Porphyromonas gingivalis Lysate Induces TLR-2/4-Dependent NF-κB Activation and Inflammatory Damage in the Human Placental Barrier
Abstract
1. Introduction
2. Results
2.1. P. gingivalis Lysate Induces Dose-Dependent Histopathological Alterations in HPEs
2.2. P. gingivalis Lysate Modulates TLR-2 Expression and Induces Soluble TLR-2 Release in HPEs
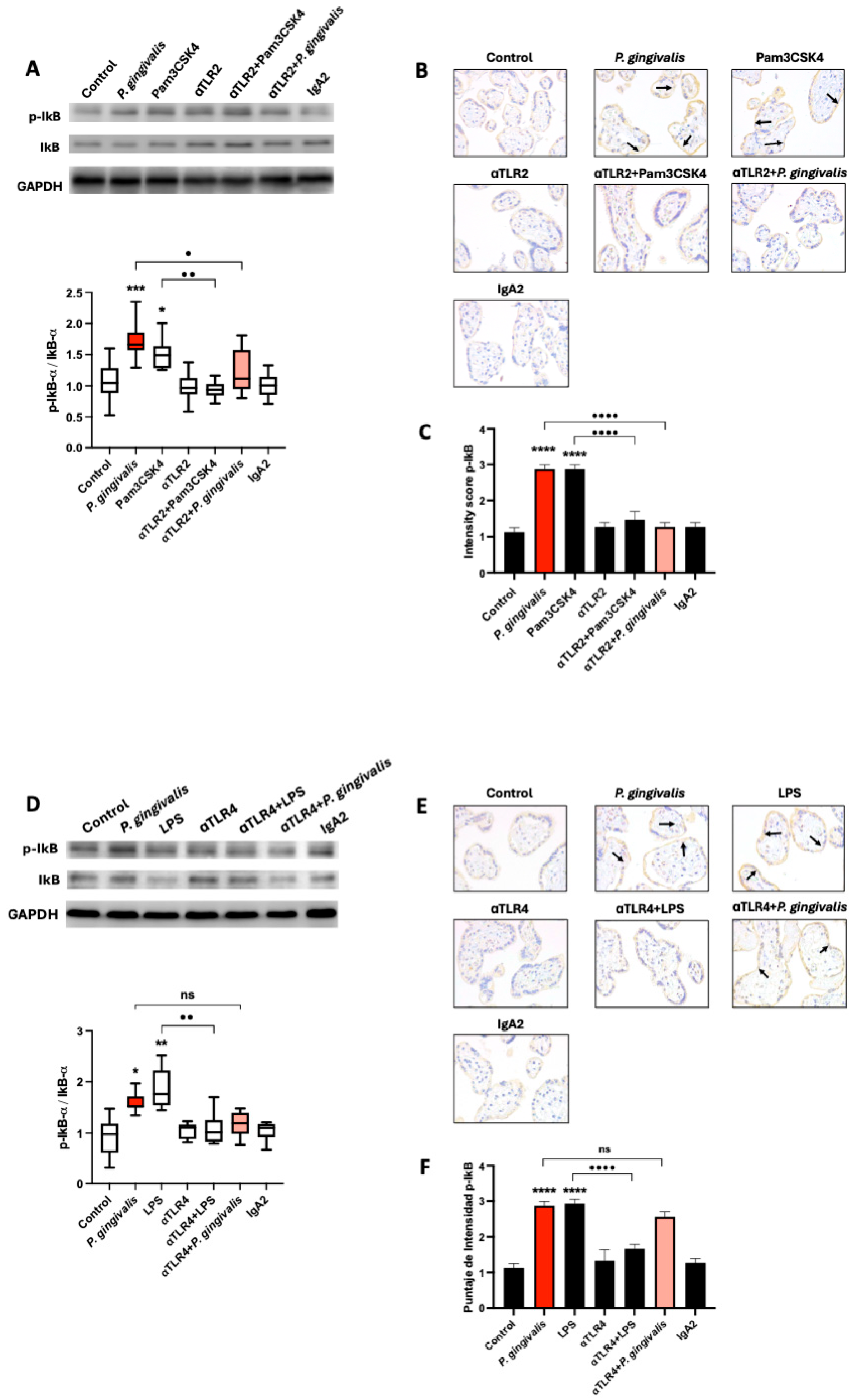
2.3. P. gingivalis Activates the Canonical NF-κB Pathway Signaling via TLR-2, but Not TLR-4, in HPEs
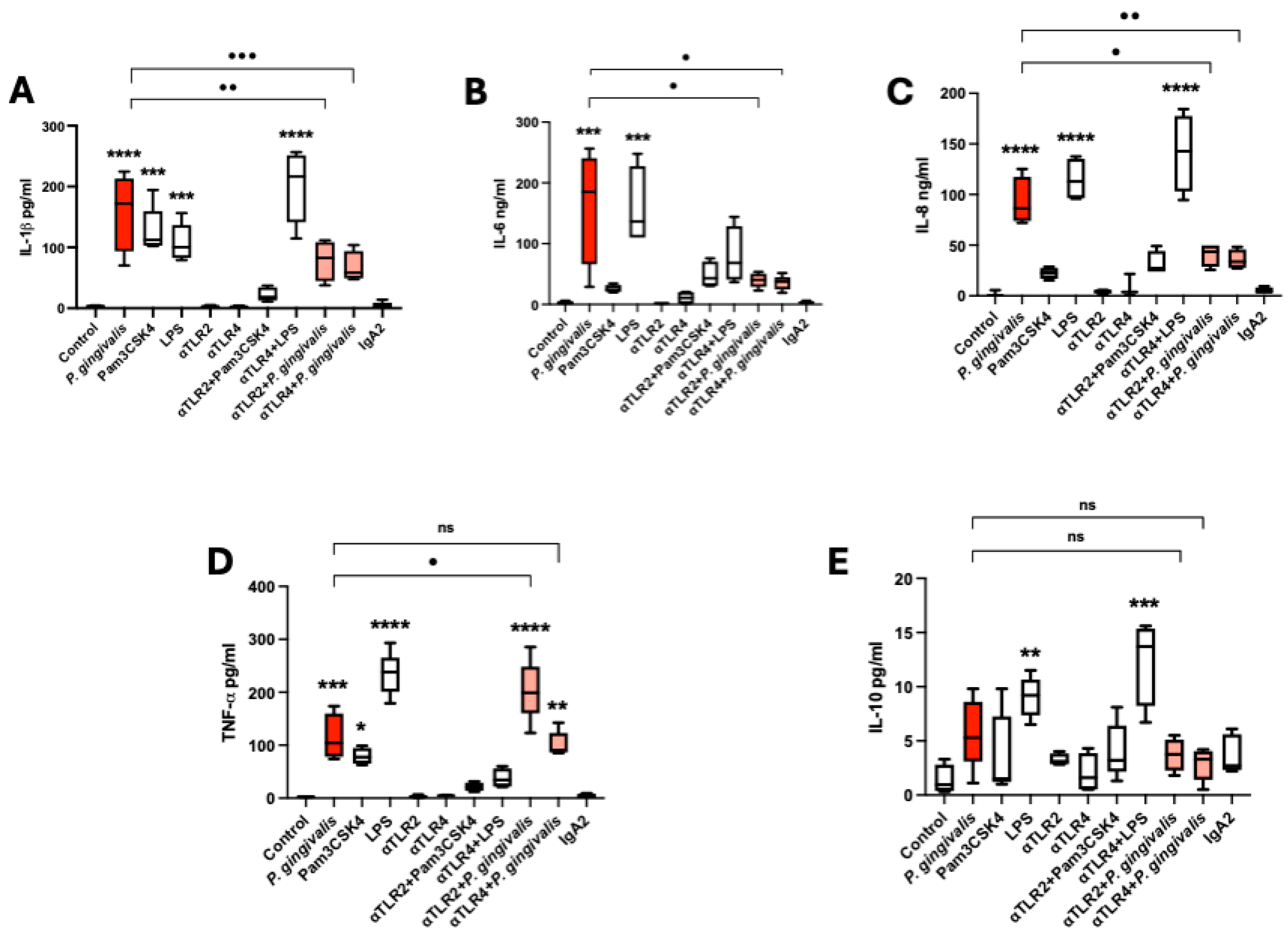
2.4. P. gingivalis Lysate Induces a TLR-2/4-Dependent Proinflammatory Cytokine Response in HPEs
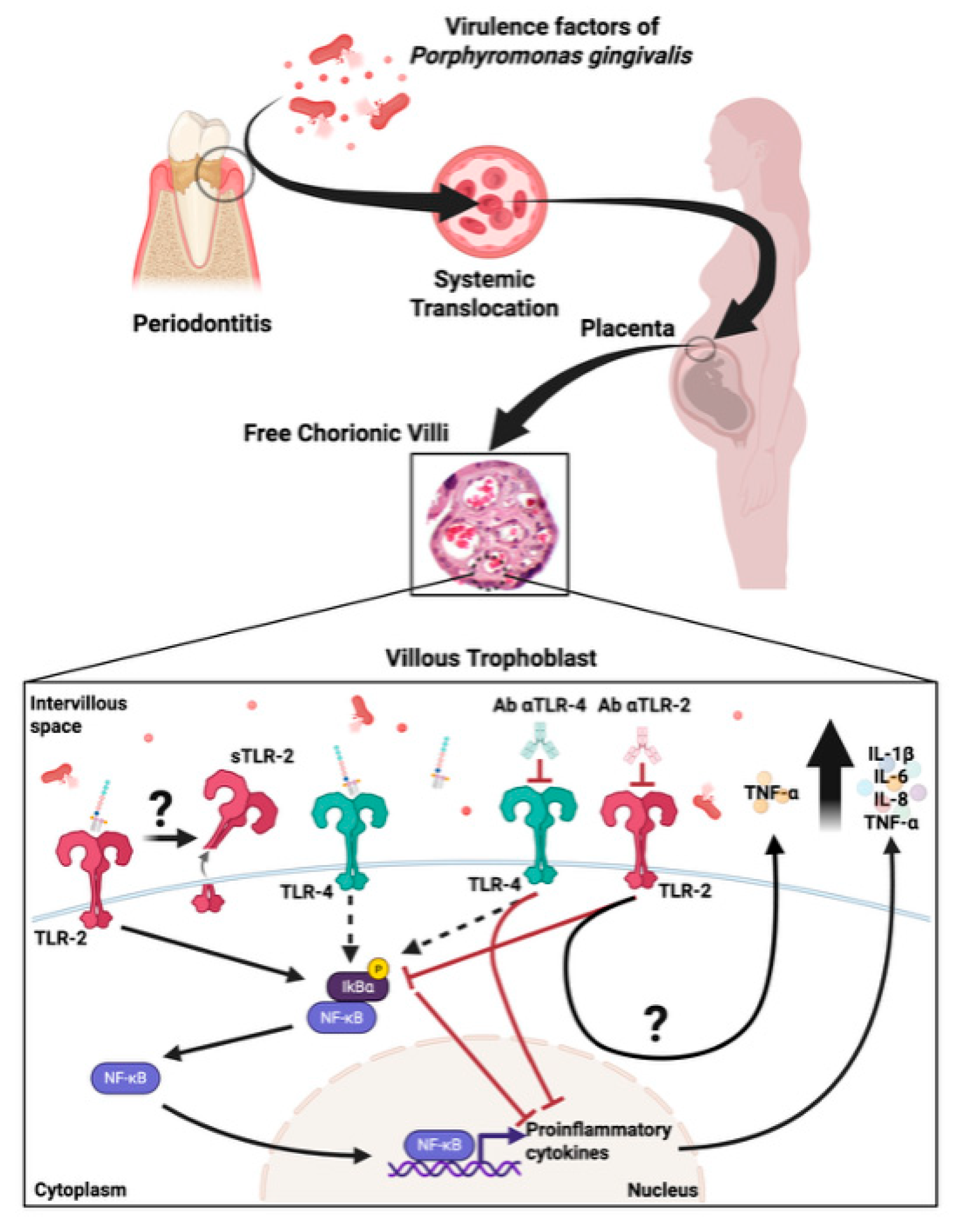
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Lysate Preparation
4.2. Human Placental Explants (HPEs) Collection and Culture
4.3. Experimental Design and Treatments
4.4. Histological and Immunohistochemical Analysis
4.5. Western Blot Analysis
4.6. Cytokine Quantification by ELISA and Cytometric Bead Array
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPEs | Human placental explants |
| P. gingivalis | Porphyromonas gingivalis |
| NF-kB | Nuclear Factor kappa B |
| TLRs | Toll-like Receptors |
| TLR-2 | Toll-like Receptor-2 |
| TLR-4 | Toll-like Receptor-4 |
| sTLR2 | Soluble TLR-2 |
| IL1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| IL-10 | Interleukin-10 |
| TNF-α | Tumor necrosis Factor alpha |
| DNA | Deoxyribonucleic acid |
| LPS | Lipopolysaccharides |
| IVS | Intervillous space |
| STB | Syncytiotrophoblast |
| CTB | Cytotrophoblast |
| VS | Villous stroma |
| PRRs | Pattern recognition receptors |
| PAMPs | Pathogen-associated molecular patterns |
| SD | Standard deviation |
| Pam3CSK4 | N-Palmitoyl-S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]- [R]-cysteinyl-[S]- seryl-[S]-lysyl-[S]-lysyl-[S]-lysyl-[S]-lysine |
| E. coli | Escherichia coli |
| p-IκBα | Phospho-I-kappa-B-alpha |
| IκBα | I-kappa-B-alpha |
| IgA2 | Immunoglobulin A2 |
| PPAD | Peptidylarginine deiminase |
| ATCC | American Type Culture Collection |
| BHI | Brain Heart Infusion |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| DL-BAPNA | Nα-Benzoyl-DL-arginine 4-nitroanilide hydrochloride |
| FBS | Fetal bovine serum |
| CO2 | Carbon dioxide |
| H&E | Hematoxylin and eosin |
| HRP | Horseradish peroxidase |
| DAB | Diaminobenzidine |
| BCA | Bicinchoninic acid |
| PVDF | Polyvinylidene fluoride |
| GAPDH | Glyceraldehyde-3-phosphate Dehydrogenase |
References
- Baergen, R.N.; Burton, G.J.; Kaplan, C.G. (Eds.) Benirschke’s Pathology of the Human Placenta; Springer International Publishing: Cham, Switzerland, 2022; ISBN 978-3-030-84724-1. [Google Scholar]
- Christian, C.; Ana, L.; Alejandro, F.-M.; Jesús, G.-M.; Sebastian, A.; Gabriela, C.; Sebastián, A.; Christian, G.; Juan Diego, M.; Marioly, M.; et al. Congenital Chagas Disease: The Importance of Trypanosoma cruzi-Placenta Interactions. Placenta 2025. [Google Scholar] [CrossRef]
- Ding, J.; Maxwell, A.; Adzibolosu, N.; Hu, A.; You, Y.; Liao, A.; Mor, G. Mechanisms of Immune Regulation by The Placenta: Role of Type I Interferon and Interferon Stimulated Genes Signaling During Pregnancy. Immunol. Rev. 2022, 308, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Aldo, P.; Alvero, A.B. The Unique Immunological and Microbial Aspects of Pregnancy. Nat. Rev. Immunol. 2017, 17, 469–482. [Google Scholar] [CrossRef]
- Ander, S.E.; Diamond, M.S.; Coyne, C.B. Immune Responses at the Maternal-Fetal Interface. Sci. Immunol. 2019, 4, eaat6114. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, S.-Q.; Zhao, L.; Ren, Z.-H.; Hu, C.-Y. Global, Regional, and National Burden of Periodontitis from 1990 to 2019: Results from the Global Burden of Disease Study 2019. J. Periodontol. 2022, 93, 1445–1454. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Liang, S.; Payne, M.A.; Hashim, A.; Jotwani, R.; Eskan, M.A.; McIntosh, M.L.; Alsam, A.; Kirkwood, K.L.; Lambris, J.D.; et al. Low-Abundance Biofilm Species Orchestrates Inflammatory Periodontal Disease through the Commensal Microbiota and Complement. Cell Host Microbe 2011, 10, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Herath, T.D.K.; Darveau, R.P.; Seneviratne, C.J.; Wang, C.-Y.; Wang, Y.; Jin, L. Tetra- and Penta-Acylated Lipid A Structures of Porphyromonas gingivalis LPS Differentially Activate TLR4-Mediated NF-κB Signal Transduction Cascade and Immuno-Inflammatory Response in Human Gingival Fibroblasts. PLoS ONE 2013, 8, e58496. [Google Scholar] [CrossRef] [PubMed]
- How, K.Y.; Song, K.P.; Chan, K.G. Porphyromonas gingivalis: An Overview of Periodontopathic Pathogen below the Gum Line. Front. Microbiol. 2016, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Widziolek, M.; Mieszkowska, A.; Marcinkowska, M.; Salamaga, B.; Folkert, J.; Rakus, K.; Chadzinska, M.; Potempa, J.; Stafford, G.P.; Prajsnar, T.K.; et al. Gingipains Protect Porphyromonas gingivalis from Macrophage-Mediated Phagocytic Clearance. PLoS Pathog. 2025, 21, e1012821. [Google Scholar] [CrossRef]
- Nannan, M.; Xiaoping, L.; Ying, J. Periodontal Disease in Pregnancy and Adverse Pregnancy Outcomes: Progress in Related Mechanisms and Management Strategies. Front. Med. 2022, 9, 963956. [Google Scholar] [CrossRef]
- Vanterpool, S.F.; Been, J.V.; Houben, M.L.; Nikkels, P.G.J.; De Krijger, R.R.; Zimmermann, L.J.I.; Kramer, B.W.; Progulske-Fox, A.; Reyes, L. Porphyromonas gingivalis within Placental Villous Mesenchyme and Umbilical Cord Stroma Is Associated with Adverse Pregnancy Outcome. PLoS ONE 2016, 11, e0146157. [Google Scholar] [CrossRef] [PubMed]
- Bobetsis, Y.A.; Ide, M.; Gürsoy, M.; Madianos, P.N. Periodontal Diseases and Adverse Pregnancy Outcomes. Present and Future. Periodontology 2000 2020, 83, 154–174. [Google Scholar] [CrossRef] [PubMed]
- Reyes, L.; Phillips, P.; Wolfe, B.; Golos, T.G.; Walkenhorst, M.; Progulske-Fox, A.; Brown, M. Porphyromonas gingivalis and Adverse Pregnancy Outcome. J. Oral Microbiol. 2017, 10, 1374153. [Google Scholar] [CrossRef]
- Chopra, A.; Radhakrishnan, R.; Sharma, M. Porphyromonas gingivalis and Adverse Pregnancy Outcomes: A Review on Its Intricate Pathogenic Mechanisms. Crit. Rev. Microbiol. 2020, 46, 213–236. [Google Scholar] [CrossRef]
- Takii, R.; Kadowaki, T.; Tsukuba, T.; Yamamoto, K. Inhibition of Gingipains Prevents Porphyromonas gingivalis-Induced Preterm Birth and Fetal Death in Pregnant Mice. Eur. J. Pharmacol. 2018, 824, 48–56. [Google Scholar] [CrossRef]
- Castillo, C.; Díaz-Luján, C.; Liempi, A.; Fretes, R.; Kemmerling, U. Mammalian Placental Explants: A Tool for Studying Host-Parasite Interactions and Placental Biology. Placenta 2024, 166, 62–70. [Google Scholar] [CrossRef]
- Koga, K.; Izumi, G.; Mor, G.; Fujii, T.; Osuga, Y. Toll-like Receptors at the Maternal-Fetal Interface in Normal Pregnancy and Pregnancy Complications. Am. J. Reprod. Immunol. 2014, 72, 192–205. [Google Scholar] [CrossRef]
- Liempi, A.; Castillo, C.; Medina, L.; Rojas, M.; Maya, J.D.; Parraguez, V.H.; Kemmerling, U. Ex Vivo Infection of Human Placental Explants with Trypanosoma cruzi and Toxoplasma gondii: Differential Activation of NF Kappa B Signaling Pathways. Acta Trop. 2019, 199, 105153. [Google Scholar] [CrossRef]
- Kim, Y.M.; Romero, R.; Oh, S.Y.; Kim, C.J.; Kilburn, B.A.; Armant, D.R.; Nien, J.K.; Gomez, R.; Mazor, M.; Saito, S.; et al. Toll-like Receptor 4: A Potential Link between “Danger Signals,” the Innate Immune System, and Preeclampsia? Am. J. Obs. Gynecol. 2005, 193, 921–927. [Google Scholar] [CrossRef]
- Langjahr, P.; Díaz-Jiménez, D.; De la Fuente, M.; Rubio, E.; Golenbock, D.; Bronfman, F.C.; Quera, R.; González, M.-J.; Hermoso, M.A. Metalloproteinase-Dependent TLR2 Ectodomain Shedding Is Involved in Soluble Toll-like Receptor 2 (sTLR2) Production. PLoS ONE 2014, 9, e104624. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Katagiri, S.; Miyasaka, N.; Kobayashi, H.; Khemwong, T.; Nagasawa, T.; Izumi, Y. The Periodontopathic Bacteria in Placenta, Saliva and Subgingival Plaque of Threatened Preterm Labor and Preterm Low Birth Weight Cases: A Longitudinal Study in Japanese Pregnant Women. Clin. Oral Investig. 2020, 24, 4261–4270. [Google Scholar] [CrossRef]
- Hajishengallis, G. Immunomicrobial Pathogenesis of Periodontitis: Keystones, Pathobionts, and Host Response. Trends Immunol. 2014, 35, 3–11. [Google Scholar] [CrossRef]
- Wielento, A.; Bereta, G.P.; Łagosz-Ćwik, K.B.; Eick, S.; Lamont, R.J.; Grabiec, A.M.; Potempa, J. TLR2 Activation by Porphyromonas gingivalis Requires Both PPAD Activity and Fimbriae. Front. Immunol. 2022, 13, 823685. [Google Scholar] [CrossRef]
- Kaczynska, A.; Klosinska, M.; Janeczek, K.; Zarobkiewicz, M.; Emeryk, A. Promising Immunomodulatory Effects of Bacterial Lysates in Allergic Diseases. Front. Immunol. 2022, 13, 907149. [Google Scholar] [CrossRef]
- Di Gioacchino, M.; Santilli, F.; Pession, A. Is There a Role for Immunostimulant Bacterial Lysates in the Management of Respiratory Tract Infection? Biomolecules 2024, 14, 1249. [Google Scholar] [CrossRef]
- Dieterle, M.P.; Husari, A.; Steinberg, T.; Wang, X.; Ramminger, I.; Tomakidi, P. From the Matrix to the Nucleus and Back: Mechanobiology in the Light of Health, Pathologies, and Regeneration of Oral Periodontal Tissues. Biomolecules 2021, 11, 824. [Google Scholar] [CrossRef]
- Andrian, E.; Grenier, D.; Rouabhia, M. In Vitro Models of Tissue Penetration and Destruction by Porphyromonas gingivalis. Infect. Immun. 2004, 72, 4689–4698. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, M.N.; Wang, G.D.; Wu, Q.; Zhao, Y.F. Cyclosporin A Suppresses Porphyromonas gingivalis Lipopolysaccharide Induced Matrix Metalloproteinases Activities in the Co-Culture of Human Gingival Fibroblasts and Monocyte Cell Line THP-1. Growth Factors 2020, 38, 65–74. [Google Scholar] [CrossRef]
- Rosini, A.M.; Teixeira, S.C.; Milian, I.C.B.; Silva, R.J.; de Souza, G.; Luz, L.C.; Gomes, A.O.; Mineo, J.R.; Mineo, T.W.P.; Ferro, E.A.V.; et al. LPS-Mediated Activation of TLR4 Controls Toxoplasma Gondii Growth in Human Trophoblast Cell (BeWo) and Human Villous Explants in a Dependent-Manner of TRIF, MyD88, NF-κB and Cytokines. Tissue Cell 2022, 78, 101907. [Google Scholar] [CrossRef]
- LeBouder, E.; Rey-Nores, J.E.; Rushmere, N.K.; Grigorov, M.; Lawn, S.D.; Affolter, M.; Griffin, G.E.; Ferrara, P.; Schiffrin, E.J.; Morgan, B.P.; et al. Soluble Forms of Toll-like Receptor (TLR)2 Capable of Modulating TLR2 Signaling Are Present in Human Plasma and Breast Milk. J. Immunol. 2003, 171, 6680–6689. [Google Scholar] [CrossRef]
- Napetschnig, J.; Wu, H. Molecular Basis of NF-κB Signaling. Annu. Rev. Biophys. 2013, 42, 443–468. [Google Scholar] [CrossRef]
- Liu, B.; Wang, J.; Cheng, L.; Liang, J. Role of JNK and NF-κB Pathways in Porphyromonas gingivalis LPS-Induced Vascular Cell Adhesion Molecule-1 Expression in Human Aortic Endothelial Cells. Mol. Med. Rep. 2013, 8, 1594–1600. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kramer, B.W.; Kaemmerer, U.; Kapp, M.; Herbst, D.; Marx, A.; Berg, D.; Groneck, P.A.; Speer, C.P. Decreased Expression of Angiogenic Factors in Placentas with Chorioamnionitis after Preterm Birth. Pediatr. Res. 2005, 58, 607–612. [Google Scholar] [CrossRef] [PubMed]
- ATCC 33277. Available online: https://www.atcc.org/products/33277?matchtype=&network=x&device=c&adposition=&keyword=&gad_source=1&gad_campaignid=17725151935&gclid=EAIaIQobChMIv5H5gfb_jwMVEZaDBx274SyWEAAYAiAAEgJ5UfD_BwE (accessed on 28 September 2025).
- Veloso, P.; Fernández, A.; Astorga, J.; González-Quintanilla, D.; Castro, A.; Escobar, A.; Hoare, A.; Hernández, M. Lipopolysaccharide from Porphyromonas gingivalis, but Not from Porphyromonas Endodontalis, Induces Macrophage M1 Profile. Int. J. Mol. Sci. 2022, 23, 10011. [Google Scholar] [CrossRef]
- Fernández-Moya, A.; Oviedo, B.; Liempi, A.I.; Guerrero-Muñoz, J.; Rivas, C.; Arregui, R.; Araneda, S.; Cornet-Gomez, A.; Maya, J.D.; Müller, M.; et al. Trypanosoma cruzi-Derived Exovesicles Contribute to Parasite Infection, Tissue Damage, and Apoptotic Cell Death during Ex Vivo Infection of Human Placental Explants. Front. Cell. Infect. Microbiol. 2024, 14, 1437339. [Google Scholar] [CrossRef]
- Castillo, C.; Muñoz, L.; Carrillo, I.; Liempi, A.; Gallardo, C.; Galanti, N.; Maya, J.D.; Kemmerling, U. Ex Vivo Infection of Human Placental Chorionic Villi Explants with Trypanosoma cruzi and Toxoplasma gondii Induces Different Toll-like Receptor Expression and Cytokine/Chemokine Profiles. Am. J. Reprod. Immunol. 2017, 78, e12660. [Google Scholar] [CrossRef]
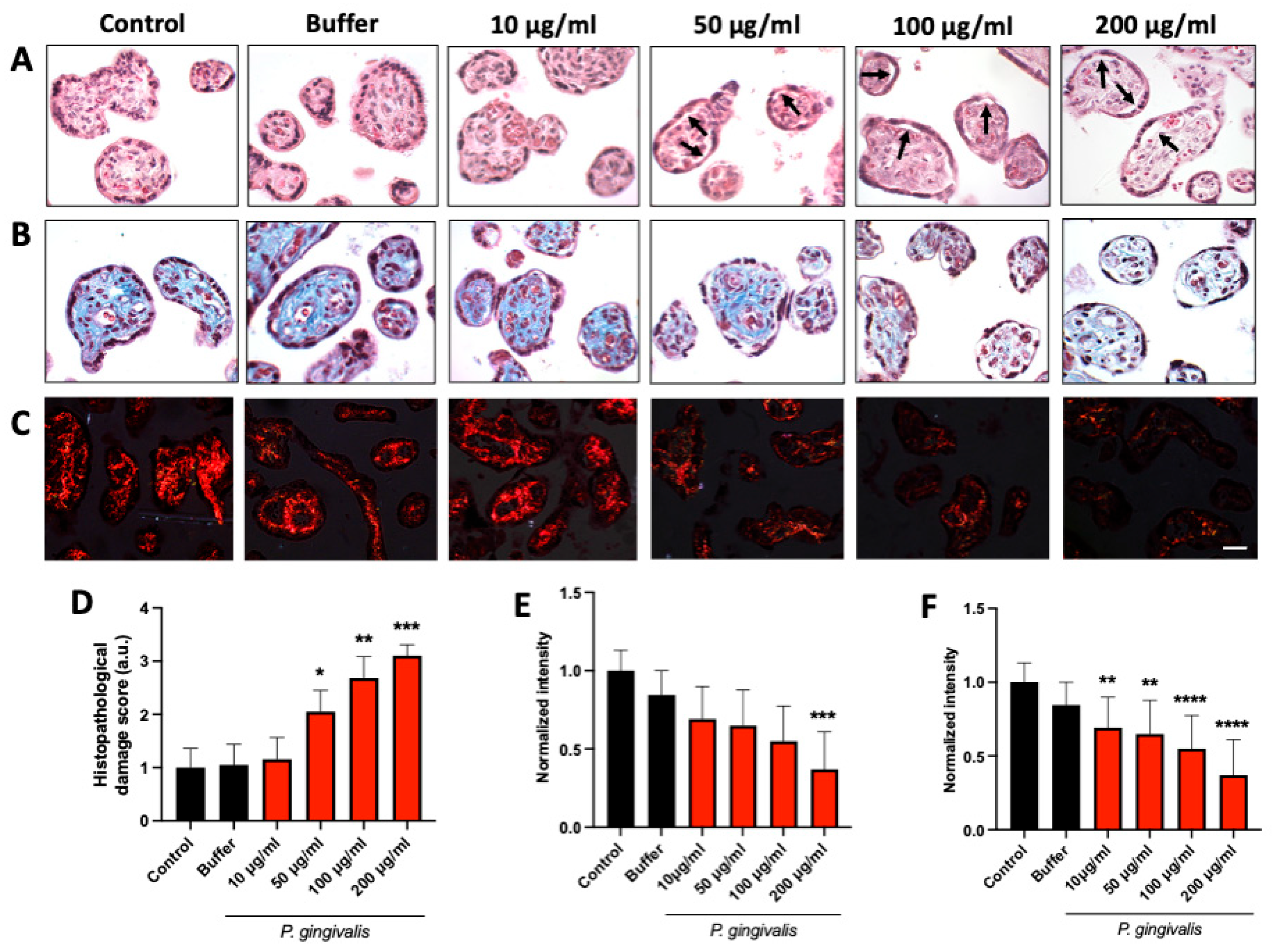

| Score | Histopathology Damage |
|---|---|
| 1 | Attached trophoblast, intact fetal connective tissue |
| 2 | Slight trophoblast detachment and/or fetal connective tissue disorganization |
| 3 | Almost complete trophoblast detachment and/or fetal connective tissue disorganization |
| 4 | Complete trophoblast detachment and disorganization or destruction of the fetal connective tissue |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araneda-Rojas, S.; Castillo, C.; Liempi, A.; Fernández-Moya, A.; Guerrero-Muñoz, J.; Alfaro, S.; Gallardo, C.; Arregui, R.; Hoare, A.; Gleisner, M.A.; et al. Porphyromonas gingivalis Lysate Induces TLR-2/4-Dependent NF-κB Activation and Inflammatory Damage in the Human Placental Barrier. Int. J. Mol. Sci. 2025, 26, 9558. https://doi.org/10.3390/ijms26199558
Araneda-Rojas S, Castillo C, Liempi A, Fernández-Moya A, Guerrero-Muñoz J, Alfaro S, Gallardo C, Arregui R, Hoare A, Gleisner MA, et al. Porphyromonas gingivalis Lysate Induces TLR-2/4-Dependent NF-κB Activation and Inflammatory Damage in the Human Placental Barrier. International Journal of Molecular Sciences. 2025; 26(19):9558. https://doi.org/10.3390/ijms26199558
Chicago/Turabian StyleAraneda-Rojas, Sebastián, Christian Castillo, Ana Liempi, Alejandro Fernández-Moya, Jesús Guerrero-Muñoz, Sebastián Alfaro, Christian Gallardo, Rocío Arregui, Anilei Hoare, Maria Alejandra Gleisner, and et al. 2025. "Porphyromonas gingivalis Lysate Induces TLR-2/4-Dependent NF-κB Activation and Inflammatory Damage in the Human Placental Barrier" International Journal of Molecular Sciences 26, no. 19: 9558. https://doi.org/10.3390/ijms26199558
APA StyleAraneda-Rojas, S., Castillo, C., Liempi, A., Fernández-Moya, A., Guerrero-Muñoz, J., Alfaro, S., Gallardo, C., Arregui, R., Hoare, A., Gleisner, M. A., Hernández, M., & Kemmerling, U. (2025). Porphyromonas gingivalis Lysate Induces TLR-2/4-Dependent NF-κB Activation and Inflammatory Damage in the Human Placental Barrier. International Journal of Molecular Sciences, 26(19), 9558. https://doi.org/10.3390/ijms26199558









