Sex Hormones and Metabolic Dysfunction-Associated Steatotic Liver Disease
Abstract
1. Introduction
Method of Bibliographic Research
2. Molecular Physiopathology of Sex Hormones and Their Receptors in the Context of MASLD
2.1. The Liver as a Sexually Dimorphic Organ
2.2. Molecular Mechanisms of Sex-Specific Action of Testosterone on the Liver
2.2.1. Lipid Droplets
2.2.2. Ferroptosis
2.3. Molecular Mechanisms of Estradiol Action on the Liver
2.3.1. Estradiol Interacts with Genetic Risk Variants
2.3.2. Effects on Gluco-Lipidic Homeostasis, Lipotoxicity, Inflammation, Mitochondrial Function, and Oxidative Stress
2.3.3. Effects on the Metabolism of Fatty Acids, Fibrogenesis, and Gut Dysbiosis
2.3.4. Masculinization of the Liver in Post-Menopausal Women
2.4. Role of Sex Hormones in the Biogenesis of MASLD-Related Liver Tumors
2.5. Molecular Mechanisms of Action of Progesterone on the Liver
2.6. Role of GH in Explaining Sex Differences in MASLD
2.7. Potential Interaction of Sex Hormones and Thyroid Hormones
3. Lesson from Epidemiological and Meta-Analytical Studies
3.1. Testosterone and Estrogens in Men and Women
3.2. Sex Hormones, Liver Enzymes, and Cardiometabolic Factors
4. Evidence from Mendelian Randomization Studies
5. Sex Hormones and MASLD in Pregnancy, Lactation, and Menopause
5.1. Pregnancy
5.2. Lactation
5.3. Menopause
6. MASLD in Male and Female Hypogonadism and Effects on MASLD of Sex Hormone Replacement Therapy and Contraceptive Use
6.1. Testosterone Replacement Therapy in Men
6.2. Efficacy and Safety of Testosterone Replacement Therapy in Women
6.3. Estradiol Replacement Therapy in Women
6.3.1. Estradiol Replacement Therapy in Menopause
6.3.2. Estradiol Replacement Therapy in Transgender Women
6.3.3. Hormonal Replacement Therapy in Turner Syndrome
6.4. Hepatic Effects of Progesterone Treatment
6.5. Safety of Sex Hormone Replacement Therapy and Contraceptives Among Those with Chronic Liver Disease
6.6. Lessons from Primary Biliary Cholangitis (PBC) and Autoimmune Hepatitis (AIH)
7. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFLP | Acute fatty liver of pregnancy |
| AMH | Anti-Müllerian hormone |
| BCL6 | B-cell CLL/lymphoma 6 |
| CCA | Cholangiocarcinoma |
| CHC | Combined hormonal contraception |
| DNL | De novo lipogenesis |
| ERR | Estrogen-related receptor |
| ERRα | Estrogen-related receptor alpha |
| ER(s) | estrogen receptor(s) |
| ERT | Estradiol replacement therapy |
| GAHT | Gender affirming hormone therapy |
| GH | Growth hormone |
| GHRH | Growth hormone-releasing hormone |
| GWAS | Genome-wide association study |
| HCC | Hepatocellular carcinoma |
| HRT | Hormone replacement therapy |
| HNF4a | Hepatocyte nuclear factor 4 alpha |
| HSC(s) | Hepatic stellate cell(s) |
| ICC | Intrahepatic cholangiocarcinoma |
| KC(s) | Kupffer cell(s) |
| LA | Linoleic acid |
| LD(s) | Lipid droplet(s) |
| MASH | Metabolic dysfunction-associated steatohepatitis |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| MHT | Menopausal hormone therapy |
| MPTP | Mitochondrial permeability transition pore |
| MR | Mendelian randomization |
| OVX | Bilateral oophorectomy |
| PCOS | Polycystic ovary syndrome |
| PGC1A | PPARG coactivator 1α |
| PPG | Postprandial glucose |
| PR | Progesterone receptor |
| ROS | Reactive oxygen species |
| SCFA | Short-chain fatty acids |
| SHBG | Sex hormone-binding globulin |
| SLD | Steatotic liver disease |
| STAT5b | Signal transducer and activator of transcription 5b |
| TH(s) | Thyroid hormone(s) |
| T2D | Type 2 diabetes |
| T3 | Triiodothyronine |
| TRT | Testosterone replacement therapy |
| TS | Turner Syndrome |
| TT | Total testosterone |
| ZHX2 | Zinc fingers and homeoboxes 2 |
References
- Chan, W.K.; Chuah, K.H.; Rajaram, R.B.; Lim, L.L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J. Obes. Metab. Syndr. 2023, 32, 197–213. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Kalligeros, M.; Henry, L. Epidemiology of metabolic dysfunction-associated steatotic liver disease. Clin. Mol. Hepatol. 2025, 31, S32–S50. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Martín-Rodríguez, A.; Martínez-Guardado, I.; Navarro-Jiménez, E.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. The Role of Adipokines in Health and Disease. Biomedicines 2023, 11, 1290. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Lugari, S.; Ballestri, S.; Nascimbeni, F.; Baldelli, E.; Maurantonio, M. A round trip from nonalcoholic fatty liver disease to diabetes: Molecular targets to the rescue? Acta Diabetol. 2019, 56, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Sechi, L.A.; Navarese, E.P.; Casu, G.; Vidili, G. Metabolic dysfunction-associated steatotic liver disease and cardiovascular risk: A comprehensive review. Cardiovasc. Diabetol. 2024, 23, 346. [Google Scholar] [CrossRef]
- Lonardo, A.; Carani, C.; Carulli, N.; Loria, P. ‘Endocrine NAFLD’ a hormonocentric perspective of nonalcoholic fatty liver disease pathogenesis. J. Hepatol. 2006, 44, 1196–1207. [Google Scholar] [CrossRef]
- Sanders, F.W.; Griffin, J.L. De novo lipogenesis in the liver in health and disease: More than just a shunting yard for glucose. Biol. Rev. Camb. Philos. Soc. 2016, 91, 452–468. [Google Scholar] [CrossRef]
- Hutchison, A.L.; Tavaglione, F.; Romeo, S.; Charlton, M. Endocrine aspects of metabolic dysfunction-associated steatotic liver disease (MASLD): Beyond insulin resistance. J. Hepatol. 2023, 79, 1524–1541. [Google Scholar] [CrossRef]
- Kawano, Y.; Cohen, D.E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 2013, 48, 434–441. [Google Scholar] [CrossRef]
- Naguib, G.; Morris, N.; Yang, S.; Fryzek, N.; Haynes-Williams, V.; Huang, W.A.; Norman-Wheeler, J.; Rotman, Y. Dietary fatty acid oxidation is decreased in non-alcoholic fatty liver disease: A palmitate breath test study. Liver Int. 2020, 40, 590–597. [Google Scholar] [CrossRef]
- Charlton, M.; Sreekumar, R.; Rasmussen, D.; Lindor, K.; Nair, K.S. Apolipoprotein synthesis in nonalcoholic steatohepatitis. Hepatology 2002, 35, 898–904. [Google Scholar] [CrossRef]
- Meda, C.; Dolce, A.; Della Torre, S. Metabolic dysfunction-associated steatotic liver disease across women’s reproductive lifespan and issues. Clin. Mol. Hepatol. 2025, 31, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, M.; Patel, P.; Dunn-Valadez, S.; Dao, C.; Khan, V.; Ali, H.; El-Serag, L.; Hernaez, R.; Sisson, A.; Thrift, A.P.; et al. Women Have a Lower Risk of Nonalcoholic Fatty Liver Disease but a Higher Risk of Progression vs Men: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2021, 19, 61–71.e15. [Google Scholar] [CrossRef]
- Lonardo, A.; Nascimbeni, F.; Ballestri, S.; Fairweather, D.; Win, S.; Than, T.A.; Abdelmalek, M.F.; Suzuki, A. Sex differences in nonalcoholic fatty liver disease: State of the art and identification of research gaps. Hepatology 2019, 70, 1457–1469. [Google Scholar] [CrossRef]
- Cherubini, A.; Della Torre, S.; Pelusi, S.; Valenti, L. Sexual dimorphism of metabolic dysfunction-associated steatotic liver disease. Trends Mol. Med. 2024, 30, 1126–1136. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Bairey Merz, N.; Barnes, P.J.; Brinton, R.D.; Carrero, J.J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and gender: Modifiers of health, disease, and medicine. Lancet 2020, 396, 565–582, Erratum in Lancet 2020, 396, 668. https://doi.org/10.1016/S0140-6736(20)31827-4. [Google Scholar] [CrossRef]
- Lonardo, A.; Suzuki, A. Sexual Dimorphism of NAFLD in Adults. Focus on Clinical Aspects and Implications for Practice and Translational Research. J. Clin. Med. 2020, 9, 1278. [Google Scholar] [CrossRef]
- Suzuki, A.; Diehl, A.M. Nonalcoholic Steatohepatitis. Annu. Rev. Med. 2017, 68, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Burra, P.; Zanetto, A.; Germani, G. Sex bias in clinical trials in gastroenterology and hepatology. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 413–414. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Ballestri, S.; Mantovani, A.; Targher, G.; Bril, F. Endpoints in NASH Clinical Trials: Are We Blind in One Eye? Metabolites 2024, 14, 40. [Google Scholar] [CrossRef]
- Matz-Soja, M.; Berg, T.; Kietzmann, T. Sex-Related Variations in Liver Homeostasis and Disease: From Zonation Dynamics to Clinical Implications. J. Hepatol. 2025, in press. [CrossRef]
- Trefts, E.; Gannon, M.; Wasserman, D.H. The liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef]
- Della Torre, S. Beyond the X Factor: Relevance of Sex Hormones in NAFLD Pathophysiology. Cells 2021, 10, 2502. [Google Scholar] [CrossRef]
- Zhang, J.W.; Zhang, N.; Lyu, Y.; Zhang, X.F. Influence of Sex in the Development of Liver Diseases. Semin. Liver Dis. 2025, 45, 15–32. [Google Scholar] [CrossRef]
- Peterfi, Z.; McGinty, D.; Sarai, E.; Szymusiak, R. Growth hormone-releasing hormone activates sleep regulatory neurons of the rat preoptic hypothalamus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R147–R156. [Google Scholar] [CrossRef]
- Brie, B.; Ramirez, M.C.; De Winne, C.; Lopez Vicchi, F.; Villarruel, L.; Sorianello, E.; Catalano, P.; Ornstein, A.M.; Becu-Villalobos, D. Brain Control of Sexually Dimorphic Liver Function and Disease: The Endocrine Connection. Cell. Mol. Neurobiol. 2019, 39, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Wauthier, V.; Sugathan, A.; Meyer, R.D.; Dombkowski, A.A.; Waxman, D.J. Intrinsic sex differences in the early growth hormone responsiveness of sex-specific genes in mouse liver. Mol. Endocrinol. 2010, 24, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Conforto, T.L.; Waxman, D.J. Sex-specific mouse liver gene expression: Genome-wide analysis of developmental changes from pre-pubertal period to young adulthood. Biol. Sex Differ. 2012, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Laz, E.V.; Sugathan, A.; Waxman, D.J. Dynamic in vivo binding of STAT5 to growth hormone-regulated genes in intact rat liver. Sex-specific binding at low- but not high-affinity STAT5 sites. Mol. Endocrinol. 2009, 23, 1242–1254. [Google Scholar] [CrossRef]
- Martinez, C.S.; Piazza, V.G.; Ratner, L.D.; Matos, M.N.; González, L.; Rulli, S.B.; Miquet, J.G.; Sotelo, A.I. Growth hormone STAT5-mediated signaling and its modulation in mice liver during the growth period. Growth Horm. IGF Res. 2013, 23, 19–28. [Google Scholar] [CrossRef]
- Creasy, K.T.; Jiang, J.; Ren, H.; Peterson, M.L.; Spear, B.T. Zinc Fingers and Homeoboxes 2 (Zhx2) Regulates Sexually Dimorphic Cyp Gene Expression in the Adult Mouse Liver. Gene Expr. 2016, 17, 7–17. [Google Scholar] [CrossRef]
- Fernández-Pérez, L.; de Mirecki-Garrido, M.; Guerra, B.; Díaz, M.; Díaz-Chico, J.C. Sex steroids and growth hormone interactions. Endocrinol. Nutr. 2016, 63, 171–180. [Google Scholar] [CrossRef]
- Roelfsema, F.; Veldhuis, J.D. Growth hormone dynamics in healthy adults are related to age and sex and strongly dependent on body mass index. Neuroendocrinology 2016, 103, 335–344. [Google Scholar] [CrossRef]
- Cvitanovic Tomaš, T.; Urlep, Ž.; Moškon, M.; Mraz, M.; Rozman, D. LiverSex Computational Model: Sexual Aspects in Hepatic Metabolism and Abnormalities. Front. Physiol. 2018, 9, 360. [Google Scholar] [CrossRef] [PubMed]
- Montefusco, D.; Jamil, M.; Maczis, M.A.; Schroeder, W.; Levi, M.; Ranjit, S.; Allegood, J.; Bandyopadhyay, D.; Retnam, R.; Spiegel, S.; et al. Sphingosine kinase 1 mediates sexual dimorphism in fibrosis in a mouse model of NASH. Mol. Metab. 2022, 62, 101523. [Google Scholar] [CrossRef] [PubMed]
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef]
- Gluchowski, N.L.; Becuwe, M.; Walther, T.C.; Farese, R.V., Jr. Lipid droplets and liver disease: From basic biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 343–355. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Barjesteh, F.; Azedi, F.; Alipourfard, I.; Rezaei, Z.; Bahreini, E. Comparative analysis of β-Estradiol and testosterone on lipid droplet accumulation, and regulatory protein expression in palmitate/oleate-induced fatty HepG2 cells. BMC Gastroenterol. 2025, 25, 263. [Google Scholar] [CrossRef] [PubMed]
- Peleman, C.; Hellemans, S.; Veeckmans, G.; Arras, W.; Zheng, H.; Koeken, I.; Van San, E.; Hassannia, B.; Walravens, M.; Kayirangwa, E.; et al. Ferroptosis is a targetable detrimental factor in metabolic dysfunction-associated steatotic liver disease. Cell Death Differ. 2024, 31, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ren, Y.; Deng, L.; Lv, D.; Chen, J.; Ling, Y.; Tu, J.; Xu, X.; Wang, D.; Cai, Z. Testosterone deficiency aggravates diet-induced non-alcoholic fatty liver disease by inducing hepatocyte ferroptosis via targeting BMAL1 in mice. Int. Immunopharmacol. 2025, 144, 113641. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Lonardo, A. PNPLA3 as a driver of steatotic liver disease: Navigating from pathobiology to the clinics via epidemiology. J. Transl. Genet. Genom. 2024, 8, 355–377. [Google Scholar] [CrossRef]
- Cherubini, A.; Rosso, C.; Della Torre, S. Sex-specific effects of PNPLA3 I148M. Liver Int. 2025, 45, e16088. [Google Scholar] [CrossRef]
- Cherubini, A.; Casirati, E.; Pelusi, S.; Valenti, L. Estrogen-ER-α axis induces PNPLA3 p.I148M protein variant to promote steatotic liver disease susceptibility in women. Clin. Transl. Med. 2024, 14, e1524. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Pirola, C.J.; Sookoian, S.; Wilson, L.A.; Liang, T.; Chalasani, N. The Protection Conferred by HSD17B13 rs72613567 Polymorphism on Risk of Steatohepatitis and Fibrosis May Be Limited to Selected Subgroups of Patients With NAFLD. Clin. Transl. Gastroenterol. 2021, 12, e00400. [Google Scholar] [CrossRef]
- Araujo, L.C.C.; Cruz, A.G.; Camargo, F.N.; Sucupira, F.G.; Moreira, G.V.; Matos, S.L.; Amaral, A.G.; Murata, G.M.; Carvalho, C.R.O.; Camporez, J.P. Estradiol Protects Female ApoE KO Mice against Western-Diet-Induced Non-Alcoholic Steatohepatitis. Int. J. Mol. Sci. 2023, 24, 9845. [Google Scholar] [CrossRef] [PubMed]
- Galmés-Pascual, B.M.; Martínez-Cignoni, M.R.; Morán-Costoya, A.; Bauza-Thorbrügge, M.; Sbert-Roig, M.; Valle, A.; Proenza, A.M.; Lladó, I.; Gianotti, M. 17β-estradiol ameliorates lipotoxicity-induced hepatic mitochondrial oxidative stress and insulin resistance. Free Radic. Biol. Med. 2020, 150, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Besse-Patin, A.; Léveillé, M.; Oropeza, D.; Nguyen, B.N.; Prat, A.; Estall, J.L. Estrogen Signals Through Peroxisome Proliferator-Activated Receptor-γ Coactivator 1α to Reduce Oxidative Damage Associated With Diet-Induced Fatty Liver Disease. Gastroenterology 2017, 152, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Li, Y.; Zeng, N.; He, L.; Zhang, X.; Tu, T.; Tang, Q.; Alba, M.; Mir, S.; Stiles, E.X.; et al. Inhibition of Estrogen-Related Receptor α Blocks Liver Steatosis and Steatohepatitis and Attenuates Triglyceride Biosynthesis. Am. J. Pathol. 2021, 191, 1240–1254. [Google Scholar] [CrossRef]
- Yang, M.; Liu, Q.; Huang, T.; Tan, W.; Qu, L.; Chen, T.; Pan, H.; Chen, L.; Liu, J.; Wong, C.W.; et al. Dysfunction of estrogen-related receptor alpha-dependent hepatic VLDL secretion contributes to sex disparity in NAFLD/NASH development. Theranostics 2020, 10, 10874–10891. [Google Scholar] [CrossRef]
- Tian, Y.; Xie, Y.; Hong, X.; Guo, Z.; Yu, Q. 17β-Estradiol protects female rats from bilateral oophorectomy-induced nonalcoholic fatty liver disease induced by improving linoleic acid metabolism alteration and gut microbiota disturbance. Heliyon 2024, 10, e29013. [Google Scholar] [CrossRef]
- Saigo, Y.; Sasase, T.; Uno, K.; Shinozaki, Y.; Maekawa, T.; Sano, R.; Toriniwa, Y.; Miyajima, K.; Ohta, T. Establishment of a new nonalcoholic steatohepatitis model; Ovariectomy exacerbates nonalcoholic steatohepatitis-like pathology in diabetic rats. J. Pharmacol. Toxicol. Methods. 2022, 116, 107190. [Google Scholar] [CrossRef] [PubMed]
- Meda, C.; Barone, M.; Mitro, N.; Lolli, F.; Pedretti, S.; Caruso, D.; Maggi, A.; Della Torre, S. Hepatic ERα accounts for sex differences in the ability to cope with an excess of dietary lipids. Mol. Metab. 2020, 32, 97–108. [Google Scholar] [CrossRef]
- Meda, C.; Dolce, A.; Vegeto, E.; Maggi, A.; Della Torre, S. ERα-Dependent Regulation of Adropin Predicts Sex Differences in Liver Homeostasis during High-Fat Diet. Nutrients 2022, 14, 3262. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fu, Q.; Li, T.; Shao, K.; Zhu, X.; Cong, Y.; Zhao, X. Gut microbiota and butyrate contribute to nonalcoholic fatty liver disease in premenopause due to estrogen deficiency. PLoS ONE 2022, 17, e0262855. [Google Scholar] [CrossRef] [PubMed]
- Meda, C.; Benedusi, V.; Cherubini, A.; Valenti, L.; Maggi, A.; Della Torre, S. Hepatic estrogen receptor alpha drives masculinization in post-menopausal women with metabolic dysfunction-associated steatotic liver disease. JHEP Rep. 2024, 6, 101143. [Google Scholar] [CrossRef]
- Toniutto, P.; Shalaby, S.; Mameli, L.; Morisco, F.; Gambato, M.; Cossiga, V.; Guarino, M.; Marra, F.; Brunetto, M.R.; Burra, P.; et al. Role of sex in liver tumor occurrence and clinical outcomes: A comprehensive review. Hepatology 2024, 79, 1141–1157. [Google Scholar] [CrossRef]
- Naugler, W.E.; Sakurai, T.; Kim, S.; Maeda, S.; Kim, K.; Elsharkawy, A.M.; Karin, M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 2007, 317, 121–124, Erratum in Science 2009, 326, 1346. [Google Scholar] [CrossRef]
- Shi, L.; Feng, Y.; Lin, H.; Ma, R.; Cai, X. Role of estrogen in hepatocellular carcinoma: Is inflammation the key? J. Transl. Med. 2014, 12, 93. [Google Scholar] [CrossRef]
- Ma, W.L.; Hsu, C.L.; Wu, M.H.; Wu, C.T.; Wu, C.C.; Lai, J.J.; Jou, Y.S.; Chen, C.W.; Yeh, S.; Chang, C. Androgen receptor is a new potential therapeutic target for the treatment of hepatocellular carcinoma. Gastroenterology 2008, 135, 947–955.e5, Erratum in Gastroenterology 2008, 135, 1805. [Google Scholar] [CrossRef]
- Petrick, J.L.; Florio, A.A.; Zhang, X.; Zeleniuch-Jacquotte, A.; Wactawski-Wende, J.; Van Den Eeden, S.K.; Stanczyk, F.Z.; Simon, T.G.; Sinha, R.; Sesso, H.D.; et al. Associations Between Prediagnostic Concentrations of Circulating Sex Steroid Hormones and Liver Cancer Among Postmenopausal Women. Hepatology 2020, 72, 535–547. [Google Scholar] [CrossRef]
- Jackson, S.S.; Pfeiffer, R.M.; Gabbi, C.; Anderson, L.; Gadalla, S.M.; Koshiol, J. Menopausal hormone therapy and risk of biliary tract cancers. Hepatology 2022, 75, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Kasarinaite, A.; Sinton, M.; Saunders, P.T.K.; Hay, D.C. The influence of sex hormones in liver function and disease. Cells 2023, 12, 1604. [Google Scholar] [CrossRef]
- Jeong, K.J.; Mukae, M.; Lee, S.R.; Kim, S.Y.; Kim, S.H.; Cho, Y.E.; An, B.S.; Ko, J.W.; Kwun, H.J.; Baek, I.J.; et al. Progesterone increases hepatic lipid content and plasma lipid levels through PR- B-mediated lipogenesis. Biomed. Pharmacother. 2024, 172, 116281. [Google Scholar] [CrossRef]
- Fedotcheva, N.I.; Teplova, V.V.; Fedotcheva, T.A.; Rzheznikov, V.M.; Shimanovskii, N.L. Effect of progesterone and its synthetic analogues on the activity of mitochondrial permeability transition pore in isolated rat liver mitochondria. Biochem. Pharmacol. 2009, 78, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Nunes, V.A.; Portioli-Sanches, E.P.; Rosim, M.P.; Araujo, M.S.; Praxedes-Garcia, P.; Valle, M.M.; Roma, L.P.; Hahn, C.; Gurgul-Convey, E.; Lenzen, S.; et al. Progesterone induces apoptosis of insulin-secreting cells: Insights into the molecular mechanism. J. Endocrinol. 2014, 221, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Hori, S.; Sugiyama, M.; Fujisawa, E.; Nakano, T.; Tsuneki, H.; Nagira, K.; Saito, S.; Sasaoka, T. Progesterone inhibits glucose uptake by affecting diverse steps of insulin signaling in 3T3-L1 adipocytes. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E881–E888. [Google Scholar] [CrossRef]
- Czogalla, B.; Kahaly, M.; Mayr, D.; Schmoeckel, E.; Niesler, B.; Hester, A.; Zeder-Göß, C.; Kolben, T.; Burges, A.; Mahner, S.; et al. Correlation of NRF2 and progesterone receptor and its effects on ovarian cancer biology. Cancer Manag. Res. 2019, 11, 7673–7684. [Google Scholar] [CrossRef]
- Oztekin, E.; Tiftik, A.M.; Baltaci, A.K.; Mogulkoc, R. Lipid peroxidation in liver tissue of ovariectomized and pinealectomized rats: Effect of estradiol and progesterone supplementation. Cell Biochem. Funct. 2007, 25, 401–405. [Google Scholar] [CrossRef]
- Lorenzo, M.; Roncero, C.; Benito, M. The role of prolactin and progesterone in the regulation of lipogenesis in maternal and foetal rat liver in vivo and in isolated hepatocytes during the last day of gestation. Biochem. J. 1986, 239, 135–139. [Google Scholar] [CrossRef]
- Lee, S.R.; Kwon, S.W.; Kaya, P.; Lee, Y.H.; Lee, J.G.; Kim, G.; Lee, G.S.; Baek, I.J.; Hong, E.J. Loss of progesterone receptor membrane component 1 promotes hepatic steatosis via the induced de novo lipogenesis. Sci. Rep. 2018, 8, 15711. [Google Scholar] [CrossRef]
- Jaffe, C.A.; Ocampo-Lim, B.; Guo, W.; Krueger, K.; Sugahara, I.; DeMott-Friberg, R.; Bermann, M.; Barkan, A.L. Regulatory mechanisms of growth hormone secretion are sexually dimorphic. J. Clin. Investig. 1998, 102, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, C.; Fontanaud, P.; He, Z.; Lafont, C.; Meunier, A.C.; Schaeffer, M.; Carmignac, D.; Molino, F.; Coutry, N.; Bonnefont, X.; et al. Pituitary growth hormone network responses are sexually dimorphic and regulated by gonadal steroids in adulthood. Proc. Natl. Acad. Sci. USA 2010, 107, 21878–21883. [Google Scholar] [CrossRef]
- Meyer, R.D.; Laz, E.V.; Su, T.; Waxman, D.J. Male-specific hepatic Bcl6: Growth hormone-induced block of transcription elongation in females and binding to target genes inversely coordinated with STAT5. Mol. Endocrinol. 2009, 23, 1914–1926. [Google Scholar] [CrossRef]
- Rampersaud, A.; Connerney, J.; Waxman, D.J. Plasma Growth Hormone Pulses Induce Male-biased Pulsatile Chromatin Opening and Epigenetic Regulation in Adult Mouse Liver. bioRxiv 2023. Update in Elife 2023, 12, RP91367. https://doi.org/10.7554/eLife.91367. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Borrego, M.C.; Del Rio-Moreno, M.; Kineman, R.D. Towards Understanding the Direct and Indirect Actions of Growth Hormone in Controlling Hepatocyte Carbohydrate and Lipid Metabolism. Cells 2021, 10, 2532. [Google Scholar] [CrossRef]
- Costa, D.N.; Santosa, S.; Jensen, M.D. Sex differences in the metabolism of glucose and fatty acids by adipose tissue and skeletal muscle in humans. Physiol. Rev. 2025, 105, 897–934. [Google Scholar] [CrossRef]
- List, E.O.; Basu, R.; Berryman, D.E.; Duran-Ortiz, S.; Martos-Moreno, G.Á.; Kopchick, J.J. Common and Uncommon Mouse Models of Growth Hormone Deficiency. Endocr. Rev. 2024, 45, 818–842. [Google Scholar] [CrossRef]
- Kineman, R.D.; Del Rio-Moreno, M.; Waxman, D.J. Liver-specific actions of GH and IGF1 that protect against MASLD. Nat. Rev. Endocrinol. 2025, 21, 105–117. [Google Scholar] [CrossRef]
- Dutta, D.; Nagendra, L.; Mohindra, R.; Bhattacharya, S.; Joshi, A.; Kamrul-Hasan, A. Role of Growth Hormone Therapy in Metabolic-Dysfunction-Associated Steatotic Liver Disease: A Systematic Review and Meta-Analysis. Indian J. Endocrinol. Metab. 2024, 28, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, I.; Gautam, M.; Abosheaishaa, H.; Hussain, S.; Kumar, K.; Kotak, A.; Baugh, M.; Qureshi, R.; Jaber, F.; Dahiya, D.S.; et al. Growth hormone augmentation in metabolic dysfunction-associated steatotic liver disease: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Gastroenterol. Hepatol. 2024, 36, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.; Krusenstjerna-Hafstrøm, T.; Møller, L.; Christensen, B.; Vendelbo, M.H.; Pedersen, S.B.; Frystyk, J.; Jessen, N.; Hansen, T.K.; Stødkilde-Jørgensen, H.; et al. Fat content in liver and skeletal muscle changes in a reciprocal manner in patients with acromegaly during combination therapy with a somatostatin analog and a GH receptor antagonist: A randomized clinical trial. J. Clin. Endocrinol. Metab. 2012, 97, 1227–1235. [Google Scholar] [CrossRef]
- Koutsou-Tassopoulou, A.; Papapostoli-Sklavounou, I.; Krawczyk, M.; Friesenhahn-Ochs, B.; Weber, S.N.; Lammert, F.; Stokes, C.S. Hepatic steatosis in patients with acromegaly. Endocrinol. Diabetes Metab. 2019, 2, e00090. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.K.; O’Sullivan, A.J.; Burt, M.G. The physiology of growth hormone (GH) in adults: Translational journey to GH replacement therapy. J. Endocrinol. 2023, 257, e220197. [Google Scholar] [CrossRef] [PubMed]
- Nijenhuis-Noort, E.C.; Berk, K.A.; Neggers, S.J.C.M.M.; Lely, A.J.V. The Fascinating Interplay between Growth Hormone, Insulin-Like Growth Factor-1, and Insulin. Endocrinol. Metab. 2024, 39, 83–89. [Google Scholar] [CrossRef]
- Fröhlich, E.; Wahl, R. Insight into potential interactions of thyroid hormones, sex hormones and their stimulating hormones in the development of non-alcoholic fatty liver disease. Metabolites 2022, 12, 718. [Google Scholar] [CrossRef] [PubMed]
- Köhrle, J.; Frädrich, C. Deiodinases control local cellular and systemic thyroid hormone availability. Free Radic. Biol. Med. 2022, 193 Pt 1, 59–79. [Google Scholar] [CrossRef]
- Flood, D.E.; Fernandino, J.I.; Langlois, V.S. Thyroid hormones in male reproductive development: Evidence for direct crosstalk between the androgen and thyroid hormone axes. Gen. Comp. Endocrinol. 2013, 192, 2–14. [Google Scholar] [CrossRef]
- Brown, E.D.L.; Obeng-Gyasi, B.; Hall, J.E.; Shekhar, S. The Thyroid Hormone Axis and Female Reproduction. Int. J. Mol. Sci. 2023, 24, 9815. [Google Scholar] [CrossRef]
- Hatziagelaki, E.; Paschou, S.A.; Schön, M.; Psaltopoulou, T.; Roden, M. NAFLD and thyroid function: Pathophysiological and therapeutic considerations. Trends Endocrinol. Metab. 2022, 33, 755–768. [Google Scholar] [CrossRef]
- Delitala, A.P.; Steri, M.; Pilia, M.G.; Dei, M.; Lai, S.; Delitala, G.; Schlessinger, D.; Cucca, F. Menopause modulates the association between thyrotropin levels and lipid parameters: The SardiNIA study. Maturitas 2016, 92, 30–34. [Google Scholar] [CrossRef]
- Fan, H.; Ren, Q.; Sheng, Z.; Deng, G.; Li, L. The role of the thyroid in polycystic ovary syndrome. Front. Endocrinol. 2023, 14, 1242050. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Abdelmalek, M.F.; Pang, H.; Guy, C.D.; Smith, A.D.; Diehl, A.M.; Suzuki, A. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology 2014, 59, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Klair, J.S.; Yang, J.D.; Abdelmalek, M.F.; Guy, C.D.; Gill, R.M.; Yates, K.; Unalp-Arida, A.; Lavine, J.E.; Clark, J.M.; Diehl, A.M.; et al. A longer duration of estrogen deficiency increases fibrosis risk among postmenopausal women with nonalcoholic fatty liver disease. Hepatology 2016, 64, 85–91. [Google Scholar] [CrossRef]
- Sarkar, M.; Wellons, M.; Cedars, M.I.; VanWagner, L.; Gunderson, E.P.; Ajmera, V.; Torchen, L.; Siscovick, D.; Carr, J.J.; Terry, J.G.; et al. Testosterone Levels in Pre-Menopausal Women are Associated With Nonalcoholic Fatty Liver Disease in Midlife. Am. J. Gastroenterol. 2017, 112, 755–762. [Google Scholar] [CrossRef]
- Yang, J.D.; Abdelmalek, M.F.; Guy, C.D.; Gill, R.M.; Lavine, J.E.; Yates, K.; Klair, J.; Terrault, N.A.; Clark, J.M.; Unalp-Arida, A.; et al. Patient Sex, Reproductive Status, and Synthetic Hormone Use Associate With Histologic Severity of Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2017, 15, 127–131.e2. [Google Scholar] [CrossRef] [PubMed]
- Minato, S.; Sakane, N.; Kotani, K.; Nirengi, S.; Hayashi, I.; Suganuma, A.; Yamaguchi, K.; Takakura, K.; Nagai, N. Prevalence and risk factors of elevated liver enzymes in Japanese women with polycystic ovary syndrome. J. Clin. Med. Res. 2018, 10, 904–910. [Google Scholar] [CrossRef]
- Mueller, N.T.; Liu, T.; Mitchel, E.B.; Yates, K.P.; Suzuki, A.; Behling, C.; Lavine, J.E. Sex hormone relations to histologic severity of pediatric nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 2020, 105, 3496–3504. [Google Scholar] [CrossRef]
- Sarkar, M.; Yates, K.; Suzuki, A.; Lavine, J.; Gill, R.; Ziegler, T.; Terrault, N.; Dhindsa, S. Low testosterone is associated with nonalcoholic steatohepatitis and fibrosis severity in men. Clin. Gastroenterol. Hepatol. 2021, 19, 400–402.e2. [Google Scholar] [CrossRef]
- Wang, J.; Wu, A.H.; Stanczyk, F.Z.; Porcel, J.; Noureddin, M.; Terrault, N.A.; Wilkens, L.R.; Setiawan, V.W. Associations between reproductive and hormone-related factors and risk of nonalcoholic fatty liver disease in a multiethnic population. Clin. Gastroenterol. Hepatol. 2021, 19, 1258–1266.e1. [Google Scholar] [CrossRef]
- Dilimulati, D.; Cai, M.; Lin, Z.; Zhang, Y.; Du, L.; Zhou, D.; Zhu, J.; Su, L.; Wang, Y.; Zhang, M.; et al. Correlation between sex hormones and non-alcoholic fatty liver disease before and after laparoscopic sleeve gastrectomy. Obes. Surg. 2021, 31, 4901–4910. [Google Scholar] [CrossRef]
- Mo, M.Q.; Huang, Z.C.; Yang, Z.H.; Liao, Y.H.; Xia, N.; Pan, L. Relationship between total testosterone, sex hormone-binding globulin levels and the severity of non-alcoholic fatty liver disease in males: A meta-analysis. Ther. Adv. Endocrinol. Metab. 2022, 13, 20420188221106879. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Xu, Y.; Shen, Y.; Wang, Y.; Ma, X.; Bao, Y. Associations between sex hormones and metabolic-associated fatty liver disease in a middle-aged and elderly community. Endocr. J. 2022, 69, 1007–1014. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, J.; Liu, Q.; Ye, Y.; Guo, W.; Cui, J.; He, Q.; Feng, W.; Liu, M. Low Serum total testosterone is associated with non-alcoholic fatty liver disease in men but not in women with type 2 diabetes mellitus. Int. J. Endocrinol. 2022, 2022, 8509204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, C.; Wang, Y.; Wang, N.; Chen, Y.; Lu, Y.; Xia, F. The associations of total testosterone with probable nonalcoholic steatohepatitis and nonalcoholic fatty liver disease fibrotic progression in men with type 2 diabetes: A cross-sectional study. Eur. J. Med. Res. 2022, 27, 307. [Google Scholar] [CrossRef]
- Yang, L.J.; Zhou, J.Z.; Zheng, Y.F.; Hu, X.; He, Z.Y.; Du, L.J.; Gu, X.; Huang, X.Y.; Li, J.; Li, Y.Q.; et al. Association of non-alcoholic fatty liver disease with total testosterone in non-overweight/obese men with type 2 diabetes mellitus. J. Endocrinol. Investig. 2023, 46, 1565–1572. [Google Scholar] [CrossRef]
- De Herdt, C.; De Block, C.; Francque, S.; Verrijken, A.; Van Dessel, K.; Van Gaal, L.; Van Cauwenberghe, J.; Dirinck, E. A cross-sectional analysis of the association between testosterone and biopsy-proven non-alcoholic fatty liver disease in men with obesity. Endocrine 2023, 80, 54–63. [Google Scholar] [CrossRef]
- Apostolov, R.; Wong, D.; Low, E.; Vaz, K.; Spurio, J.; Worland, T.; Liu, D.; Chan, R.K.; Gow, P.; Grossmann, M.; et al. Testosterone is lower in men with non-alcoholic fatty liver disease and alcohol-related cirrhosis and is associated with adverse clinical outcomes. Scand. J. Gastroenterol. 2023, 58, 1328–1334. [Google Scholar] [CrossRef]
- Cai, X.; Thorand, B.; Hohenester, S.; Prehn, C.; Cecil, A.; Adamski, J.; Zeller, T.; Dennis, A.; Banerjee, R.; Peters, A.; et al. Association of sex hormones and sex hormone-binding globulin with liver fat in men and women: An observational and Mendelian randomization study. Front. Endocrinol. 2023, 14, 1223162. [Google Scholar] [CrossRef]
- Maldonado, S.S.; Cedars, M.I.; Yates, K.P.; Wilson, L.A.; Gill, R.; Terrault, N.A.; Suzuki, A.; Sarkar, M.A. Antimullerian hormone, a marker of ovarian reserve, is protective against presence and severity of NASH in premenopausal women. Clin. Gastroenterol. Hepatol. 2024, 22, 339–346.e5. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.; Shao, Z.; Xiao, M.; Song, M.; Zhao, Y.; Li, A.; Pang, Y.; Huang, T.; Yu, C.; Lv, J.; et al. Association of sex hormones with non-alcoholic fatty liver disease: An observational and Mendelian randomization study. Liver Int. 2024, 44, 1154–1166. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, S.; Zhang, S.; Yang, Y.; Li, S.; Zhang, M.; Li, X.; Bai, H.; Luo, P.; Yuan, Y. The value of sex hormones and sex hormone-binding globulin in metabolic dysfunction-associated fatty liver disease among boys with obesity. Front. Endocrinol. 2025, 16, 1446049. [Google Scholar] [CrossRef]
- Christ, J.P.; Cedars, M.I. Current Guidelines for Diagnosing PCOS. Diagnostics 2023, 13, 1113. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, J.; Lu, Y.; Wu, L. Association of metabolic-dysfunction associated steatotic liver disease with polycystic ovary syndrome. iScience 2024, 27, 108783. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Lonardo, A. The Ovary–Liver Axis: Molecular Science and Epidemiology. Int. J. Mol. Sci. 2025, 26, 6382. [Google Scholar] [CrossRef]
- Watling, C.Z.; Kelly, R.K.; Watts, E.L.; Graubard, B.I.; Petrick, J.L.; Matthews, C.E.; McGlynn, K.A. Total testosterone, sex hormone-binding globulin, and free testosterone concentrations and risk of primary liver cancer: A prospective analysis of 200,000 men and 180,000 postmenopausal women. Int. J. Cancer 2025, 156, 1518–1528. [Google Scholar] [CrossRef]
- Hiyoshi, T.; Fujiwara, M.; Yao, Z. Postprandial hyperglycemia and postprandial hypertriglyceridemia in type 2 diabetes. J. Biomed. Res. 2017, 33, 1–16. [Google Scholar] [CrossRef]
- Masango, B.; Goedecke, J.H.; Ramsay, M.; Storbeck, K.H.; Micklesfield, L.K.; Chikowore, T. Postprandial glucose variability and clusters of sex hormones, liver enzymes, and cardiometabolic factors in a South African cohort of African ancestry. BMJ Open Diabetes Res. Care 2024, 12, e003927. [Google Scholar] [CrossRef] [PubMed]
- Lorek, D.; Łupina, K.; Bisaga, W.; Malicki, D.; Stępień, W.; Kumor, L.; Janczura, J. The socioeconomic and environmental determinants of metabolic dysfunction-associated steatotic liver disease: Understanding inequalities in prevalence and outcomes. Korean J. Fam. Med. 2025, 46, 61–69. [Google Scholar] [CrossRef]
- Bagheri Lankarani, K.; Jamalinia, M.; Zare, F.; Heydari, S.T.; Ardekani, A.; Lonardo, A. Liver-kidney-metabolic health, sex, and menopause impact total scores and monovessel vs. multivessel coronary artery calcification. Adv. Ther. 2025, 42, 1729–1744. [Google Scholar] [CrossRef] [PubMed]
- Jamalinia, M.; Zare, F.; Mantovani, A.; Targher, G.; Lonardo, A. Metabolic dysfunction-associated steatotic liver disease and sex-specific risk of fatal and non-fatal cardiovascular events: A meta-analysis. Diabetes Obes. Metab. 2025, 27, 5171–5181. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.; Mitchell, B.D. A Guide to understanding Mendelian randomization studies. Arthritis Care Res. 2024, 76, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Gao, X.; Pan, X.F.; Zhou, T.; Zhu, C.; Li, F.; Fan, J.G.; Targher, G.; Zhao, J. The hepato-ovarian axis: Genetic evidence for a causal association between non-alcoholic fatty liver disease and polycystic ovary syndrome. BMC Med. 2023, 21, 62. [Google Scholar] [CrossRef]
- Grimbert, S.; Fisch, C.; Deschamps, D.; Berson, A.; Fromenty, B.; Feldmann, G.; Pessayre, D. Effects of female sex hormones on mitochondria: Possible role in acute fatty liver of pregnancy. Am. J. Physiol. 1995, 268 Pt 1, G107–G115. [Google Scholar] [CrossRef]
- Sarkar, M.; Kushner, T. Metabolic dysfunction-associated steatotic liver disease and pregnancy. J. Clin. Investig. 2025, 135, e186426. [Google Scholar] [CrossRef]
- Shao, S.; Yao, Z.; Lu, J.; Song, Y.; He, Z.; Yu, C.; Zhou, X.; Zhao, L.; Zhao, J.; Gao, L. Ablation of prolactin receptor increases hepatic triglyceride accumulation. Biochem. Biophys. Res. Commun. 2018, 498, 693–699. [Google Scholar] [CrossRef]
- Physiology, Lactation—StatPearls—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499981/ (accessed on 22 September 2025).
- Lonardo, A.; Suzuki, A. Concise review: Breastfeeding, lactation, and NAFLD. An updated view of cross-generational disease transmission and prevention. Metab. Target. Organ. Damage 2023, 3, 16. [Google Scholar] [CrossRef]
- Jaroenlapnopparat, A.; Charoenngam, N.; Ponvilawan, B.; Mariano, M.; Thongpiya, J.; Yingchoncharoen, P. Menopause is associated with increased prevalence of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Menopause 2023, 30, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Park, Y.E.; Lee, J.; Choi, J.H.; Heo, N.Y.; Park, J.; Kim, T.O.; Moon, Y.S.; Kim, H.K.; Jang, H.J.; et al. Lack of association between early menopause and non-alcoholic fatty liver disease in postmenopausal women. Climacteric 2020, 23, 173–177. [Google Scholar] [CrossRef]
- Wegermann, K.; Garrett, M.E.; Zheng, J.; Coviello, A.; Moylan, C.A.; Abdelmalek, M.F.; Chow, S.C.; Guy, C.D.; Diehl, A.M.; Ashley-Koch, A.; et al. Sex and Menopause Modify the Effect of Single Nucleotide Polymorphism Genotypes on Fibrosis in NAFLD. Hepatol. Commun. 2021, 5, 598–607. [Google Scholar] [CrossRef]
- Bassil, N.; Alkaade, S.; Morley, J.E. The benefits and risks of testosterone replacement therapy: A review. Ther. Clin. Risk Manag. 2009, 5, 427–448. [Google Scholar] [CrossRef]
- Apostolov, R.; Gianatti, E.; Wong, D.; Kutaiba, N.; Gow, P.; Grossmann, M.; Sinclair, M. Testosterone therapy reduces hepatic steatosis in men with type 2 diabetes and low serum testosterone concentrations. World J. Hepatol. 2022, 14, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.; Brady, C.W.; Fleckenstein, J.; Forde, K.A.; Khungar, V.; Molleston, J.P.; Afshar, Y.; Terrault, N.A. Reproductive Health and Liver Disease: Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021, 73, 318–365. [Google Scholar] [CrossRef] [PubMed]
- Tapper, E.B.; Chen, X.; Parikh, N.D. Testosterone Replacement Reduces Morbidity and Mortality for Most Patients With Cirrhosis. Clin. Gastroenterol. Hepatol. 2025, in press. [CrossRef]
- Lonardo, A.; Weiskirchen, R. Testosterone rescue for failing livers? Target-trial signals survival gains in hypogonadic men with cirrhosis. Adv. Transl. Med. 2025, 4, 1–4. [Google Scholar] [CrossRef]
- Raina, R.; Pahlajani, G.; Khan, S.; Gupta, S.; Agarwal, A.; Zippe, C.D. Female sexual dysfunction: Classification, pathophysiology, and management. Fertil. Steril. 2007, 88, 1273–1284. [Google Scholar] [CrossRef]
- British Menopause Society. Tools for Clinicians. Available online: https://thebms.org.uk/wp-content/uploads/2022/12/08-BMS-TfC-Testosterone-replacement-in-menopause-DEC2022-A.pdf (accessed on 22 September 2025).
- Kim, S.E.; Min, J.S.; Lee, S.; Lee, D.Y.; Choi, D. Different effects of menopausal hormone therapy on non-alcoholic fatty liver disease based on the route of estrogen administration. Sci. Rep. 2023, 13, 15461. [Google Scholar] [CrossRef]
- Lake, J.E.; Hyatt, A.N.; Feng, H.; Miao, H.; Somasunderam, A.; Utay, N.S.; Corey, K.E. Transgender Women with HIV Demonstrate Unique Non-Alcoholic Fatty Liver Disease Profiles. Transgend. Health 2024, 9, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Steiger, A.; VanderVeen, N.T.; Kang, E.H.; Weimer, A.K. Resolution of metabolic dysfunction-associated steatohepatitis with estradiol in a transgender female: A case report. JPGN Rep. 2024, 5, 223–227. [Google Scholar] [CrossRef]
- Gravholt, C.H.; Andersen, N.H.; Conway, G.S.; Dekkers, O.M.; Geffner, M.E.; Klein, K.O.; Lin, A.E.; Mauras, N.; Quigley, C.A.; Rubin, K.; et al. Clinical practice guidelines for the care of girls and women with Turner syndrome: Proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur. J. Endocrinol. 2017, 177, G1–G70. [Google Scholar] [CrossRef]
- Fedor, I.; Zold, E.; Barta, Z. Liver Abnormalities in Turner Syndrome: The Importance of Estrogen Replacement. J. Endocr. Soc. 2022, 6, bvac124. [Google Scholar] [CrossRef]
- Singh, I.; Noel, G.; Barker, J.M.; Chatfield, K.C.; Furniss, A.; Khanna, A.D.; Nokoff, N.J.; Patel, S.; Pyle, L.; Nahata, L.; et al. Hepatic abnormalities in youth with Turner syndrome. Liver Int. 2022, 42, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Twohig, P.; Li, L.; Danford, D.; Craft, M.; Yetman, A.T. Prevalence of hepatic steatosis and fibrosis in Turner syndrome: A prospective case-control study. Liver Int. 2024, 44, 1309–1315. [Google Scholar] [CrossRef]
- Ridder, L.O.R.; Just, J.; Hvas, C.L.; Nielsen, M.M.; Møller, H.J.; Grønbæk, H.; Gravholt, C.H. Elevated Liver Enzymes in Turner Syndrome: The Role of Low-grade Inflammation and Hormonal Imbalances. J. Endocr. Soc. 2025, 9, bvaf059. [Google Scholar] [CrossRef] [PubMed]
- Progestins—LiverTox—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548237/ (accessed on 22 September 2025).
- Garriga, M.; Zhang, G.; Sarkar, M. Contraception in patients with liver disease and liver transplant. Clin. Liver Dis. 2024, 23, e0104. [Google Scholar] [CrossRef]
- Floreani, A.; Gabbia, D.; De Martin, S. Are gender differences important for autoimmune liver diseases? Life 2024, 14, 500. [Google Scholar] [CrossRef]
- Khan, D.; Ansar Ahmed, S. The immune system is a natural target for estrogen action: Opposing effects of estrogen in two prototypical autoimmune diseases. Front. Immunol. 2016, 6, 635. [Google Scholar] [CrossRef]
- Henze, L.; Will, N.; Lee, D.; Haas, V.; Casar, C.; Meyer, J.; Stein, S.; Mangler, F.; Steinmann, S.; Poch, T.; et al. Testosterone affects female CD4+ T cells in healthy individuals and autoimmune liver diseases. JCI Insight 2025, 10, e184544. [Google Scholar] [CrossRef]
- Erikainen, S.; Chan, S. Contested futures: Envisioning “Personalized,” “Stratified,” and “Precision” medicine. New Genet. Soc. 2019, 38, 308–330. [Google Scholar] [CrossRef]
- Hochmuth, L.; Körner, C.; Ott, F.; Volke, D.; Cokan, K.B.; Juvan, P.; Brosch, M.; Hofmann, U.; Hoffmann, R.; Rozman, D.; et al. Sex-dependent dynamics of metabolism in primary mouse hepatocytes. Arch. Toxicol. 2021, 95, 3001–3013. [Google Scholar] [CrossRef]
- Dileo, E.; Saba, F.; Parasiliti-Caprino, M.; Rosso, C.; Bugianesi, E. Impact of Sexual Dimorphism on Therapy Response in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease: From Conventional and Nutritional Approaches to Emerging Therapies. Nutrients 2025, 17, 477. [Google Scholar] [CrossRef] [PubMed]
- Burnside, J.; Cinque, F.; Sebastiani, G.; Ramji, A.; Patel, K.; Swain, M.; Saeed, S. Sex differences in the prevalence and cardiometabolic risk profiles of steatotic liver disease: A Canadian Longitudinal Study on Aging analysis. Can. J. Public Health 2025. [Google Scholar] [CrossRef]
- Wang, C.; Ma, H.; Yang, H.; Nie, Q.; Zhu, L.; Yin, J.; Zhou, L. Sex differences in the association between total energy intake and all-cause mortality among patients with metabolic dysfunction-associated steatotic liver disease. Sci. Rep. 2025, 15, 19176. [Google Scholar] [CrossRef]
- Penmetsa, R.; Kapil, S.; VanWagner, L.B. Sex and gender differences in metabolic dysfunction-associated liver disease. Indian J Gastroenterol. 2025. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef] [PubMed]
- Sohn, W.; Lee, Y.S.; Kim, S.S.; Kim, J.H.; Jin, Y.J.; Kim, G.A.; Sung, P.S.; Yoo, J.J.; Chang, Y.; Lee, E.J.; et al. KASL clinical practice guidelines for the management of metabolic dysfunction-associated steatotic liver disease 2025. Clin. Mol. Hepatol. 2025, 31, S1–S31. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.A.; Arab, J.P.; Idalsoaga, F.; Perelli, J.; Vega, J.; Dirchwolf, M.; Carreño, J.; Samith, B.; Valério, C.; Moreira, R.O.; et al. Updated recommendations for the management of metabolic dysfunction-associated steatotic liver disease (MASLD) by the Latin American working group. Ann. Hepatol. 2025, 30, 101903. [Google Scholar] [CrossRef]
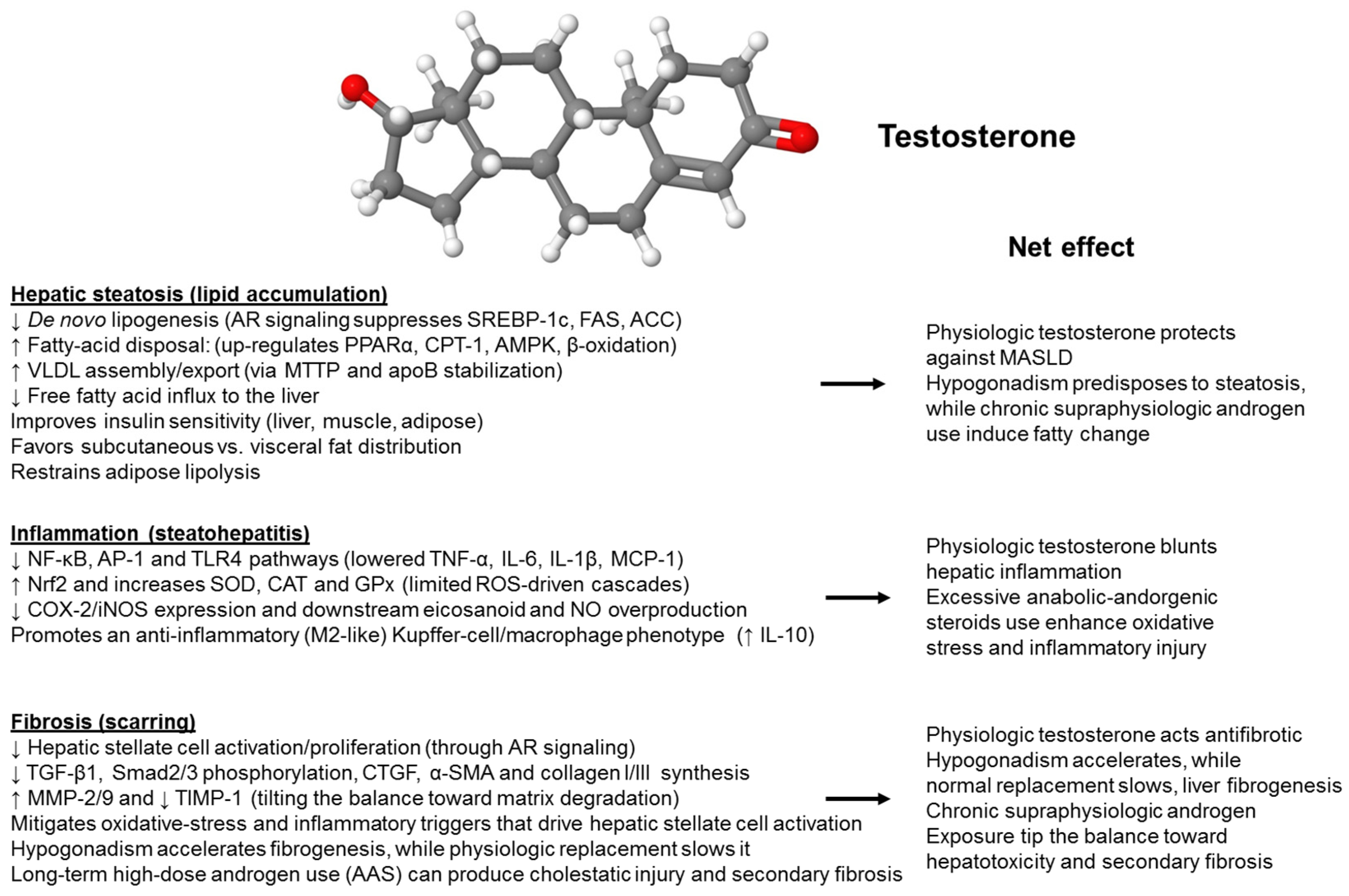
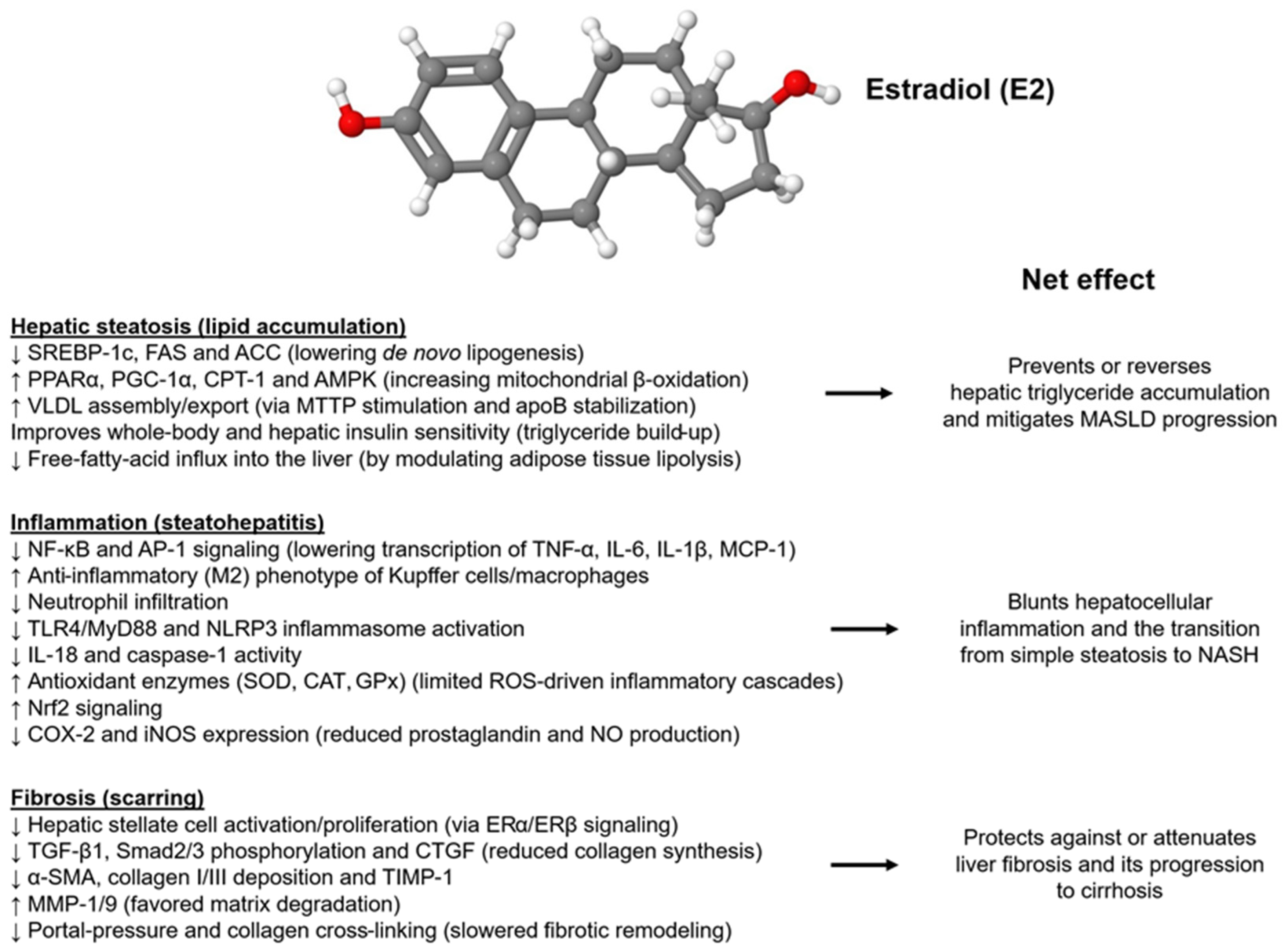
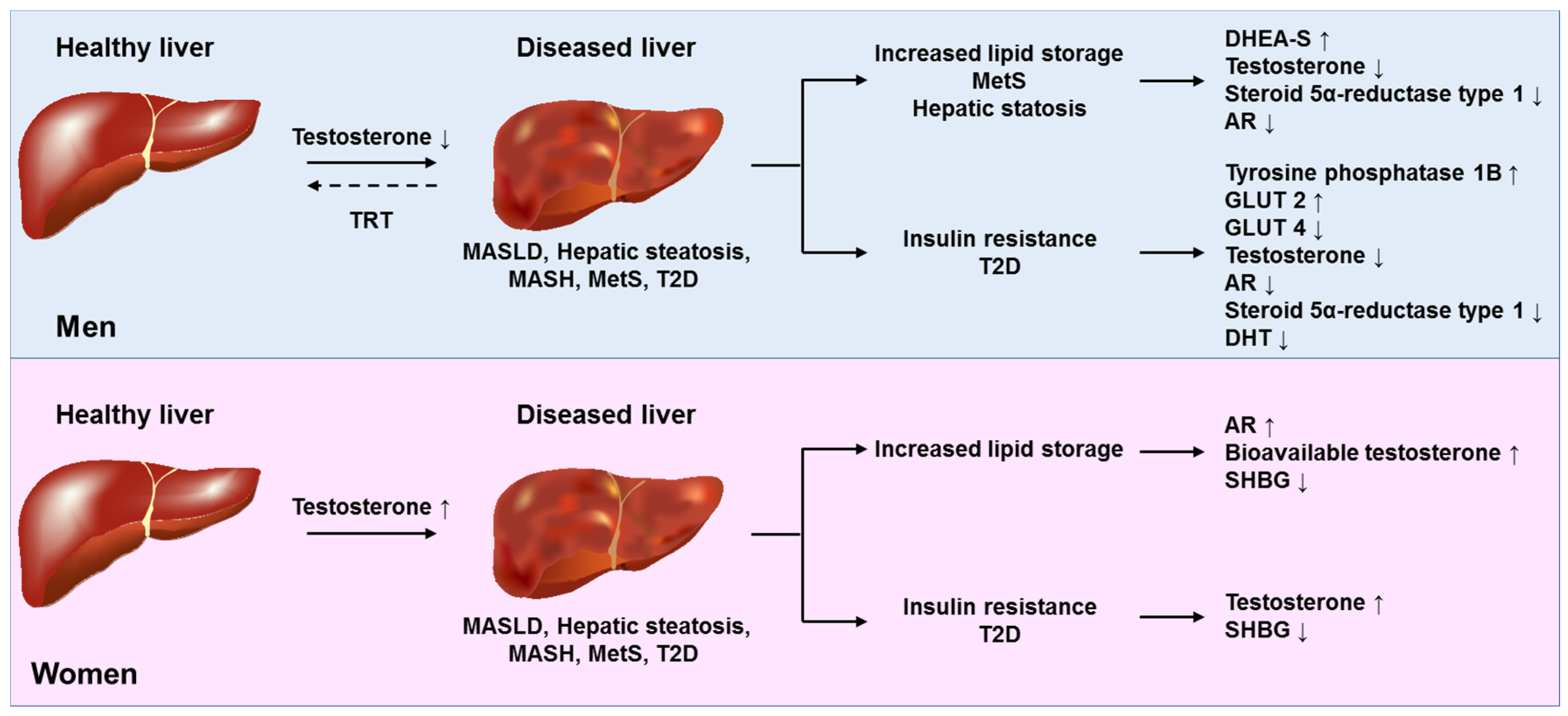
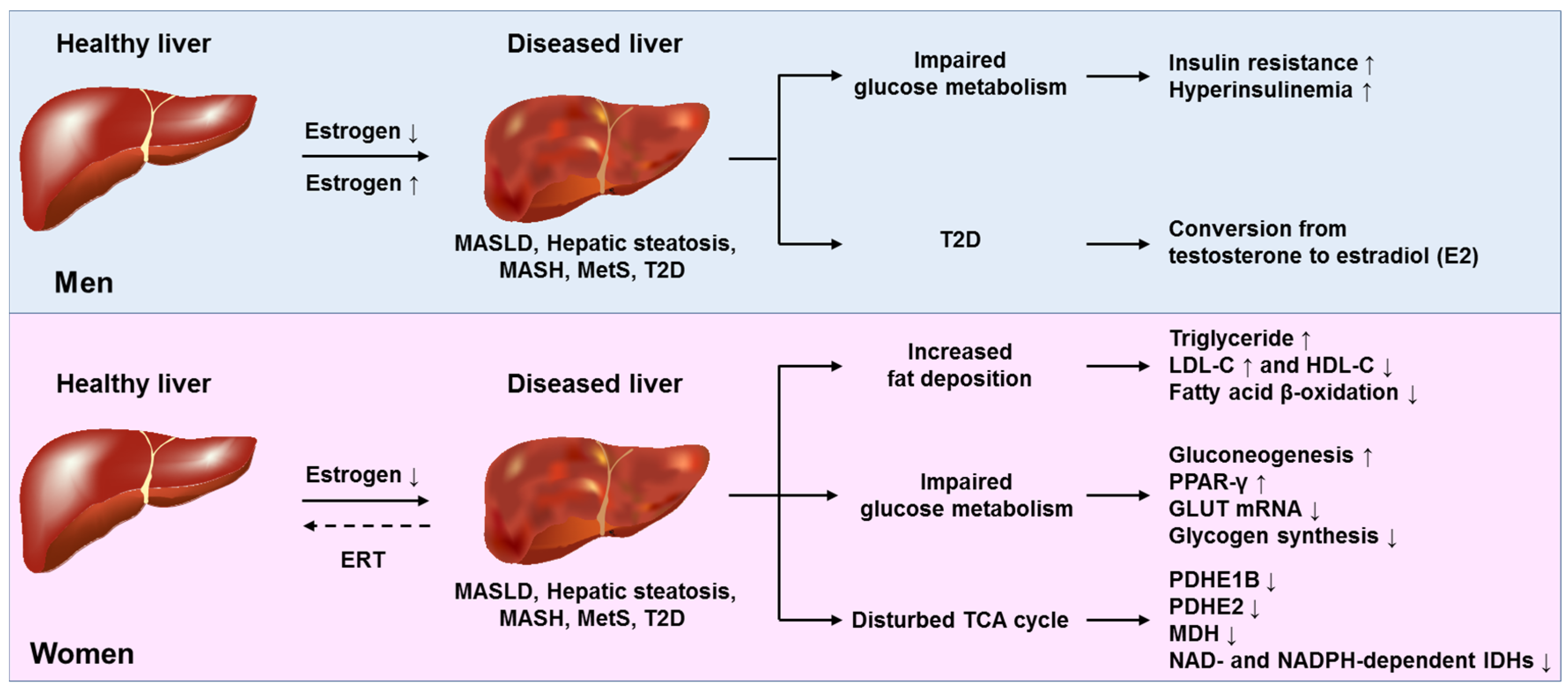
| Author, Year [Ref] | MASLD/MASH Diagnosis | Origin | Patient Population | Findings | Conclusions |
|---|---|---|---|---|---|
| Yang JD, 2014 [92] | LB | USA | 541 adults with MASH. | ACOR and 95% CI for more severe fibrosis were 1.4 (0.9, 2.1) (p = 0.17) for postmenopausal women and 1.6 (1.0, 2.5) (p = 0.03) for men, with premenopausal women as the reference. The ACOR and 95% CI of having more severe fibrosis in men than women were 1.8 (1.1, 2.9) for patients below 50 years (p = 0.02) and 1.2 (0.7, 2.1) for patients over 50 years (p = 0.59). | Men have higher odds of more severe fibrosis than pre-menopausal women; post-menopausal women have liver fibrosis of severity like men. |
| Klair JS, 2016 [93] | LB | USA | 488 women in post-menopause with MASLD. | After adjusting for multiple confounding factors, premature menopause was a risk factor for more severe fibrosis. Time from menopause was associated with more severe fibrosis. | In MASLD women, the duration of postmenopausal estrogen deficiency increases the odds of liver fibrosis. |
| Sarkar M, 2017 [94] | Non-contrast abdominal CT scan with liver attenuation ≤ 40 HU after excluding competing causes of SLD. | USA | 1052 women participating in the prospective population-based multicenter CARDIA study, whether cFT levels measured at year 2 were associated with prevalent MASLD at year 25. | Increasing quintiles of cFT were associated with the prevalence of MASLD at Year 25, regardless of confounders. This association was confirmed among 955 women who did not have any androgen excess and was partially mediated by VAT volume. | Increasing cFT is associated with the prevalence of MASLD in middle-aged women, even in the absence of androgen excess, mediated by visceral adiposity. |
| Yang JD, 2017 [95] | LB | USA | 1112 patients with MASLD participating in 3 large U.S. studies. | Premenopausal women, compared to men, had a higher risk of LOBI, hepatocyte ballooning, and Mallory-Denk bodies. Compared to postmenopausal women, they also had an increased risk of LOBI and Mallory-Denk bodies. In premenopausal women, oral contraceptives were associated with an increased risk of LOBI and Mallory-Denk bodies. In postmenopausal women, HRT was associated with an increased risk of LOBI. | Being a premenopausal woman or a female user of synthetic hormones is associated with increased histologic severity of hepatocyte injury and inflammation among patients with MASLD. |
| Minato S, 2018 [96] | Surrogate biomarkers | Japan | Retrospective analysis of 102 reproductive-aged women with a confirmed diagnosis of PCOS (ICD-10 codes). | Raised liver enzymes were found in 33.3% of cases. PCOS subjects had significantly higher BMI values than those with normal liver enzymes. In ROC analyses, T proved to be related to SLD. | An algorithm using BMI, glycemia, and testosterone levels may predict raised liver enzymes in PCOS women. |
| Mueller NT, 2020 [97] | LB | USA | 573 children and adolescents aged 18 or younger. | In both sexes, lower SHBG was inversely associated with steatosis severity and with portal inflammation in girls. Higher T was associated with improved steatosis and fibrosis in boys but was detrimental in girls. In both sexes, higher estrone, estradiol, and T were associated with a lower grade of portal inflammation; higher estradiol was positively associated with the severity of ballooning; DHEAS was inversely associated with ballooning and MASH severity. | Sex hormones are associated with MASLD histological features in children and adolescents. |
| Sarkar M, 2021 [98] | LB | USA | 159 random men, participating in the MASH CRN database. | Low cFT was associated with MASH, independent of age, WC, insulin resistance, and TG, and higher liver fibrosis stages | In men, low cFT is independently associated with MASH presence and severity. |
| Wamg J, 2021 [99] | Cases were identified using Medicare claims data; controls were selected among participants without liver disease. | USA | Nested case–control study with 1861 cases and 17,664 controls in the Multiethnic Cohort Study. | There was an inverse relationship between later age at menarche and MASLD (Ptrend = 0.01). Parity was associated with an increased risk of NAFLD. The use of oral contraceptives was associated with a higher odds of MASLD. Duration of use, women with oophorectomy or hysterectomy had a higher MASLD risk than women with natural menopause. A longer duration of menopause hormone therapy (only estrogen therapy) was associated with an increasing risk of MASLD. | Menstrual and reproductive factors, along with exogenous hormones, are associated with the risk of MASLD. |
| Dilimulati D, 2021 [100] | FibroScan | China | 360 adults with obesity were enrolled, with follow-up data available for 132 individuals who underwent LSG. | In the preoperative cohort, lower TT was associated with higher CAP and LSM in men. In women, higher TT was associated with higher CAP. In the postoperative cohort, changes in TT levels at 3 months after surgery were negatively correlated with changes in CAP values in men, and in women, changes in TT levels at 6 months post-surgery were positively correlated with variations in CAP values. After adjustment for confounding factors, the variations in TT levels were independently associated with CAP variations in both sexes. | TT concentrations are involved in pre-operative MASLD and post-operative disease regression in both sexes. |
| Mo MQ, 2022 [101] | USG in most studies and LB in three studies. | China | Meta-analysis of 2995 MASLD patients from 10 published cross-sectional studies. | Among men with MASLD, those with moderate-severe disease had lower TT than those with mild liver disease. TT and SHBG were significantly associated with moderate-severe MASLD. Among men older than 50, SHBG levels were lower in those with moderate-severe disease; among men with BMI > 27 kg/m2, moderate-severe MASLD was associated with higher SHBG levels than those with mild disease. | In men, lower TT is associated with more severe MASLD. However, the relationship of SHBG with MASLD severity or MASLD remains uncertain. |
| Cao W, 2022 [102] | USG | China | 732 participants aged 50–80 years were enrolled from communities. | After adjusting for confounders, LRA found a negative correlation of SHBG with MAFLD in men. Among women, SHBG and FSH had a negative correlation with MAFLD. In multivariate linear regression analysis, SHBG was a negative factor for LFC in both sexes. In women, FSH was a borderline significant negative factor for LFC. SHBG was negatively correlated with MAFLD in middle-aged and elderly individuals of both sexes. In women, FSH was negatively correlated, and bioactive testosterone was positively correlated with MAFLD. | Sex hormones are associated with MAFLD. |
| Zhang X, 2022 [103] | USG | China | 1155 subjects with T2D. | In men with T2D, increasing TT values were associated with decreased odds of MASLD. There were no statistically significant correlations observed between rising concentrations of androgen precursors and the likelihood of MASLD (all p values > 0.05). Among women with T2D, no significant associations were found between TT, androstenedione, DHEA, and DHEAS, with the risk of MASLD. | Serum TT is strongly associated with MASLD in men with T2D. |
| Zhang Z, 2022 [104] | Probable MASH was defined by concurrent NAFLD and MetS. | China | Cross-sectional study enrolling 1782 men with T2D. | TT quartiles were associated in a negative manner with probable MASH and disease inflammatory progression, but positively with fibrotic progression. In stratified analyses, the interactions of age, duration of T2D, and dyslipidemia were significant for inflammatory progression rather than for fibrotic progression. | In men, TT exhibits variable relationships with inflammatory and fibrotic components in MASLD, implying that this hormone has different roles in the individual features of MASLD histology. |
| Yang LJ, 103 [105] | USG | China | Cross-sectional study involving 1005 men with T2D. | After adjustments for confounders, the top TT tertile, compared to the lowest tertile, was associated with a reduced prevalence of MASLD. TT and MASLD were more strongly associated in lean individuals than among subjects with overweight/obesity. A significant interaction of TT with overweight/obesity (p for interaction = 0.018 for MASLD) was found. | Higher serum concentrations of TT were associated with a significantly lower prevalence of MASLD among men with T2D. The association of TT with MASLD was stronger in lean subjects. |
| De Herdt C, 2023 [106] | LB | Europe | Retrospective analysis of 134 men who underwent metabolic-hepatological work-up and liver biopsy. | No significant differences were found in concentrations of TT and cFT between MASL and MASH, and steatosis and ballooning. cFT was significantly lower in a higher stage of fibrosis (p = 0.013), not TT, and this difference did not persist after controlling for metabolic onfounders. A higher stage of LOBI was associated with lower TT concentrations (p = 0.033), not cFT, and this difference did not persist after controlling for VAT surface and HOMA-IR. | No association has been found between testosterone levels and MASLD, histological subgroups, or fibrosis. The lower levels of cFT observed among subjects with higher liver fibrosis stages and the association of TT with LOBI are driven by metabolic dysfunction. |
| Apostolov R, 2023 [107] | Cirrhosis was confirmed by a hepatologist through a combination of clinical, biochemical, radiological, and pathological findings. | Australia | Monocentric retrospective survey of 766 men with cirrhosis, with ALD and MASLD accounting for 33.3% and 11.9% of cases, respectively, in whom the determination of TT levels was available. | Low TT levels and cFT levels were found in 53.3% and 79.6% of cases, respectively. Median TT was lower in men with ALD and MASLD than in cirrhosis owing to other etiologies, irrespective of age and MELD score. TT was associated in an inverse manner with 1-year mortality or transplant and liver decompensation. | Hypotestosteronemia is common among men with cirrhosis and is associated with unfavourable clinical outcomes. Subjects with ALD and MASLD exhibit significantly lower TT serum concentrations compared to other causes of cirrhosis. |
| Cai X, 2023 [108] | FLI | Europe | Observational study involving 2239 participants followed up for an average of 6.5 years. | In this observational study, in men, TT, DHT, progesterone, and 17-OHP were inversely associated with FLI. Among women, free T was positively associated with FLI. SHBG was inversely associated with FLI across sexes. At MR analysis, no causal association was identified between genetically determined sex hormones and LFC. However, higher genetically determined SHBG was related to lower LFC in women. | In women, SHBG helps protect against liver fat accumulation. |
| Maldonado SS, 2024 [109] | LB | USA | 205 MASLD participants in the CRN. | After adjustment for confounders, higher AMH quartiles were inversely associated with MASLD histological features, including prevalent MASH, NAS ≥ 5, Mallory hyaline, and higher fibrosis stage. | Aging of the reproductive system is associated with the histologic severity of MASLD in women. |
| Weng C, 2024 [110] | ICD-9 (571.8) and ICD-10 (K76.0, K75.8) from the hospital admissions and death records | China | 187,395 men and 170,193 women from the UK Biobank followed up for 12.49 years using linear and nonlinear Cox regression models and MR analysis to test associations. | During follow-up, 2209 men and 1886 women with MASLD were identified. Elevated SHBG levels were linearly associated with a reduced risk of MASLD in women, but not in men. Higher BAT levels were associated with a reduced risk of MASLD in men and an increased disease in women. Genetically determined SHBG and BAT levels were linearly associated with MASLD risk in women; in men, an “L-shaped” MR association between SHBG levels and MASLD risk was found. Bidirectional MR analysis confirmed that MASLD was causally associated with SHBG and BAT levels in either sex. | In women, lower SHBG and higher BAT levels confer an increased risk of MASLD, both at conventional analysis and with MR assessment. In men, SHBG acts in a nonlinear manner. MASLD affects SHBG and BAT levels. |
| Wang Y, 2025 [111] | USG | China | 155 male children with obesity, with a mean age of 11.07 ± 1.53 years. | Children with MAFLD had statistically higher BMI, fasting insulin, HOMA-IR, fasting C-peptide, WBC, HbA1c, ALT, and AST, and lower levels of HDL, T, and SHBG than controls with simple obesity. At LR BMI, testosterone, and SHBG independently predicted MAFLD in boys, and these variables are of potential value in the early diagnosis of MAFLD as indicated by ROC curve analysis. | Among boys with obesity, BMI, testosterone, and SHBG independently predict MAFLD. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weiskirchen, R.; Lonardo, A. Sex Hormones and Metabolic Dysfunction-Associated Steatotic Liver Disease. Int. J. Mol. Sci. 2025, 26, 9594. https://doi.org/10.3390/ijms26199594
Weiskirchen R, Lonardo A. Sex Hormones and Metabolic Dysfunction-Associated Steatotic Liver Disease. International Journal of Molecular Sciences. 2025; 26(19):9594. https://doi.org/10.3390/ijms26199594
Chicago/Turabian StyleWeiskirchen, Ralf, and Amedeo Lonardo. 2025. "Sex Hormones and Metabolic Dysfunction-Associated Steatotic Liver Disease" International Journal of Molecular Sciences 26, no. 19: 9594. https://doi.org/10.3390/ijms26199594
APA StyleWeiskirchen, R., & Lonardo, A. (2025). Sex Hormones and Metabolic Dysfunction-Associated Steatotic Liver Disease. International Journal of Molecular Sciences, 26(19), 9594. https://doi.org/10.3390/ijms26199594







