Protective Effect of Aromatic Plant Essential Oil Administration on Brain Tissue of PTZ-Treated and Non-Treated Mice
Abstract
1. Introduction
2. Results
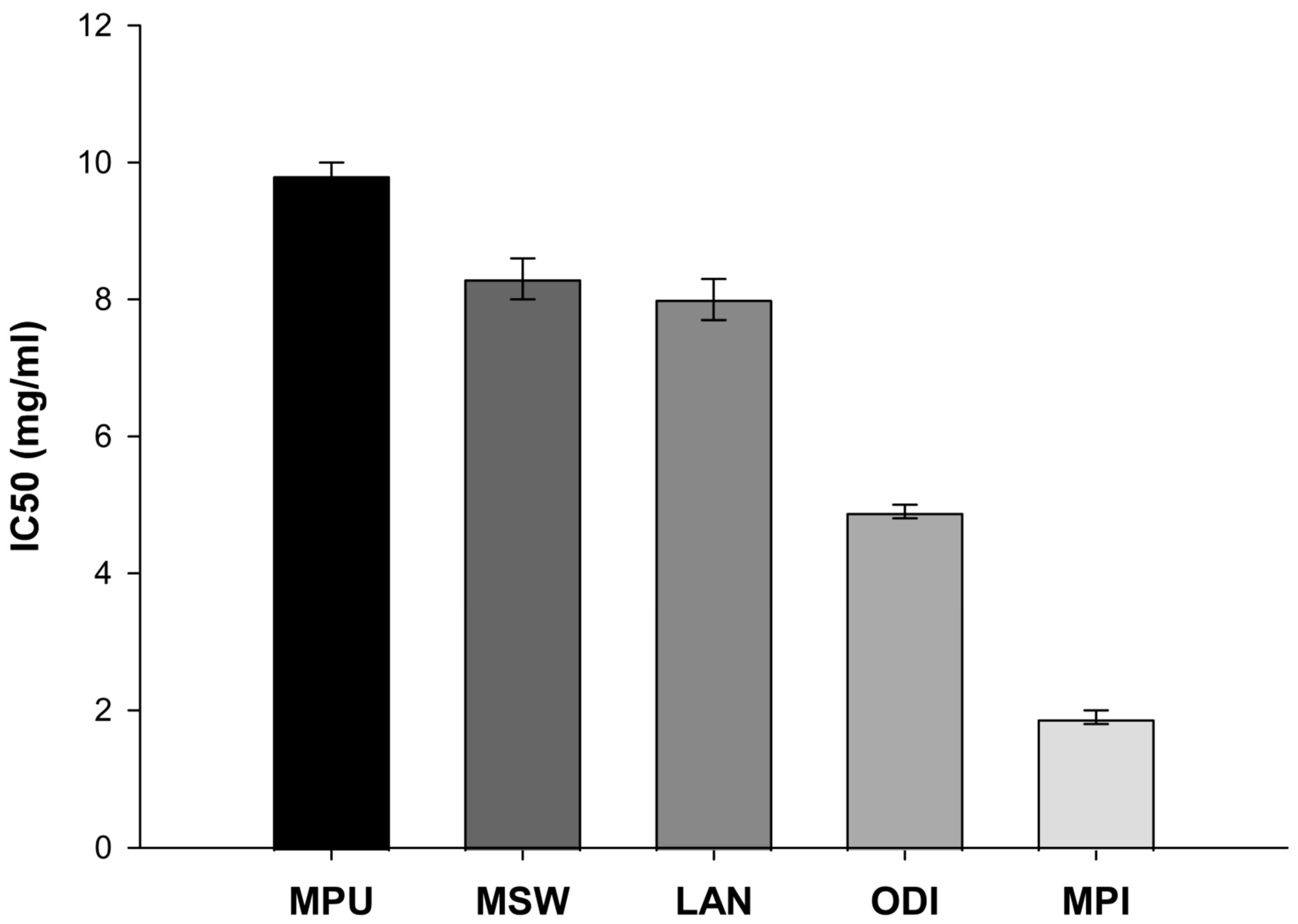
2.1. Evaluation of the Cell-Free Radical Scavenging Capacity Through DPPH Assay
2.2. Antioxidant Power Study
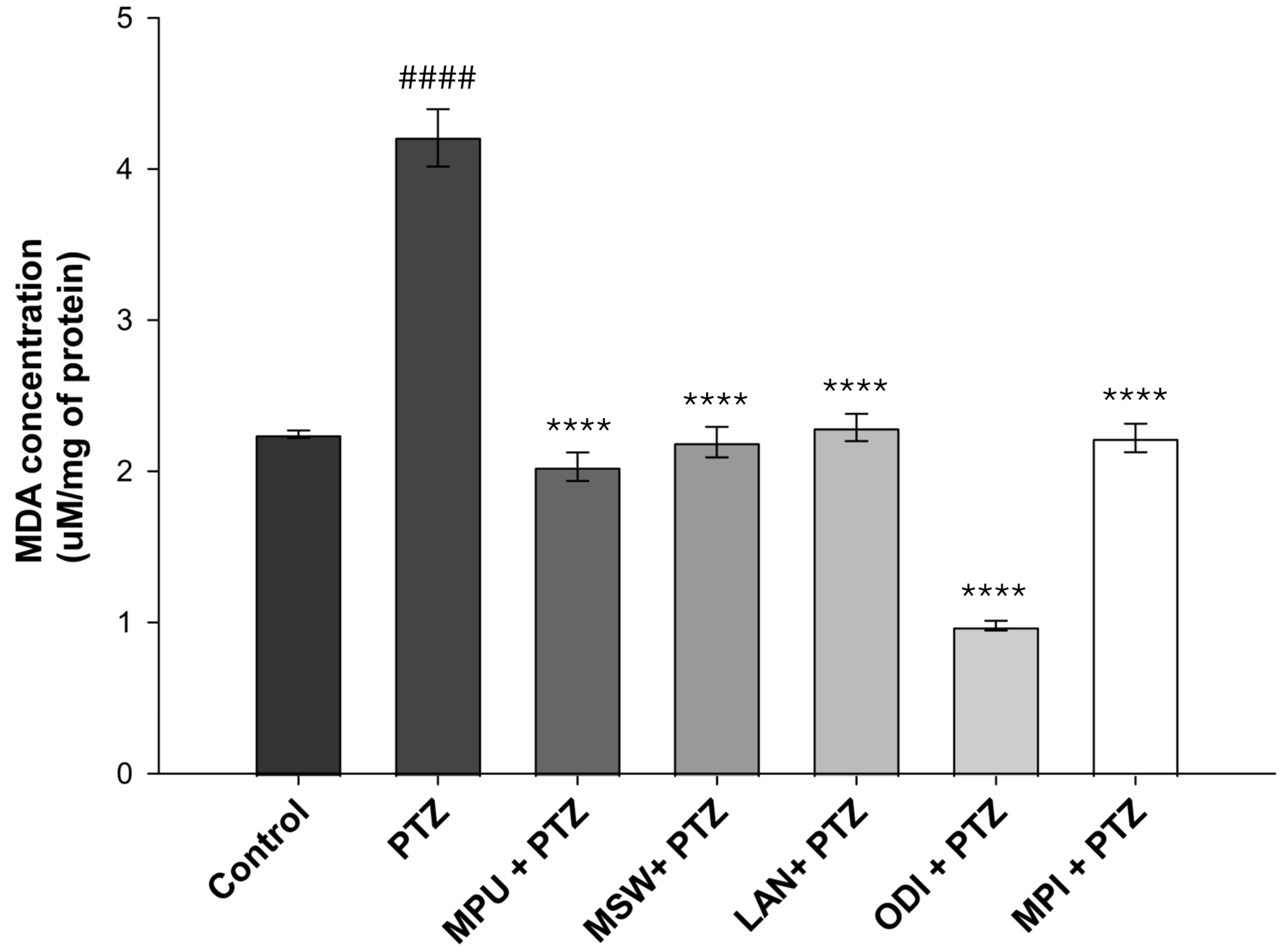
2.3. Evaluation of Lipid Peroxidation via Malondialdehyde (MDA) Levels
2.4. Immunohistochemical Study
3. Discussion
4. Materials and Methods
4.1. Extraction of EOs
4.2. Evaluation of Free Radical Scavenging Activity by DPPH Assay
4.3. Archived Animal Samples
4.4. Antioxidant Power Study
4.5. Evaluation of Lipid Peroxidation via Malondialdehyde (MDA) Levels
4.6. Immunohistochemistry
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EOs | Essential Oils |
| PTZ | Pentylenetetrazol |
| ROS | Reactive Oxygen Species |
| DSC | Differential Scanning Calorimetry |
| MDA | Malondialdehyde |
| LPO | Lipid Peroxidation |
| AOP | Antioxidant Power |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| FFPEs | Formalin-Fixed, Paraffin-Embedded Tissues |
References
- Dai, P.; Xu, L.; Zhang, P.; Liang, Z.; Chu, Y.; Yu, Z.; Cao, L.; Sun, P.; Li, X.; Kukula-Koch, W.; et al. Protective effects of curcumin on epileptic rodent models by alleviating oxidative stress and inflammation: A meta-analysis and mechanism exploration. Front. Pharmacol. 2025, 16, 1602716. [Google Scholar] [CrossRef] [PubMed]
- Dash, U.C.; Bhol, N.K.; Swain, S.K.; Samal, R.R.; Nayak, P.K.; Raina, V.; Panda, S.K.; Kerry, R.G.; Duttaroy, A.K.; Jena, A.B. Oxidative stress and inflammation in the pathogenesis of neurological disorders: Mechanisms and implications. Acta Pharm. Sin. B 2025, 15, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.C.B.; Costa, I.M.; Freire, M.A.M.; Lima, F.O.V.; Neta, F.I.; de Souza Lucena, E.E.; Alves, R.D.; Cavalcanti, J.R.L.P.; Pinheiro, F.I.; de Azevedo, E.P.; et al. Essential Oils in Experimental Models of Neuropsychiatric Disorders: A Systematic Review. Curr. Neuropharmacol. 2021, 19, 1738–1759. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; Van Emde Boas, W.; Blume, W.; Elger, C.; Genton, P.; Lee, P.; Engel, J. Epileptic seizures and epilepsy: Definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005, 46, 470–472. [Google Scholar] [CrossRef]
- Sucher, N.J.; Carles, M.C. A pharmacological basis of herbal medicines for epilepsy. Epilepsy Behav. 2015, 52, 308–318. [Google Scholar] [CrossRef]
- Avola, R.; Furnari, A.G.; Graziano, A.C.E.; Russo, A.; Cardile, V. Management of the Brain: Essential Oils as Promising Neuroinflammation Modulator in Neurodegenerative Diseases. Antioxidants 2024, 13, 178. [Google Scholar] [CrossRef]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Salama, A.; Mohasib, R.M.M.; Shalaby, E.S. Neuroprotective role of Origanum majorana essential oil loaded in mixed surfactants based nano emulsion against lipopolysaccharide-induced Alzheimer in mice. J. Pharm. Investig. 2025, 55, 265–281. [Google Scholar] [CrossRef]
- Lahlou, R.A.; Gonçalves, A.C.; Bounechada, M.; Nunes, A.R.; Soeiro, P.; Alves, G.; Moreno, D.A.; Garcia-Viguera, C.; Raposo, C.; Silvestre, S.; et al. Antioxidant, Phytochemical, and Pharmacological Properties of Algerian Mentha aquatica Extracts. Antioxidants 2024, 13, 1512. [Google Scholar] [CrossRef]
- Tafrihi, M.; Imran, M.; Tufail, T.; Gondal, T.A.; Caruso, G.; Sharma, S.; Sharma, R.; Atanassova, M.; Atanassov, L.; Fokou, P.V.T.; et al. The Wonderful Activities of the Genus Mentha: Not Only Antioxidant Properties. Molecules 2021, 26, 1118. [Google Scholar] [CrossRef]
- Hirata, M.; Fornari Laurindo, L.; Dogani Rodrigues, V.; Cristina Castilho Caracio, F.; Valenti, V.E.; Pereira, E.d.S.B.M.; Haber Mellem, R.; Penteado Detregiachi, C.R.; dos Santos Bueno, M.; Guissoni Campos, L.M.; et al. Investigating the Health Potential of Mentha Species Against Gastrointestinal Disorders—A Systematic Review of Clinical Evidence. Pharmaceuticals 2025, 18, 693. [Google Scholar] [CrossRef]
- Mohammadi, F.; Rahimi, K.; Ahmadi, A.; Hooshmandi, Z.; Amini, S.; Mohammadi, A. Anti-inflammatory effects of Mentha pulegium L. extract on human peripheral blood mononuclear cells are mediated by TLR-4 and NF-κB suppression. Heliyon 2024, 10, e24040. [Google Scholar] [CrossRef]
- El Menyiy, N.; Mrabti, H.N.; El Omari, N.; Bakili, A.E.I.; Bakrim, S.; Mekkaoui, M.; Balahbib, A.; Amiri-Ardekani, E.; Ullah, R.; Alqahtani, A.S.; et al. Medicinal Uses, Phytochemistry, Pharmacology, and Toxicology of Mentha spicata. Evidence-Based Complement. Altern. Med. 2022, 2022, 7990508. [Google Scholar] [CrossRef] [PubMed]
- Hudz, N.; Kobylinska, L.; Pokajewicz, K.; Horčinová Sedláčková, V.; Fedin, R.; Voloshyn, M.; Myskiv, I.; Brindza, J.; Wieczorek, P.P.; Lipok, J. Mentha piperita: Essential Oil and Extracts, Their Biological Activities, and Perspectives on the Development of New Medicinal and Cosmetic Products. Molecules 2023, 28, 7444. [Google Scholar] [CrossRef]
- Koutroumanidou, E.; Kimbaris, A.; Kortsaris, A.; Bezirtzoglou, E.; Polissiou, M.; Charalabopoulos, K.; Pagonopoulou, O. Increased seizure latency and decreased severity of pentylenetetrazol-induced seizures in mice after essential oil administration. Epilepsy Res. Treat. 2013, 2013, 532657. [Google Scholar] [CrossRef] [PubMed]
- Mitropoulou, G.; Fitsiou, E.; Stavropoulou, E.; Papavassilopoulou, E.; Vamvakias, M.; Pappa, A.; Oreopoulou, A.; Kourkoutas, Y. Composition, antimicrobial, antioxidant, and antiproliferative activity of Origanum dictamnus (dittany) essential oil. Microb. Ecol. Health Dis. 2015, 26, 26543. [Google Scholar] [CrossRef] [PubMed]
- Letsiou, S.; Trapali, M.; Vougiouklaki, D.; Tsakni, A.; Antonopoulos, D.; Houhoula, D. Antioxidant Profile of Origanum dictamnus L. Exhibits Antiaging Properties against UVA Irradiation. Cosmetics 2023, 10, 124. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Sudha, T.; Darwish, N.H.E.; Chader, H.; Belkadi, A.; Rajabi, M.; Houche, A.; Benkebailli, F.; Oudjida, F.; Mousa, S.A. A new eucalyptol-rich lavender (Lavandula stoechas L.) essential oil: Emerging potential for therapy against inflammation and cancer. Molecules 2020, 25, 3671. [Google Scholar] [CrossRef]
- Romano, R.; De Luca, L.; Aiello, A.; Pagano, R.; Di Pierro, P.; Pizzolongo, F.; Masi, P. Basil (Ocimum basilicum L.) Leaves as a Source of Bioactive Compounds. Foods 2022, 11, 3212. [Google Scholar] [CrossRef]
- Muzio, L.; Viotti, A.; Martino, G. Microglia in Neuroinflammation and Neurodegeneration: From Understanding to Therapy. Front. Neurosci. 2021, 15, 742065. [Google Scholar] [CrossRef]
- Hakimi Naeini, S.; Rajabi-Maham, H.; Azizi, V.; Hosseini, A. Anticonvulsant effect of glycitin in pentylenetetrazol induced male Wistar rat model by targeting oxidative stress and Nrf2/HO-1 signaling. Front. Pharmacol. 2024, 15, 1392325. [Google Scholar] [CrossRef]
- Demyashkin, G.; Blinova, E.; Grigoryan, M.; Parshenkov, M.; Skovorodko, P.; Ius, V.; Lebed, A.; Shegay, P.; Kaprin, A. Neuroprotective Effects of Myricetin on PTZ-Induced Seizures in Mice: Evaluation of Oxidation, Neuroinflammation and Metabolism, and Apoptosis in the Hippocampus. Curr. Issues Mol. Biol. 2024, 46, 8914–8944. [Google Scholar] [CrossRef] [PubMed]
- Azim, M.S.; Agarwal, N.B.; Vohora, D. Effects of agomelatine on pentylenetetrazole-induced kindling, kindling-associated oxidative stress, and behavioral despair in mice and modulation of its actions by luzindole and 1-(m-chlorophenyl) piperazine. Epilepsy Behav. 2017, 72, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Łukawski, K.; Czuczwar, S.J. Oxidative Stress and Neurodegeneration in Animal Models of Seizures and Epilepsy. Antioxidants 2023, 12, 1049. [Google Scholar] [CrossRef] [PubMed]
- Zavileyskiy, L.G.; Aleshin, V.A.; Kaehne, T.; Karlina, I.S.; Artiukhov, A.V.; Maslova, M.V.; Graf, A.V.; Bunik, V.I. The Brain Protein Acylation System Responds to Seizures in the Rat Model of PTZ-Induced Epilepsy. Int. J. Mol. Sci. 2022, 23, 12302. [Google Scholar] [CrossRef]
- Kandratavicius, L.; Alves Balista, P.; Lopes-Aguiar, C.; Ruggiero, R.N.; Umeoka, E.H.; Garcia-Cairasco, N.; Bueno-Junior, L.S.; Leite, J.P. Animal models of epilepsy: Use and limitations. Neuropsychiatr. Dis. Treat. 2014, 10, 1693–1705. [Google Scholar] [CrossRef]
- Jirovetz, L.; Wlcek, K.; Buchbauer, G.; Stoilova, I.; Atanasova, T.; Stoyanova, A.; Krastanov, A.; Schmidt, E. Chemical composition, olfactory evaluation and antioxidant effects of essential oil from Mentha canadensis. Nat. Prod. Commun. 2009, 4, 1011–1016. [Google Scholar] [CrossRef]
- Ramkissoon, J.S.; Mahomoodally, M.F.; Ahmed, N.; Subratty, A.H. Antioxidant and anti-glycation activities correlates with phenolic composition of tropical medicinal herbs. Asian Pac. J. Trop. Med. 2013, 6, 561–569. [Google Scholar] [CrossRef]
- Mimica-Dukić, N.; Božin, B.; Soković, M.; Mihajlović, B.; Matavulj, M. Antimicrobial and antioxidant activities of three Mentha species essential oils. Planta Med. 2003, 69, 413–419. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Adamczak, A.; Ożarowski, M. Radioprotective Effects of Plants from the Lamiaceae Family. Anticancer. Agents Med. Chem. 2020, 22, 4–19. [Google Scholar] [CrossRef]
- Bellassoued, K.; Ben Hsouna, A.; Athmouni, K.; Van Pelt, J.; Makni Ayadi, F.; Rebai, T.; Elfeki, A. Protective effects of Mentha piperita L. leaf essential oil against CCl4 induced hepatic oxidative damage and renal failure in rats. Lipids Health Dis. 2018, 17, 9. [Google Scholar] [CrossRef]
- Kamkar, A.; Javan, A.J.; Asadi, F.; Kamalinejad, M. The antioxidative effect of Iranian Mentha pulegium extracts and essential oil in sunflower oil. Food Chem. Toxicol. 2010, 48, 1796–1800. [Google Scholar] [CrossRef]
- Messaoudi, M.; Rebiai, A.; Sawicka, B.; Atanassova, M.; Ouakouak, H.; Larkem, I.; Egbuna, C.; Awuchi, C.G.; Boubekeur, S.; Ferhat, M.A.; et al. Effect of extraction methods on polyphenols, flavonoids, mineral elements, and biological activities of essential oil and extracts of Mentha pulegium L. Molecules 2022, 27, 11. [Google Scholar] [CrossRef]
- Egbuna, C.; Patrick-Iwuanyanwu, K.C.; Onyeike, E.N.; Khan, J.; Palai, S.; Patel, S.B.; Parmar, V.K.; Kushwaha, G.; Singh, O.; Jeevanandam, J.; et al. Phytochemicals and bioactive compounds effective against acute myeloid leukemia: A systematic review. Food Sci. Nutr. 2023, 11, 4191–4210. [Google Scholar] [CrossRef] [PubMed]
- Turkoglu, I. Assessment of wild mint from Tunceli as source of bioactive compounds, and its antioxidant Activity. Cell Mol Biol. 2018, 64, 42–46. [Google Scholar] [CrossRef]
- Hosseinimehr, S.J.; Pourmorad, F.; Shahabimajd, N.; Shahrbandy, K.; Hosseinzadeh, R. In vitro antioxidant activity of Polygonium hyrcanicum, Centaurea depressa, Sambucus ebulus, Mentha spicata and Phytolacca americana. Pakistan J. Biol. Sci. 2007, 10, 637–640. [Google Scholar] [CrossRef]
- Alpsoy, L.; Şahin, H.; Karaman, Ş. Anti-oxidative and anti-genotoxic effects of methanolic extract of Mentha pulegium on human lympocyte culture. Toxicol. Ind. Health 2011, 27, 647–654. [Google Scholar] [CrossRef]
- Couladis, M.; Tzakou, O.; Verykokidou, E.; Harvala, C. Screening of some Greek aromatic plants for antioxidant activity. Phyther. Res. 2003, 17, 194–195. [Google Scholar] [CrossRef]
- Gortzi, O.; Lalas, S.; Chinou, I.; Tsaknis, J. Evaluation of the antimicrobial and antioxidant activities of Origanum dictamnus extracts before and after encapsulation in liposomes. Molecules 2007, 12, 932–945. [Google Scholar] [CrossRef]
- Amaghnouje, A.; Mechchate, H.; Es-Safi, I.; Boukhira, S.; Aliqahtani, A.S.; Noman, O.M.; Nasr, F.A.; Conte, R.; Calarco, A.; Bousta, D. Subacute assessment of the toxicity and antidepressant-like effects of Origanum majorana L. Polyphenols in Swiss albino mice. Molecules 2020, 25, 5653. [Google Scholar] [CrossRef]
- Canli, K.; Bozyel, M.E.; Turu, D.; Benek, A.; Simsek, O.; Altuner, E.M. Biochemical, Antioxidant Properties and Antimicrobial Activity of Steno-Endemic Origanum onites. Microorganisms 2023, 11, 1987. [Google Scholar] [CrossRef]
- Yang, S.A.; Jeon, S.K.; Lee, E.J.; Shim, C.H.; Lee, I.S. Comparative study of the chemical composition and antioxidant activity of six essential oils and their components. Nat. Prod. Res. 2010, 24, 140–151. [Google Scholar] [CrossRef]
- Molina-Tijeras, J.A.; Ruiz-Malagón, A.J.; Hidalgo-García, L.; Diez-Echave, P.; Rodríguez-Sojo, M.J.; Cádiz-Gurrea, M.d.l.L.; Segura-Carretero, A.; del Palacio, J.P.; González-Tejero, M.R.; Rodríguez-Cabezas, M.E.; et al. The Antioxidant Properties of Lavandula multifida Extract Contribute to Its Beneficial Effects in High-Fat Diet-Induced Obesity in Mice. Antioxidants 2023, 12, 832. [Google Scholar] [CrossRef] [PubMed]
- Blažeković, B.; Vladimir-Knežević, S.; Brantner, A.; Štefan, M.B. Evaluation of antioxidant potential of Lavandula x intermedia Emeric ex Loisel. “Budrovka”: A comparative study with L. angustifolia Mill. Molecules 2010, 15, 5971–5987. [Google Scholar] [CrossRef] [PubMed]
- Hancianu, M.; Cioanca, O.; Mihasan, M.; Hritcu, L. Neuroprotective effects of inhaled lavender oil on scopolamine-induced dementia via anti-oxidative activities in rats. Phytomedicine 2013, 20, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.; Long, Y.; Ma, Y.; Yu, S.; Li, D.; Deng, J.; Wen, J.; Li, X.; Wu, Y.; He, X.; et al. Natural essential oils derived from herbal medicines: A promising therapy strategy for treating cognitive impairment. Front. Aging Neurosci. 2023, 15, 1104269. [Google Scholar] [CrossRef]
- Abdulsahib, W.K.; Kathem, S.H.; Al-Radeef, M.Y.; Jasim, L.S. Mentha piperita Oil Exerts an Antiepileptic Effect in Pilocarpine and Pentylenetetrazol-Induced Seizures in Mice. Vet. Med. Int. 2022, 2022, 4431317. [Google Scholar] [CrossRef]
- Anjum, R.; Raza, C.; Faheem, M.; Ullah, A.; Chaudhry, M. Neuroprotective potential of Mentha piperita extract prevents motor dysfunctions in mouse model of Parkinson’s disease through anti-oxidant capacities. PLoS ONE 2024, 19, e0302102. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412. [Google Scholar] [CrossRef]
- Jung, M.E.; Lal, H.; Gatch, M.B. The discriminative stimulus effects of pentylenetetrazol as a model of anxiety: Recent developments. Neurosci. Biobehav. Rev. 2002, 26, 429–439. [Google Scholar] [CrossRef]
- Monteiro, Á.B.; Alves, A.F.; Ribeiro Portela, A.C.; Oliveira Pires, H.F.; Pessoa de Melo, M.; Medeiros Vilar Barbosa, N.M.; Bezerra Felipe, C.F. Pentylenetetrazole: A review. Neurochem. Int. 2024, 180, 105841. [Google Scholar] [CrossRef]
- Gillis, K.; Stevens, K.K.; Bell, E.; Patel, R.K.; Jardine, A.G.; Morris, S.T.W.; Schneider, M.P.; Delles, C.; Mark, P.B. Ascorbic acid lowers central blood pressure and asymmetric dimethylarginine in chronic kidney disease. Clin. Kidney J. 2018, 11, 532–539. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Tian, J.; Brown, L.A.S.; Jones, D.P.; Levin, M.S.; Wang, L.; Rubin, D.C.; Ziegler, T.R. Intestinal Redox Status of Major Intracellular Thiols in a Rat Model of Chronic Alcohol Consumption. JPEN J. Parenter. Enteral Nutr. 2009, 33, 662. [Google Scholar] [CrossRef]
- Chi, V.; Chandy, K.G. Immunohistochemistry: Paraffin sections using the Vectastain ABC kit from vector labs. J. Vis. Exp. 2007, 8, 308. [Google Scholar] [CrossRef]


| Name | n | Treatments |
|---|---|---|
| Control | 20 | No treatment |
| PTZ | 20 | PTZ administration |
| MPU | 10 | Treatment with Mentha pulegium |
| MPU + PTZ | 10 | Pre-treatment with Mentha pulegium prior to PTZ administration |
| MSW | 10 | Treatment with Mentha spicata wild |
| MSW + PTZ | 10 | Pre-treatment with Mentha spicata wild prior to PTZ administration |
| LAN | 10 | Treatment with Lavandula angustifolia |
| LAN + PTZ | 10 | Pre-treatment with Lavandula angustifolia prior to PTZ administration |
| ODI | 10 | Treatment with Origanum dictamnus |
| ODI + PTZ | 10 | Pre-treatment with Origanum dictamnus prior to PTZ administration |
| MPI | 10 | Treatment with Mentha piperita |
| MPI + PTZ | 10 | Pre-treatment with Mentha piperita prior to PTZ administration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagonopoulou, O.; Koutroumanidou, E.; Mitrakas, A.; Pappa, A.; Voulgaridou, G.-P.; Vasiloudi, D.; Alexopoulou, S.-P.; Alexiadis, T.; Lambropoulou, M. Protective Effect of Aromatic Plant Essential Oil Administration on Brain Tissue of PTZ-Treated and Non-Treated Mice. Int. J. Mol. Sci. 2025, 26, 9618. https://doi.org/10.3390/ijms26199618
Pagonopoulou O, Koutroumanidou E, Mitrakas A, Pappa A, Voulgaridou G-P, Vasiloudi D, Alexopoulou S-P, Alexiadis T, Lambropoulou M. Protective Effect of Aromatic Plant Essential Oil Administration on Brain Tissue of PTZ-Treated and Non-Treated Mice. International Journal of Molecular Sciences. 2025; 26(19):9618. https://doi.org/10.3390/ijms26199618
Chicago/Turabian StylePagonopoulou, Olga, Eleni Koutroumanidou, Achilleas Mitrakas, Aglaia Pappa, Georgia-Persephoni Voulgaridou, Despoina Vasiloudi, Sofia-Panagiota Alexopoulou, Triantafyllos Alexiadis, and Maria Lambropoulou. 2025. "Protective Effect of Aromatic Plant Essential Oil Administration on Brain Tissue of PTZ-Treated and Non-Treated Mice" International Journal of Molecular Sciences 26, no. 19: 9618. https://doi.org/10.3390/ijms26199618
APA StylePagonopoulou, O., Koutroumanidou, E., Mitrakas, A., Pappa, A., Voulgaridou, G.-P., Vasiloudi, D., Alexopoulou, S.-P., Alexiadis, T., & Lambropoulou, M. (2025). Protective Effect of Aromatic Plant Essential Oil Administration on Brain Tissue of PTZ-Treated and Non-Treated Mice. International Journal of Molecular Sciences, 26(19), 9618. https://doi.org/10.3390/ijms26199618









