Involvement of Vitamin D Receptor Gene Polymorphism in Increased Cardiovascular Risk Disease in the Algerian Population
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
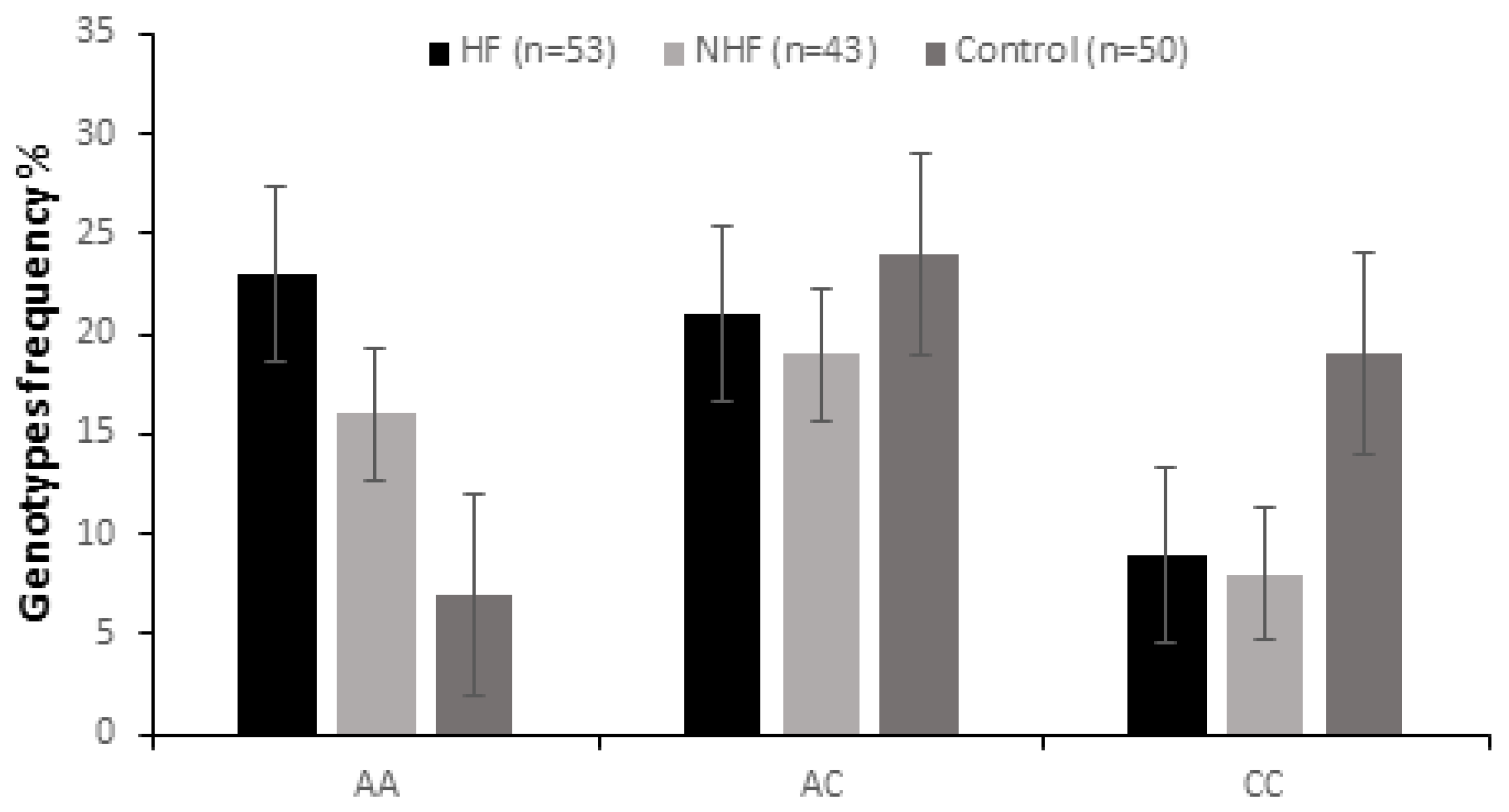
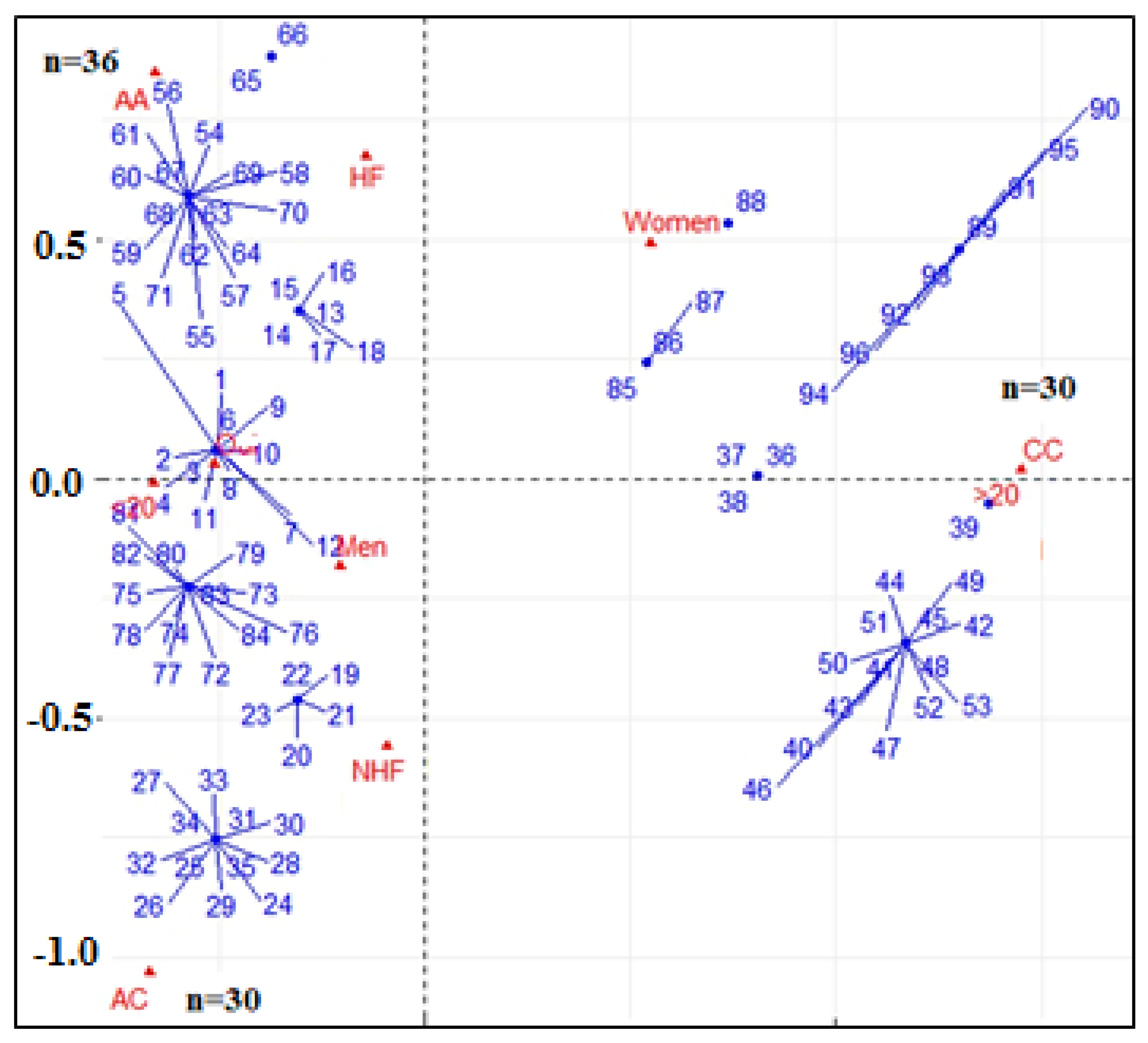
2.2. Genotypic and Allelic Analysis
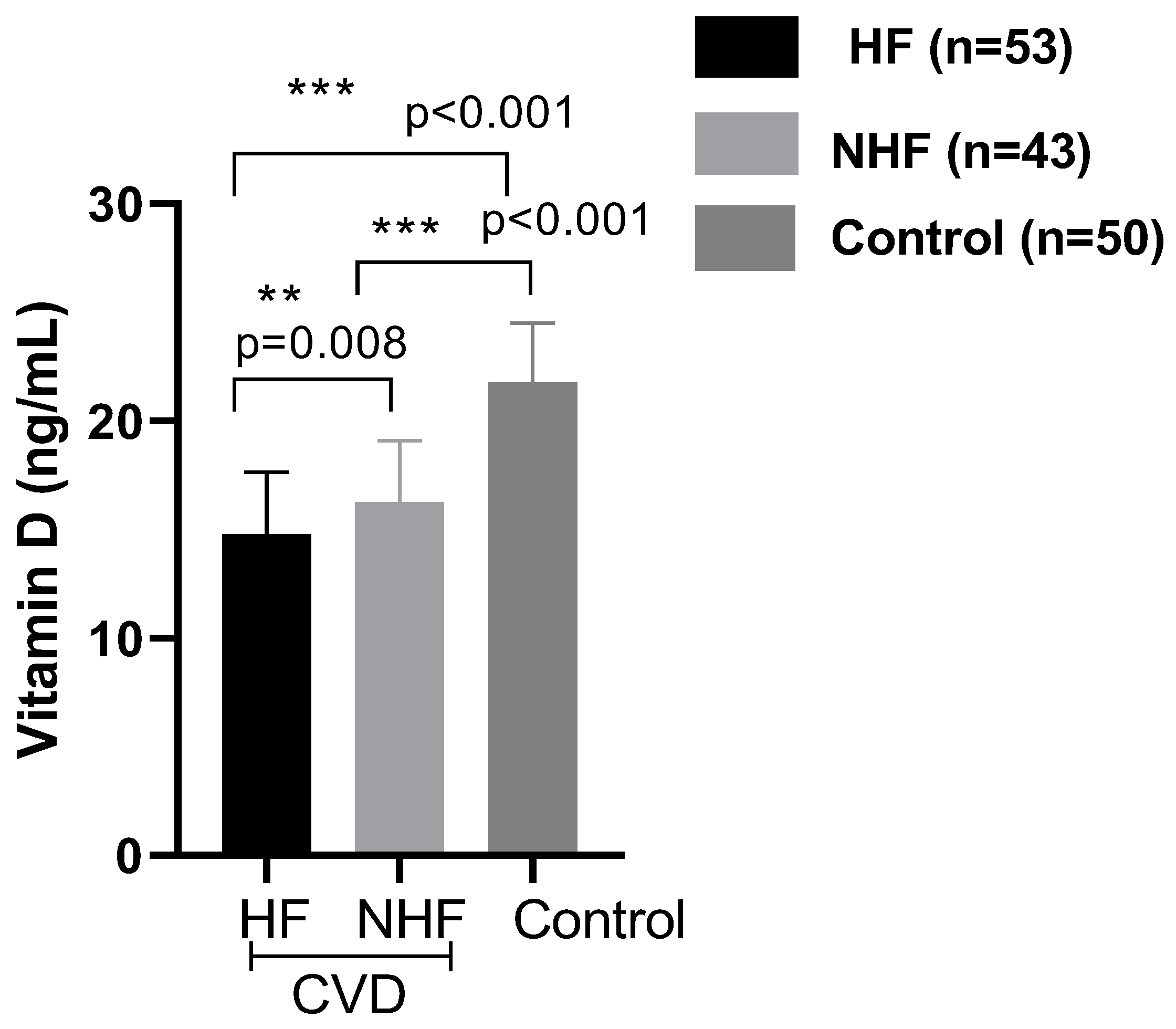
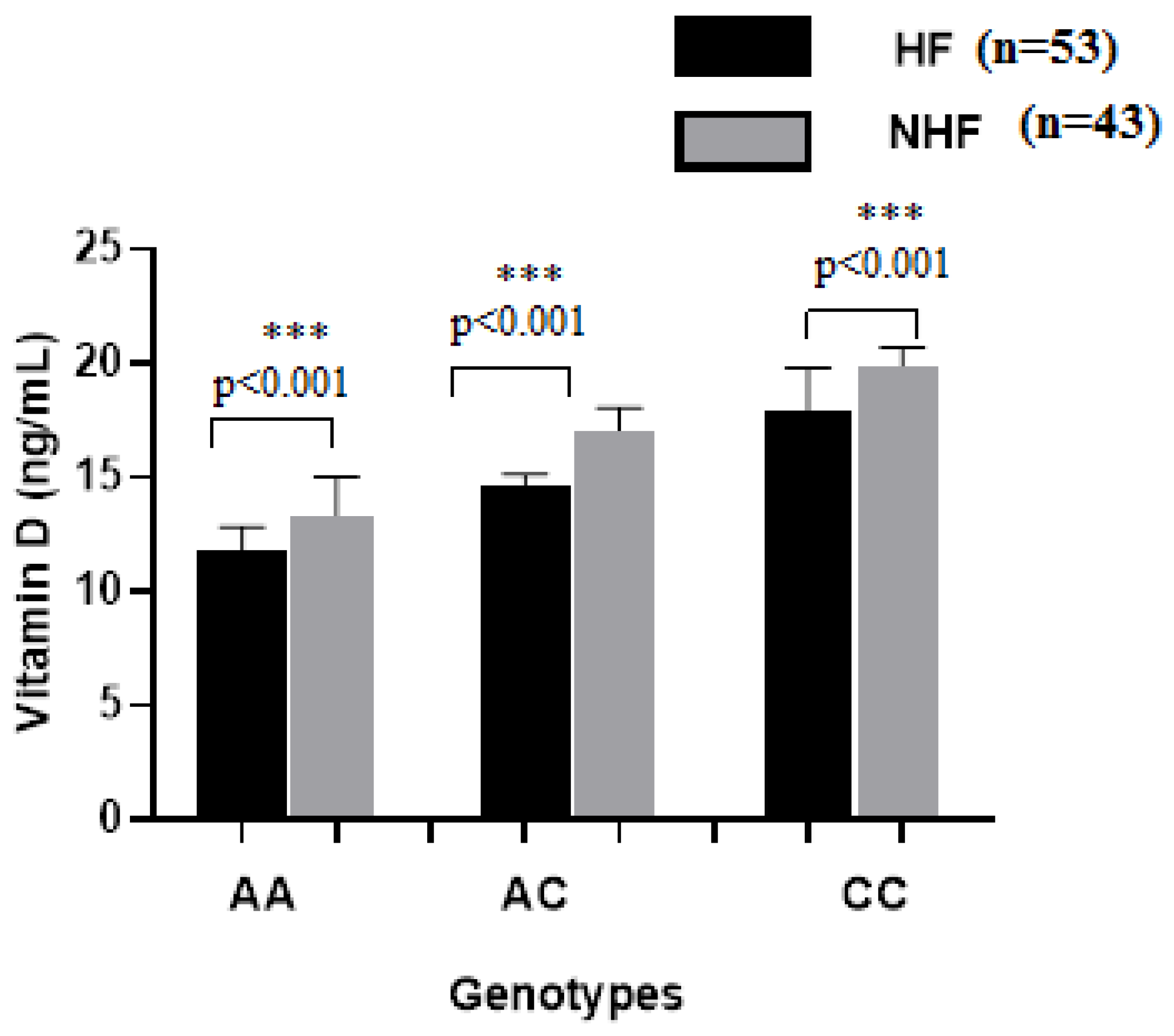
2.3. Biochemical Analysis
3. Discussion
4. Materials and Methods
4.1. Study Subjects
4.2. Genetic Analyses
4.3. Biochemical Analysis
4.3.1. Measurement of 25-Hydroxyvitamin D (25(OH)D)
4.3.2. Measurement of NT-proBNP
4.4. Statistical Analysis
5. Conclusions
6. Limitations of This Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eweida, S.M.; Salem, A.; Shaker, Y.M.; Samy, N.; Yassen, I.; Mohamed, R.S. Vitamin D levels and vitamin D receptor genetic variants in Egyptian cardiovascular disease patients with and without diabetes. Egypt. J. Med. Hum. Genet. 2021, 22, 55. [Google Scholar] [CrossRef]
- Gálvez-Navas, J.M.; Márquez-Pete, N.; Paiva-Chaves, M.; Rojo-Tolosa, S.; Pineda-Lancheros, L.E.; Cura, Y.; Membrive-Jiménez, C.; Marangoni-Iglecias, L.M.; Fernández-Alonso, A.; Ramírez-Tortosa, M.C.; et al. Molecular study of vitamin D metabolism-related single nucleotide polymorphisms in cardiovascular risk: A case-control study. J. Physiol. Biochem. 2025, 81, 347–357. [Google Scholar] [CrossRef]
- Rojo, P.G.; Ramírez, C.P.; Gálvez-Navas, J.M.; Lancheros, L.E.P.; Rojo-Tolosa, S.; Ramírez-Tortosa, M.C.; Jiménez-Morales, A. Vitamin D-related single nucleotide polymorphisms as risk biomarker of cardiovascular disease. Int. J. Mol. Sci. 2022, 23, 8686. [Google Scholar] [CrossRef]
- Akhlaghi, B.; Firouzabadi, N.; Foroughinia, F.; Nikparvar, M.; Dehghani, P. Impact of vitamin D receptor gene polymorphisms (TaqI and BsmI) on the incidence and severity of coronary artery disease: A report from southern Iran. BMC Cardiovasc. Disord. 2023, 23, 113. [Google Scholar] [CrossRef] [PubMed]
- Schwinger, R.H.G. Pathophysiology of heart failure. Cardiovasc. Diagn. Ther. 2021, 11, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Gtif, I.; Abdelhedi, R.; Ouarda, W.; Bouzid, F.; Charfeddine, S.; Zouari, F.; Abid, L.; Rebai, A.; Kharrat, N. Oxidative stress markers-driven prognostic model to predict post-discharge mortality in heart failure with reduced ejection fraction. Front. Cardiovasc. Med. 2022, 9, 1017673. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, Y.; Zhang, J. Epidemiology and burden of heart failure in Asia. JACC Asia 2022, 4, 249–264. [Google Scholar] [CrossRef]
- Pocock, S.J.; Ferreira, J.P.; Gregson, J.; Anker, S.D.; Butler, J.; Filippatos, G.; Gollop, N.D.; Iwata, T.; Brueckmann, M.; Januzzi, J.L.; et al. Novel biomarker-driven prognostic models to predict morbidity and mortality in chronic heart failure: The EMPEROR-Reduced trial. Eur. Heart J. 2021, 42, 4455–4464. [Google Scholar] [CrossRef]
- Fiaz, H.; Khan, A.R.; Abbas, S.; Bilal, A.; Khan, H.N.; Hussain, M.; Awan, F.R. Association of vitamin D receptor polymorphisms with cardiometabolic conditions in Pakistani population. Int. J. Vitam. Nutr. Res. 2024, 94, 45–53. [Google Scholar] [CrossRef]
- Kashyap, J.; Chhabra, A.; Rizvi, S.; Tyagi, R.K. Vitamin D receptor in human health and disease: An Overview. J. Endocrinol. Reprod. 2022, 26, 1–23. [Google Scholar]
- Abouzid, M.; Kruszyna, M.; Burchardt, P.; Kruszyna, L.; Główka, F.K.; Karaźniewicz-Łada, M. Vitamin D receptor gene polymorphism and vitamin D status in population of patients with cardiovascular disease—A Preliminary Study. Nutrients 2021, 13, 3117. [Google Scholar] [CrossRef]
- Rozmus, D.; Płomiński, J.; Augustyn, K.; Cieślińska, A. rs7041 and rs4588 Polymorphisms in vitamin D binding protein gene (VDBP) and the risk of diseases. Int. J. Mol. Sci. 2022, 23, 933. [Google Scholar] [CrossRef]
- Ruiz-Ballesteros, A.I.; Meza-Meza, M.R.; Vizmanos-Lamotte, B.; Parra-Rojas, I.; de la Cruz-Mosso, U. Association of vitamin D metabolism gene polymorphisms with autoimmunity: Evidence in population genetic studies. Int. J. Mol. Sci. 2020, 21, 9626. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, M.; Alourfi, Z.; Haddad, S. Relationship between vitamin D receptor gene FokI polymorphism and 25-hydroxyvitamin D levels in apparently healthy Syrians. Meta Gene 2021, 29, 100945. [Google Scholar] [CrossRef]
- de Souza Freitas, R.; Fratelli, C.F.; de Souza Silva, C.M.; de Lima, L.R.; Stival, M.M.; da Silva, I.C.R.; Funghetto, S.S. Association of vitamin D with the TaqI polymorphism of the VDR gene in older women atending the basic health unit of the federal district, DF (Brazil). J. Aging Res. 2020, 2020, 7145193. [Google Scholar] [CrossRef] [PubMed]
- Al-Barazenji, T.; Allouch, A.; Al Husaini, N.; Yousef, S.; Ibrahim, N.I.; Al-Haidose, A.; Zayed, H.; Abdallah, A.M. Association between vitamin D receptor BsmI polymorphism and low bone mineral density in postmenopausal women in the MENA region. Pathophysiology 2025, 32, 6. [Google Scholar] [CrossRef]
- Li, Q.; Li, W.; Chen, M.; Chai, Y.; Guan, L.; Chen, Y. Association of vitamin D receptor gene polymorphism with the risk of sepsis: A systematic review and meta-analysis. Medicine 2023, 102, e35130. [Google Scholar] [CrossRef]
- Rai, T.; Sushma, M.; Jadeppa, G.; Suma, M.N.; Swetha, N.K. Exploring the vitamin D receptor gene polymorphisms: Implications for disease susceptibility. Int. J. Health Allied Sci. 2024, 13, 2. [Google Scholar] [CrossRef]
- Śledzińsk, K.; Kloska, A.; Jakóbkiewicz-Banecka, J.; Landowski, P.; Oppmann, A.; Wilczynski, S.; Zagierska, A.; Kamińska, B.; Żmijewski, M.A.; Liberek, A. The role of vitamin D and vitamin D receptor gene polymorphisms in the course of inflammatory bowel disease in children. Nutrients 2024, 16, 2261. [Google Scholar] [CrossRef]
- Zmijewski, M.A.; Carlberg, C. Vitamin D receptor(s): In the nucleus but also at membranes? Exp. Dermatol. 2020, 29, 876–884. [Google Scholar] [CrossRef]
- Feng, P.; Qin, J.; Chai, Z.; Wei, D.; Zhang, Y.; Wang, P.; Zhao, M.; He, B.; Ling, Z.; Li, X. Distribution characteristics and screening reference values of NT-proBNP in high cardiovascular risk population. Nutr. Metab. Cardiovasc. Dis. 2025, 35, 104029. [Google Scholar] [CrossRef]
- Santos, B.R.; Mascarenhas, L.P.G.; Satler, F.; Boguszewski, M.C.S.; Spritzer, P.M. Vitamin D deficiency in girls from south brazil: A cross-sectional study on prevalence and association with vitamin D receptor gene variants. BMC Pediatr. 2012, 12, 62. [Google Scholar] [CrossRef]
- Divanoglou, N.; Komninou, D.; Stea, E.A.; Argiriou, A.; Papatzikas, G.; Tsakalof, A.; Pazaitou-Panayiotou, K.; Georgakis, M.K.; Petridou, E. Association of vitamin D receptor gene polymorphisms with serum vitamin D levels in a greek rural population (Velestino Study). Lifestyle Genom. 2021, 14, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Calton, E.K.; Keane, K.N.; Newsholme, P.; Soares, M.J. The impact of vitamin D levels on inflammatory status: A systematic review of immune cell studies. PLoS ONE 2015, 10, e0141770. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.; Mora, C.; Prado-Uribe, M.; Cueto-Manzano, A.; Qureshi, A.R.; Lindholm, B.; Bernal Amador, A.S.; Paniagua, R. Inflammation and vitamin D receptor polymorphism: Impact on All-Cause and cardiovascular mortality in Mexican women on dialysis. Biomedicine 2024, 12, 1990. [Google Scholar] [CrossRef]
- Nwia, S.N.; Leite, A.P.O.; Li, X.C.; Zhuo, J.L. Sex differences in the renin-angiotensin-aldosterone system and its roles in hypertension, cardiovascular, and kidney diseases. Front. Cardiovasc. Med. 2023, 19, 1198090. [Google Scholar] [CrossRef] [PubMed]
- Foxa, H.; Seegerc, F.H.; von Quernheimd, Q.F.; Ramos-Lopez, E. itamin D gene polymorphisms and risk of acute cardiovascular events. Clin. Epidemiol. Glob. Health 2020, 8, 1371–1376. [Google Scholar] [CrossRef]
- Ferrarezi, D.A.; Bellili-Muñoz, N.; Dubois-Laforgue, D.; Cheurfa, N.; Lamri, A.; Reis, A.; Le Feuvre, C.; Roussel, R.; Fumeron, F.; Timsit, J.; et al. Allelic variations of the vitamin D receptor (VDR) gene are associated with increased risk of coronary artery disease in type 2 diabetics: The DIABHYCAR prospective study. Diabetes Metab. 2013, 39, 263–270. [Google Scholar] [CrossRef]
- Huang, Y.; Peng, Q.; Bao, M.; Liu, C.; Wu, K.; Zhou, S. Biochemical metabolic levels and vitamin D receptor FokI gene polymorphisms in Uyghur children with urolithiasis. PLoS ONE 2019, 14, e0212183. [Google Scholar]
- Jonas, M.I.; Kurylowicz, A.A.; Bartoszewicz, Z.; Lisik, W.; Jonas, M.; Kozniewski, K.; Puzianowska-Kuznicka, M. Vitamin D Receptor Gene Expression in Adipose Tissue of Obese Individuals Is Regulated by miRNA and Correlates with the Pro-Inflammatory Cytokine Level. Int. J. Mol. Sci. 2019, 20, 5272. [Google Scholar] [CrossRef]
- Ni, W.; Watts, S.W.; Ng, M.; Chen, S.; Glenn, D.J.; Gardne, D.G. Elimination of vitamin D receptor in vascular endothelial cells alters vascular function. Hypertension 2014, 64, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Law, C.S.; Grigsby, C.L.; Olsen, K.; Hong, T.T.; Zhang, Y.; Yeghiazarians, Y.; Gardner, D.G. Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation 2011, 124, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Tabaei, S.; Motallebnezhad, M.; Tabaee, S.S. Vitamin D receptor (VDR) gene polymorphisms and risk of coronary artery disease (CAD): Systematic review and meta-analysis. Biochem. Genet. 2021, 59, 813–836. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.M.; Li, D.R.; Yang, L.; Wang, E.-Y.; Chen, T.-Y.; Liu, Y.-J.; Liu, M.; Liao, Z.-G. No association between vitamin D receptor polymorphisms and coronary artery disease in a Chinese population. DNA Cell Biol. 2009, 28, 521–525. [Google Scholar] [CrossRef]
- Lizadeh, S.; Djafarian, K.; Alizadeh, H.; Mohseni, R.; Shab-Bidar, S. A common variants of vitamin D receptor gene polymorphisms and susceptibility to coronary artery disease: A systematic review and meta-analysis. Nutr. Nutr. 2017, 10, 9–18. [Google Scholar]
- Ortlepp, J.R.; Von Korff, A.; Hanrath, P.; Zerres, K.; Hoffmann, R. Vitamin D receptor gene polymorphism BsmI is not associated with the prevalence and severity of CAD in a large-scale angiographic cohort of 3441 patients. Eur. J. Clin. Investig. 2003, 33, 106–109. [Google Scholar] [CrossRef]
- Van Schooten, F.J.; Hirvonen, A.; Maas, L.M.; De Mol, B.A.; Kleinjans, J.C.S.; Bell, D.A.; Durrer, J.D. Putative susceptibility markers of coronary artery disease: Association between VDR genotype, smoking, and aromatic DNA adduct levels in human fight atrial tissue. FASEB J. 1998, 12, 1409–1417. [Google Scholar] [CrossRef]
- Lu, S.; Guo, S.; Hu, F.; Guo, Y.; Yan, L.; Ma, W.; Wang, Y.; Wei, Y.; Zhang, Z.; Wang, Z. The Associations Between the Polymorphisms of Vitamin D Receptor and Coronary Artery Disease. A Systematic Review and Meta-Analysis. Medicine 2016, 95, pe3467. [Google Scholar] [CrossRef]
- Fronczek, A.; Strzelczyk, J.K.; Osadnik, T.; Biernacki, K.; Ostrowska, Z. VDR Gene Polymorphisms in Healthy Individuals with Family History of Premature Coronary Artery Disease. Dis. Markers 2021, 2021, 8832478. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Gebhard, C. Gender medicine: Effects of sex and gender on cardiovascular disease manifestation and outcomes. Nat. Rev. Cardiol. 2023, 20, 236–247. [Google Scholar] [CrossRef]
- Betai, D.; Ahmed, A.S.; Saxena, P.; Rashid, H.; Patel, H.; Shahzadi, A.; Mowo-wale, A.G.; Nazir, Z. Gender disparities in cardiovascular disease and their management: A Review. Cureus 2024, 16, e59663. [Google Scholar] [CrossRef] [PubMed]
- Man, J.J.; Beckman, J.A.; Jaffe, I.Z. Sex as a Biological Variable in Atherosclerosis. Circ. Res. 2020, 126, 1297–1319. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Cushman, M.; Stampfer, M.J.; Tracy, R.P.; Hennekens, C.H. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N. Engl. J. Med. 1997, 336, 973–979, Correction in N. Engl. J. Med. 1997, 7, 356. [Google Scholar] [CrossRef]
- Sagris, M.; Antonopoulos, A.S.; Theofilis, P.; Oikonomou, E.; Siasos, G.; Tsalamandris, S.; Antoniades, C.; Brilakis, E.S.; Kaski, J.C.; Tousoulis, D. Risk factors profile of young and older patients with myocardial infarction. Cardiovasc. Res. 2022, 118, 2281–2292. [Google Scholar] [CrossRef] [PubMed]
- Bergami, M.; Scarpone, M.; Bugiardini, R.; Cenko, E.; Manfrini, O. Sex beyond cardiovascular risk factors and clinical biomarkers of cardiovascular disease. Rev. Cardiovasc. Med. 2022, 23, 19. [Google Scholar] [CrossRef]
- Shaik, M.V.; Kuragayala, S.D.; Madhuri, S.; Shaik, M.; BabuL, S.; Gangapatnam, S. Association of vitamin D receptor genetic variant Fok1, Bsm1, Apa1, and Taq1 polymorphism and vitamin D deficiency with increased incidence of coronary artery disease. Biomed. Biotechnol. Res. J. 2023, 7, 303–311. [Google Scholar] [CrossRef]
- Ionova, H.I.; Sergeeva, E.G.; Berkovich, O.A. Genetic and epigenetic factors regulating the expression and function of the vitamin D receptor in patients with coronary artery disease. Russ. J. Cardiol. 2021, 26, 4251. [Google Scholar] [CrossRef]
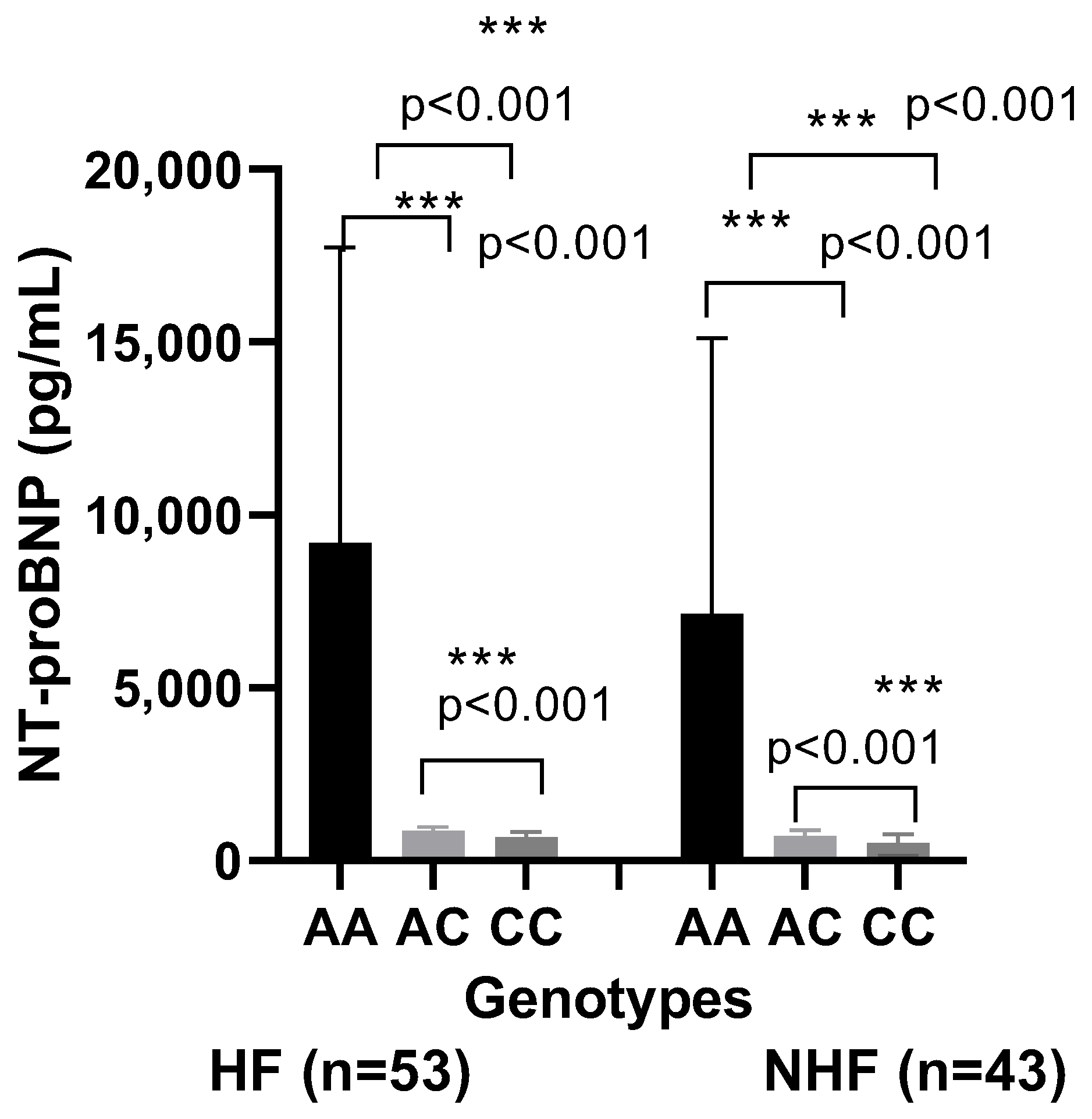
| Parameters | CVD with HF (N = 53) | CVD Without HF (N = 43) | Control (N = 50) |
|---|---|---|---|
| Gender | |||
| Male | 38 | 32 | 27 |
| Female | 15 | 11 | 23 |
| Age (y) | 60 ± 18.51 | 66 ± 10.37 | 58 ± 11.22 |
| BMI | 29.03 ± 0.94 | 28.5 ± 0.28 | 23.6 ± 1.20 |
| Systolic BP (mmHg) | 170.35 ± 26.6 | 162.17 ± 33.1 | 119 ± 15 |
| Diastolic BP (mmHg) | 104.68 ± 9.19 | 97.93 ± 7.39 | 77 ± 10 |
| Hypertension | 38 | 35 | 00 |
| Diabetes | 15 | 08 | 00 |
| ALT(U/L) | 33.14 ± 29.03 | 32.10 ± 24.02 | 22.3 ± 11.5 |
| AST(U/L) | 39.25 ± 12.05 | 35.24 ± 15.2 | 30.10 ± 17.2 |
| Uric acid (mg/L) | 70.6 ± 2.6 | 69.4 ± 1.8 | 37.2 ± 15.6 |
| Creatinin (mg/dL) | 1.41 ± 0.80 | 0.85 ± 0.70 | 0.78 ± 0.25 |
| Urea (mg/dL) | 67 ± 4 | 62 ± 3.5 | 35 ± 9 |
| Glucose (g/L) | 1.37 ± 0.68 | 1.58 ± 0.21 | 0.96 ± 0.21 |
| Troponin (pg/mL) | 2740.6 ± 12.6 | 24.6 ± 12.7 | 6.35 ± 1.75 |
| Genotypes | CVD (N = 96) | Control (N = 50) | |||||
|---|---|---|---|---|---|---|---|
| Group 1 (N = 53) | p | OR (95% IC) | Group 2 (N = 43) | p | OR (95% IC) | ||
| ApaI | |||||||
| AA | 23 (43.4%) | 0.001 | 4.71 (1.66–14.51) | 16 (37.20%) | 0.01 | 3.64 (1.21–11.74) | 7 (14%) |
| AC | 21 (39.62%) | 0.003 | 4.03 (1.41–12.49) | 19 (44.18%) | 0.71 | 0.86 (0.35–2.10) | 24 (48.0%) |
| CC | 9 (16.98%) | 0.01 | 0.33 (0.12–0.91) | 8 (18.60%) | 0.04 | 0.37 (0.12–1.06) | 19 (38%) |
| FokI | |||||||
| TT | 8 (15.09%) | 0.36 | 0.63 (0.20–1.93) | 9 (20.93%) | 0.90 | 0.94 (0.44–2.08) | 11 (22%) |
| CT | 23 (43.40%) | 0.72 | 1.15 (0.49–2.71) | 20 (46.51%) | 0.52 | 1.30 (0.53–3.22) | 20 (40%) |
| CC | 22 (41.51%) | 0.71 | 1.16 (0.49–2.75) | 14 (32.55%) | 0.58 | 0.79 (0.30–2.01) | 19 (38%) |
| BsmI | |||||||
| GG | 18 (33.96%) | 0.83 | 1.09 (0.44–2.71) | 15 (34.88%) | 0.76 | 1.14 (0.47–2.73) | 16 (32%) |
| GA | 23 (43.40%) | 0.63 | 0.83 (0.36–1.94) | 20 (46.51%) | 0.88 | 0.94 (0.38–2.3) | 24 (48%) |
| AA | 12 (22.64%) | 0.74 | 1.17 (0.41–3.40) | 8 (18.6%) | 0.86 | 0.91 (0.41–2.08) | 10 (20%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galleze, A.; Djaballah-Ider, F.Z.; Gouaref, I.; Atmani, S.M.; Allal, K.; Touil-Boukoffa, C.; Belguendouz, H. Involvement of Vitamin D Receptor Gene Polymorphism in Increased Cardiovascular Risk Disease in the Algerian Population. Int. J. Mol. Sci. 2025, 26, 9627. https://doi.org/10.3390/ijms26199627
Galleze A, Djaballah-Ider FZ, Gouaref I, Atmani SM, Allal K, Touil-Boukoffa C, Belguendouz H. Involvement of Vitamin D Receptor Gene Polymorphism in Increased Cardiovascular Risk Disease in the Algerian Population. International Journal of Molecular Sciences. 2025; 26(19):9627. https://doi.org/10.3390/ijms26199627
Chicago/Turabian StyleGalleze, Assia, Fatma Zohra Djaballah-Ider, Ines Gouaref, Sara Mimi Atmani, Karima Allal, Chafia Touil-Boukoffa, and Houda Belguendouz. 2025. "Involvement of Vitamin D Receptor Gene Polymorphism in Increased Cardiovascular Risk Disease in the Algerian Population" International Journal of Molecular Sciences 26, no. 19: 9627. https://doi.org/10.3390/ijms26199627
APA StyleGalleze, A., Djaballah-Ider, F. Z., Gouaref, I., Atmani, S. M., Allal, K., Touil-Boukoffa, C., & Belguendouz, H. (2025). Involvement of Vitamin D Receptor Gene Polymorphism in Increased Cardiovascular Risk Disease in the Algerian Population. International Journal of Molecular Sciences, 26(19), 9627. https://doi.org/10.3390/ijms26199627





