Pro-Dermcidin as an Emerging Regulator of Innate Immunity in Sepsis
Abstract
1. Introduction
2. Dermcidin as a Sweat Gland-Derived Antimicrobial Peptide
3. Pro-DCD as an Inducible Protein
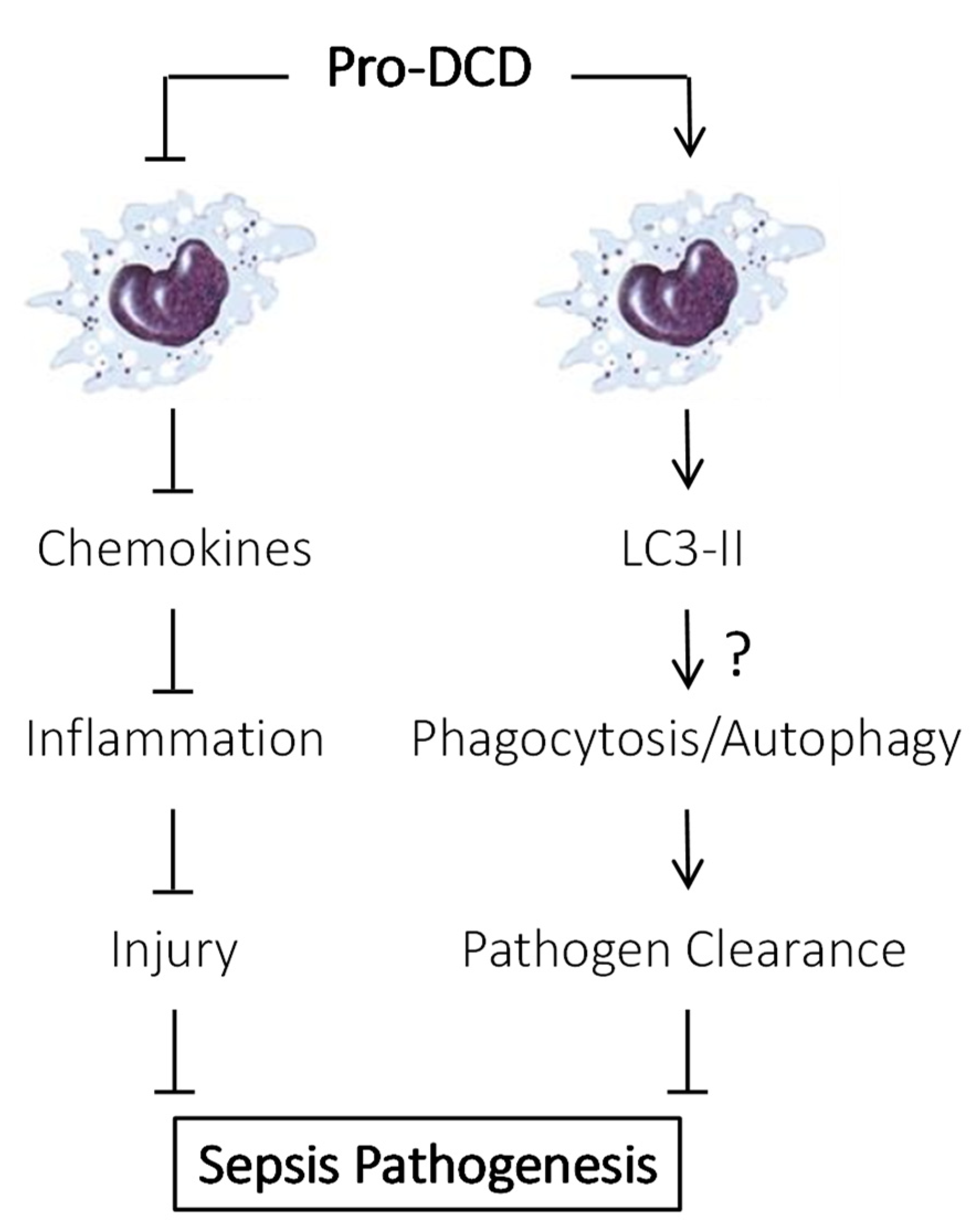
4. Pro-DCD as a Protective Protein in Sepsis
5. Therapeutic Efficacy of Pro-DCD and Derivatives in Sepsis
6. PEG-Pro-DCD-C34S Reduced Sepsis-Induced Inflammation and Bacterial Dissemination
7. Pro-DCD Induced LC3 Activation in Innate Immune Cells
8. Future Research Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| ALT | Alanine Aminotransferase |
| AMP | Antimicrobial Peptide |
| AST | Aspartate Aminotransferase |
| BM-MSCs | Bone Marrow-derived Mesenchymal Stromal Cells |
| C | Cysteine |
| CLP | Cecal Ligation and Puncture |
| DCD | Dermcidin |
| EGFR | Epidermal Growth Factor Receptor |
| G-CSF | Granulocyte Colony Stimulating Factor |
| IL-6 | Interleukin 6 |
| IgG | Immunoglobulin G |
| KC | Keratinocytes-derived Chemokine |
| LC3 | microtubule-associated protein 1A/1B-light chain 3 |
| LPS | Lipopolysaccharide |
| MCP-1 | Monocyte Chemoattractant Protein 1 |
| MIP-2 | Macrophage Inflammatory Protein-2 |
| pAbs | Polyclonal Antibodies |
| PBMCs | Peripheral Blood Mononuclear Cells |
| PEG | PEGylation |
| S | Serine |
| ST2 | Suppression of Tumorigenicity 2 |
| sTNFRI | Soluble Tumor Necrosis Factor Receptor I |
References
- Li, J.; Zhu, C.S.; He, L.; Qiang, X.; Chen, W.; Wang, H. A two-decade journey in identifying high mobility group box 1 (HMGB1) and procathepsin L (pCTS-L) as potential therapeutic targets for sepsis. Expert Opin. Ther. Targets 2023, 27, 575–591. [Google Scholar] [CrossRef]
- Buchman, T.G.; Simpson, S.Q.; Sciarretta, K.L.; Finne, K.P.; Sowers, N.; Collier, M.; Chavan, S.; Oke, I.; Pennini, M.E.; Santhosh, A.; et al. Sepsis Among Medicare Beneficiaries: 1. The Burdens of Sepsis, 2012–2018. Crit. Care Med. 2020, 48, 276–288. [Google Scholar] [CrossRef]
- Tindal, E.W.; Armstead, B.E.; Monaghan, S.F.; Heffernan, D.S.; Ayala, A. Emerging therapeutic targets for sepsis. Expert Opin. Ther. Targets 2021, 25, 175–189. [Google Scholar] [CrossRef]
- Reinhart, K.; Daniels, R.; Kissoon, N.; Machado, F.R.; Schachter, R.D.; Finfer, S. Recognizing Sepsis as a Global Health Priority—A WHO Resolution. N. Engl. J. Med. 2017, 377, 414–417. [Google Scholar] [CrossRef]
- Li, J.; Lou, L.; Chen, W.; Qiang, X.; Zhu, C.; Wang, H. Connexin 43 and Pannexin 1 hemichannels as endogenous regulators of innate immunity in sepsis. Front. Immunol. 2024, 15, 1523306. [Google Scholar] [CrossRef]
- Schittek, B.; Hipfel, R.; Sauer, B.; Bauer, J.; Kalbacher, H.; Stevanovic, S.; Schirle, M.; Schroeder, K.; Blin, N.; Meier, F.; et al. Dermcidin: A novel human antibiotic peptide secreted by sweat glands. Nat. Immunol. 2001, 2, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Daci, D.; Altrichter, S.; Grillet, F.M.; Dib, S.; Mouna, A.; Suresh Kumar, S.; Terhorst-Molawi, D.; Maurer, M.; Günzel, D.; Scheffel, J. Altered Sweat Composition Due to Changes in Tight Junction Expression of Sweat Glands in Cholinergic Urticaria Patients. Int. J. Mol. Sci. 2024, 25, 4658. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Gu, S.; Xi, W.; Wang, W.; Xu, J.; An, N.; Zhang, L.; Xin, J.; Hu, X.; Chen, Y.; et al. Anti-bacterial activity of dermcidin in human platelets: Suppression of methicillin-resistant Staphylococcus aureus growth. Microbiol. Spectr. 2025, 13, e0327324. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Qiang, X.; Zhu, C.S.; Li, J.; Lou, L.; Wang, P.; Tracey, K.J.; Wang, H. Pro-dermcidin and derivatives as potential therapeutics for lethal experimental sepsis. Front. Immunol. 2025, 16, e1621633. [Google Scholar] [CrossRef]
- Lousada, M.B.; Edelkamp, J.; Lachnit, T.; Fehrholz, M.; Pastar, I.; Jimenez, F.; Erdmann, H.; Bosch, T.C.G.; Paus, R. Spatial Distribution and Functional Impact of Human Scalp Hair Follicle Microbiota. J. Investig. Dermatol. 2024, 144, 1353–1367. [Google Scholar] [CrossRef]
- Schittek, B. The multiple facets of dermcidin in cell survival and host defense. J. Innate Immun. 2012, 4, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Berscheid, A.; Straetener, J.; Schilling, N.A.; Ruppelt, D.; Konnerth, M.C.; Schittek, B.; Krismer, B.; Peschel, A.; Steinem, C.; Grond, S.; et al. The microbiome-derived antibacterial lugdunin acts as a cation ionophore in synergy with host peptides. mBio 2024, 15, e0057824. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.; De Souza, G.A.; Salte, T.; Wiker, H.G.; Asjo, B. HIV induces both a down-regulation of IRAK-4 that impairs TLR signalling and an up-regulation of the antibiotic peptide dermcidin in monocytic cells. Scand. J. Immunol. 2009, 70, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Su, X.; Lu, P.; Kang, X.; Hu, M.; Li, C.; Wang, S.; Lu, D.; Shen, S.; Huang, H.; et al. Bone Marrow Mesenchymal Stem Cell-Derived Dermcidin-Containing Migrasomes enhance LC3-Associated Phagocytosis of Pulmonary Macrophages and Protect against Post-Stroke Pneumonia. Adv. Sci. 2023, 10, e2206432. [Google Scholar] [CrossRef]
- Landgraf, P.; Sieg, F.; Wahle, P.; Meyer, G.; Kreutz, M.R.; Pape, H.C. A maternal blood-borne factor promotes survival of the developing thalamus. FASEB J. 2005, 19, 225–227. [Google Scholar] [CrossRef]
- Esposito, G.; Schiattarella, G.G.; Perrino, C.; Cattaneo, F.; Pironti, G.; Franzone, A.; Gargiulo, G.; Magliulo, F.; Serino, F.; Carotenuto, G.; et al. Dermcidin: A skeletal muscle myokine modulating cardiomyocyte survival and infarct size after coronary artery ligation. Cardiovasc. Res. 2015, 107, 431–441. [Google Scholar] [CrossRef][Green Version]
- Qiang, X.; Li, J.; Zhu, S.; He, M.; Chen, W.; Al-Abed, Y.; Brenner, M.; Tracey, K.J.; Wang, P.; Wang, H. Human Dermcidin Protects Mice Against Hepatic Ischemia-Reperfusion-Induced Local and Remote Inflammatory Injury. Front. Immunol. 2021, 12, 821154. [Google Scholar] [CrossRef]
- Chen, W.; Qiang, X.; Zhu, C.S.; Li, J.; Lou, L.; Wang, P.; Tracey, K.J.; Wang, H. Identification of pro-dermcidin and derivatives as potential therapeutics for lethal experimental sepsis. Shock 2025, 64, S32–S33. Available online: https://journals.lww.com/shockjournal/toc/2025/08001 (accessed on 3 August 2025).
- Lee Motoyama, J.P.; Kim-Motoyama, H.; Kim, P.; Nakagama, H.; Miyagawa, K.; Suzuki, K. Identification of dermcidin in human gestational tissue and characterization of its proteolytic activity. Biochem. Biophys. Res. Commun. 2007, 357, 828–833. [Google Scholar] [CrossRef]
- Ghosh, R.; Karmohapatra, S.K.; Bhattacharyya, M.; Bhattacharya, R.; Bhattacharya, G.; Sinha, A.K. The appearance of dermcidin isoform 2, a novel platelet aggregating agent in the circulation in acute myocardial infarction that inhibits insulin synthesis and the restoration by acetyl salicylic acid of its effects. J. Thromb. Thrombolysis 2011, 31, 13–21. [Google Scholar] [CrossRef]
- Corasolla Carregari, V.; Monforte, M.; Di Maio, G.; Pieroni, L.; Urbani, A.; Ricci, E.; Tasca, G. Proteomics of Muscle Microdialysates Identifies Potential Circulating Biomarkers in Facioscapulohumeral Muscular Dystrophy. Int. J. Mol. Sci. 2020, 22, 290. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, F.; Lage, S.; Rasero, J.; Díaz-Ramón, J.L.; Apraiz, A.; Pérez-Yarza, G.; Ezkurra, P.A.; Penas, C.; Sánchez-Diez, A.; García-Vazquez, M.D.; et al. Serum markers improve current prediction of metastasis development in early-stage melanoma patients: A machine learning-based study. Mol. Oncol. 2020, 14, 1705–1718. [Google Scholar] [CrossRef] [PubMed]
- Kohli, M.; Sharma, S.K.; Upadhyay, V.; Varshney, S.; Sengupta, S.; Basak, T.; Sreenivas, V. Urinary EPCR and dermcidin as potential novel biomarkers for severe adult OSA patients. Sleep Med. 2019, 64, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Qiang, X.; Li, J.; Zhu, S.; Wang, P. The in Vitro Immune-Modulating Properties of a Sweat Gland-Derived Antimicrobial Peptide Dermcidin. Shock 2016, 45, 28–32. [Google Scholar] [CrossRef]
- Li, W.; Zhu, S.; Li, J.; D’Amore, J.; D’Angelo, J.; Yang, H.; Wang, P.; Tracey, K.J.; Wang, H. Serum Amyloid A Stimulates PKR Expression and HMGB1 Release Possibly through TLR4/RAGE Receptors. Mol. Med. 2015, 21, 515–525. [Google Scholar] [CrossRef]
- Mohanty, T.; Milicevic, K.; Gothert, H.; Tillmann, A.; Padra, M.; Papareddy, P.; Herwald, H. Balancing inflammation: The specific roles of serum amyloid A proteins in sterile and infectious diseasese. Front. Immunol. 2025, 16, 1544085. [Google Scholar] [CrossRef]
- Heuer, J.G.; Sharma, G.R.; Gerlitz, B.; Zhang, T.; Bailey, D.L.; Ding, C.; Berg, D.T.; Perkins, D.; Stephens, E.J.; Holmes, K.C.; et al. Evaluation of protein C and other biomarkers as predictors of mortality in a rat cecal ligation and puncture model of sepsis. Crit. Care Med. 2004, 32, 1570–1578. [Google Scholar] [CrossRef]
- Li, W.; Ashok, M.; Li, J.; Yang, H.; Sama, A.E.; Wang, H. A Major Ingredient of Green Tea Rescues Mice from Lethal Sepsis Partly by Inhibiting HMGB1. PLoS ONE 2007, 2, e1153. [Google Scholar] [CrossRef]
- Bozza, F.A.; Salluh, J.I.; Japiassu, A.M.; Soares, M.; Assis, E.F.; Gomes, R.N.; Bozza, M.T.; Castro-Faria-Neto, H.C.; Bozza, P.T. Cytokine profiles as markers of disease severity in sepsis: A multiplex analysis. Crit. Care 2007, 11, R49. [Google Scholar] [CrossRef]
- Osuchowski, M.F.; Welch, K.; Siddiqui, J.; Remick, D.G. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J. Immunol. 2006, 177, 1967–1974. [Google Scholar] [CrossRef]
- Chen, W.; Brenner, M.; Aziz, M.; Chavan, S.S.; Deutschman, C.S.; Diamond, B.; Pavlov, V.A.; Sherry, B.; Wang, P.; Tracey, K.J.; et al. Buprenorphine Markedly Elevates a Panel of Surrogate Markers in a Murine Model of Sepsis. Shock 2019, 52, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bloom, O.; Zhang, M.; Vishnubhakat, J.M.; Ombrellino, M.; Che, J.; Frazier, A.; Yang, H.; Ivanova, S.; Borovikova, L.; et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999, 285, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Weichbrodt, C.; Salnikov, E.S.; Dynowski, M.; Forsberg, B.O.; Bechinger, B.; Steinem, C.; de Groot, B.L.; Zachariae, U.; Zeth, K. Crystal structure and functional mechanism of a human antimicrobial membrane channel. Proc. Natl. Acad. Sci. USA 2013, 110, 4586–4591. [Google Scholar] [CrossRef] [PubMed]
- Burian, M.; Schittek, B. The secrets of dermcidin action. Int. J. Med. Microbiol. 2015, 305, 283–286. [Google Scholar] [CrossRef]
- Zeth, K.; Sancho-Vaello, E. The Human Antimicrobial Peptides Dermcidin and LL-37 Show Novel Distinct Pathways in Membrane Interactions. Front. Chem. 2017, 5, 86. [Google Scholar] [CrossRef]
- Che, D.; Jia, T.; Zhang, X.; Zhang, L.; Du, X.; Zheng, Y.; Zhou, T.; Song, X.; Geng, S. Dermcidin-derived polypeptides: DCD(86–103) induced inflammatory reaction in the skin by activation of mast cells via ST2. Immunol. Lett. 2022, 251, 29–37. [Google Scholar] [CrossRef]
- Chopra, D.; Arens, R.A.; Amornpairoj, W.; Lowes, M.A.; Tomic-Canic, M.; Strbo, N.; Lev-Tov, H.; Pastar, I. Innate immunity and microbial dysbiosis in hidradenitis suppurativa—Vicious cycle of chronic inflammation. Front. Immunol. 2022, 13, 960488. [Google Scholar] [CrossRef]
- Sağmak Tartar, A.; Uğur, K.; Tuncer Kara, K.; Akbulut, A.; Demirdağ, K.; Aydin, S. Association Between Dermcidin, Salusin-α, Salusin-β Molecules and Diabetic Foot Infections. Int. J. Low. Extrem. Wounds 2024, 23, 450–454. [Google Scholar] [CrossRef]
- Cipáková, I.; Gasperík, J.; Hostinová, E. Expression and purification of human antimicrobial peptide, dermcidin, in Escherichia coli. Protein Expr. Purif. 2006, 45, 269–274. [Google Scholar] [CrossRef]
- Nakagawa, I.; Amano, A.; Mizushima, N.; Yamamoto, A.; Yamaguchi, H.; Kamimoto, T.; Nara, A.; Funao, J.; Nakata, M.; Tsuda, K.; et al. Autophagy defends cells against invading group A Streptococcus. Science 2004, 306, 1037–1040. [Google Scholar] [CrossRef]
- Gutierrez, M.G.; Master, S.S.; Singh, S.B.; Taylor, G.A.; Colombo, M.I.; Deretic, V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 2004, 119, 753–766. [Google Scholar] [CrossRef]
- Sanjuan, M.A.; Dillon, C.P.; Tait, S.W.; Moshiach, S.; Dorsey, F.; Connell, S.; Komatsu, M.; Tanaka, K.; Cleveland, J.L.; Withoff, S.; et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 2007, 450, 1253–1257. [Google Scholar] [CrossRef]
- Alonso, S.; Pethe, K.; Russell, D.G.; Purdy, G.E. Lysosomal killing of Mycobacterium mediated by ubiquitin-derived peptides is enhanced by autophagy. Proc. Natl. Acad. Sci. USA 2007, 104, 6031–6036. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N. The pleiotropic role of autophagy: From protein metabolism to bactericide. Cell Death Differ. 2005, 12, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Checroun, C.; Wehrly, T.D.; Fischer, E.R.; Hayes, S.F.; Celli, J. Autophagy-mediated reentry of Francisella tularensis into the endocytic compartment after cytoplasmic replication. Proc. Natl. Acad. Sci. USA 2006, 103, 14578–14583. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Ita, M.; Zhou, H.; Zhao, H.; Hassan, F.; Bai, Z.; O’Leary, D.P.; Li, Y.; Redmond, H.P.; Wang, J.H.; et al. Autophagy induced by taurolidine protects against polymicrobial sepsis by promoting both host resistance and disease tolerance. Proc. Natl. Acad. Sci. USA 2022, 119, e2121244119. [Google Scholar] [CrossRef]
- Kim, J.J.; Lee, H.M.; Shin, D.M.; Kim, W.; Yuk, J.M.; Jin, H.S.; Lee, S.H.; Cha, G.H.; Kim, J.M.; Lee, Z.W.; et al. Host cell autophagy activated by antibiotics is required for their effective antimycobacterial drug action. Cell Host Microbe 2012, 11, 457–468. [Google Scholar] [CrossRef]
- Wang, J.; Feng, X.; Zeng, Y.; Fan, J.; Wu, J.; Li, Z.; Liu, X.; Huang, R.; Huang, F.; Yu, X.; et al. Lipopolysaccharide (LPS)-induced autophagy is involved in the restriction of Escherichia coli in peritoneal mesothelial cells. BMC Microbiol. 2013, 13, 255. [Google Scholar] [CrossRef]
- Giraud-Gatineau, A.; Coya, J.M.; Maure, A.; Biton, A.; Thomson, M.; Bernard, E.M.; Marrec, J.; Gutierrez, M.G.; Larrouy-Maumus, G.; Brosch, R.; et al. The antibiotic bedaquiline activates host macrophage innate immune resistance to bacterial infection. eLife 2020, 9, e55692. [Google Scholar] [CrossRef]
- Trzoss, L.; Fukuda, T.; Costa-Lotufo, L.V.; Jimenez, P.; La Clair, J.J.; Fenical, W. Seriniquinone, a selective anticancer agent, induces cell death by autophagocytosis, targeting the cancer-protective protein dermcidin. Proc. Natl. Acad. Sci. USA 2014, 111, 14687–14692. [Google Scholar] [CrossRef]
- Peláez Coyotl, E.A.; Barrios Palacios, J.; Muciño, G.; Moreno-Blas, D.; Costas, M.; Montiel Montes, T.; Diener, C.; Uribe-Carvajal, S.; Massieu, L.; Castro-Obregón, S.; et al. Antimicrobial Peptide against Mycobacterium Tuberculosis That Activates Autophagy Is an Effective Treatment for Tuberculosis. Pharmaceutics 2020, 12, 1071. [Google Scholar] [CrossRef]
- Sun, Y.; Aliyari, S.R.; Parvatiyar, K.; Wang, L.; Zhen, A.; Sun, W.; Han, X.; Zhang, A.; Kato, E.; Shi, H.; et al. STING directly interacts with PAR to promote apoptosis upon acute ionizing radiation-mediated DNA damage. Cell Death Differ. 2025, 32, 1167–1179. [Google Scholar] [CrossRef]
- Belizario, J.E. Role of skin antimicrobial peptides in the pathogenesis of psoriasis, atopic dermatitis and hidradenitis suppurative: Highlights on dermcidin. Clin. Immunol. Commun. 2025, 7, 18–26. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, L.; Li, J.; Chen, W.; Zhu, C.S.; Qiang, X.; Wang, H. Pro-Dermcidin as an Emerging Regulator of Innate Immunity in Sepsis. Int. J. Mol. Sci. 2025, 26, 7643. https://doi.org/10.3390/ijms26157643
Lou L, Li J, Chen W, Zhu CS, Qiang X, Wang H. Pro-Dermcidin as an Emerging Regulator of Innate Immunity in Sepsis. International Journal of Molecular Sciences. 2025; 26(15):7643. https://doi.org/10.3390/ijms26157643
Chicago/Turabian StyleLou, Li, Jianhua Li, Weiqiang Chen, Cassie Shu Zhu, Xiaoling Qiang, and Haichao Wang. 2025. "Pro-Dermcidin as an Emerging Regulator of Innate Immunity in Sepsis" International Journal of Molecular Sciences 26, no. 15: 7643. https://doi.org/10.3390/ijms26157643
APA StyleLou, L., Li, J., Chen, W., Zhu, C. S., Qiang, X., & Wang, H. (2025). Pro-Dermcidin as an Emerging Regulator of Innate Immunity in Sepsis. International Journal of Molecular Sciences, 26(15), 7643. https://doi.org/10.3390/ijms26157643







