Antioxidant and Antiviral Potential of Cold-Brewed and Cold-Concentrated Plant Extracts
Abstract
1. Introduction
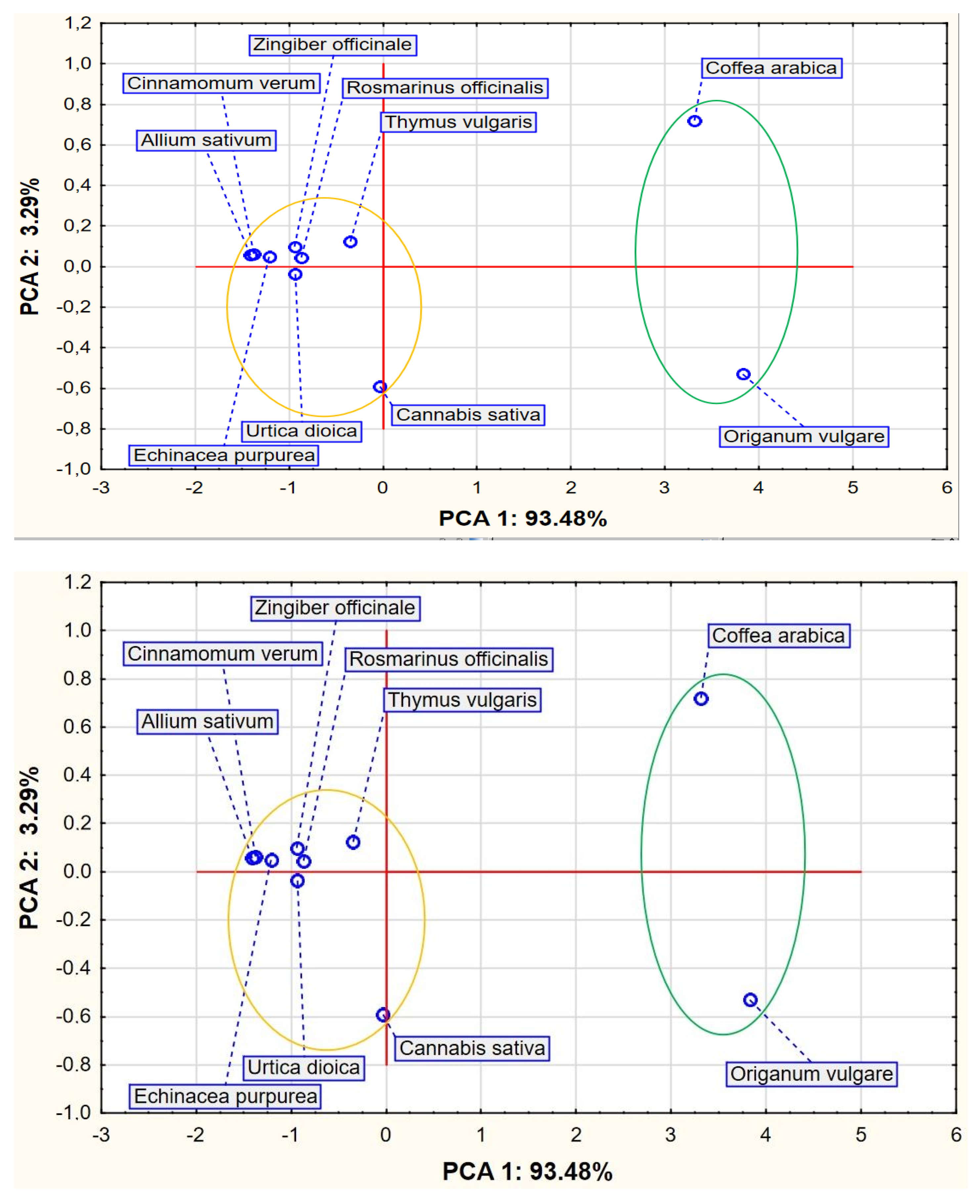
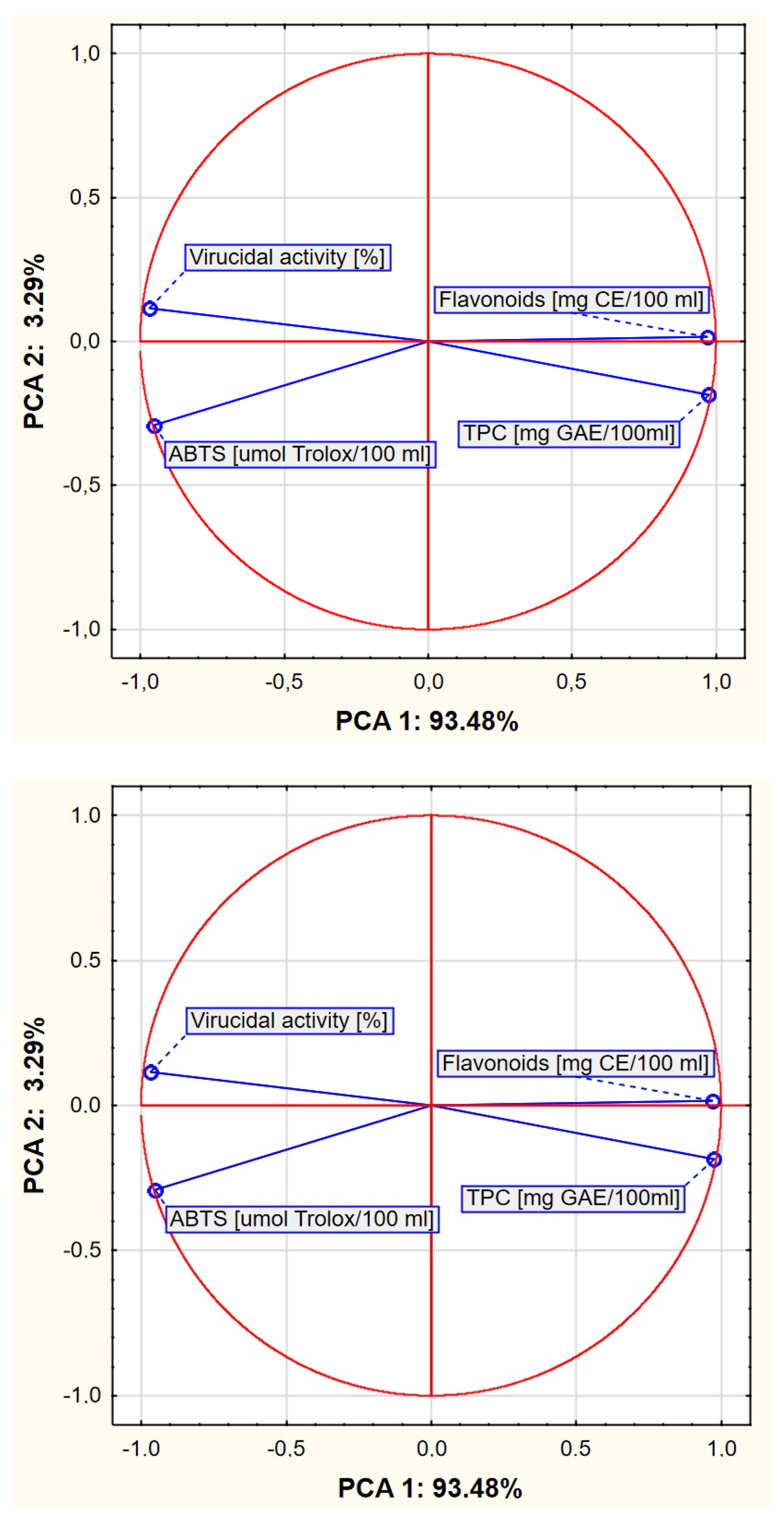
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Extracts
4.1.1. Cold Brewing Technique
4.1.2. Cryoconcentration (Cold-Concentration)
4.1.3. Total Dissolved Solids (TDSs)
4.1.4. Total Phenolic Content (TPC)
4.1.5. Flavonoid Content
4.1.6. Antioxidant Activity
4.2. Virological Tests
4.2.1. Cell Culture for Virological Test
4.2.2. Virus Propagation
4.3. Cytotoxicity Assay
4.4. Antiviral Potential
4.5. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pasrija, D.; Anandharamakrishnan, C. Techniques for Extraction of Green Tea Polyphenols: A Review. Food Bioprocess Technol. 2015, 8, 935–950. [Google Scholar] [CrossRef]
- Squeo, M.; Mondelli, G.; Cappello, F.; Colonna, G.; Logrieco, A.; Summo, G. Comparative analysis of hot and cold infusions of single-estate teas (Camellia sinensis) from Europe: A novel specialty product. Antioxidants 2023, 12, 61306. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, Y.; Zong, Y.; Wu, J.; Lao, F. Comparative Decoding of Physicochemical and Flavor Profiles of Coffee Prepared by High-Pressure Carbon Dioxide, Ice Drip, and Traditional Cold Brew. Foods 2025, 14, 2840. [Google Scholar] [CrossRef] [PubMed]
- Koszowska, A.; Dittfeld, A.; Puzoń-Brończyk, A.; Nowak, J.; Zubelewicz-Szkodzińska, B. Polyphenols in the prevention of lifestyle diseases. Postępy Fitoter. 2013, 4, 263–266. [Google Scholar]
- Williams, C.A.; Grayer, R.J. Anthocyanins and other flavonoids. Nat. Prod. Rep. 2004, 21, 539–573. [Google Scholar] [CrossRef] [PubMed]
- Hasler, C.M. The changing face of functional foods. J. Am. Coll. Nutr. 2000, 19 (Suppl. 5), 499S–506S. [Google Scholar] [CrossRef]
- Li, Y.; Quan, H.; Liang, L.L.; Yang, T.; Feng, L.; Mao, X.; Wang, Y. Nontargeted metabolomics reveals the discrimination of Cyclocarya paliurus leaves brewed by different methods. Food Res. Int. 2021, 142, 110221. [Google Scholar] [CrossRef]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Tenore, G.C.; Novellino, E. Resveratrol as a novel anti-herpes simplex virus nutraceutical agent: An overview. Viruses 2018, 10, 473. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Sodagari, H.R.; Farzaei, M.H.; Abdolghaffari, A.H.; Gooshe, M.; Rezaei, N. The preventive and therapeutic potential of natural polyphenols on influenza. Expert Rev. Anti-Infect. Ther. 2015, 14, 57–80. [Google Scholar] [CrossRef]
- Das, J.; Ramani, R.; Suraju, M.O. Polyphenol compounds and pkc signaling. Biochim. Biophys. Acta (BBA) Gen. Subj. 2016, 1860, 2107–2121. [Google Scholar] [CrossRef]
- Wawer, I.; Różański, H. Rośliny zielarskie, kosmetyki naturalne i żywność funkcjonalna. In Monografia Naukowa VII Konferencji: Rośliny Lecznicze i Leki Ziołowe w Terapii i Profilaktyce Chorób Wirusowych. Naturalne Substancje Przeciwwirusowe; Karpacka Państwowa Uczelnia w Krośnie: Krosno, Poland, 2022; pp. 21–29. [Google Scholar]
- Asensio, C.M.; Grosso, N.R.; Juliani, H.R. Quality characters, chemical composition and biological activities of oregano (Origanum spp.) essential oils from central and southern Argentina. Ind. Crop. Prod. 2015, 63, 203–213. [Google Scholar] [CrossRef]
- Patay, É.B.; Bencsik, T.; Papp, N. Phytochemical overview and medicinal importance of Coffea species from the past until now. Asian Pac. J. Trop. Med. 2016, 9, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Belayneh, A.; Bussa, N.F. Ethnomedicinal plants used to treat human ailments in the prehistoric place of Harla and Dengego valleys, eastern Ethiopia. J. Ethnobiol. Ethnomed. 2014, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Lowe, H.; Toyang, N.; Steele, B.; Bryant, J.; Ngwa, W.; Nedamat, K. The Current and Potential Application of Medicinal Cannabis Products in Dentistry. Dent. J. 2021, 9, 106. [Google Scholar] [CrossRef]
- Chacon, F.T.; Raup-Konsavage, W.M.; Vrana, K.E.; Kellogg, J.J. Secondary terpenes in Cannabis sativa l.: Synthesis and synergy. Biomedicines 2022, 10, 3142. [Google Scholar] [CrossRef]
- Baghiani, A. Determination of Total Phenolics Contents, Antioxidant Capacity of Thymus vulgaris Extracts using Electrochemical and Spectrophotometric methods. Int. J. Electrochem. Sci. 2018, 13, 7882–7893. [Google Scholar] [CrossRef]
- Disler, M.; Ivemeyer, S.; Hamburger, M.; Vogl, C.R.; Tesic, A.; Klarer, F.; Beat, M.; Walkenhorst, M. Ethnoveterinary herbal remedies used by farmers in four north-eastern swiss cantons (st. Gallen, thurgau, appenzell innerrhoden and Appenzell Ausserrhoden). J. Ethnobiol. Ethnomed. 2014, 10, 32. [Google Scholar] [CrossRef]
- Francišković, M.; González-Pérez, R.; Orčić, D.; de Medina, F.S.; Martínez-Augustin, O.; Svirčev, E.; Mimica–Dukić, N. Chemical composition and immuno-modulatory effects of Urtica dioica l. (stinging nettle) extracts. Phytother. Res. 2017, 31, 1183–1191. [Google Scholar] [CrossRef]
- Singh, H. Ethnobotanical Studies on Urtica dioica Linn. among the Bhotias of ChaMoli Garhwal, U.P. J. Econ. Taxon. Bot. 1989, 13, 719–724. [Google Scholar]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Popa, C.V.; Farcasanu, I.; Jipa, S.; Zaharescu, T.; Danet, A. Chemiluminescence Determination of the Total Antioxidant Capacity of Rosemary Extract. Rev. Chim. 2012, 63, 715–719. [Google Scholar] [CrossRef]
- Bitari, A.; Oualdi, I.; Touzani, R.; Bitari, A.; Legssyer, A. Zingiber officinale Roscoe: A comprehensive review of clinical properties. Mater. Proc. 2023, 72, 3757–3767. [Google Scholar] [CrossRef]
- Hudson, J.B. Applications of the phytomedicine Echinacea purpurea (Purple Coneflower) in infectious diseases. BioMed Res. Int. 2012, 2012, 769896. [Google Scholar] [CrossRef]
- Nyalambisa, M.; Oyemitan, I.A.; Matewu, R.; Oyedeji, O.O.; Oluwafemi, O.S.; Songca, S.P.; Nkeh-Chungag, B.N.; Oyedeji, A.O. Volatile constituents and biological activities of the leaf and root of Echinacea species from South Africa. Saudi Pharm. J. 2017, 25, 381–386. [Google Scholar] [CrossRef]
- Abe, K.; Hori, Y.; Myoda, T. Characterization of key aroma compounds in aged garlic extract. Food Chem. 2019, 312, 126081. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, P.; Pigera, S.; Premakumara, G.A.; Galappaththy, P.; Constantine, G.R.; Katulanda, P. Medicinal properties of ‘true’ cinnamon (Cinnamomum zeylanicum): A systematic review. BMC Complement. Altern. Med. 2013, 13, 275. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, C.; Seamon, E.; Windsor, R.C.; Armbruester, N.; Bryan, J.K.; Costa, D.; Giese, N.; Gruenwald, J.; Iovin, R.; Isaac, R.; et al. An evidence-based systematic review of cinnamon (Cinnamomum spp.) by the Natural Standard Research Collaboration. J. Diet. Suppl. 2011, 8, 378–454. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.K.; Hoq, M.O.; Uddin, M.S. Medicinal plant Allium sativum–A review. J. Med. Plant Stud. 2016, 4, 72–79. [Google Scholar]
- Velíšek, J.; Kubec, R.; Davídek, J. Chemical composition and classification of culinary and pharmaceutical garlic based products. Z. Lebensm. Unters. Forsch. A 1997, 204, 161–164. [Google Scholar] [CrossRef]
- Singh, R.; Singh, K. Garlic: A spice with wide medicinal actions. J. Pharmacogn. Phytochem. 2019, 8, 1349–1355. [Google Scholar]
- Ahmed, S.M.; Hall, A.J.; Robinson, A.E.; Verhoef, L.; Premkumar, P.; Parashar, U.D.; Koopmans, M.; Lopman, B.A. Global prevalence of norovirus in cases of gastroenteritis: A systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 725–730. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, M.; Wang, K.; Estes, M.K. Sequence and genomic organization of Norwalk virus. Virology 1993, 195, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Begall, L.F.; Mauroy, A.; Thiry, E. Noroviruses-The State of the Art, Nearly Fifty Years after Their Initial Discovery. Viruses 2021, 13, 1541. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.M.; Widdowson, M.A.; Glass, R.I.; Akazawa, K.; Vinjé, J.; Parashar, U.D. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg. Infect. Dis. 2008, 14, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, S.M.; Lopman, B.A.; Ozawa, S.; Hall, A.J.; Lee, B.Y. Global Economic Burden of Norovirus Gastroenteritis. PLoS ONE 2016, 11, e0151219. [Google Scholar] [CrossRef]
- Oszmiański, J.; Kolniak-Ostek, J.; Lachowicz, S.; Gorzelany, J.; Matłok, N. Effect of Dried Powder Preparation Process on Polyphenolic Content and Antioxidant Capacity of Cranberry (Vaccinium macrocarpon L.). Ind. Crops Prod. 2015, 77, 658–665. [Google Scholar] [CrossRef]
- Hayashi, K.; Komatsu, S.; Kuno, H.; Asai, S.; Matsuura, I.; Kudkyal, V.; Kawahara, T. Virucidal and immunostimulating activities of monogalactosyl diacylglyceride from Coccomyxa sp. kj, a green microalga, against murine norovirus and feline calicivirus. Mar. Drugs 2022, 20, 131. [Google Scholar] [CrossRef]
- Gilling, D.H.; Kitajima, M.; Torrey, J.T.; Bright, K.R. Antiviral efficacy and mechanisms of action of oregano essential oil and its primary component carvacrol against murine norovirus. J. Appl. Microbiol. 2014, 116, 1149–1163. [Google Scholar] [CrossRef]
- Lim, C.; Kim, H.; Chung, M. ; Chung, M. Mori cortex radicis extract inhibits human norovirus surrogate in simulated digestive conditions. Food Sci. Biotechnol. 2021, 30, 1243–1248. [Google Scholar] [CrossRef]
- Joshi, S.; Su, X.; D’Souza, D. Antiviral effects of grape seed extract against feline calicivirus, murine norovirus, and hepatitis a virus in model food systems and under gastric conditions. Food Microbiol. 2015, 52, 1–10. [Google Scholar] [CrossRef]
- Iloghalu, U.; Holmes, B.; Khatiwada, J.; Williams, L. Selected plant extracts show antiviral effects against murine norovirus surrogate. Adv. Microbiol. 2019, 9, 372–384. [Google Scholar] [CrossRef]
- Kim, K.; Park, M.; Eom, S.; Lim, K.; Kim, J.; Lee, D.; Lee, M. Antiviral activity of seaweed extracts against feline calicivirus. Fish. Aquat. Sci. 2010, 13, 96–101. [Google Scholar] [CrossRef]
- Eom, S.; Moon, S.; Lee, D.; Kim, H.; Park, K.; Lee, E.; Kim, Y. In vitro antiviral activity of dieckol and phlorofucofuroeckol-a isolated from edible brown alga Eisenia bicyclis against murine norovirus. Algae 2015, 30, 241–246. [Google Scholar] [CrossRef]
- Cacace, J.E.; Mazza, G. Mass transfer process during extraction of phenolic compounds from milled berries. J. Food Eng. 2003, 59, 379–389. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Silva, S.; Henriques, M.; Ferreira, I.C.F.R. Decoction, infusion and hydroalcoholic extract of cultivated thyme: Antioxidant and antibacterial activities, and phenolic characterisation. Food Chem. 2015, 167, 131–137. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Paixão, N.; Perestrelo, R.; Marques, J.C.; Câmara, J.S. Relationship between Antioxidant Capacity and Total Phenolic Content of Red, Rosé and White Wines. Food Chem. 2007, 105, 204–214. [Google Scholar] [CrossRef]
- Atanassova, M.; Georgieva, S.; Ivancheva, K. Total phenolic and total flavonoid contents, antioxidant capacity and biological contaminants in medicinal herbs. J. Univ. Chem. Technol. Metall. 2011, 46, 81–88. [Google Scholar]
- Tirado-Kulieva, V.A.; Hernández-Martínez, E.; Choque-Rivera, T.J. Phenolic compounds versus SARS-CoV-2: An update on the main findings against COVID-19. Heliyon 2022, 8, e10702. [Google Scholar] [CrossRef]
- Córdoba, N.; Moreno, F.L.; Osorio, C.; Velásquez, S.; Ruiz, Y. Chemical and sensory evaluation of cold brew coffees using different roasting profiles and brewing methods. Food Res. Int. 2021, 141, 110141. [Google Scholar] [CrossRef]
- Correa, L.J.; Ruiz, R.Y.; Moreno, F.L. Effect of falling-film freeze concentration on bioactive compounds in aqueous coffee extract. J. Food Process Eng. 2018, 41, e12606. [Google Scholar] [CrossRef]
- Sepahpour, S.; Selamat, J.; Abdul Manap, M.Y.; Khatib, A.; Abdull Razis, A.F. Comparative Analysis of Chemical Composition, Antioxidant Activity and Quantitative Characterization of Some Phenolic Compounds in Selected Herbs and Spices in Different Solvent Extraction Systems. Molecules 2018, 23, 402. [Google Scholar] [CrossRef] [PubMed]
- Majewska, M.; Czeczot, H. Flawonoidy w profilaktyce i terapii. Farm. Pol. 2009, 65, 369–377. [Google Scholar]
- Hosu, A.; Cristea, V.M.; Cimpoiu, C. Analysis of total phenolic, flavonoids, anthocyanins and tannins content in Romanian red wines: Prediction of antioxidant activities and classification of wines using artificial neural networks. Food Chem. 2014, 150, 113–118. [Google Scholar] [CrossRef]
- Rana, S.; Gupta, S.; Rana, A.; Bhushan, S. Functional properties, phenolic constituents and antioxidant potential of industrial apple pomace for utilization as active food ingredient. Food Sci. Hum. Wellnes 2015, 4, 180–187. [Google Scholar] [CrossRef]
- D’Abrosca, B.; Pacifico, S.; Cefarelli, G.; Mastellone, C.; Fiorentino, A. ‘Limoncella’ apple, an Italian apple cultivar: Phenolic and flavonoid contents and antioxidant activity. Food Chem. 2007, 104, 1333–1337. [Google Scholar] [CrossRef]
- Lopes, G.R.; Passos, C.P.; Rodrigues, C.; Teixeira, J.A.; Coimbra, M.A. Impact of microwave-assisted extraction on roasted coffee carbohydrates, caffeine, chlorogenic acids and coloured compounds. Food Res. Int. 2020, 129, 108864. [Google Scholar] [CrossRef]
- Ikawa, M.; Schaper, T.D.; Dollard, C.A.; Sasner, J.J. Utilization of Folin−Ciocalteu phenol reagent for the detection of certain nitrogen compounds. J. Agric. Food Chem. 2003, 51, 1811–1815. [Google Scholar] [CrossRef]
- Ziarno, M.; Kozłowska, M.; Ratusz, K.; Hasalliu, R. Effect of the Addition of Selected Herbal Extracts on the Quality Characteristics of Flavored Cream and Butter. Foods 2023, 12, 471. [Google Scholar] [CrossRef]
- Skotti, E.; Anastasaki, E.; Kanellou, G.; Polissiou, M.; Tarantilis, P.A. Total phenolic content, antioxidant activity and toxicity of aqueous extracts from selected Greek medicinal and aromatic plants. Ind. Crops Prod. 2014, 53, 46–54. [Google Scholar] [CrossRef]
- Reis, S.F.; Rai, D.K.; Abu-Ghannam, N. Water at room temperature as a solvent for the extraction of apple pomace phenolic compounds. Food Chem. 2012, 135, 1991–1998. [Google Scholar] [CrossRef]
- Habib, H.I.; Omar, S.K.; Mohamed, H.S. Estimation of rutin and ascorbic acid in some Libyan herbal plants by RP-HPLC. Med. Aromat. Plants 2016, 5, 255–259. [Google Scholar]
- Parliment, T.H. An Overview of Coffee Roasting. Caffeinated Beverages. ACS Symp. Ser. Am. Chem. Soc. 2000, 20, 188–201. [Google Scholar] [CrossRef]
- Sukoco, A.; Novenda, I.; Maryanto; Kuswardhani, N.; Sari, P. Chemical compounds and antioxidant activity in caffeinated and decaffeinated green robusta coffee beans enriched with ginger extract. IOP Conf. Ser. Earth Environ. Sci. 2021, 709, 12–35. [Google Scholar] [CrossRef]
- Rao, N.Z.; Fuller, M. Acidity and Antioxidant Activity of Cold Brew Coffee. Sci. Rep. 2018, 8, 16030. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Kawabata, R.; Irie, T.; Nakai, Y.; Tohya, Y.; Sakaguchi, T. Inactivation of pathogenic viruses by plant-derived tannins: Strong effects of extracts from persimmon (Diospyros kaki) on a broad range of viruses. PLoS ONE 2013, 8, e55343. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, H.; Ichinose, M.; Ikeda, K.; Uozaki, M.; Morishita, J.; Kuwahara, T.; Koyama, A.H.; Yamasaki, H. Inhibition by caffeic acid of the influenza A virus multiplication in vitro. Int. J. Mol. Med. 2014, 34, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, Pharmacology and Health Benefits of Anthocyanins. Phytother. Res. 2016, 30, 1265–1286. [Google Scholar] [CrossRef]
- Judžentienė, A.; Garjonytė, R.; Būdienė, J. Phytochemical Composition and Antioxidant Activity of Various Extracts of Fibre Hemp (Cannabis sativa L.). Cultiv. Lithuania. Mol. 2023, 28, 4928. [Google Scholar] [CrossRef]
- Abdelfadel, M.M.; Khalaf, H.H.; Sharoba, A.M.; Assous, M.T.M. Effect of extraction methods on antioxidant and antimicrobial activities of some spices and herbs extracts. J. Food Technol. Nutr. Sci. 2016, 1, 1–14. [Google Scholar]
- Köksal, E.; Bursal, E.; Gülçın, İ.; Korkmaz, M.; Çağlayan, C.; Gören, A.; Polat, A.; Uysal, S.; Alwasel, S. Antioxidant activity and polyphenol content of Turkish thyme (Thymus vulgaris) monitored by liquid chromatography and tandem mass spectrometry. Int. J. Food Prop 2016, 20, 514–525. [Google Scholar] [CrossRef]
- Kőszegi, K.; Békássy-Molnár, E.; Koczka, N.; Kerner, T.; Stefanovits-Bányai, E. Changes in Total Polyphenol Content and Antioxidant Capacity of Stinging Nettle (Urtica dioica L.) from Spring to Autumn. Period. Polytech. Chem. Eng. 2020, 64, 548–554. [Google Scholar] [CrossRef]
- Khan, M.I.; Ullah, N.F.; Khan, M.I.; Khan, M.A. Antioxidant activity of rosemary (Rosmarinus officinalis L.) extracts: A comprehensive review. Antioxidants 2019, 8, 546. [Google Scholar] [CrossRef]
- Irakli, M.; Skendi, A.; Bouloumpasi, E.; Christaki, S.; Biliaderis, C.G.; Chatzopoulou, P. Sustainable recovery of phenolic compounds from distilled rosemary by-product using green extraction methods: Optimization, comparison, and antioxidant activity. Molecules 2023, 28, 6669. [Google Scholar] [CrossRef] [PubMed]
- Sida, S.; Samakradhamrongthai, R.S.; Utama-Ang, N. Influence of maturity and drying temperature on antioxi-dant activity and chemical compositions in ginger. Curr. Appl. Sci. Technol. 2019, 19, 28–42. [Google Scholar] [CrossRef]
- Dvorackova, E.; Snoblova, M.; Chromcova, L.; Hrdlicka, P. Effects of extraction methods on the phenolic com-pounds contents and antioxidant capacities of cinnamon extracts. Food Sci Biotechnol. 2015, 24, 1201–1207. [Google Scholar] [CrossRef]
- Zhai, X.; Yang, M.; Zhang, J.; Zhang, L.; Tian, Y.; Li, C.; Bao, L.; Ma, C.; Abd El-Aty, A.M. Feasibility of Ultrasound-Assisted Extraction for Accelerated Cold Brew Coffee Processing: Characterization and Comparison with Conventional Brewing Methods. Front. Nutr. 2022, 9, 849811. [Google Scholar] [CrossRef]
- Wijewardhana, U.S.; Gunathilaka, U.G.S.; Navaratne, S.B. Determination of total phenolic content, radical scavenging activity and total antioxidant capacity of cinnamon bark, black cumin sees and garlic. Int. Res. J. Adv. Eng. Sci. 2019, 4, 55–57. Available online: http://dr.lib.sjp.ac.lk/handle/123456789/12092 (accessed on 3 May 2025).
- Schön, C.; Mödinger, Y.; Krüger, F.; Doebis, C.; Pischel, I.; Bonnländer, B. A new high-quality elderberry plant extract exerts antiviral and immunomodulatory effects in vitro and ex vivo. Food Agric. Immunol. 2021, 32, 650–662. [Google Scholar] [CrossRef]
- Pachura, N.; Włodarczyk, M.; Bazanów, B.; Pogorzelska, A.; Gebarowski, T.; Kupczynski, R.; Szumny, A. Antiviral and Cytotoxic Activities of Ilex aquifolium Silver Queen in the Context of Chemical Profiling of Two Ilex Species. Molecules 2024, 29, 3231. [Google Scholar] [CrossRef]
- Ross, I.A. Medicinal Plants of the World; Humana Press Inc.: Totowa, NJ, USA, 2005; Volume 3, pp. 155–184. [Google Scholar]
- Barclay, L.; Park, G.; Vega, E.; Hall, A.J.; Parashar, U.D.; Vinjé, J.; Lopman, B.A. Infection control for norovirus. Clin. Microbiol. Infection. 2014, 20, 731–740. [Google Scholar] [CrossRef]
- Tsai, H.; Yune, P.S.; Rao, M. Norovirus disease among older adults. Ther. Adv. Infect. Dis. 2022, 9, 204993612211367. [Google Scholar] [CrossRef]
- Hosmillo, M.; Chaudhry, Y.; Nayak, K.; Sorgeloos, F.; Koo, B.; Merenda, A.; Lillestol, R.; Drumright, L.; Zilbauer, M.; Goodfellow, I. Norovirus replication in human intestinal epithelial cells is restricted by the interferon-induced jak/stat signaling pathway and rna polymerase ii-mediated transcriptional responses. mBio 2020, 11, 10–1128. [Google Scholar] [CrossRef]
- Chojnacka, K.; Skrzypczak, D.; Izydorczyk, G.; Mikula, K.; Szopa, D.; Witek-Krowiak, A. Antiviral properties of polyphenols from plants. Foods 2021, 10, 2277. [Google Scholar] [CrossRef] [PubMed]
- El-Toumy, S.A.; Salib, J.Y.; El-Kashak, W.A.; Marty, C.; Bedoux, G.; Bourgougnon, N. Antiviral effect of polyphenol rich plant extracts on herpes simplex virus type 1. Food Sci. Hum. Wellness 2018, 7, 91–101. [Google Scholar] [CrossRef]
- Diaz, P.; Jeong, S.C.; Lee, S.; Khoo, C.; Koyyalamudi, S.R. Antioxidant and anti-inflammatory activities of selected medicinal plants and fungi containing phenolic and flavonoid compounds. Chin. Med. 2012, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.M.; Davidorf, F.H.; Abdel-Rahman, M.H. In vitro anti-melanoma activity of phenolic compounds from the Egyptian medicinal plant Acacia nilotica. Phytotherapy 2011, 82, 1279–1284. [Google Scholar] [CrossRef]
- Yu, M.S.; Lee, J.; Lee, J.M.; Kim, Y.; Chin, Y.W.; Jee, J.G.; Keum, Y.S.; Jeong, Y.J. Identification of myricetin and scutellarein as novel chemical inhibitors of the SARS coronavirus helicase, nsP13. Med. Bioorganiczna Chem. 2012, 22, 4049–4054. [Google Scholar] [CrossRef]
- Hendrayana, M.A.; Jawi, I.M.; Sukrama, D.M.; Suprapta, D.N. The potential of polyphenols from natural ingredients against SARS-CoV-2 infection: A review. Indones. J. Pharmacol. Ther. 2021, 2, 10. [Google Scholar] [CrossRef]
- Vázquez-Calvo, Á.; Martín-Acebes, M.Á.; Blázquez, A.B.; Saiz, J.C.; Moreno, J. Antiviral properties of the natural polyphenols delphinidin and epigallocatechin gallate against the flaviviruses West Nile virus, Zika virus, and Dengue virus. Antivir. Res. 2017, 146, 1314. [Google Scholar] [CrossRef]
- Dias, L.K.M.; Medeiros, G.C.B.S.; Silva, A.K.N.; Morais, A.H.D.A.; Silva-Maia, J.K. Can polyphenols improve the gut health status in pre-clinical study with diet-induced obesity? Medicine 2021, 100, e28162. [Google Scholar] [CrossRef]
- Gilling, D.; Kitajima, M.; Torrey, J.; Bright, K. Mechanisms of antiviral action of plant antimicrobials against murine norovirus. Appl. Environ. Microbiol. 2014, 80, 4898–4910. [Google Scholar] [CrossRef]
- Cozzi, L.; Vicenza, T.; Battistini, R.; Masotti, C.; Suffredini, E.; Di Pasquale, S.; Fauconnier, M.-L.; Ercolini, C.; Serracca, L. Effects of Essential Oils and Hydrolates on the Infectivity of Murine Norovirus. Viruses 2023, 15, 682. [Google Scholar] [CrossRef]
- Azizkhani, M.; Tooryan, F. Effect of cinnamon, rosemary and zataria essential oils on food-borne gastroenteritis viruses. J. Maz. Univ. Med. Sci. 2016, 26, 8–15. [Google Scholar]
- Hamada, A.A.; Andrew, N.; Nhungoc, T.L.; Shivani, A.; Sobhy, A.A.E.; Mohammed, M.Y.; Sagar, M.G. In Vitro Antiviral Activity of Clove and Ginger Aqueous Extracts against Feline Calicivirus, a Surrogate for Human norovirus. J. Food Prot. 2016, 79, 1001–1012. [Google Scholar]
- Aydın, H.B.; Korkmaz, S.; Omurtag Korkmaz, B.I. Antibacterial and antiviral activities of stinging nettle (Urtica dioica l.) Leaf extract on norovirus and campylobacter jejuni as foodborne pathogens. J. Microbiol. Biotechnol. Food Sci. 2023, 13, e9768. [Google Scholar] [CrossRef]
- Castañeda-Rodríguez, R.; Mulík, S.; Ozuna, C. Brewing temperature and particle size affect extraction kinetics of cold brew coffee in terms of its physicochemical, bioactive, and antioxidant properties. J. Culin. Sci. Technol. 2020, 20, 366–387. [Google Scholar] [CrossRef]
- Maksimowski, D. Opracowanie Metody Otrzymywania Napojów Instant Cold Brew Coffee Typu Liquid Opartych na Kawie o Zwiększonych Właściwościach Sensorycznych i Prozdrowotnych [Development of a Method for Producing Instant Liquid Cold Brew Coffee Based on Coffee with Enhanced Sensory and Health-Promoting Properties]. Ph.D. Thesis, Uniwersytet Przyrodniczy we Wrocławiu, Wrocław, Poland, 2024. [Google Scholar]
- Zakaria, N.H.; Whanmek, K.; Thangsiri, S.; Chathiran, W.; Srichamnong, W.; Suttisansanee, U.; Santivarangkna, C. Optimization of Cold Brew Coffee Using Central Composite Design and Its Properties Compared with Hot Brew Coffee. Foods 2023, 12, 2412. [Google Scholar] [CrossRef]
- Muzykiewicz-Szymańska, A.; Nowak, A.; Wira, D.; Klimowicz, A. The effect of brewing process parameters on antioxidant activity and caffeine content in infusions of roasted and unroasted arabica coffee beans originated from different countries. Molecules 2021, 26, 3681. [Google Scholar] [CrossRef]
- EN 14476; Chemical Disinfectants and Antiseptics Quantitative Suspension Test for the Evaluation of Virucidal Activity in the Medical Area. Test Method and Requirements (Phase 2/Step 1). European Committee for Standardization: Brussels, Belgium, 2013.
| Plant Extract Source | TDS Extract | TDS After Concentration | Ratio TDS (After Concentration:Extract) |
|---|---|---|---|
| [%] | |||
| Origanum vulgare L. Oregano leaves | 0.48 ± 0.07 d | 0.97 ± 0.12 b,c | 2.02 |
| Coffea arabica L. Arabica coffee roasted beans | 0.53 ± 0.04 c | 0.99 ± 0.06 a | 1.86 |
| Cannabis sativa L. Hemp seeds and leaves | 0.56 ±0.02 b | 1.01 ± 0.06 a | 1.80 |
| Thymus vulgaris L. Common thyme leaves | 0.49 ± 0.08 d | 0.93 ± 0.02 c | 1.89 |
| Urtica dioica L. Common nettle leaves | 0.41 ± 0.05 e | 0.77 ± 0.07 e | 1.87 |
| Rosmarinus officinalis L. Rosemary leaves | 0.52 ± 0.04 c | 0.94 ± 0.01 c | 1.81 |
| Zingiber officinale Roscoe Ginger root | 0.49 ± 0.08 d | 0.81 ± 0.04 d | 1.65 |
| Echinacea purpurea L. Purple coneflower | 0.54 ± 0.05 b,c | 0.96 ± 0.02 b,c | 1.77 |
| Cinnamomum verum J. Presl Ceylon cinnamon bark | 0.63 ± 0.03 a | 0.94 ± 0.10 c | 1.49 |
| Allium sativum L. Garlic bulb | 0.51 ± 0.06 c | 0.95 ± 0.04 c | 1.86 |
| Plant Extract Source | TPC [mg GAE/100 mL] | Flavonoid [mg CE/100 mL] | ABTS [µmol Trolox/100 mL] | Virus-Inactivating Activity [%] |
|---|---|---|---|---|
| Origanum vulgare L. Oregano leaves | 105.73 a ± 1.57 | 1217.94 a ± 2.12 | 574.56 c ± 4.34 | 99.90 |
| Coffea arabica L. Arabica coffee roasted beans | 77.67 b ± 3.14 | 955.28 a ± 0.56 | 451.15 d ± 5.21 | 99.91 |
| Cannabis sativa L. Hemp seeds and leaves | 47.26 c ± 17.81 | 268.58 d ± 2.76 | 791.31 b ± 5.21 | 99.95 |
| Thymus vulgaris L. Common thyme leaves | 37.69 c ± 1.32 | 455.28 b ± 1.14 | 762.45 b ± 0.87 | 99.99 |
| Urtica dioica L. Common nettle leaves | 25.53 d ± 2.15 | 335.03 c ± 4.7 | 818.32 a ± 3.47 | 99.99 |
| Rosmarinus officinalis L. Rosemary leaves | 24.83 d ± 3.14 | 357.18 c ± 7.01 | 807.27 a ± 5.21 | 99.99 |
| Zingiber officinale Roscoe Ginger root | 24.13 d ± 2.81 | 293.89 d ± 2.94 | 798.67 a ± 3.49 | 99.99 |
| Echinacea purpurea L. Purple coneflower | 14.54 d ± 0.5 | 300.22 d ± 3.01 | 832.44 a ± 4.34 | 99.99 |
| Cinnamomum verum J. Presl Ceylon cinnamon bark | 9.63 e ± 0.33 | 271.74 d ± 5.56 | 842.88 a ± 6.95 | 99.99 |
| Allium sativum L. Garlic bulb | 9.11 e ± 0.16 | 259.08 d ± 2.54 | 844.72 a ± 6.09 | 99.99 |
| Substance Used for Testing | Concentration of the Test Substance (%) | Dilution that Did Not Produce Cytotoxic Effects |
|---|---|---|
| Origanum vulgare L. Oregano leaves | 18 | 10−3 |
| Coffea arabica L. Arabica coffee roasted beans | 18 | 10−3 |
| Cannabis sativa L. Hemp seeds and leaves | 18 | 10−3 |
| Thymus vulgaris L. Common thyme leaves | 18 | 10−3 |
| Urtica dioica L. Common nettle leaves | 18 | 10−3 |
| Rosmarinus officinalis L. Rosemary leaves | 18 | 10−3 |
| Zingiber officinale Roscoe Ginger root | 18 | 10−3 |
| Echinacea purpurea L. Purple coneflower | 18 | 10−3 |
| Cinnamomum verum J. Presl Ceylon cinnamon bark | 18 | 10−3 |
| Allium sativum L. Garlic bulb | 18 | 10−3 |
| Eicatechin (EPI) | 10 | 10−2 |
| Cavoylquinic acid (CQA) | 10 | 10−2 |
| Substance Used for Testing | Extract Concentration % (w/v) | Concentration Used in Antiviral Tests (mg/mL) | The Volumes Used in EN 14476 Mixes | Solvent |
|---|---|---|---|---|
| Origanum vulgare L. Oregano leaves | 2.04 | 9.7 | 800 μL | Dulbecco’s Modified Eagle’s Medium (DMEM) |
| Coffea arabica L. Arabica coffee roasted beans | 2.04 | 9.9 | 800 μL | DMEM |
| Cannabis sativa L. Hemp seeds and leaves | 2.04 | 10.1 | 800 μL | DMEM |
| Thymus vulgaris L. Common thyme leaves | 2.04 | 9.3 | 800 μL | DMEM |
| Urtica dioica L. Common nettle leaves | 2.04 | 7.7 | 800 μL | DMEM |
| Rosmarinus officinalis L. Rosemary leaves | 2.04 | 9.4 | 800 μL | DMEM |
| Zingiber officinale Roscoe Ginger root | 2.04 | 8.1 | 800 μL | DMEM |
| Echinacea purpurea L. Purple coneflower | 2.04 | 9.6 | 800 μL | DMEM |
| Cinnamomum verum J. Presl Ceylon cinnamon bark | 2.04 | 9.4 | 800 μL | DMEM |
| Allium sativum L. Garlic bulb | 2.04 | 9.5 | 800 μL | DMEM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janicka, P.; Maksimowski, D.; Chwirot, A.; Oziembłowski, M.; Michalczyk, K.; Nawirska-Olszańska, A.; Poręba, P.; Baluta, S.; Kaczmar, E.; Stygar, D.; et al. Antioxidant and Antiviral Potential of Cold-Brewed and Cold-Concentrated Plant Extracts. Int. J. Mol. Sci. 2025, 26, 9617. https://doi.org/10.3390/ijms26199617
Janicka P, Maksimowski D, Chwirot A, Oziembłowski M, Michalczyk K, Nawirska-Olszańska A, Poręba P, Baluta S, Kaczmar E, Stygar D, et al. Antioxidant and Antiviral Potential of Cold-Brewed and Cold-Concentrated Plant Extracts. International Journal of Molecular Sciences. 2025; 26(19):9617. https://doi.org/10.3390/ijms26199617
Chicago/Turabian StyleJanicka, Paulina, Damian Maksimowski, Aleksandra Chwirot, Maciej Oziembłowski, Katarzyna Michalczyk, Agnieszka Nawirska-Olszańska, Piotr Poręba, Sylwia Baluta, Ewa Kaczmar, Dominika Stygar, and et al. 2025. "Antioxidant and Antiviral Potential of Cold-Brewed and Cold-Concentrated Plant Extracts" International Journal of Molecular Sciences 26, no. 19: 9617. https://doi.org/10.3390/ijms26199617
APA StyleJanicka, P., Maksimowski, D., Chwirot, A., Oziembłowski, M., Michalczyk, K., Nawirska-Olszańska, A., Poręba, P., Baluta, S., Kaczmar, E., Stygar, D., & Bażanów, B. (2025). Antioxidant and Antiviral Potential of Cold-Brewed and Cold-Concentrated Plant Extracts. International Journal of Molecular Sciences, 26(19), 9617. https://doi.org/10.3390/ijms26199617









