Critical Requirement of Senescence-Associated CCN3 Expression in CD44-Positive Stem Cells for Osteoarthritis Progression
Abstract
1. Introduction
2. Results
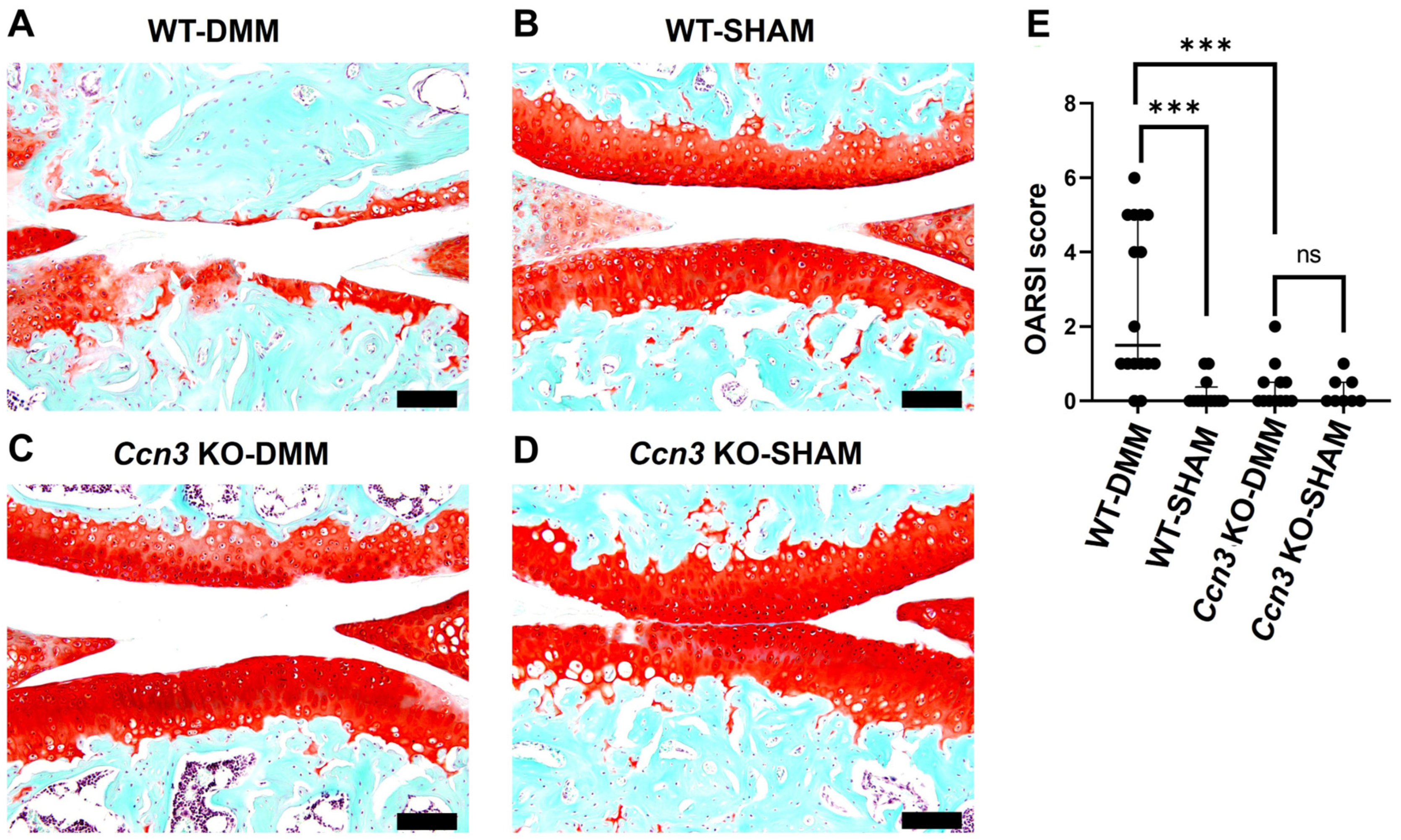
2.1. Ccn3 WT (DMM) Exhibits Severe Cartilage Degradation, While Ccn3 KO (DMM) Demonstrates Protective Effects
2.2. Ccn3 Deletion Attenuates the Expression of Cartilage-Degrading Enzymes in DMM-OA
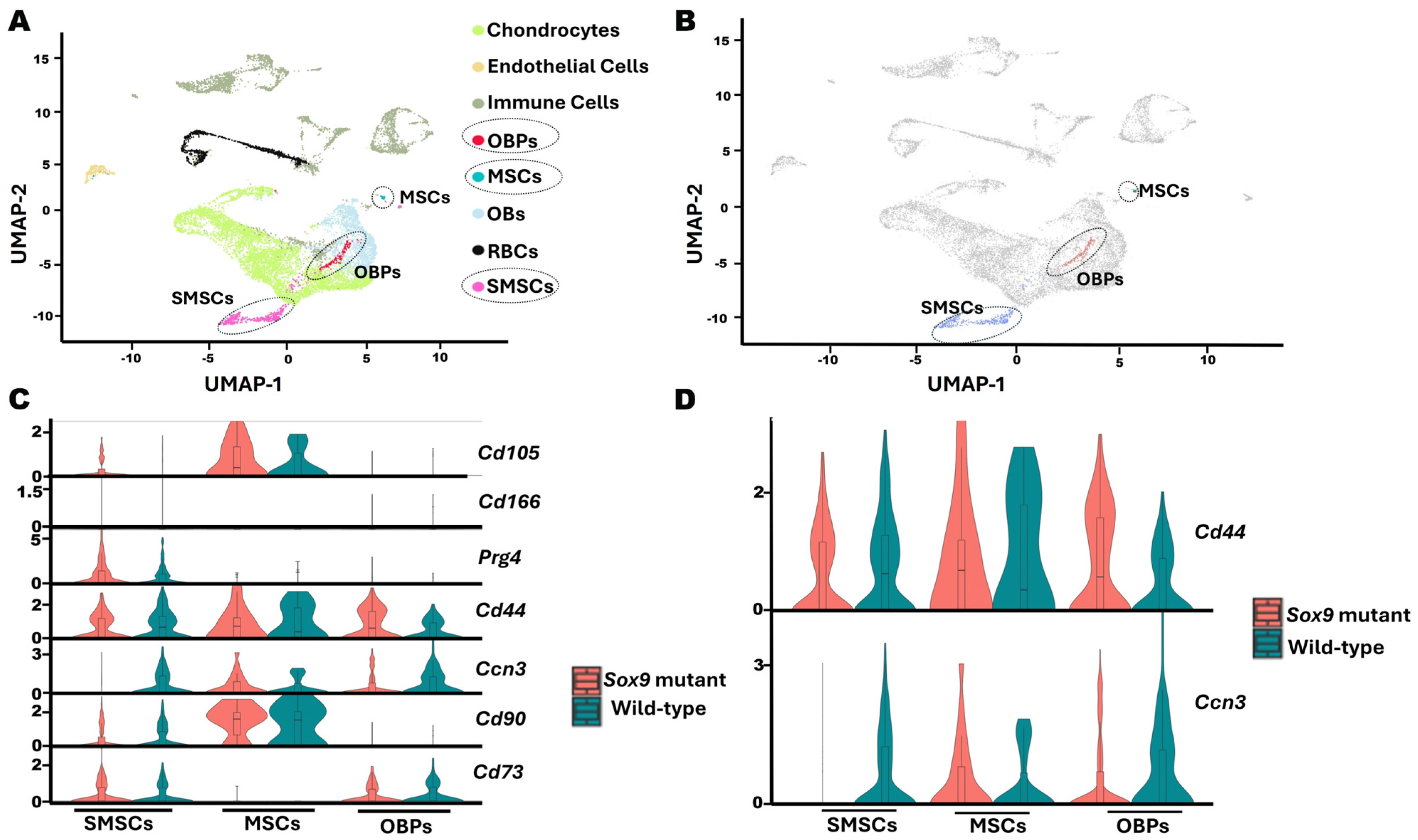
2.3. Single-Cell RNA Sequencing Reveals That Ccn3-Expressing Clusters Co-Express Cd44 in Postnatal Day 13 Mouse Cartilage
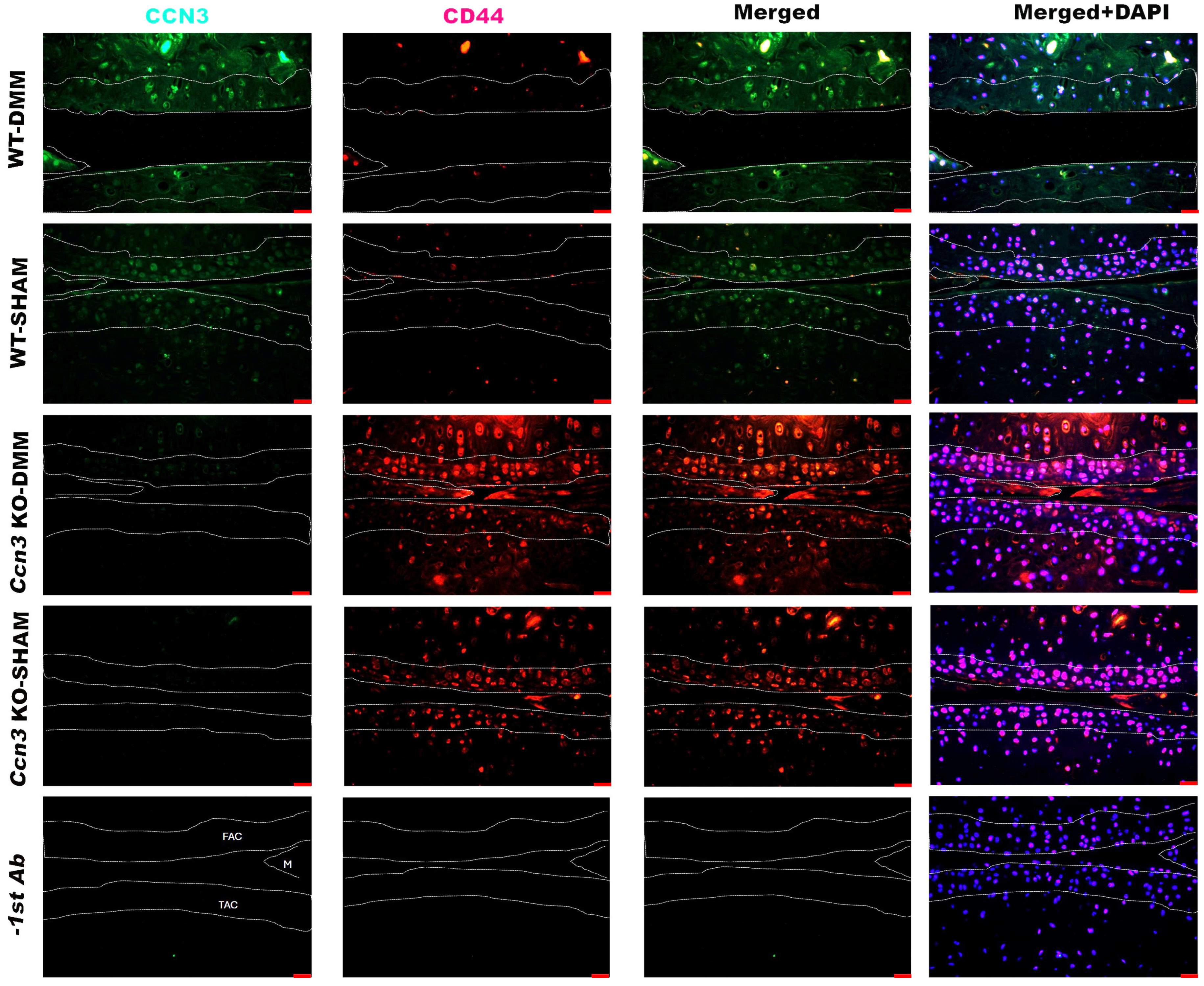
2.4. Colocalization of CCN3 and CD44 in Ccn3 WT-DMM Samples
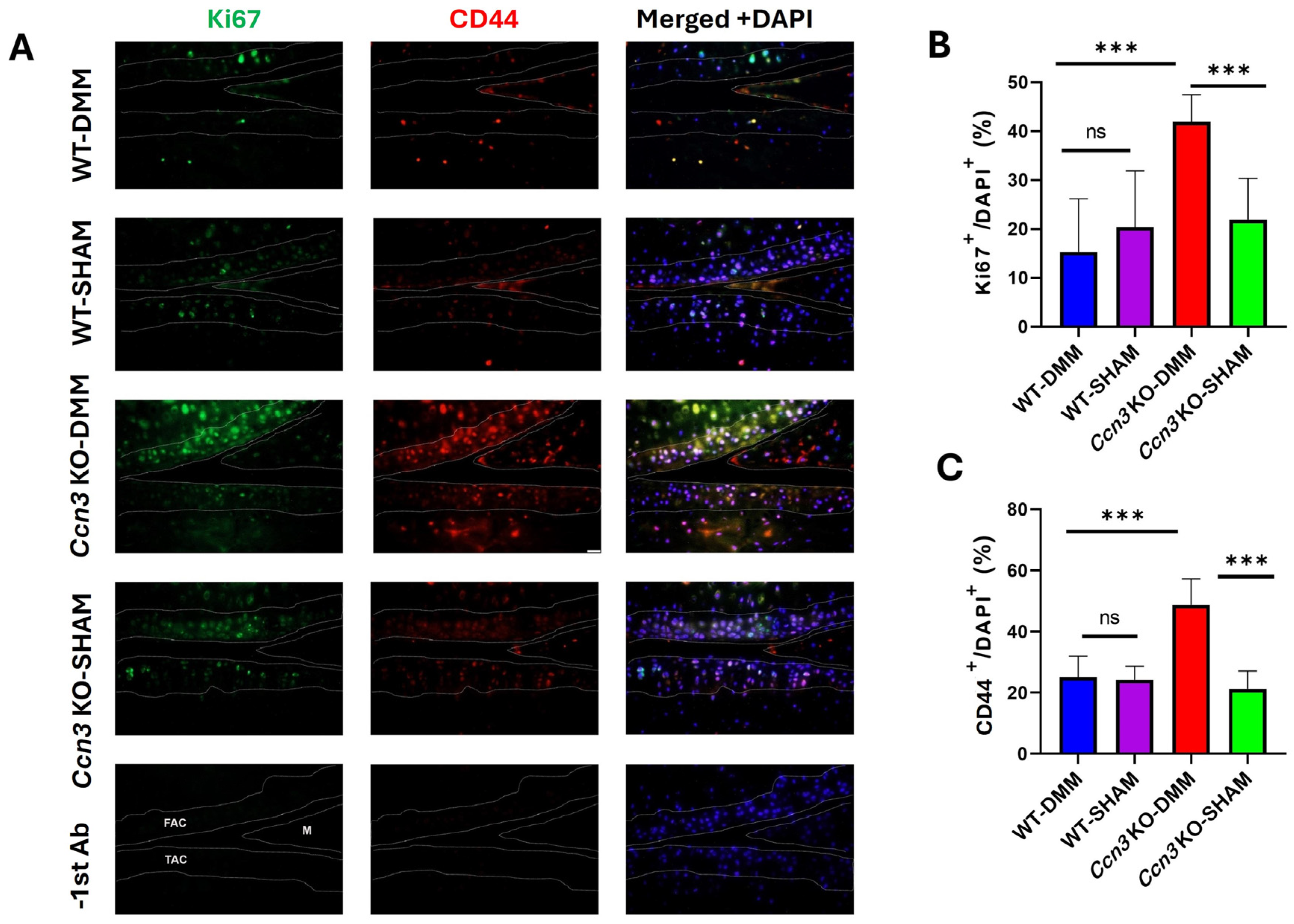
2.5. Ccn3 KO-DMM Displays Higher Chondrocyte Proliferation than Ccn3 WT-DMM
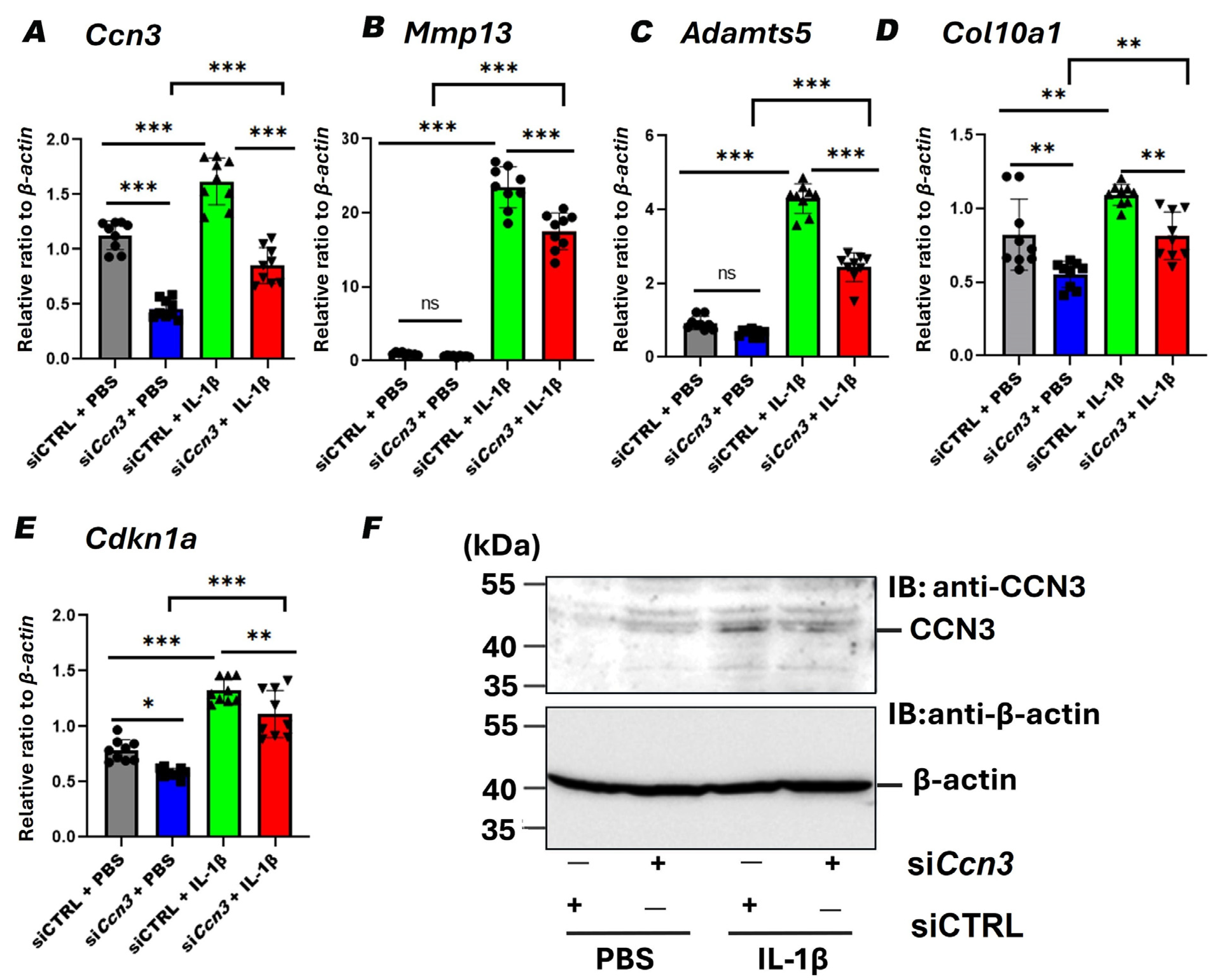
2.6. Ccn3 Knockdown Attenuates IL-1β-Induced Expression of Cartilage Degradation and Senescence Markers in RCS Cells
3. Discussion
Limitations
4. Materials and Methods
4.1. Animal Model and Experimental Groups
4.2. Histology and Safranin O Staining
4.3. Immunofluorescence and Immunohistochemistry
4.4. Single-Cell RNA Sequencing Data Processing and Clustering
4.5. RNA Extraction from Articular Cartilage
4.6. Cell Culture
4.7. Western Blot Analysis
4.8. Reverse Transcription and Quantitative Real-Time PCR
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.J. Osteoarthritis: Toward a Comprehensive Understanding of Pathological Mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef] [PubMed]
- Mathiessen, A.; Conaghan, P.G. Synovitis in Osteoarthritis: Current Understanding with Therapeutic Implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Manocchio, N.; Ljoka, C.; Piacentini, N.; Sorge, R.; Vita, G.; Foti, C. Intra-Articular Injections with Carboxymethyl-Chitosan in Patients Affected by Knee Osteoarthritis Non-Responders to Hyaluronic Acid: A Pilot Study. Eur. J. Transl. Myol. 2024, 34, 31–38. [Google Scholar] [CrossRef]
- Cao, M.; Ou, Z.; Sheng, R.; Wang, Q.; Chen, X.; Zhang, C.; Dai, G.; Wang, H.; Li, J.; Zhang, X.; et al. Efficacy and Safety of Mesenchymal Stem Cells in Knee Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Stem Cell Res. Ther. 2025, 16, 122. [Google Scholar] [CrossRef]
- Kon, E.; Di Matteo, B.; Verdonk, P.; Drobnic, M.; Dulic, O.; Gavrilovic, G.; Patrascu, J.M.; Zaslav, K.; Kwiatkowski, G.; Altschuler, N.; et al. Aragonite-Based Scaffold for the Treatment of Joint Surface Lesions in Mild to Moderate Osteoarthritic Knees: Results of a 2-Year Multicenter Prospective Study. Am. J. Sports Med. 2021, 49, 588–598. [Google Scholar] [CrossRef]
- Tong, L.; Yu, H.; Huang, X.; Shen, J.; Xiao, G.; Chen, L.; Wang, H.; Xing, L.; Chen, D. Current Understanding of Osteoarthritis Pathogenesis and Relevant New Approaches. Bone Res. 2022, 10, 60. [Google Scholar] [CrossRef]
- Loeser, R.F.; Collins, J.A.; Diekman, B.O. Ageing and the Pathogenesis of Osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 412–420. [Google Scholar] [CrossRef]
- Hirose, K.; Kuwahara, M.; Nakata, E.; Tetsunaga, T.; Yamada, K.; Saiga, K.; Takigawa, M.; Ozaki, T.; Kubota, S.; Hattori, T. Elevated Expression of CCN3 in Articular Cartilage Induces Osteoarthritis in Hip Joints Irrespective of Age and Weight Bearing. Int. J. Mol. Sci. 2022, 23, 15311. [Google Scholar] [CrossRef]
- Tokuhiro, T.; Matsumae, G.; Endo, T.; Ogawa, Y.; Ogawa, T.; Liyile, C.; Nishida, Y.; Alhasan, H.; Kobayashi, H.; Ebata, T.; et al. Cellular Communication Network Factor 3 Contributes to the Pathological Process of Rheumatoid Arthritis through Promoting Cell Senescence and Osteoclastogenesis in the Joint. J. Autoimmun. 2024, 149, 103334. [Google Scholar] [CrossRef]
- Kubota, S.; Kawaki, H.; Perbal, B.; Kawata, K.; Hattori, T.; Nishida, T. Cellular Communication Network Factor 3 in Cartilage Development and Maintenance. J. Cell Commun. Signal. 2021, 15, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wei, Y.; Shao, Y.; Li, Y.; Liu, N.; Duan, L. The Emerging Roles of CCN3 Protein in Immune-Related Diseases. Mediators Inflamm. 2021, 2021, 5576059. [Google Scholar] [CrossRef]
- Roddy, K.A.; Boulter, C.A. Targeted Mutation of NOV/CCN3 in Mice Disrupts Joint Homeostasis and Causes Osteoarthritis-like Disease. Osteoarthr. Cartil. 2015, 23, 607–615. [Google Scholar] [CrossRef]
- Lin, C.G.; Leu, S.J.; Chen, N.; Tebeau, C.M.; Lin, S.X.; Yeung, C.Y.; Lau, L.F. CCN3 (NOV) Is a Novel Angiogenic Regulator of the CCN Protein Family. J. Biol. Chem. 2003, 278, 24200–24208. [Google Scholar] [CrossRef]
- Kubota, S.; Takigawa, M. The CCN Family Acting throughout the Body: Recent Research Developments. Biomol. Concepts 2013, 4, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Z.; Bi, D.; Yuan, X.; Liu, X.; Ding, S.; Lu, J.; Niu, Z. CCN3 (NOV) Regulates Proliferation, Adhesion, Migration and Invasion in Clear Cell Renal Cell Carcinoma. Oncol. Lett. 2012, 3, 1099–1104. [Google Scholar] [CrossRef]
- Kuwahara, M.; Kadoya, K.; Kondo, S.; Fu, S.; Miyake, Y.; Ogo, A.; Ono, M.; Furumatsu, T.; Nakata, E.; Sasaki, T.; et al. CCN3 (NOV) Drives Degradative Changes in Aging Articular Cartilage. Int. J. Mol. Sci. 2020, 21, 7556. [Google Scholar] [CrossRef]
- Wei, Y.; Peng, L.; Li, Y.; Zhang, N.; Shang, K.; Duan, L.; Zhong, J.; Chen, J. Higher Serum CCN3 Is Associated with Disease Activity and Inflammatory Markers in Rheumatoid Arthritis. J. Immunol. Res. 2020, 2020, 3891425. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.; Kamel, H.; Zaghloul, M.I.; Elsharaby, R.M.; Hussein, M.I. Evaluation of CCN3 as an Inflammatory Marker of Disease Activity in Rheumatoid Arthritis Patients. Egypt. J. Hosp. Med. 2023, 92, 6185–6190. [Google Scholar] [CrossRef]
- Huang, X.; Ni, B.; Mao, Z.; Xi, Y.; Chu, X.; Zhang, R.; Ma, X.; You, H. NOV/CCN3 Induces Cartilage Protection by Inhibiting PI3K/AKT/MTOR Pathway. J. Cell. Mol. Med. 2019, 23, 7525–7534. [Google Scholar] [CrossRef] [PubMed]
- Janune, D.; Kubota, S.; Lazar, N.; Perbal, B.; Iida, S.; Takigawa, M. CCN3-Mediated Promotion of Sulfated Proteoglycan Synthesis in Rat Chondrocytes from Developing Joint Heads. J. Cell Commun. Signal. 2011, 5, 167–171. [Google Scholar] [CrossRef]
- Knudson, W.; Loeser, R.F. CD44 and Integrin Matrix Receptors Participate in Cartilage Homeostasis. Cell Mol. Life Sci. 2002, 59, 36–44. [Google Scholar] [CrossRef]
- Wu, S.C.; Chen, C.H.; Chang, J.K.; Fu, Y.C.; Wang, C.K.; Eswaramoorthy, R.; Lin, Y.S.; Wang, Y.H.; Lin, S.Y.; Wang, G.J.; et al. Hyaluronan Initiates Chondrogenesis Mainly via CD44 in Human Adipose-Derived Stem Cells. J. Appl. Physiol. 2013, 114, 1610–1618. [Google Scholar] [CrossRef] [PubMed]
- Grogan, S.P.; Miyaki, S.; Asahara, H.; D’Lima, D.D.; Lotz, M.K. Mesenchymal Progenitor Cell Markers in Human Articular Cartilage: Normal Distribution and Changes in Osteoarthritis. Arthritis Res. Ther. 2009, 11, R85. [Google Scholar] [CrossRef] [PubMed]
- Mazor, M.; Cesaro, A.; Ali, M.; Best, T.M.; Lespessaille, E.; Toumi, H. Progenitor Cells from Cartilage: Grade Specific Differences in Stem Cell Marker Expression. Int. J. Mol. Sci. 2017, 18, 1759. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.F.; Gulati, G.S.; Sinha, R.; Tompkins, J.V.; Lopez, M.; Carter, A.C.; Ransom, R.C.; Reinisch, A.; Wearda, T.; Murphy, M.; et al. Identification of the Human Skeletal Stem Cell. Cell 2018, 175, 43–56.e21. [Google Scholar] [CrossRef]
- Ramos, T.L.; Sánchez-Abarca, L.I.; Muntión, S.; Preciado, S.; Puig, N.; López-Ruano, G.; Hernández-Hernández, Á.; Redondo, A.; Ortega, R.; Rodríguez, C.; et al. MSC Surface Markers (CD44, CD73, and CD90) Can Identify Human MSC-Derived Extracellular Vesicles by Conventional Flow Cytometry. Cell Commun. Signal. 2016, 14, 2. [Google Scholar] [CrossRef]
- Samvelyan, H.J.; Madi, K.; Törnqvist, A.E.; Javaheri, B.; Staines, K.A. Characterisation of Growth Plate Dynamics in Murine Models of Osteoarthritis. Front. Endocrinol. 2021, 12, 734988. [Google Scholar] [CrossRef]
- Gamer, L.W.; Shi, R.R.; Gendelman, A.; Mathewson, D.; Gamer, J.; Rosen, V. Identification and Characterization of Adult Mouse Meniscus Stem/Progenitor Cells. Connect. Tissue Res. 2017, 58, 238–245. [Google Scholar] [CrossRef]
- Hao, R.C.; Li, Z.L.; Wang, F.Y.; Tang, J.; Li, P.L.; Yin, B.F.; Li, X.T.; Han, M.Y.; Mao, N.; Liu, B.; et al. Single-Cell Transcriptomic Analysis Identifies a Highly Replicating Cd168+ Skeletal Stem/Progenitor Cell Population in Mouse Long Bones. J. Genet. Genom. 2023, 50, 702–712. [Google Scholar] [CrossRef]
- Karlsen, T.A.; Sundaram, A.Y.M.; Brinchmann, J.E. Single-Cell RNA Sequencing of In Vitro Expanded Chondrocytes: MSC-Like Cells With No Evidence of Distinct Subsets. Cartilage 2021, 13, 774S–784S. [Google Scholar] [CrossRef]
- Zhang, P.; Dong, J.; Fan, X.; Yong, J.; Yang, M.; Liu, Y.; Zhang, X.; Lv, L.; Wen, L.; Qiao, J.; et al. Characterization of Mesenchymal Stem Cells in Human Fetal Bone Marrow by Single-Cell Transcriptomic and Functional Analysis. Signal Transduct. Target. Ther. 2023, 8, 126. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, N.; Huang, R.; Wang, L.; Ke, Q.; Cai, L.; Wu, S. Single-Cell Rna Seq Analysis Identifies the Biomarkers and Differentiation of Chondrocyte in Human Osteoarthritis. Am. J. Transl. Res. 2020, 12, 7326–7339. [Google Scholar]
- Wu, C.L.; Dicks, A.; Steward, N.; Tang, R.; Katz, D.B.; Choi, Y.R.; Guilak, F. Single Cell Transcriptomic Analysis of Human Pluripotent Stem Cell Chondrogenesis. Nat. Commun. 2021, 12, 362. [Google Scholar] [CrossRef]
- Huynh, N.P.T.; Kelly, N.H.; Katz, D.; Pham, M.; Guilak, F. Single cell RNA sequencing reveals heterogeneity of human MSC chondrogenesis: Lasso regularized logistic regression to identify gene and regulatory signatures. bioRxiv 2019, 854406. [Google Scholar] [CrossRef]
- Tong, W.; Geng, Y.; Huang, Y.; Shi, Y.; Xiang, S.; Zhang, N.; Qin, L.; Shi, Q.; Chen, Q.; Dai, K.; et al. In Vivo Identification and Induction of Articular Cartilage Stem Cells by Inhibiting NF-ΚB Signaling in Osteoarthritis. Stem Cells 2015, 33, 3125–3137. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Cai, H. The Role of Chondrocyte Senescence in Osteoarthritis Pathogenesis and Therapeutic Implications. Exp. Gerontol. 2025, 208, 112828. [Google Scholar] [CrossRef] [PubMed]
- Toh, W.S.; Brittberg, M.; Farr, J.; Foldager, C.B.; Gomoll, A.H.; Hui, J.H.P.; Richardson, J.B.; Roberts, S.; Spector, M. Cellular Senescence in Aging and Osteoarthritis: Implications for Cartilage Repair. Acta Orthop. 2016, 87, 6–14. [Google Scholar] [CrossRef]
- Hu, N.; Qiu, J.; Xu, B.; Zhang, S.; Guo, Z.; Xie, J.; Yang, W. The Role of Cartilage Stem/Progenitor Cells in Cartilage Repair in Osteoarthritis. Curr. Stem Cell Res. Ther. 2022, 18, 892–903. [Google Scholar] [CrossRef] [PubMed]
- Maleki, M.; Ghanbarvand, F.; Behvarz, M.R.; Ejtemaei, M.; Ghadirkhomi, E. Comparison of Mesenchymal Stem Cell Markers in Multiple Human Adult Stem Cells. Int. J. Stem Cells 2014, 7, 118–126. [Google Scholar] [CrossRef]
- Marinova-Mutafchieva, L.; Taylor, P.; Funa, K.; Maini, R.N.; Zvaifler, N.J. Mesenchymal Cells Expressing Bone Morphogenetic Protein Receptors Are Present in the Rheumatoid Arthritis Joint. Arthritis Rheum. 2000, 43, 2046–2055. [Google Scholar] [CrossRef]
- Zhu, H.; Mitsuhashi, N.; Klein, A.; Barsky, L.W.; Weinberg, K.; Barr, M.L.; Demetriou, A.; Wu, G.D. The Role of the Hyaluronan Receptor CD44 in Mesenchymal Stem Cell Migration in the Extracellular Matrix. Stem Cells 2006, 24, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Fard, S.B.; Mazaheri, Z.; Ghorbanmehr, N.; Movahedin, M.; Fard, M.B.; Gholampour, M.A. Analysis of MiRNA-17 and MiRNA-146 Expression during Differentiation of Spermatogonial Stem like Cells Derived from Mouse Bone Marrow Mesenchymal Stem Cells. Int. J. Mol. Cell. Med. 2019, 8, 14–23. [Google Scholar] [CrossRef]
- Grässel, S.; Zaucke, F.; Madry, H. Osteoarthritis: Novel Molecular Mechanisms Increase Our Understanding of the Disease Pathology. J. Clin. Med. 2021, 10, 1938. [Google Scholar] [CrossRef]
- Cho, Y.; Jeong, S.; Kim, H.; Kang, D.; Lee, J.; Kang, S.B.; Kim, J.H. Disease-Modifying Therapeutic Strategies in Osteoarthritis: Current Status and Future Directions. Exp. Mol. Med. 2021, 53, 1689–1696. [Google Scholar] [CrossRef]
- Coryell, P.R.; Diekman, B.O.; Loeser, R.F. Mechanisms and Therapeutic Implications of Cellular Senescence in Osteoarthritis. Nat. Rev. Rheumatol. 2021, 17, 47–57. [Google Scholar] [CrossRef]
- Gupta, N.; Wang, H.; McLeod, T.L.; Naus, C.C.G.; Kyurkchiev, S.; Advani, S.; Yu, J.; Perbal, B.; Weichselbaum, R.R. Inhibition of Glioma Cell Growth and Tumorigenic Potential by CCN3 (NOV). Mol. Pathol. 2001, 54, 293–299. [Google Scholar] [CrossRef]
- Fukunaga-Kalabis, M.; Martinez, G.; Telson, S.M.; Liu, Z.J.; Balint, K.; Juhasz, I.; Elder, D.E.; Perbal, B.; Herlyn, M. Downregulation of CCN3 Expression as a Potential Mechanism for Melanoma Progression. Oncogene 2008, 27, 2552–2560. [Google Scholar] [CrossRef]
- Knudson, C.B.; Knudson, W. Hyaluronan and CD44: Modulators of Chondrocyte Metabolism. Clin. Orthop. Relat. Res. 2004, 427, 152–162. [Google Scholar] [CrossRef]
- Zhang, F.-J.; Luo, W.; Gao, S.-G.; Su, D.-Z.; Li, Y.-S.; Zeng, C.; Lei, G.-H. Expression of CD44 in Articular Cartilage Is Associated with Disease Severity in Knee Osteoarthritis. Mod. Rheumatol. 2013, 23, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Ostergaard, K.; Salter, D.M.; Andersen, C.B.; Petersen, J.; Bendtzen, K. CD44 Expression Is Up-Regulated in the Deep Zone of Osteoarthritic Cartilage from Human Femoral Heads. Histopathology 1997, 31, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Ishida, O.; Tanaka, Y.; Morimoto, I.; Takigawa, M.; Eto, S. Chondrocytes Are Regulated by Cellular Adhesion through CD44 and Hyaluronic Acid Pathway. J. Bone Miner. Res. 1997, 12, 1657–1663. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, A.; Kc, R.; Angelozzi, M.; de Charleroy, C.; Rux, D.; Tower, R.J.; Yao, L.; da Silva, R.P.; Pacifici, M.; Qin, L.; et al. SOX9 Keeps Growth Plates and Articular Cartilage Healthy by Inhibiting Chondrocyte Dedifferentiation/Osteoblastic Redifferentiation. Proc. Natl. Acad. Sci. USA 2021, 118, e2019152118. [Google Scholar] [CrossRef]
- Zhang, Q.; Ji, Q.; Wang, X.; Kang, L.; Fu, Y.; Yin, Y.; Li, Z.; Liu, Y.; Xu, X.; Wang, Y. SOX9 Is a Regulator of ADAMTSs-Induced Cartilage Degeneration at the Early Stage of Human Osteoarthritis. Osteoarthr. Cartil. 2015, 23, 2259–2268. [Google Scholar] [CrossRef]
- Liang, B.; Mamidi, M.K.; Samsa, W.E.; Chen, Y.; Lee, B.; Zheng, Q.; Zhou, G. Targeted and Sustained Sox9 Expression in Mouse Hypertrophic Chondrocytes Causes Severe and Spontaneous Osteoarthritis by Perturbing Cartilage Homeostasis. Am. J. Transl. Res. 2020, 12, 1056–1069. [Google Scholar]
- Vousden, K.H.; Prives, C. Blinded by the Light: The Growing Complexity of P53. Cell 2009, 137, 413–431. [Google Scholar] [CrossRef]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular Senescence in Ageing: From Mechanisms to Therapeutic Opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Campisi, J.; d’Adda di Fagagna, F. Cellular Senescence: When Bad Things Happen to Good Cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef]
- Matsushita, Y.; Sakamoto, K.; Tamamura, Y.; Shibata, Y.; Minamizato, T.; Kihara, T.; Ito, M.; Katsube, K.I.; Hiraoka, S.; Koseki, H.; et al. CCN3 Protein Participates in Bone Regeneration as an Inhibitory Factor. J. Biol. Chem. 2013, 288, 19973–19985. [Google Scholar] [CrossRef]
- Wang, K.; Mitoh, Y.; Horie, K.; Yoshida, R. Exploring the Role of Ccn3 in Type III Cell of Mice Taste Buds. J. Neurochem. 2025, 169, e16291. [Google Scholar] [CrossRef] [PubMed]
- Glasson, S.S.; Blanchet, T.J.; Morris, E.A. The Surgical Destabilization of the Medial Meniscus (DMM) Model of Osteoarthritis in the 129/SvEv Mouse. Osteoarthr. Cartil. 2007, 15, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Tu, P.; Xu, Q.; Zhou, X.; Villa-Roel, N.; Kumar, S.; Dong, N.; Jo, H.; Ou, C.; Lin, Z. Myeloid CCN3 Protects against Aortic Valve Calcification. Cell Commun. Signal. 2023, 21, 14. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habumugisha, J.; Okuda, R.; Hirose, K.; Kuwahara, M.; Wang, Z.; Ono, M.; Kamioka, H.; Kubota, S.; Hattori, T. Critical Requirement of Senescence-Associated CCN3 Expression in CD44-Positive Stem Cells for Osteoarthritis Progression. Int. J. Mol. Sci. 2025, 26, 9630. https://doi.org/10.3390/ijms26199630
Habumugisha J, Okuda R, Hirose K, Kuwahara M, Wang Z, Ono M, Kamioka H, Kubota S, Hattori T. Critical Requirement of Senescence-Associated CCN3 Expression in CD44-Positive Stem Cells for Osteoarthritis Progression. International Journal of Molecular Sciences. 2025; 26(19):9630. https://doi.org/10.3390/ijms26199630
Chicago/Turabian StyleHabumugisha, Janvier, Ryuichiro Okuda, Kazuki Hirose, Miho Kuwahara, Ziyi Wang, Mitsuaki Ono, Hiroshi Kamioka, Satoshi Kubota, and Takako Hattori. 2025. "Critical Requirement of Senescence-Associated CCN3 Expression in CD44-Positive Stem Cells for Osteoarthritis Progression" International Journal of Molecular Sciences 26, no. 19: 9630. https://doi.org/10.3390/ijms26199630
APA StyleHabumugisha, J., Okuda, R., Hirose, K., Kuwahara, M., Wang, Z., Ono, M., Kamioka, H., Kubota, S., & Hattori, T. (2025). Critical Requirement of Senescence-Associated CCN3 Expression in CD44-Positive Stem Cells for Osteoarthritis Progression. International Journal of Molecular Sciences, 26(19), 9630. https://doi.org/10.3390/ijms26199630





