Attenuation Effect of Withania somnifera Extract on Restraint Stress-Induced Anxiety-like Behavior and Hippocampal Alterations in Mice
Abstract
1. Introduction
2. Results
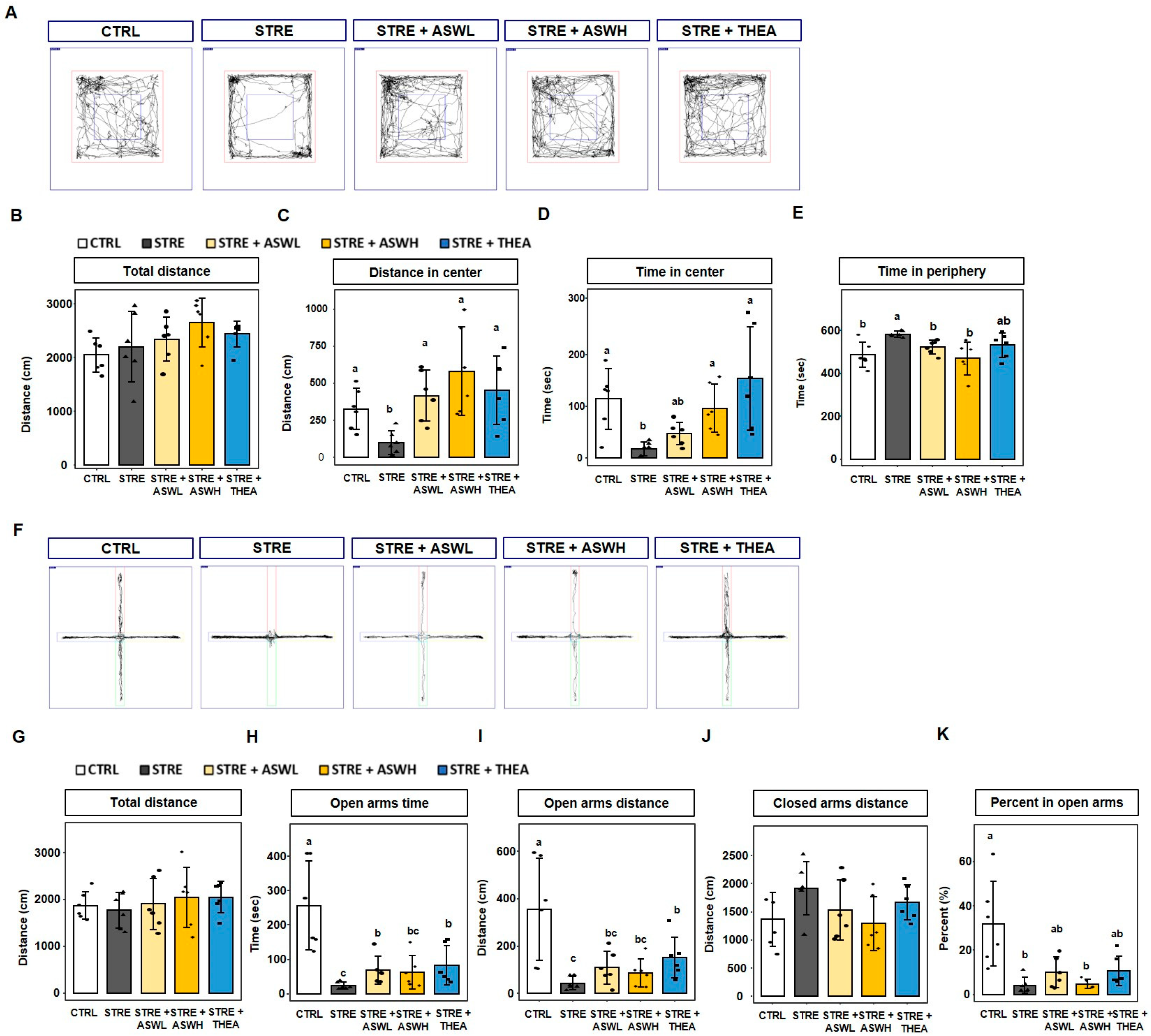
2.1. Effect of W. somniferous Extract on Behavior in Restraint Stress-Exposed Mice
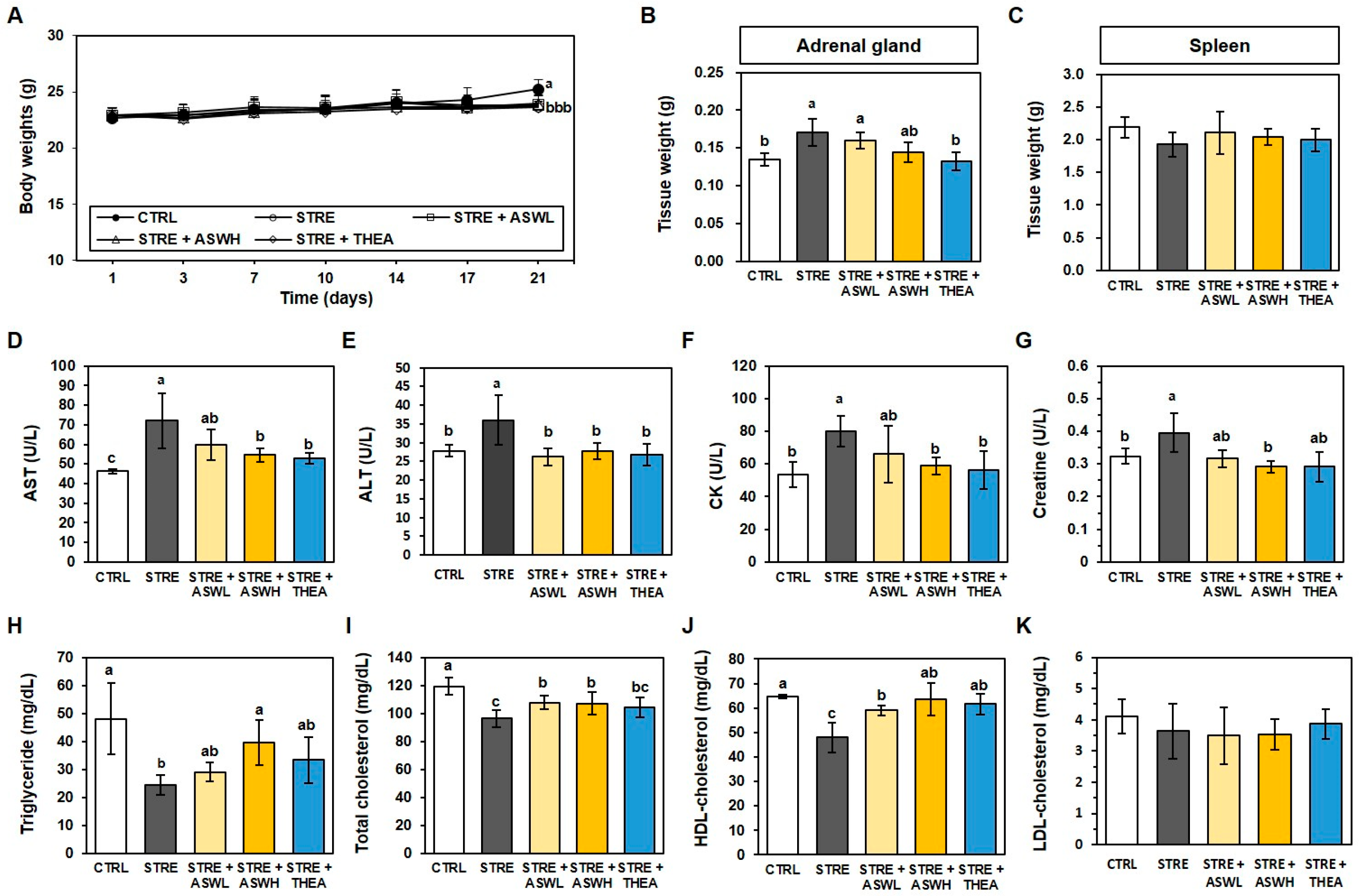
2.2. Effect of W. somniferous Extract on Tissue Weight and Biochemical Parameters in Restraint Stress-Exposed Mice
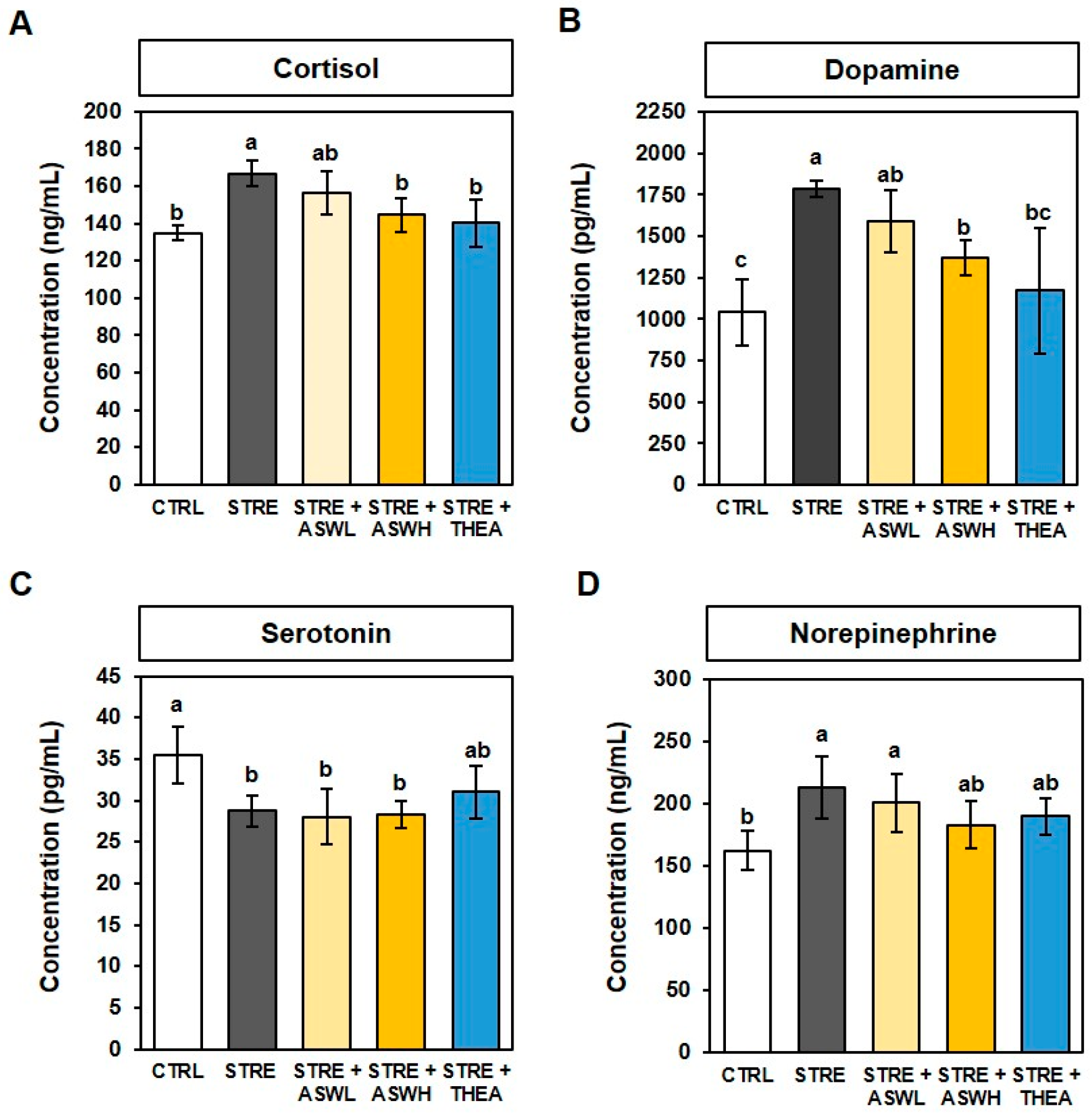
2.3. Effect of W. somniferous Extract on Serum Stress Hormone Levels in Restraint Stress-Exposed Mice
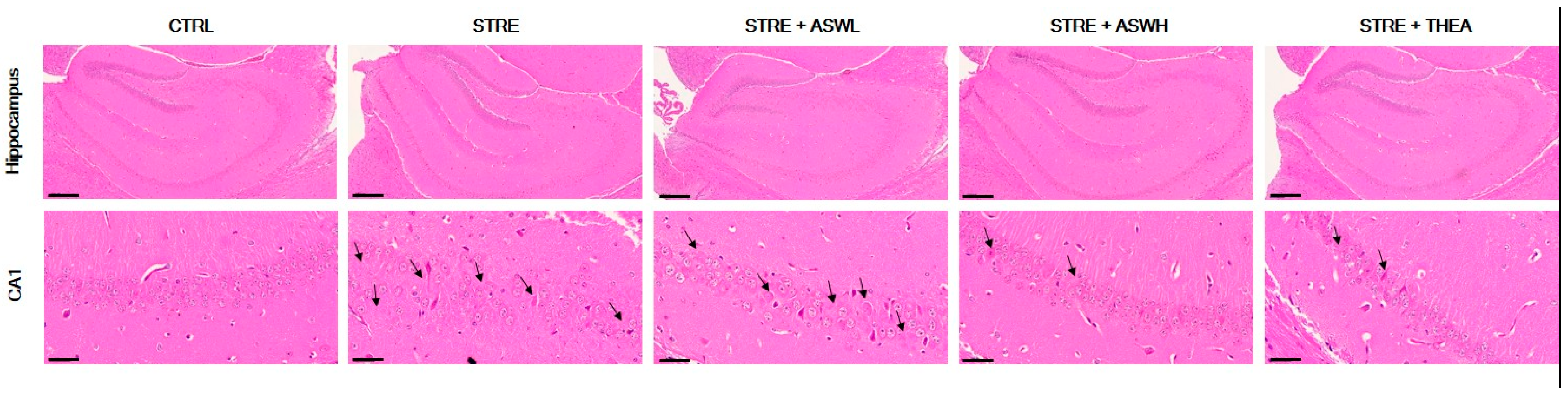
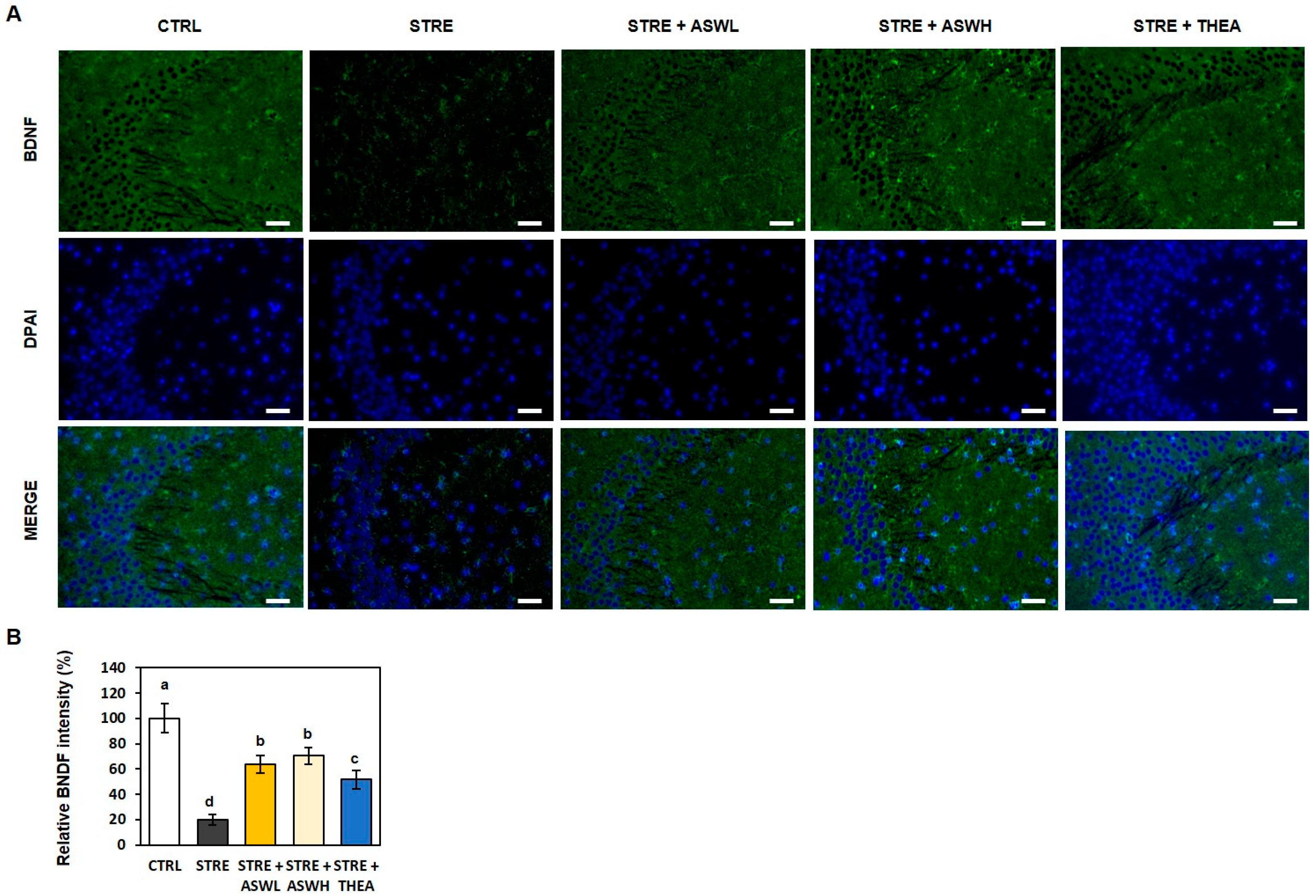
2.4. Effect of W. somniferous on Hippocampal Formation and BDNF Expression in the Hippocampus of Restraint Stress-Exposed Mice
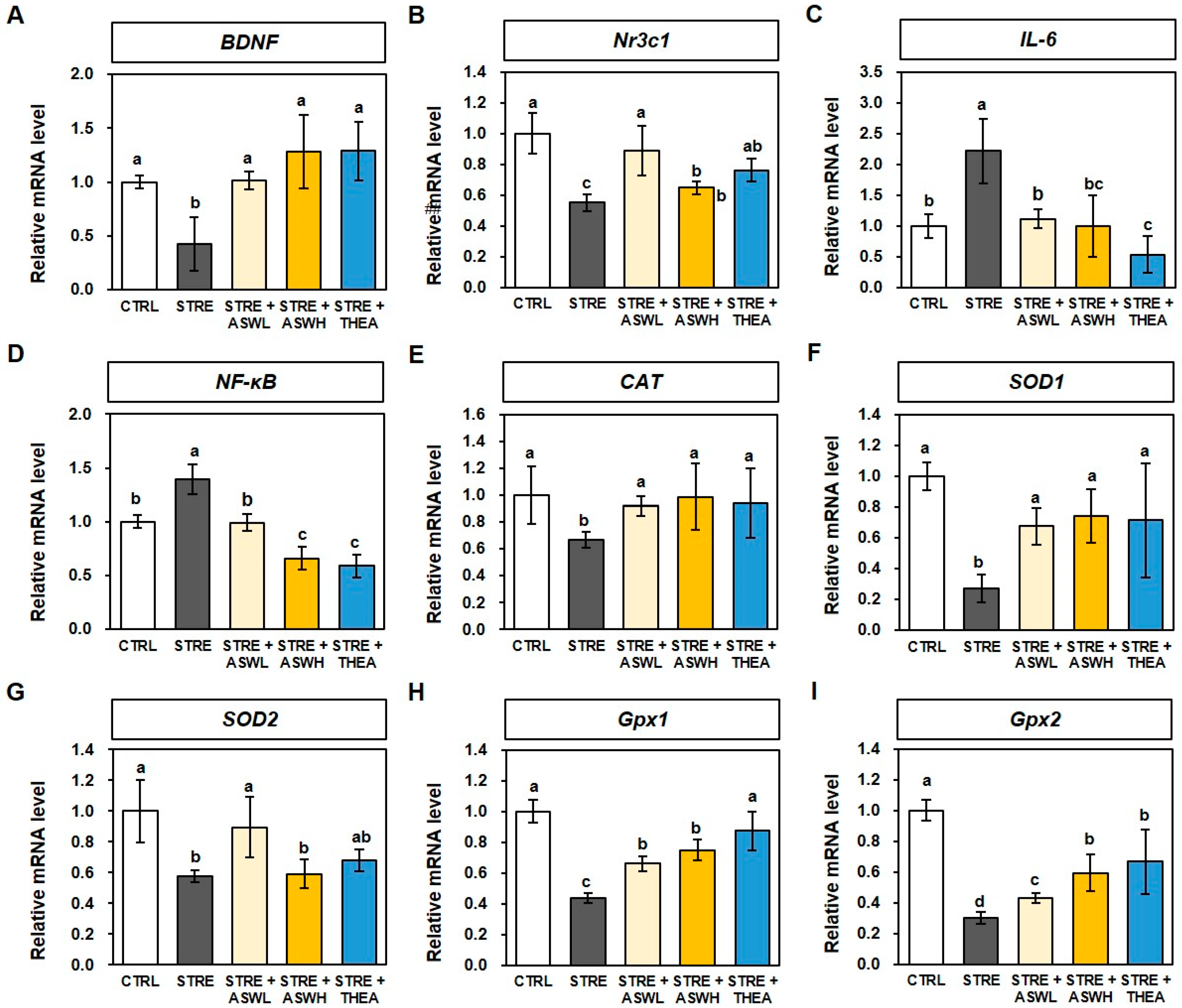
2.5. Effect of W. somniferous Extract on mRNA Levels for BDNF, Inflammation, and Antioxidants in Restraint Stress-Exposed Mice
3. Discussion
4. Materials and Methods
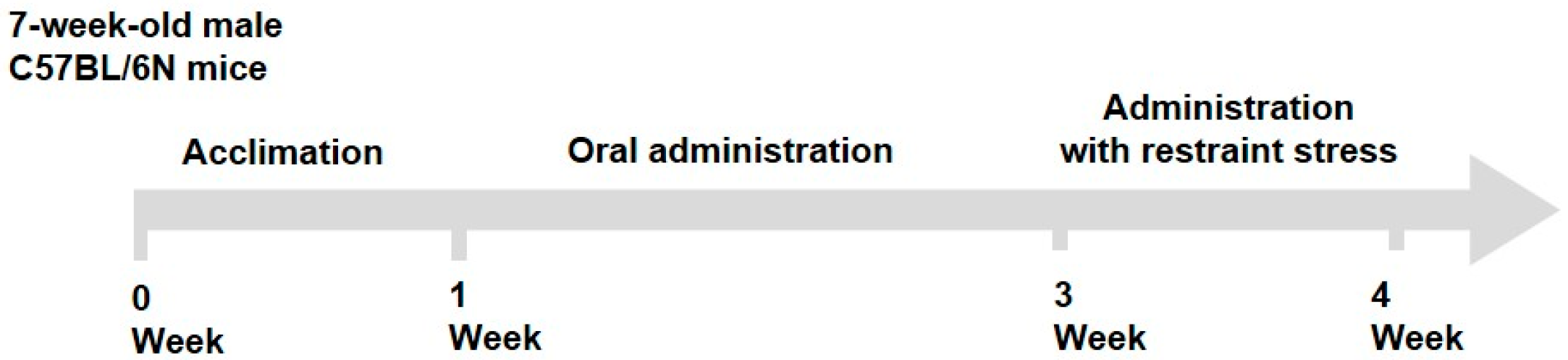
4.1. Animals and Experimental Design
4.2. Behavioral Tests
4.2.1. OFT
4.2.2. EPM Test
4.3. Analysis of Biological Parameters
4.3.1. Measurement of Biochemical Components in Serum
4.3.2. Measurement of Cortisol, Dopamine, Serotonin, and Norepinephrine in Serum
4.3.3. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) Analysis
4.4. Histological and Immunofluorescence (IF) Analyses
4.4.1. Hematoxylin and Eosin (H&E) Staining
4.4.2. IF Staining
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAT | catalase |
| SOD | superoxide dismutase |
| BDNF | brain-derived neurotrophic factor |
| CTRL | control mice treated with saline |
| STRE | restraint-stressed mice treated with saline |
| STRE + ASWL | restraint-stressed mice treated with W. somniferous extract (20 mg/kg/day) |
| STRE + ASWH | restraint-stressed mice treated with W. somniferous extract (40 mg/kg/day) |
| STRE + THEA | restraint-stressed mice treated with L-theanine (50 mg/kg/day, positive control) |
| OFT | open field test |
| EPM | elevated plus maze |
| AST | aspartate aminotransferase |
| ALT | alanine aminotransferase |
| CK | creatine kinase |
| HDL | high-density lipoprotein |
| LDL | low-density lipoprotein |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| Nr3c1 | nuclear receptor subfamily 3C member 1 |
| IL-6 | interleukin-6 |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| GPx | glutathione peroxidase |
| H&E | hematoxylin and eosin |
References
- Mariotti, A. The effects of chronic stress on health: New insights into the molecular mechanisms of brain–body communication. Futur. Sci. OA 2015, 1, FSO23. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr. Physiol. 2016, 6, 603. [Google Scholar] [CrossRef]
- Ranabir, S.; Reetu, K. Stress and hormones. Indian J. Endocrinol. Metab. 2011, 15, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Li, S.; Wang, S.; Wu, X.; Liu, Y.; Yu, W.; Wang, Y.; Tang, Y.; Xia, M.; Li, B. Major depressive disorder: Hypothesis, mechanism, prevention and treatment. Signal Transduct. Target. Ther. 2024, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Justice, N.J. The relationship between stress and Alzheimer’s disease. Neurobiol. Stress 2018, 8, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.E.; Perks, P.; Lightman, S.L.; Shanks, N. Restraint stress is associated with changes in glucocorticoid immunoregulation. Physiol. Behav. 2001, 73, 525–532. [Google Scholar] [CrossRef]
- Molina, P.; Andero, R.; Armario, A. Restraint or immobilization: A comparison of methodologies for restricting free movement in rodents and their potential impact on physiology and behavior. Neurosci. Biobehav. Rev. 2023, 151, 105224. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, T.G.; Kaur, A.; Dhiman, S.; Arora, S. Ameliorative effect of trigonelline in restraint stress-induced behavioral alterations in mice. J. Appl. Pharm. Sci. 2021, 11, 054–062. [Google Scholar] [CrossRef]
- Saveanu, R.V.; Nemeroff, C.B. Etiology of depression: Genetic and environmental factors. Psychiatr. Clin. 2012, 35, 51–71. [Google Scholar] [CrossRef]
- Philpotts, R.; Gillan, N.; Barrow, M.; Seidler, K. Stress-induced alterations in hippocampal BDNF in the pathophysiology of major depressive disorder and the antidepressant effect of saffron. J. Affect. Disord. Rep. 2023, 14, 100630. [Google Scholar] [CrossRef]
- Kim, E.J.; Pellman, B.; Kim, J.J. Stress effects on the hippocampus: A critical review. Learn. Mem. 2015, 22, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Kalpana, K.; Yap, S.; Tsuji, M.; Kawamura, A. Molecular Mechanism behind the Safe Immunostimulatory Effect of Withania somnifera. Biomolecules 2023, 13, 828. [Google Scholar] [CrossRef] [PubMed]
- Dar, N.J.; Hamid, A.; Ahmad, M. Pharmacologic overview of Withania somnifera, the Indian Ginseng. Cell. Mol. Life Sci. 2015, 72, 4445–4460. [Google Scholar] [CrossRef] [PubMed]
- Ziauddin, M.; Phansalkar, N.; Patki, P.; Diwanay, S.; Patwardhan, B. Studies on the immunomodulatory effects of Ashwagandha. J. Ethnopharmacol. 1996, 50, 69–76. [Google Scholar] [CrossRef]
- Gupta, A.; Mahdi, A.A.; Shukla, K.K.; Ahmad, M.K.; Bansal, N.; Sankhwar, P.; Sankhwar, S.N. Efficacy of Withania somnifera on seminal plasma metabolites of infertile males: A proton NMR study at 800 MHz. J. Ethnopharmacol. 2013, 149, 208–214. [Google Scholar] [CrossRef]
- Mikulska, P.; Malinowska, M.; Ignacyk, M.; Szustowski, P.; Nowak, J.; Pesta, K.; Szeląg, M.; Szklanny, D.; Judasz, E.; Kaczmarek, G. Ashwagandha (Withania somnifera)—Current research on the health-promoting activities: A narrative review. Pharmaceutics 2023, 15, 1057. [Google Scholar] [CrossRef]
- Dutta, R.; Khalil, R.; Green, R.; Mohapatra, S.S.; Mohapatra, S. Withania somnifera (Ashwagandha) and withaferin A: Potential in integrative oncology. Int. J. Mol. Sci. 2019, 20, 5310. [Google Scholar] [CrossRef]
- Paul, S.; Chakraborty, S.; Anand, U.; Dey, S.; Nandy, S.; Ghorai, M.; Saha, S.C.; Patil, M.T.; Kandimalla, R.; Proćków, J. Withania somnifera (L.) Dunal (Ashwagandha): A comprehensive review on ethnopharmacology, pharmacotherapeutics, biomedicinal and toxicological aspects. Biomed. Pharmacother. 2021, 143, 112175. [Google Scholar] [CrossRef]
- Li, M.-Y.; Liu, H.-Y.; Wu, D.-T.; Kenaan, A.; Geng, F.; Li, H.-B.; Gunaratne, A.; Li, H.; Gan, R.-Y. L-theanine: A unique functional amino acid in tea (Camellia sinensis L.) with multiple health benefits and food applications. Front. Nutr. 2022, 9, 853846. [Google Scholar] [CrossRef]
- Hidese, S.; Ota, M.; Wakabayashi, C.; Noda, T.; Ozawa, H.; Okubo, T.; Kunugi, H. Effects of chronic l-theanine administration in patients with major depressive disorder: An open-label study. Acta Neuropsychiatr. 2017, 29, 72–79. [Google Scholar] [CrossRef]
- Moshfeghinia, R.; Sanaei, E.; Mostafavi, S.; Assadian, K.; Sanaei, A.; Ayano, G. The effects of L-theanine supplementation on the outcomes of patients with mental disorders: A systematic review. BMC Psychiatry 2024, 24, 886. [Google Scholar] [CrossRef]
- Ter Horst, J.P.; de Kloet, E.R.; Schächinger, H.; Oitzl, M. Relevance of stress and female sex hormones for emotion and cognition. Cell. Mol. Neurobiol. 2012, 32, 725–735. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Lee, D.H.; Kang, S.S. Effects of chronic restraint stress on body weight, food intake, and hypothalamic gene expressions in mice. Endocrinol. Metab. 2013, 28, 288. [Google Scholar] [CrossRef] [PubMed]
- Dallman, M.F.; Akana, S.F.; Scribner, K.A.; Bradbury, M.J.; Walker, C.D.; Strack, A.M.; Cascio, C.S. Stress, feedback and facilitation in the hypothalamo-pituitary-adrenal axis. J. Neuroendocrinol. 1992, 4, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, E.; Nenic, K.; Milanovic, V.; Knezevic, N.N. The role of cortisol in chronic stress, neurodegenerative diseases, and psychological disorders. Cells 2023, 12, 2726. [Google Scholar] [CrossRef]
- Chao, A.M.; Jastreboff, A.M.; White, M.A.; Grilo, C.M.; Sinha, R. Stress, cortisol, and other appetite-related hormones: Prospective prediction of 6-month changes in food cravings and weight. Obesity 2017, 25, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, O.; Arnau, A.; Pareja, M.; Poch, E.; Ramírez, I.; Soley, M. Acute stress-induced tissue injury in mice: Differences between emotional and social stress. Cell Stress Chaperones 2002, 7, 36. [Google Scholar] [CrossRef]
- Dziurkowska, E.; Wesolowski, M. Cortisol as a biomarker of mental disorder severity. J. Clin. Med. 2021, 10, 5204. [Google Scholar] [CrossRef]
- Finsterwald, C.; Alberini, C.M. Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: From adaptive responses to psychopathologies. Neurobiol. Learn. Mem. 2014, 112, 17–29. [Google Scholar] [CrossRef]
- Trainor, B.C. Stress responses and the mesolimbic dopamine system: Social contexts and sex differences. Horm. Behav. 2011, 60, 457–469. [Google Scholar] [CrossRef]
- Saal, D.; Dong, Y.; Bonci, A.; Malenka, R.C. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron 2003, 37, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, M.A.; McCutcheon, R.A.; Kempton, M.; Freeman, T.P.; Howes, O. The effects of psychosocial stress on dopaminergic function and the acute stress response. Elife 2019, 8, e46797. [Google Scholar] [CrossRef] [PubMed]
- Stein-Behrens, B.; Mattson, M.; Chang, I.; Yeh, M.; Sapolsky, R. Stress exacerbates neuron loss and cytoskeletal pathology in the hippocampus. J. Neurosci. 1994, 14, 5373–5380. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F. Corticotropin-releasing factor, norepinephrine, and stress. Biol. Psychiatry 1999, 46, 1167–1180. [Google Scholar] [CrossRef]
- McEwen, B.S.; Nasca, C.; Gray, J.D. Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 2016, 41, 3–23. [Google Scholar] [CrossRef]
- Hoge, J.; Kesner, R.P. Role of CA3 and CA1 subregions of the dorsal hippocampus on temporal processing of objects. Neurobiol. Learn. Mem. 2007, 88, 225–231. [Google Scholar] [CrossRef]
- Albini, M.; Krawczun-Rygmaczewska, A.; Cesca, F. Astrocytes and brain-derived neurotrophic factor (BDNF). Neurosci. Res. 2023, 197, 42–51. [Google Scholar] [CrossRef]
- Yang, T.; Nie, Z.; Shu, H.; Kuang, Y.; Chen, X.; Cheng, J.; Yu, S.; Liu, H. The role of BDNF on neural plasticity in depression. Front. Cell. Neurosci. 2020, 14, 82. [Google Scholar] [CrossRef]
- Herman, J.P. The neuroendocrinology of stress: Glucocorticoid signaling mechanisms. Psychoneuroendocrinology 2022, 137, 105641. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.; Lee, D.; Kim, J.Y.; Shim, J.J.; Bae, J.W.; Lee, J.H. Attenuation Effect of Withania somnifera Extract on Restraint Stress-Induced Anxiety-like Behavior and Hippocampal Alterations in Mice. Int. J. Mol. Sci. 2025, 26, 7317. https://doi.org/10.3390/ijms26157317
Lee K, Lee D, Kim JY, Shim JJ, Bae JW, Lee JH. Attenuation Effect of Withania somnifera Extract on Restraint Stress-Induced Anxiety-like Behavior and Hippocampal Alterations in Mice. International Journal of Molecular Sciences. 2025; 26(15):7317. https://doi.org/10.3390/ijms26157317
Chicago/Turabian StyleLee, Kippuem, Daehyeop Lee, Joo Yun Kim, Jae Jung Shim, Jae Woo Bae, and Jae Hwan Lee. 2025. "Attenuation Effect of Withania somnifera Extract on Restraint Stress-Induced Anxiety-like Behavior and Hippocampal Alterations in Mice" International Journal of Molecular Sciences 26, no. 15: 7317. https://doi.org/10.3390/ijms26157317
APA StyleLee, K., Lee, D., Kim, J. Y., Shim, J. J., Bae, J. W., & Lee, J. H. (2025). Attenuation Effect of Withania somnifera Extract on Restraint Stress-Induced Anxiety-like Behavior and Hippocampal Alterations in Mice. International Journal of Molecular Sciences, 26(15), 7317. https://doi.org/10.3390/ijms26157317





