A Targeted Blockade of Terminal C5a Is Critical to Management of Sepsis and Acute Respiratory Distress Syndrome: The Mechanism of Action of Vilobelimab
Abstract
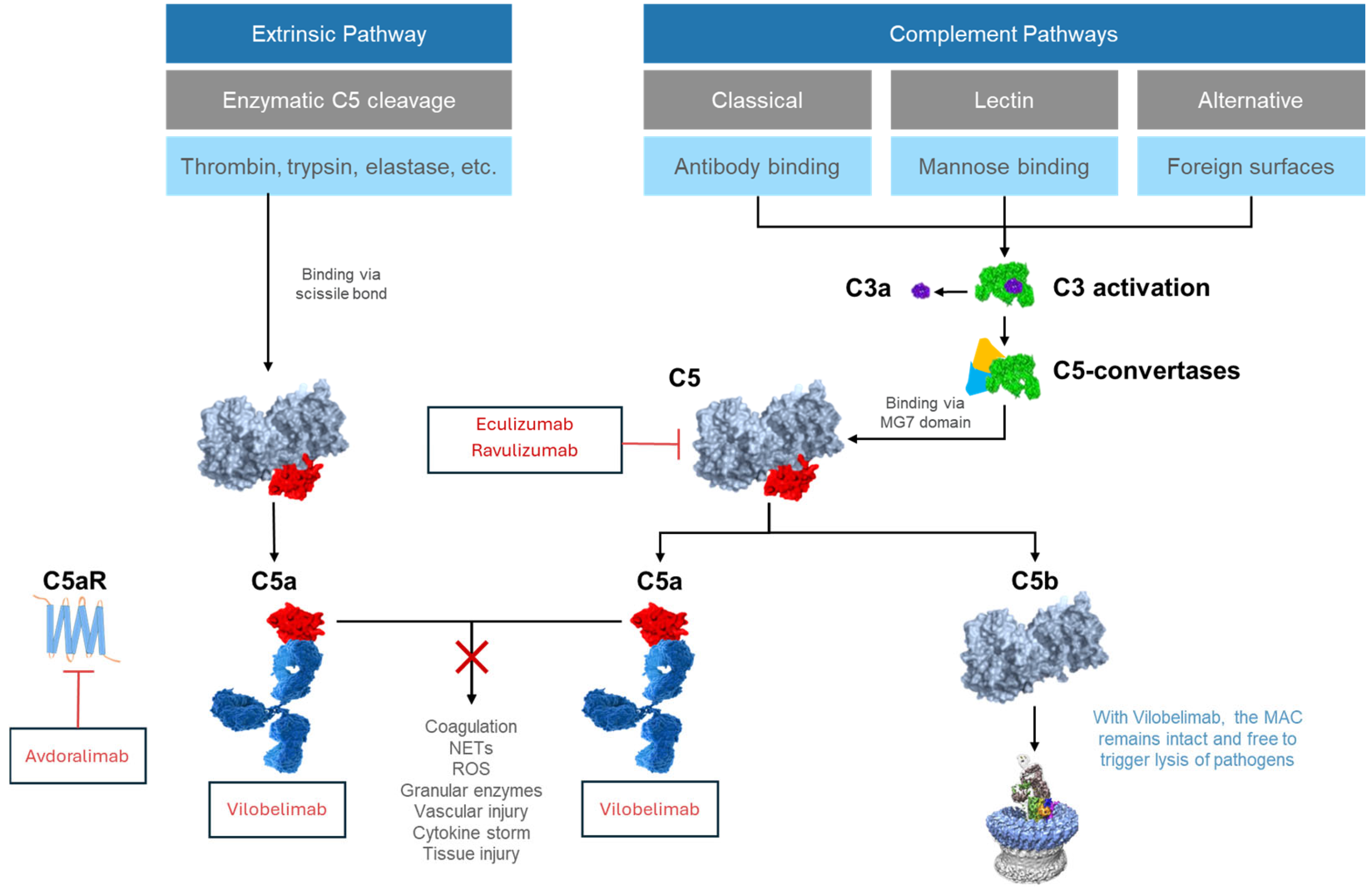
1. Introduction
2. Complement Activation
3. Downstream Effects of Activation of the C5a/C5aR1 Axis
4. C5a and C5aR1 in Sepsis and ARDS
4.1. Pre-Clinical Evidence
4.2. Clinical Evidence
5. Upstream Complement Inhibitors That Do Not Fully Block C5a
6. Vilobelimab
6.1. Mechanism of Action
6.2. Preclinical Evidence
6.3. Clinical Evidence in Sepsis
6.4. Clinical Efficacy and Safety in COVID-19 Induced ARDS
7. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Janeway, C.A., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 1989, 54 Pt 1, 1–13. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr.; Travers, P.; Walport, M. Principles of innate and adaptive immunity. In Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Leligdowicz, A.; Harhay, M.O.; Calfee, C.S. Immune Modulation in Sepsis, ARDS, and COVID-19—The Road Traveled and the Road Ahead. NEJM Evid. 2022, 1, EVIDra2200118. [Google Scholar] [CrossRef]
- DeLeo, F.R.; Allen, L.H. Phagocytosis and neutrophil extracellular traps. Fac. Rev. 2020, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.L.; Karl, I.; Giner, T.; Poppe, H.; Schmidt, M.; Presser, D.; Goebeler, M.; Bauer, B. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br. J. Dermatol. 2016, 174, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Wallach, D.; Vignon-Pennamen, M.D. From acute febrile neutrophilic dermatosis to neutrophilic disease: Forty years of clinical research. J. Am. Acad. Dermatol. 2006, 55, 1066–1071. [Google Scholar] [CrossRef]
- Xiao, H.; Schreiber, A.; Heeringa, P.; Falk, R.J.; Jennette, J.C. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am. J. Pathol. 2007, 170, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Hendrickson, K.W.; Peltan, I.D.; Brown, S.M. The Epidemiology of Acute Respiratory Distress Syndrome Before and After Coronavirus Disease 2019. Crit. Care Clin. 2021, 37, 703–716. [Google Scholar] [CrossRef]
- Kang, M.; Kempker, J.A. Definitions, Epidemiology, Clinical Risk Factors, and Health Disparities in Acute Respiratory Distress Syndrome. Semin. Respir. Crit. Care Med. 2019, 40, 3–11. [Google Scholar] [CrossRef]
- Diamond, M.; Peniston, H.L.; Sanghavi, D.K.; Mahapatra, S. Acute Respiratory Distress Syndrome; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Bardaji-Carrillo, M.; Lopez-Herrero, R.; Aguilar, G.; Arroyo-Hernantes, I.; Gomez-Sanchez, E.; Camporota, L.; Villar, J.; Tamayo, E. Epidemiological trends of mechanically ventilated acute respiratory distress syndrome in the twenty-first century: A nationwide, population-based retrospective study. J. Intensive Care 2025, 13, 9. [Google Scholar] [CrossRef]
- Zambon, M.; Vincent, J.L. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest 2008, 133, 1120–1127. [Google Scholar] [CrossRef]
- Dunkelberger, J.R.; Song, W.C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010, 20, 34–50. [Google Scholar] [CrossRef]
- Rus, H.; Cudrici, C.; Niculescu, F. The role of the complement system in innate immunity. Immunol. Res. 2005, 33, 103–112. [Google Scholar] [CrossRef]
- Sarma, J.V.; Ward, P.A. The complement system. Cell Tissue Res. 2011, 343, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Klos, A.; Wende, E.; Wareham, K.J.; Monk, P.N. International Union of Basic and Clinical Pharmacology. LXXXVII. Complement peptide C5a, C4a, and C3a receptors. Pharmacol. Rev. 2013, 65, 500–543, Erratum in: Pharmacol. Rev. 2014, 66, 466. [Google Scholar] [CrossRef] [PubMed]
- Riedemann, N.C.; Habel, M.; Ziereisen, J.; Hermann, M.; Schneider, C.; Wehling, C.; Kirschfink, M.; Kentouche, K.; Guo, R. Controlling the anaphylatoxin C5a in diseases requires a specifically targeted inhibition. Clin. Immunol. 2017, 180, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, A.; Walachowski, S.; Bosmann, M. The complement system: A key player in the host response to infections. Eur. J. Immunol. 2024, 54, e2350814. [Google Scholar] [CrossRef]
- Ma, W.; Tang, S.; Yao, P.; Zhou, T.; Niu, Q.; Liu, P.; Tang, S.; Chen, Y.; Gan, L.; Cao, Y. Advances in acute respiratory distress syndrome: Focusing on heterogeneity, pathophysiology, and therapeutic strategies. Signal Transduct. Target. Ther. 2025, 10, 75. [Google Scholar] [CrossRef]
- Szachowicz, P.J.; Wohlford-Lenane, C.; Donelson, C.J.; Ghimire, S.; Thurman, A.; Xue, B.; Boly, T.J.; Verma, A.; MašinoviĆ, L.; Bermick, J.R.; et al. Complement is primarily activated in the lung in a mouse model of severe COVID-19. iScience 2025, 28, 111930. [Google Scholar] [CrossRef]
- Guo, R.F.; Riedemann, N.C.; Ward, P.A. Role of C5a-C5aR interaction in sepsis. Shock 2004, 21, 1–7. [Google Scholar] [CrossRef]
- Lim, E.H.T.; Vlaar, A.P.J.; de Bruin, S.; Rückinger, S.; Thielert, C.; Habel, M.; Guo, R.; Burnett, B.P.; Dickinson, J.; Brouwer, M.C.; et al. Pharmacokinetic analysis of vilobelimab, anaphylatoxin C5a and antidrug antibodies in PANAMO: A phase 3 study in critically ill, invasively mechanically ventilated COVID-19 patients. Intensive Care Med. Exp. 2023, 11, 37. [Google Scholar] [CrossRef]
- Vlaar, A.P.J.; Lim, E.H.T.; de Bruin, S.; Rückinger, S.; Pilz, K.; Brouwer, M.C.; Guo, R.F.; Heunks, L.M.A.; Busch, M.H.; van Paassen, P.; et al. The anti-C5a antibody vilobelimab efficiently inhibits C5a in patients with severe COVID-19. Clin. Transl. Sci. 2022, 15, 854–858. [Google Scholar] [CrossRef] [PubMed]
- Vlaar, A.P.J.; Witzenrath, M.; van Paassen, P.; Heunks, L.M.A.; Mourvillier, B.; de Bruin, S.; Lim, E.H.T.; Brouwer, M.C.; Tuinman, P.R.; Saraiva, J.F.K.; et al. Anti-C5a antibody (vilobelimab) therapy for critically ill, invasively mechanically ventilated patients with COVID-19 (PANAMO): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2022, 10, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.J.; Argyropoulou, M.; Kanni, T.; Spyridopoulos, T.; Otto, I.; Zenker, O.; Guo, R.; Riedemann, N.C. Clinical efficacy of complement C5a inhibition by IFX-1 in hidradenitis suppurativa: An open-label single-arm trial in patients not eligible for adalimumab. Br. J. Dermatol. 2020, 183, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Merkel, P.; Hellmich, B.; Pfaff, A.; Müller, C.; Startseva, E.; Jayne, D. A Randomized, Double-Blind, Phase II Study of Glucocorticoid Replacement by Vilobelimab, an Anti-C5a Monoclonal Antibody, in ANCA-Associated Vasculitis. Arthritis Rheumatol. 2022, 74, 1054–1055. [Google Scholar]
- Markiewski, M.M.; Lambris, J.D. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am. J. Pathol. 2007, 171, 715–727. [Google Scholar] [CrossRef]
- Serna, M.; Giles, J.L.; Morgan, B.P.; Bubeck, D. Structural basis of complement membrane attack complex formation. Nat. Commun. 2016, 7, 10587. [Google Scholar] [CrossRef]
- Gerard, C.; Gerard, N.P. C5A anaphylatoxin and its seven transmembrane-segment receptor. Annu. Rev. Immunol. 1994, 12, 775–808. [Google Scholar] [CrossRef]
- Pandey, S.; Maharana, J.; Li, X.X.; Woodruff, T.M.; Shukla, A.K. Emerging Insights into the Structure and Function of Complement C5a Receptors. Trends Biochem. Sci. 2020, 45, 693–705. [Google Scholar] [CrossRef]
- Li, X.X.; Lee, J.D.; Kemper, C.; Woodruff, T.M. The Complement Receptor C5aR2: A Powerful Modulator of Innate and Adaptive Immunity. J. Immunol. 2019, 202, 3339–3348. [Google Scholar] [CrossRef]
- Seiler, D.L.; Kahler, K.H.; Kleingarn, M.; Sadik, C.D.; Bieber, K.; Kohl, J.; Ludwig, R.J.; Karsten, C.M. The complement receptor C5aR2 regulates neutrophil activation and function contributing to neutrophil-driven epidermolysis bullosa acquisita. Front. Immunol. 2023, 14, 1197709. [Google Scholar] [CrossRef]
- Müller-Eberhard, H.J. Molecular organization and function of the complement system. Annu. Rev. Biochem. 1988, 57, 321–347. [Google Scholar] [CrossRef] [PubMed]
- Skendros, P.; Mitsios, A.; Chrysanthopoulou, A.; Mastellos, D.C.; Metallidis, S.; Rafailidis, P.; Ntinopoulou, M.; Sertaridou, E.; Tsironidou, V.; Tsigalou, C.; et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J. Clin. Investig. 2020, 130, 6151–6157. [Google Scholar] [CrossRef] [PubMed]
- Ali, H. Regulation of human mast cell and basophil function by anaphylatoxins C3a and C5a. Immunol. Lett. 2010, 128, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Aiello, S.; Gastoldi, S.; Galbusera, M.; Ruggenenti, P.; Portalupi, V.; Rota, S.; Rubis, N.; Liguori, L.; Conti, S.; Tironi, M.; et al. C5a and C5aR1 are key drivers of microvascular platelet aggregation in clinical entities spanning from aHUS to COVID-19. Blood Adv. 2022, 6, 866–881, Erratum in: Blood Adv. 2022, 16, 2812. [Google Scholar] [CrossRef]
- Sacks, S.H. Complement fragments C3a and C5a: The salt and pepper of the immune response. Eur. J. Immunol. 2010, 40, 668–670. [Google Scholar] [CrossRef]
- Cao, Q.; McIsaac, S.M.; Stadnyk, A.W. Human colonic epithelial cells detect and respond to C5a via apically expressed C5aR through the ERK pathway. Am. J. Physiol.-Cell Physiol. 2012, 302, C1731–C1740. [Google Scholar] [CrossRef]
- Oikonomopoulou, K.; Ricklin, D.; Ward, P.A.; Lambris, J.D. Interactions between coagulation and complement—Their role in inflammation. Semin. Immunopathol. 2012, 34, 151–165. [Google Scholar] [CrossRef]
- Guo, R.F.; Huber-Lang, M.; Wang, X.; Sarma, V.; Padgaonkar, V.A.; Craig, R.A.; Riedemann, N.C.; McClintock, S.D.; Hlaing, T.; Shi, M.M.; et al. Protective effects of anti-C5a in sepsis-induced thymocyte apoptosis. J. Clin. Investig. 2000, 106, 1271–1280. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Grégoire, M.; Uhel, F.; Lesouhaitier, M.; Gacouin, A.; Guirriec, M.; Mourcin, F.; Dumontet, E.; Chalin, A.; Samson, M.; Berthelot, L.L.; et al. Impaired efferocytosis and neutrophil extracellular trap clearance by macrophages in ARDS. Eur. Respir. J. 2018, 52, 1702590. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Jin, J.; Lv, T.; Song, Y. A Narrative Review: The Role of NETs in Acute Respiratory Distress Syndrome/Acute Lung Injury. Int. J. Mol. Sci. 2024, 25, 1464. [Google Scholar] [CrossRef] [PubMed]
- Robbins, R.A.; Russ, W.D.; Rasmussen, J.K.; Clayton, M.M. Activation of the complement system in the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1987, 135, 651–658. [Google Scholar] [PubMed]
- de Boer, J.P.; Creasey, A.A.; Chang, A.; Roem, D.; Eerenberg, A.J.; Hack, C.E.; Taylor, F.B., Jr. Activation of the complement system in baboons challenged with live Escherichia coli: Correlation with mortality and evidence for a biphasic activation pattern. Infect. Immun. 1993, 61, 4293–4301. [Google Scholar] [CrossRef]
- Smedegård, G.; Cui, L.X.; Hugli, T.E. Endotoxin-induced shock in the rat. A role for C5a. Am. J. Pathol. 1989, 135, 489–497. [Google Scholar]
- Dahlke, K.; Wrann, C.D.; Sommerfeld, O.; Sossdorf, M.; Recknagel, P.; Sachse, S.; Winter, S.W.; Klos, A.; Stahl, G.L.; Ma, Y.X.; et al. Distinct different contributions of the alternative and classical complement activation pathway for the innate host response during sepsis. J. Immunol. 2011, 186, 3066–3075. [Google Scholar] [CrossRef]
- Windbichler, M.; Echtenacher, B.; Hehlgans, T.; Jensenius, J.C.; Schwaeble, W.; Männel, D.N. Involvement of the lectin pathway of complement activation in antimicrobial immune defense during experimental septic peritonitis. Infect. Immun. 2004, 72, 5247–5252. [Google Scholar] [CrossRef]
- Huber-Lang, M.; Sarma, J.V.; Zetoune, F.S.; Rittirsch, D.; Neff, T.A.; McGuire, S.R.; Lambris, J.D.; Warner, R.L.; Flierl, M.A.; Hoesel, L.M.; et al. Generation of C5a in the absence of C3: A new complement activation pathway. Nat. Med. 2006, 12, 682–687. [Google Scholar] [CrossRef]
- Stevens, J.H.; O’Hanley, P.; Shapiro, J.M.; Mihm, F.G.; Satoh, P.S.; Collins, J.A.; Raffin, T.A. Effects of anti-C5a antibodies on the adult respiratory distress syndrome in septic primates. J. Clin. Investig. 1986, 77, 1812–1816. [Google Scholar] [CrossRef]
- Hangen, D.H.; Bloom, R.J.; Stevens, J.H.; O’Hanley, P.; Ranchod, M.; Collins, J.; Raffin, T.A. Adult respiratory distress syndrome. A live E coli septic primate model. Am. J. Pathol. 1987, 126, 396–400. [Google Scholar]
- Keshari, R.S.; Silasi, R.; Popescu, N.I.; Patel, M.M.; Chaaban, H.; Lupu, C.; Coggeshall, K.M.; Mollnes, T.E.; DeMarco, S.J.; Lupu, F. Inhibition of complement C5 protects against organ failure and reduces mortality in a baboon model of Escherichia coli sepsis. Proc. Natl. Acad. Sci. USA 2017, 114, E6390–E6399. [Google Scholar] [CrossRef]
- Rittirsch, D.; Huber-Lang, M.S.; Flierl, M.A.; Ward, P.A. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 2009, 4, 31–36. [Google Scholar] [CrossRef]
- Deitch, E.A. Animal models of sepsis and shock: A review and lessons learned. Shock 1998, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.F.; Riedemann, N.C.; Bernacki, K.D.; Sarma, V.J.; Laudes, I.J.; Reuben, J.S.; Younkin, E.M.; Neff, T.A.; Paulauskis, J.D.; Zetoune, F.S.; et al. Neutrophil C5a receptor and the outcome in a rat model of sepsis. FASEB J. 2003, 17, 1889–1891. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.A.; Riedemann, N.C.; Guo, R.F.; Huber-Lang, M.; Sarma, J.V.; Zetoune, F.S. Anti-complement strategies in experimental sepsis. Scand. J. Infect. Dis. 2003, 35, 601–603. [Google Scholar] [CrossRef] [PubMed]
- Czermak, B.J.; Sarma, V.; Pierson, C.L.; Warner, R.L.; Huber-Lang, M.; Bless, N.M.; Schmal, H.; Friedl, H.P.; Ward, P.A. Protective effects of C5a blockade in sepsis. Nat. Med. 1999, 5, 788–792. [Google Scholar] [CrossRef]
- Huber-Lang, M.; Sarma, V.J.; Lu, K.T.; McGuire, S.R.; Padgaonkar, V.A.; Guo, R.F.; Younkin, E.M.; Kunkel, R.G.; Ding, J.; Erickson, R.; et al. Role of C5a in multiorgan failure during sepsis. J. Immunol. 2001, 166, 1193–1199. [Google Scholar] [CrossRef]
- Riedemann, N.C.; Guo, R.F.; Neff, T.A.; Laudes, I.J.; Keller, K.A.; Sarma, V.J.; Markiewski, M.M.; Mastellos, D.; Strey, C.W.; Pierson, C.L.; et al. Increased C5a receptor expression in sepsis. J. Clin. Investig. 2002, 110, 101–108. [Google Scholar] [CrossRef]
- Patel, S.N.; Berghout, J.; Lovegrove, F.E.; Ayi, K.; Conroy, A.; Serghides, L.; Min-oo, G.; Gowda, D.C.; Sarma, J.V.; Rittirsch, D.; et al. C5 deficiency and C5a or C5aR blockade protects against cerebral malaria. J. Exp. Med. 2008, 205, 1133–1143. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, G.; Song, N.; Li, P.; Chen, Y.; Guo, Y.; Li, J.; Du, L.; Jiang, S.; Guo, R.; et al. Blockade of the C5a-C5aR axis alleviates lung damage in hDPP4-transgenic mice infected with MERS-CoV. Emerg. Microbes Infect. 2018, 7, 77. [Google Scholar] [CrossRef]
- Hammerschmidt, D.E.; Weaver, L.J.; Hudson, L.D.; Craddock, P.R.; Jacob, H.S. Association of complement activation and elevated plasma-C5a with adult respiratory distress syndrome. Pathophysiological relevance and possible prognostic value. Lancet 1980, 1, 947–949. [Google Scholar] [CrossRef]
- Duchateau, J.; Haas, M.; Schreyen, H.; Radoux, L.; Sprangers, I.; Noel, F.X.; Braun, M.; Lamy, M. Complement activation in patients at risk of developing the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1984, 130, 1058–1064. [Google Scholar]
- Carvelli, J.; Demaria, O.; Vély, F.; Batista, L.; Chouaki Benmansour, N.; Fares, J.; Carpentier, S.; Thibult, M.L.; Morel, A.; Remark, R.; et al. Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis. Nature 2020, 588, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Bengtson, A.; Heideman, M. Anaphylatoxin formation in sepsis. Arch. Surg. 1988, 123, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Nakae, H.; Endo, S.; Inada, K.; Takakuwa, T.; Kasai, T.; Yoshida, M. Serum complement levels and severity of sepsis. Res. Commun. Chem. Pathol. Pharmacol. 1994, 84, 189–195. [Google Scholar] [PubMed]
- Langlois, P.F.; Gawryl, M.S. Accentuated formation of the terminal C5b-9 complement complex in patient plasma precedes development of the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1988, 138, 368–375. [Google Scholar] [CrossRef]
- Sprong, T.; Brandtzaeg, P.; Fung, M.; Pharo, A.M.; Høiby, E.A.; Michaelsen, T.E.; Aase, A.; van der Meer, J.W.; van Deuren, M.; Mollnes, T.E. Inhibition of C5a-induced inflammation with preserved C5b-9-mediated bactericidal activity in a human whole blood model of meningococcal sepsis. Blood 2003, 102, 3702–3710. [Google Scholar] [CrossRef]
- Barrett, C.D.; Hsu, A.T.; Ellson, C.D.; Miyazawa, B.Y.; Kong, Y.W.; Greenwood, J.D.; Dhara, S.; Neal, M.D.; Sperry, J.L.; Park, M.S.; et al. Blood clotting and traumatic injury with shock mediates complement-dependent neutrophil priming for extracellular ROS, ROS-dependent organ injury and coagulopathy. Clin. Exp. Immunol. 2018, 194, 103–117. [Google Scholar] [CrossRef]
- Silva, B.M.; Gomes, G.F.; Veras, F.P.; Cambier, S.; Silva, G.V.; Quadros, A.U.; Caetité, D.B.; Nascimento, D.C.; Silva, C.M.; Silva, J.C.; et al. C5aR1 signaling triggers lung immunopathology in COVID-19 through neutrophil extracellular traps. J. Clin. Investig. 2023, 133, e163105. [Google Scholar] [CrossRef]
- Middleton, E.A.; He, X.Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 2020, 136, 1169–1179. [Google Scholar] [CrossRef]
- Hamed, M.E.; Naeem, A.; Alkadi, H.; Alamri, A.A.; AlYami, A.S.; AlJuryyan, A.; Alturaiki, W.; Enani, M.; Al-Shouli, S.T.; Assiri, A.M.; et al. Elevated Expression Levels of Lung Complement Anaphylatoxin, Neutrophil Chemoattractant Chemokine IL-8, and RANTES in MERS-CoV-Infected Patients: Predictive Biomarkers for Disease Severity and Mortality. J. Clin. Immunol. 2021, 41, 1607–1620. [Google Scholar] [CrossRef]
- Annane, D.; Heming, N.; Grimaldi-Bensouda, L.; Frémeaux-Bacchi, V.; Vigan, M.; Roux, A.L.; Marchal, A.; Michelon, H.; Rottman, M.; Moine, P. Eculizumab as an emergency treatment for adult patients with severe COVID-19 in the intensive care unit: A proof-of-concept study. eClinicalMedicine 2020, 28, 100590. [Google Scholar] [CrossRef] [PubMed]
- Raghunandan, S.; Josephson, C.D.; Verkerke, H.; Linam, W.M.; Ingram, T.C.; Zerra, P.E.; Arthur, C.M.; Stowell, S.R.; Briones, M.; Chonat, S. Complement Inhibition in Severe COVID-19 Acute Respiratory Distress Syndrome. Front. Pediatr. 2020, 8, 616731. [Google Scholar] [CrossRef] [PubMed]
- Diurno, F.; Numis, F.G.; Porta, G.; Cirillo, F.; Maddaluno, S.; Ragozzino, A.; De Negri, P.; Di Gennaro, C.; Pagano, A.; Allegorico, E.; et al. Eculizumab treatment in patients with COVID-19: Preliminary results from real life ASL Napoli 2 Nord experience. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4040–4047. [Google Scholar] [CrossRef]
- Annane, D.; Pittock, S.J.; Kulkarni, H.S.; Pickering, B.W.; Khoshnevis, M.R.; Siegel, J.L.; Powell, C.A.; Castro, P.; Fujii, T.; Dunn, D.; et al. Intravenous ravulizumab in mechanically ventilated patients hospitalised with severe COVID-19: A phase 3, multicentre, open-label, randomised controlled trial. Lancet Respir. Med. 2023, 11, 1051–1063. [Google Scholar] [CrossRef]
- Hall, F.C.; Cheriyan, J.; Cope, A.P.; Galloway, J.; Wilkinson, I.; Bond, S.; Norton, S.; Banham-Hall, E.; Bayes, H.; Kostapanos, M.; et al. Efficacy and safety of baricitinib or ravulizumab in adult patients with severe COVID-19 (TACTIC-R): A randomised, parallel-arm, open-label, phase 4 trial. Lancet Respir. Med. 2023, 11, 1064–1074, Erratum in: Lancet. Respir. Med. 2024, 12, e35. [Google Scholar] [CrossRef]
- Apellis. Apellis Initiates Phase 1/2 Study of APL-9 in Patients with Severe COVID-19. 2020. Available online: https://investors.apellis.com/news-releases/news-release-details/apellis-provides-update-apl-9-severe-covid-19 (accessed on 30 June 2025).
- Bauer, M.; Weyland, A.; Marx, G.; Bloos, F.; Weber, S.; Weiler, N.; Kluge, S.; Diers, A.; Simon, T.P.; Lautenschläger, I.; et al. Efficacy and Safety of Vilobelimab (IFX-1), a Novel Monoclonal Anti-C5a Antibody, in Patients With Early Severe Sepsis or Septic Shock-A Randomized, Placebo-Controlled, Double-Blind, Multicenter, Phase IIa Trial (SCIENS Study). Crit. Care Explor. 2021, 3, e0577. [Google Scholar] [CrossRef]
- Vlaar, A.P.J.; de Bruin, S.; Busch, M.; Timmermans, S.; van Zeggeren, I.E.; Koning, R.; Ter Horst, L.; Bulle, E.B.; van Baarle, F.; van de Poll, M.C.G.; et al. Anti-C5a antibody IFX-1 (vilobelimab) treatment versus best supportive care for patients with severe COVID-19 (PANAMO): An exploratory, open-label, phase 2 randomised controlled trial. Lancet Rheumatol. 2020, 2, e764–e773. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, G.; Liu, C.; Wu, X.; Guo, Y.; Yu, H.; Song, H.; Du, L.; Jiang, S.; Guo, R.; et al. Inhibition of complement activation alleviates acute lung injury induced by highly pathogenic avian influenza H5N1 virus infection. Am. J. Respir. Cell Mol. Biol. 2013, 49, 221–230. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, G.; Liu, C.; Fan, W.; Zhou, X.; Zeng, L.; Guo, Y.; Kou, Z.; Yu, H.; Li, J.; et al. Treatment with anti-C5a antibody improves the outcome of H7N9 virus infection in African green monkeys. Clin. Infect. Dis. 2015, 60, 586–595. [Google Scholar] [CrossRef]
- Sun, S.; Jiang, Y.; Wang, R.; Liu, C.; Liu, X.; Song, N.; Guo, Y.; Guo, R.; Du, L.; Jiang, S.; et al. Treatment of Paraquat-Induced Lung Injury With an Anti-C5a Antibody: Potential Clinical Application. Crit. Care Med. 2018, 46, e419–e425. [Google Scholar] [CrossRef]
- Carlini, V.; Noonan, D.M.; Abdalalem, E.; Goletti, D.; Sansone, C.; Calabrone, L.; Albini, A. The multifaceted nature of IL-10: Regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post-COVID conditions. Front. Immunol. 2023, 14, 1161067. [Google Scholar] [CrossRef]
- Sandrock, C.E.; Song, P.X.K. Limitation of site-stratified cox regression analysis in survival data: A cautionary tale of the PANAMO phase III randomized, controlled study in critically ill COVID-19 patients. Trials 2024, 25, 822. [Google Scholar] [CrossRef]
- FDA, U.S. Emergency Use Authorization (EUA) Summary Review for Gohibic (Vilobelimab); U.S. Food and Drug Administration: Silver Spring, MD, USA, 2023. Available online: https://www.fda.gov/media/167044/download (accessed on 30 June 2025).
- EMA. Authorization of Gohibic (Vilobelimab); The Committee for Medicinal Products for Human Use (CHMP): Amsterdam, The Netherlands, 2025; Available online: https://www.ema.europa.eu/en/documents/product-information/gohibic-epar-product-information_en.pdf (accessed on 30 June 2025).
- Sandrock, C.E.; Vlaar, A.; Lim, E.H.T.; Burnett, B.P.; Chong, C.; Zink, C.; Zerbib, R.; Panas, R.; Guo, R.; Riedemann, N.; et al. Vilobelimab in combination with tocilizumab may synergistically improve mortality in critically ill COVID-19 patients: A post-hoc analysis of the phase III PANAMO study. CHEST 2024, 166, A2225–A2226. [Google Scholar] [CrossRef]
- Shorr, A.F.; Vlaar, A.; Lim, E.; Burnett, B.P.; Chong, C.; Zink, C.; Zerbib, R.; Guo, R.; Riedemann, N.; Beek, D.; et al. Vilobelimab in Combination With Tocilizumab or Baricitinib Dramatically Improves Mortality in Critically Ill COVID-19 Patients: A Subgroup Analysis. Am. J. Respir. Crit. Care Med. 2024, 209, A5511. [Google Scholar] [CrossRef]
- Chemaly, R.F.; Vlaar, A.; Lim, E.H.T.; Burnett, B.P.; Chong, C.; Rückinger, s.; Zerbib, R.; Panas, R.M.; Guo, R.; Riedemann, N.; et al. 85. Improved Survival with Dual Immunomodulator Treatment of ARDS by blocking C5a and IL-6 Activity: Subanalysis from the PANAMO Study in Critically Ill COVID-19 Patients. Open Forum Infect. Dis. 2025; 12, (Suppl. S1), ofae631.022. [Google Scholar] [CrossRef]
- Riedemann, N.C.; Guo, R.F.; Hollmann, T.J.; Gao, H.; Neff, T.A.; Reuben, J.S.; Speyer, C.L.; Sarma, J.V.; Wetsel, R.A.; Zetoune, F.S.; et al. Regulatory role of C5a in LPS-induced IL-6 production by neutrophils during sepsis. FASEB J. 2004, 18, 370–372. [Google Scholar] [CrossRef] [PubMed]
- Riedemann, N.C.; Neff, T.A.; Guo, R.F.; Bernacki, K.D.; Laudes, I.J.; Sarma, J.V.; Lambris, J.D.; Ward, P.A. Protective effects of IL-6 blockade in sepsis are linked to reduced C5a receptor expression. J. Immunol. 2003, 170, 503–507. [Google Scholar] [CrossRef]
- Li, L.; Wei, T.; Liu, S.; Wang, C.; Zhao, M.; Feng, Y.; Ma, L.; Lu, Y.; Fu, P.; Liu, J. Complement C5 activation promotes type 2 diabetic kidney disease via activating STAT3 pathway and disrupting the gut-kidney axis. J. Cell Mol. Med. 2021, 25, 960–974. [Google Scholar] [CrossRef]
- Zheng, J.M.; Zhou, H.X.; Yu, H.Y.; Xia, Y.H.; Yu, Q.X.; Qu, H.S.; Bao, J.Q. By Increasing the Expression and Activation of STAT3, Sustained C5a Stimulation Increases the Proliferation, Migration, and Invasion of RCC Cells and Promotes the Growth of Transgrafted Tumors. Cancer Manag. Res. 2021, 13, 7607–7621. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCarthy, M.W.; Chong, C.; Riedemann, N.C.; Guo, R. A Targeted Blockade of Terminal C5a Is Critical to Management of Sepsis and Acute Respiratory Distress Syndrome: The Mechanism of Action of Vilobelimab. Int. J. Mol. Sci. 2025, 26, 9628. https://doi.org/10.3390/ijms26199628
McCarthy MW, Chong C, Riedemann NC, Guo R. A Targeted Blockade of Terminal C5a Is Critical to Management of Sepsis and Acute Respiratory Distress Syndrome: The Mechanism of Action of Vilobelimab. International Journal of Molecular Sciences. 2025; 26(19):9628. https://doi.org/10.3390/ijms26199628
Chicago/Turabian StyleMcCarthy, Matthew W., Camilla Chong, Niels C. Riedemann, and Renfeng Guo. 2025. "A Targeted Blockade of Terminal C5a Is Critical to Management of Sepsis and Acute Respiratory Distress Syndrome: The Mechanism of Action of Vilobelimab" International Journal of Molecular Sciences 26, no. 19: 9628. https://doi.org/10.3390/ijms26199628
APA StyleMcCarthy, M. W., Chong, C., Riedemann, N. C., & Guo, R. (2025). A Targeted Blockade of Terminal C5a Is Critical to Management of Sepsis and Acute Respiratory Distress Syndrome: The Mechanism of Action of Vilobelimab. International Journal of Molecular Sciences, 26(19), 9628. https://doi.org/10.3390/ijms26199628




