Cardiovascular Effects of Cannabidiol: From Molecular Mechanisms to Clinical Implementation
Abstract
1. Introduction
2. Cannabidiol, Cannabinoid Receptors, and Endocannabinoids
2.1. Cannabinoid Receptors: Structure, Distribution, and Signaling Pathways
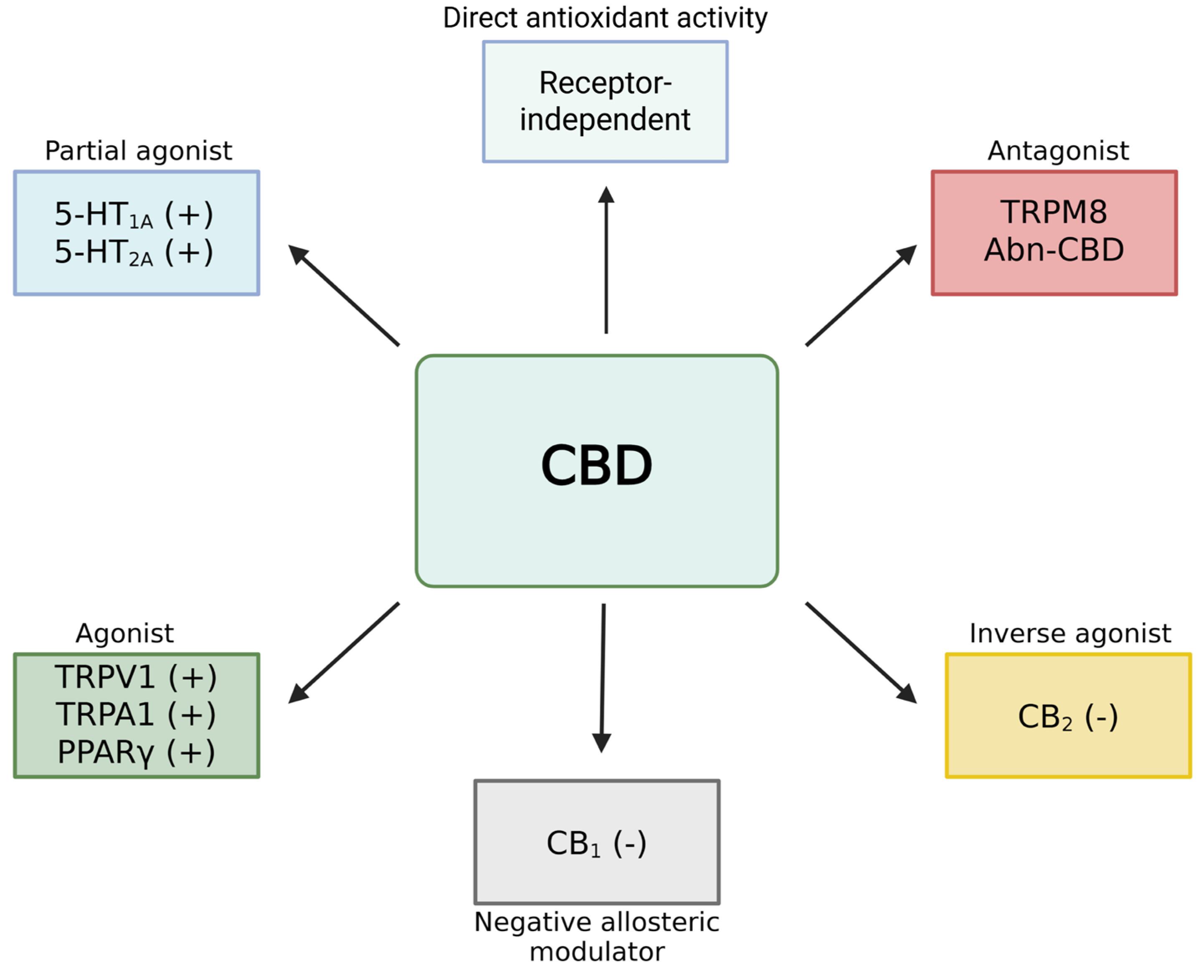
2.2. Pharmacodynamics of Cannabidiol
2.3. The Endocannabinoid System
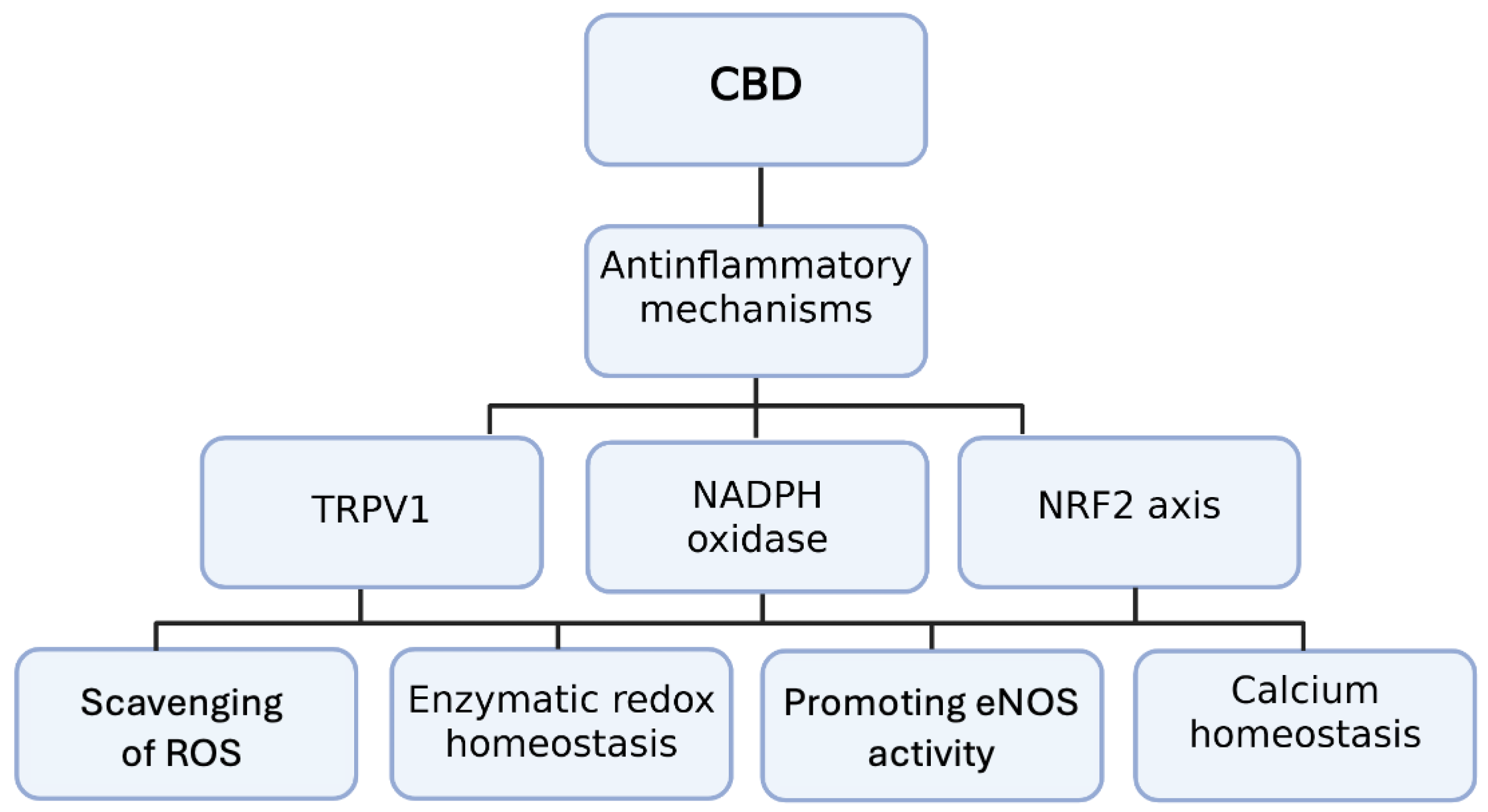
3. CB2 Receptor Activation and Anti-Inflammatory Mechanisms in Cardiovascular Diseases
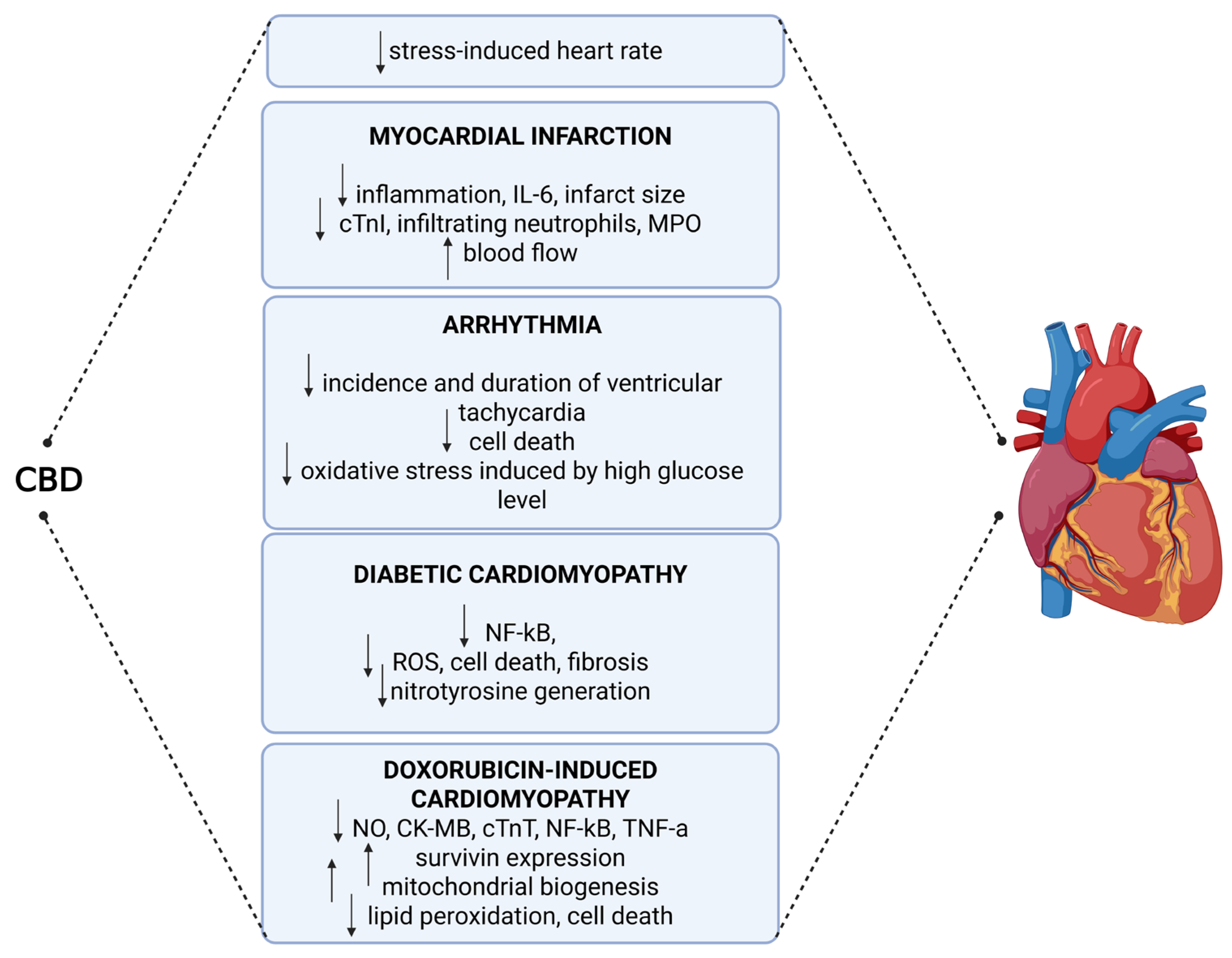
4. Molecular Effects of CBD on Cardiovascular Pathophysiology
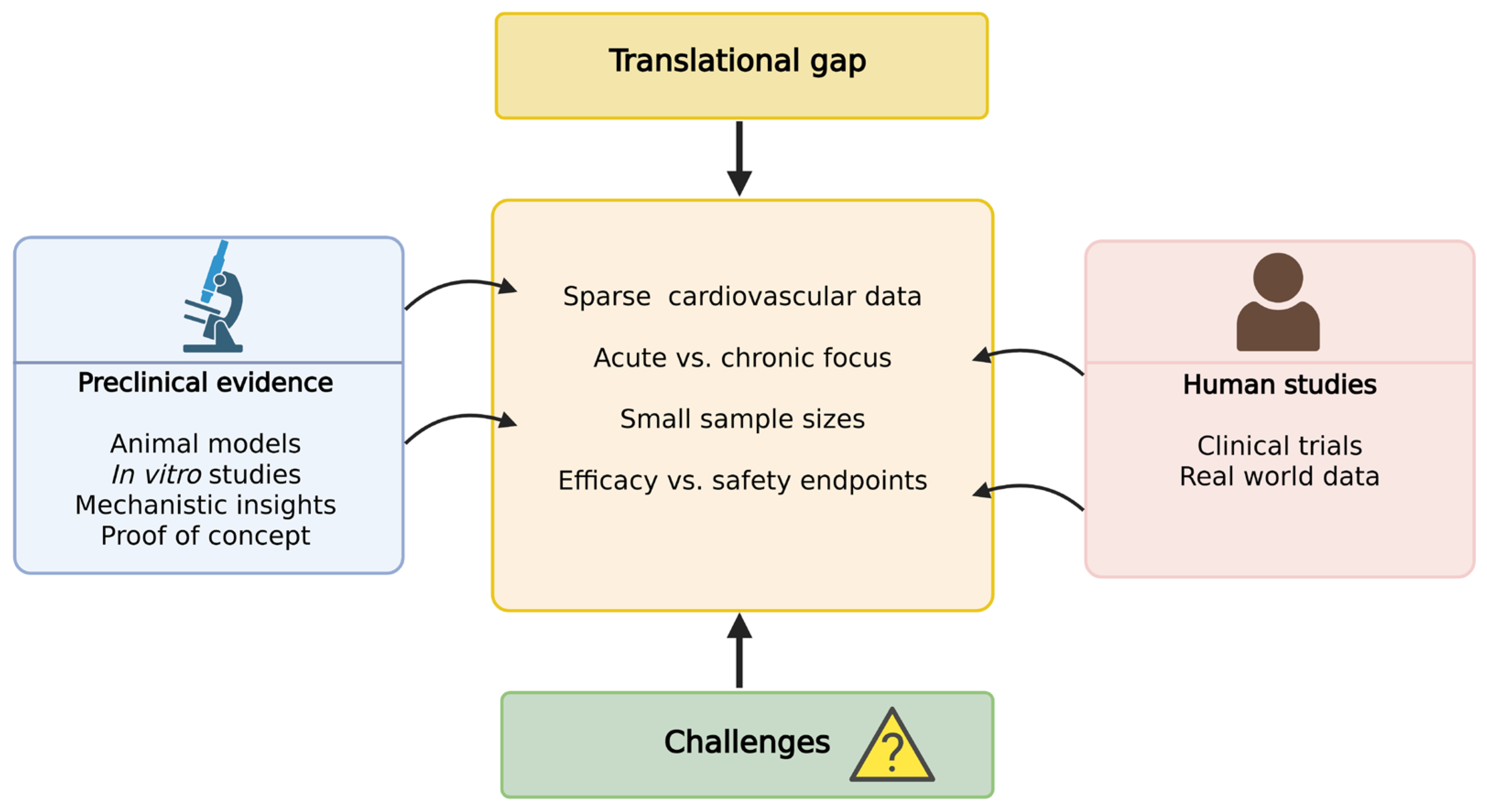
5. Translational Evidence and Therapeutic Outlook: Clinical Findings and Experimental Insights
6. Challenges, Knowledge Gaps, and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Zuardi, A.W. History of cannabis as a medicine: A review. Braz. J. Psychiatry 2006, 28, 153–157. [Google Scholar] [CrossRef]
- Guimarães, F.S. Historical perspective on the therapeutic potential of cannabidiol. Int. Rev. Neurobiol. 2024, 177, 1–9. [Google Scholar]
- Franzè, S.; Ricci, C.; Del Favero, E.; Rama, F.; Casiraghi, A.; Cilurzo, F. Micelles-in-Liposome Systems Obtained by Proliposomal Approach for Cannabidiol Delivery: Structural Features and Skin Penetration. Mol. Pharm. 2023, 20, 3393–3402. [Google Scholar] [CrossRef]
- Mechoulam, R.; Gaoni, Y.; Hashish, I.V. The isolation and structure of cannabinolic cannabidiolic and cannabigerolic acids. Tetrahedron 1965, 21, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Malfitano, A.M.; Basu, S.; Maresz, K.; Bifulco, M.; Dittel, B.N. What we know and do not know about the cannabinoid receptor 2 (CB2). Semin. Immunol. 2014, 26, 369–379. [Google Scholar] [CrossRef]
- Hu, S.S.; Mackie, K. Distribution of the Endocannabinoid System in the Central Nervous System. Handb. Exp. Pharmacol. 2015, 231, 59–93. [Google Scholar] [PubMed]
- Cabral, G.A.; Ferreira, G.A.; Jamerson, M.J. Endocannabinoids and the Immune System in Health and Disease. Handb. Exp. Pharmacol. 2015, 231, 185–211. [Google Scholar]
- Pertwee, R.G. Endocannabinoids and Their Pharmacological Actions. Handb. Exp. Pharmacol. 2015, 231, 1–37. [Google Scholar]
- Singh, J.; Budhiraja, S. Therapeutic potential of cannabinoid receptor ligands: Current status. Methods Find. Exp. Clin. Pharmacol. 2006, 28, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Huculeci, R.; et al. European Society of Cardiology: Cardiovascular disease statistics 2021: Executive Summary. Eur. Heart J. Qual. Care Clin. Outcomes 2022, 8, 377–382. [Google Scholar] [CrossRef]
- Townsend, N.; Kazakiewicz, D.; Wright, F.L.; Timmis, A.; Huculeci, R.; Torbica, A.; Gale, C.P.; Achenbach, S.; Weidinger, F.; Vardas, P. Epidemiology of cardiovascular disease in Europe. Nat. Rev. Cardiol. 2022, 19, 133–143. [Google Scholar] [CrossRef]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef]
- Ueda, N. Endocannabinoid hydrolases. Prostaglandins Other Lipid Mediat. 2002, 68–69, 521–534. [Google Scholar] [CrossRef]
- Ralevic, V. Cannabinoid modulation of peripheral autonomic and sensory neurotransmission. Eur. J. Pharmacol. 2003, 472, 1–21. [Google Scholar] [CrossRef]
- Gérard, C.; Mollereau, C.; Vassart, G.; Parmentier, M. Nucleotide sequence of a human cannabinoid receptor cDNA. Nucleic Acids Res. 1990, 18, 7142. [Google Scholar] [CrossRef]
- Quarta, C.; Bellocchio, L.; Mancini, G.; Mazza, R.; Cervino, C.; Braulke, L.J.; Fekete, C.; Latorre, R.; Nanni, C.; Bucci, M.; et al. CB(1) signaling in forebrain and sympathetic neurons is a key determinant of endocannabinoid actions on energy balance. Cell Metab. 2010, 11, 273–285. [Google Scholar] [CrossRef]
- Pi-Sunyer, F.X.; Aronne, L.J.; Heshmati, H.M.; Devin, J.; Rosenstock, J.; RIO-North America Study Group. Effect of rimonabant a cannabinoid-1 receptor blocker on weight cardiometabolic risk factors in overweight or obese patients: RIO-North America: A randomized controlled trial. JAMA 2006, 295, 761–775, Erratumin in JAMA 2006, 295, 1252. [Google Scholar] [CrossRef]
- Tonstad, S. Is rimonabant a safe and effective therapy for sustained weight loss and improved cardiometabolic risk factors? Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, 364–365, Erratumin in Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, 520. [Google Scholar] [CrossRef] [PubMed]
- Lepor, N.E. Obesity. Rimonabant trials confirm benefit. Rev. Cardiovasc. Med. 2006, 7, 102–106. [Google Scholar] [PubMed]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.C.; Mackie, K. Review of the Endocannabinoid System. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 607–615. [Google Scholar] [CrossRef]
- Galiegue, S.; Mary, S.; Marchand, J.; Dussossoy, D.; Carriere, D.; Carayon, P.; Bouaboula, M.; Shire, D.; Le Fur, G.; Casellas, P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 1995, 232, 54–61. [Google Scholar] [CrossRef]
- Carlisle, S.J.; Marciano-Cabral, F.; Staab, A.; Ludwick, C.; Cabral, G.A. Differential expression of the CB2 cannabinoid receptor by rodent macrophages and macrophage-like cells in relation to cell activation. Int. Immunopharmacol. 2002, 2, 69–82. [Google Scholar] [CrossRef]
- Eisenstein, T.K.; Meissler, J.J.; Wilson, Q.; Gaughan, J.P.; Adler, M.W. Anandamide and Delta9-tetrahydrocannabinol directly inhibit cells of the immune system via CB2 receptors. J. Neuroimmunol. 2007, 189, 17–22. [Google Scholar] [CrossRef]
- Ahn, K.H.; Scott, C.E.; Abrol, R.; Goddard, W.A., 3rd; Kendall, D.A. Computationally predicted CB1 cannabinoid receptor mutants show distinct patterns of salt-bridges that correlate with their level of constitutive activity reflected in G protein coupling levels, thermal stability, and ligand binding. Proteins 2013, 81, 1304–1317. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Mackie, K.; Martin, B.R.; et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef]
- Scott, C.E.; Abrol, R.; Ahn, K.H.; Kendall, D.A.; Goddard, W.A., 3rd. Molecular basis for dramatic changes in cannabinoid CB1 G protein-coupled receptor activation upon single and double point mutations. Protein Sci. 2013, 22, 101–113. [Google Scholar] [CrossRef]
- Ahn, K.H.; Mahmoud, M.M.; Kendall, D.A. Allosteric modulator ORG27569 induces CB1 cannabinoid receptor high affinity agonist binding state, receptor internalization, and Gi protein-independent ERK1/2 kinase activation. J. Biol. Chem. 2012, 287, 12070–12082. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.; Cavic, M.; Canela, E.I. Functional Fine-Tuning of Metabolic Pathways by the Endocannabinoid System-Implications for Health and Disease. Int. J. Mol. Sci. 2021, 22, 3661. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.S.; Paddibhatla, I.; Raghuwanshi, S.; Malleswarapu, M.; Sangeeth, A.; Kovuru, N.; Dahariya, S.; Gautam, D.K.; Pallepati, A.; Gutti, R.K. Endocannabinoid system: Role in blood cell development, neuroimmune interactions and associated disorders. J. Neuroimmunol. 2021, 353, 577501. [Google Scholar] [CrossRef]
- Zhang, L.; Simonsen, C.; Zimova, L.; Wang, K.; Moparthi, L.; Gaudet, R.; Ekoff, M.; Nilsson, G.; Hellmich, U.A.; Vlachova, V.; et al. Cannabinoid non-cannabidiol site modulation of TRPV2 structure and function. Nat. Commun. 2022, 13, 7483. [Google Scholar] [CrossRef]
- Zou, G.; Xia, J.; Han, Q.; Liu, D.; Xiong, W. The synthetic cannabinoid dehydroxylcannabidiol restores the function of a major GABAA receptor isoform in a cell model of hyperekplexia. J. Biol. Chem. 2020, 295, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Chen, S.; Shen, J.; You, H.; Yang, H.; Yan, C.; Fang, Z.; Zhang, J.; Cai, X.; Dong, X.; et al. Cannabis suppresses antitumor immunity by inhibiting JAK/STAT signaling in T cells through CNR2. Signal Transduct. Target. Ther. 2022, 7, 99. [Google Scholar] [CrossRef]
- Kappenberger, L.; Girod, G.; Schlueter, L.; Berger, A.; Graf, D.; Fivaz-Arbane, M. Cardiologie [Cardiology]. Rev. Med. Suisse 2005, 1, 105–111. [Google Scholar]
- Loprinzi, P.D.; Zou, L.; Li, H. The Endocannabinoid System as a Potential Mechanism through which Exercise Influences Episodic Memory Function. Brain Sci. 2019, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef] [PubMed]
- MacCallum, C.A.; Russo, E.B. Practical considerations in medical cannabis administration and dosing. Eur. J. Intern. Med. 2018, 49, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Lowe, H.; Toyang, N.; Steele, B.; Bryant, J.; Ngwa, W. The Endocannabinoid System: A Potential Target for the Treatment of Various Diseases. Int. J. Mol. Sci. 2021, 22, 9472. [Google Scholar] [CrossRef]
- Lu, Y.; Anderson, H.D. Cannabinoid signaling in health and disease. Can. J. Physiol. Pharmacol. 2017, 95, 311–327. [Google Scholar] [CrossRef]
- Kaur, R.; Ambwani, S.R.; Singh, S. Endocannabinoid System: A Multi-Facet Therapeutic Target. Curr. Clin. Pharmacol. 2016, 11, 110–117. [Google Scholar] [CrossRef]
- Chen, C. Inhibiting degradation of 2-arachidonoylglycerol as a therapeutic strategy for neurodegenerative diseases. Pharmacol. Ther. 2023, 244, 108394. [Google Scholar] [CrossRef]
- Powell, D.R.; Gay, J.P.; Wilganowski, N.; Doree, D.; Savelieva, K.V.; Lanthorn, T.H.; Read, R.; Vogel, P.; Hansen, G.M.; Brommage, R.; et al. Diacylglycerol lipase α knockout mice demonstrate metabolic and behavioral phenotypes similar to those of cannabinoid receptor 1 knockout mice. Front. Endocrinol. 2015, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Alharthi, N.; Christensen, P.; Hourani, W.; Ortori, C.; Barrett, D.A.; Bennett, A.J.; Chapman, V.; Alexander, S.P. n-3 polyunsaturated N-acylethanolamines are CB2 cannabinoid receptor-preferring endocannabinoids. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1433–1440. [Google Scholar] [CrossRef]
- Di Marzo, V.; Piscitelli, F. The Endocannabinoid System and its Modulation by Phytocannabinoids. Neurotherapeutics 2015, 12, 692–698. [Google Scholar] [CrossRef]
- Mock, E.D.; Gagestein, B.; van der Stelt, M. Anandamide and other N-acylethanolamines: A class of signaling lipids with therapeutic opportunities. Prog. Lipid Res. 2023, 89, 101194. [Google Scholar] [CrossRef]
- Pacher, P.; Ungvári, Z. Pleiotropic effects of the CB2 cannabinoid receptor activation on human monocyte migration: Implications for atherosclerosis and inflammatory diseases. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1133–H1134. [Google Scholar] [CrossRef] [PubMed]
- Immenschuh, S. Endocannabinoid signalling as an anti-inflammatory therapeutic target in atherosclerosis: Does it work? Cardiovasc. Res. 2009, 84, 341–342. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, Y.; Yuan, Z.; Liu, Y.; Xue, J.; Tian, Y.; Liu, W.; Zhang, W.; Shen, Y.; Xu, W.; Liang, X.; et al. Activation of cannabinoid CB2 receptor ameliorates atherosclerosis associated with suppression of adhesion molecules. J. Cardiovasc. Pharmacol. 2010, 55, 292–298. [Google Scholar] [CrossRef]
- Montecucco, F.; Lenglet, S.; Braunersreuther, V.; Burger, F.; Pelli, G.; Bertolotto, M.; Mach, F.; Steffens, S. CB2 cannabinoid receptor activation is cardioprotective in a mouse model of ischemia/reperfusion. J. Mol. Cell Cardiol. 2009, 46, 612–620. [Google Scholar] [CrossRef]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Patel, V.; Saito, K.; Matsumoto, S.; Kashiwaya, Y.; Horváth, B.; Mukhopadhyay, B.; Becker, L.; et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 2115–2125. [Google Scholar] [CrossRef]
- He, M.; Shi, J.; Xu, Y.J.; Liu, Y. Cannabidiol (CBD) Inhibits Foam Cell Formation via Regulating Cholesterol Homeostasis and Lipid Metabolism. Mol. Nutr. Food Res. 2024, 68, e2400154. [Google Scholar] [CrossRef]
- He, X.-W.; Yu, D.; Li, W.-L.; Zheng, Z.; Lv, C.-L.; Li, C.; Liu, P.; Xu, C.-Q.; Hu, X.-F.; Jin, X.-P. Anti-atherosclerotic potential of baicalin mediated by promoting cholesterol efflux from macrophages via the PPARγ-LXRα-ABCA1/ABCG1 pathway. Biomed. Pharmacother. 2016, 83, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Garza-Cervantes, J.A.; Ramos-González, M.; Lozano, O.; Jerjes-Sánchez, C.; García-Rivas, G. Therapeutic Applications of Cannabinoids in Cardiomyopathy and Heart Failure. Oxid. Med. Cell. Longev. 2020, 2020, 4587024. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, M.; Zimmer, S.; Becker, A.; Lütjohann, D.; Buchalla, R.; Zimmer, A.; Nickenig, G. Atheroprotection via cannabinoid receptor-2 is mediated by circulating and vascular cells in vivo. J. Mol. Cell Cardiol. 2011, 51, 1007–1014. [Google Scholar] [CrossRef]
- Fulmer, M.L.; Thewke, D.P. The Endocannabinoid System and Heart Disease: The Role of Cannabinoid Receptor Type 2. Cardiovasc. Hematol. Disord. Targets 2018, 18, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Dhopeshwarkar, A.; Mackie, K. CB2 Cannabinoid receptors as a therapeutic target-what does the future hold? Mol. Pharmacol. 2014, 86, 430–437. [Google Scholar] [CrossRef]
- Stanley, C.P.; Hind, W.H.; O’Sullivan, S.E. Is the cardiovascular system a therapeutic target for cannabidiol? Br. J. Clin. Pharmacol. 2013, 75, 313–322. [Google Scholar] [CrossRef]
- Weiss, L.; Zeira, M.; Reich, S.; Slavin, S.; Raz, I.; Mechoulam, R.; Gallily, R. Cannabidiol arrests onset of autoimmune diabetes in NOD mice. Neuropharmacology 2008, 54, 244–249. [Google Scholar] [CrossRef]
- Griendling, K.K.; Camargo, L.L.; Rios, F.J.; Alves-Lopes, R.; Montezano, A.C.; Touyz, R.M. Oxidative Stress and Hypertension. Circ. Res. 2021, 128, 993–1020. [Google Scholar] [CrossRef]
- Haffner, S.M. The metabolic syndrome: Inflammation, diabetes mellitus, and cardiovascular disease. Am. J. Cardiol. 2006, 97, 3–11. [Google Scholar] [CrossRef]
- Pirillo, A.; Norata, G.D.; Catapano, A.L. LOX-1, OxLDL, and atherosclerosis. Mediat. Inflamm. 2013, 2013, 152786. [Google Scholar] [CrossRef]
- Khoukaz, H.B.; Ji, Y.; Braet, D.J.; Vadali, M.; Abdelhamid, A.A.; Emal, C.D.; Lawrence, D.A.; Fay, W.P. Drug Targeting of Plasminogen Activator Inhibitor-1 Inhibits Metabolic Dysfunction and Atherosclerosis in a Murine Model of Metabolic Syndrome. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1479–1490. [Google Scholar] [CrossRef]
- Bryk, D.; Olejarz, W.; Zapolska-Downar, D. The role of oxidative stress and NADPH oxidase in the pathogenesis of atherosclerosis. Postepy Higieny i Medycyny Doswiadczalnej 2017, 71, 57–68. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xuan, Y.; Zhu, B.; Wang, X.; Tian, X.; Zhao, L.; Wang, Y.; Jiang, X.; Wen, N.; Khan, A. Protective Effects of Cannabidiol on Chemotherapy-Induced Oral Mucositis via the Nrf2/Keap1/ARE Signaling Pathways. Oxid. Med. Cell Longev. 2022, 2022, 4619760. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzi, J.F.; Silva-Amaral, D.; Issy, A.C.; Gomes, F.V.; Crippa, J.A.; Guimarães, F.S.; Del Bel, E. Cannabidiol attenuates prepulse inhibition disruption by facilitating TRPV1 and 5-HT1A receptor-mediated neurotransmission. Pharmacol. Biochem. Behav. 2024, 245, 173879. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, P.; Biernacki, M.; Domian, N.; Žarković, N.; Skrzydlewska, E. Influence of Inhibition of COX-2-Dependent Lipid Metabolism on Regulation of UVB-Induced Keratinocytes Apoptosis by Cannabinoids. Biomolecules 2022, 12, 842. [Google Scholar] [CrossRef]
- Henshaw, F.R.; Dewsbury, L.S.; Lim, C.K.; Steiner, G.Z. The Effects of Cannabinoids on Pro- and Anti-Inflammatory Cytokines: A Systematic Review of In Vivo Studies. Cannabis Cannabinoid Res. 2021, 6, 177–195. [Google Scholar] [CrossRef]
- Zaiachuk, M.; Suryavanshi, S.V.; Pryimak, N.; Kovalchuk, I.; Kovalchuk, O. The Anti-Inflammatory Effects of Cannabis sativa Extracts on LPS-Induced Cytokines Release in Human Macrophages. Molecules 2023, 28, 4991. [Google Scholar] [CrossRef]
- Begg, M.; Pacher, P.; Batkai, S.; Oseihyiaman, D.; Offertaler, L.; Mo, F.; Liu, J.; Kunos, G. Evidence for novel cannabinoid receptors. Pharmacol. Ther. 2005, 106, 133–145. [Google Scholar] [CrossRef]
- Bomfim, A.J.L.; Zuze, S.M.F.; Fabrício, D.M.; Pessoa, R.M.P.; Crippa, J.A.S.; Chagas, M.H.N. Effects of the Acute and Chronic Administration of Cannabidiol on Cognition in Humans and Animals: A Systematic Review. Cannabis Cannabinoid Res. 2023, 8, 955–973. [Google Scholar] [CrossRef]
- Remiszewski, P.; Jarocka-Karpowicz, I.; Biernacki, M.; Jastrzab, A.; Schlicker, E.; Toczek, M.; Harasim-Symbor, E.; Pędzińska-Betiuk, A.; Malinowska, B. Chronic Cannabidiol Administration Fails to Diminish Blood Pressure in Rats with Primary Secondary Hypertension Despite Its Effects on Cardiac Plasma Endocannabinoid System Oxidative Stress Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 1295. [Google Scholar] [CrossRef]
- Sultan, S.R.; Millar, S.A.; England, T.J.; O’Sullivan, S.E. A Systematic Review and Meta-Analysis of the Haemodynamic Effects of Cannabidiol. Front. Pharmacol. 2017, 8, 81. [Google Scholar] [CrossRef]
- Flôr, A.F.L.; Duarte-Maia, S.; Fernandes-Costa, F.; de Souza, R.M.P.; Braga, V.d.A.; Amaral, S.L.D.; Mascarenhas, S.R.; Brito-Alves, J.L.; Colombari, D.S.A.; Cruz, J.C. Chronic cannabidiol treatment induces cardiovascular improvement in renovascular hypertensive rats. J. Hypertens. 2025, 43, 98–108. [Google Scholar] [CrossRef]
- Baranowska-Kuczko, M.; Kozłowska, H.; Kloza, M.; Kusaczuk, M.; Harasim-Symbor, E.; Biernacki, M.; Kasacka, I.; Malinowska, B. Vasoprotective Endothelial Effects of Chronic Cannabidiol Treatment and Its Influence on the Endocannabinoid System in Rats with Primary and Secondary Hypertension. Pharmaceuticals 2021, 14, 1120. [Google Scholar] [CrossRef]
- Walsh, S.K.; Hepburn, C.Y.; Kane, K.A.; Wainwright, C.L. Acute administration of cannabidiol in vivo suppresses ischaemia-induced cardiac arrhythmias and reduces infarct size when given at reperfusion. Br. J. Pharmacol. 2010, 160, 1234–1242. [Google Scholar] [CrossRef]
- Durst, R.; Danenberg, H.; Gallily, R.; Mechoulam, R.; Meir, K.; Grad, E.; Beeri, R.; Pugatsch, T.; Tarsish, E.; Lotan, C. Cannabidiol, a nonpsychoactive Cannabis constituent, protects against myocardial ischemic reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3602–H3607. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, O.; Baranowska-Kuczko, M.; Gromotowicz-Popławska, A.; Biernacki, M.; Kicman, A.; Malinowska, B.; Kasacka, I.; Krzyżewska, A.; Kozłowska, H. Cannabidiol Ameliorates Monocrotaline-Induced Pulmonary Hypertension in Rats. Int. J. Mol. Sci. 2020, 21, 7077. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Franco-Vadillo, A.; Toledo-Blass, M.; Rivera-Herrera, Z.; Guevara-Balcazar, G.; Orihuela-Rodriguez, O.; Morales-Carmona, J.A.; Kormanovski-Kovzova, A.; Lopez-Sanchez, P.; Rubio-Gayosso, I.; Castillo-Hernandez, M.d.C. Cannabidiol-mediated RISK PI3K/AKT and MAPK/ERK pathways decreasing reperfusion myocardial damage. Pharmacol. Res. Perspect. 2021, 9, e00784. [Google Scholar] [CrossRef] [PubMed]
- Jadoon, K.A.; Tan, G.D.; O’Sullivan, S.E. A single dose of cannabidiol reduces blood pressure in healthy volunteers in a randomized crossover study. J. Clin. Investig. 2017, 2, e93760. [Google Scholar] [CrossRef]
- Sultan, S.R.; O’Sullivan, S.E.; England, T.J. The effects of acute and sustained cannabidiol dosing for seven days on the haemodynamics in healthy men: A randomised controlled trial. Br. J. Clin. Pharmacol. 2020, 86, 1125–1138. [Google Scholar] [CrossRef]
- Patrician, A.; Versic-Bratincevic, M.; Mijacika, T.; Banic, I.; Marendic, M.; Sutlović, D.; Dujić, Ž.; Ainslie, P.N. Examination of a New Delivery Approach for Oral Cannabidiol in Healthy Subjects: A Randomized, Double-Blinded, Placebo-Controlled Pharmacokinetics Study. Adv. Ther. 2019, 36, 3196–3210. [Google Scholar] [CrossRef]
- Dragun, T.; Brown, C.V.; Tulppo, M.P.; Obad, A.; Dujić, Ž. The Influence of Oral Cannabidiol on 24-h Ambulatory Blood Pressure and Arterial Stiffness in Untreated Hypertension: A Double-Blind, Placebo-Controlled, Cross-Over Pilot Study. Adv. Ther. 2023, 40, 3495–3511. [Google Scholar] [CrossRef]
- Dujic, G.; Kumric, M.; Vrdoljak, J.; Dujic, Z.; Bozic, J. Chronic Effects of Oral Cannabidiol Delivery on 24-h Ambulatory Blood Pressure in Patients with Hypertension (HYPER-H21-4): A Randomized, Placebo-Controlled, and Crossover Study. Cannabis Cannabinoid Res. 2024, 9, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Kumric, M.; Dujic, G.; Vrdoljak, J.; Svagusa, K.; Kurir, T.T.; Supe-Domic, D.; Dujic, Z.; Bozic, J. CBD supplementation reduces arterial blood pressure via modulation of the sympatho-chromaffin system: A substudy from the HYPER-H21-4 trial. Biomed. Pharmacother. 2023, 160, 114387. [Google Scholar] [CrossRef]
- Kumric, M.; Dujic, G.; Vrdoljak, J.; Supe-Domic, D.; Bilopavlovic, N.; Dolic, K.; Dujic, Z.; Bozic, J. Effects of CBD supplementation on ambulatory blood pressure and serum urotensin-II concentrations in Caucasian patients with essential hypertension: A sub-analysis of the HYPER-H21-4 trial. Biomed. Pharmacother. 2023, 164, 115016. [Google Scholar] [CrossRef] [PubMed]
- Batinic, A.; Sutlović, D.; Kuret, S.; Matana, A.; Kumric, M.; Bozic, J.; Dujic, Z. Trial of a Novel Oral Cannabinoid Formulation in Patients with Hypertension: A Double-Blind, Placebo-Controlled Pharmacogenetic Study. Pharmaceuticals 2023, 16, 645. [Google Scholar] [CrossRef]
- Dujic, G.; Kumric, M.; Vrdoljak, J.; Sutlovic, D.; Dujic, Z.; Bozic, J. Chronic Cannabidiol Administration Mitigates Excessive Daytime Sleepiness and Fatigue in Patients with Primary Hypertension: Insights from a Randomized Crossover Trial. Cannabis Cannabinoid Res. 2024, 10, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Iffland, K.; Grotenhermen, F. An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies. Cannabis Cannabinoid Res. 2017, 2, 139–154. [Google Scholar] [CrossRef]
- Landa, E.; Vigandt, E.; Andreev, A.; Malyshev, Y.; Sahni, S. Cannabis-induced Acute Coronary Syndrome: A Coincidence or Not? Cureus 2019, 11, e5696. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.; Shahinas, J. Dosage, Efficacy and Safety of Cannabidiol Administration in Adults: A Systematic Review of Human Trials. J. Clin. Med. Res. 2020, 12, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Kutanzi, K.R.; Ewing, L.E.; Skinner, C.M.; Quick, C.M.; Kennon-McGill, S.; McGill, M.R.; Walker, L.A.; ElSohly, M.A.; Gurley, B.J.; Koturbash, I. Safety and Molecular-Toxicological Implications of Cannabidiol-Rich Cannabis Extract and Methylsulfonylmethane Co-Administration. Int. J. Mol. Sci. 2020, 21, 7808. [Google Scholar] [CrossRef]
- Huestis, M.A.; Solimini, R.; Pichini, S.; Pacifici, R.; Carlier, J.; Busardò, F.P. Cannabidiol Adverse Effects and Toxicity. Curr. Neuropharmacol. 2019, 17, 974–989. [Google Scholar] [CrossRef]
- Lo, L.A.; Christiansen, A.; Eadie, L.; Strickland, J.C.; Kim, D.D.; Boivin, M.; Barr, A.M.; MacCallum, C.A. Cannabidiol-associated hepatotoxicity: A systematic review and meta-analysis. J. Intern. Med. 2023, 293, 724–752. [Google Scholar] [CrossRef]
- Kumric, M.; Bozic, J.; Dujic, G.; Vrdoljak, J.; Dujic, Z. Chronic Effects of Effective Oral Cannabidiol Delivery on 24-h Ambulatory Blood Pressure and Vascular Outcomes in Treated and Untreated Hypertension (HYPER-H21-4): Study Protocol for a Randomized, Placebo-Controlled, and Crossover Study. J. Pers. Med. 2022, 12, 1037. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Beers, J.L.; Jackson, K.D.; Zhou, Z. CBD and THC in Special Populations: Pharmacokinetics and Drug-Drug Interactions. Pharmaceutics 2024, 16, 484. [Google Scholar] [CrossRef]
- Batinic, A.; Sutlovic, D.; Kuret, S.; Burcul, F.; Kalajzic, N.; Matana, A.; Dujic, G.; Vrdoljak, J.; Kumric, M.; Bozic, J.; et al. Differences in Plasma Cannabidiol Concentrations in Women and Men: A Randomized, Placebo-Controlled, Crossover Study. Int. J. Mol. Sci. 2023, 24, 10273. [Google Scholar] [CrossRef]
- Rajesh, M.; Mukhopadhyay, P.; Haskó, G.; Huffman, J.W.; Mackie, K.; Pacher, P. CB2 cannabinoid receptor agonists attenuate TNF-alpha-induced human vascular smooth muscle cell proliferation and migration. Br. J. Pharmacol. 2008, 153, 347–357. [Google Scholar] [CrossRef]
- Babayeva, M.; Loewy, Z.G. Cannabis Pharmacogenomics: A Path to Personalized Medicine. Curr. Issues Mol. Biol. 2023, 45, 3479–3514. [Google Scholar] [CrossRef]
- Godlewski, G.; Alapafuja, S.O.; Bátkai, S.; Nikas, S.P.; Cinar, R.; Offertáler, L.; Osei-Hyiaman, D.; Liu, J.; Mukhopadhyay, B.; Harvey-White, J.; et al. Inhibitor of fatty acid amide hydrolase normalizes cardiovascular function in hypertension without adverse metabolic effects. Chem. Biol. 2010, 17, 1256–1266. [Google Scholar] [CrossRef]
- McCartney, D.; Benson, M.J.; Desbrow, B.; Irwin, C.; Suraev, A.; McGregor, I.S. Cannabidiol and Sports Performance: A Narrative Review of Relevant Evidence and Recommendations for Future Research. Sports Med. Open 2020, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Swenson, K. Beyond the hype: A comprehensive exploration of CBD’s biological impacts and mechanisms of action. J. Cannabis Res. 2025, 7, 24. [Google Scholar] [CrossRef]
- Eddin, L.B.; Meeran, M.F.N.; Subramanya, S.B.; Jha, N.K.; Ojha, S. Therapeutic potential of agents targeting cannabinoid type 2 receptors in organ fibrosis. Pharmacol. Res. Perspect. 2024, 12, e1219. [Google Scholar] [CrossRef]
- Turcotte, C.; Blanchet, M.R.; Laviolette, M.; Flamand, N. The CB2 receptor and its role as a regulator of inflammation. Cell Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef]
- Li, J.; Carvajal, R.; Bruner, L.; Kaminski, N.E. The current understanding of the benefits, safety, and regulation of cannabidiol in consumer products. Food Chem. Toxicol. 2021, 157, 112600. [Google Scholar] [CrossRef]
- Paudel, K.S.; Hammell, D.C.; Agu, R.U.; Valiveti, S.; Stinchcomb, A.L. Cannabidiol bioavailability after nasal and transdermal application: Effect of permeation enhancers. Drug Dev. Ind. Pharm. 2010, 36, 1088–1097. [Google Scholar] [CrossRef]
- Dasram, M.H.; Walker, R.B.; Khamanga, S.M. Recent Advances in Endocannabinoid System Targeting for Improved Specificity: Strategic Approaches to Targeted Drug Delivery. Int. J. Mol. Sci. 2022, 23, 13223. [Google Scholar] [CrossRef]
- Naya, N.M.; Kelly, J.; Hogwood, A.; Abbate, A.; Toldo, S. Therapeutic potential of cannabidiol (CBD) in the treatment of cardiovascular diseases. Expert. Opin. Investig. Drugs 2024, 33, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.H.; Navaravong, L.; Sirilak, T.; Prasitwarachot, R.; Nathisuwan, S.; Page, R.L.; Chaiyakunapruk, N. A systematic review and meta-analysis of randomized controlled trials of cardiovascular toxicity of medical cannabinoids. J. Am. Pharm. Assoc. 2021, 61, e1–e13. [Google Scholar] [CrossRef] [PubMed]
- Kicman, A.; Toczek, M. The Effects of Cannabidiol, a Non-Intoxicating Compound of Cannabis, on the Cardiovascular System in Health and Disease. Int. J. Mol. Sci. 2020, 21, 6740. [Google Scholar] [CrossRef] [PubMed]
- Millar, S.A.; Stone, N.L.; Yates, A.S.; O’Sullivan, S.E. A Systematic Review on the Pharmacokinetics of Cannabidiol in Humans. Front. Pharmacol. 2018, 9, 1365. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Zamarripa, C.A.; Spindle, T.R.; Weerts, E.M.; Thummel, K.E.; Vandrey, R.; Paine, M.F.; Unadkat, J.D. Evaluation of Cytochrome P450-Mediated Cannabinoid-Drug Interactions in Healthy Adult Participants. Clin. Pharmacol. Ther. 2023, 114, 693–703. [Google Scholar] [CrossRef] [PubMed]
| Study | Animal Model | Disease | Findings |
|---|---|---|---|
| [75] | Male Wistar rats | Renovascular hypertension | CBD loweredarterial pressure, improved baroreflex sensitivity, and reduced vascular oxidative stress |
| [76] | Wistar–Kyoto rats | Primary and secondary hypertension | CBD vasoprotective effects in hypertensive rats, via inducing local vascular changes in the ECS |
| [77] | Male Sprague-Dawley rats | Acute ischemia–reperfusion model | CBD reduced ventricular arrhythmias and attenuated infarct size |
| [78] | Ligating-induced MI in rats | Heart failure after MI | CBD improved cardiac function and reduced infarct size via anti-inflammatory pathways |
| [80] | Male Wistar rats | Monocrotaline- induced PAH | CBD improved endothelial efficiency and function, reduced RVSP and pulmonary vascular remodeling, normalized hemostatic alterations |
| Study | Population (N) | Condition | CBD Dosage | Findings |
|---|---|---|---|---|
| [81] | 9 healthy male volunteers | Without documented diseases | 600 mg orally (single dose) | CBD reduced resting systolic blood pressure and attenuated blood pressure response to stress |
| [82] | 10 healthy volunteers | Stress-related cardiovascular response | 600 mg orally for seven days | CBD reduced arterial stiffness and improved endothelial function after repeated dosing in response to stress |
| [84] | 16 patients with untreated Grade 1 and Grade 2 hypertension | Primary hypertension | 150 mg every 8 h orally | CBD lowers systolic and mean BP and arterial stiffness |
| [85] | 64 patients with mild or moderate hypertension, untreated or receiving standard of care therapy | Primary hypertension | CBD orally (225–450 mg) for 5 weeks | CBD reduced ambulatory BP and improved daytime alertness |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urlić, H.; Kumrić, M.; Pavlović, N.; Dujić, G.; Dujić, Ž.; Božić, J. Cardiovascular Effects of Cannabidiol: From Molecular Mechanisms to Clinical Implementation. Int. J. Mol. Sci. 2025, 26, 9610. https://doi.org/10.3390/ijms26199610
Urlić H, Kumrić M, Pavlović N, Dujić G, Dujić Ž, Božić J. Cardiovascular Effects of Cannabidiol: From Molecular Mechanisms to Clinical Implementation. International Journal of Molecular Sciences. 2025; 26(19):9610. https://doi.org/10.3390/ijms26199610
Chicago/Turabian StyleUrlić, Hrvoje, Marko Kumrić, Nikola Pavlović, Goran Dujić, Željko Dujić, and Joško Božić. 2025. "Cardiovascular Effects of Cannabidiol: From Molecular Mechanisms to Clinical Implementation" International Journal of Molecular Sciences 26, no. 19: 9610. https://doi.org/10.3390/ijms26199610
APA StyleUrlić, H., Kumrić, M., Pavlović, N., Dujić, G., Dujić, Ž., & Božić, J. (2025). Cardiovascular Effects of Cannabidiol: From Molecular Mechanisms to Clinical Implementation. International Journal of Molecular Sciences, 26(19), 9610. https://doi.org/10.3390/ijms26199610







