Predictive Factors for Cervical Intraepithelial Neoplasia in Women with Abnormal Cytology According to Human Papillomavirus Genotype: An Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Population and Eligibility Criteria
2.3. Study Variables
- HPV genotype: categorized as negative, HPV16, HPV18, or other high-risk HPV genotypes.
- Cytology: classified as ASC-US, ASC-H, AGC, LSIL, or HSIL.
- Age: grouped into three categories: 20–29, 30–59, and ≥60 years.
2.4. Diagnostic Procedures
2.5. Statistical Analysis
2.6. Sample Size Calculation
2.7. Ethical Considerations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| HPV | Human papillomavirus |
| hrHPV | High-risk human papillomavirus |
| CIN | Cervical intraepithelial neoplasia |
| CIN2–3 | Cervical intraepithelial neoplasia grades 2 and 3 |
| ASC-US | Atypical squamous cells of undetermined significance |
| ASC-H | Atypical squamous cells—cannot exclude HSIL |
| AGC | Atypical glandular cells |
| LSIL | Low-grade squamous intraepithelial lesion |
| HSIL | High-grade squamous intraepithelial lesion |
| aOR | Adjusted odds ratio |
| OR | Odds ratio |
| CI | Confidence interval |
| GLM | Generalized linear model |
| LLETZ | Large loop excision of the transformation zone |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| AIC | Akaike information criterion |
| ASCCP | American Society for Colposcopy and Cervical Pathology |
References
- World Health Organization. Cervical Cancer [Internet]. Geneva: World Health Organization. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed on 15 July 2025).
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Zur Hausen, H. Papillomaviruses and cancer: From basic studies to clinical application. Nat. Rev. Cancer 2002, 2, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, N.; Bosch, F.X.; de Sanjosé, S.; Herrero, R.; Castellsagué, X.; Shah, K.V.; Snijders, P.J.; Meijer, C.J. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Lindsay, L.; Hoots, B.; Keys, J.; Franceschi, S.; Winer, R.; Clifford, G.M. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: A meta-analysis update. Int. J. Cancer 2007, 121, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Castellsagué, X. Natural history and epidemiology of HPV infection and cervical cancer. Gynecol. Oncol. 2008, 110 (Suppl. 2), S4–S7. [Google Scholar] [CrossRef]
- Koliopoulos, G.; Nyaga, V.N.; Santesso, N.; Bryant, A.; Martin-Hirsch, P.P.; Mustafa, R.A.; Schünemann, H.; Paraskevaidis, E.; Arbyn, M. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst. Rev. 2017, 8, CD008587. [Google Scholar] [CrossRef]
- Schiffman, M.; Wentzensen, N.; Wacholder, S.; Kinney, W.; Gage, J.C.; Castle, P.E. Human papillomavirus testing in the prevention of cervical cancer. J. Natl. Cancer Inst. 2011, 103, 368–383. [Google Scholar] [CrossRef]
- Khan, M.J.; Castle, P.E.; Lorincz, A.T.; Wacholder, S.; Sherman, M.; Scott, D.R.; Rush, B.B.; Glass, A.G.; Schiffman, M. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J. Natl. Cancer Inst. 2005, 97, 1072–1079. [Google Scholar] [CrossRef]
- Schiffman, M.; Castle, P.E.; Jeronimo, J.; Rodriguez, A.C.; Wacholder, S. Human papillomavirus and cervical cancer. Lancet 2007, 370, 890–907. [Google Scholar] [CrossRef]
- Egemen, D.; Cheung, L.C. Chen Risk Estimates Supporting the 2019 ASCCP Risk-Based Management Consensus Guidelines. J. Low. Genit. Tract Dis. 2020, 24, 132–143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aguilar Luna, J.T.; Ortiz Tejedor, J.G.; Vizhñay Guzmán, M.G. Genotipos de alto riesgo del Virus del Papiloma Humano en mujeres de América Latina y el Caribe. Vive Rev. Salud 2024, 7, 788–802. Available online: https://revistavive.org/index.php/revistavive/article/view/518 (accessed on 16 September 2025). [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Cobas 4800 HPV Test (Package Insert); Roche Molecular Systems: Branchburg, NJ, USA. Available online: http://e-labdoc.roche.com (accessed on 1 March 2025).
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef] [PubMed]
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical carcinoma and reproductive factors: Collaborative reanalysis of individual data on 16,563 women with cervical carcinoma and 33,542 women without cervical carcinoma. Int. J. Cancer 2006, 119, 1108–1124. [Google Scholar] [CrossRef]
- Bruni, L.; Diaz, M.; Castellsagué, X.; Ferrer, E.; Bosch, F.X.; de Sanjosé, S. Cervical human papillomavirus prevalence in 5 continents: Meta-analysis of 1 million women with normal cytological findings. J. Infect. Dis. 2010, 202, 1789–1799. [Google Scholar] [CrossRef]
- Wentzensen, N.; Schiffman, M.; Palmer, T.; Arbyn, M. Triage of HPV positive women in cervical cancer screening. J. Clin. Virol. 2016, 76 (Suppl. 1), S49–S55. [Google Scholar] [CrossRef]
- De Sanjosé, S.; Quint, W.G.; Alemany, L.; Geraets, D.T.; Klaustermeier, J.E.; Lloveras, B.; Tous, S.; Felix, A.; Bravo, L.E.; Shin, H.-R. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010, 11, 1048–1056. [Google Scholar] [CrossRef]
- Rodríguez, A.C.; Schiffman, M.; Herrero, R.; Hildesheim, A.; Bratti, C.; Sherman, M.E.; Solomon, D.; Guillén, D.; Alfaro, M.; Morales, J. Longitudinal study of HPV persistence and cervical intraepithelial neoplasia grade 2/3: Critical role of duration of infection. J. Natl. Cancer Inst. 2010, 102, 315–324. [Google Scholar] [CrossRef]
- Rositch, A.F.; Koshiol, J.; Hudgens, M.G.; Razzaghi, H.; Backes, D.M.; Pimenta, J.M.; Franco, E.L.; Poole, C.; Smith, J.S. Patterns of persistent genital human papillomavirus infection among women worldwide: A literature review and meta-analysis. Int. J. Cancer 2013, 133, 1271–1285. [Google Scholar] [CrossRef]
- Stevens, M.P.; Garland, S.M.; Tabrizi, S.N. Human papillomavirus genotypes in women with abnormal pap smears in Melbourne, Australia. J. Med. Virol. 2009, 81, 1283–1291. [Google Scholar] [CrossRef]
- Hanley, S.J.B.; Fujii, T.; Konno, R.; Takehara, K.; Nishio, H.; Banno, K. HPV genotyping for risk stratification in women with normal cytology: The COMPACT study. Int. J. Cancer 2021, 148, 1975–1987. [Google Scholar]
- Song, F.; Du, H.; Wang, C.; Yang, Y.; Wang, Y.; Jiang, S. Human papillomavirus type-specific prevalence in women with abnormal cervical cytology in China: A multicenter study. J. Cancer 2020, 11, 5078–5085. [Google Scholar]
- Mo, B.; Sun, P.; Guo, H.; Fang, J.; Zhang, J.; Han, C. Prevalence of human papillomavirus infection and genotype distribution among women in Sichuan Province, China. Cancer Med. 2023, 12, 14978–14988. [Google Scholar]
- Schiffman, M.; Castle, P.E. The promise of global cervical cancer prevention. N. Engl. J. Med. 2005, 353, 2101–2104. [Google Scholar] [CrossRef] [PubMed]
- Katki, H.A.; Schiffman, M.; Castle, P.E.; Fetterman, B.; Poitras, N.E.; Lorey, T.; Cheung, L.C.; Raine-Bennett, T.; Gage, J.C.; Kinney, W.K. Five-year risk of CIN3+ and cervical cancer among women with HPV testing of ASC-US Pap results. J. Low. Genit. Tract Dis. 2013, 17 (Suppl. 1), S36–S42. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.M.; Smith, J.S.; Aguado, T.; Franceschi, S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: A meta-analysis. Br. J. Cancer 2003, 89, 101–105. [Google Scholar] [CrossRef]
- Bosch, F.X.; Lorincz, A.; Muñoz, N.; Meijer, C.J.; Shah, K.V. The causal relation between human papillomavirus and cervical cancer. J. Clin. Pathol. 2002, 55, 244–265. [Google Scholar] [CrossRef]
- Cho, H.W.; So, K.A.; Lee, J.K.; Hong, J.H. Type-specific persistence or regression of human papillomavirus genotypes in women with cervical intraepithelial neoplasia 1: A prospective cohort study. Obstet. Gynecol. Sci. 2015, 58, 40–45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonde, J.H.; Sandri, M.T.; Gary, D.S.; Andrews, J.C. Clinical utility of human papillomavirus genotyping in cervical cancer screening: A systematic review. J. Low. Genit. Tract Dis. 2020, 24, 1–13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cho, E.H.; Park, M.S.; Woo, H.Y.; Park, H.; Kwon, M.J. Evaluation of clinical usefulness of HPV-16 and HPV-18 genotyping for cervical cancer screening. J. Gynecol. Oncol. 2024, 35, e72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chu, Y.; Chen, Q.; Liu, R.; Zhou, X.; Bao, M.; Wang, H.; Lin, Y. Analysis of factors affecting the accuracy of colposcopic diagnosis of cervical lesions: A retrospective cohort study. Front. Med. 2024, 11, 1462079. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moscicki, A.B.; Schiffman, M.; Kjaer, S.; Villa, L.L. Chapter 5: Updating the natural history of HPV and anogenital cancer. Vaccine. 2006, 24 (Suppl. 3), S42–S51. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, H.; Zheng, J.; Cai, L.; Chen, Z.; Li, J.; Yu, L. Epidemiological study of HPV infection in 40,693 women in Putian: A population study based on screening for high-risk HPV infection. BMC Infect. Dis. 2022, 22, 893. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.; Zhao, Y.; Xiang, F.; Zhang, X.; Chen, Z.; Zhang, M.; Kang, X.; Wu, R. Evaluation of the diagnostic performance of colposcopy in the detection of cervical high-grade squamous intraepithelial lesions among women with transformation zone type 3. BMC Cancer 2024, 24, 381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koc, S.; Yuksel, D.; Kinay, T.; Burkankulu, D.; Kayikcioglu, F. Histological results of HPV genotyping from a colposcopy center. Arch. Gynecol. Obstet. 2023, 308, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Mühr, L.S.; Lagheden, C.; Hultin, E.; Eklund, C.; Adami, H.O.; Dillner, J.; Sundström, K. The HPV16 genome is stable in women who progress to in situ or invasive cervical cancer: A prospective population-based study. Cancer Res. 2019, 79, 4532–4538. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ding, H.; Jin, H.; Tang, Y. HPV genotype distribution and cervical lesions in Chongqing: A comprehensive analysis of 229,770 females (2015–2023). BMC Infect. Dis. 2025, 25, 760. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kawano, K.; Tsuda, N.; Nasu, H.; Tasaki, S.; Park, J.; Tasaki, K.; Terada, A.; Nishio, S.; Ushijima, K. Human papillomavirus genotyping predicts residual/recurrent disease after local treatment for cervical intraepithelial neoplasia better than viral DNA testing. J. Obstet. Gynaecol. Res. 2021, 47, 3628–3633. [Google Scholar] [CrossRef] [PubMed]
- Osmani, V.; Rossiter, M.; Hörner, L.; Nkurunziza, T.; Rank, S.; Tanaka, L.F.; Klug, S.J. Worldwide burden of cervical human papillomavirus (HPV) in women over 50 years with abnormal cytology: A systematic review and meta-analysis. BMJ Glob. Health 2025, 10, e017309. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Possati-Resende, J.C.; Fritsch, T.Z.; Souza, K.C.B. Risk profile of high-grade cervical lesions and cervical cancer considering the combination of cytology, HPV genotype, and age among women undergoing colposcopy. Rev. Bras. Ginecol. Obstet. 2023, 45, e689–e698. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Castellsagué, X.; Díaz, M.; de Sanjosé, S.; Muñoz, N.; Herrero, R.; Franceschi, S.; Peeling, R.W.; Ashley, R.; Smith, J.S.; Snijders, P.J.F. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: Implications for screening and prevention. J. Natl. Cancer Inst. 2006, 98, 303–315. [Google Scholar] [CrossRef]
- Mortaki, A.; Douligeris, A.; Daskalaki, M.A.; Bikouvaraki, E.S.; Louizou, E.; Daskalakis, G.; Rodolakis, A.; Grigoriadis, T.; Pappa, K.I. The role of HPV genotyping, cytology, and methylation in the triage of high-risk HPV-positive patients. Biomedicines 2025, 13, 1139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

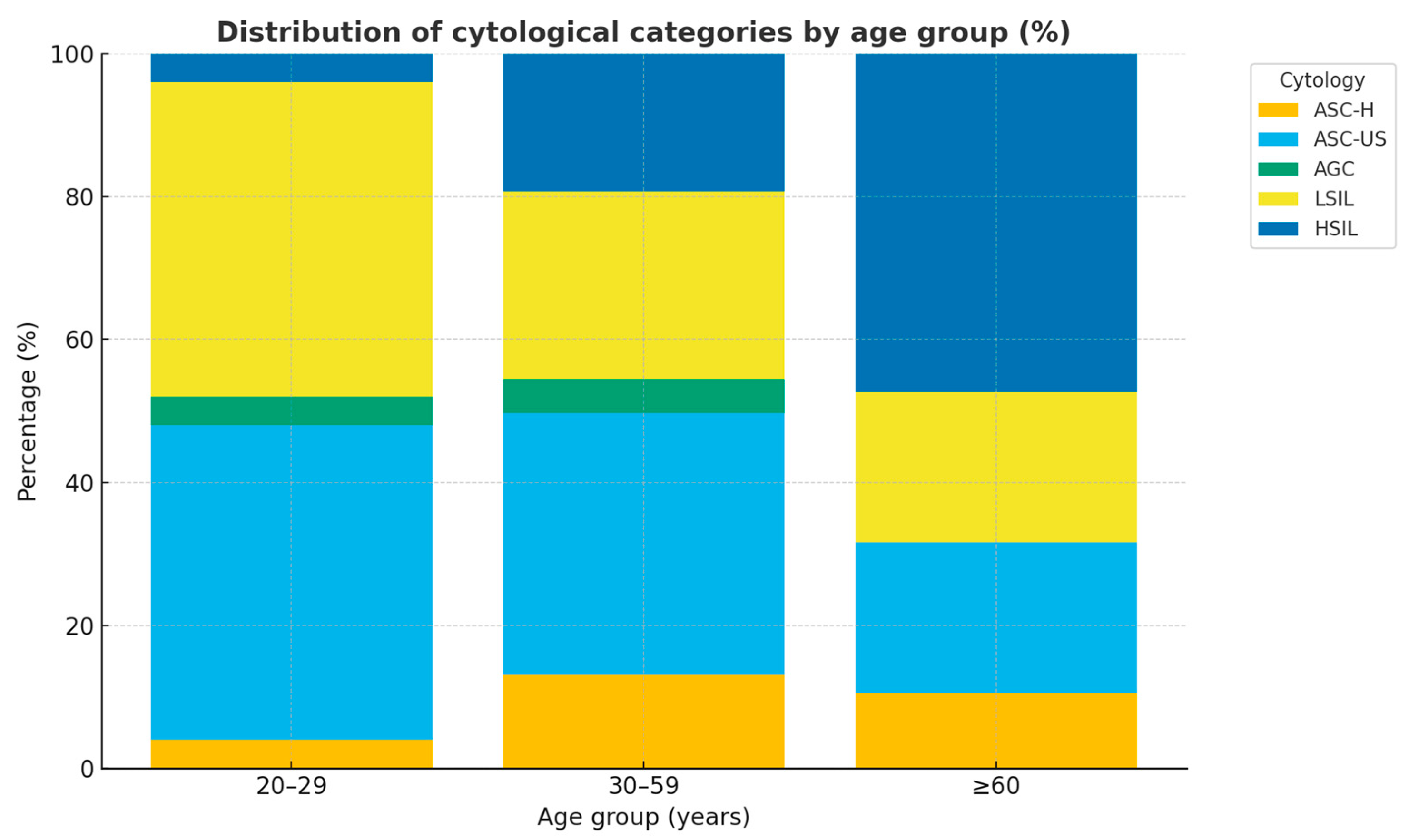
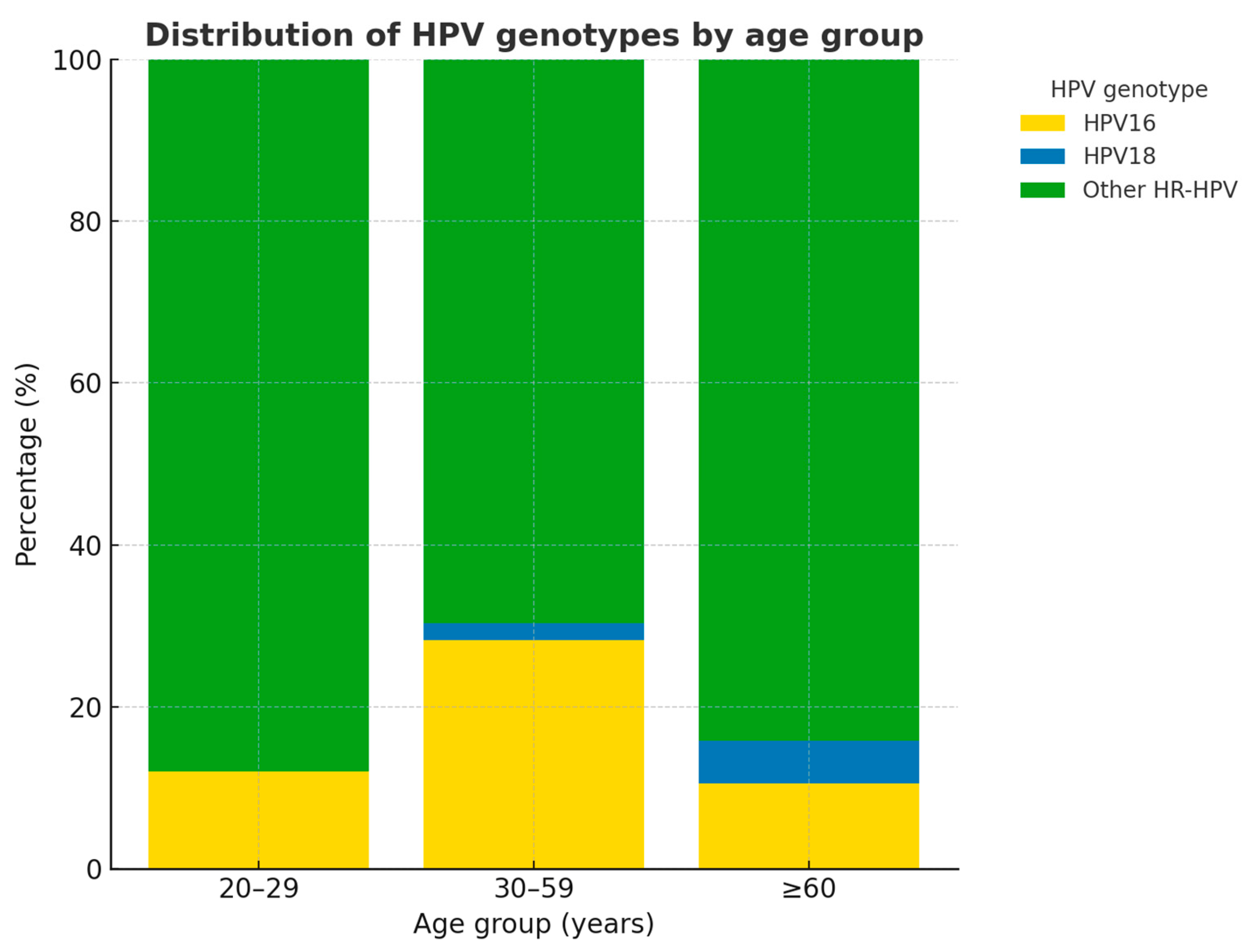
| Variable | n (%) |
|---|---|
| Age group (years) | |
| 20–29 | 25 (13.2) |
| 30–59 | 145 (76.7) |
| ≥60 | 19 (10.1) |
| Parity | |
| Nulliparous | 42 (22.2) |
| Multiparous | 147 (77.8) |
| Cytology | |
| ASC-H | 22 (11.6) |
| ASC-US | 68 (36.0) |
| AGC | 8 (4.2) |
| HSIL | 38 (20.1) |
| LSIL | 53 (28.0) |
| HPV status | |
| HPV16 | 46 (24.3) |
| HPV18 | 4 (2.1) |
| Other HR-HPV | 139 (73.6) |
| Total | 189 (100) |
| Category | HPV16, n (%) | HPV18, n (%) | Other HR-HPV, n (%) | Total, n (%) | * p-Value |
|---|---|---|---|---|---|
| Cytology | |||||
| ASC-H | 5 (22.7) | 1 (4.5) | 16 (72.7) | 22 (100) | |
| ASC-US | 13 (19.1) | 1 (1.5) | 54 (79.4) | 68 (100) | |
| AGC | 0 (0.0) | 0 (0.0) | 8 (100.0) | 8 (100) | |
| HSIL | 18 (47.4) | 2 (5.3) | 18 (47.4) | 38 (100) | |
| LSIL | 10 (18.9) | 0 (0.0) | 43 (81.1) | 53 (100) | |
| Subtotal | 46 (24.3) | 4 (2.1) | 139 (73.5) | 189 (100) | 0.009 |
| Biopsy | |||||
| Negative | 18 (16.2) | 2 (1.8) | 91 (82.0) | 111 (100) | |
| CIN1 | 5 (20.0) | 0 (0.0) | 20 (80.0) | 25 (100) | |
| CIN2 | 6 (40.0) | 0 (0.0) | 9 (60.0) | 15 (100) | |
| CIN3 | 17 (44.7) | 2 (5.3) | 19 (50.0) | 38 (100) | |
| Subtotal | 46 (24.3) | 4 (2.1) | 139 (73.5) | 189 (100) | 0.005 |
| Age Group (years) | Negative, n (%) | CIN1, n (%) | CIN2, n (%) | CIN3, n (%) | Total, N (%) | * p-Value |
|---|---|---|---|---|---|---|
| 20–29 | 18 (72.0) | 4 (16.0) | 2 (8.0) | 1 (4.0) | 25 (100) | |
| 30–59 | 84 (57.9) | 19 (13.1) | 11 (7.6) | 31 (21.4) | 145 (100) | |
| ≥60 | 9 (47.4) | 2 (10.5) | 2 (10.5) | 6 (31.6) | 19 (100) | |
| Total | 111 (58.7) | 25 (13.2) | 15 (7.9) | 38 (20.1) | 189 (100) | 0.401 |
| Cytology Category | Negative, n (%) | CIN1, n (%) | CIN2, n (%) | CIN3, n (%) | Total, n (%) | p-Value |
|---|---|---|---|---|---|---|
| ASC-H | 14 (63.6) | 1 (4.5) | 1 (4.5) | 6 (27.3) | 22 (100) | |
| ASC-US | 55 (80.9) | 10 (14.7) | 1 (1.5) | 2 (2.9) | 68 (100) | |
| AGC | 3 (37.5) | 4 (50.0) | 0 (0.0) | 1 (12.5) | 8 (100) | |
| HSIL | 2 (5.3) | 0 (0.0) | 9 (23.7) | 27 (71.1) | 38 (100) | |
| LSIL | 37 (69.8) | 10 (18.9) | 4 (7.5) | 2 (3.8) | 53 (100) | |
| Total | 111 (58.7) | 25 (13.2) | 15 (7.9) | 38 (20.1) | 189 (100) | <0.001 |
| Factor | Univariable OR (95% CI) | p-Value | Multivariable aOR (95% CI) | p-Value |
|---|---|---|---|---|
| Age ≥ 30 | 3.65 (1.66–8.01) | 0.001 | 4.50 (1.90–10.65) | 0.001 |
| HPV16+ | 3.63 (1.76–7.50) | <0.001 | 4.19 (1.95–9.00) | <0.001 |
| HPV18+ | 3.30 (0.45–24.1) | 0.239 | – | – |
| Other HR-HPV+ | 0.28 (0.13–0.56) | <0.001 | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina Bueno, G.A.; Jaramillo Saavedra, E.A.; Torres Rendón, N.; Huareccallo Suni, D.D. Predictive Factors for Cervical Intraepithelial Neoplasia in Women with Abnormal Cytology According to Human Papillomavirus Genotype: An Observational Study. Int. J. Mol. Sci. 2025, 26, 9612. https://doi.org/10.3390/ijms26199612
Medina Bueno GA, Jaramillo Saavedra EA, Torres Rendón N, Huareccallo Suni DD. Predictive Factors for Cervical Intraepithelial Neoplasia in Women with Abnormal Cytology According to Human Papillomavirus Genotype: An Observational Study. International Journal of Molecular Sciences. 2025; 26(19):9612. https://doi.org/10.3390/ijms26199612
Chicago/Turabian StyleMedina Bueno, Gonzalo Arturo, Enrique Adolfo Jaramillo Saavedra, Natalia Torres Rendón, and Damaris Diana Huareccallo Suni. 2025. "Predictive Factors for Cervical Intraepithelial Neoplasia in Women with Abnormal Cytology According to Human Papillomavirus Genotype: An Observational Study" International Journal of Molecular Sciences 26, no. 19: 9612. https://doi.org/10.3390/ijms26199612
APA StyleMedina Bueno, G. A., Jaramillo Saavedra, E. A., Torres Rendón, N., & Huareccallo Suni, D. D. (2025). Predictive Factors for Cervical Intraepithelial Neoplasia in Women with Abnormal Cytology According to Human Papillomavirus Genotype: An Observational Study. International Journal of Molecular Sciences, 26(19), 9612. https://doi.org/10.3390/ijms26199612








