Creatinine-to-Cystatin C Ratio Combined with FIB-4 and ELF for Noninvasive Fibrosis Assessment in MASLD
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Histology
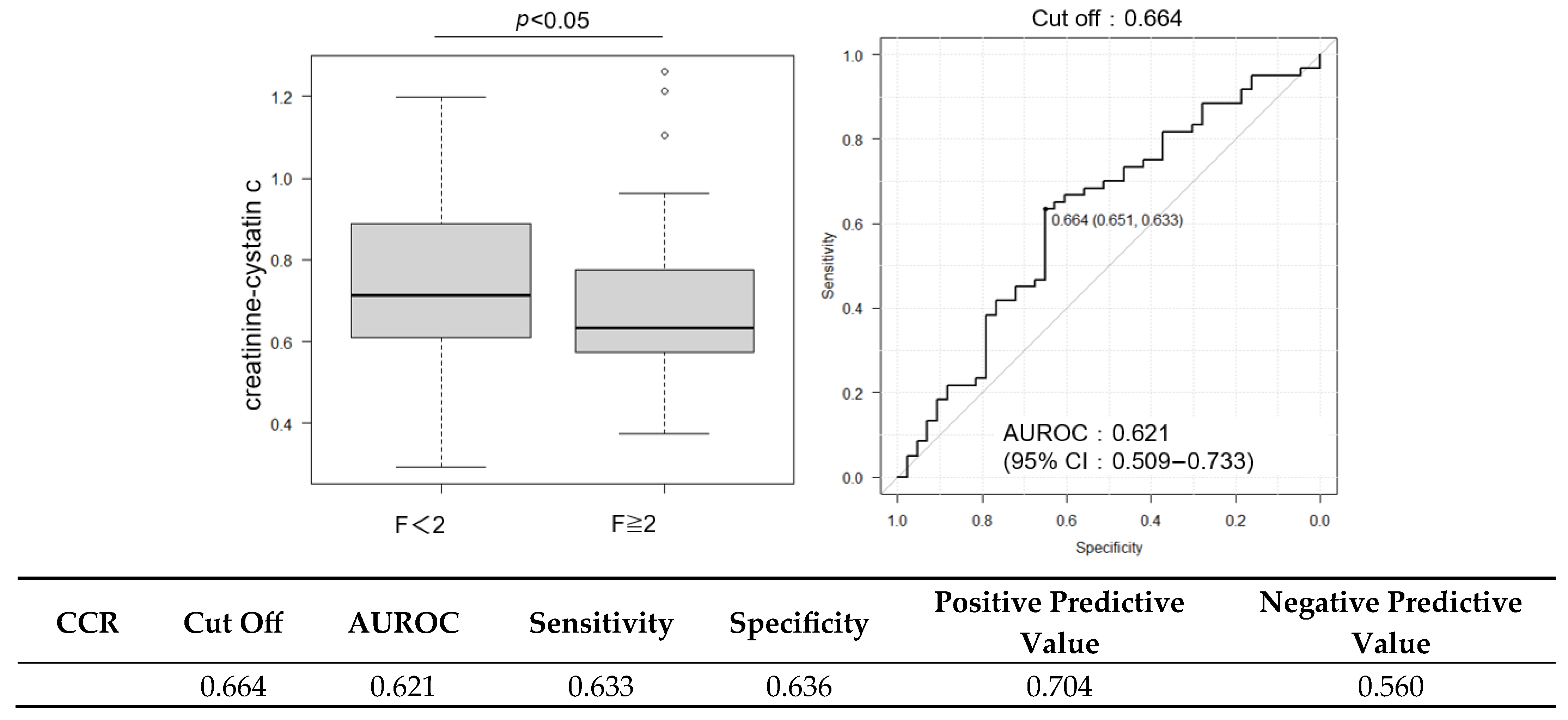
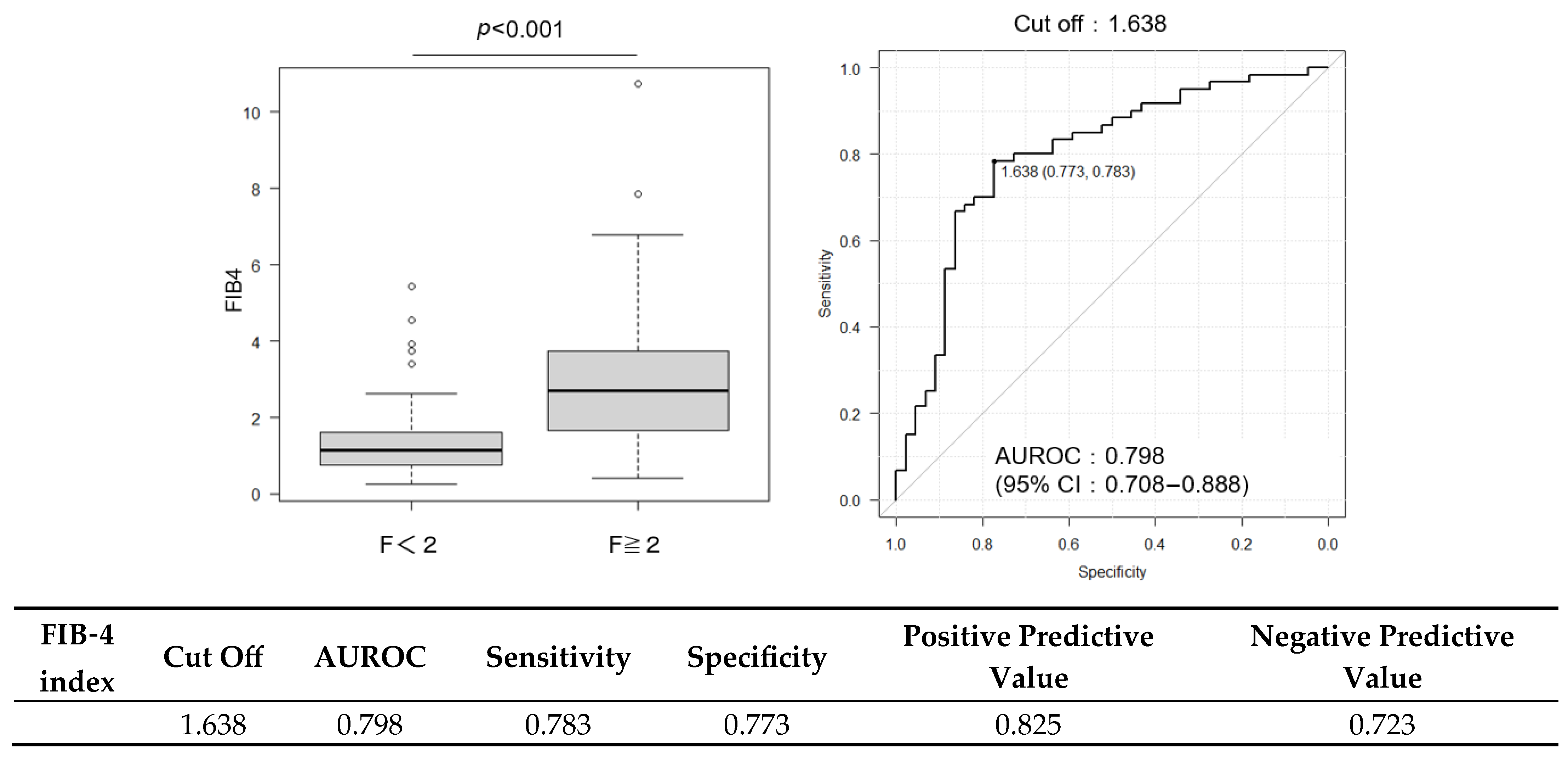
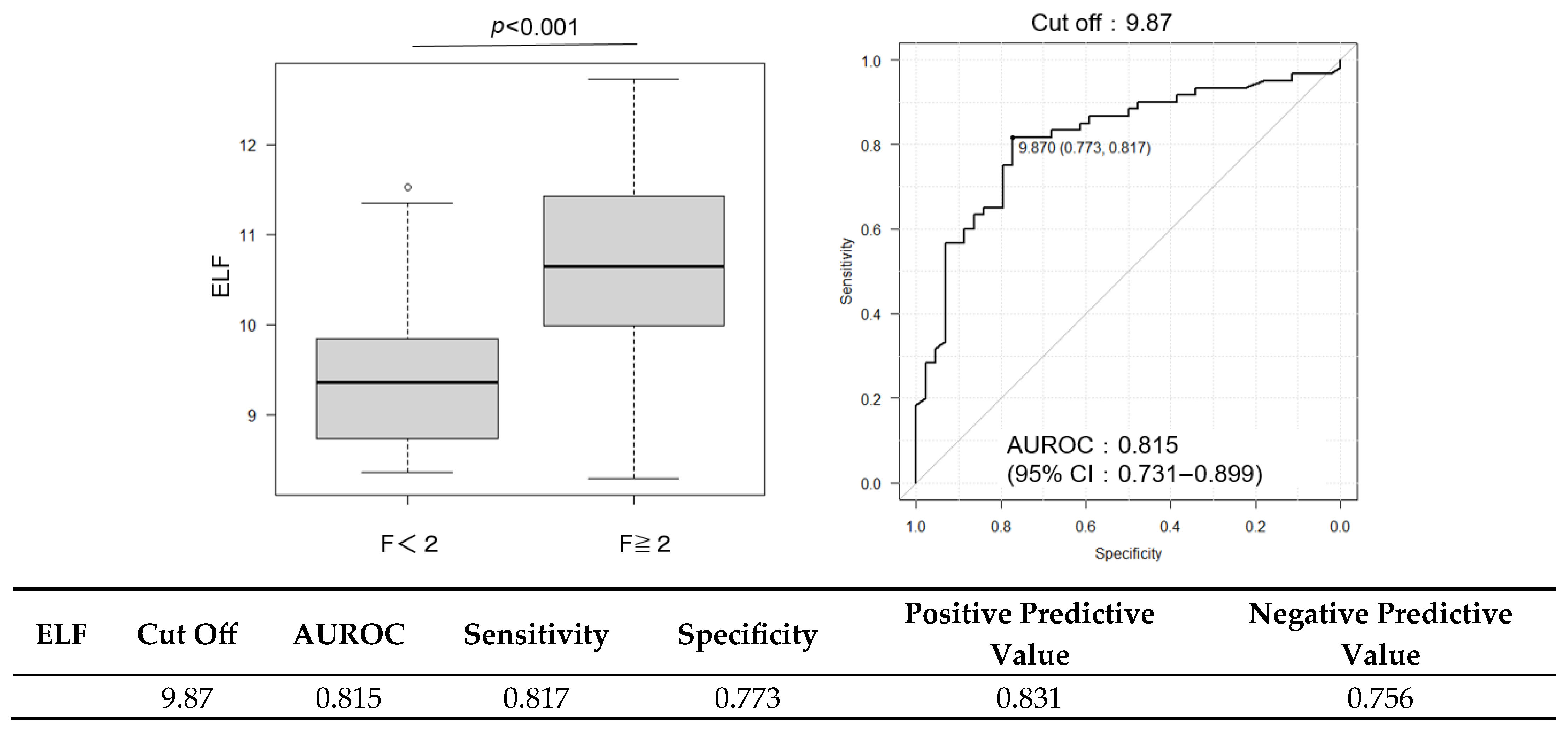
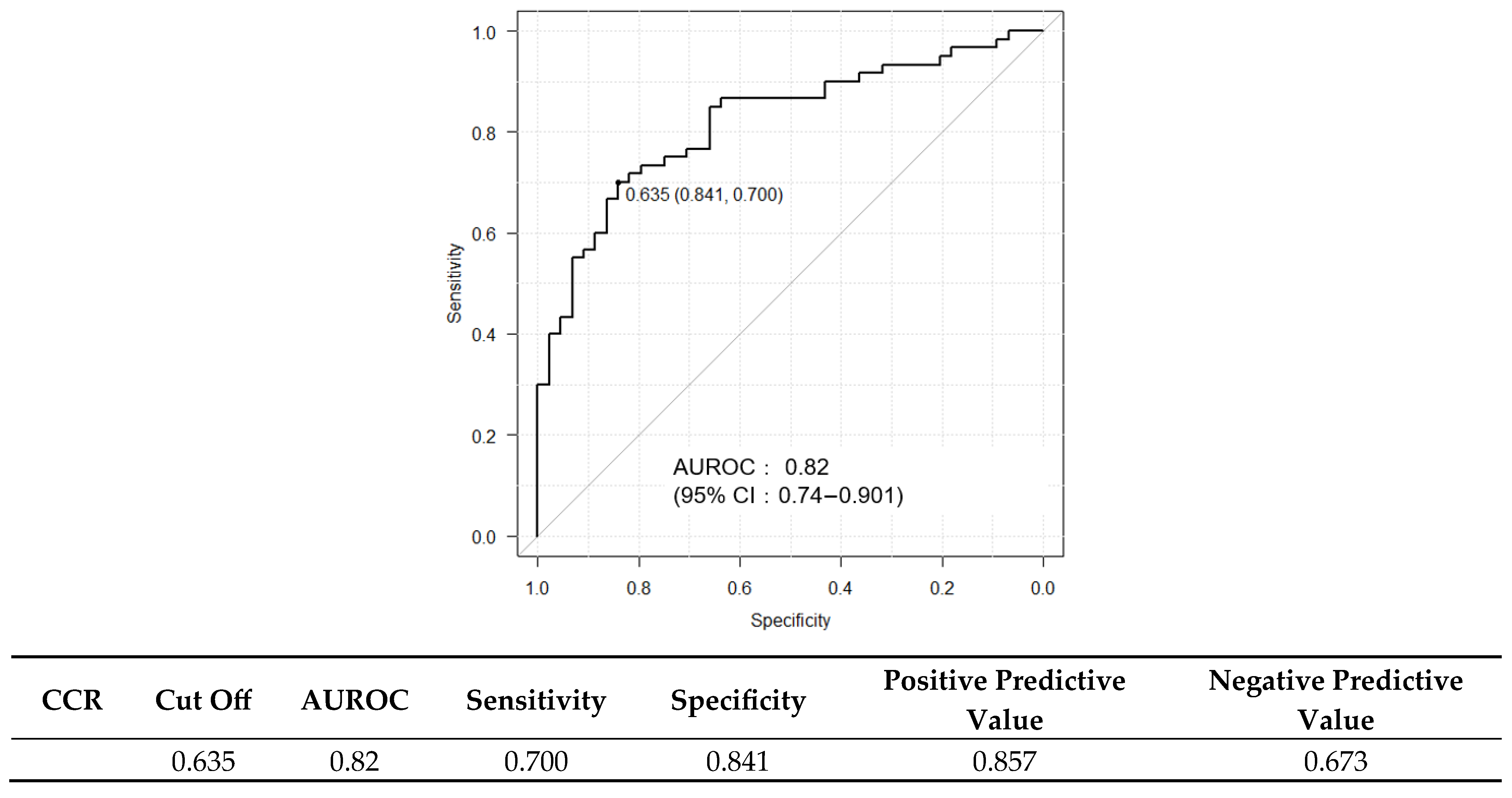
2.3. Diagnostic Performance of FIB-4 and ELF Scores and Their Combination with CCR
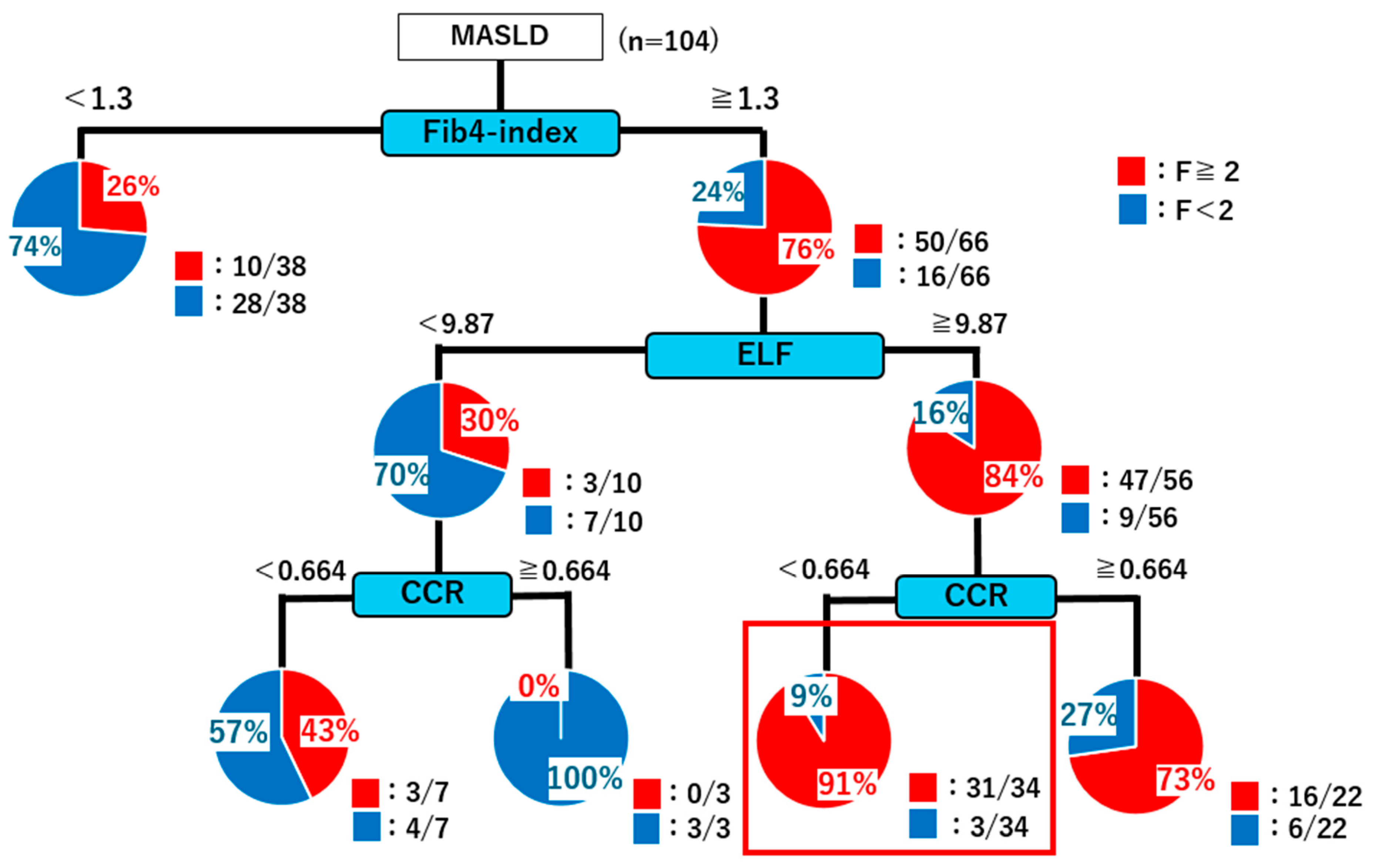
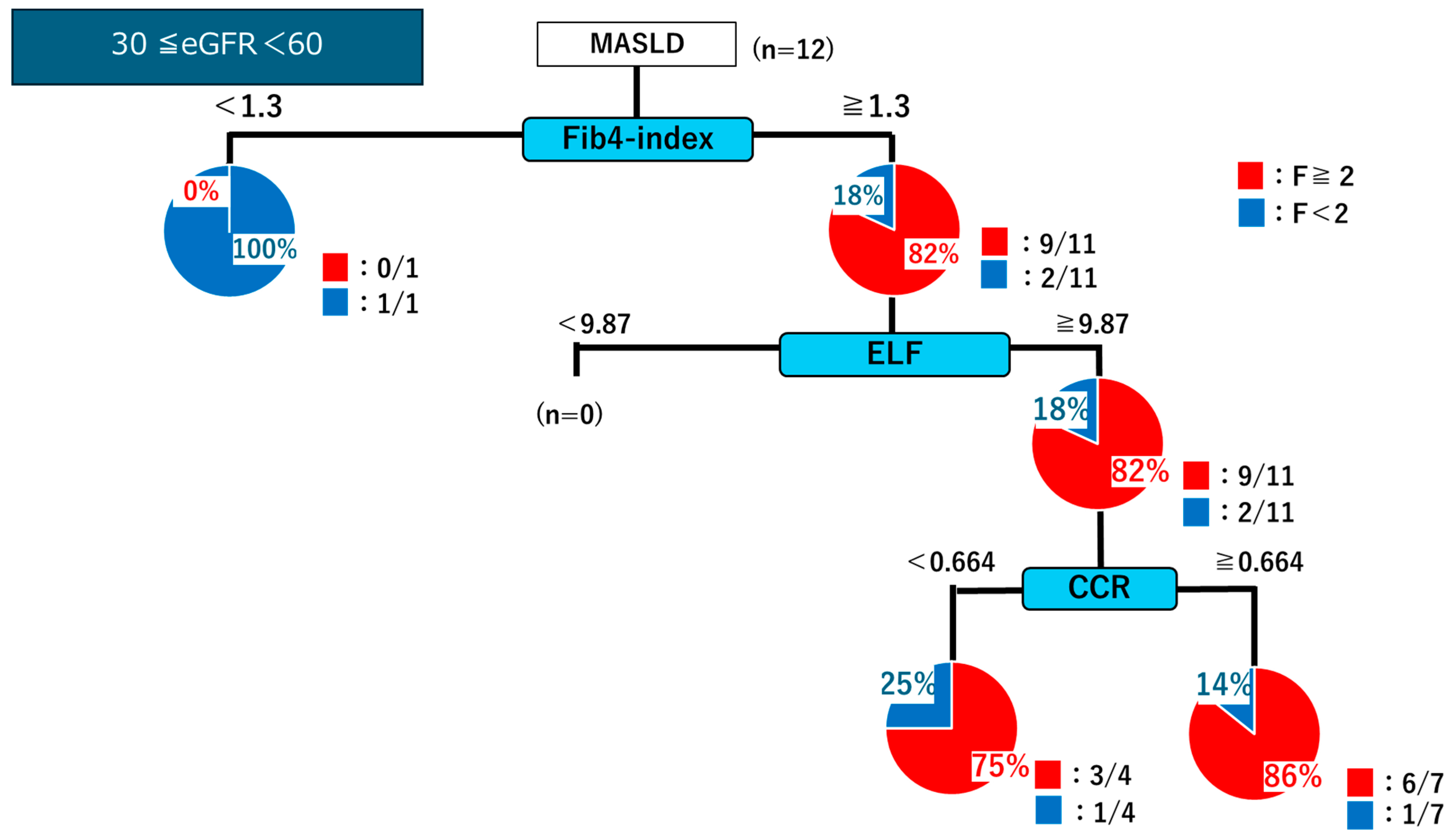
2.4. Decision Tree Analysis
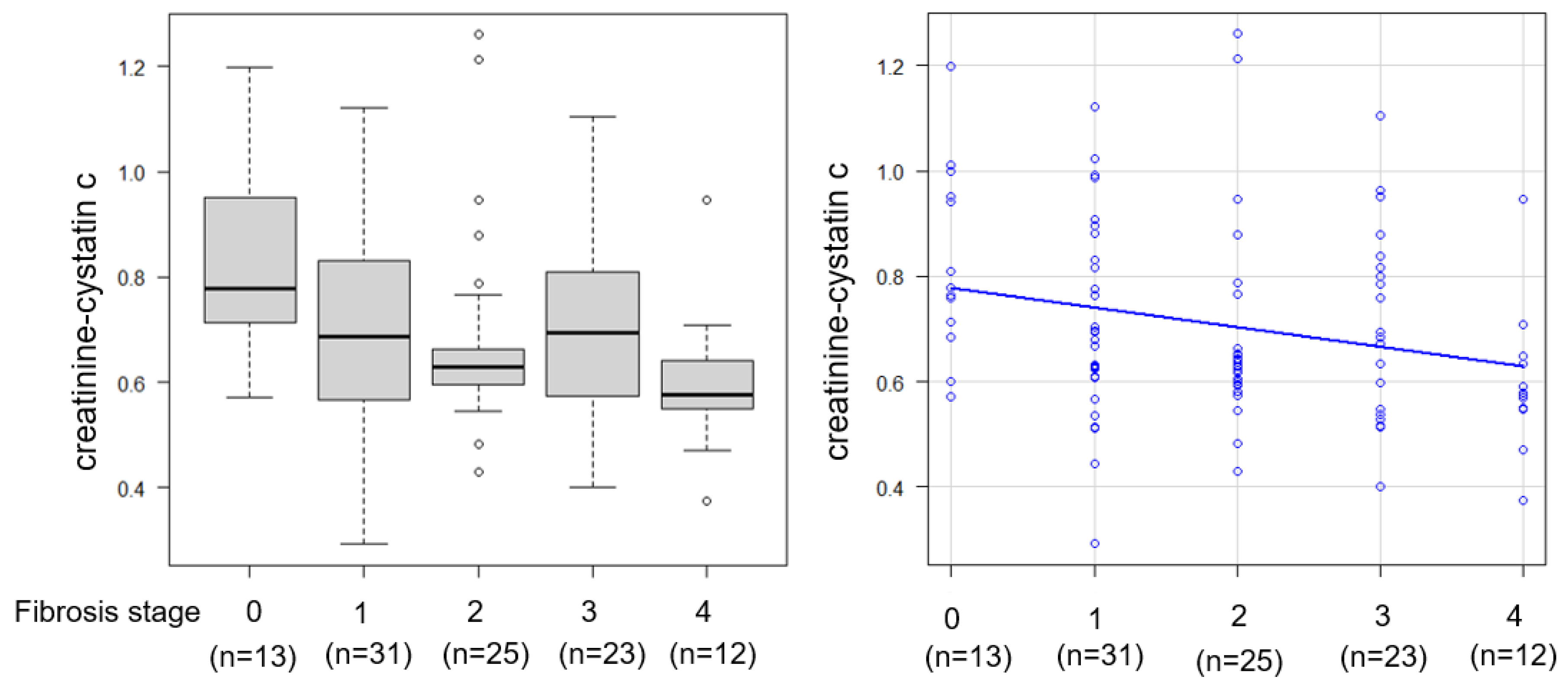
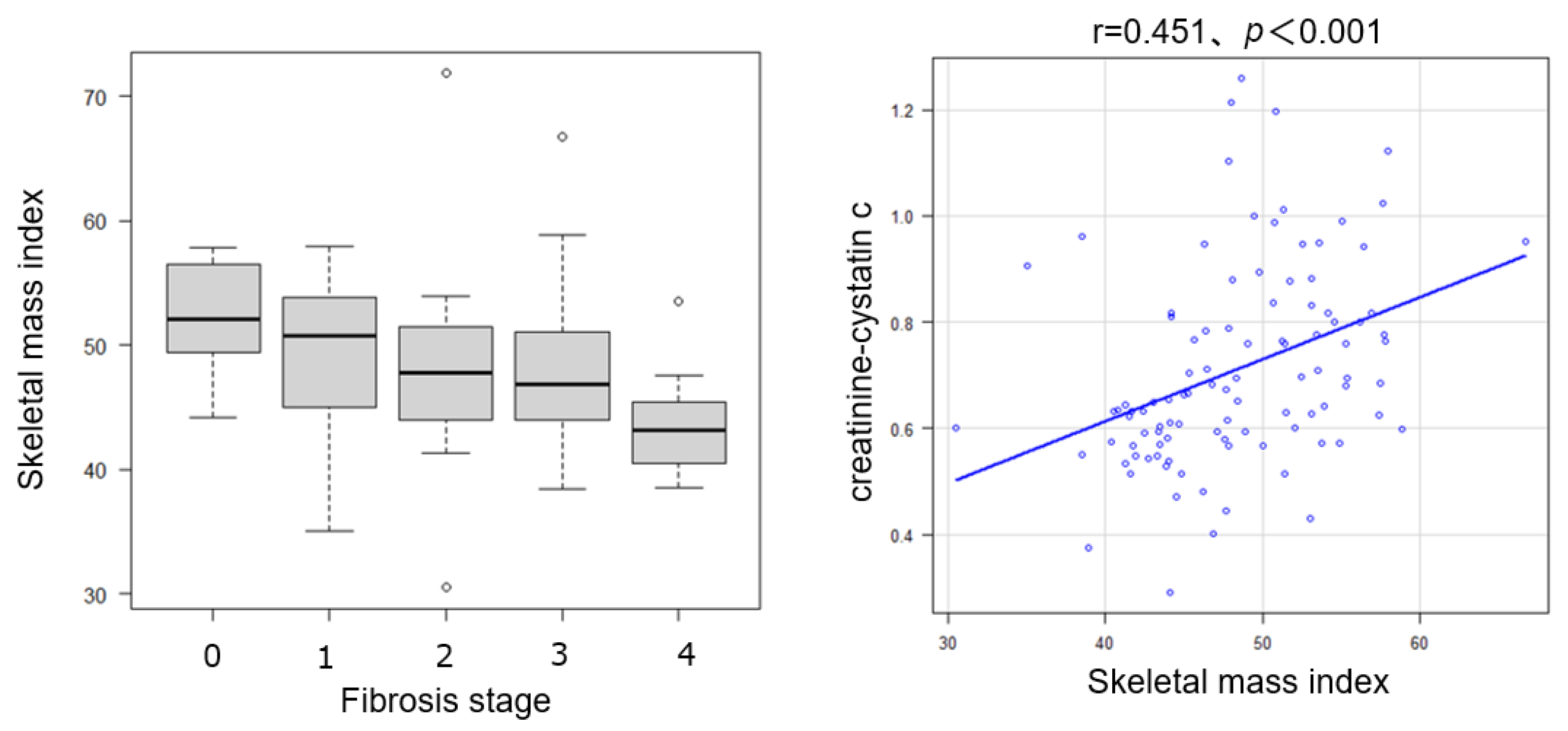
2.5. Relationship Between Skeletal Muscle Mass, Steatosis/Inflammation, and CCR
2.6. Relationship Between NAS and Cystatin C
3. Discussion
4. Materials and Methods
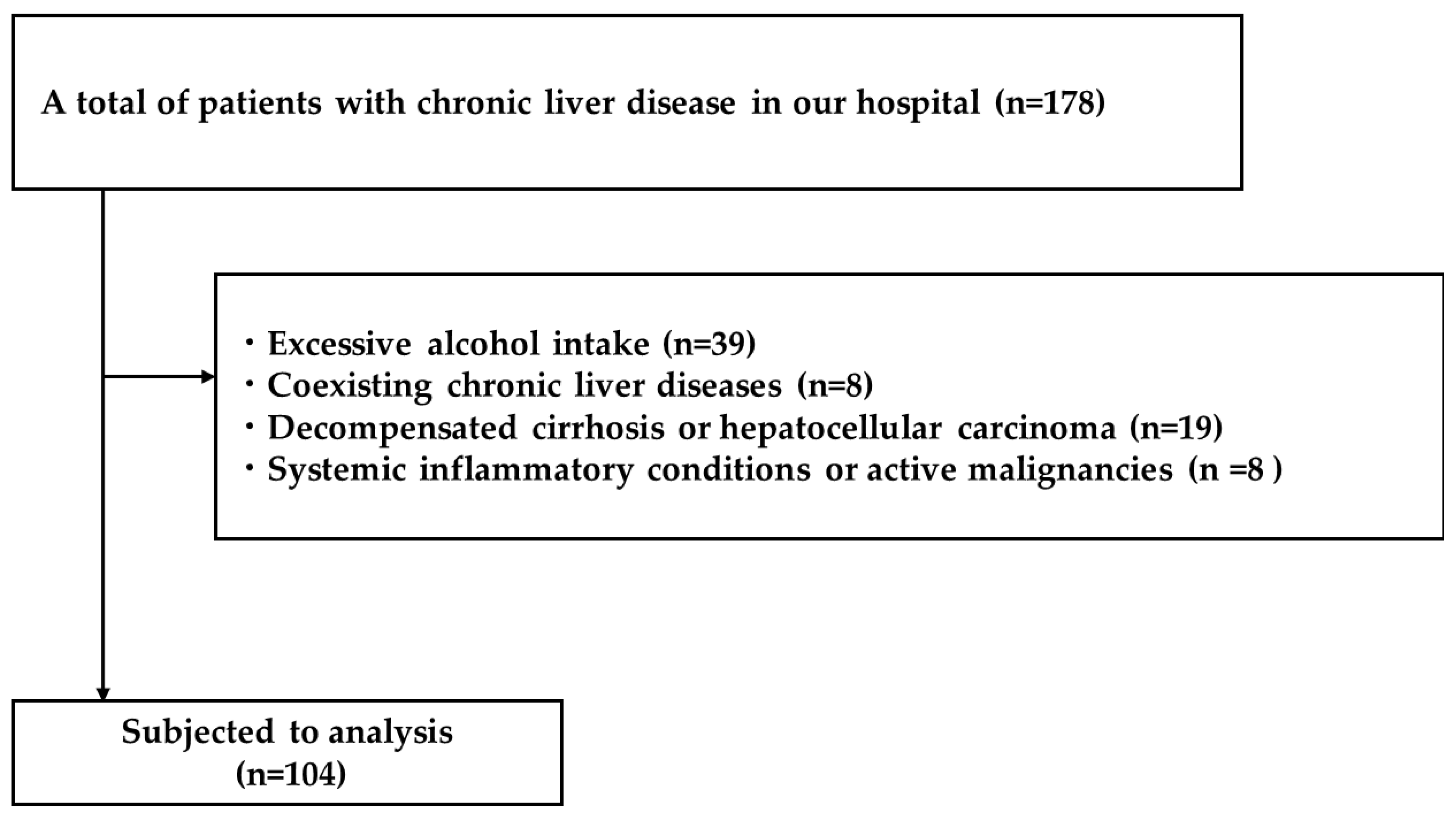
4.1. Study Design and Patients
4.2. Clinical and Laboratory Data Collection
4.3. Liver Histology Assessment
4.4. Assessment of Skeletal Muscle Mass
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUROC | Area under the ROC curve |
| CCR | Cystatin C ratio |
| CI | Confidence interval |
| ELF | Enhanced liver fibrosis |
| HA | Hyaluronic acid |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| NAS | NAFLD activity score |
| ROC | Receiver operating characteristic |
| SMI | Skeletal muscle index |
References
- Hashim, M.M.A.; Khan, M.A.M.; Ashraf, M.U.; Mohsin, S.; Zahoor, K.; Niazi, J.; Khan, A.; Muzaffar, S.; Makhdumi, M.; Ibad, O.A. Pathological evolution and internal medicine management of nonalcoholic fatty liver disease (NAFLD) in the era of metabolic dysfunction-associated steatotic liver disease (MASLD). Cureus 2025, 17, 86963. [Google Scholar] [CrossRef]
- Chan, W.-K.; Chuah, K.-H.; Rajaram, R.B.; Lim, L.-L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic dysfunction-associated steatotic liver disease (MASLD): A state-of-the-art review. J. Obes. Metab. Syndr. 2023, 32, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Jiang, L.; An, Y.; Liu, J.; Wang, G.; Wang, Y.; Yang, N. Association of sensitivity to thyroid hormones and non-alcoholic fatty liver disease and the severity of liver fibrosis in euthyroid adults: A retrospective study. Diabetes Metab. Syndr. Obes. 2025, 18, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Targher, G.; Byrne, C.D.; Cao, Y.Y.; Zheng, M.H. Current status and future trends of the global burden of MASLD. Trends Endocrinol. Metab. 2024, 35, 697–707. [Google Scholar] [CrossRef]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef]
- Herrmann, E.; de Lédinghen, V.; Cassinotto, C.; Chu, W.C.; Leung, V.Y.; Ferraioli, G.; Filice, C.; Castera, L.; Vilgrain, V.; Ronot, M.; et al. Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: An individual patient data-based meta-analysis. Hepatology 2018, 67, 260–272. [Google Scholar] [CrossRef]
- Sumida, Y.; Nakajima, A.; Itoh, Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World. J. Gastroenterol. 2014, 20, 475. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, D.; Zeng, C.; Zhang, L.; Gao, X.; Wang, X. Impact of sarcopenia on the survival of patients undergoing liver transplantation for decompensated liver cirrhosis. J. Cachexia Sarcopenia Muscle 2023, 14, 2602–2612. [Google Scholar] [CrossRef] [PubMed]
- Sandireddy, R.; Sakthivel, S.; Gupta, P.; Behari, J.; Tripathi, M.; Singh, B.K. Systemic impacts of metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH) on heart, muscle, and kidney related diseases. Front. Cell Dev. Biol. 2024, 12, 1433857. [Google Scholar] [CrossRef]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef]
- Al-Nimer, M.S. Sarcopenia and metabolic dysfunction-associated steatotic liver disease: The role of exercise-related biomarkers. World J. Hepatol. 2025, 17, 101165. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.H.; Zhu, Y.B.; Yao, Y.; Huang, H.B. Serum creatinine/cystatin C ratio as a muscle mass evaluating tool and prognostic indicator for hospitalized patients: A meta-analysis. Front. Med. 2022, 9, 1058464. [Google Scholar]
- Ning, X.; Xie, C.; Kong, Y. Serum creatinine- and cystatin C-based indices are associated with the risk of subsequent sarcopenia: Evidence from the China Health and Retirement Longitudinal Study. Front. Nutr. 2024, 11, 1471068. [Google Scholar] [CrossRef]
- Shi, S.; Jiang, Y.; Chen, W.; Chen, K.; Liao, Y.; Huang, K. Diagnostic and prognostic value of the Creatinine/Cystatin C ratio for low muscle mass evaluation among US adults. Front. Nutr. 2022, 9, 897774. [Google Scholar] [CrossRef]
- Ding, P.; Guo, H.; Sun, C.; Chen, S.; Yang, P.; Tian, Y.; Lowe, S.; Zhao, Q. Serum creatinine/cystatin C ratio is a systemic marker of sarcopenia in patients with gastrointestinal stromal tumours. Front. Nutr. 2022, 9, 963265. [Google Scholar] [CrossRef]
- Bai, A.; Xu, J.; Xu, W.; Cao, J.; Zhao, B. Creatinine and cystatin C-based indices for predicting sarcopenia, frailty and disability in older community-dwelling adults. J. Nutr. Health Aging 2025, 29, 100635. [Google Scholar] [CrossRef]
- He, Q.; Jiang, J.; Xie, L.; Zhang, L.; Yang, M. A sarcopenia index based on serum creatinine and cystatin C cannot accurately detect either low muscle mass or sarcopenia in urban community-dwelling older people. Sci. Rep. 2018, 8, 11534. [Google Scholar] [CrossRef]
- Matsuzawa, R.; Nagai, K.; Takahashi, K.; Mori, T.; Onishi, M.; Tsuji, S.; Hashimoto, K.; Tamaki, K.; Wada, Y.; Kusunoki, H.; et al. Serum creatinine–cystatin C based screening of sarcopenia in community dwelling older adults: A cross-sectional analysis. J. Frailty Aging 2024, 13, 116–124. [Google Scholar] [CrossRef]
- Deng, L.; Zheng, X.; Chen, Y.; Liu, C.; Shi, J.; Bu, Z.; Liu, X.; Zhao, H.; Li, S.; Yin, B.; et al. The predictive power of the cystatin C-creatinine score in assessing frailty. J. Cachexia Sarcopenia Muscle 2025, 16, e70040. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, L.; Hong, C.; Cui, H.; Wang, W.; Zhu, H.; Li, Q.; Li, Y.; Li, R.; He, J. Lower creatinine to cystatin C ratio is associated with an increased risk of MASLD: A cross-sectional and prospective study of 368,634 UK biobank participants. Clin. Endocrinol. 2024, 100, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, J.; Patel, J.; Caldwell, H.; Davies, S.; Hebditch, V.; Hollywood, C.; Hubscher, S.; Karkhanis, S.; Lester, W.; Roslund, N. Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut 2020, 69, 1382–1403. [Google Scholar] [CrossRef]
- Zambon Azevedo, V.; Silaghi, C.A.; Maurel, T.; Silaghi, H.; Ratziu, V.; Pais, R. Impact of sarcopenia on the severity of the liver damage in patients with non-alcoholic fatty liver disease. Front. Nutr. 2021, 8, 774030. [Google Scholar] [CrossRef] [PubMed]
- Isakov, V. Metabolic dysfunction-associated steatotic liver disease: A story of muscle and mass. World J. Gastroenterol. 2025, 31, 105346. [Google Scholar] [CrossRef] [PubMed]
- Tabara, Y.; Kohara, K.; Okada, Y.; Ohyagi, Y.; Igase, M. Creatinine-to-cystatin C ratio as a marker of skeletal muscle mass in older adults: J-SHIPP study. Clin. Nutr. 2020, 39, 1857–1862. [Google Scholar] [CrossRef]
- Qiu, J.; Thapaliya, S.; Runkana, A.; Yang, Y.; Tsien, C.; Mohan, M.L.; Narayanan, A.; Eghtesad, B.; Mozdziak, P.E.; McDonald, C.; et al. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-kappaB-mediated mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, 18162–18167. [Google Scholar] [CrossRef]
- Chen, D.C.; Potok, O.A.; Rifkin, D.; Estrella, M.M. Advantages, limitations, and clinical considerations in using cystatin C to estimate GFR. Kidney360 2022, 3, 1807–1814. [Google Scholar] [CrossRef]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Hara, N.; Moriya, K.; Hino, K.; Koike, K. Reduced handgrip strength predicts poorer survival in chronic liver diseases: A large multicenter study in Japan. Hepatol. Res. 2021, 51, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Chiu, W.C.; Kao, T.W.; Peng, T.C. Prevalence of sarcopenia in asian older adults: A comparison of nine diagnostic criteria across different regions. Exp. Gerontol. 2025, 202, 112721. [Google Scholar] [CrossRef]
- Aleknavičiūtė-Valienė, G.; Banys, V. Clinical importance of laboratory biomarkers in liver fibrosis. Biochem. Med. 2022, 32, 030501. [Google Scholar]
- Tong, X.F.; Wang, Q.Y.; Zhao, X.Y.; Sun, Y.M.; Wu, X.N.; Yang, L.L.; Lu, Z.Z.; Ou, X.J.; Jia, J.D.; You, H. Histological assessment based on liver biopsy: The value and challenges in NASH drug development. Acta Pharmacol. Sin. 2022, 43, 1200–1209. [Google Scholar] [CrossRef]
- Brunt, E.M.; Kleiner, D.E.; Wilson, L.A.; Belt, P.; Neuschwander-Tetri, B.A.; Network NCR. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: Distinct clinicopathologic meanings. Hepatology 2011, 53, 810–820. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Vasdev, N.; Kakati, A.G.; Saigal, S.; Nayak, N.C. Spectrum of histological features in non-alcoholic fatty liver disease. Natl. Med. J. India 2007, 20, 282–287. [Google Scholar] [PubMed]
- Kunzler, I.L.; Da Croce, M.A.; Fornari, F. Alcohol-associated liver disease increases the risk of muscle loss and mortality in patients with cirrhosis. J. Gastroenterol. 2024, 59, 1143. [Google Scholar] [CrossRef] [PubMed]

| All (n = 104) | F ≥ 2 (n = 60) | F < 2 (n = 44) | p Value | |
|---|---|---|---|---|
| Age (years) | 62.9 (50.0–67.8) | 64.9 (55.9–68.1) | 53.3 (34.9–65.0) | <0.001 |
| Sex (M/F) | 44/60 | 20/40 | 24/20 | <0.05 |
| BMI (kg/m2) | 27.3 (24.6–31.2) | 27.8 (24.4–31.9) | 26.6 (25.1–30.0) | 0.396 |
| Plt (104×/μL) | 19 (15.8–24.1) | 18.1 (14.7–21.1) | 23.5 (18.2–27.7) | <0.001 |
| AST (U/L) | 48 (34–73) | 54.5 (43.8–75.5) | 38 (29.8–66.3) | <0.05 |
| ALT (U/L) | 64 (40–95) | 61 (42.3–95) | 65 (40–87) | 0.508 |
| γ-GT (U/L) | 54 (37–91) | 68.5 (45.8–95.5) | 41.5 (27.8–65.3) | <0.05 |
| T-bil (mg/dL) | 0.8 (0.7–1.1) | 0.8 (0.7–1.1) | 0.8 (0.7–1.1) | 0.573 |
| ALB (g/dL) | 4.3 (4.1–4.4) | 4.2 (4–4.4) | 4.4 (4.2–4.5) | <0.05 |
| BUN (mg/dL) | 14 (12–16) | 14 (12–16) | 13 (11–15) | 0.0648 |
| Cr (mg/dL) | 0.69 (0.59–0.84) | 0.68 (0.57–0.81) | 0.71 (0.60–0.87) | 0.394 |
| Cysctatin C (mg/L) | 1.03 (0.89–1.16) | 1.04 (0.93–1.16) | 1.01 (0.84–1.15) | 0.185 |
| HbA1c (%) | 6.3 (5.7–7.0) | 6.4 (5.8–7.2) | 6.1 (5.6–6.9) | 0.191 |
| FIB-4 index | 1.79 (1.08–3.19) | 2.71 (1.66–3.70) | 1.14 (0.75–2.04) | <0.001 |
| Hypertension (no/yes) | 48/56 | 24/36 | 24/20 | 0.145 |
| Dyslipidemia (no/yes) | 64/40 | 33/27 | 31/13 | 0.112 |
| Diabetes (no/yes) | 44/60 | 23/37 | 21/19 | 0.342 |
| ELF score | 10.06 (9.22–11.0) | 10.65 (9.99–11.42) | 9.35 (8.74–9.84) | <0.001 |
| Cr/CysC (CCR) | 0.658 (0.581–0.816) | 0.634 (0.574–0.770) | 0.736 (0.610–0.900) | <0.05 |
| Fibrosis Stage | F0/F1/F2:F3/F4 | F2/F3/F4:25/23/12 | F0/F1:13/31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyama, M.; Namisaki, T.; Shibamoto, A.; Iwai, S.; Takami, M.; Tsuji, Y.; Fujinaga, Y.; Takaya, H.; Inoue, T.; Nishimura, N.; et al. Creatinine-to-Cystatin C Ratio Combined with FIB-4 and ELF for Noninvasive Fibrosis Assessment in MASLD. Int. J. Mol. Sci. 2025, 26, 9560. https://doi.org/10.3390/ijms26199560
Oyama M, Namisaki T, Shibamoto A, Iwai S, Takami M, Tsuji Y, Fujinaga Y, Takaya H, Inoue T, Nishimura N, et al. Creatinine-to-Cystatin C Ratio Combined with FIB-4 and ELF for Noninvasive Fibrosis Assessment in MASLD. International Journal of Molecular Sciences. 2025; 26(19):9560. https://doi.org/10.3390/ijms26199560
Chicago/Turabian StyleOyama, Masafumi, Tadashi Namisaki, Akihiko Shibamoto, Satoshi Iwai, Masayoshi Takami, Yuki Tsuji, Yukihisa Fujinaga, Hiroaki Takaya, Takashi Inoue, Norihisa Nishimura, and et al. 2025. "Creatinine-to-Cystatin C Ratio Combined with FIB-4 and ELF for Noninvasive Fibrosis Assessment in MASLD" International Journal of Molecular Sciences 26, no. 19: 9560. https://doi.org/10.3390/ijms26199560
APA StyleOyama, M., Namisaki, T., Shibamoto, A., Iwai, S., Takami, M., Tsuji, Y., Fujinaga, Y., Takaya, H., Inoue, T., Nishimura, N., Sato, S., Kitagawa, K., Kaji, K., Mitoro, A., Asada, K., Masuda, H., Hanatani, J., & Yoshiji, H. (2025). Creatinine-to-Cystatin C Ratio Combined with FIB-4 and ELF for Noninvasive Fibrosis Assessment in MASLD. International Journal of Molecular Sciences, 26(19), 9560. https://doi.org/10.3390/ijms26199560






