Comparative Analysis of CT and MRI Combined with RNA Sequencing for Radiogenomic Staging of Bladder Cancer
Abstract
1. Introduction
2. Results
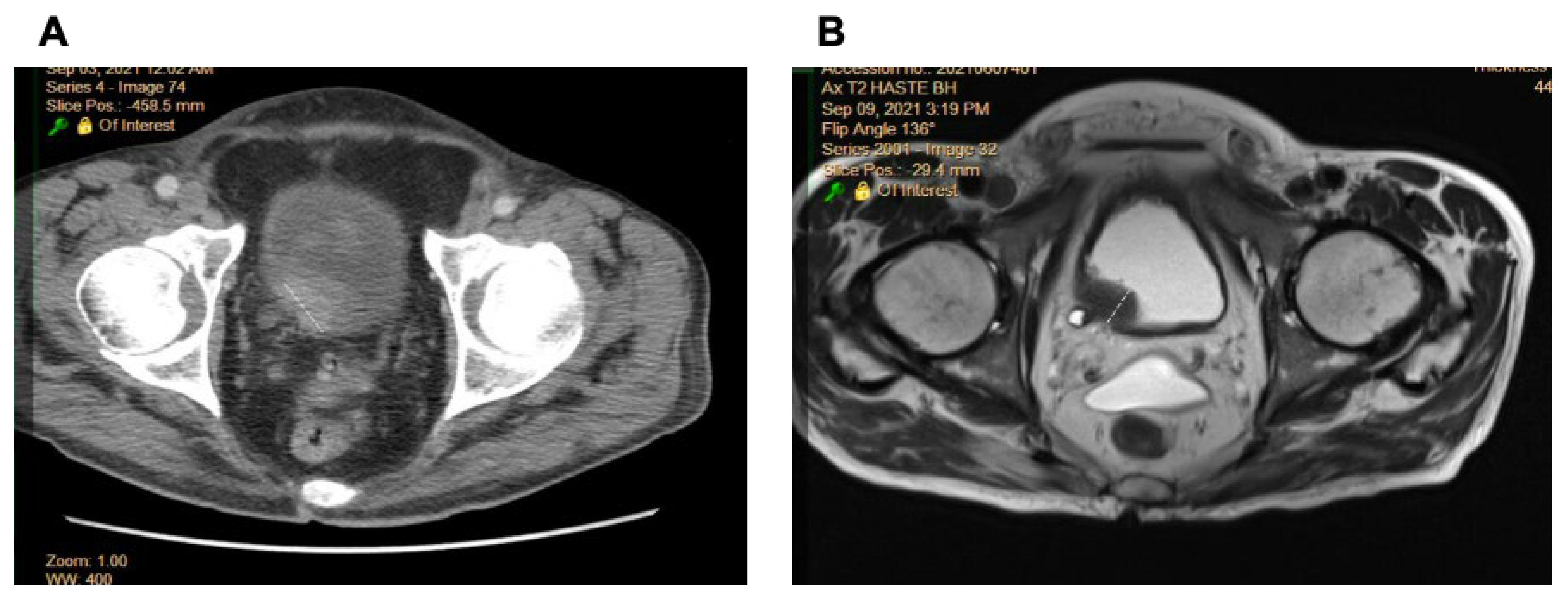
2.1. Comparison of Radiologic Interpretation on CT and MR Images
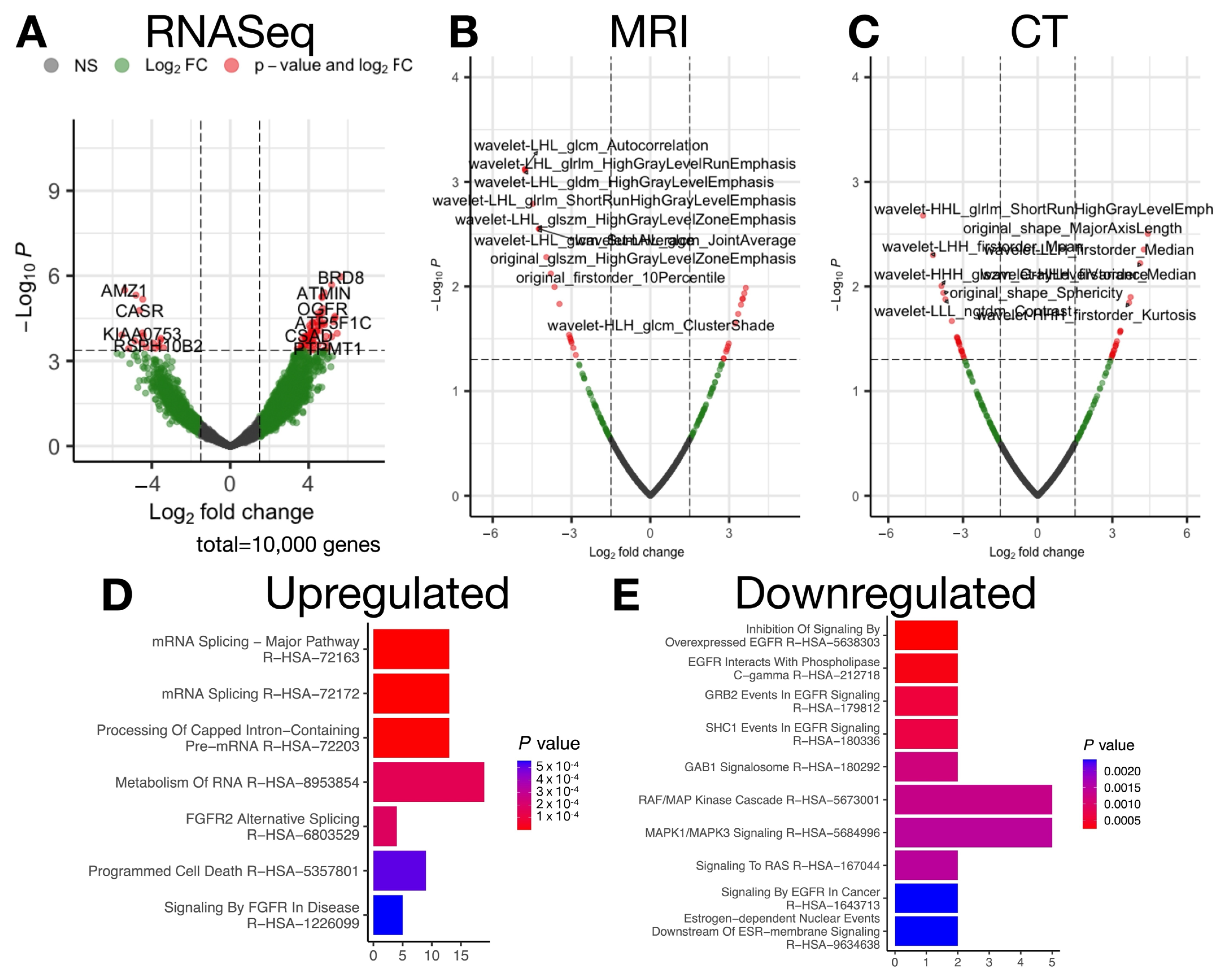
2.2. Transcriptional and Radiomic Correlates of Advanced Stage
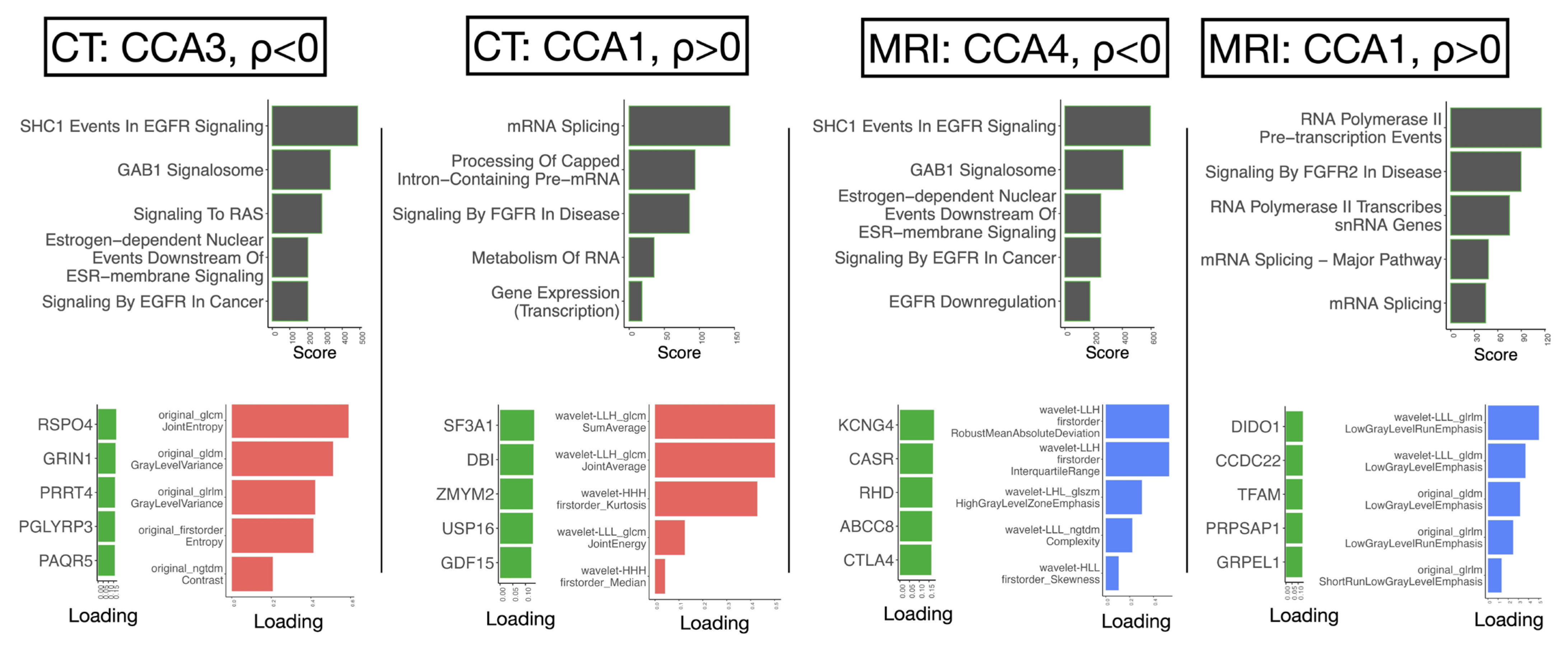
2.3. Canonical Correlation Uncovers Biological Pathways Underlying Progression-Related Radiomic Features
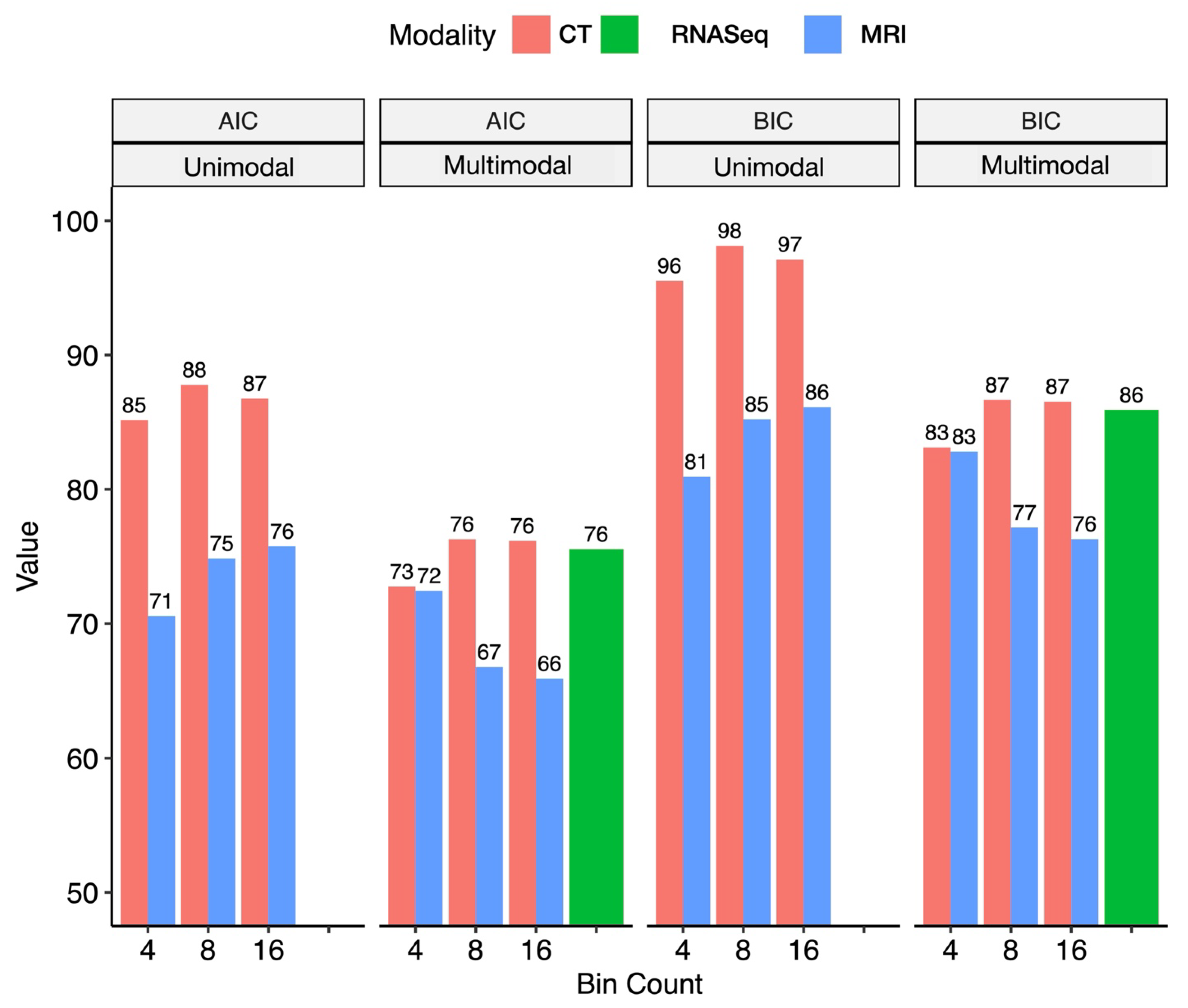
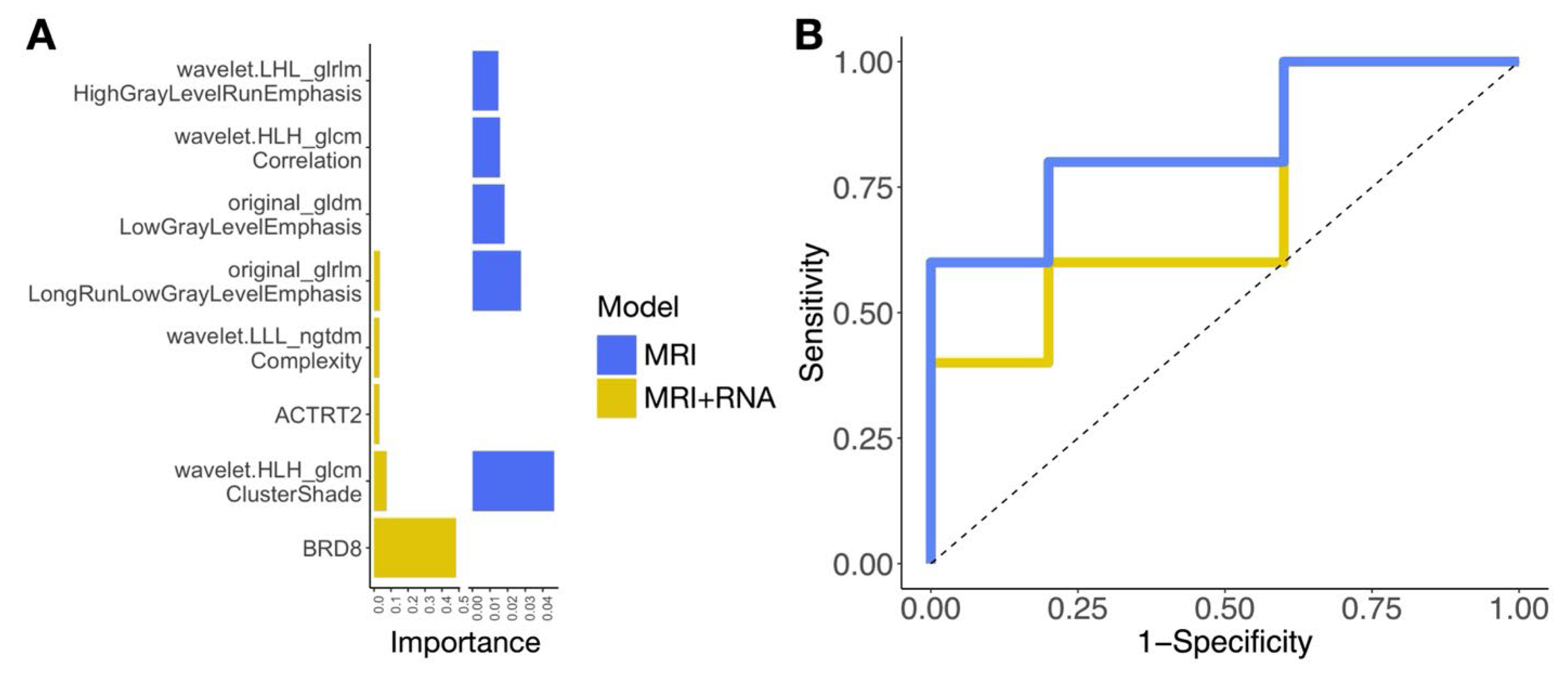
2.4. A Pilot Study Developing Workflow for Radiogenomic Models
3. Discussion
4. Materials and Methods
4.1. Retrospective Study Cohort
4.2. Prospective Study Cohort
4.3. Radiologic Staging of BCa
4.4. Radiomics Analysis of CT and MR Images
4.5. RNASeq Data Analysis
4.6. Identifying Radiomics Features Underlying Prognostic Biologic Pathways of Advanced Stage
4.7. Comparison of Radiogenomic Modeling Approaches for BCa Staging in a Retrospective Cohort for Selection of Radiomics Approach for Signature Development
4.8. Development of Radiogenomics MR Signature in a Retrospective Cohort
4.9. Validation of Radiogenomics Signatures in a Prospective Cohort
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCa | bladder cancer |
| CT | computed tomography |
| MRI | magnetic resonance imaging |
| RNASeq | RNA sequencing |
| NMIBC | non-muscle-invasive bladder cancer |
| MIBC | muscle-invasive bladder cancer |
| TURBT | transurethral resection of bladder tumor |
| mpMRI | multiparametric MRI |
| IRB | Institutional Review Board |
| CCA | canonical correlation analysis |
| AIC/BIC | Akaike and Bayesian information criteria |
| ROC | receiver operating characteristic |
| AUC | area under the curve |
| FGFR | fibroblast growth factor receptor |
| EGFR | epidermal growth factor receptor |
| RAS | rat sarcoma virus |
| MAPK | mitogen-activated protein kinase |
| EMT | epithelial–mesenchymal transition |
| LoG | Laplacian of Gaussian |
| PCA | principal component analysis |
| LASSO | least absolute shrinkage and selection operator |
| DV200 | percentage of RNA fragments >200 nucleotides |
References
- Burger, M.; Catto, J.W.; Dalbagni, G.; Grossman, H.B.; Herr, H.; Karakiewicz, P.; Kassouf, W.; Kiemeney, L.A.; La Vecchia, C.; Shariat, S.; et al. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 2013, 63, 234–241. [Google Scholar] [CrossRef]
- Cahn, D.B.; Handorf, E.A.; Ghiraldi, E.M.; Ristau, B.T.; Geynisman, D.M.; Churilla, T.M.; Horwitz, E.M.; Sobczak, M.L.; Chen, D.Y.T.; Viterbo, R.; et al. Contemporary use trends and survival outcomes in patients undergoing radical cystectomy or bladder-preservation therapy for muscle-invasive bladder cancer. Cancer 2017, 123, 4337–4345. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.S.; Shan, B.L.; Shan, L.L.; Chin, P.; Murray, S.; Ahmadi, N.; Saxena, A. A systematic review and meta-analysis of quality of life outcomes after radical cystectomy for bladder cancer. Surg. Oncol. 2016, 25, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Mak, R.H.; Hunt, D.; Shipley, W.U.; Efstathiou, J.A.; Tester, W.J.; Hagan, M.P.; Kaufman, D.S.; Heney, N.M.; Zietman, A.L. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: A pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J. Clin. Oncol. 2014, 32, 3801–3809, Erratum in J. Clin. Oncol. 2015, 33, 814. [Google Scholar] [CrossRef] [PubMed]
- Cancer Facts and Figures 2020. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf (accessed on 15 December 2020).
- Adamczyk, P.; Poblocki, P.; Kadlubowski, M.; Ostrowski, A.; Wrobel, A.; Mikolajczak, W.; Adamowicz, J.; Drewa, T.; Juszczak, K. A Comprehensive Approach to Clinical Staging of Bladder Cancer. J. Clin. Med. 2022, 11, 761. [Google Scholar] [CrossRef]
- Bodalal, Z.; Trebeschi, S.; Nguyen-Kim, T.D.L.; Schats, W.; Beets-Tan, R. Radiogenomics: Bridging imaging and genomics. Abdom. Radiol. (NY) 2019, 44, 1960–1984. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.; Granton, P.; Zegers, C.M.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef]
- Roth, S.C. What is genomic medicine? J. Med. Libr. Assoc. 2019, 107, 442–448. [Google Scholar] [CrossRef]
- West, C.; Rosenstein, B.S. Establishment of a radiogenomics consortium. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1295–1296. [Google Scholar] [CrossRef]
- He, W.; Huang, W.; Zhang, L.; Wu, X.; Zhang, S.; Zhang, B. Radiogenomics: Bridging the gap between imaging and genomics for precision oncology. MedComm 2024, 5, e722. [Google Scholar] [CrossRef]
- Alhussaini, A.J.; Veluchamy, A.; Jawli, A.; Kernohan, N.; Tang, B.; Palmer, C.N.A.; Steele, J.D.; Nabi, G. Radiogenomics Pilot Study: Association Between Radiomics and Single Nucleotide Polymorphism-Based Microarray Copy Number Variation in Diagnosing Renal Oncocytoma and Chromophobe Renal Cell Carcinoma. Int. J. Mol. Sci. 2024, 25, 12512. [Google Scholar] [CrossRef]
- Tortora, M.; Cordelli, E.; Sicilia, R.; Nibid, L.; Ippolito, E.; Perrone, G.; Ramella, S.; Soda, P. RadioPathomics: Multimodal Learning in Non-Small Cell Lung Cancer for Adaptive Radiotherapy. IEEE Access 2023, 11, 47563–47578. [Google Scholar] [CrossRef]
- Lin, P.; Wen, D.Y.; Chen, L.; Li, X.; Li, S.H.; Yan, H.B.; He, R.Q.; Chen, G.; He, Y.; Yang, H. A radiogenomics signature for predicting the clinical outcome of bladder urothelial carcinoma. Eur. Radiol. 2020, 30, 547–557. [Google Scholar] [CrossRef]
- Ye, F.; Hu, Y.; Gao, J.; Liang, Y.; Liu, Y.; Ou, Y.; Cheng, Z.; Jiang, H. Radiogenomics Map Reveals the Landscape of m6A Methylation Modification Pattern in Bladder Cancer. Front. Immunol. 2021, 12, 722642. [Google Scholar] [CrossRef]
- Xu, C.; Cao, J.; Zhou, T. Radiogenomics uncovers an interplay between angiogenesis and clinical outcomes in bladder cancer. Environ. Toxicol. 2024, 39, 1374–1387. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, T.A.; Chen, X.; Xie, Y.; Murakami, K.; Sakatani, T.; Kita, Y.; Kobayashi, T.; Miyake, M.; Knott, S.R.V.; Li, D.; et al. MRI/RNA-Seq-Based Radiogenomics and Artificial Intelligence for More Accurate Staging of Muscle-Invasive Bladder Cancer. Int. J. Mol. Sci. 2023, 25, 88. [Google Scholar] [CrossRef] [PubMed]
- Malayeri, A.A.; Pattanayak, P.; Apolo, A.B. Imaging muscle-invasive and metastatic urothelial carcinoma. Curr. Opin. Urol. 2015, 25, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Virarkar, M.; Szklaruk, J.; Diab, R.; Bassett, R.; Bhosale, P. Diagnostic value of 3.0 T versus 1.5 T MRI in staging prostate cancer: Systematic review and meta-analysis. Pol. J. Radiol. 2022, 87, e421–e429. [Google Scholar] [CrossRef]
- Ullrich, T.; Quentin, M.; Oelers, C.; Dietzel, F.; Sawicki, L.M.; Arsov, C.; Rabenalt, R.; Albers, P.; Antoch, G.; Blondin, D.; et al. Magnetic resonance imaging of the prostate at 1.5 versus 3.0T: A prospective comparison study of image quality. Eur. J. Radiol. 2017, 90, 192–197. [Google Scholar] [CrossRef]
- Barentsz, J.O.; Richenberg, J.; Clements, R.; Choyke, P.; Verma, S.; Villeirs, G.; Rouviere, O.; Logager, V.; Futterer, J.J.; European Society of Urogenital, Radiology. ESUR prostate MR guidelines 2012. Eur. Radiol. 2012, 22, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Kanesvaran, R.; Castro, E.; Wong, A.; Fizazi, K.; Chua, M.L.K.; Zhu, Y.; Malhotra, H.; Miura, Y.; Lee, J.L.; Chong, F.L.T.; et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the diagnosis, treatment and follow-up of patients with prostate cancer. ESMO Open 2022, 7, 100518. [Google Scholar] [CrossRef] [PubMed]
- Mohler, J.L.; Antonarakis, E.S. NCCN Guidelines Updates: Management of Prostate Cancer. J. Natl. Compr. Cancer Netw. 2019, 17, 583–586. [Google Scholar] [CrossRef]
- Woo, S.; Suh, C.H.; Kim, S.Y.; Cho, J.Y.; Kim, S.H. Diagnostic performance of MRI for prediction of muscle-invasiveness of bladder cancer: A systematic review and meta-analysis. Eur. J. Radiol. 2017, 95, 46–55. [Google Scholar] [CrossRef]
- Huang, L.; Kong, Q.; Liu, Z.; Wang, J.; Kang, Z.; Zhu, Y. The Diagnostic Value of MR Imaging in Differentiating T Staging of Bladder Cancer: A Meta-Analysis. Radiology 2018, 286, 502–511. [Google Scholar] [CrossRef]
- Ficarra, V.; Dalpiaz, O.; Alrabi, N.; Novara, G.; Galfano, A.; Artibani, W. Correlation between clinical and pathological staging in a series of radical cystectomies for bladder carcinoma. BJU Int. 2005, 95, 786–790. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, S.Y.; Ahn, H.J.; Kim, C.S.; Cho, K.S. Bladder cancer: Analysis of multi-detector row helical CT enhancement pattern and accuracy in tumor detection and perivesical staging. Radiology 2004, 231, 725–731. [Google Scholar] [CrossRef]
- Cai, L.; Yang, X.; Yu, J.; Shao, Q.; Wang, G.; Yuan, B.; Zhuang, J.; Li, K.; Wu, Q.; Liu, P.; et al. Deep learning on T2WI to predict the muscle-invasive bladder cancer: A multi-center clinical study. Sci. Rep. 2025, 15, 9942. [Google Scholar] [CrossRef]
- Li, M.; Jiang, Z.; Shen, W.; Liu, H. Deep learning in bladder cancer imaging: A review. Front. Oncol. 2022, 12, 930917. [Google Scholar] [CrossRef]
- Bizzarri, F.P.; Nelson, A.W.; Colquhoun, A.J.; Lobo, N. Utility of Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Detecting Lymph Node Involvement in Comparison to Conventional Imaging in Patients with Bladder Cancer with Variant Histology. Eur. Urol. Oncol. 2025. [Google Scholar] [CrossRef]
- Kozikowski, M.; Suarez-Ibarrola, R.; Osiecki, R.; Bilski, K.; Gratzke, C.; Shariat, S.F.; Miernik, A.; Dobruch, J. Role of Radiomics in the Prediction of Muscle-invasive Bladder Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Focus 2022, 8, 728–738. [Google Scholar] [CrossRef]
- Ozdemir, H.; Azamat, S.; Sam Ozdemir, M. Can Only the Shape Feature in Radiomics Help Machine Learning Show That Bladder Cancer Has Invaded Muscles? Cureus 2023, 15, e45488. [Google Scholar] [CrossRef] [PubMed]
- Boca, B.; Caraiani, C.; Telecan, T.; Pintican, R.; Lebovici, A.; Andras, I.; Crisan, N.; Pavel, A.; Diosan, L.; Balint, Z.; et al. MRI-Based Radiomics in Bladder Cancer: A Systematic Review and Radiomics Quality Score Assessment. Diagnostics 2023, 13, 2300. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Wang, Y.; Xiang, W.; Yao, J.; Liu, J.; Song, B. Radiomic Analysis of Quantitative T2 Mapping and Conventional MRI in Predicting Histologic Grade of Bladder Cancer. J. Clin. Med. 2023, 12, 5900. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Ma, L.; Yu, Y.; Zhang, C.; Ouyang, J.; Mao, C.; Zhang, Z. Development of a radiomics and clinical feature-based nomogram for preoperative prediction of pathological grade in bladder cancer. Front. Oncol. 2025, 15, 1661979. [Google Scholar] [CrossRef]
- Prip, F.; Lamy, P.; Lindskrog, S.V.; Strandgaard, T.; Nordentoft, I.; Birkenkamp-Demtroder, K.; Birkbak, N.J.; Kristjansdottir, N.; Kjaer, A.; Andreasen, T.G.; et al. Comprehensive genomic characterization of early-stage bladder cancer. Nat. Genet. 2025, 57, 115–125. [Google Scholar] [CrossRef]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017, 171, 540–556.e25, Erratum in Cell 2018, 174, 1033. [Google Scholar] [CrossRef]
- Kamoun, A.; de Reynies, A.; Allory, Y.; Sjodahl, G.; Robertson, A.G.; Seiler, R.; Hoadley, K.A.; Groeneveld, C.S.; Al-Ahmadie, H.; Choi, W.; et al. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. Eur. Urol. 2020, 77, 420–433. [Google Scholar] [CrossRef]
- Yang, W.; Fan, Z.; Tuli, R.; Deng, Z.; Pang, J.; Wachsman, A.; Reznik, R.; Sandler, H.; Li, D.; Fraass, B.A. Four-Dimensional Magnetic Resonance Imaging With 3-Dimensional Radial Sampling and Self-Gating-Based K-Space Sorting: Early Clinical Experience on Pancreatic Cancer Patients. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 1136–1143. [Google Scholar] [CrossRef]
- Rajesh, A.; Sokhi, H.K.; Fung, R.; Mulcahy, K.A.; Bankart, M.J. Bladder cancer: Evaluation of staging accuracy using dynamic MRI. Clin. Radiol. 2011, 66, 1140–1145. [Google Scholar] [CrossRef]
- Barentsz, J.O.; Jager, G.J.; Witjes, J.A.; Ruijs, J.H. Primary staging of urinary bladder carcinoma: The role of MRI and a comparison with CT. Eur. Radiol. 1996, 6, 129–133. [Google Scholar] [CrossRef]
- van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef]
- Ewels, P.A.; Peltzer, A.; Fillinger, S.; Patel, H.; Alneberg, J.; Wilm, A.; Garcia, M.U.; Di Tommaso, P.; Nahnsen, S. The nf-core framework for community-curated bioinformatics pipelines. Nat. Biotechnol. 2020, 38, 276–278. [Google Scholar] [CrossRef]
| (A) | ||||
| Pathologic Outcome | ||||
| ≤pT1 | pT2 | ≥pT3 | ||
| Correct | 3 | 6 | 5 | |
| Wrong | 2 | 6 | 9 | |
| 3/5 = 60% | 6/12 = 50% | 5/14 = 35% | 14/31 = 45% | |
| (B) | ||||
| Pathologic Outcome | ||||
| ≤pT1 | pT2 | ≥pT3 | ||
| Correct | 3 | 6 | 8 | |
| Wrong | 2 | 6 | 6 | |
| 3/5 = 60% | 6/12 = 50% | 8/14 = 57% | 17/31 = 54% | |
| Retrospective Cohort (Cross-Validation) | |||
| Predictors | AUC ± SE | Sensitivity ± SE | Specificity ± SE |
| MRI + RNASeq | 0.80 ± 0.14 | 0.88 ± 0.13 | 0.83 ± 0.17 |
| MRI | 0.77 ± 0.14 | 0.78 ± 0.2 | 0.83 ± 0.2 |
| Prospective Cohort (Held-Out Test) | |||
| Predictors | AUC ± SE | Sensitivity ± SE | Specificity ± SE |
| MRI + RNASeq | 0.75 ± 0.22 | 0.8 ± 0.19 | 0.78 ± 0.2 |
| MRI | 0.84 ± 0.22 | 0.84 ± 0.17 | 0.89 ± 0.16 |
| Features | BCa (n = 31) |
|---|---|
| Age, years, mean (range) | 76.3 (58–88) |
| <65 years, n (%) | 4 (12.9) |
| ≥65 years, n (%) | 27 (87.1) |
| Male/female ratio (% male) | 28:3 (90.3% male) |
| Race, n (%) | |
| Asian | 31 (100) |
| Primary tumor stage, n (%) * | |
| Intra-vesical (Ta, Tis, or T1–T2) | 17 (54.8) |
| Extra-vesical (T3–T4) | 14 (45.2) |
| Grade, n (%) | |
| Low | 0 (0) |
| High | 31 (100) |
| Features | BCa (n = 10) |
|---|---|
| Age, years, mean (range) | 64.1 (21–81) |
| <65 years, n (%) | 3 (30) |
| ≥65 years, n (%) | 7 (70) |
| Male/female ratio (% male) | 6:4 (60% male) |
| Race, n (%) | |
| White | 7 (70) |
| Hispanic | 2 (20) |
| Asian | 1 (10) |
| Primary tumor stage, n (%) * | |
| NMIBC (Ta, Tis, or T1) ** | 5 (50) |
| MIBC (T2) ** | 5 (50) |
| Grade, n (%) | |
| Low | 1 (10) |
| High | 9 (90) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levy, J.; Sakatani, T.; Murakami, K.; Kita, Y.; Kobayashi, T.; Win, S.; Manoukian, S.; Rosser, C.J.; Furuya, H. Comparative Analysis of CT and MRI Combined with RNA Sequencing for Radiogenomic Staging of Bladder Cancer. Int. J. Mol. Sci. 2025, 26, 9570. https://doi.org/10.3390/ijms26199570
Levy J, Sakatani T, Murakami K, Kita Y, Kobayashi T, Win S, Manoukian S, Rosser CJ, Furuya H. Comparative Analysis of CT and MRI Combined with RNA Sequencing for Radiogenomic Staging of Bladder Cancer. International Journal of Molecular Sciences. 2025; 26(19):9570. https://doi.org/10.3390/ijms26199570
Chicago/Turabian StyleLevy, Joshua, Toru Sakatani, Kaoru Murakami, Yuki Kita, Takashi Kobayashi, Susan Win, Saro Manoukian, Charles J. Rosser, and Hideki Furuya. 2025. "Comparative Analysis of CT and MRI Combined with RNA Sequencing for Radiogenomic Staging of Bladder Cancer" International Journal of Molecular Sciences 26, no. 19: 9570. https://doi.org/10.3390/ijms26199570
APA StyleLevy, J., Sakatani, T., Murakami, K., Kita, Y., Kobayashi, T., Win, S., Manoukian, S., Rosser, C. J., & Furuya, H. (2025). Comparative Analysis of CT and MRI Combined with RNA Sequencing for Radiogenomic Staging of Bladder Cancer. International Journal of Molecular Sciences, 26(19), 9570. https://doi.org/10.3390/ijms26199570







