTOR, AMPK, SIRT, IGF-1, autophagy) and/or biomarkers (epigenetic, transcriptomic, metabolomic, or clinically relevant).
- Animal studies without clinical or pathophysiological extrapolation to humans.
- Opinion papers, editorials, letters to the editor, and conference abstracts.
- Reviews lacking a critical component or comparative analysis between CR, IF, and mimetics.
- Articles focused exclusively on other diets (e.g., ketogenic, DASH, Mediterranean) without molecular links to longevity mechanisms.
2.4. Selection Process
- Level of evidence and type of population studied.
- Methodological quality, replicability, and control of confounding variables.
- Clinical relevance of outcomes and biomarkers.
- Consistency between described molecular mechanisms and therapeutic applicability.
- Suitability for current clinical contexts and potential for intervention personalization.
2.5. Methodological Limitations
- High heterogeneity among study designs, populations, and outcomes.
- Limited availability of longitudinal studies with robust clinical biomarkers.
- Lack of standardization in the definition of interventions (CR, IF, mimetics).
- Possible overlap of interventions or outcomes among studies.
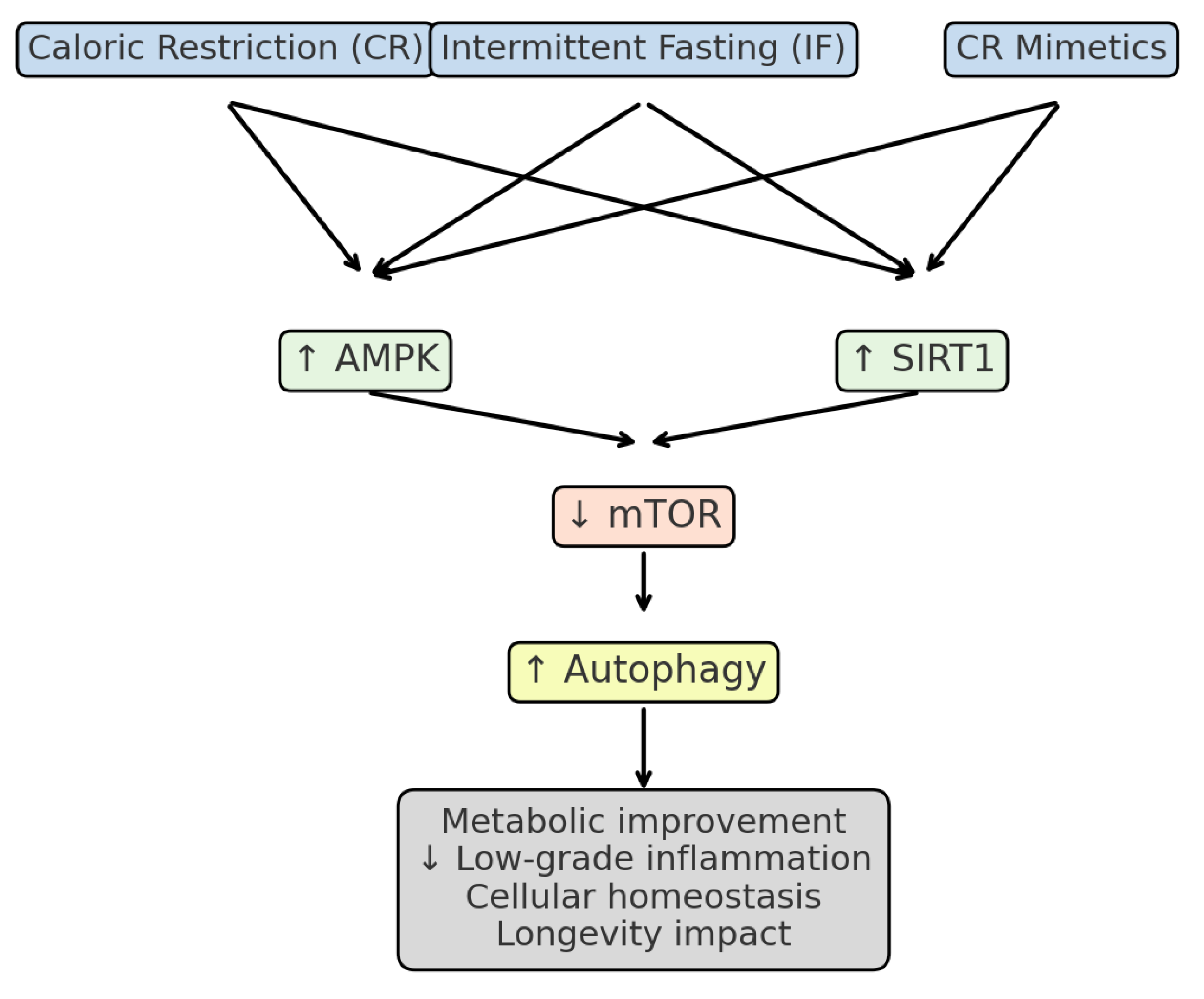
3. Synthesis of the Evidence
3.1. Caloric Restriction (CR)
3.2. Clinical Evidence of Intermittent Fasting (IF)
3.3. Clinical Evidence of Caloric Restriction Mimetics (CR-Mimetics)
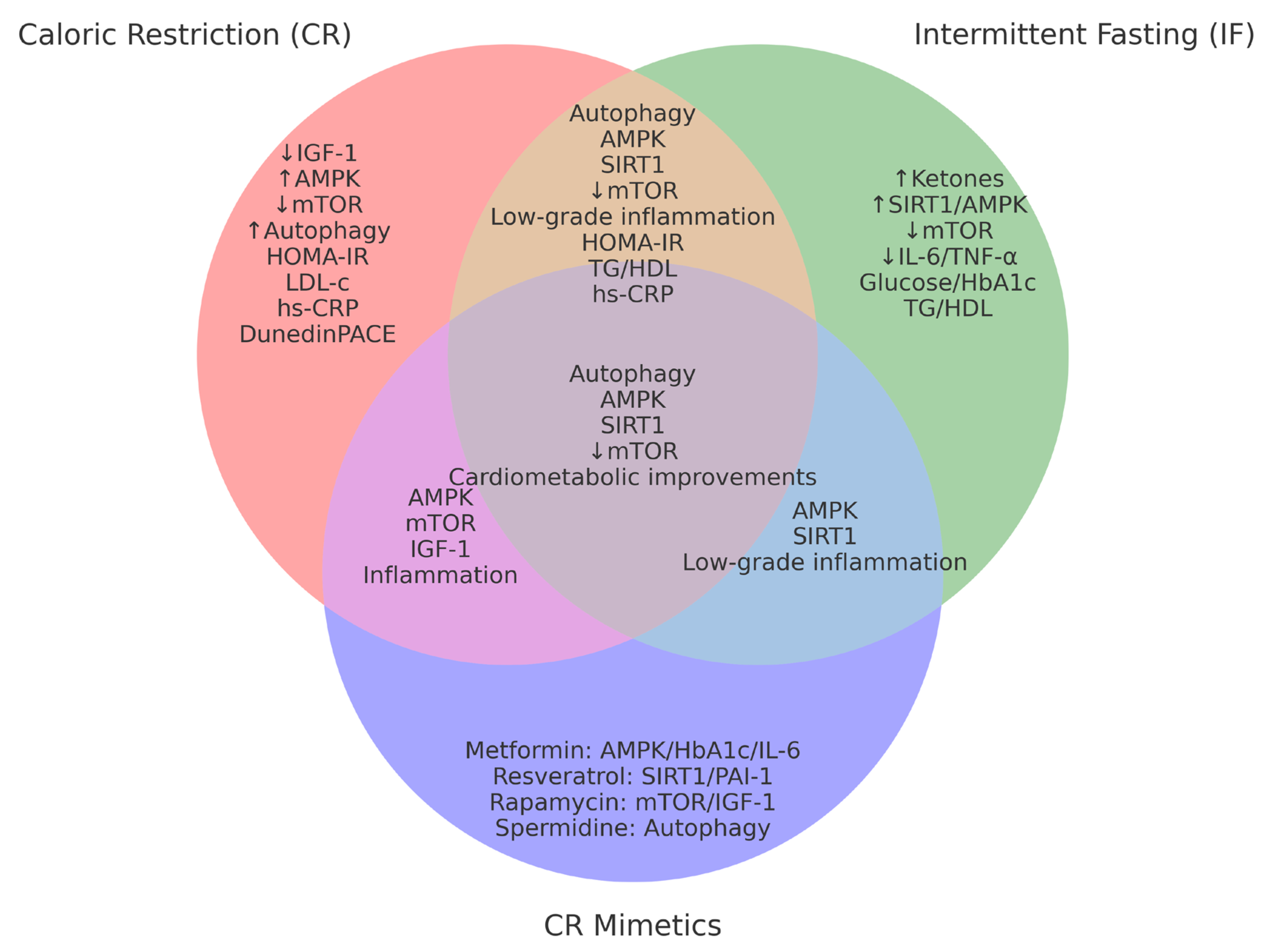
3.4. Cross-Sectional Comparison of CR, IF, and CR-Mimetics
4. Discussion
4.1. Clinical Applicability and Translational Biomarkers
- (a)
- Molecular mechanisms: limited extrapolation to humans
- Human baseline longevity is already high, precluding trials with direct survival endpoints.
- Comorbidities, polypharmacy, psychosocial environment, and genetic diversity complicate the reproduction of effects observed in animals [33].
- In older humans, IGF-1 inhibition—associated with longevity in mice—may be linked to muscle loss or frailty [58].
- (b)
- Longevity biomarkers: utility and limits
- Telomere length has yielded ambiguous results, with a possible accelerated loss in the early phases of CR intervention [60].
- Transcriptomic biomarkers derived from muscle biopsies have demonstrated changes in proteostasis, mitochondrial biogenesis, and apoptosis pathways, correlated with functional improvements [61].
- Composite measures of biological age (e.g., Klemera–Doubal, homeostatic dysregulation) have also shown slowing after CR, independent of weight loss [62].
- (c)
- Clinical endpoints: an unmet need
- Reduction in hospitalizations or all-cause mortality.
- Sustained improvement in physical or cognitive function.
- (d)
- Structural and contextual barriers
- Limited training in nutritional medicine, chronobiology, and geroscience among clinical professionals [64].
- Lack of structured tools for behavioral support, digital monitoring, or individualized biofeedback.
- Fragmented healthcare systems that hinder multidisciplinary approaches (nutritionists, psychologists, physicians, geriatricians).
- (e)
- Emerging perspectives: integrated biomarkers and artificial intelligence for longevity medicine
4.2. Current Gaps and Future Directions
- (a)
- Insufficient clinical trials: duration, sample size, and endpoints
- Have a duration ≤12 months, which prevents the evaluation of clinically relevant outcomes such as frailty, sustained physical function, major cardiovascular events, or healthy longevity.
- Focused on particular populations (young healthy adults), with insufficient representation of:
- –
- Adults over 70 years,
- –
- Individuals with comorbidities or polypharmacy,
- –
- Subjects in socioeconomically or ethnically vulnerable contexts.
- Assess intermediate outcomes (biomarkers) instead of hard clinical outcomes, such as mortality reduction, functional decline, or loss of independence.
- (b)
- Limited integration of multidimensional biomarkers
- Validated epigenetic clocks (DunedinPACE, PhenoAge, GrimAge) as primary outcomes.
- Transcriptomic, metabolomic, and proteomic profiling to characterize responders vs. non-responders.
- Immunological cluster analyses linked to cellular senescence, immune resilience, and functional capacity.
- (c)
- Insufficient personalization: from population average to clinical phenotype
- Phenotypic classification by chronotype, inflammatory pattern, anabolic resistance, and microbiota profile.
- Adaptive and dynamic protocols, adjusted according to clinical evolution and treatment response.
- (d)
- Adherence: the underestimated barrier
- Mobile applications with real-time feedback, wearable sensors (glucose, HRV, physical activity), and individualized remote monitoring.
- Behavioral motivation techniques (MI), positive reinforcement, and nutritional coaching with psychosocial support.
- (e)
- Clinical implementation and healthcare sustainability
- Cost-effectiveness evaluation of nutritional and metabolic longevity programs.
- Specific training of healthcare professionals in longevity medicine, microbiota, and applied AI.
4.3. Clinical–Translational Proposal: Rationale and Justification
- Level 1:
- Personalized basal intervention (primary prevention)
- Level 2:
- Combined bioactive intervention (secondary prevention)
- Level 3:
- Advanced personalized intervention (tertiary prevention/clinical longevity)
- Operational deployment
- Applied examples
- A 78-year-old man with frailty, elevated IL-6, and microbiota poor in butyrate may benefit from an anti-inflammatory diet with mild IF, resveratrol supplementation, and weekly telemonitoring support.
- A 36-year-old woman with HPA axis dysfunction and high estrogen sensitivity may require extended fasting windows, psychological support, and hormonal adjustments without strict CR [77].
4.4. Integrative Synthesis
4.4.1. Type 2 Diabetes (T2D)
4.4.2. Non-Alcoholic Fatty Liver Disease (NAFLD)
4.4.3. Metabolic Syndrome (MetS)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADF | Alternate-Day Fasting |
| AMAL | Active Management of Aging and Longevity |
| AMPK | AMP-Activated Protein Kinase |
| BP | Blood Pressure |
| CR | Caloric Restriction |
| CRM | Caloric Restriction Mimetics |
| CRP | C-Reactive Protein |
| FMD | Fasting-Mimicking Diet |
| FTO | Fat Mass and Obesity-Associated Gene |
| HPA | Hypothalamic–Pituitary–Adrenal Axis |
| IF | Intermittent Fasting |
| IGF | Insulin-like Growth Factor |
| IL | Interleukin |
| LDL | Low-Density Lipoprotein |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| OR | Odds Ratio |
| RCT | Randomized Controlled Trial |
| TNF | Tumor Necrosis Factor |
References
- United Nations. Ageing. Available online: https://www.un.org/es/global-issues/ageing (accessed on 30 March 2025).
- World Health Organization. Integrated Care for Older People: Guidelines on Community-Level Interventions to Manage Declines in Intrinsic Capacity. WHO: Geneva, Switzerland, 2017; Available online: https://www.who.int/publications/i/item/9789241513500 (accessed on 30 March 2025).
- World Health Organization. Decade of Healthy Ageing: Proposal for a Decade of Healthy Ageing 2020–2030. WHO: Geneva, Switzerland, 2020; Available online: https://cdn.who.int/media/docs/default-source/decade-of-healthy-ageing/decade-proposal-final-apr2020rev-es.pdf (accessed on 30 March 2025).
- Moskalev, A.A.; Shaposhnikov, M.V.; Plyusnina, E.N.; Zhavoronkov, A.; Budovsky, A.; Yanai, H.; Fraifeld, V.E. The role of DNA damage and repair in aging through the prism of Koch-like criteria. Ageing Res. Rev. 2013, 12, 661–684. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Barzilai, N.; Ferrucci, L. Insulin resistance and aging: A cause or a protective response? J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 1329–1331. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.K.; Roth, G.S. Calorie restriction mimetics: Can you have your cake and eat it, too? Ageing Res. Rev. 2015, 20, 46–62. [Google Scholar] [CrossRef]
- Guan, L.; Liu, R. The Role of Diet and Gut Microbiota Interactions in Metabolic Homeostasis. Adv. Biol. 2023, 7, e2300100. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Partridge, L.; Fuentealba, M.; Kennedy, B.K. The quest to slow ageing through drug discovery. Nat. Rev. Drug Discov. 2020, 19, 513–532. [Google Scholar] [CrossRef]
- Duan, H.; Pan, J.; Guo, M.; Li, J.; Yu, L.; Fan, L. Dietary strategies with anti-aging potential: Dietary patterns and supplements. Food Res. Int. 2022, 158, 111501. [Google Scholar] [CrossRef]
- Senior, A.M.; Nakagawa, S.; Raubenheimer, D.; Simpson, S.J. Global associations between macronutrient supply and age-specific mortality. Proc. Natl. Acad. Sci. USA 2020, 117, 30824–30835. [Google Scholar] [CrossRef]
- Green, C.L.; Lamming, D.W.; Fontana, L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat. Rev. Mol. Cell Biol. 2022, 23, 56–73. [Google Scholar] [CrossRef]
- Martucci, M.; Ostan, R.; Biondi, F.; Bellavista, E.; Fabbri, C.; Bertarelli, C.; Salvioli, S.; Capri, M.; Franceschi, C.; Santoro, A. Mediterranean diet and inflammaging within the hormesis paradigm. Nutr. Rev. 2017, 75, 442–455. [Google Scholar] [CrossRef]
- Weiss, E.P.; Racette, S.B.; Villareal, D.T.; Fontana, L.; Steger-May, K.; Schechtman, K.B.; Klein, S.; Holloszy, J.O.; Washington University School of Medicine CALERIE Group. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: A randomized controlled trial. Am. J. Clin. Nutr. 2006, 84, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Most, J.; Gilmore, L.A.; Smith, S.R.; Han, H.; Ravussin, E.; Redman, L.M. Significant improvement in cardiometabolic health in healthy non-obese individuals during caloric restriction-induced weight loss and weight loss maintenance. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E396–E405. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.K.; Bhapkar, M.; Pittas, A.G.; Pieper, C.F.; Das, S.K.; Williamson, D.A.; Scott, T.; Redman, L.M.; Stein, R.; Gilhooly, C.H.; et al. Effect of Calorie Restriction on Mood, Quality of Life, Sleep, and Sexual Function in Healthy Non-obese Adults: The CALERIE 2 Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 743–752. [Google Scholar] [CrossRef]
- Longo, V.D.; Panda, S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Manoogian, E.N.C.; Panda, S. Circadian rhythms, time-restricted feeding, and healthy aging. Ageing Res. Rev. 2017, 39, 59–67. [Google Scholar] [CrossRef]
- Kahleova, H.; Lloren, J.I.; Mashchak, A.; Hill, M.; Fraser, G.E. Meal Frequency and Timing Are Associated with Changes in Body Mass Index in Adventist Health Study 2. J. Nutr. 2017, 147, 1722–1728. [Google Scholar] [CrossRef]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef]
- Dorn, G.W., II. Evolving Concepts of Mitochondrial Dynamics. Annu. Rev. Physiol. 2019, 81, 1–17. [Google Scholar] [CrossRef]
- Waziry, R.; Ryan, C.P.; Corcoran, D.L.; Huffman, K.M.; Kobor, M.S.; Kothari, M.; Graf, G.H.; Kraus, V.B.; Kraus, W.E.; Lin, D.T.S.; et al. Effect of long-term caloric restriction on DNA methylation measures of biological aging in healthy adults from the CALERIE trial. Nat. Aging 2023, 3, 248–257. [Google Scholar] [CrossRef]
- Chen, Y.E.; Tsai, H.L.; Tu, Y.K.; Chen, L.W. Effects of different types of intermittent fasting on metabolic outcomes: An umbrella review and network meta-analysis. BMC Med. 2024, 22, 529. [Google Scholar] [CrossRef]
- Alfahl, S.O. Evaluation of the effectiveness of intermittent fasting versus caloric restriction in weight loss and improving cardiometabolic health: A systematic review and meta-analysis. J. Taibah Univ. Med. Sci. 2025, 20, 159–168. [Google Scholar] [CrossRef]
- Moel, M.; Harinath, G.; Lee, V.; Nyquist, A.; Morgan, S.L.; Isman, A.; Zalzala, S. Influence of rapamycin on safety and healthspan metrics after one year: Results from the PEARL randomized controlled trial. Aging 2025, 17, 16024–16042. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, C.; Stekovic, S.; Wirth, M.; Benson, G.; Royer, P.; Sigrist, S.J.; Pieber, T.; Dammbrueck, C.; Magnes, C.; Eisenberg, T.; et al. Safety and tolerability of spermidine supplementation in older adults with subjective cognitive decline: A phase IIa randomized controlled trial. Aging 2022, 14, 2456–2473. [Google Scholar]
- Belsky, D.W.; Caspi, A.; Arseneault, L.; Baccarelli, A.; Corcoran, D.L.; Gao, X.; Hannon, E.; Harrington, H.L.; Rasmussen, L.J.H.; Houts, R.; et al. Quantification of the pace of biological aging in humans through a blood test, the DunedinPoAm DNA methylation algorithm. eLife 2020, 9, e54870. [Google Scholar] [CrossRef] [PubMed]
- Surendran, P.; Stewart, I.D.; Au Yeung, V.P.W.; Pietzner, M.; Raffler, J.; Wörheide, M.A.; Li, C.; Smith, R.F.; Wittemans, L.B.L.; Bomba, L.; et al. Rare and common genetic determinants of metabolic individuality and their effects on human health. Nat. Med. 2022, 28, 2321–2332. [Google Scholar] [CrossRef]
- López-Otín, C.; Kroemer, G. Hallmarks of Health. Cell 2021, 184, 33–63. [Google Scholar] [CrossRef]
- Madeo, F.; Carmona-Gutierrez, D.; Hofer, S.J.; Kroemer, G. Caloric restriction mimetics against age-associated disease: Targets, mechanisms, and therapeutic potential. Cell Metab. 2019, 29, 592–610. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Gubbi, S.; Barzilai, N. Benefits of metformin in attenuating the hallmarks of aging. Cell Metab. 2020, 32, 15–30. [Google Scholar] [CrossRef]
- Levine, M.E.; Lu, A.T.; Quach, A.; Chen, B.H.; Assimes, T.L.; Bandinelli, S.; Hou, L.; Baccarelli, A.A.; Stewart, J.D.; Li, Y.; et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging 2018, 10, 573–591. [Google Scholar] [CrossRef]
- Mattison, J.A.; Roth, G.S.; Beasley, T.M.; Tilmont, E.M.; Handy, A.M.; Herbert, R.L.; Longo, D.L.; Allison, D.B.; Young, J.E.; Bryant, M.; et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 2012, 489, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Anton, S.D.; Lee, S.A.; Donahoo, W.T.; McLaren, C.; Manini, T.; Leeuwenburgh, C.; Pahor, M. The Effects of Time Restricted Feeding on Overweight, Older Adults: A Pilot Study. Nutrients 2019, 11, 1500. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.-L.; Yao, W.; Wang, X.-Y.; Gao, S.; Varady, K.A.; Forslund, S.K.; Zhang, M.; Shi, Z.-Y.; Cao, F.; Zou, B.-J.; et al. Intermittent fasting and health outcomes: An umbrella review of systematic reviews and meta-analyses of randomised controlled trials. eClinicalMedicine 2024, 70, 102519. [Google Scholar] [CrossRef] [PubMed]
- Stekovic, S.; Hofer, S.J.; Tripolt, N.; Aon, M.A.; Royer, P.; Pein, L.; Stadler, J.T.; Pendl, T.; Prietl, B.; Url, J.; et al. Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell Metab. 2020, 31, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmi de Toledo, F.; Grundler, F.; Bergouignan, A.; Drinda, S.; Michalsen, A. Safety, health improvement and well-being during a 4 to 21-day fasting period in an observational study including 1422 subjects. PLoS ONE 2019, 14, e0209353. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span—From yeast to humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef]
- López-Otín, C.; Galluzzi, L.; Freije, J.M.P.; Madeo, F.; Kroemer, G. Metabolic control of longevity. Cell 2016, 166, 802–821. [Google Scholar] [CrossRef]
- Waziry, R.; Levine, M.E.; Belsky, D.W.; Newman, J.C.; Wang, J.; Smith, C.J.; Redman, L.M.; Das, S.K.; Huffman, K.M.; Kobor, M.S.; et al. Caloric restriction slows epigenetic aging in humans: The CALERIE randomized controlled trial. Nat. Aging 2023, 3, 178–186. [Google Scholar] [CrossRef]
- Das, J.K.; Banskota, N.; Candia, J.; Griswold, M.E.; Orenduff, M.; de Cabo, R.; Corcoran, D.L.; Das, S.K.; De, S.; Huffman, K.M.; et al. Calorie restriction modulates the transcription of genes related to stress response and longevity in human muscle: The CALERIE study. Aging Cell 2023, 22, e13963. [Google Scholar] [CrossRef]
- Hastings, W.J.; Ye, Q.; Wolf, S.E.; Ryan, C.P.; Das, S.K.; Huffman, K.M.; Kobor, M.S.; Kraus, W.E.; MacIsaac, J.L.; Martin, C.K.; et al. Effect of long-term caloric restriction on telomere length in healthy adults: CALERIE™ 2 trial analysis. Aging Cell 2024, 23, e14149. [Google Scholar] [CrossRef]
- Ramaker, M.E.; Corcoran, D.L.; Apsley, A.T.; Kobor, M.S.; Kraus, V.B.; Kraus, W.E.; Lin, D.T.S.; Orenduff, M.C.; Pieper, C.F.; Waziry, R.; et al. Epigenome-wide Association Study Analysis of Calorie Restriction in Humans, CALERIE™ Trial Analysis. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 2395–2401. [Google Scholar] [CrossRef] [PubMed]
- Dorling, J.L.; Belsky, D.W.; Racette, S.B.; Das, S.K.; Ravussin, E.; Redman, L.M.; Höchsmann, C.; Huffman, K.M.; Kraus, W.E.; Kobor, M.S.; et al. Association between the FTO rs9939609 single nucleotide polymorphism and dietary adherence during a 2-year caloric restriction intervention: Exploratory analyses from CALERIE™ phase 2. Exp. Gerontol. 2021, 155, 111555. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Mi, J.; Jiang, Q.M.; Xu, J.M.; Tang, Y.Y.; Tian, G.; Wang, B. Metformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus. Clin. Exp. Pharmacol. Physiol. 2014, 41, 650–656. [Google Scholar] [CrossRef]
- García-Martínez, B.I.; Ruiz-Ramos, M.; Pedraza-Chaverri, J.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Effect of Resveratrol on Markers of Oxidative Stress and Sirtuin 1 in Elderly Adults with Type 2 Diabetes. Int. J. Mol. Sci. 2023, 24, 7422. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kraus, W.E.; Bhapkar, M.; Huffman, K.M.; Pieper, C.F.; Das, S.K.; Redman, L.M.; Villareal, D.T.; Rochon, J.; Roberts, S.B.; Ravussin, E.; et al. 2 years of calorie restriction and cardiometabolic risk (CALERIE): Exploratory outcomes of a multicentre, phase 2, randomized controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 673–683. [Google Scholar] [CrossRef]
- Lowe, D.A.; Wu, N.; Rohdin-Bibby, L.; Moore, A.H.; Kelly, N.; Liu, Y.E.; Philip, E.; Vittinghoff, E.; Heymsfield, S.B.; Olgin, J.E.; et al. Effects of Time-Restricted Eating on Weight Loss and Other Metabolic Parameters in Women and Men With Overweight and Obesity: The TREAT Randomized Clinical Trial. JAMA Intern. Med. 2020, 180, 1491–1499, Erratum in: JAMA Intern. Med. 2020, 180, 1555. https://doi.org/10.1001/jamainternmed.2020.6728. Erratum in: JAMA Intern. Med. 2021, 181, 883. https://doi.org/10.1001/jamainternmed.2020.8941. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Templeman, I.; Gonzalez, J.T.; Thompson, D.; Betts, J.A. The role of intermittent fasting and meal timing in weight management and metabolic health. Proc. Nutr. Soc. 2020, 79, 76–87. [Google Scholar] [CrossRef]
- Wei, W.; Ji, S.; Shang, G.; Zhang, R. Caloric restriction mimetics: Natural and synthetic compounds that mimic calorie restriction. Nutrients 2024, 16, 342. [Google Scholar]
- Kraus, D.; Yang, Q.; Kong, D. Rapamycin and gut microbiota: The emerging links. Aging Cell 2023, 22, e13899. [Google Scholar] [CrossRef]
- Ros, M.; Carrascosa, J.M. Current nutritional and pharmacological anti-aging interventions. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165612. [Google Scholar] [CrossRef]
- Racette, S.B.; Rochon, J.; Uhrich, M.L.; Villareal, D.T.; Das, S.K.; Fontana, L.; Bhapkar, M.; Martin, C.K.; Redman, L.M.; Fuss, P.J.; et al. Effects of Two Years of Calorie Restriction on Aerobic Capacity and Muscle Strength. Med. Sci. Sports Exerc. 2017, 49, 2240–2249. [Google Scholar] [CrossRef]
- Barzilai, N.; Crandall, J.P.; Kritchevsky, S.B.; Espeland, M.A. Metformin as a tool to target aging. Cell Metab. 2016, 23, 1060–1065. [Google Scholar] [CrossRef]
- Colman, R.J.; Anderson, R.M.; Johnson, S.C.; Kastman, E.K.; Kosmatka, K.J.; Beasley, T.M.; Allison, D.B.; Cruzen, C.; Simmons, H.A.; Kemnitz, J.W.; et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 2009, 325, 201–204. [Google Scholar] [CrossRef]
- Lehallier, B.; Gate, D.; Schaum, N.; Nanasi, T.; Lee, S.E.; Yosef, H.; Moran, P.; Bernidk, D.; Keller, A.; Verghese, J.; et al. Undulating changes in human plasma proteome profiles across the lifespan. Nat. Med. 2019, 25, 1843–1850. [Google Scholar] [CrossRef]
- Belsky, D.W.; Caspi, A.; Corcoran, D.L.; Sugden, K.; Poulton, R.; Arseneault, L.; Baccarelli, A.; Chamarti, K.; Gao, X.; Hannon, E.; et al. DunedinPACE, a DNA methylation biomarker of the pace of aging. eLife 2022, 11, e73420. [Google Scholar] [CrossRef]
- Marioni, R.E.; Shah, S.; McRae, A.F.; von Zglinicki, T.S.J.; Martinez-Ruiz, C.; Wray, N.R.; Visscher, P.M.; Deary, I.J. The epigenetic clock and telomere length are independently associated with chronological age and mortality. Int. J. Epidemiol. 2018, 47, 356–365. [Google Scholar] [CrossRef]
- Fang, E.F.; Scheibye-Knudsen, M.; Jahn, H.J.; Li, J.; Ling, L.; Guo, H.; Zhu, X.; Preedy, V.; Lu, H.; Borh, V.A.; et al. A research agenda for aging in China in the 21st century. Ageing Res. Rev. 2015, 24, 197–205. [Google Scholar] [CrossRef]
- Hagg, S.; Jylhava, J. Sex differences in biological aging with a focus on human studies. eLife 2021, 10, e63425. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; de Godoi Rezende Costa Molino, C.; Rival, S.; Vellas, B.; Rizzoli, R.; Kressig, R.W.; Kanis, J.A.; Manson, J.E.; Dawson-Hughes, B.; Orav, E.J.; et al. DO-HEALT: Vitamin D3-Omega-3-Home exercise-Healthy aging and longevity trial-Design of a multinational clinical trial on healthy aging among European seniors. Contemp. Clin. Trials 2021, 100, 106124. [Google Scholar] [CrossRef] [PubMed]
- Meer, M.V.; Podolskiy, D.I.; Tyshkovskiy, A.; Gladyshev, V.N. A whole lifespan mouse multi- tiss ue DNA methylation clock. eLife 2018, 7, e40675. [Google Scholar] [CrossRef] [PubMed]
- Trudel-Fitzgerald, C.; Poole, E.M.; Rosner, B.; Tworoger, S.S.; Kubzansky, L.D. DNA methylation-based age acceleration and risk of colorectal cancer: Data from three prospective cohort studies. J. Natl. Cancer Inst. 2020, 112, 1055–1064. [Google Scholar] [CrossRef]
- Redman, L.M.; Ravussin, E. Caloric restriction in humans: Impact on physiological, psychological, and behavioral outcomes. Cell Metab. 2011, 14, 291–302. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Most, J.; Tosti, V.; Redman, L.M.; Fontana, L. Calorie restriction in humans: An update. Ageing Res. Rev. 2017, 39, 36–45. [Google Scholar] [CrossRef]
- Zierer, J.; Jackson, M.A.; Kastenmüller, G.; Mangino, M.; Long, T.; Telenti, A.; Mohney, R.P.; Small, K.S.; Bell, J.T.; Steves, C.J.; et al. The fecal metabolome as a functional readout of the gut microbiome. Nat. Genet. 2018, 50, 790–795. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [PubMed]
- Bafei, S.E.C.; Shen, C. Biomarkers selection and mathematical modeling in biological age estimation. NPJ Aging 2023, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Topol, E.J. Deep Medicine: How Artificial Intelligence Can Make Healthcare Human Again; Basic Books: New York, NY, USA, 2019. [Google Scholar]
- Bohn, B.; Herbst, A.; Pfeifer, M.; Krakow, D.; Zimny, S.; Kopp, F.; Melmer, A.; Steinacker, J.M.; Holl, R.W.; DPV Initiative. Impact of Physical Activity on Glycemic Control and Prevalence of Cardiovascular Risk Factors in Adults With Type 1 Diabetes: A Cross-sectional Multicenter Study of 18,028 Patients. Diabetes Care 2015, 38, 1536–1543. [Google Scholar] [CrossRef]
- Wells, R.; Cimino, J.J.; Shortliffe, E.H. Clinical decision support systems in healthcare: Overview and future directions. Yearb. Med. Inform. 2020, 29, 105–111. [Google Scholar] [CrossRef][Green Version]
- Bailey, S.R.; O’Malley, J.P.; Gold, R.; Heintzman, J.; Likumahuwa, S.; DeVoe, J.E. Receipt of diabetes preventive services differs by insurance status at visit. Am. J. Prev. Med. 2015, 48, 229–233. [Google Scholar] [CrossRef]
- Manoogian, E.N.C.; Chow, L.S.; Taub, P.R.; Laferrère, B.; Panda, S. Time-restricted eating for the prevention and management of metabolic diseases. Endocr. Rev. 2022, 43, 405–436. [Google Scholar] [CrossRef] [PubMed]
- Mannick, J.B.; Del Giudice, G.; Lattanzi, M.; Valiante, N.M.; Praestgaard, J.; Huang, B.; Lonetto, M.A.; Maecker, H.T.; Kovarik, J.; Carson, S.; et al. mTOR inhibition improves immune function in the elderly. Sci. Transl. Med. 2014, 6, 268ra179. [Google Scholar] [CrossRef]
- Santoro, N.; Epperson, C.N.; Mathews, S.B. Menopausal symptoms and their management. Endocrinol. Metab. Clin. N. Am. 2015, 44, 497–515. [Google Scholar] [CrossRef]
- Mattson, M.P.; Moehl, K.; Ghena, N.; Schmaedick, M.; Cheng, A. Intermittent metabolic switching, neuroplasticity and brain health. Nat. Rev. Neurosci. 2018, 19, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Gonzalez-Freire, M.; Fabbri, E.; Simonsick, E.; Tanaka, T.; Moore, Z.; Salimi, S.; Sierra, F.; de Cabo, R. Measuring biological aging in humans: A quest. Aging Cell 2020, 19, e13080. [Google Scholar] [CrossRef] [PubMed]
| Author(s) | Brief Title | Main Topic | Study Type | Key Finding or Relevance | Year |
|---|---|---|---|---|---|
| Kennedy BK et al. [10] | Geroscience link to chronic disease | Conceptual aging framework | Review | Establishes aging as the root of chronic diseases | 2014 |
| [5] | Molecular hallmarks of aging | Aging biology | Review | Defines nine molecular hallmarks of aging | 2013 |
| [30] | Hallmarks of health | Health and resilience pathways | Review | Extends the model to health-protective mechanisms | 2021 |
| [31] | CR mimetics | Molecular targets, therapy | Review | Spermidine, resveratrol, and others as mimetics | 2019 |
| [13] | CR mechanisms across species | Comparative metabolism | Review | Differences in CR impact by species | 2022 |
| [32] | CR and mimetics | Integrated perspective on longevity | Review | Combines metabolic and molecular insights | 2021 |
| [19] | Chrononutrition | Circadian–metabolic interaction | Animal study | Diet rhythm modulates metabolic pathways | 2017 |
| [33] | Epigenetic clocks | Biomarkers of aging | Clinical study | GrimAge validated for lifespan and healthspan | 2018 |
| [34] | Caloric restriction | Non-human primate longevity | Experimental study | CR improves survival and function in monkeys | 2017 |
| [31] | Fasting-mimicking diet | Periodic restriction | Clinical trial | FMD improves IGF-1, glucose, regeneration | 2019 |
| [35] | Intermittent fasting | Metabolic reprogramming | Review | Switch to fat oxidation, metabolic flexibility | 2018 |
| [36] | IF and metabolic markers | Cardiometabolic health | Review | IF improves insulin, glucose, and lipids | 2024 |
| [16] | CR and NAFLD | Visceral fat and liver | Clinical trial | CR reduces hepatic fat in non-obese adults | 2018 |
| [37] | Alternate-day fasting | Body composition | Clinical trial | ADF reduces fat mass and improves lipids | 2020 |
| [19] | Circadian IF | Chronobiology and metabolism | Review | Aligning meals to rhythms boosts IF effects | 2017 |
| [38] | Prolonged fasting | Safety and tolerability | Human observational | Safe in a large cohort with improved well-being | 2019 |
| [39] | CR translation across species | Translational medicine | Review | Bridges preclinical and human evidence | 2015 |
| [40] | Metabolic control of longevity | Mitochondrial networks | Review | Metabolism is central to lifespan modulation | 2016 |
| [41] | CR and epigenetics | DNA methylation clocks | RCT (CALERIE) | CR slows epigenetic aging (PhenoAge, GrimAge) | 2023 |
| [42] | CR and transcriptomics | Muscle stress and longevity genes | RCT (CALERIE) | CR shifts gene expression toward resilience | 2023 |
| [43] | CR and telomere biology | Cellular senescence | RCT (CALERIE) | CR preserves telomere length | 2024 |
| [44] | CR and EWAS | Epigenomic modulation | RCT (CALERIE) | CR alters aging-related CpG methylation | 2022 |
| [23] | CR and biological pace | DunedinPACE biomarker | RCT (CALERIE) | CR slows the molecular aging rate | 2017 |
| [45] | FTO polymorphism and CR | Genetic determinants of adherence | RCT (CALERIE) | FTO SNPs linked to lower CR adherence | 2021 |
| [46] | Metformin and cognition | Cognitive performance in T2D | RCT | Improves memory, linked to HbA1c drop | 2014 |
| [47] | Resveratrol in aging adults | SIRT1 and oxidative stress | RCT | ↑ SIRT1, antioxidant capacity in the elderly | 2023 |
| [36] | Intermittent fasting meta-review | Health outcomes | Umbrella review | Consistent benefits on glucose, weight, and lipids | 2024 |
| Study/Author | Design/Sample | Duration | Biomarkers Evaluated | Main Findings |
|---|---|---|---|---|
| [48] | RCT, 218 adults, 25% CR | 24 months | Weight, glucose, insulin, CRP, IGF-1 | Reduced weight, inflammation, and improved insulin sensitivity |
| [23] | CALERIE epigenetic substudy, 197 participants | 24 months | Epigenetic clocks | Slowed epigenetic aging (~2–3%) |
| [45] | CALERIE follow-up, 105 participants | 6–12 months | Glucose, lipids, and insulin sensitivity | Maintained cardiometabolic benefits |
| Study/Author | Design/Sample | Duration | Biomarkers Evaluated | Main Findings |
|---|---|---|---|---|
| [49] | RCT, 116 overweight adults, 16:8 regimen | 12 weeks | Weight, glucose, insulin, BP | Weight loss; no major insulin changes |
| [25] | RCT, obese adults, 5:2 vs. IF | 12 weeks | BMI, lipids, glucose | Reduced fat mass, improved cardiometabolic markers |
| [50] | Trial, older adults with metabolic syndrome | 8 weeks | CRP, IL-6, TNFα, glucose | Improved inflammation and metabolic profile |
| [36] | Systematic review of 25 RCTs | 4–52 weeks | Glucose, HbA1c, cholesterol, BP | IF improves metabolic markers |
| Study/Author | Design/Sample | Duration | Biomarkers Evaluated | Main Findings |
|---|---|---|---|---|
| [51] | RCT, 124 adults, placebo-controlled, oral resveratrol | 6 months | BP, TAC, GPx, SH/GSSG, TG, cholesterol, HOMA-IR, SIRT1, insulin, glucose | Resveratrol improved SIRT1, SIRT1, TAC, GPx, ↓ TG; no significant change in BP |
| [52] | RCT, older adults, metformin vs. placebo | 6 months | IL-6, TNFα, glucose, cognition | Metformin reduced inflammation, improved metabolism |
| [51] | RCT, prediabetes patients, rapamycin | 10 weeks | IGF-1, mTOR, HbA1c, microbiota | Rapamycin reduced IGF-1, improved insulin sensitivity |
| [51] | Systematic review of 18 studies | 8–52 weeks | AMPK, mTOR, sirtuins, glucose, lipids | Mimetics replicate CR molecular effects |
| Characteristic | CR (Caloric Restriction) | IF (Intermittent Fasting) | CR Mimetics |
|---|---|---|---|
| Intervention type | Continuous caloric reduction | Restricted feeding windows | Use of compounds activating longevity pathways |
| Main mechanisms | ↓ IGF-1, ↑ AMPK, ↓ mTOR, ↑ autophagy | ↑ Ketone bodies, ↑ SIRT1, ↑ AMPK, ↓ mTOR | ↑ SIRT1, ↓ mTOR, ↑ autophagy, ↓ inflammation |
| Duration in trials | 6–24 months | 8–12 weeks | 8–24 weeks (pilot trials) |
| Biomarkers evaluated | IGF-1, CRP, glucose, DNA methylation | Glucose, IL-6, ketones, TNF-α | AMPK, IGF-1, HbA1c, autophagy, epigenetics |
| Clinical advantages | High efficacy, strong evidence base | Well tolerated, adaptable | Potential pharmacological application |
| Limitations | Low adherence, lean mass loss risk | Variable adherence, heterogeneous effects | Side effects, lack of long-term studies |
| Translational applicability | High (requires clinical supervision) | High (personalized by chronotype, age) | Moderate (under clinical research) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murillo-Cancho, A.F.; Lozano-Paniagua, D.; Nievas-Soriano, B.J. Dietary and Pharmacological Modulation of Aging-Related Metabolic Pathways: Molecular Insights, Clinical Evidence, and a Translational Model. Int. J. Mol. Sci. 2025, 26, 9643. https://doi.org/10.3390/ijms26199643
Murillo-Cancho AF, Lozano-Paniagua D, Nievas-Soriano BJ. Dietary and Pharmacological Modulation of Aging-Related Metabolic Pathways: Molecular Insights, Clinical Evidence, and a Translational Model. International Journal of Molecular Sciences. 2025; 26(19):9643. https://doi.org/10.3390/ijms26199643
Chicago/Turabian StyleMurillo-Cancho, Antonio Fernando, David Lozano-Paniagua, and Bruno José Nievas-Soriano. 2025. "Dietary and Pharmacological Modulation of Aging-Related Metabolic Pathways: Molecular Insights, Clinical Evidence, and a Translational Model" International Journal of Molecular Sciences 26, no. 19: 9643. https://doi.org/10.3390/ijms26199643
APA StyleMurillo-Cancho, A. F., Lozano-Paniagua, D., & Nievas-Soriano, B. J. (2025). Dietary and Pharmacological Modulation of Aging-Related Metabolic Pathways: Molecular Insights, Clinical Evidence, and a Translational Model. International Journal of Molecular Sciences, 26(19), 9643. https://doi.org/10.3390/ijms26199643









