Antioxidant System Response of Yarrowia lipolytica Cells Under Oxidative Stress
Abstract
1. Introduction
2. Results
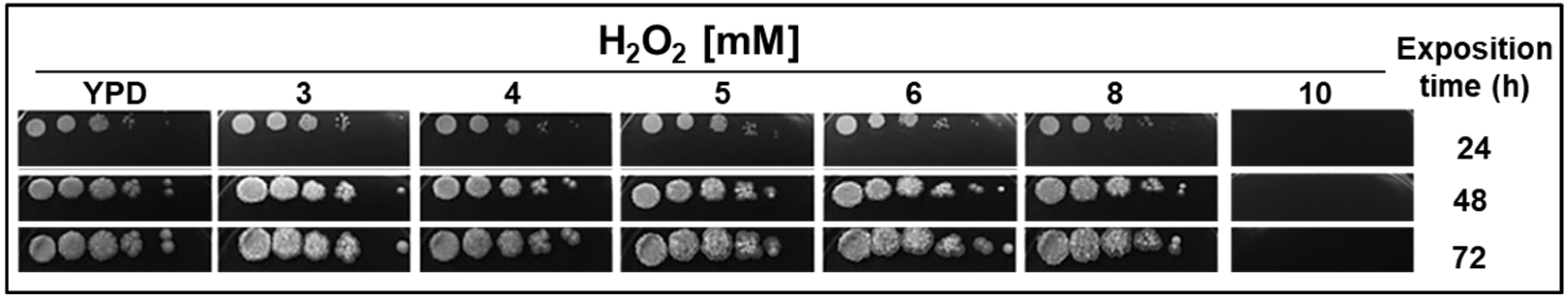
2.1. H2O2 Affects Y. lipolytica Cell Growth
2.2. Y. lipolytica Cell Growth: Identification of Different Life Stages
2.3. Oxidative Conditions Modified the Gene Expression Profile
2.4. The Gene Expression of the Antioxidant Response Is Higher in Young Y. lipolytica Cells
3. Discussion
4. Materials and Methods
4.1. Identification of Sequences That Encode the Genes of Interest
4.2. Microorganisms and Culture Conditions
4.3. Yarrowia lipolytica Growth Curve
4.4. Effect of Different H2O2 Concentrations on Cell Growth
4.5. Oxidative Stress Induction and Nucleic Acid Extraction
4.6. mRNA Purification and cDNA Synthesis
4.7. Gene Expression Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flohé, L. Looking back at the early stages of redox biology. Antioxidants 2020, 9, 1254. [Google Scholar] [CrossRef] [PubMed]
- Rowe, L.A.; Degtyareva, N.; Doetsch, P.W. DNA damage-induced reactive oxygen species (ROS) stress response in Saccharomyces cerevisiae. Free Radic. Biol. Med. 2008, 45, 1167–1177. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Eustress and distress in redox homeostasis. In Stress: Physiology, Biochemistry, and Pathology; Fink, G., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 153–163. [Google Scholar] [CrossRef]
- Wahlqvist, M.L. Antioxidant relevance to human health. Asia Pac. J. Clin. Nutr. 2013, 22, 171–176. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.; Davies, K.; Dick, T.; Finkel, T.; Forman, H.; Jassen-Heininger, Y.; Gems, D.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metabol. 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Ojaimi, C.; Kinugawa, S.; Recchia, F.A.; Hintze, T.H. Oxidant-NO dependent gene regulation in dogs with type I diabetes: Impact on cardiac function and metabolism. Cardiovasc. Diabetol. 2010, 9, 43. [Google Scholar] [CrossRef]
- Miller, V.J.; Villamena, F.A.; Volek, J.S. Nutritional ketosis and mitohormesis: Potential implications for mitochondrial function and human health. J. Nutr. Metab. 2018, 2018, 5157645. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eu-stress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Marchi, S.; Simoes, I.C.M.; Ren, Z.; Morciano, G.; Perrone, M.; Patalas-Krawczyk, P.; Borchard, S.; Jedrak, P.; Pierzynowska, K.; et al. Mitochondria and reactive oxygen species in aging and age-related diseases. Int. Rev. Cell Mol. Biol. 2018, 340, 209–344. [Google Scholar] [CrossRef]
- Rahman, K. Studies on free radicals, antioxidants, and co-factors. Clin. Interv. Aging 2007, 2, 219–236. [Google Scholar] [PubMed]
- Xiao, H.; Jedrychowski, M.; Schweppe, D.; Huttlin, E.; Yu, Q.; Heppner, D.; Li, J.; Long, J.; Millis, E.; Szpyt, J.; et al. A quantitative tissue-specific landscape of protein redox regulation during aging. Cell 2020, 180, 968–983.e24. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.; Meade, J.; Peace, T.; Zhou, T. Inflammaging and oxidative stress in human diseases: From molecular mechanisms to novel treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef]
- Cheeseman, K.H.; Slater, T.F. An introduction to free radical biochemistry. Br. Med. Bull. 1993, 49, 481–493. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709, Erratum in Nat. Rev. Drug Discov. 2021, 20, 652. [Google Scholar] [CrossRef]
- Pitocco, D.; Zaccardi, F.; Di Stasio, E.; Romitelli, F.; Santini, S.; Zuppi, C.; Ghirlanda, G. Oxidative stress, nitric oxide, and diabetes. Rev. Diabet. Stud. 2010, 7, 15–25. [Google Scholar] [CrossRef]
- Brieger, K.; Schiavone, S.; Miller, F.J.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Chuang, L.M. The role of oxidative stress in the pathogenesis of type 2 diabetes: From molecular mechanism to clinical implication. Am. J. Transl. Res. 2010, 2, 316–331. [Google Scholar] [PubMed]
- Kawahito, S.; Kitahata, H.; Oshita, S. Problems associated with glucose toxicity: Role of hyperglycemia-induced oxidative stress. World J. Gastroenterol. 2009, 15, 4137–4142. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdul, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Shao, H.; Tu, Y.; Wang, Y.; Jiang, C.; Ma, L.; Hu, Z.; Wang, J.; Zeng, B.; He, B. Oxidative stress response of Aspergillus oryzae induced by hydrogen peroxide and menadione sodium bisulfite. Microorganisms 2019, 7, 225. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defense antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defense grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Mulford, K.E.; Fassler, J.S. Association of the Skn7 and Yap1 transcription factors in the Saccharomyces cerevisiae oxidative stress response. Eukaryot. Cell 2011, 10, 761–769. [Google Scholar] [CrossRef]
- Dawes, I.W.; Perrone, G.G. Stress and ageing in yeast. FEMS Yeast Res. 2020, 20, foz085. [Google Scholar] [CrossRef] [PubMed]
- Pusev, M.S.; Klein, O.I.; Gessler, N.N.; Bachurina, G.; Filippovich, S.; Isakova, E.; Deryabina, Y. Effect of dihydroquercetin during long-last growth of Yarrowia lipolytica yeast: Anti-aging potential and hormetic properties. Int. J. Mol. Sci. 2024, 25, 12574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Kennedy, B.K. Investigating the biology of yeast aging by single-cell RNA-seq. Aging 2023, 15, 7340–7342. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y.; Huang, Y.; An, Y.; Fu, X.; Yang, D.; Wang, Y.; Zhang, J.; Mitchell, L.; Bader, J.; et al. The de novo design and synthesis of yeast chromosome XIII facilitates investigations on aging. Nat. Commun. 2024, 15, 10139. [Google Scholar] [CrossRef]
- Sofianovich, O.; Willis-Urena, K.; Dong, Y.; Ignea, C. Bioengineered yeast for preventing age-related diseases. Trends Biotechnol. 2024, 43, 586–600. [Google Scholar] [CrossRef]
- Ledesma-Amaro, R.; Nicaud, J.M. Yarrowia lipolytica as a biotechnological chassis to produce usual and unusual fatty acids. Prog. Lipid Res. 2016, 61, 40–50. [Google Scholar] [CrossRef]
- Lopes, M.; Mota, M.; Belo, I. Comparison of Yarrowia lipolytica and Pichia pastoris cellular response to different agents of oxidative stress. Appl. Biochem. Biotechnol. 2013, 170, 448–458. [Google Scholar] [CrossRef]
- Biryukova, E.N.; Medentsev, A.G.; Arinbasarova, A.Y.; Akimenko, V.K. Adaptation of the yeast Yarrowia lipolytica to heat shock. Microbiology 2007, 76, 158–163. [Google Scholar] [CrossRef]
- Arinbasarova, A.Y.; Biryukova, E.N.; Medentsev, A.G. Antistress systems of the yeast Yarrowia lipolytica (review). Prikl Biokhim Mikrobiol. 2015, 51, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, M.A.; Workman, C.T. Oxidative stress response pathways: Fission yeast as archetype. Crit. Rev. Microbiol. 2015, 41, 520–535. [Google Scholar] [CrossRef]
- Kavitha, S.; Chandra, T.S. Oxidative stress protection and glutathione metabolism in response to hydrogen peroxide and menadione in riboflavinogenic fungus Ashbya gossypii. Appl. Biochem. Biotechnol. 2014, 174, 2307–2325. [Google Scholar] [CrossRef] [PubMed]
- Quiñones-González, C.A.; Arredondo-Mendoza, G.I.; Jiménez-Salas, Z.; Larriba-Calle, G.; Ruiz-Herrera, J.; Campos-Góngora, E. Genotoxic effect of caffeine in Yarrowia lipolytica cells deficient in DNA repair mechanisms. Arch. Microbiol. 2019, 201, 991–998. [Google Scholar] [CrossRef] [PubMed]
- De Sá, R.A.; de Castro, F.A.; Eleutherio, E.C.; de Souza, R.M.; da Silva, J.F.; Pereira, M. Brazilian propolis protects Saccharomyces cerevisiae cells against oxidative stress. Braz. J. Microbiol. 2013, 44, 993–1000. [Google Scholar] [CrossRef]
- Wu, M.J.; O’Doherty, P.J.; Fernandez, H.R.; Lyons, V.; Rogers, P.J.; Dawes, I.W.; Higgins, V.J. An antioxidant screening assay based on oxidant-induced growth arrest in Saccharomyces cerevisiae. FEMS Yeast Res. 2011, 11, 379–387. [Google Scholar] [CrossRef]
- Liu, J.; Wisniewski, M.; Droby, S.; Norelli, J.; Hershkovitz, V.; Tian, S.; Farrell, R. Increase in antioxidant gene transcripts, stress tolerance and biocontrol efficacy of Candida oleophila following sublethal oxidative stress exposure. FEMS Microbiol. Ecol. 2012, 80, 578–590. [Google Scholar] [CrossRef]
- Martins, D.; English, A.M. Catalase activity is stimulated by H2O2 in rich culture medium and is required for H2O2 resistance and adaptation in yeast. Redox Biol. 2014, 2, 308–313. [Google Scholar] [CrossRef]
- Sui, Y.; Wisniewski, M.; Droby, S.; Liu, J. Responses of yeast biocontrol agents to environmental stress. Appl. Environ. Microbiol. 2015, 81, 2968–2975. [Google Scholar] [CrossRef]
- Gao, Y.; Fang, L.; Wang, X.; Lan, R.; Wang, M.; Du, G.; Guan, W.; Liu, J.; Brennan, M.; Guo, H.; et al. Antioxidant activity evaluation of dietary flavonoid hyperoside using Saccharomyces cerevisiae as a model. Molecules 2019, 24, 788. [Google Scholar] [CrossRef]
- Vázquez, J.; Grillitsch, K.; Daum, G.; Mas, A.; Torija, M.J.; Beltran, G. Melatonin minimizes the impact of oxidative stress induced by hydrogen peroxide in Saccharomyces and non-conventional yeast. Front. Microbiol. 2018, 9, 1933. [Google Scholar] [CrossRef]
- Tsang, C.K.; Liu, Y.; Thomas, J.; Zhang, Y.; Zheng, X.F. Superoxide dismutase 1 acts as a nuclear transcription factor to regulate oxidative stress resistance. Nat. Commun. 2014, 5, 3446. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Ozumi, K.; Kim, H.W.; Nakagawa, O.; McKinney, R.D.; Folz, R.J.; Zelko, I.N.; Ushio-Fukai, M.; Fukai, T. Novel mechanism for regulation of extracellular SOD transcription and activity by copper: Role of antioxidant-1. Free Radic. Biol. Med. 2009, 46, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.M.; Torres, A.S.; Doan, P.E.; O’Halloran, T.V. Oxygen and the copper chaperone CCS regulate posttranslational activation of Cu, Zn superoxide dismutase. Proc. Natl. Acad. Sci. USA 2004, 101, 5518–5523. [Google Scholar] [CrossRef] [PubMed]
- Canizal-García, M.; Olmos-Orizaba, B.E.; Moreno-Jiménez, M.; Calderón-Cortés, E.; Saavedra-Molina, A.; Cortés-Rojo, C. Glutathione peroxidase 2 (Gpx2) preserves mitochondrial function and decreases ROS levels in chronologically aged yeast. Free Radic. Res. 2021, 55, 165–175. [Google Scholar] [CrossRef]
- Vázquez, J.; González, B.; Sempere, V.; Mas, A.; Torija, M.J.; Beltran, G. Melatonin reduces oxidative stress damage induced by hydrogen peroxide in Saccharomyces cerevisiae. Front. Microbiol. 2017, 8, 1066. [Google Scholar] [CrossRef]
- Ivan, A.; Lukinich-Gruia, A.T.; Cristea, I.M.; Pricop, M.A.; Calma, C.L.; Simina, A.G.; Tatu, C.A.; Galuscan, A.; Păunescu, V. Quercetin and mesenchymal stem cell metabolism: A comparative analysis of young and senescent states. Molecules 2024, 29, 5755. [Google Scholar] [CrossRef]
- Biryukova, E.N.; Medentsev, A.G.; Arinbasarova, A.Y.; Akimenko, V.K. Tolerance of the yeast Yarrowia lipolytica to oxidative stress. Microbiology 2006, 75, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.L.; Magalhães-Guedes, K.T. Preparing Yeast Suspension Through Serial Dilution for Enumeration. In Detection and Enumeration of Bacteria, Yeast, Viruses, and Protozoan in Foods and Freshwater; Magnani, M., Ed.; Springer Protocols; Humana Press: New York, NY, USA, 2021; pp. 109–116. [Google Scholar] [CrossRef]
- Sherman, F. Getting started with yeast. Methods Enzymol. 2002, 350, 3–41. [Google Scholar] [CrossRef]
- Thomas, P.; Sekhar, A.C.; Upreti, R.; Mujawar, M.M.; Pasha, A.S. Optimization of single plate-serial dilution spotting (SP-SDS) with sample anchoring as an assured method for bacterial and yeast CFU enumeration and single colony isolation from diverse samples. Biotechnol. Rep. 2015, 8, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, C.S.; Winston, F. Ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for the transformation of Escherichia coli. Gene 1987, 57, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Teste, M.A.; Duquenne, M.; François, J.M.; Parrou, J.-L. Validation of reference genes for quantitative expression analysis by real-time RT-PCR in Saccharomyces cerevisiae. BMC Molecular Biol. 2009, 10, 99. [Google Scholar] [CrossRef] [PubMed]


| Gene | NCBI-RS | Primer Sequence (5′→3′) | Annealing Temperature (°C) | Amplicon Size (bp) |
|---|---|---|---|---|
| CCS | YALI0F30877g | F: CACTGGAACTTCTGCTCCGTCCC R: CTGGACGTCCTTTCGCTCCTCC | 60 | 547 |
| SOD | YALI0E12133g | F: CTTCGAGGAGATTCCAAGGTCTCC R: CTTGAGAGAGTCGGCATGGCC | 60 | 390 |
| GPX | YALI0E02310g | F: CCGCTTTCTACAACCTCGCTCC R: CGACGTTACCGTGCTTATCAACC | 60 | 411 |
| CAT1 | YALI0E34265g | F: CCACCACCGTGCGATTTTCTACC R: CATGGTCTGAAGGGAAACGGTCC | 60 | 539 |
| CAT2 | YALI0E34749g | F: CCATGCAAAGGGAGGAGGAGCC R: CCGTCCACGAGGGGTAATCCC | 60 | 623 |
| CAT3 | YALI0F30987g | F: CAAGACCTTCACTCGATTCTCCACC R: CGTCATTGGTGAGGTTCTTGATGCC | 60 | 425 |
| UBC6 | YALI0E30173g | F: CCGCGAAACCAGCAGGAACAATCTCC R: CCGAGGAATCTAGCTGCCACAATCC | 60 | 598 |
| Gene | Young Cells | Aged Cells | p-Value |
|---|---|---|---|
| CCS | 7.66 ± 5.22 | 4.43 ± 1.13 | 0.2695 |
| SOD | 26.09 ± 2.76 | 16.97 ± 5.24 | 0.0800 |
| GPX | 19.35 ± 7.13 | 13.20 ± 6.10 | 0.1818 |
| CAT1 | 13.81 ± 2.97 * | 6.48 ± 3.06 * | 0.005 |
| CAT2 | 23.86 ± 2.37 ** | 11.30 ± 2.54 ** | 0.0001 |
| CAT3 | 27.62 ± 4.92 | 27.77 ± 1.80 | 0.9595 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arredondo-Mendoza, G.I.; Castillo-Roque, M.; Miranda-Roblero, H.O.; Desentis-Desentis, M.F.; Teniente, S.L.; Jiménez-Salas, Z.; Campos-Góngora, E. Antioxidant System Response of Yarrowia lipolytica Cells Under Oxidative Stress. Int. J. Mol. Sci. 2025, 26, 9629. https://doi.org/10.3390/ijms26199629
Arredondo-Mendoza GI, Castillo-Roque M, Miranda-Roblero HO, Desentis-Desentis MF, Teniente SL, Jiménez-Salas Z, Campos-Góngora E. Antioxidant System Response of Yarrowia lipolytica Cells Under Oxidative Stress. International Journal of Molecular Sciences. 2025; 26(19):9629. https://doi.org/10.3390/ijms26199629
Chicago/Turabian StyleArredondo-Mendoza, Gerardo Ismael, Maripaz Castillo-Roque, Hipólito Otoniel Miranda-Roblero, María Fernanda Desentis-Desentis, Sandra Lucía Teniente, Zacarías Jiménez-Salas, and Eduardo Campos-Góngora. 2025. "Antioxidant System Response of Yarrowia lipolytica Cells Under Oxidative Stress" International Journal of Molecular Sciences 26, no. 19: 9629. https://doi.org/10.3390/ijms26199629
APA StyleArredondo-Mendoza, G. I., Castillo-Roque, M., Miranda-Roblero, H. O., Desentis-Desentis, M. F., Teniente, S. L., Jiménez-Salas, Z., & Campos-Góngora, E. (2025). Antioxidant System Response of Yarrowia lipolytica Cells Under Oxidative Stress. International Journal of Molecular Sciences, 26(19), 9629. https://doi.org/10.3390/ijms26199629







