Pertussis—A Re-Emerging Threat Despite Immunization: An Analysis of Vaccine Effectiveness and Antibiotic Resistance
Abstract
1. Introduction
2. Methods
3. Virulence Factors of Bordetella pertussis
4. Clinical Manifestation of Pertussis
5. Types of Vaccines
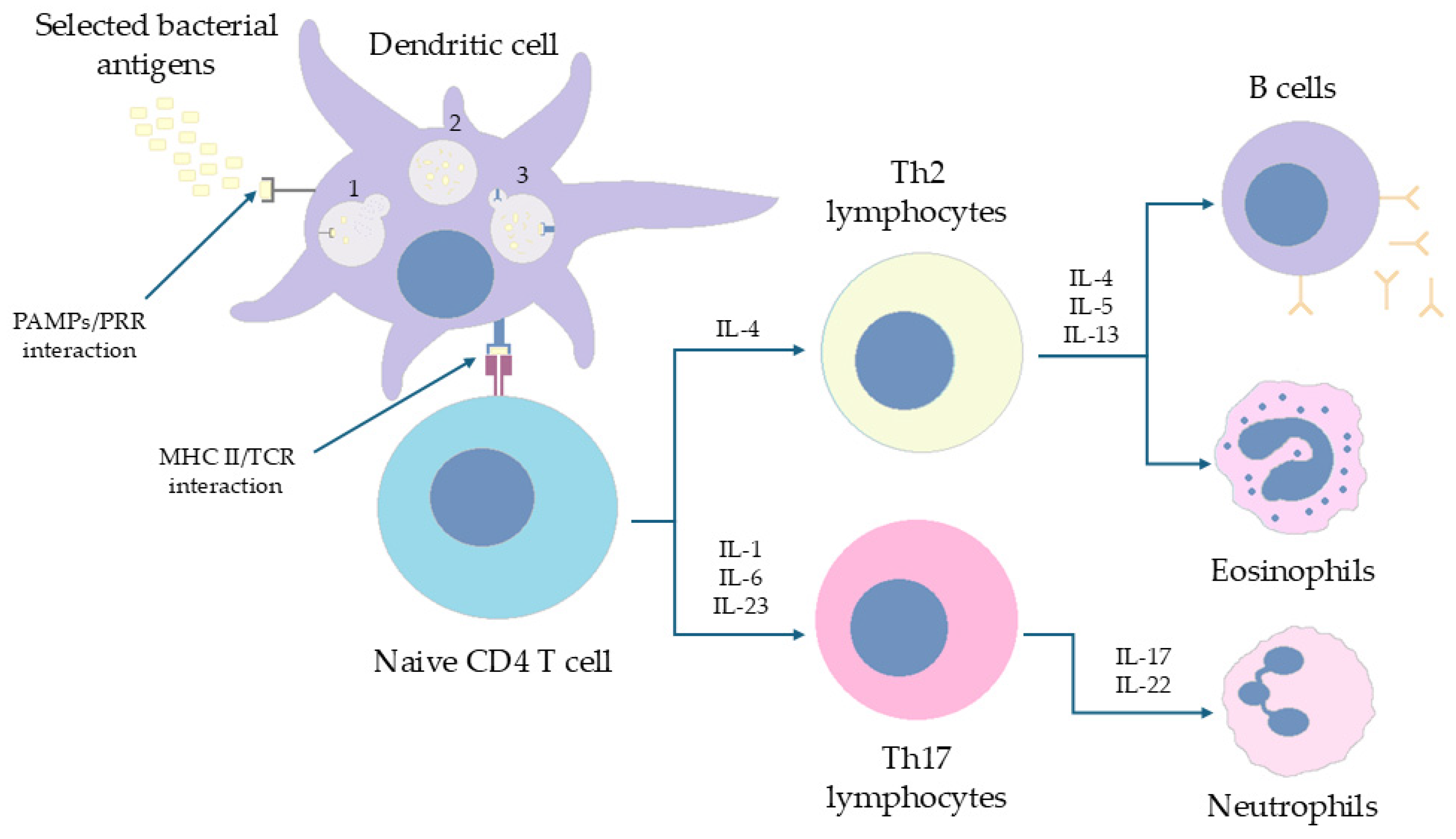
6. The Characteristics of Immunological System Response to wP/aP Vaccination
6.1. The Immune Response of CD4+ T Cells in Humans Is Polarized (Th1/Th17 vs. Th2) Depending on the Form of the Vaccine Administered in Childhood
6.2. The Absence of B. pertussis Carriers on the Nasal Mucosa During Vaccination with wP Is Possible Due to Local Proliferation of TRM Cells and Shaping Local Immune Memory
6.2.1. Murine Model of Infection
6.2.2. Baboons Model of Infection
6.3. Mechanisms of Innate Immunity Against B. pertussis Play Role as Factors Critically Limiting Th1/Th17—Dependent Polarization of Lymphocytes
6.4. Plasma Cell Activity and Immunoglobulin Synthesis Profile Are Determined by the Form of Vaccination
7. Possible Reasons for Increasing Pertussis Prevalence Worldwide
Phenotypic and Genotypic Changes in B. pertussis-Limitation Factors of aP Vaccine Efficiency
8. Vaccine-Dependent Factors Diminish Its Sufficiency
8.1. Silent Infections Promotes Asymptomatic Colonization and Spreading of Pertussis in Populations
8.2. Original Antigenic Sin Phenomena May Decrease Efficacy of aP Vaccine
8.3. Blunting Phenomenon Leads to Impaired Immunity Against Pertussis in Paediatric Populations
9. Antibiotic Resistance Among Bordetella pertussis Strains
10. Discussion
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Immunological Basis for Immunization Series: Module 4: Pertussis, Update 2017. Available online: https://www.who.int/publications/i/item/the-immunological-basis-for-immunization-series-module-4-pertussis-update-2017 (accessed on 15 May 2025).
- Prygiel, M.; Mosiej, E.; Zasada, A.A. Causes of Pertussis Incidence Increase. Postęp. Mikrobiol.—Adv. Microbiol. 2020, 59, 11–24. [Google Scholar] [CrossRef]
- Ucieklak, K. Krztusiec I “Efekt Czerwonej Królowej”. Postępy Mikrobiol.—Adv. Microbiol. 2022, 61, 133–145. [Google Scholar] [CrossRef]
- Belcher, T.; Dubois, V.; Rivera-Millot, A.; Locht, C.; Jacob-Dubuisson, F. Pathogenicity and Virulence of Bordetella pertussis and Its Adaptation to Its Strictly Human Host. Virulence 2021, 12, 2608–2632. [Google Scholar] [CrossRef] [PubMed]
- Lauria, A.M.; Zabbo, C.P. Pertussis; StatPearls Publishing: St. Petersburg, Russia, 2025. [Google Scholar]
- Weyrich, L.S.; Feaga, H.A.; Park, J.; Muse, S.J.; Safi, C.Y.; Rolin, O.Y.; Young, S.E.; Harvill, E.T. Resident Microbiota Affect Bordetella pertussis Infectious Dose and Host Specificity. J. Infect. Dis. 2014, 209, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Deen, J.L.; Mink, C.M.; Cherry, J.D.; Christenson, P.D.; Pineda, E.F.; Lewis, K.; Blumberg, D.A.; Ross, L.A. Household Contact Study of Bordetella pertussis Infections Materials and Methods Subjects and Procedures. Clin. Infect. Dis. 1995, 21, 1211–1220. [Google Scholar] [CrossRef]
- Kline, J.M.; Lewis, W.D.; Smith, E.A.; Tracy, L.R.; Moerschel, S.K. Pertussis: A Reemerging Infection. Am. Fam. Physician 2013, 88, 507–514. [Google Scholar]
- Kardos, P.; Correia de Sousa, J.; Heininger, U.; Konstantopoulos, A.; MacIntyre, C.R.; Middleton, D.; Nolan, T.; Papi, A.; Rendon, A.; Rizzo, A.; et al. Understanding the Impact of Adult Pertussis and Current Approaches to Vaccination: A Narrative Review and Expert Panel Recommendations. Hum. Vaccines Immunother. 2024, 20, 2324547. [Google Scholar] [CrossRef]
- What Is the 100-Day Cough? Available online: https://www.unicef.org/eca/stories/what-100-day-cough (accessed on 15 May 2025).
- Pertussis (Whooping Cough): For Health Professionals. Available online: https://www.canada.ca/en/public-health/services/immunization/vaccine-preventable-diseases/pertussis-whooping-cough/health-professionals.html (accessed on 15 May 2025).
- WHO. Laboratory Manual for the Diagnosis of Whooping Cough Caused by Bordetella pertussis-Bordetella parapertussis. Update 2014; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Wang, S.; Zhang, S.; Liu, J. Resurgence of Pertussis: Epidemiological Trends, Contributing Factors, Challenges, and Recommendations for Vaccination and Surveillance. Hum. Vaccines Immunother. 2025, 21, 2513729. [Google Scholar] [CrossRef]
- Mehr Fälle von Keuchhusten. 2024. Available online: https://www.bundestag.de/presse/hib/kurzmeldungen-1082540 (accessed on 1 September 2025).
- Kerr, J.R.; Matthews, R.C. Bordetella pertussis Infection: Pathogenesis, Diagnosis, Management, and the Role of Protective Immunity. Eur. J. Clin. Microbiol. Infect. Dis. 2000, 19, 77–88. [Google Scholar] [CrossRef]
- Klein, N.P. Licensed Pertussis Vaccines in the United States. Hum. Vaccines Immunother. 2014, 10, 2684–2690. [Google Scholar] [CrossRef]
- Hellenbrand, W.; Beier, D.; Jensen, E.; Littmann, M.; Meyer, C.; Oppermann, H.; von König, C.H.W.; Reiter, S. The Epidemiology of Pertussis in Germany: Past and Present. BMC Infect. Dis. 2009, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Millward, G. Vaccinating Britain Mass Vaccination and the Public Since the Second World War; Manchester University Press: Manchester, UK, 2019. [Google Scholar]
- Krztusiec—Dlaczego Jest Groźny. Available online: https://pacjent.gov.pl/aktualnosc/krztusiec-dlaczego-jest-grozny (accessed on 1 September 2025).
- Ross, P.J.; Sutton, C.E.; Higgins, S.; Allen, A.C.; Walsh, K.; Misiak, A.; Lavelle, E.C.; McLoughlin, R.M.; Mills, K.H.G. Relative Contribution of Th1 and Th17 Cells in Adaptive Immunity to Bordetella pertussis: Towards the Rational Design of an Improved Acellular Pertussis Vaccine. PLoS Pathog. 2013, 9, e1003264. [Google Scholar] [CrossRef] [PubMed]
- Higgs, R.; Higgins, S.C.; Ross, P.J.; Mills, K.H.G. Immunity to the Respiratory Pathogen Bordetella pertussis. Mucosal Immunol. 2012, 5, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Bouchez, V.; Soares, A.; Trombert-Paolanton, S.; El Belghiti, F.A.; Cohen, J.F.; Armatys, N.; Landier, A.; Blanchot, T.; Hervo, M.; et al. Resurgence of Bordetella pertussis, Including One Macrolide-Resistant Isolate, France, 2024. Eurosurveillance 2024, 29, 2400459. [Google Scholar] [CrossRef]
- Gabutti, G.; Azzari, C.; Bonanni, P.; Prato, R.; Tozzi, A.E.; Zanetiti, A.; Zuccotti, G. Pertussis Current Perspectives on Epidemiology and Prevention. Hum. Vaccines Immunother. 2015, 11, 108–117. [Google Scholar] [CrossRef]
- Heininger, U.; Martini, H.; Eeuwijk, J.; Prokić, I.; Guignard, A.P.; Turriani, E.; Duchenne, M.; Berlaimont, V. Pertactin Deficiency of Bordetella pertussis: Insights into Epidemiology, and Perspectives on Surveillance and Public Health Impact. Hum. Vaccines Immunother. 2024, 20, 2435134. [Google Scholar] [CrossRef]
- Chen, Z.; He, Q. Immune Persistence after Pertussis Vaccination. Hum. Vaccines Immunother. 2017, 13, 744–756. [Google Scholar] [CrossRef]
- Marcellini, V.; Mortari, E.P.; Fedele, G.; Gesualdo, F.; Pandolfi, E.; Midulla, F.; Leone, P.; Stefanelli, P.; Tozzi, A.E.; Carsetti, R.; et al. Protection against Pertussis in Humans Correlates to Elevated Serum Antibodies and Memory B Cells. Front. Immunol. 2017, 8, 1158. [Google Scholar] [CrossRef]
- Althouse, B.M.; Scarpino, S.V. Asymptomatic Transmission and the Resurgence of Bordetella pertussis. BMC Med. 2015, 13, 146. [Google Scholar] [CrossRef]
- Edelman, K.; He, Q.; Mäkinen, J.; Sahlberg, A.; Haanperä, M.; Schuerman, L.; Wolter, J.; Mertsola, J. Immunity to Pertussis 5 Years after Booster Immunization during Adolescence. Clin. Infect. Dis. 2007, 44, 1271–1277. [Google Scholar] [CrossRef]
- Esposito, S.; Stefanelli, P.; Fry, N.K.; Fedele, G.; He, Q.; Paterson, P.; Tan, T.; Knuf, M.; Rodrigo, C.; Weil Olivier, C. Pertussis Prevention: Reasons for Resurgence, and Differences in the Current Acellular Pertussis Vaccines. Front. Immunol. 2019, 10, 1344. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Lau, E.H.Y.; Cowling, B.J. Waning Immunity after Receipt of Pertussis, Diphtheria, Tetanus, and Polio-Related Vaccines: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2022, 225, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Dimeas, I.E.; Kotsiou, O.S.; Salgkami, P.; Poulakida, I.; Boutlas, S.; Daniil, Z.; Papadamou, G.; Gourgoulianis, K.I. Real-Life Insights into Pertussis Diagnosis: High Yield of PCR Testing and Clinical Outcomes—An Emerging Old Enemy or Just a Sign of PCR Times? J. Pers. Med. 2024, 14, 1116. [Google Scholar] [CrossRef] [PubMed]
- Paireau, J.; Guillot, S.; Aït El Belghiti, F.; Matczak, S.; Trombert-Paolantoni, S.; Jacomo, V.; Taha, M.K.; Salje, H.; Brisse, S.; Lévy-Bruhl, D.; et al. Effect of Change in Vaccine Schedule on Pertussis Epidemiology in France: A Modelling and Serological Study. Lancet Infect. Dis. 2022, 22, 265–273. [Google Scholar] [CrossRef]
- Leininger, E.; Roberts, M.; Kenimer, J.G.; Charles, I.G.; Fairweather, N.; Novotny, P.; Brennan, M.J. Pertactin, an Arg-Gly-Asp-Containing Bordetella pertussis Surface Protein That Promotes Adherence of Mammalian Cells. Proc. Natl. Acad. Sci. USA 1991, 88, 345–349. [Google Scholar] [CrossRef]
- Tuomanen, E.; Weiss, A. Characterization of Two Adhesins of Bordetella pertussis for Human Ciliated Respiratory-Epithelial Cells. J. Infect. Dis. 1985, 152, 118–125. [Google Scholar] [CrossRef]
- Scanlon, K.; Skerry, C.; Carbonetti, N. Association of Pertussis Toxin with Severe Pertussis Disease. Toxins 2019, 11, 373. [Google Scholar] [CrossRef]
- Van Meijeren, C.E.; Vleeming, W.; Van De Kuil, T.; Gerards, A.L.; Hendriksen, C.F.M.; De Wildt, D.J. Pertussis Toxin-Induced Histamine Sensitisation: An Aspecific Phenomenon Independent from the Nitric Oxide System? Eur. J. Pharmacol. 2004, 493, 139–150. [Google Scholar] [CrossRef]
- Zhao, A.; Ohara-Imaizumi, M.; Brissova, M.; Benninger, R.K.P.; Xu, Y.; Hao, Y.; Abramowitz, J.; Boulay, G.; Powers, A.C.; Piston, D.; et al. Gαo Represses Insulin Secretion by Reducing Vesicular Docking in Pancreatic β-Cells. Diabetes 2010, 59, 2522–2529. [Google Scholar] [CrossRef]
- Pauza, C.D.; Hinds, P.W.; Yin, C.; McKechnie, T.S.; Hinds, S.B.; Salvato, M.S. The Lymphocytosis-Promoting Agent Pertussis Toxin Affects Virus Burden and Lymphocyte Distribution in the SIV-Infected Rhesus Macaque. AIDS Res. Hum. Retroviruses 1997, 13, 87–95. [Google Scholar] [CrossRef]
- Finger, H.; von Koenig, C.H.W. Bordetella. In Medical Microbiology; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Belcher, C.E.; Drenkow, J.; Kehoe, B.; Gingeras, T.R.; McNamara, N.; Lemjabbar, H.; Basbaum, C.; Relman, D.A. The Transcriptional Responses of Respiratory Epithelial Cells to Bordetella pertussis Reveal Host Defensive and Pathogen Counter-Defensive Strategies. Proc. Natl. Acad. Sci. USA 2000, 97, 13847–13852. [Google Scholar] [CrossRef]
- Malandra, A.; Rahman, W.U.; Klimova, N.; Streparola, G.; Holubova, J.; Osickova, A.; Bariselli, S.; Sebo, P.; Osicka, R. Bordetella Adenylate Cyclase Toxin Elicits Airway Mucin Secretion through Activation of the Camp Response Element Binding Protein. Int. J. Mol. Sci. 2021, 22, 9064. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Ma, S.; Zhou, Q.; Xu, J. Pertussis Resurgence: Epidemiological Trends, Pathogenic Mechanisms, and Preventive Strategies. Front. Immunol. 2025, 16, 1618883. [Google Scholar] [CrossRef] [PubMed]
- Clinical Features of Pertussis. Available online: https://www.cdc.gov/pertussis/hcp/clinical-signs/index.html (accessed on 30 May 2025).
- Clinical Overview of Pertussis. Available online: https://www.cdc.gov/pertussis/hcp/clinical-overview/index.html (accessed on 30 May 2025).
- Bush, L.M. Pertussis. Available online: https://www.merckmanuals.com/professional/infectious-diseases/gram-negative-bacilli/pertussis (accessed on 30 May 2025).
- Whooping Cough. Available online: https://www.nhs.uk/conditions/whooping-cough/ (accessed on 1 June 2025).
- Havers, F.P.; Moro, P.L.; Hariri, S.; Skoff, T. Chapter 16: Pertussis; Health Care Providers; 2024. Available online: https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-16-pertussis.html (accessed on 1 June 2025).
- Treatment of Pertussis. Available online: https://www.cdc.gov/pertussis/hcp/clinical-care/index.html (accessed on 24 July 2025).
- Mcnamara, L.A.; Preventable, V.; Branch, D.; Diseases, R.; Skoff, T.; Preventable, V.; Branch, D.; Diseases, R.; Faulkner, A.; Preventable, V.; et al. Reduced severity of pertussis in persons with age-appropriate pertussis vaccination—United States, 2010–2012. Clin. Infect. Dis. 2018, 65, 811–818. [Google Scholar] [CrossRef] [PubMed]
- De Colsa-Ranero, A.; Macías-Parra, M.; Hernández-Porras, M.; Xochihua-Díaz, L.; Galindo-Fraga, A.; Moreno-Espinosa, S.; Solórzano-Santos, F. An Expert Panel Opinion on Protection against Pertussis in Mexico: The Role of Acellular and Whole-Cell Vaccines. Bol. Med. Hosp. Infant. Mex. 2022, 79, 152–160. [Google Scholar] [CrossRef]
- Schmitt, P.; Borkner, L.; Jazayeri, S.D.; McCarthy, K.N.; Mills, K.H. Nasal Vaccines for Pertussis. Curr. Opin. Immunol. 2023, 84, 102355. [Google Scholar] [CrossRef]
- Alghounaim, M.; Alsaffar, Z.; Alfraij, A.; Bin-Hasan, S.; Hussain, E. Whole-Cell and Acellular Pertussis Vaccine: Reflections on Efficacy. Med. Princ. Pract. 2022, 31, 313–321. [Google Scholar] [CrossRef]
- Decker, M.D.; Edwards, K.M. Pertussis (Whooping Cough). J. Infect. Dis. 2021, 224, S310–S320. [Google Scholar] [CrossRef]
- Szwejser-Zawislak, E.; Wilk, M.M.; Piszczek, P.; Krawczyk, J.; Wilczyńska, D.; Hozbor, D. Evaluation of Whole-Cell and Acellular Pertussis Vaccines in the Context of Long-Term Herd Immunity. Vaccines 2023, 11, 1. [Google Scholar] [CrossRef]
- Leontari, K.; Lianou, A.; Tsantes, A.G.; Filippatos, F.; Iliodromiti, Z.; Boutsikou, T.; Paliatsou, S.; Chaldoupis, A.E.; Ioannou, P.; Mpakosi, A.; et al. Pertussis in Early Infancy: Diagnostic Challenges, Disease Burden, and Public Health Implications Amidst the 2024 Resurgence, with Emphasis on Maternal Vaccination Strategies. Vaccines 2025, 13, 276. [Google Scholar] [CrossRef]
- Ausiello, C.M.; Urbani, F.; la Sala, A.; Lande, R.; Cassone, A. Vaccine- and Antigen-Dependent Type 1 and Type 2 Cytokine Induction after Primary Vaccination of Infants with Whole-Cell or Acellular Pertussis Vaccines. Infect. Immun. 1997, 65, 2168–2174. [Google Scholar] [CrossRef]
- Ryan, E.J.; Nilsson, L.; Kjellman, N.I.M.; Gothefors, L.; Mills, K.H.G. Booster Immunization of Children with an Acellular Pertussis Vaccine Enhances Th2 Cytokine Production and Serum IgE Responses against Pertussis Toxin but Not against Common Allergens. Clin. Exp. Immunol. 2000, 121, 193–200. [Google Scholar] [CrossRef]
- Mascart, F.; Verscheure, V.; Malfroot, A.; Hainaut, M.; Pierard, D.; Temerman, S.; Peltier, A.; Debrie, A.-S.; Levy, J.; Del Giudice, G. Bordetella pertussis Infection in 2-Month-Old Infants Promotes Type 1 T Cell Responses. J. Immunol. 2003, 170, 1504–1509. [Google Scholar] [CrossRef] [PubMed]
- Schure, R.-M.; Hendrikx, L.H.; de Rond, L.G.H.; Öztürk, K.; Sanders, E.A.M.; Berbers, G.A.M.; Buisman, A.-M. T-Cell Responses before and after the Fifth Consecutive Acellular Pertussis Vaccination in 4-Year-Old Dutch Children. Clin. vaccine Immunol. 2012, 19, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Mascart, F.; Hainaut, M.; Peltier, A.; Verscheure, V.; Levy, J.; Locht, C. Modulation of the Infant Immune Responses by the First Pertussis Vaccine Administrations. Vaccine 2007, 25, 391–398. [Google Scholar] [CrossRef] [PubMed]
- van der Lee, S.; Hendrikx, L.H.; Sanders, E.A.M.; Berbers, G.A.M.; Buisman, A.-M. Whole-Cell or Acellular Pertussis Primary Immunizations in Infancy Determines Adolescent Cellular Immune Profiles. Front. Immunol. 2018, 9, 51. [Google Scholar] [CrossRef]
- van der Lee, S.; Sanders, E.A.M.; Berbers, G.A.M.; Buisman, A.-M. Whole-Cell or Acellular Pertussis Vaccination in Infancy Determines IgG Subclass Profiles to DTaP Booster Vaccination. Vaccine 2018, 36, 220–226. [Google Scholar] [CrossRef]
- Redpath, S.; Michaelsen, T.; Sandlie, I.; Clark, M.R. Activation of Complement by Human IgG1 and Human IgG3 Antibodies against the Human Leucocyte Antigen CD52. Immunology 1998, 93, 595–600. [Google Scholar] [CrossRef]
- Bancroft, T.; Dillon, M.B.C.; da Silva Antunes, R.; Paul, S.; Peters, B.; Crotty, S.; Lindestam Arlehamn, C.S.; Sette, A. Th1 versus Th2 T Cell Polarization by Whole-Cell and Acellular Childhood Pertussis Vaccines Persists upon Re-Immunization in Adolescence and Adulthood. Cell. Immunol. 2016, 304–305, 35–43. [Google Scholar] [CrossRef]
- Dirix, V.; Verscheure, V.; Goetghebuer, T.; Hainaut, M.; Debrie, A.-S.; Locht, C.; Mascart, F. Cytokine and Antibody Profiles in 1-Year-Old Children Vaccinated with Either Acellular or Whole-Cell Pertussis Vaccine during Infancy. Vaccine 2009, 27, 6042–6047. [Google Scholar] [CrossRef]
- Schure, R.-M.; Hendrikx, L.H.; de Rond, L.G.H.; Öztürk, K.; Sanders, E.A.M.; Berbers, G.A.M.; Buisman, A.-M. Differential T-and B-Cell Responses to Pertussis in Acellular Vaccine-Primed versus Whole-Cell Vaccine-Primed Children 2 Years after Preschool Acellular Booster Vaccination. Clin. Vaccine Immunol. 2013, 20, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- da Silva Antunes, R.; Babor, M.; Carpenter, C.; Khalil, N.; Cortese, M.; Mentzer, A.J.; Seumois, G.; Petro, C.D.; Purcell, L.A.; Vijayanand, P.; et al. Th1/Th17 Polarization Persists Following Whole-Cell Pertussis Vaccination despite Repeated Acellular Boosters. J. Clin. Investig. 2018, 128, 3853–3865. [Google Scholar] [CrossRef] [PubMed]
- Rieber, N.; Graf, A.; Hartl, D.; Urschel, S.; Belohradsky, B.H.; Liese, J. Acellular Pertussis Booster in Adolescents Induces Th1 and Memory CD8+ T Cell Immune Response. PLoS ONE 2011, 6, e17271. [Google Scholar] [CrossRef] [PubMed]
- Lambert, E.E.; Corbière, V.; van Gaans-van den Brink, J.A.M.; Duijst, M.; Venkatasubramanian, P.B.; Simonetti, E.; Huynen, M.; Diavatopoulos, D.D.; Versteegen, P.; Berbers, G.A.M.; et al. Uncovering Distinct Primary Vaccination-Dependent Profiles in Human Bordetella pertussis Specific CD4+ T-Cell Responses Using a Novel Whole Blood Assay. Vaccines 2020, 8, 225. [Google Scholar] [CrossRef]
- da Silva Antunes, R.; Quiambao, L.G.; Soldevila, F.; Sutherland, A.; Peters, B.; Sette, A. Lack of Evidence Supporting a Role of IFN-β and TGF-β in Differential Polarization of Bordetella pertussis Specific-T Cell Responses. Cytokine 2021, 137, 155313. [Google Scholar] [CrossRef]
- Sheridan, S.L.; Ware, R.S.; Keith Grimwood, M.; Stephen, B. Lambert Number and Order of Whole Cell Pertussis Vaccines in Infancy and Disease Protection. JAMA—J. Am. Med. Assoc. 2012, 308, 454–456. [Google Scholar] [CrossRef]
- Klein, N.P.; Bartlett, J.; Fireman, B.; Baxter, R. Waning Tdap Effectiveness in Adolescents. Pediatrics 2016, 137, e20153326. [Google Scholar] [CrossRef]
- McGirr, A.; Fisman, D.N. Duration of Pertussis Immunity After DTaP Immunization: A Meta-Analysis. Pediatrics 2015, 135, 331–343. [Google Scholar] [CrossRef]
- Domenech de Cellès, M.; Rohani, P.; King, A.A. Duration of Immunity and Effectiveness of Diphtheria-Tetanus–Acellular Pertussis Vaccines in Children. JAMA Pediatr. 2019, 173, 588. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Lewis, M.; Kenyon, C.; White, K.; Osborn, A.; Liko, J.; Zell, E.; Martin, S.; Messonnier, N.E.; Clark, T.A.; et al. Waning Immunity to Pertussis Following 5 Doses of DTaP. Pediatrics 2013, 131, e1047–e1052. [Google Scholar] [CrossRef]
- Witt, M.A.; Arias, L.; Katz, P.H.; Truong, E.T.; Witt, D.J. Reduced Risk of Pertussis Among Persons Ever Vaccinated With Whole Cell Pertussis Vaccine Compared to Recipients of Acellular Pertussis Vaccines in a Large US Cohort. Clin. Infect. Dis. 2013, 56, 1248–1254. [Google Scholar] [CrossRef]
- Valeri, V.; Sochon, A.; Cousu, C.; Chappert, P.; Lecoeuche, D.; Blanc, P.; Weill, J.-C.; Reynaud, C.-A. The Whole-Cell Pertussis Vaccine Imposes a Broad Effector B Cell Response in Mouse Heterologous Prime-Boost Settings. JCI Insight 2022, 7, e157034. [Google Scholar] [CrossRef] [PubMed]
- Calvo, A.E.; Tristán Urrutia, A.G.; Vargas-Zambrano, J.C.; López Castillo, H. Pertussis Vaccine Effectiveness Following Country-Wide Implementation of a Hexavalent Acellular Pertussis Immunization Schedule in Infants and Children in Panama. Hum. Vaccines Immunother. 2024, 20, 2389577. [Google Scholar] [CrossRef] [PubMed]
- Mosley, Y.-Y.C.; Lu, F.; HogenEsch, H. Differences in Innate IFNγ and IL-17 Responses to Bordetella pertussis between BALB/c and C57BL/6 Mice: Role of ΓδT Cells, NK Cells, and Dendritic Cells. Immunol. Res. 2017, 65, 1139–1149. [Google Scholar] [CrossRef]
- Zachariadis, O.; Cassidy, J.P.; Brady, J.; Mahon, B.P. Γδ T Cells Regulate the Early Inflammatory Response to Bordetella pertussis Infection in the Murine Respiratory Tract. Infect. Immun. 2006, 74, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Wilk, M.M.; Borkner, L.; Misiak, A.; Curham, L.; Allen, A.C.; Mills, K.H.G. Immunization with Whole Cell but Not Acellular Pertussis Vaccines Primes CD4 T RM Cells That Sustain Protective Immunity against Nasal Colonization with Bordetella pertussis. Emerg. Microbes Infect. 2019, 8, 169–185. [Google Scholar] [CrossRef]
- Brummelman, J.; Helm, K.; Hamstra, H.-J.; van der Ley, P.; Boog, C.J.P.; Han, W.G.H.; van Els, C.A.C.M. Modulation of the CD4+ T Cell Response after Acellular Pertussis Vaccination in the Presence of TLR4 Ligation. Vaccine 2015, 33, 1483–1491. [Google Scholar] [CrossRef]
- De Rond, L.; Schure, R.-M.; Öztürk, K.; Berbers, G.; Sanders, E.; Van Twillert, I.; Carollo, M.; Mascart, F.; Ausiello, C.M.; Van Els, C.A.C.M. Identification of Pertussis-Specific Effector Memory T Cells in Preschool Children. Clin. Vaccine Immunol. 2015, 22, 561–569. [Google Scholar] [CrossRef]
- Raffaella, P.; Maria, C.; Manuela, B.; Giorgio, F.; Ilaria, S.; Elisabetta, P.; Villani, A.; Tozzi Alberto, E.; Francoise, M.; Ausiello Clara, M. Persistence of T-Cell Immune Response Induced by Two Acellular Pertussis Vaccines in Children Five Years after Primary Vaccination. New Microbiol. 2016, 39, 35–47. [Google Scholar]
- Smits, K.; Pottier, G.; Smet, J.; Dirix, V.; Vermeulen, F.; De Schutter, I.; Carollo, M.; Locht, C.; Ausiello, C.M.; Mascart, F. Different T Cell Memory in Preadolescents after Whole-Cell or Acellular Pertussis Vaccination. Vaccine 2013, 32, 111–118. [Google Scholar] [CrossRef]
- Wilk, M.M.; Misiak, A.; McManus, R.M.; Allen, A.C.; Lynch, M.A.; Mills, K.H.G. Lung CD4 Tissue-Resident Memory T Cells Mediate Adaptive Immunity Induced by Previous Infection of Mice with Bordetella pertussis. J. Immunol. 2017, 199, 233–243. [Google Scholar] [CrossRef]
- Misiak, A.; Wilk, M.M.; Raverdeau, M.; Mills, K.H.G. IL-17–Producing Innate and Pathogen-Specific Tissue Resident Memory Γδ T Cells Expand in the Lungs of Bordetella pertussis–Infected Mice. J. Immunol. 2017, 198, 363–374. [Google Scholar] [CrossRef]
- Holubová, J.; Staněk, O.; Brázdilová, L.; Mašín, J.; Bumba, L.; Gorringe, A.R.; Alexander, F.; Šebo, P. Acellular Pertussis Vaccine Inhibits Bordetella pertussis Clearance from the Nasal Mucosa of Mice. Vaccines 2020, 8, 695. [Google Scholar] [CrossRef]
- Warfel, J.M.; Merkel, T.J. Bordetella pertussis Infection Induces a Mucosal IL-17 Response and Long-Lived Th17 and Th1 Immune Memory Cells in Nonhuman Primates. Mucosal Immunol. 2013, 6, 787–796. [Google Scholar] [CrossRef]
- Warfel, J.M.; Edwards, K.M. Pertussis Vaccines and the Challenge of Inducing Durable Immunity. Curr. Opin. Immunol. 2015, 35, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Warfel, J.M.; Papin, J.F.; Wolf, R.F.; Zimmerman, L.I.; Merkel, T.J. Maternal and Neonatal Vaccination Protects Newborn Baboons from Pertussis Infection. J. Infect. Dis. 2014, 210, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Warfel, J.M.; Beren, J.; Merkel, T.J. Airborne Transmission of Bordetella pertussis. J. Infect. Dis. 2012, 206, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Warfel, J.M.; Beren, J.; Kelly, V.K.; Lee, G.; Merkel, T.J. Nonhuman Primate Model of Pertussis. Infect. Immun. 2012, 80, 1530–1536. [Google Scholar] [CrossRef]
- Warfel, J.M.; Zimmerman, L.I.; Merkel, T.J. Comparison of Three Whole-Cell Pertussis Vaccines in the Baboon Model of Pertussis. Clin. Vaccine Immunol. 2016, 23, 47–54. [Google Scholar] [CrossRef]
- Warfel, J.M.; Zimmerman, L.I.; Merkel, T.J. Acellular Pertussis Vaccines Protect against Disease but Fail to Prevent Infection and Transmission in a Nonhuman Primate Model. Proc. Natl. Acad. Sci. USA 2014, 111, 787–792. [Google Scholar] [CrossRef]
- Higgins, S.C.; Jarnicki, A.G.; Lavelle, E.C.; Mills, K.H.G. TLR4 Mediates Vaccine-Induced Protective Cellular Immunity to Bordetella pertussis: Role of IL-17-Producing T Cells. J. Immunol. 2006, 177, 7980–7989. [Google Scholar] [CrossRef]
- Allen, A.C.; Wilk, M.M.; Misiak, A.; Borkner, L.; Murphy, D.; Mills, K.H.G. Sustained Protective Immunity against Bordetella pertussis Nasal Colonization by Intranasal Immunization with a Vaccine-Adjuvant Combination That Induces IL-17-Secreting TRM Cells. Mucosal Immunol. 2018, 11, 1763–1776. [Google Scholar] [CrossRef]
- Dunne, A.; Mielke, L.A.; Allen, A.C.; Sutton, C.E.; Higgs, R.; Cunningham, C.C.; Higgins, S.C.; Mills, K.H.G. A Novel TLR2 Agonist from Bordetella pertussis Is a Potent Adjuvant That Promotes Protective Immunity with an Acellular Pertussis Vaccine. Mucosal Immunol. 2015, 8, 607–617. [Google Scholar] [CrossRef]
- Borkner, L.; Curham, L.M.; Wilk, M.M.; Moran, B.; Mills, K.H.G. IL-17 Mediates Protective Immunity against Nasal Infection with Bordetella pertussis by Mobilizing Neutrophils, Especially Siglec-F+ Neutrophils. Mucosal Immunol. 2021, 14, 1183–1202. [Google Scholar] [CrossRef]
- Weaver, K.L.; Blackwood, C.B.; Horspool, A.M.; Pyles, G.M.; Sen-Kilic, E.; Grayson, E.M.; Huckaby, A.B.; Witt, W.T.; DeJong, M.A.; Wolf, M.A.; et al. Long-Term Analysis of Pertussis Vaccine Immunity to Identify Potential Markers of Vaccine-Induced Memory Associated With Whole Cell But Not Acellular Pertussis Immunization in Mice. Front. Immunol. 2022, 13, 838504. [Google Scholar] [CrossRef] [PubMed]
- Hendrikx, L.H.; Schure, R.-M.; Öztürk, K.; de Rond, L.G.H.; de Greeff, S.C.; Sanders, E.A.M.; Berbers, G.A.M.; Buisman, A.-M. Different IgG-Subclass Distributions after Whole-Cell and Acellular Pertussis Infant Primary Vaccinations in Healthy and Pertussis Infected Children. Vaccine 2011, 29, 6874–6880. [Google Scholar] [CrossRef] [PubMed]
- Raeven, R.H.M.; van der Maas, L.; Tilstra, W.; Uittenbogaard, J.P.; Bindels, T.H.E.; Kuipers, B.; van der Ark, A.; Pennings, J.L.A.; van Riet, E.; Jiskoot, W.; et al. Immunoproteomic Profiling of Bordetella pertussis Outer Membrane Vesicle Vaccine Reveals Broad and Balanced Humoral Immunogenicity. J. Proteome Res. 2015, 14, 2929–2942. [Google Scholar] [CrossRef]
- Diks, A.M.; Versteegen, P.; Teodosio, C.; Groenland, R.J.; de Mooij, B.; Buisman, A.-M.; Torres-Valle, A.; Pérez-Andrés, M.; Orfao, A.; Berbers, G.A.M.; et al. Age and Primary Vaccination Background Influence the Plasma Cell Response to Pertussis Booster Vaccination. Vaccines 2022, 10, 136. [Google Scholar] [CrossRef]
- Locht, C.; Papin, J.F.; Lecher, S.; Debrie, A.-S.; Thalen, M.; Solovay, K.; Rubin, K.; Mielcarek, N. Live Attenuated Pertussis Vaccine BPZE1 Protects Baboons Against Bordetella pertussis Disease and Infection. J. Infect. Dis. 2017, 216, 117–124. [Google Scholar] [CrossRef]
- Solans, L.; Debrie, A.-S.; Borkner, L.; Aguiló, N.; Thiriard, A.; Coutte, L.; Uranga, S.; Trottein, F.; Martín, C.; Mills, K.H.G.; et al. IL-17-Dependent SIgA-Mediated Protection against Nasal Bordetella pertussis Infection by Live Attenuated BPZE1 Vaccine. Mucosal Immunol. 2018, 11, 1753–1762. [Google Scholar] [CrossRef]
- Lin, A.; Apostolovic, D.; Jahnmatz, M.; Liang, F.; Ols, S.; Tecleab, T.; Wu, C.; van Hage, M.; Solovay, K.; Rubin, K.; et al. Live Attenuated Pertussis Vaccine BPZE1 Induces a Broad Antibody Response in Humans. J. Clin. Investig. 2020, 130, 2332–2346. [Google Scholar] [CrossRef]
- Creech, C.B.; Leguia, M.; Goll, J.B.; Howard, L.M.; Vila-Sanjurjo, A.; Yoder, S.; Juarez, D.; Garcia-Glaessner, A.; Gelber, C.E.; Jimenez-Truque, N.; et al. Immunologic Profiling of the Infant Immune Response to Whole-Cell and Acellular Pertussis Vaccines. npj Vaccines 2025, 10, 189. [Google Scholar] [CrossRef]
- Brandal, L.T.; Vestrheim, D.F.; Bruvik, T.; Roness, R.B.; Bjørnstad, M.L.; Greve-Isdahl, M.; Steens, A.; Brynildsrud, O.B. Evolution of Bordetella pertussis in the Acellular Vaccine Era in Norway, 1996 to 2019. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 913–924. [Google Scholar] [CrossRef]
- Advani, A.; Gustafsson, L.; Åhrén, C.; Mooi, F.R.; Hallander, H.O. Appearance of Fim3 and PtxP3-Bordetella pertussis Strains, in Two Regions of Sweden with Different Vaccination Programs. Vaccine 2011, 29, 3438–3442. [Google Scholar] [CrossRef] [PubMed]
- Octavia, S.; Sintchenko, V.; Gilbert, G.L.; Lawrence, A.; Keil, A.D.; Hogg, G.; Lan, R. Newly Emerging Clones of Bordetella pertussis Carrying Prn2 and PtxP3 Alleles Implicated in Australian Pertussis Epidemic in 2008–2010. J. Infect. Dis. 2012, 205, 1220–1224. [Google Scholar] [CrossRef] [PubMed]
- Mooi, F.R.; Van Loo, I.H.M.; Van Gent, M.; He, Q.; Bart, M.J.; Heuvelman, K.J.; De Greeff, S.C.; Diavatopoulos, D.; Teunis, P.; Nagelkerke, N. Bordetella pertussis Strains with Increased Toxin Production Associated with Pertussis Resurgence. Emerg. Infect. Dis. 2009, 15, 1206. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.F.; Dalby, T.; Dragsted, D.M.; Mooi, F.; Lambertsen, L. Temporal Trends in Bordetella pertussis Populations, Denmark, 1949–2010. Emerg. Infect. Dis. 2012, 18, 767. [Google Scholar] [CrossRef]
- Gutiérrez-Ferman, J.L.; Villarreal-Treviño, L.; Ramírez-Aranda, J.M.; Camacho-Ortiz, A.; Ballesteros-Elizondo, M.R.; Moreno-Juárez, M.R.; Mendoza-Olazarán, S.; de la O Cavazos, M.E.; Villarreal-Pérez, J.Z.; Gómez-Govea, M.A.; et al. Emerging of PtxP3 Lineage in Bordetella pertussis Strains Circulating in a Population in Northeastern Mexico. Epidemiol. Infect. 2018, 146, 2096–2101. [Google Scholar] [CrossRef]
- de Gouw, D.; Hermans, P.W.M.; Bootsma, H.J.; Zomer, A.; Heuvelman, K.; Diavatopoulos, D.A.; Mooi, F.R. Differentially Expressed Genes in Bordetella pertussis Strains Belonging to a Lineage Which Recently Spread Globally. PLoS ONE 2014, 9, e84523. [Google Scholar] [CrossRef]
- Marr, N.; Shah, N.R.; Lee, R.; Kim, E.J.; Fernandez, R.C. Bordetella pertussis Autotransporter Vag8 Binds Human C1 Esterase Inhibitor and Confers Serum Resistance. PLoS ONE 2011, 6, e20585. [Google Scholar] [CrossRef]
- Safarchi, A.; Saedi, S.; Octavia, S.; Sedaghatpour, M.; Bolourchi, N.; Tay, C.Y.; Lamichhane, B.; Shahcheraghi, F.; Lan, R. Evolutionary Genomics of Recent Clinical Bordetella pertussis Isolates from Iran: Wide Circulation of Multiple PtxP3 Lineages and Report of the First PtxP3 Filamentous Hemagglutinin-Negative B. Pertussis. Infect. Genet. Evol. 2021, 93, 104970. [Google Scholar] [CrossRef]
- Luu, L.D.W.; Octavia, S.; Zhong, L.; Raftery, M.J.; Sintchenko, V.; Lan, R. Comparison of the Whole Cell Proteome and Secretome of Epidemic Bordetella pertussis Strains from the 2008–2012 Australian Epidemic under Sulfate-Modulating Conditions. Front. Microbiol. 2018, 9, 2851. [Google Scholar] [CrossRef]
- Martin, S.W.; Pawloski, L.; Williams, M.; Weening, K.; DeBolt, C.; Qin, X.; Reynolds, L.; Kenyon, C.; Giambrone, G.; Kudish, K. Pertactin-Negative Bordetella pertussis Strains: Evidence for a Possible Selective Advantage. Clin. Infect. Dis. 2015, 60, 223–227. [Google Scholar] [CrossRef]
- Safarchi, A.; Octavia, S.; Luu, L.D.W.; Tay, C.Y.; Sintchenko, V.; Wood, N.; Marshall, H.; McIntyre, P.; Lan, R. Pertactin Negative Bordetella pertussis Demonstrates Higher Fitness under Vaccine Selection Pressure in a Mixed Infection Model. Vaccine 2015, 33, 6277–6281. [Google Scholar] [CrossRef] [PubMed]
- Jayasundara, D.; Lee, E.; Octavia, S.; Lan, R.; Tanaka, M.M.; Wood, J.G. Emergence of Pertactin-Deficient Pertussis Strains in Australia Can Be Explained by Models of Vaccine Escape. Epidemics 2020, 31, 100388. [Google Scholar] [CrossRef] [PubMed]
- Polak, M.; Zasada, A.A.; Mosiej, E.; Krysztopa-Grzybowska, K.; Witkowski, L.; Rzeczkowska, M.; Piekarska, K.; Lutyńska, A. Pertactin-Deficient Bordetella pertussis Isolates in Poland—A Country with Whole-Cell Pertussis Primary Vaccination. Microbes Infect. 2019, 21, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xiao, F.; Hou, Y.; Jia, B.; Zhuang, J.; Cao, Y.; Ma, J.; Zhao, J.; Xu, Z.; Jia, Z.; et al. Genomic Epidemiology and Evolution of Bordetella pertussis under the Vaccination Pressure of Acellular Vaccines in Beijing, China, 2020–2023. Emerg. Microbes Infect. 2025, 14, 2447611. [Google Scholar] [CrossRef]
- Leite, D.; Camargo, C.H.; Kashino, S.S.; Polatto, R.; Martins, L.M.; Pereira, J.C.; Pawloski, L.; Tondella, M.L.; de Oliveira, R.S.; Rehder de Andrade Vaz de Lima, L. Prevalence and Characterization of Pertactin Deficient Bordetella pertussis Strains in Brazil, a Whole-Cell Vaccine Country. Vaccine X 2021, 8, 100103. [Google Scholar] [CrossRef]
- Weigand, M.R.; Pawloski, L.C.; Peng, Y.; Ju, H.; Burroughs, M.; Cassiday, P.K.; Davis, J.K.; DuVall, M.; Johnson, T.; Juieng, P.; et al. Screening and Genomic Characterization of Filamentous Hemagglutinin-Deficient Bordetella pertussis. Infect. Immun. 2018, 86, e00869-17. [Google Scholar] [CrossRef]
- Clarke, M.; McIntyre, P.B.; Blyth, C.C.; Wood, N.; Octavia, S.; Sintchenko, V.; Giles, L.; Quinn, H.; Hill, V.; Hanly, G. The Relationship between Bordetella pertussis Genotype and Clinical Severity in Australian Children with Pertussis. J. Infect. 2016, 72, 171–178. [Google Scholar] [CrossRef]
- Barkoff, A.-M.; Mertsola, J.; Guillot, S.; Guiso, N.; Berbers, G.; He, Q. Appearance of Bordetella pertussis Strains Not Expressing the Vaccine Antigen Pertactin in Finland. Clin. Vaccine Immunol. 2012, 19, 1703–1704. [Google Scholar] [CrossRef] [PubMed]
- Payne, M.; Xu, Z.; Hu, D.; Kaur, S.; Octavia, S.; Sintchenko, V.; Lan, R. Genomic Epidemiology and Multilevel Genome Typing of Bordetella pertussis. Emerg. Microbes Infect. 2023, 12, 2239945. [Google Scholar] [CrossRef] [PubMed]
- Lesne, E.; Cavell, B.E.; Freire-Martin, I.; Persaud, R.; Alexander, F.; Taylor, S.; Matheson, M.; van Els, C.A.C.M.; Gorringe, A. Acellular Pertussis Vaccines Induce Anti-Pertactin Bactericidal Antibodies Which Drives the Emergence of Pertactin-Negative Strains. Front. Microbiol. 2020, 11, 2108. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.M.; Sen, K.; Weigand, M.R.; Skoff, T.H.; Cunningham, V.A.; Halse, T.A.; Tondella, M.L.; Group, C.D.C.P.W. Bordetella pertussis Strain Lacking Pertactin and Pertussis Toxin. Emerg. Infect. Dis. 2016, 22, 319. [Google Scholar] [CrossRef]
- van Gent, M.; Bart, M.J.; van der Heide, H.G.J.; Heuvelman, K.J.; Mooi, F.R. Small Mutations in Bordetella pertussis Are Associated with Selective Sweeps. PLoS ONE 2012, 7, e46407. [Google Scholar] [CrossRef]
- Bart, M.J.; Harris, S.R.; Advani, A.; Arakawa, Y.; Bottero, D.; Bouchez, V.; Cassiday, P.K.; Chiang, C.-S.; Dalby, T.; Fry, N.K.; et al. Global Population Structure and Evolution of Bordetella pertussis and Their Relationship with Vaccination. MBio 2014, 5, e01074. [Google Scholar] [CrossRef]
- Schmidtke, A.J.; Boney, K.O.; Martin, S.W.; Skoff, T.H.; Tondella, M.L.; Tatti, K.M. Population Diversity among Bordetella pertussis Isolates, United States, 1935–2009. Emerg. Infect. Dis. 2012, 18, 1248–1255. [Google Scholar] [CrossRef]
- Lefrancq, N.; Bouchez, V.; Fernandes, N.; Barkoff, A.-M.; Bosch, T.; Dalby, T.; Åkerlund, T.; Darenberg, J.; Fabianova, K.; Vestrheim, D.F.; et al. Global Spatial Dynamics and Vaccine-Induced Fitness Changes of Bordetella pertussis. Sci. Transl. Med. 2022, 14, eabn3253. [Google Scholar] [CrossRef]
- Bouchez, V.; Hegerle, N.; Strati, F.; Njamkepo, E.; Guiso, N. New Data on Vaccine Antigen Deficient Bordetella pertussis Isolates. Vaccines 2015, 3, 751–770. [Google Scholar] [CrossRef]
- de Paula, V.G.; de Sousa, R.S.; da Silva, R.C.M.R.; Alves, E.G.; Caetano, A.R.; Ianella, P.; de Campos, T.A. Fim3–24/PtxP-3 Genotype Is Associated to Whooping Cough Outbreak in Brazilian Midwest: The Selection of Bordetella pertussis Strains Driven by Vaccine Immunization. Infect. Genet. Evol. 2024, 121, 105599. [Google Scholar] [CrossRef]
- Zeddeman, A.; van Schuppen, E.; Kok, K.E.; van Gent, M.; Heuvelman, K.J.; Bart, M.J.; van der Heide, H.G.J.; Gillard, J.; Simonetti, E.; Eleveld, M.J. Effect of FHA and Prn on Bordetella pertussis Colonization of Mice Is Dependent on Vaccine Type and Anatomical Site. PLoS ONE 2020, 15, e0237394. [Google Scholar] [CrossRef]
- Xu, Z.; Octavia, S.; Luu, L.D.W.; Payne, M.; Timms, V.; Tay, C.Y.; Keil, A.D.; Sintchenko, V.; Guiso, N.; Lan, R. Pertactin-Negative and Filamentous Hemagglutinin-Negative Bordetella pertussis, Australia, 2013–2017. Emerg. Infect. Dis. 2019, 25, 1196. [Google Scholar] [CrossRef]
- Bart, M.J.; van der Heide, H.G.J.; Zeddeman, A.; Heuvelman, K.; van Gent, M.; Mooi, F.R. Complete Genome Sequences of 11 Bordetella pertussis Strains Representing the Pandemic PtxP3 Lineage. Genome Announc. 2015, 3, e01394-15. [Google Scholar] [CrossRef]
- Hegerle, N.; Paris, A.-S.; Brun, D.; Dore, G.; Njamkepo, E.; Guillot, S.; Guiso, N. Evolution of French Bordetella pertussis and Bordetella Parapertussis Isolates: Increase of Bordetellae Not Expressing Pertactin. Clin. Microbiol. Infect. 2012, 18, E340–E346. [Google Scholar] [CrossRef] [PubMed]
- Jongerius, I.; Schuijt, T.J.; Mooi, F.R.; Pinelli, E. Complement Evasion by Bordetella pertussis: Implications for Improving Current Vaccines. J. Mol. Med. 2015, 93, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Brookes, C.; Freire-Martin, I.; Cavell, B.; Alexander, F.; Taylor, S.; Persaud, R.; Fry, N.; Preston, A.; Diavatopoulos, D.; Gorringe, A. Bordetella pertussis Isolates Vary in Their Interactions with Human Complement Components. Emerg. Microbes Infect. 2018, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hovingh, E.S.; van Gent, M.; Hamstra, H.-J.; Demkes, M.; Mooi, F.R.; Pinelli, E. Emerging Bordetella pertussis Strains Induce Enhanced Signaling of Human Pattern Recognition Receptors TLR2, NOD2 and Secretion of IL-10 by Dendritic Cells. PLoS ONE 2017, 12, e0170027. [Google Scholar] [CrossRef]
- Nagamatsu, K.; Kuwae, A.; Konaka, T.; Nagai, S.; Yoshida, S.; Eguchi, M.; Watanabe, M.; Mimuro, H.; Koyasu, S.; Abe, A. Bordetella Evades the Host Immune System by Inducing IL-10 through a Type III Effector, BopN. J. Exp. Med. 2009, 206, 3073–3088. [Google Scholar] [CrossRef]
- Kelly, A.M.; McCarthy, K.N.; Claxton, T.J.; Carlile, S.R.; O’Brien, E.C.; Vozza, E.G.; Mills, K.H.G.; McLoughlin, R.M. IL-10 Inhibition during Immunization Improves Vaccine-Induced Protection against Staphylococcus Aureus Infection. JCI Insight 2024, 9, e178216. [Google Scholar] [CrossRef]
- Bone, M.A.; Wilk, A.J.; Perault, A.I.; Marlatt, S.A.; Scheller, E.V.; Anthouard, R.; Chen, Q.; Stibitz, S.; Cotter, P.A.; Julio, S.M. Bordetella PlrSR Regulatory System Controls BvgAS Activity and Virulence in the Lower Respiratory Tract. Proc. Natl. Acad. Sci. USA 2017, 114, E1519–E1527. [Google Scholar] [CrossRef]
- Dorji, D.; Mooi, F.; Yantorno, O.; Deora, R.; Graham, R.M.; Mukkur, T.K. Bordetella pertussis Virulence Factors in the Continuing Evolution of Whooping Cough Vaccines for Improved Performance. Med. Microbiol. Immunol. 2018, 207, 3–26. [Google Scholar] [CrossRef]
- Moon, K.; Bonocora, R.P.; Kim, D.D.; Chen, Q.; Wade, J.T.; Stibitz, S.; Hinton, D.M. The BvgAS Regulon of Bordetella pertussis. MBio 2017, 8, e00694-18. [Google Scholar] [CrossRef]
- Melvin, J.A.; Scheller, E.V.; Miller, J.F.; Cotter, P.A. Bordetella pertussis Pathogenesis: Current and Future Challenges. Nat. Rev. Microbiol. 2014, 12, 274–288. [Google Scholar] [CrossRef] [PubMed]
- King, A.J.; van der Lee, S.; Mohangoo, A.; van Gent, M.; van der Ark, A.; van de Waterbeemd, B. Genome-Wide Gene Expression Analysis of Bordetella pertussis Isolates Associated with a Resurgence in Pertussis: Elucidation of Factors Involved in the Increased Fitness of Epidemic Strains. PLoS ONE 2013, 8, e66150. [Google Scholar] [CrossRef]
- Karataev, G.I.; Sinyashina, L.N.; Medkova, A.Y.; Semin, E.G.; Shevtsova, Z.V.; Matua, A.Z.; Kondzariya, I.G.; Amichba, A.A.; Kubrava, D.T.; Mikvabia, Z.Y. Insertional Inactivation of Virulence Operon in Population of Persistent Bordetella pertussis Bacteria. Russ. J. Genet. 2016, 52, 370–377. [Google Scholar] [CrossRef]
- Jones, A.M.; Boucher, P.E.; Williams, C.L.; Stibitz, S.; Cotter, P.A. Role of BvgA Phosphorylation and DNA Binding Affinity in Control of Bvg-mediated Phenotypic Phase Transition in Bordetella pertussis. Mol. Microbiol. 2005, 58, 700–713. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, Y.; Yoshino, S.; Yamamura, Y.; Otsuka, N.; Shibayama, K.; Watanabe, M.; Kamachi, K. The Proline Residue at Position 319 of BvgS Is Essential for BvgAS Activation in Bordetella pertussis. Pathog. Dis. 2017, 75, ftx011. [Google Scholar] [CrossRef]
- Dai, H.; He, H.; Xu, J.; Zhu, Y.; Fu, T.; Chen, B.; Li, J.; Gao, Y.; Qin, A.; Zhang, M.; et al. Underestimated Incidence Rate of Pertussis in the Community: Results from Active Population-Based Surveillance in Yiwu, China. Microorganisms 2024, 12, 2186. [Google Scholar] [CrossRef]
- Long, S.S.; Welkon, C.J.; Clark, J.L. Widespread Silent Transmission of Pertussis in Families: Antibody Correlates of Infection and Symptomatology. J. Infect. Dis. 1990, 161, 480–486. [Google Scholar] [CrossRef]
- Crowcroft, N.S. Estimating the Burden of Bordetella pertussis Infection Presenting to Paediatric Intensive Care Units and Wards in London to Inform Vaccination Policy in the United Kingdom; University of Cambridge: Cambridge, UK, 2005. [Google Scholar]
- Grimprel, E.; Njamkepo, E.; Bégué, P.; Guiso, N. Rapid Diagnosis of Pertussis in Young Infants: Comparison of Culture, PCR, and Infant’s and Mother’s Serology. Clin. Diagn. Lab. Immunol. 1997, 4, 723–726. [Google Scholar] [CrossRef]
- De Schutter, I.; Malfroot, A.; Dab, I.; Hoebrekx, N.; Muyldermans, G.; Piérard, D.; Lauwers, S. Molecular Typing of Bordetella pertussis Isolates Recovered from Belgian Children and Their Household Members. Clin. Infect. Dis. 2003, 36, 1391–1396. [Google Scholar] [CrossRef]
- Wendelboe, A.M.; Njamkepo, E.; Bourillon, A.; Floret, D.D.; Gaudelus, J.; Gerber, M.; Grimprel, E.; Greenberg, D.; Halperin, S.; Liese, J. Transmission of Bordetella pertussis to Young Infants. Pediatr. Infect. Dis. J. 2007, 26, 293–299. [Google Scholar] [CrossRef]
- Fedele, G.; Carollo, M.; Palazzo, R.; Stefanelli, P.; Pandolfi, E.; Gesualdo, F.; Tozzi, A.E.; Carsetti, R.; Villani, A.; Nicolai, A. Parents as Source of Pertussis Transmission in Hospitalized Young Infants. Infection 2017, 45, 171–178. [Google Scholar] [CrossRef]
- Kara, E.O.; Campbell, H.; Ribeiro, S.; Fry, N.K.; Litt, D.; Eletu, S.; Amirthalingam, G. Survey of Household Contacts of Infants with Laboratory-Confirmed Pertussis Infection during a National Pertussis Outbreak in England and Wales. Pediatr. Infect. Dis. J. 2017, 36, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Raeven, R.H.M.; Rockx-Brouwer, D.; Kanojia, G.; van der Maas, L.; Bindels, T.H.E.; ten Have, R.; van Riet, E.; Metz, B.; Kersten, G.F.A. Intranasal Immunization with Outer Membrane Vesicle Pertussis Vaccine Confers Broad Protection through Mucosal IgA and Th17 Responses. Sci. Rep. 2020, 10, 7396. [Google Scholar] [CrossRef] [PubMed]
- Jahnmatz, M.; Richert, L.; al-Tawil, N.; Storsaeter, J.; Colin, C.; Bauduin, C.; Thalen, M.; Solovay, K.; Rubin, K.; Mielcarek, N.; et al. Safety and Immunogenicity of the Live Attenuated Intranasal Pertussis Vaccine BPZE1: A Phase 1b, Double-Blind, Randomised, Placebo-Controlled Dose-Escalation Study. Lancet Infect. Dis. 2020, 20, 1290–1301, Correction in Lancet Infect. Dis. 2020, 20, e215. https://doi.org/10.1016/S1473-3099(20)30274-7. [Google Scholar] [CrossRef]
- Keech, C.; Miller, V.E.; Rizzardi, B.; Hoyle, C.; Pryor, M.J.; Ferrand, J.; Solovay, K.; Thalen, M.; Noviello, S.; Goldstein, P. Immunogenicity and Safety of BPZE1, an Intranasal Live Attenuated Pertussis Vaccine, versus Tetanus–Diphtheria–Acellular Pertussis Vaccine: A Randomised, Double-Blind, Phase 2b Trial. Lancet 2023, 401, 843–855. [Google Scholar] [CrossRef]
- Cherry, J.D. Pertussis: Challenges Today and for the Future. PLoS Pathog. 2013, 9, 9–12. [Google Scholar] [CrossRef]
- Eberhardt, C.S.; Siegrist, C.A. What Is Wrong with Pertussis Vaccine Immunity? Inducing and Recalling Vaccine-Specific Immunity. Cold Spring Harb. Perspect. Biol. 2017, 9, a029629. [Google Scholar] [CrossRef]
- Kim, J.H.; Skountzou, I.; Compans, R.; Jacob, J. Original Antigenic Sin Responses to Influenza Viruses. J. Immunol. 2009, 183, 3294–3301. [Google Scholar] [CrossRef]
- Sutherland, J.N.; Chang, C.; Yoder, S.M.; Rock, M.T.; Maynard, J.A. Antibodies Recognizing Protective Pertussis Toxin Epitopes Are Preferentially Elicited by Natural Infection versus Acellular Immunization. Clin. Vaccine Immunol. 2011, 18, 954–962. [Google Scholar] [CrossRef]
- Merdrignac, L.; Acosta, L.; Habington, A.; Garcìa Cenoz, M.; Pandolfi, E.; Fabiánová, K.; Jordan, I.; O’Sullivan, N.; Navasués, A.; Tozzi, A.E.; et al. Effectiveness of Pertussis Vaccination in Pregnancy to Prevent Hospitalisation in Infants Aged <2 Months and Effectiveness of Both Primary Vaccination and Mother’s Vaccination in Pregnancy in Infants Aged 2–11 Months. Vaccine 2022, 40, 6374–6382. [Google Scholar] [CrossRef]
- Baxter, R.; Bartlett, J.; Fireman, B.; Lewis, E.; Klein, N.P. Effectiveness of Vaccination during Pregnancy to Prevent Infant Pertussis. Pediatrics 2017, 139, e20164091. [Google Scholar] [CrossRef] [PubMed]
- Quinn, H.E.; Comeau, J.L.; Marshall, H.S.; Elliott, E.J.; Crawford, N.W.; Blyth, C.C.; Kynaston, J.A.; Snelling, T.L.; Richmond, P.C.; Francis, J.R. Pertussis Disease and Antenatal Vaccine Effectiveness in Australian Children. Pediatr. Infect. Dis. J. 2022, 41, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Amirthalingam, G.; Andrews, N.; Campbell, H.; Ribeiro, S.; Kara, E.; Donegan, K.; Fry, N.K.; Miller, E.; Ramsay, M. Effectiveness of Maternal Pertussis Vaccination in England: An Observational Study. Lancet 2014, 384, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Amirthalingam, G.; Campbell, H.; Ribeiro, S.; Stowe, J.; Tessier, E.; Litt, D.; Fry, N.K.; Andrews, N. Optimization of Timing of Maternal Pertussis Immunization from 6 Years of Postimplementation Surveillance Data in England. Clin. Infect. Dis. 2023, 76, e1129–e1139. [Google Scholar] [CrossRef]
- Kandeil, W.; Savic, M.; Ceregido, M.A.; Guignard, A.; Kuznetsova, A.; Mukherjee, P. Immune Interference (Blunting) in the Context of Maternal Immunization with Tdap-Containing Vaccines: Is It a Class Effect? Expert Rev. Vaccines 2020, 19, 341–352. [Google Scholar] [CrossRef]
- Siegrist, C.-A.; Aspinall, R. B-Cell Responses to Vaccination at the Extremes of Age. Nat. Rev. Immunol. 2009, 9, 185–194. [Google Scholar] [CrossRef]
- Knuutila, A.; Barkoff, A.M.; Ivaska, L.; Tenhu, E.; Teräsjärvi, J.; van Gageldonk, P.; Buisman, A.; Mertsola, J.; He, Q. Effect of Immunization during Pregnancy and Pre-Existing Immunity on Diphtheria-Tetanus-Acellular Pertussis Vaccine Responses in Infants. Emerg. Microbes Infect. 2023, 12, 2204146. [Google Scholar] [CrossRef]
- Voysey, M.; Kelly, D.F.; Fanshawe, T.R.; Sadarangani, M.; O’Brien, K.L.; Perera, R.; Pollard, A.J. The Influence of Maternally Derived Antibody and Infant Age at Vaccination on Infant Vaccine Responses. JAMA Pediatr. 2017, 171, 637. [Google Scholar] [CrossRef]
- Wanlapakorn, N.; Maertens, K.; Vongpunsawad, S.; Puenpa, J.; Tran, T.M.P.; Hens, N.; Van Damme, P.; Thiriard, A.; Raze, D.; Locht, C.; et al. Quantity and Quality of Antibodies After Acellular Versus Whole-Cell Pertussis Vaccines in Infants Born to Mothers Who Received Tetanus, Diphtheria, and Acellular Pertussis Vaccine During Pregnancy: A Randomized Trial. Clin. Infect. Dis. 2020, 71, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Abu-Raya, B.; Maertens, K.; Munoz, F.M.; Zimmermann, P.; Curtis, N.; Halperin, S.A.; Rots, N.; Barug, D.; Holder, B.; Kampmann, B.; et al. The Effect of Tetanus-Diphtheria-Acellular-Pertussis Immunization During Pregnancy on Infant Antibody Responses: Individual-Participant Data Meta-Analysis. Front. Immunol. 2021, 12, 689394. [Google Scholar] [CrossRef]
- Sapuan, S.; Andrews, N.; Hallis, B.; Hole, L.; Jones, C.E.; Matheson, M.; Miller, E.; Snape, M.D.; Heath, P.T. An Observational, Cohort, Multi-Centre, Open Label Phase IV Extension Study Comparing Preschool DTAP-IPV Booster Vaccine Responses in Children Whose Mothers Were Randomised to One of Two Pertussis-Containing Vaccines or Received No Pertussis-Containing Vaccine in Pregnancy in England. Vaccine 2022, 40, 7050–7056. [Google Scholar] [CrossRef] [PubMed]
- Ivaska, L.; Barkoff, A.M.; Mertsola, J.; He, Q. Macrolide Resistance in Bordetella pertussis: Current Situation and Future Challenges. Antibiotics 2022, 11, 1570. [Google Scholar] [CrossRef]
- Kilgore, P.E.; Salim, A.M.; Zervos, M.J.; Schmitt, H.J. Pertussis: Microbiology, Disease, Treatment, and Prevention. Clin. Microbiol. Rev. 2016, 29, 449–486. [Google Scholar] [CrossRef]
- Cherry, J.D. Treatment of Pertussis—2017. J. Pediatr. Infect. Dis. Soc. 2018, 7, e123–e125. [Google Scholar] [CrossRef]
- Feng, Y.; Chiu, C.H.; Heininger, U.; Hozbor, D.F.; Tan, T.Q.; von König, C.H.W. Emerging Macrolide Resistance in Bordetella pertussis in Mainland China: Findings and Warning from the Global Pertussis Initiative. Lancet Reg. Health—West. Pacific 2021, 8, 100098. [Google Scholar] [CrossRef] [PubMed]
- Fong, W.; Timms, V.; Sim, E.; Pey, K.; Nguyen, T.; Sintchenko, V. Genomic and Transcriptomic Variation in Bordetella Spp. Following Induction of Erythromycin Resistance. J. Antimicrob. Chemother. 2022, 77, 3016–3025. [Google Scholar] [CrossRef]
- Bartkus, J.M.; Juni, B.A.; Ehresmann, K.; Miller, C.A.; Sanden, G.N.; Cassiday, P.K.; Saubolle, M.; Lee, B.; Long, J.; Harrison, A.R.; et al. Identification of a Mutation Associated with Erythromycin Resistance in Bordetella pertussis: Implications for Surveillance of Antimicrobial Resistance. J. Clin. Microbiol. 2003, 41, 1167–1172. [Google Scholar] [CrossRef]
- Fu, P.; Zhou, J.; Yang, C.; Nijiati, Y.; Zhou, L.; Yan, G.; Lu, G.; Zhai, X.; Wang, C. Molecular Evolution and Increasing Macrolide Resistance of Bordetella pertussis, Shanghai, China, 2016–2022. Emerg. Infect. Dis. 2024, 30, 29. [Google Scholar] [CrossRef]
- Li, L.; Deng, J.; Ma, X.; Zhou, K.; Meng, Q.; Yuan, L.; Shi, W.; Wang, Q.; Li, Y.; Yao, K. High Prevalence of Macrolide-Resistant Bordetella pertussis and PtxP1 Genotype, Mainland China, 2014-2016. Emerg. Infect. Dis. 2019, 25, 2205–2214. [Google Scholar] [CrossRef]
- Vermeulen, F.; Verscheure, V.; Damis, E.; Vermeylen, D.; Leloux, G.; Dirix, V.; Locht, C.; Mascart, F. Cellular Immune Responses of Preterm Infants after Vaccination with Whole-Cell or Acellular Pertussis Vaccines. Clin. Vaccine Immunol. 2010, 17, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Kollmann, T.R.; Kampmann, B.; Mazmanian, S.K.; Marchant, A.; Levy, O. Protecting the Newborn and Young Infant from Infectious Diseases: Lessons from Immune Ontogeny. Immunity 2017, 46, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Slack, M.H.; Schapira, D.; Thwaites, R.J.; Burrage, M.; Southern, J.; Goldblatt, D.; Miller, E. Responses to a Fourth Dose of Haemophilus Influenzae Type B Conjugate Vaccine in Early Life. J. Epidemiol. Community Health 2004, 58, 622. [Google Scholar] [CrossRef] [PubMed]
- Van Twillert, I.; Van Gaans-van Den Brink, J.A.M.; Poelen, M.C.M.; Helm, K.; Kuipers, B.; Schipper, M.; Boog, C.J.P.; Verheij, T.J.M.; Versteegh, F.G.A.; Van Els, C.A.C.M. Age Related Differences in Dynamics of Specific Memory B Cell Populations after Clinical Pertussis Infection. PLoS ONE 2014, 9, e85227. [Google Scholar] [CrossRef]
- Frasca, D.; Diaz, A.; Romero, M.; Mendez, N.V.; Landin, A.M.; Blomberg, B.B. Effects of Age on H1N1-Specific Serum IgG1 and IgG3 Levels Evaluated during the 2011–2012 Influenza Vaccine Season. Immun. Ageing 2013, 10, 14. [Google Scholar] [CrossRef]
- Bouche, F.B.; Ertl, O.T.; Muller, C.P. Neutralizing B Cell Response in Measles. Viral Immunol. 2002, 15, 451–471. [Google Scholar] [CrossRef]
- Yuen, C.T.; Asokanathan, C.; Cook, S.; Lin, N.; Xing, D. Effect of Different Detoxification Procedures on the Residual Pertussis Toxin Activities in Vaccines. Vaccine 2016, 34, 2129–2134. [Google Scholar] [CrossRef]
- Englund, J.A.; Anderson, E.L.; Reed, G.F.; Decker, M.D.; Edwards, K.M.; Pichichero, M.E.; Steinhoff, M.C.; Rennels, M.B.; Deforest, A.; Meade, B.D. The Effect of Maternal Antibody on the Serologic Response and the Incidence of Adverse Reactions after Primary Immunization with Acellular and Whole-Cell Pertussis Vaccines Combined with Diphtheria and Tetanus Toxoids. Pediatrics 1995, 96, 580–584. [Google Scholar]
- Amirthalingam, G.; Campbell, H.; Ribeiro, S.; Fry, N.K.; Ramsay, M.; Miller, E.; Andrews, N. Sustained Effectiveness of the Maternal Pertussis Immunization Program in England 3 Years Following Introduction. Clin. Infect. Dis. 2016, 63, S236–S243. [Google Scholar] [CrossRef]
- Ma, L.; Caulfield, A.; Dewan, K.K.; Harvill, E.T. Pertactin-Deficient Bordetella pertussis, Vaccine-Driven Evolution, and Reemergence of Pertussis. Emerg. Infect. Dis. 2021, 27, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Mir-Cros, A.; Moreno-Mingorance, A.; Martín-Gómez, M.T.; Abad, R.; Bloise, I.; Campins, M.; González-Praetorius, A.; Gutiérrez, M.N.; Martín-González, H.; Muñoz-Almagro, C. Pertactin-Deficient Bordetella pertussis with Unusual Mechanism of Pertactin Disruption, Spain, 1986–2018. Emerg. Infect. Dis. 2022, 28, 967. [Google Scholar] [CrossRef] [PubMed]
- Barkoff, A.-M.; Mertsola, J.; Pierard, D.; Dalby, T.; Hoegh, S.V.; Guillot, S.; Stefanelli, P.; van Gent, M.; Berbers, G.; Vestrheim, D. Pertactin-Deficient Bordetella pertussis Isolates: Evidence of Increased Circulation in Europe, 1998 to 2015. Eurosurveillance 2019, 24, 1700832. [Google Scholar] [CrossRef] [PubMed]
- Visser, L.J.; van Veldhuizen, J.; Tromp, V.; Noomen, R.; Bonačić-Harpal, A.; Mariman, R. Resurgence of Bordetella pertussis in the Netherlands in 2023-2024 Reveals Novel Mutation in T3SS Translocator Gene BopD. medRxiv 2025. [Google Scholar] [CrossRef]
- Mi, Y.-M.; Deng, J.-K.; Zhang, T.; Cao, Q.; Wang, C.-Q.; Ye, S.; Chen, Y.-H.; He, H.-Q.; Wu, B.-B.; Liu, Y.; et al. Expert Consensus for Pertussis in Children: New Concepts in Diagnosis and Treatment. World J. Pediatr. 2024, 20, 1209–1222. [Google Scholar] [CrossRef]
- See, K.C. Pertussis Vaccination for Adults: An Updated Guide for Clinicians. Vaccines 2025, 13, 60. [Google Scholar] [CrossRef]


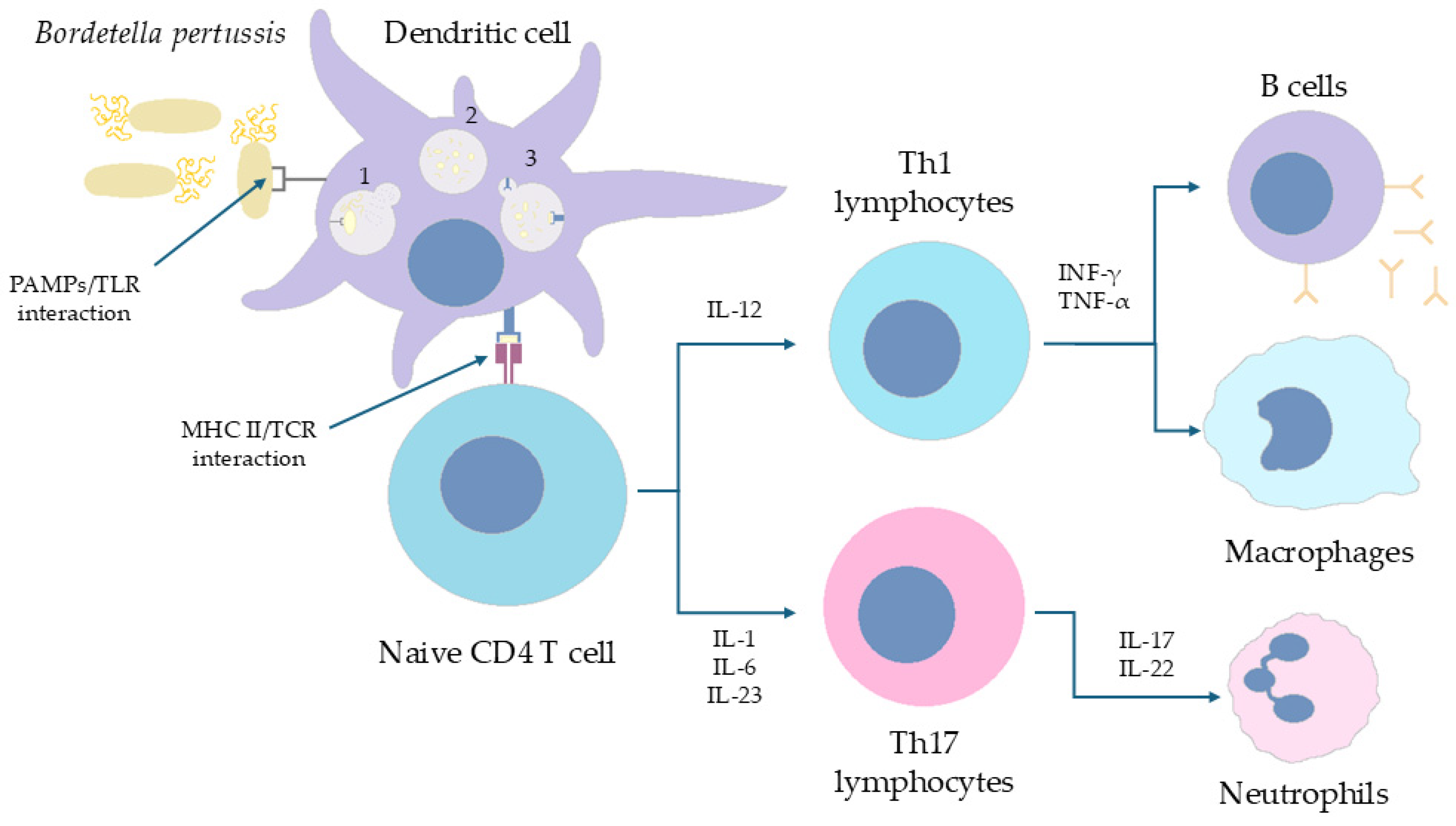
| Type of Vaccine | wP Vaccine | aP Vaccine |
|---|---|---|
| Efficacy and effectiveness | better protection even against colonization | poorer protection |
| Duration of protection | longer | shorter |
| Reactogenicity | higher | lower |
| Cost | more cost-effective when disease prevention is the priority | more cost-effective, when healthcare visits related to vaccines are included |
| References | [52,54] | |
| Name of the Vaccine | Producer | Composition |
|---|---|---|
| Whole-cell pertussis vaccines (wP) | ||
| Quintanrix | GlaxoSmithKline, London, UK | Inactivated B. pertussis strain—not less than 4 international units. |
| Acellular pertussis vaccines (aP) | ||
| Infantrix; Boostrix; Pediatrix; Kinrix | GlaxoSmithKline, London, UK | Always contain detoxified Ptx. May include FHA, PRN, fimbriae (not uniform, but generally present in combinations). |
| Daptacel; Adacel; Quadracel; Pentacel; Pediacel; Repevax; Hexacima | Sanofi Pasteur Inc., Paris, France | |
| Vaxelis | Merck & Co. Inc., Rahway, NJ, USA and Sanofi Pasteur Inc., Paris, France | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duda-Madej, A.; Łabaz, J.; Topola, E.; Bazan, H.; Viscardi, S. Pertussis—A Re-Emerging Threat Despite Immunization: An Analysis of Vaccine Effectiveness and Antibiotic Resistance. Int. J. Mol. Sci. 2025, 26, 9607. https://doi.org/10.3390/ijms26199607
Duda-Madej A, Łabaz J, Topola E, Bazan H, Viscardi S. Pertussis—A Re-Emerging Threat Despite Immunization: An Analysis of Vaccine Effectiveness and Antibiotic Resistance. International Journal of Molecular Sciences. 2025; 26(19):9607. https://doi.org/10.3390/ijms26199607
Chicago/Turabian StyleDuda-Madej, Anna, Jakub Łabaz, Ewa Topola, Hanna Bazan, and Szymon Viscardi. 2025. "Pertussis—A Re-Emerging Threat Despite Immunization: An Analysis of Vaccine Effectiveness and Antibiotic Resistance" International Journal of Molecular Sciences 26, no. 19: 9607. https://doi.org/10.3390/ijms26199607
APA StyleDuda-Madej, A., Łabaz, J., Topola, E., Bazan, H., & Viscardi, S. (2025). Pertussis—A Re-Emerging Threat Despite Immunization: An Analysis of Vaccine Effectiveness and Antibiotic Resistance. International Journal of Molecular Sciences, 26(19), 9607. https://doi.org/10.3390/ijms26199607






