Translational Insights into NK Immunophenotyping: Comparative Surface Marker Analysis and Circulating Immune Cell Profiling in Cancer Immunotherapy
Abstract
1. Introduction
2. Approaches to Assess the Overall State of the Immune System Using Peripheral Blood
3. Some Examples of Changes in Immune Cell Populations in Cancer
4. Principle Criteria for the Identification of Immune Cells
4.1. T Cells or T Lymphocytes
4.2. B Cells or B Lymphocytes
4.3. Monocytes
Macrophages and Dendritic Cells of Peripheral Blood
4.4. Neutrophils
4.5. NKT Cells
4.6. Natural Killer (NK) Cells
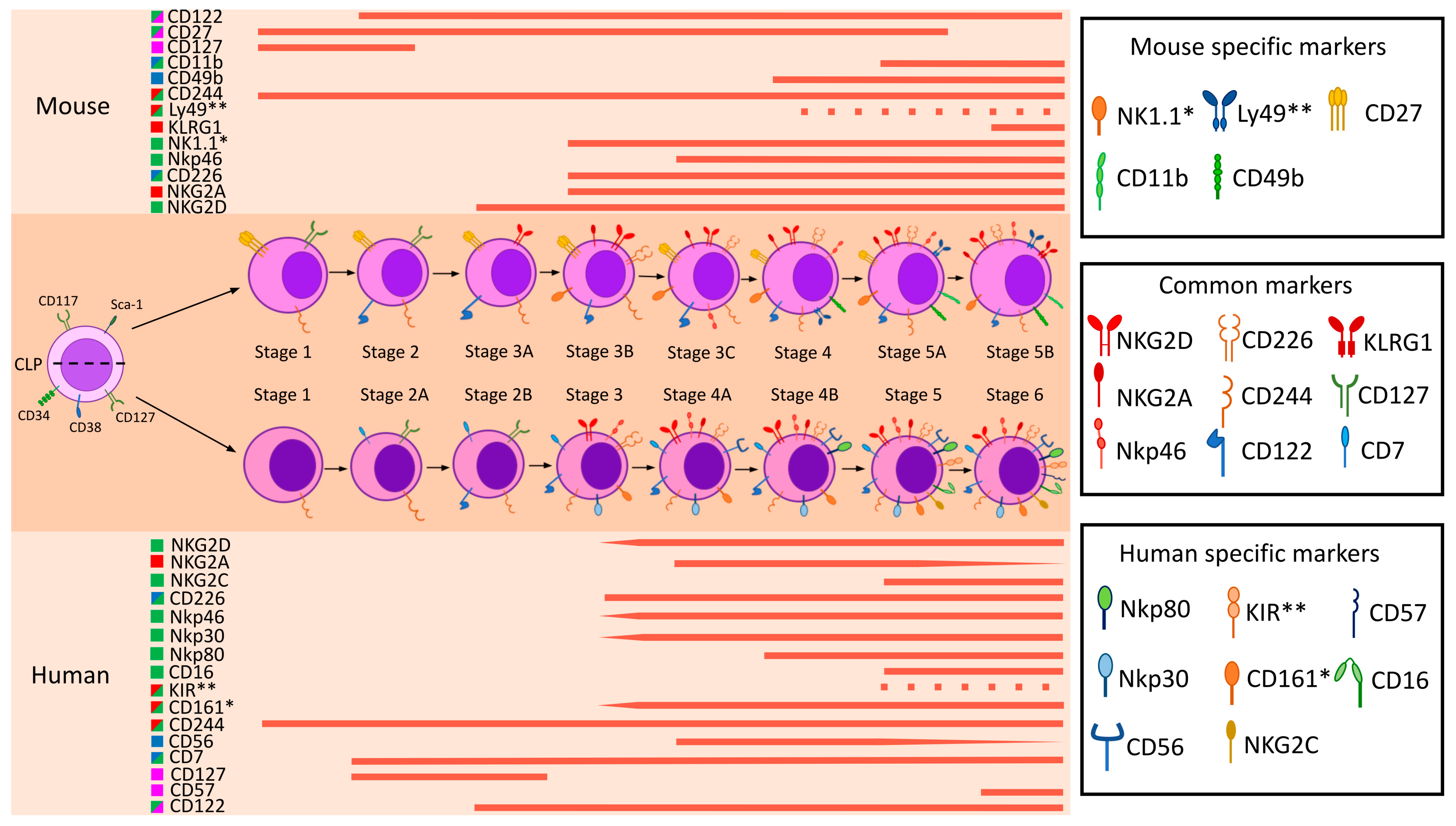
5. A Brief Outline of Human and Mouse NK Cell Maturation
6. Comparison of Human and Mouse Surface NK Markers
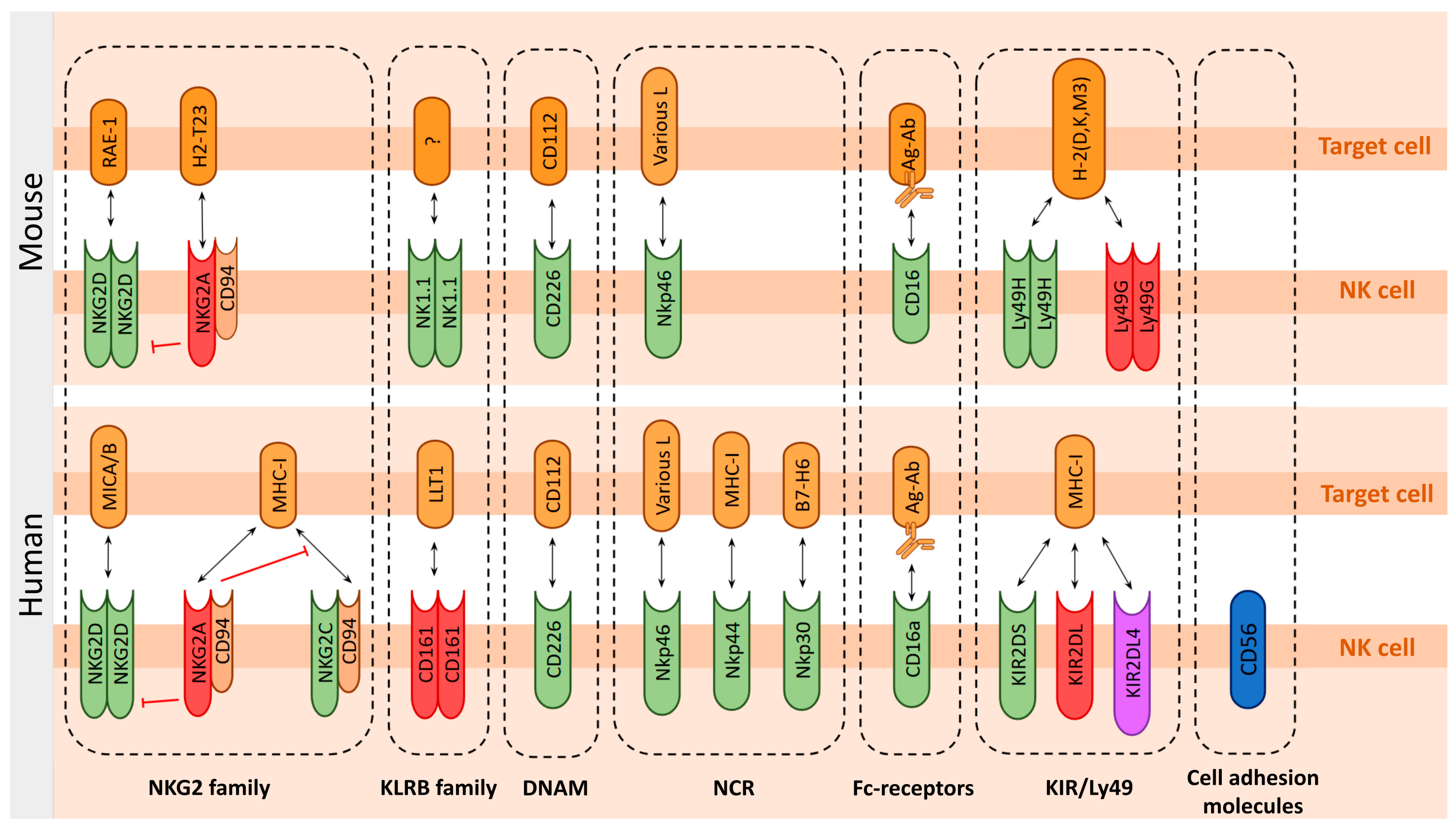
6.1. Function-Determining Molecules of the NK Cells
6.1.1. Death Ligands in NK Cell-Mediated Cytotoxicity
6.1.2. Lectins and Lectin-like Receptors
NKG2 Family
NKRP1 Family—Human KLRB1 vs. Mice NK1.1 Receptors
6.1.3. DNAM-1 (CD226)
6.1.4. Natural Cytotoxic Receptors: NCR Family
6.1.5. CD16 (FcγRIII) IgG Receptor
6.1.6. KIRs and Ly49—Mutually Exclusive Human and Mouse Receptor Families
Killer Cell Immunoglobulin-like Receptors (KIRs) Family
Ly49 Family Receptors
6.2. Degranulation Markers
6.3. Other Characteristic Markers of NK Cell
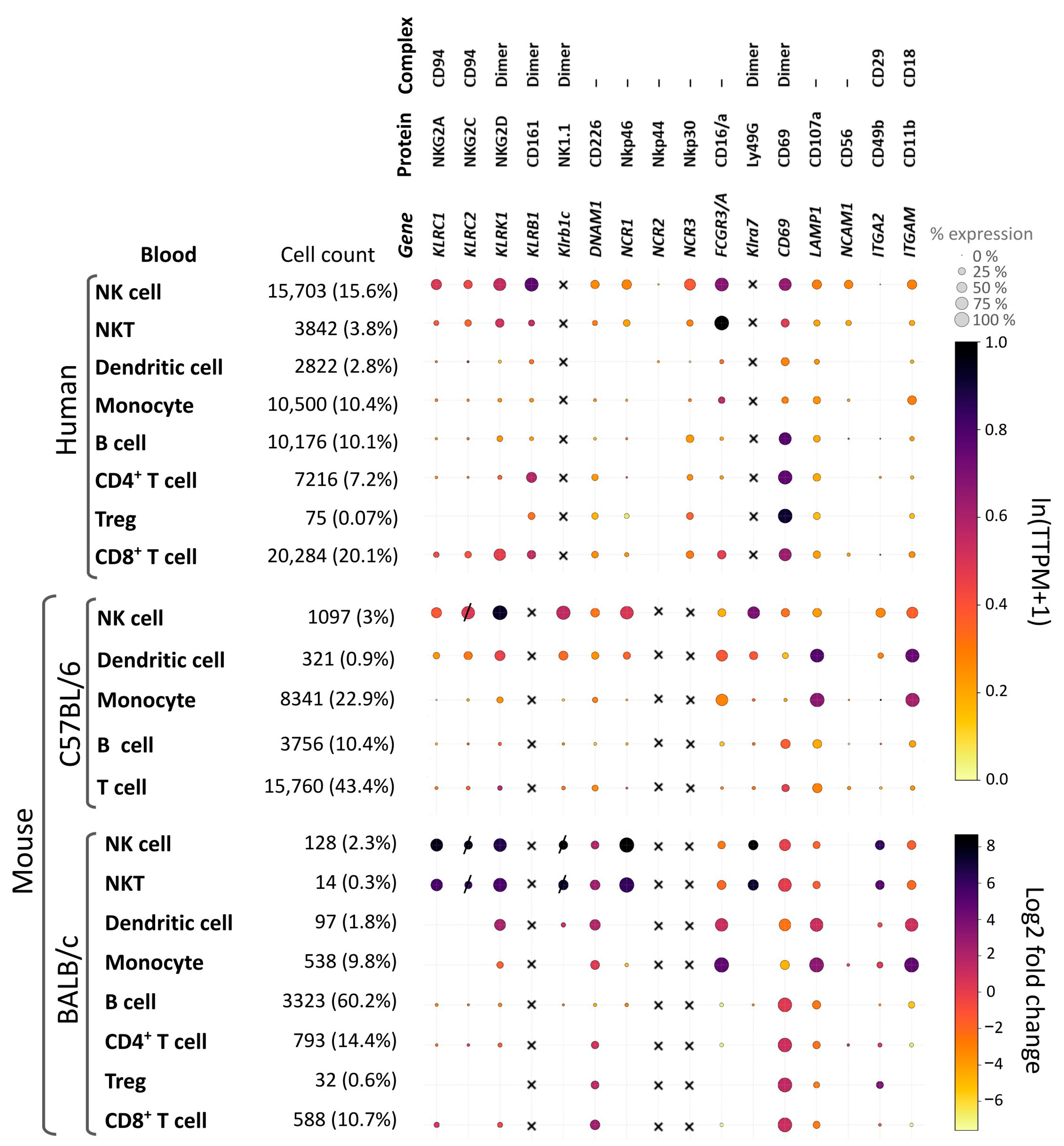
6.4. Systematic Comparison of Key NK Cell Surface Markers to Access Their Translational Relevance
7. Approaches to the Assessment of Host Immune Status by Analysis of Cell Populations
- Standardized immunoprofiling is essential for translational relevance. Prioritizing conserved surface markers across species in a workflow enables the direct correlation of preclinical findings with clinical outcomes.
- NK Cell Identification: In mouse models, NK cells are usually identified as CD3−NK1.1+ in the C57BL/6 strain and as CD3−Nkp46+ in the BALB/c strain. Additional markers, such as CD49b, are sometimes used. In humans, NK cells are identified as CD3−CD56+ populations. Accurate analysis requires careful exclusion of T and B cell populations.
- Priority to Activating Markers: The primary markers of interest are NKG2D, CD107a, and CD69 because they indicate activation status and functional potential. An alternative approach is to examine inhibitory receptors (NKG2A and PD-1) to identify functionally suppressed NK cell subsets that may affect treatment outcomes.
- Longitudinal monitoring of circulating immune cells provides a dynamic readout of therapy efficacy. This approach captures systemic immune activation, distinguishes responders from nonresponders, and aligns with the principles of minimally invasive liquid biopsy.
- A phased translational pipeline—from mechanistic validation in murine models to biomarker confirmation in clinical trials—is critical for developing predictive signatures. This strategy ensures that immune profiles are biologically grounded and clinically actionable for patient stratification.
8. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADCC | antibody-dependent cellular cytotoxicity |
| BC | breast cancer |
| ctDNA | circulating tumor DNA |
| CTCs | circulating tumor clusters |
| CTL | CD8+ cytotoxic T lymphocyte |
| CLEC | C-type lectin receptor |
| CTLR | C-type lectin-like receptor |
| CTLD | C-type lectin-like domain |
| CRC | colorectal cancer |
| DC | dendritic cell |
| DP T cell | double-positive T cell |
| GZM | granzyme |
| HSC | hematopoietic stem cell |
| ITAM | immunoreceptor tyrosine-based activation motif |
| ITIM | immunoreceptor tyrosine-based inhibitory motifs |
| KIR | killer cell immunoglobulin-like receptor |
| LPS | lipopolysaccharide |
| MHC | major histocompatibility complex |
| MF | macrophage |
| NCR | natural cytotoxicity receptors |
| NK | natural killer |
| NSCLC | non-small-cell lung cancer |
| PBMC | peripheral blood mononuclear cell |
| PRF | perforin |
| TCR | T cell receptor |
| Th | CD4+ T helper |
| TME | tumor microenvironment |
| Treg | regulatory T cell |
References
- Mamessier, E.; Pradel, L.C.; Thibult, M.L.; Drevet, C.; Zouine, A.; Jacquemier, J.; Houvenaeghel, G.; Bertucci, F.; Birnbaum, D.; Olive, D. Peripheral blood NK cells from breast cancer patients are tumor-induced composite subsets. J. Immunol. 2013, 190, 2424–2436. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Sausville, E.A.; Hollingshead, M. Mouse Models in Cancer Drug Discovery and Development. In Handbook of Anticancer Pharmacokinetics and Pharmacodynamics; Figg, W.D., McLeod, H.L., Eds.; Humana Press: Totowa, NJ, USA, 2004; pp. 45–55. [Google Scholar]
- Guo, H.; Xu, X.; Zhang, J.; Du, Y.; Yang, X.; He, Z.; Zhao, L.; Liang, T.; Guo, L. The Pivotal Role of Preclinical Animal Models in Anti-Cancer Drug Discovery and Personalized Cancer Therapy Strategies. Pharmaceuticals 2024, 17, 1048. [Google Scholar] [CrossRef]
- Coenon, L.; Geindreau, M.; Ghiringhelli, F.; Villalba, M.; Bruchard, M. Natural Killer cells at the frontline in the fight against cancer. Cell Death Dis. 2024, 15, 614. [Google Scholar] [CrossRef]
- Chan, I.S.; Ewald, A.J. The changing role of natural killer cells in cancer metastasis. J. Clin. Investig. 2022, 132, e143762. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y. The Function of NK Cells in Tumor Metastasis and NK Cell-Based Immunotherapy. Cancers 2023, 15, 2323. [Google Scholar] [CrossRef]
- Masmoudi, D.; Villalba, M.; Alix-Panabieres, C. Natural killer cells: The immune frontline against circulating tumor cells. J. Exp. Clin. Cancer Res. 2025, 44, 118. [Google Scholar] [CrossRef] [PubMed]
- Kyrysyuk, O.; Wucherpfennig, K.W. Designing Cancer Immunotherapies That Engage T Cells and NK Cells. Annu. Rev. Immunol. 2023, 41, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Maskalenko, N.A.; Zhigarev, D.; Campbell, K.S. Harnessing natural killer cells for cancer immunotherapy: Dispatching the first responders. Nat. Rev. Drug Discov. 2022, 21, 559–577. [Google Scholar] [CrossRef]
- Morcillo-Martin-Romo, P.; Valverde-Pozo, J.; Ortiz-Bueno, M.; Arnone, M.; Espinar-Barranco, L.; Espinar-Barranco, C.; Garcia-Rubino, M.E. The Role of NK Cells in Cancer Immunotherapy: Mechanisms, Evasion Strategies, and Therapeutic Advances. Biomedicines 2025, 13, 857. [Google Scholar] [CrossRef]
- Yang, Y.L.; Yang, F.; Huang, Z.Q.; Li, Y.Y.; Shi, H.Y.; Sun, Q.; Ma, Y.; Wang, Y.; Zhang, Y.; Yang, S.; et al. T cells, NK cells, and tumor-associated macrophages in cancer immunotherapy and the current state of the art of drug delivery systems. Front. Immunol. 2023, 14, 1199173. [Google Scholar] [CrossRef]
- Liu, S.; Galat, V.; Galat, Y.; Lee, Y.K.A.; Wainwright, D.; Wu, J. NK cell-based cancer immunotherapy: From basic biology to clinical development. J. Hematol. Oncol. 2021, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, J.M.; Buzdin, A.A.; Mohammad, T.; Rakitina, O.A.; Didych, D.A.; Pleshkan, V.V.; Alekseenko, I.V. Molecules promoting circulating clusters of cancer cells suggest novel therapeutic targets for treatment of metastatic cancers. Front. Immunol. 2023, 14, 1099921. [Google Scholar] [CrossRef]
- Zhu, Z.; Hu, E.; Shen, H.; Tan, J.; Zeng, S. The functional and clinical roles of liquid biopsy in patient-derived models. J. Hematol. Oncol. 2023, 16, 36. [Google Scholar] [CrossRef]
- Cheung, A.H.; Chow, C.; To, K.F. Latest development of liquid biopsy. J. Thorac. Dis. 2018, 10 (Suppl. S14), S1645–S1651. [Google Scholar] [CrossRef]
- Huang, H.M.; Li, H.X. Tumor heterogeneity and the potential role of liquid biopsy in bladder cancer. Cancer Commun. 2021, 41, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Guo, H.; Zhao, Y.; Liu, Z.; Wang, C.; Bu, J.; Sun, T.; Wei, J. Liquid biopsy in cancer current: Status, challenges and future prospects. Signal Transduct. Target. Ther. 2024, 9, 336. [Google Scholar] [CrossRef]
- Trapani, J.A.; Smyth, M.J. Functional significance of the perforin/granzyme cell death pathway. Nat. Rev. Immunol. 2002, 2, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.J.; Cretney, E.; Kelly, J.M.; Westwood, J.A.; Street, S.E.; Yagita, H.; Takeda, K.; van Dommelen, S.L.; Degli-Esposti, M.A.; Hayakawa, Y. Activation of NK cell cytotoxicity. Mol. Immunol. 2005, 42, 501–510. [Google Scholar] [CrossRef]
- Rooney, M.S.; Shukla, S.A.; Wu, C.J.; Getz, G.; Hacohen, N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 2015, 160, 48–61. [Google Scholar] [CrossRef]
- Wakiyama, H.; Masuda, T.; Motomura, Y.; Hu, Q.; Tobo, T.; Eguchi, H.; Sakamoto, K.; Hirakawa, M.; Honda, H.; Mimori, K. Cytolytic Activity (CYT) Score Is a Prognostic Biomarker Reflecting Host Immune Status in Hepatocellular Carcinoma (HCC). Anticancer Res. 2018, 38, 6631–6638. [Google Scholar] [CrossRef]
- Zhang, X.; Jing, J. Effect of Peripheral Blood Lymphocytes on Prognosis of Multiple Cancers. Cancer Control 2023, 30, 10732748231202921. [Google Scholar] [CrossRef]
- Li, M.; Yao, D.; Zeng, X.; Kasakovski, D.; Zhang, Y.; Chen, S.; Zha, X.; Li, Y.; Xu, L. Age related human T cell subset evolution and senescence. Immun. Ageing 2019, 16, 24. [Google Scholar] [CrossRef]
- Amadori, A.; Zamarchi, R.; De Silvestro, G.; Forza, G.; Cavatton, G.; Danieli, G.A.; Clementi, M.; Chieco-Bianchi, L. Genetic control of the CD4/CD8 T-cell ratio in humans. Nat. Med. 1995, 1, 1279–1283. [Google Scholar] [CrossRef] [PubMed]
- Callahan, J.E.; Kappler, J.W.; Marrack, P. Unexpected expansions of CD8-bearing cells in old mice. J. Immunol. 1993, 151, 6657–6669. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.M.; Park, H.J.; Choi, E.A.; Jung, K.C.; Lee, J.I. Cellular heterogeneity of circulating CD4+CD8+ double-positive T cells characterized by single-cell RNA sequencing. Sci. Rep. 2021, 11, 23607. [Google Scholar] [CrossRef]
- Hagen, M.; Pangrazzi, L.; Rocamora-Reverte, L.; Weinberger, B. Legend or Truth: Mature CD4+CD8+ Double-Positive T Cells in the Periphery in Health and Disease. Biomedicines 2023, 11, 2702. [Google Scholar] [CrossRef]
- Schad, S.E.; Chow, A.; Mangarin, L.; Pan, H.; Zhang, J.; Ceglia, N.; Caushi, J.X.; Malandro, N.; Zappasodi, R.; Gigoux, M.; et al. Tumor-induced double positive T cells display distinct lineage commitment mechanisms and functions. J. Exp. Med. 2022, 219, e20212169. [Google Scholar] [CrossRef]
- Bohner, P.; Chevalier, M.F.; Cesson, V.; Rodrigues-Dias, S.C.; Dartiguenave, F.; Burruni, R.; Tawadros, T.; Valerio, M.; Lucca, I.; Nardelli-Haefliger, D.; et al. Double Positive CD4+CD8+ T Cells Are Enriched in Urological Cancers and Favor T Helper-2 Polarization. Front. Immunol. 2019, 10, 622. [Google Scholar] [CrossRef]
- Clenet, M.L.; Gagnon, F.; Moratalla, A.C.; Viel, E.C.; Arbour, N. Peripheral human CD4+CD8+ T lymphocytes exhibit a memory phenotype and enhanced responses to IL-2, IL-7 and IL-15. Sci. Rep. 2017, 7, 11612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, X.; Zu, S.; Lu, Y. Characteristics of circulating adaptive immune cells in patients with colorectal cancer. Sci. Rep. 2022, 12, 18166. [Google Scholar] [CrossRef]
- Chen, H.K.; Chen, Y.L.; Chung, W.P.; Loh, Z.J.; Lee, K.T.; Hsu, H.P. Circulating CD3+CD8+ T Lymphocytes as Indicators of Disease Status in Patients with Early Breast Cancer. Cancer Med. 2025, 14, e70547. [Google Scholar] [CrossRef]
- Koh, J.; Hur, J.Y.; Lee, K.Y.; Kim, M.S.; Heo, J.Y.; Ku, B.M.; Sun, J.M.; Lee, S.H.; Ahn, J.S.; Park, K.; et al. Regulatory (FoxP3+) T cells and TGF-beta predict the response to anti-PD-1 immunotherapy in patients with non-small cell lung cancer. Sci. Rep. 2020, 10, 18994. [Google Scholar] [CrossRef] [PubMed]
- Strayer, D.R.; Carter, W.A.; Brodsky, I. Familial occurrence of breast cancer is associated with reduced natural killer cytotoxicity. Breast Cancer Res. Treat. 1986, 7, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Ashouri, E.; Rajalingam, K.; Barani, S.; Farjadian, S.; Ghaderi, A.; Rajalingam, R. Coexistence of inhibitory and activating killer-cell immunoglobulin-like receptors to the same cognate HLA-C2 and Bw4 ligands confer breast cancer risk. Sci. Rep. 2021, 11, 7932. [Google Scholar] [CrossRef]
- Ozturk, O.G.; Gun, F.D.; Polat, G. Killer cell immunoglobulin-like receptor genes in patients with breast cancer. Med. Oncol. 2012, 29, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Hematian Larki, M.; Barani, S.; Talei, A.R.; Ghaderi, A. Diversity of KIRs in invasive breast cancer patients and healthy controls along with the clinical significance in ER/PR/HER2+ patients. Genes Immun. 2020, 21, 380–389. [Google Scholar] [CrossRef]
- Tembhurne, A.K.; Maheshwari, A.; Warke, H.; Chaudhari, H.; Kerkar, S.C.; Deodhar, K.; Rekhi, B.; Mania-Pramanik, J. Killer cell immunoglobulin-like receptor (KIR) gene contents: Are they associated with cervical cancer? J. Med. Virol. 2023, 95, e27873. [Google Scholar] [CrossRef]
- Hernandez, E.G.; Partida-Rodriguez, O.; Camorlinga-Ponce, M.; Nieves-Ramirez, M.; Ramos-Vega, I.; Torres, J.; Perez-Rodriguez, M. Genotype B of Killer Cell Immunoglobulin-Like Receptor is Related with Gastric Cancer Lesions. Sci. Rep. 2018, 8, 6104. [Google Scholar] [CrossRef]
- Imai, K.; Matsuyama, S.; Miyake, S.; Suga, K.; Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet 2000, 356, 1795–1799. [Google Scholar] [CrossRef]
- Tang, Y.P.; Xie, M.Z.; Li, K.Z.; Li, J.L.; Cai, Z.M.; Hu, B.L. Prognostic value of peripheral blood natural killer cells in colorectal cancer. BMC Gastroenterol. 2020, 20, 31. [Google Scholar] [CrossRef]
- Xu, Y.; Mao, Y.; Lv, Y.; Tang, W.; Xu, J. B cells in tumor metastasis: Friend or foe? Int. J. Biol. Sci. 2023, 19, 2382–2393. [Google Scholar] [CrossRef]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv. Sci. 2021, 8, e2004433. [Google Scholar] [CrossRef]
- Lee, J.Y.; Hong, S.H. Hematopoietic Stem Cells and Their Roles in Tissue Regeneration. Int. J. Stem Cells 2020, 13, 1–12. [Google Scholar] [CrossRef]
- Belyavsky, A.; Petinati, N.; Drize, N. Hematopoiesis during Ontogenesis, Adult Life, and Aging. Int. J. Mol. Sci. 2021, 22, 9231. [Google Scholar] [CrossRef]
- Rheinlander, A.; Schraven, B.; Bommhardt, U. CD45 in human physiology and clinical medicine. Immunol. Lett. 2018, 196, 22–32. [Google Scholar] [CrossRef]
- Dean, L. Blood Groups and Red Cell Antigens; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2005.
- Chetty, R.; Gatter, K. CD3: Structure, function, and role of immunostaining in clinical practice. J. Pathol. 1994, 173, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Kuhns, M.S.; Davis, M.M.; Garcia, K.C. Deconstructing the form and function of the TCR/CD3 complex. Immunity 2006, 24, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Rudensky, A.Y. Regulatory T cells and Foxp3. Immunol. Rev. 2011, 241, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Syeda, M.Z.; Hong, T.; Huang, C.; Huang, W.; Mu, Q. B cell memory: From generation to reactivation: A multipronged defense wall against pathogens. Cell Death Discov. 2024, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Uchida, J.; Lee, Y.; Hasegawa, M.; Liang, Y.; Bradney, A.; Oliver, J.A.; Bowen, K.; Steeber, D.A.; Haas, K.M.; Poe, J.C.; et al. Mouse CD20 expression and function. Int. Immunol. 2004, 16, 119–129. [Google Scholar] [CrossRef]
- Klasener, K.; Jellusova, J.; Andrieux, G.; Salzer, U.; Bohler, C.; Steiner, S.N.; Albinus, J.B.; Cavallari, M.; Suss, B.; Voll, R.E.; et al. CD20 as a gatekeeper of the resting state of human B cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2021342118. [Google Scholar] [CrossRef]
- Benitez, A.; Weldon, A.J.; Tatosyan, L.; Velkuru, V.; Lee, S.; Milford, T.A.; Francis, O.L.; Hsu, S.; Nazeri, K.; Casiano, C.M.; et al. Differences in mouse and human nonmemory B cell pools. J. Immunol. 2014, 192, 4610–4619. [Google Scholar] [CrossRef]
- Suryani, S.; Fulcher, D.A.; Santner-Nanan, B.; Nanan, R.; Wong, M.; Shaw, P.J.; Gibson, J.; Williams, A.; Tangye, S.G. Differential expression of CD21 identifies developmentally and functionally distinct subsets of human transitional B cells. Blood 2010, 115, 519–529. [Google Scholar] [CrossRef]
- Xiao, Y.; Hendriks, J.; Langerak, P.; Jacobs, H.; Borst, J. CD27 is acquired by primed B cells at the centroblast stage and promotes germinal center formation. J. Immunol. 2004, 172, 7432–7441. [Google Scholar] [CrossRef]
- Hoffmann, R. Gene expression patterns in human and mouse B cell development. In Chronic Lymphocytic Leukemia; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2005; Volume 294, pp. 19–29. [Google Scholar] [CrossRef]
- Ichii, M.; Oritani, K.; Kanakura, Y. Early B lymphocyte development: Similarities and differences in human and mouse. World J. Stem Cells 2014, 6, 421–431. [Google Scholar] [CrossRef]
- Austermann, J.; Roth, J.; Barczyk-Kahlert, K. The Good and the Bad: Monocytes’ and Macrophages’ Diverse Functions in Inflammation. Cells 2022, 11, 1979. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Chen, N.; Hu, Y.; Huang, L.; Peng, J.; Yang, M.; Shen, X.; Song, Y.; Xu, L. Elevated peripheral absolute monocyte count related to clinicopathological features and poor prognosis in solid tumors: Systematic review, meta-analysis, and meta-regression. Cancer Med. 2021, 10, 1690–1714. [Google Scholar] [CrossRef]
- Boyette, L.B.; Macedo, C.; Hadi, K.; Elinoff, B.D.; Walters, J.T.; Ramaswami, B.; Chalasani, G.; Taboas, J.M.; Lakkis, F.G.; Metes, D.M. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS ONE 2017, 12, e0176460. [Google Scholar] [CrossRef] [PubMed]
- Sommer, K.; Garibagaoglu, H.; Paap, E.M.; Wiendl, M.; Muller, T.M.; Atreya, I.; Kronke, G.; Neurath, M.F.; Zundler, S. Discrepant Phenotyping of Monocytes Based on CX3CR1 and CCR2 Using Fluorescent Reporters and Antibodies. Cells 2024, 13, 819. [Google Scholar] [CrossRef] [PubMed]
- Cormican, S.; Griffin, M.D. Human Monocyte Subset Distinctions and Function: Insights From Gene Expression Analysis. Front. Immunol. 2020, 11, 1070. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Shi, M.; Lou, J.; Huang, Q.; Li, C.; Huang, Y.; Xu, L. Heterogeneity of monocytes in cancer. Am. J. Cancer Res. 2025, 15, 3359–3375. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Guilliams, M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity 2016, 44, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.P.; Gupta, S.; Sarangi, P.P. Monocytes and macrophages: Origin, homing, differentiation, and functionality during inflammation. Heliyon 2024, 10, e29686. [Google Scholar] [CrossRef]
- Kinoshita, M.; Uchida, T.; Sato, A.; Nakashima, M.; Nakashima, H.; Shono, S.; Habu, Y.; Miyazaki, H.; Hiroi, S.; Seki, S. Characterization of two F4/80-positive Kupffer cell subsets by their function and phenotype in mice. J. Hepatol. 2010, 53, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Hamann, J.; Lin, H.H.; Stacey, M. F4/80 and the related adhesion-GPCRs. Eur. J. Immunol. 2011, 41, 2472–2476. [Google Scholar] [CrossRef]
- Frafjord, A.; Skarshaug, R.; Hammarstrom, C.; Stankovic, B.; Dorg, L.T.; Aamodt, H.; Woldbaek, P.R.; Helland, A.; Brustugun, O.T.; Oynebraten, I.; et al. Antibody combinations for optimized staining of macrophages in human lung tumours. Scand. J. Immunol. 2020, 92, e12889. [Google Scholar] [CrossRef]
- Mizuno, N.; Yanagawa, Y. Tofacitinib enhances interferon-gamma-induced expression of major histocompatibility complex class II in macrophages. Eur. J. Pharmacol. 2022, 915, 174564. [Google Scholar] [CrossRef]
- Wei, Q.; Deng, Y.; Yang, Q.; Zhan, A.; Wang, L. The markers to delineate different phenotypes of macrophages related to metabolic disorders. Front. Immunol. 2023, 14, 1084636. [Google Scholar] [CrossRef]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.; Liu, Y.J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef]
- Boltjes, A.; van Wijk, F. Human dendritic cell functional specialization in steady-state and inflammation. Front. Immunol. 2014, 5, 131. [Google Scholar] [CrossRef]
- Adachi, Y.; Toki, J.; Ikebukuro, K.; Tomita, M.; Kaneda, H.; Tanabe, A.; Jun, L.; Minamino, K.; Suzuki, Y.; Taketani, S.; et al. Immature dendritic cells (CD11c+CD3−B220− cells) present in mouse peripheral blood. Immunobiology 2002, 206, 354–367. [Google Scholar] [CrossRef]
- Lakschevitz, F.S.; Hassanpour, S.; Rubin, A.; Fine, N.; Sun, C.; Glogauer, M. Identification of neutrophil surface marker changes in health and inflammation using high-throughput screening flow cytometry. Exp. Cell Res. 2016, 342, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Liu, Y.; Takashima, A. Intravital Imaging of Neutrophil Priming Using IL-1beta Promoter-driven DsRed Reporter Mice. J. Vis. Exp. 2016, 112, e54070. [Google Scholar] [CrossRef]
- Moller, M.; Orth, V.; Umansky, V.; Hetjens, S.; Braun, V.; Reissfelder, C.; Hardt, J.; Seyfried, S. Myeloid-derived suppressor cells in peripheral blood as predictive biomarkers in patients with solid tumors undergoing immune checkpoint therapy: Systematic review and meta-analysis. Front. Immunol. 2024, 15, 1403771. [Google Scholar] [CrossRef]
- Gapin, L.; Matsuda, J.L.; Surh, C.D.; Kronenberg, M. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat. Immunol. 2001, 2, 971–978. [Google Scholar] [CrossRef]
- Godfrey, D.I.; Stankovic, S.; Baxter, A.G. Raising the NKT cell family. Nat. Immunol. 2010, 11, 197–206. [Google Scholar] [CrossRef]
- Balato, A.; Unutmaz, D.; Gaspari, A.A. Natural killer T cells: An unconventional T-cell subset with diverse effector and regulatory functions. J. Investig. Dermatol. 2009, 129, 1628–1642. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, J.L.; Naidenko, O.V.; Gapin, L.; Nakayama, T.; Taniguchi, M.; Wang, C.R.; Koezuka, Y.; Kronenberg, M. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 2000, 192, 741–754. [Google Scholar] [CrossRef]
- Sidobre, S.; Kronenberg, M. CD1 tetramers: A powerful tool for the analysis of glycolipid-reactive T cells. J. Immunol. Methods 2002, 268, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Boyson, J.E.; Rybalov, B.; Koopman, L.A.; Exley, M.; Balk, S.P.; Racke, F.K.; Schatz, F.; Masch, R.; Wilson, S.B.; Strominger, J.L. CD1d and invariant NKT cells at the human maternal-fetal interface. Proc. Natl. Acad. Sci. USA 2002, 99, 13741–13746. [Google Scholar] [CrossRef]
- Exley, M.A.; Wilson, S.B.; Balk, S.P. Isolation and Functional Use of Human NKT Cells. Curr. Protoc. Immunol. 2017, 119, 14.11.1–14.11.20. [Google Scholar] [CrossRef]
- Lenart, M.; Pyrc, K.; Siedlar, M. Can we define CD3+CD56+ cells as NKT cells with impunity? Clin. Immunol. 2021, 226, 108708. [Google Scholar] [CrossRef]
- Slauenwhite, D.; Johnston, B. Regulation of NKT Cell Localization in Homeostasis and Infection. Front. Immunol. 2015, 6, 255. [Google Scholar] [CrossRef]
- Heller, N.M.; Berga-Bolanos, R.; Naler, L.; Sen, J.M. Natural Killer T (NKT) Cells in Mice and Men. In Signaling Mechanisms Regulating T Cell Diversity and Function; Soboloff, J., Kappes, D.J., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2018; Chapter 8. [Google Scholar] [CrossRef]
- Erick, T.K.; Brossay, L. Phenotype and functions of conventional and non-conventional NK cells. Curr. Opin. Immunol. 2016, 38, 67–74. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, H.; Jounaidi, Y. Comprehensive snapshots of natural killer cells functions, signaling, molecular mechanisms and clinical utilization. Signal Transduct. Target. Ther. 2024, 9, 302. [Google Scholar] [CrossRef]
- Melsen, J.E.; van Ostaijen-Ten Dam, M.M.; Schoorl, D.J.A.; Schol, P.J.; van den Homberg, D.A.L.; Lankester, A.C.; Lugthart, G.; Schilham, M.W. Single-cell transcriptomics in bone marrow delineates CD56dimGranzymeK+ subset as intermediate stage in NK cell differentiation. Front. Immunol. 2022, 13, 1044398. [Google Scholar] [CrossRef]
- Cocker, A.T.H.; Liu, F.; Djaoud, Z.; Guethlein, L.A.; Parham, P. CD56-negative NK cells: Frequency in peripheral blood, expansion during HIV-1 infection, functional capacity, and KIR expression. Front. Immunol. 2022, 13, 992723. [Google Scholar] [CrossRef] [PubMed]
- Cocker, A.T.H.; Guethlein, L.A.; Parham, P. The CD56-CD16+ NK cell subset in chronic infections. Biochem. Soc. Trans. 2023, 51, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.J.; Zhang, X.C.; Wan, L.Y.; Li, Q.Y.; Mu, X.Y.; Guo, A.L.; Zhou, M.J.; Shen, L.L.; Zhang, C.; Fan, X.; et al. Immune Dysfunctions of CD56(neg) NK Cells Are Associated With HIV-1 Disease Progression. Front. Immunol. 2021, 12, 811091. [Google Scholar] [CrossRef]
- Portillo, A.L.; Rojas, E.A.; Mehboob, M.; Moinuddin, A.; Balint, E.; Feng, E.; Silvestri, C.; Vahedi, F.; Ritchie, T.M.; Mansour, A.J.; et al. CD56 does not contribute to the antitumor, tissue homing, and glycolytic capacity of human NK cells. J. Leukoc. Biol. 2025, 117, qiae227. [Google Scholar] [CrossRef] [PubMed]
- Bozzano, F.; Marras, F.; Ascierto, M.L.; Cantoni, C.; Cenderello, G.; Dentone, C.; Di Biagio, A.; Orofino, G.; Mantia, E.; Boni, S.; et al. Emergency exit’ of bone-marrow-resident CD34+DNAM-1brightCXCR4+-committed lymphoid precursors during chronic infection and inflammation. Nat. Commun. 2015, 6, 8109. [Google Scholar] [CrossRef]
- Abe, S.; Asahi, T.; Hara, T.; Cui, G.; Shimba, A.; Tani-Ichi, S.; Yamada, K.; Miyazaki, K.; Miyachi, H.; Kitano, S.; et al. Hematopoietic cell-derived IL-15 supports NK cell development in scattered and clustered localization within the bone marrow. Cell Rep. 2023, 42, 113127. [Google Scholar] [CrossRef]
- Ma, S.; Caligiuri, M.A.; Yu, J. A four-stage model for murine natural killer cell development in vivo. J. Hematol. Oncol. 2022, 15, 31. [Google Scholar] [CrossRef]
- Hegewisch-Solloa, E.; Nalin, A.P.; Freud, A.G.; Mace, E.M. Deciphering the localization and trajectory of human natural killer cell development. J. Leukoc. Biol. 2023, 114, 487–506. [Google Scholar] [CrossRef] [PubMed]
- Bourayou, E.; Golub, R. Inflammatory-driven NK cell maturation and its impact on pathology. Front. Immunol. 2022, 13, 1061959. [Google Scholar] [CrossRef] [PubMed]
- Sparano, C.; Solis-Sayago, D.; Zangger, N.S.; Rindlisbacher, L.; Van Hove, H.; Vermeer, M.; Westermann, F.; Mussak, C.; Rallo, E.; Dergun, S.; et al. Autocrine TGF-beta1 drives tissue-specific differentiation and function of resident NK cells. J. Exp. Med. 2025, 222, e20240930. [Google Scholar] [CrossRef]
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front. Immunol. 2018, 9, 1869. [Google Scholar] [CrossRef]
- Mace, E.M. Human natural killer cells: Form, function, and development. J. Allergy Clin. Immunol. 2023, 151, 371–385. [Google Scholar] [CrossRef]
- Schorr, C.; Krishnan, M.S.; Capitano, M. Deficits in our understanding of natural killer cell development in mouse and human. Curr. Opin. Hematol. 2023, 30, 106–116. [Google Scholar] [CrossRef]
- Russick, J.; Torset, C.; Sun, D.; Marmier, S.; Merle, N.; Voilin, E.; Josseaume, N.; Meylan, M.; Hernandez, I.; Foy, P.E.; et al. Tumor stage-driven disruption of NK cell maturation in human and murine tumors. iScience 2024, 27, 111233. [Google Scholar] [CrossRef] [PubMed]
- Medjouel Khlifi, H.; Guia, S.; Vivier, E.; Narni-Mancinelli, E. Role of the ITAM-Bearing Receptors Expressed by Natural Killer Cells in Cancer. Front. Immunol. 2022, 13, 898745. [Google Scholar] [CrossRef] [PubMed]
- Kusnierczyk, P. Killer cell immunoglobulin-like receptor gene associations with autoimmune and allergic diseases, recurrent spontaneous abortion, and neoplasms. Front. Immunol. 2013, 4, 8. [Google Scholar] [CrossRef]
- Faure, M.; Long, E.O. KIR2DL4 (CD158d), an NK cell-activating receptor with inhibitory potential. J. Immunol. 2002, 168, 6208–6214. [Google Scholar] [CrossRef]
- Sambrook, J.G.; Beck, S. Evolutionary vignettes of natural killer cell receptors. Curr. Opin. Immunol. 2007, 19, 553–560. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 1989, 54 Pt 1, 1–13. [Google Scholar] [CrossRef]
- Matzinger, P. The danger model: A renewed sense of self. Science 2002, 296, 301–305. [Google Scholar] [CrossRef]
- Scur, M.; Parsons, B.D.; Dey, S.; Makrigiannis, A.P. The diverse roles of C-type lectin-like receptors in immunity. Front. Immunol. 2023, 14, 1126043. [Google Scholar] [CrossRef]
- Radaev, S.; Sun, P.D. Structure and function of natural killer cell surface receptors. Annu. Rev. Biophys. Biomol. Struct. 2003, 32, 93–114. [Google Scholar] [CrossRef]
- Willemze, R.; Ruggeri, A.; Purtill, D.; Rodrigues, C.A.; Gluckman, E.; Rocha, V.; on behalf of Eurocord and of the European Group of Blood and Marrow Transplantation. Is there an impact of killer cell immunoglobulin-like receptors and KIR-ligand incompatibilities on outcomes after unrelated cord blood stem cell transplantation? Best. Pr. Res. Clin. Haematol. 2010, 23, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, C.L.; Chalupny, N.J.; Cosman, D. The UL16-binding proteins, a novel family of MHC class I-related ligands for NKG2D, activate natural killer cell functions. Immunol. Rev. 2001, 181, 185–192. [Google Scholar] [CrossRef]
- Cerwenka, A.; Bakker, A.B.; McClanahan, T.; Wagner, J.; Wu, J.; Phillips, J.H.; Lanier, L.L. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity 2000, 12, 721–727. [Google Scholar] [CrossRef]
- Djelloul, M.; Popa, N.; Pelletier, F.; Raguenez, G.; Boucraut, J. RAE-1 expression is induced during experimental autoimmune encephalomyelitis and is correlated with microglia cell proliferation. Brain Behav. Immun. 2016, 58, 209–217. [Google Scholar] [CrossRef]
- Siemaszko, J.; Marzec-Przyszlak, A.; Bogunia-Kubik, K. NKG2D Natural Killer Cell Receptor-A Short Description and Potential Clinical Applications. Cells 2021, 10, 1420. [Google Scholar] [CrossRef] [PubMed]
- Wensveen, F.M.; Jelencic, V.; Polic, B. NKG2D: A Master Regulator of Immune Cell Responsiveness. Front. Immunol. 2018, 9, 441. [Google Scholar] [CrossRef]
- Creelan, B.C.; Antonia, S.J. The NKG2A immune checkpoint—A new direction in cancer immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 277–278. [Google Scholar] [CrossRef]
- Petrie, E.J.; Clements, C.S.; Lin, J.; Sullivan, L.C.; Johnson, D.; Huyton, T.; Heroux, A.; Hoare, H.L.; Beddoe, T.; Reid, H.H.; et al. CD94-NKG2A recognition of human leukocyte antigen (HLA)-E bound to an HLA class I leader sequence. J. Exp. Med. 2008, 205, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Goodall, K.J.; Nguyen, A.; Sullivan, L.C.; Andrews, D.M. The expanding role of murine class Ib MHC in the development and activation of Natural Killer cells. Mol. Immunol. 2019, 115, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Andre, P.; Denis, C.; Soulas, C.; Bourbon-Caillet, C.; Lopez, J.; Arnoux, T.; Blery, M.; Bonnafous, C.; Gauthier, L.; Morel, A.; et al. Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells. Cell 2018, 175, 1731–1743.e13. [Google Scholar] [CrossRef]
- Siemaszko, J.; Marzec-Przyszlak, A.; Bogunia-Kubik, K. Activating NKG2C Receptor: Functional Characteristics and Current Strategies in Clinical Applications. Arch. Immunol. Ther. Exp. 2023, 71, 9. [Google Scholar] [CrossRef]
- Kusumi, M.; Yamashita, T.; Fujii, T.; Nagamatsu, T.; Kozuma, S.; Taketani, Y. Expression patterns of lectin-like natural killer receptors, inhibitory CD94/NKG2A, and activating CD94/NKG2C on decidual CD56bright natural killer cells differ from those on peripheral CD56dim natural killer cells. J. Reprod. Immunol. 2006, 70, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Saez-Borderias, A.; Romo, N.; Magri, G.; Guma, M.; Angulo, A.; Lopez-Botet, M. IL-12-dependent inducible expression of the CD94/NKG2A inhibitory receptor regulates CD94/NKG2C+ NK cell function. J. Immunol. 2009, 182, 829–836. [Google Scholar] [CrossRef]
- Murad, S.; Michen, S.; Becker, A.; Fussel, M.; Schackert, G.; Tonn, T.; Momburg, F.; Temme, A. NKG2C+ NK Cells for Immunotherapy of Glioblastoma Multiforme. Int. J. Mol. Sci. 2022, 23, 5857. [Google Scholar] [CrossRef] [PubMed]
- de Dios, O.; Ramirez-Gonzalez, M.A.; Gomez-Soria, I.; Segura-Collar, B.; Manosalva, J.; Megias, D.; De Andrea, C.E.; Fernandez-Rubio, L.; Hernandez-Lain, A.; Sepulveda-Sanchez, J.M.; et al. NKG2C/KLRC2 tumor cell expression enhances immunotherapeutic efficacy against glioblastoma. J. Immunother. Cancer 2024, 12, e009210. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Botet, M.; Muntasell, A.; Vilches, C. The CD94/NKG2C+ NK-cell subset on the edge of innate and adaptive immunity to human cytomegalovirus infection. Semin. Immunol. 2014, 26, 145–151. [Google Scholar] [CrossRef]
- Riese, P.; Trittel, S.; Pathirana, R.D.; Klawonn, F.; Cox, R.J.; Guzman, C.A. Responsiveness to Influenza Vaccination Correlates with NKG2C-Expression on NK Cells. Vaccines 2020, 8, 281. [Google Scholar] [CrossRef]
- Lopez-Botet, M.; De Maria, A.; Muntasell, A.; Della Chiesa, M.; Vilches, C. Adaptive NK cell response to human cytomegalovirus: Facts and open issues. Semin. Immunol. 2023, 65, 101706. [Google Scholar] [CrossRef]
- Ataya, M.; Redondo-Pachon, D.; Llinas-Mallol, L.; Yelamos, J.; Alari-Pahissa, E.; Perez-Saez, M.J.; Altadill, M.; Raich-Regue, D.; Vilches, C.; Pascual, J.; et al. Long-Term Evolution of the Adaptive NKG2C+ NK Cell Response to Cytomegalovirus Infection in Kidney Transplantation: An Insight on the Diversity of Host-Pathogen Interaction. J. Immunol. 2021, 207, 1882–1890. [Google Scholar] [CrossRef]
- Rapaport, A.S.; Schriewer, J.; Gilfillan, S.; Hembrador, E.; Crump, R.; Plougastel, B.F.; Wang, Y.; Le Friec, G.; Gao, J.; Cella, M.; et al. The Inhibitory Receptor NKG2A Sustains Virus-Specific CD8+ T Cells in Response to a Lethal Poxvirus Infection. Immunity 2015, 43, 1112–1124. [Google Scholar] [CrossRef]
- Rozbesky, D.; Ivanova, L.; Hernychova, L.; Grobarova, V.; Novak, P.; Cerny, J. Nkrp1 family, from lectins to protein interacting molecules. Molecules 2015, 20, 3463–3478. [Google Scholar] [CrossRef]
- Sovova, Z.; Kopecky, V., Jr.; Pazderka, T.; Hofbauerova, K.; Rozbesky, D.; Vanek, O.; Bezouska, K.; Ettrich, R. Structural analysis of natural killer cell receptor protein 1 (NKR-P1) extracellular domains suggests a conserved long loop region involved in ligand specificity. J. Mol. Model. 2011, 17, 1353–1370. [Google Scholar] [CrossRef]
- Balaji, G.R.; Aguilar, O.A.; Tanaka, M.; Shingu-Vazquez, M.A.; Fu, Z.; Gully, B.S.; Lanier, L.L.; Carlyle, J.R.; Rossjohn, J.; Berry, R. Recognition of host Clr-b by the inhibitory NKR-P1B receptor provides a basis for missing-self recognition. Nat. Commun. 2018, 9, 4623. [Google Scholar] [CrossRef]
- Poggi, A.; Costa, P.; Tomasello, E.; Moretta, L. IL-12-induced up-regulation of NKRP1A expression in human NK cells and consequent NKRP1A-mediated down-regulation of NK cell activation. Eur. J. Immunol. 1998, 28, 1611–1616. [Google Scholar] [CrossRef]
- Pozo, D.; Vales-Gomez, M.; Mavaddat, N.; Williamson, S.C.; Chisholm, S.E.; Reyburn, H. CD161 (human NKR-P1A) signaling in NK cells involves the activation of acid sphingomyelinase. J. Immunol. 2006, 176, 2397–2406. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Zhou, Y. Deciphering the role of KLRB1: A novel prognostic indicator in hepatocellular carcinoma. BMC Gastroenterol. 2024, 24, 210. [Google Scholar] [CrossRef]
- Zhang, Z.; Bahabayi, A.; Liu, D.; Hasimu, A.; Zhang, Y.; Guo, S.; Liu, R.; Zhang, K.; Li, Q.; Xiong, Z.; et al. KLRB1 defines an activated phenotype of CD4+ T cells and shows significant upregulation in patients with primary Sjogren’s syndrome. Int. Immunopharmacol. 2024, 133, 112072. [Google Scholar] [CrossRef] [PubMed]
- Duurland, C.L.; Santegoets, S.J.; Abdulrahman, Z.; Loof, N.M.; Sturm, G.; Wesselink, T.H.; Arens, R.; Boekestijn, S.; Ehsan, I.; van Poelgeest, M.I.E.; et al. CD161 expression and regulation defines rapidly responding effector CD4+ T cells associated with improved survival in HPV16-associated tumors. J. Immunother. Cancer 2022, 10, e003995. [Google Scholar] [CrossRef]
- Lenart, M.; Gorecka, M.; Bochenek, M.; Barreto-Duran, E.; Szczepanski, A.; Galuszka-Bulaga, A.; Mazur-Panasiuk, N.; Weglarczyk, K.; Siwiec-Kozlik, A.; Korkosz, M.; et al. SARS-CoV-2 infection impairs NK cell functions via activation of the LLT1-CD161 axis. Front. Immunol. 2023, 14, 1123155. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Cao, Y.; Wang, X.; Cheng, L.; Liu, Y.; Lei, J.; Peng, W.; Shi, D. Systematic Pan-Cancer Analysis of KLRB1 with Prognostic Value and Immunological Activity across Human Tumors. J. Immunol. Res. 2022, 2022, 5254911. [Google Scholar] [CrossRef]
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, Z.; Cui, H.; Yang, J.; Liu, A. Biological characteristics and immune responses of NK Cells in commonly used experimental mouse models. Front. Immunol. 2024, 15, 1478323. [Google Scholar] [CrossRef]
- Carlyle, J.R.; Mesci, A.; Ljutic, B.; Belanger, S.; Tai, L.H.; Rousselle, E.; Troke, A.D.; Proteau, M.F.; Makrigiannis, A.P. Molecular and genetic basis for strain-dependent NK1.1 alloreactivity of mouse NK cells. J. Immunol. 2006, 176, 7511–7524. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xu, R.; Pan, L.; Bhattarai, U.; Liu, X.; Zeng, H.; Chen, J.X.; Hall, M.E.; Chen, Y. Inhibition of NK1.1 signaling attenuates pressure overload-induced heart failure, and consequent pulmonary inflammation and remodeling. Front. Immunol. 2023, 14, 1215855. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, S.W.; Park, Y.H.; Kim, T.C.; Van Kaer, L.; Hong, S. CD1d-independent NK1.1+ Treg cells are IL2-inducible Foxp3+ T cells co-expressing immunosuppressive and cytotoxic molecules. Front. Immunol. 2022, 13, 951592. [Google Scholar] [CrossRef]
- Landa-Saldivar, C.; Resendiz-Mora, A.; Sanchez-Barbosa, S.; Sotelo-Rodriguez, A.; Barrera-Aveleida, G.; Nevarez-Lechuga, I.; Galarce-Sosa, I.; Taniguchi-Ponciano, K.; Cruz-Guzman, O.D.R.; Wong-Baeza, I.; et al. Liposomes Bearing Non-Bilayer Phospholipid Arrangements Induce Specific IgG Anti-Lipid Antibodies by Activating NK1.1+, CD4+ T Cells in Mice. Membranes 2022, 12, 643. [Google Scholar] [CrossRef]
- Ishihara, S.; Nieda, M.; Kitayama, J.; Osada, T.; Yabe, T.; Ishikawa, Y.; Nagawa, H.; Muto, T.; Juji, T. CD8+NKR-P1A+T cells preferentially accumulate in human liver. Eur. J. Immunol. 1999, 29, 2406–2413. [Google Scholar] [CrossRef]
- Reichlin, A.; Yokoyama, W.M. Natural killer cell proliferation induced by anti-NK1.1 and IL-2. Immunol. Cell Biol. 1998, 76, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Vahlne, G.; Becker, S.; Brodin, P.; Johansson, M.H. IFN-gamma production and degranulation are differentially regulated in response to stimulation in murine natural killer cells. Scand. J. Immunol. 2008, 67, 1–11. [Google Scholar] [CrossRef]
- Cifaldi, L.; Melaiu, O.; Giovannoni, R.; Benvenuto, M.; Focaccetti, C.; Nardozi, D.; Barillari, G.; Bei, R. DNAM-1 chimeric receptor-engineered NK cells: A new frontier for CAR-NK cell-based immunotherapy. Front. Immunol. 2023, 14, 1197053. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Chen, L.; Fei, Q.; Shao, X.; Lv, W.; Yang, J.; Xu, F.; Shi, J. Upregulation of CD226 on subsets of T cells and NK cells is associated with upregulated adhesion molecules and cytotoxic factors in patients with tuberculosis. Int. Immunopharmacol. 2023, 120, 110360. [Google Scholar] [CrossRef]
- Seth, S.; Georgoudaki, A.M.; Chambers, B.J.; Qiu, Q.; Kremmer, E.; Maier, M.K.; Czeloth, N.; Ravens, I.; Foerster, R.; Bernhardt, G. Heterogeneous expression of the adhesion receptor CD226 on murine NK and T cells and its function in NK-mediated killing of immature dendritic cells. J. Leukoc. Biol. 2009, 86, 91–101. [Google Scholar] [CrossRef]
- Wagner, A.K.; Kadri, N.; Snall, J.; Brodin, P.; Gilfillan, S.; Colonna, M.; Bernhardt, G.; Hoglund, P.; Karre, K.; Chambers, B.J. Expression of CD226 is associated to but not required for NK cell education. Nat. Commun. 2017, 8, 15627. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Mariuzza, R.A. Structure of the human activating natural cytotoxicity receptor NKp30 bound to its tumor cell ligand B7-H6. J. Exp. Med. 2011, 208, 703–714. [Google Scholar] [CrossRef]
- Siewiera, J.; Gouilly, J.; Hocine, H.R.; Cartron, G.; Levy, C.; Al-Daccak, R.; Jabrane-Ferrat, N. Natural cytotoxicity receptor splice variants orchestrate the distinct functions of human natural killer cell subtypes. Nat. Commun. 2015, 6, 10183. [Google Scholar] [CrossRef]
- Bryceson, Y.T.; March, M.E.; Ljunggren, H.G.; Long, E.O. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol. Rev. 2006, 214, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.L.; Nagasaki, K.; Martinez, O.M.; Krams, S.M. NKp30 is a functional activation receptor on a subset of rat natural killer cells. Eur. J. Immunol. 2006, 36, 2170–2180. [Google Scholar] [CrossRef] [PubMed]
- Memmer, S.; Weil, S.; Koch, J. Reconstitution of a ligand-binding competent murine NKp30 receptor. Immunogenetics 2018, 70, 185–194. [Google Scholar] [CrossRef]
- Hollyoake, M.; Campbell, R.D.; Aguado, B. NKp30 (NCR3) is a pseudogene in 12 inbred and wild mouse strains, but an expressed gene in Mus caroli. Mol. Biol. Evol. 2005, 22, 1661–1672. [Google Scholar] [CrossRef] [PubMed]
- Walzer, T.; Jaeger, S.; Chaix, J.; Vivier, E. Natural killer cells: From CD3−NKp46+ to post-genomics meta-analyses. Curr. Opin. Immunol. 2007, 19, 365–372. [Google Scholar] [CrossRef]
- Tomasello, E.; Yessaad, N.; Gregoire, E.; Hudspeth, K.; Luci, C.; Mavilio, D.; Hardwigsen, J.; Vivier, E. Mapping of NKp46+ Cells in Healthy Human Lymphoid and Non-Lymphoid Tissues. Front. Immunol. 2012, 3, 344. [Google Scholar] [CrossRef]
- Stewart, C.A.; Walzer, T.; Robbins, S.H.; Malissen, B.; Vivier, E.; Prinz, I. Germ-line and rearranged Tcrd transcription distinguish bona fide NK cells and NK-like gammadelta T cells. Eur. J. Immunol. 2007, 37, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Walzer, T.; Blery, M.; Chaix, J.; Fuseri, N.; Chasson, L.; Robbins, S.H.; Jaeger, S.; Andre, P.; Gauthier, L.; Daniel, L.; et al. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc. Natl. Acad. Sci. USA 2007, 104, 3384–3389. [Google Scholar] [CrossRef] [PubMed]
- Naeim, F. Chapter 2—Principles of Immunophenotyping. In Hematopathology; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar] [CrossRef]
- Wong, J.K.M.; McCulloch, T.R.; Alim, L.; Omer, N.; Mehdi, A.M.; Tuong, Z.K.; Bonfim-Melo, A.; Chung, E.; Nicol, A.; Simpson, F.; et al. TGF-beta signalling limits effector function capacity of NK cell anti-tumour immunity in human bladder cancer. EBioMedicine 2024, 104, 105176. [Google Scholar] [CrossRef]
- Alderson, K.L.; Sondel, P.M. Clinical cancer therapy by NK cells via antibody-dependent cell-mediated cytotoxicity. J. Biomed. Biotechnol. 2011, 2011, 379123. [Google Scholar] [CrossRef]
- Wu, J.; Mishra, H.K.; Walcheck, B. Role of ADAM17 as a regulatory checkpoint of CD16A in NK cells and as a potential target for cancer immunotherapy. J. Leukoc. Biol. 2019, 105, 1297–1303. [Google Scholar] [CrossRef]
- Roberts, J.T.; Barb, A.W. A single amino acid distorts the Fc gamma receptor IIIb/CD16b structure upon binding immunoglobulin G1 and reduces affinity relative to CD16a. J. Biol. Chem. 2018, 293, 19899–19908. [Google Scholar] [CrossRef]
- Coenon, L.; Villalba, M. From CD16a Biology to Antibody-Dependent Cell-Mediated Cytotoxicity Improvement. Front. Immunol. 2022, 13, 913215. [Google Scholar] [CrossRef]
- Bruhns, P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood 2012, 119, 5640–5649. [Google Scholar] [CrossRef]
- Nimmerjahn, F.; Ravetch, J.V. Fcgamma receptors: Old friends and new family members. Immunity 2006, 24, 19–28. [Google Scholar] [CrossRef]
- Aguilar, O.A.; Gonzalez-Hinojosa, M.D.R.; Arakawa-Hoyt, J.S.; Millan, A.J.; Gotthardt, D.; Nabekura, T.; Lanier, L.L. The CD16 and CD32b Fc-gamma receptors regulate antibody-mediated responses in mouse natural killer cells. J. Leukoc. Biol. 2023, 113, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, F.; Bruhns, P.; Horiuchi, K.; Ravetch, J.V. FcgammaRIV: A novel FcR with distinct IgG subclass specificity. Immunity 2005, 23, 41–51. [Google Scholar] [CrossRef]
- Aguilar, O.A.; Fong, L.K.; Ishiyama, K.; DeGrado, W.F.; Lanier, L.L. The CD3zeta adaptor structure determines functional differences between human and mouse CD16 Fc receptor signaling. J. Exp. Med. 2022, 219, e20220022. [Google Scholar] [CrossRef]
- Hirano, M.; Davis, R.S.; Fine, W.D.; Nakamura, S.; Shimizu, K.; Yagi, H.; Kato, K.; Stephan, R.P.; Cooper, M.D. IgEb immune complexes activate macrophages through FcgammaRIV binding. Nat. Immunol. 2007, 8, 762–771. [Google Scholar] [CrossRef]
- Mancardi, D.A.; Iannascoli, B.; Hoos, S.; England, P.; Daeron, M.; Bruhns, P. FcgammaRIV is a mouse IgE receptor that resembles macrophage FcepsilonRI in humans and promotes IgE-induced lung inflammation. J. Clin. Investig. 2008, 118, 3738–3750. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J.; Newton, R.; Bahaie, N.S.; Long, C.; Walcheck, B. ADAM17 cleaves CD16b (FcgammaRIIIb) in human neutrophils. Biochim. Biophys. Acta 2013, 1833, 680–685. [Google Scholar] [CrossRef]
- Matson, A.W.; Hullsiek, R.; Dixon, K.J.; Wang, S.; Lindstedt, A.J.; Friess, R.R.; Phung, S.K.; Freedman, T.S.; Felices, M.; Truckenbrod, E.N.; et al. Enhanced IL-15-mediated NK cell activation and proliferation by an ADAM17 function-blocking antibody involves CD16A, CD137, and accessory cells. J. Immunother. Cancer 2024, 12, e008959. [Google Scholar] [CrossRef]
- Caligiuri, M.A. Human natural killer cells. Blood 2008, 112, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Feng, J.; Chen, S.; Yang, H.; Dong, Z. Synergized regulation of NK cell education by NKG2A and specific Ly49 family members. Nat. Commun. 2019, 10, 5010. [Google Scholar] [CrossRef]
- Rahim, M.M.; Tu, M.M.; Mahmoud, A.B.; Wight, A.; Abou-Samra, E.; Lima, P.D.; Makrigiannis, A.P. Ly49 receptors: Innate and adaptive immune paradigms. Front. Immunol. 2014, 5, 145. [Google Scholar] [CrossRef] [PubMed]
- Mora-Bitria, L.; Asquith, B. Innate receptors modulating adaptive T cell responses: KIR-HLA interactions and T cell-mediated control of chronic viral infections. Immunogenetics 2023, 75, 269–282. [Google Scholar] [CrossRef]
- Pende, D.; Falco, M.; Vitale, M.; Cantoni, C.; Vitale, C.; Munari, E.; Bertaina, A.; Moretta, F.; Del Zotto, G.; Pietra, G.; et al. Killer Ig-Like Receptors (KIRs): Their Role in NK Cell Modulation and Developments Leading to Their Clinical Exploitation. Front. Immunol. 2019, 10, 1179. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Long, E.O. KIR2DL4 (CD158d): An activation receptor for HLA-G. Front. Immunol. 2012, 3, 258. [Google Scholar] [CrossRef]
- Scarpellino, L.; Oeschger, F.; Guillaume, P.; Coudert, J.D.; Levy, F.; Leclercq, G.; Held, W. Interactions of Ly49 family receptors with MHC class I ligands in trans and cis. J. Immunol. 2007, 178, 1277–1284. [Google Scholar] [CrossRef]
- Morris, M.A.; Koulich, E.; Liu, J.; Arora, V.; George, T.C.; Schatzle, J.D.; Kumar, V.; Bennett, M. Definition of additional functional ligands for Ly49I(B6) using FVBLy49I(B6) transgenic mice and B6 natural killer cell effectors. Transplantation 2002, 74, 1449–1454. [Google Scholar] [CrossRef]
- Chung, D.H.; Natarajan, K.; Boyd, L.F.; Tormo, J.; Mariuzza, R.A.; Yokoyama, W.M.; Margulies, D.H. Mapping the ligand of the NK inhibitory receptor Ly49A on living cells. J. Immunol. 2000, 165, 6922–6932. [Google Scholar] [CrossRef]
- Sullivan, L.C.; Berry, R.; Sosnin, N.; Widjaja, J.M.; Deuss, F.A.; Balaji, G.R.; LaGruta, N.L.; Mirams, M.; Trapani, J.A.; Rossjohn, J.; et al. Recognition of the Major Histocompatibility Complex (MHC) Class Ib Molecule H2-Q10 by the Natural Killer Cell Receptor Ly49C. J. Biol. Chem. 2016, 291, 18740–18752. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.K.; Dewar, K.; Goulet, M.L.; Leveque, G.; Makrigiannis, A.P. Complete elucidation of a minimal class I MHC natural killer cell receptor haplotype. Genes Immun. 2005, 6, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Millan, A.J.; Hom, B.A.; Libang, J.B.; Sindi, S.; Manilay, J.O. Evidence for Prescribed NK Cell Ly-49 Developmental Pathways in Mice. J. Immunol. 2021, 206, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
- Forbes, C.A.; Scalzo, A.A.; Degli-Esposti, M.A.; Coudert, J.D. Ly49C Impairs NK Cell Memory in Mouse Cytomegalovirus Infection. J. Immunol. 2016, 197, 128–140. [Google Scholar] [CrossRef]
- Makrigiannis, A.P.; Rousselle, E.; Anderson, S.K. Independent control of Ly49g alleles: Implications for NK cell repertoire selection and tumor cell killing. J. Immunol. 2004, 172, 1414–1425. [Google Scholar] [CrossRef]
- Barao, I.; Alvarez, M.; Ames, E.; Orr, M.T.; Stefanski, H.E.; Blazar, B.R.; Lanier, L.L.; Anderson, S.K.; Redelman, D.; Murphy, W.J. Mouse Ly49G2+ NK cells dominate early responses during both immune reconstitution and activation independently of MHC. Blood 2011, 117, 7032–7041. [Google Scholar] [CrossRef]
- Osman, M.S.; Silver, E.T.; Varghese, J.C.; Chang, C.S.; Gong, D.E.; Audette, G.F.; Hazes, B.; Kane, K.P. Epitope mapping of Ly-49G and G-like receptors: CK-1 antibody defines a polymorphic site of functional interaction with class I ligand. J. Leukoc. Biol. 2005, 77, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Murugin, V.V.; Zuikova, I.N.; Murugina, N.E.; Shulzhenko, A.E.; Pinegin, B.V.; Pashenkov, M.V. Reduced degranulation of NK cells in patients with frequently recurring herpes. Clin. Vaccine Immunol. 2011, 18, 1410–1415. [Google Scholar] [CrossRef]
- Cohnen, A.; Chiang, S.C.; Stojanovic, A.; Schmidt, H.; Claus, M.; Saftig, P.; Janssen, O.; Cerwenka, A.; Bryceson, Y.T.; Watzl, C. Surface CD107a/LAMP-1 protects natural killer cells from degranulation-associated damage. Blood 2013, 122, 1411–1418. [Google Scholar] [CrossRef]
- Alter, G.; Malenfant, J.M.; Altfeld, M. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 2004, 294, 15–22. [Google Scholar] [CrossRef]
- Bryceson, Y.T.; March, M.E.; Barber, D.F.; Ljunggren, H.G.; Long, E.O. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J. Exp. Med. 2005, 202, 1001–1012. [Google Scholar] [CrossRef]
- Betts, M.R.; Brenchley, J.M.; Price, D.A.; De Rosa, S.C.; Douek, D.C.; Roederer, M.; Koup, R.A. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 2003, 281, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Casazza, J.P.; Betts, M.R.; Price, D.A.; Precopio, M.L.; Ruff, L.E.; Brenchley, J.M.; Hill, B.J.; Roederer, M.; Douek, D.C.; Koup, R.A. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J. Exp. Med. 2006, 203, 2865–2877. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.R.; Davis, D.M. Segregation of HLA-C from ICAM-1 at NK cell immune synapses is controlled by its cell surface density. J. Immunol. 2006, 177, 6904–6910. [Google Scholar] [CrossRef] [PubMed]
- Sague, S.L.; Tato, C.; Pure, E.; Hunter, C.A. The regulation and activation of CD44 by natural killer (NK) cells and its role in the production of IFN-gamma. J. Interferon Cytokine Res. 2004, 24, 301–309. [Google Scholar] [CrossRef]
- Van Acker, H.H.; Capsomidis, A.; Smits, E.L.; Van Tendeloo, V.F. CD56 in the Immune System: More Than a Marker for Cytotoxicity? Front. Immunol. 2017, 8, 892. [Google Scholar] [CrossRef] [PubMed]
- Kozlova, I.; Sah, S.; Keable, R.; Leshchyns’ka, I.; Janitz, M.; Sytnyk, V. Cell Adhesion Molecules and Protein Synthesis Regulation in Neurons. Front. Mol. Neurosci. 2020, 13, 592126. [Google Scholar] [CrossRef]
- Gagliani, N.; Magnani, C.F.; Huber, S.; Gianolini, M.E.; Pala, M.; Licona-Limon, P.; Guo, B.; Herbert, D.R.; Bulfone, A.; Trentini, F.; et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat. Med. 2013, 19, 739–746. [Google Scholar] [CrossRef]
- Fan, X.; Moltedo, B.; Mendoza, A.; Davydov, A.N.; Faire, M.B.; Mazutis, L.; Sharma, R.; Pe’er, D.; Chudakov, D.M.; Rudensky, A.Y. CD49b defines functionally mature Treg cells that survey skin and vascular tissues. J. Exp. Med. 2018, 215, 2796–2814. [Google Scholar] [CrossRef]
- Melssen, M.M.; Lindsay, R.S.; Stasiak, K.; Rodriguez, A.B.; Briegel, A.M.; Cyranowski, S.; Rutkowski, M.R.; Conaway, M.R.; Melief, C.J.M.; van der Burg, S.H.; et al. Differential Expression of CD49a and CD49b Determines Localization and Function of Tumor-Infiltrating CD8+ T Cells. Cancer Immunol. Res. 2021, 9, 583–597. [Google Scholar] [CrossRef]
- Li, W.; Zhou, J.; Wang, X.; Wu, Y.; Ye, L.; Wei, H.; Sun, R.; Tian, Z.; Peng, H. CD49a+CD49b+ NK cells induced by viral infection reflect an activated state of conventional NK cells. Sci. China Life Sci. 2020, 63, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Garrod, K.R.; Wei, S.H.; Parker, I.; Cahalan, M.D. Natural killer cells actively patrol peripheral lymph nodes forming stable conjugates to eliminate MHC-mismatched targets. Proc. Natl. Acad. Sci. USA 2007, 104, 12081–12086. [Google Scholar] [CrossRef] [PubMed]
- Arase, H.; Saito, T.; Phillips, J.H.; Lanier, L.L. Cutting edge: The mouse NK cell-associated antigen recognized by DX5 monoclonal antibody is CD49b (alpha 2 integrin, very late antigen-2). J. Immunol. 2001, 167, 1141–1144. [Google Scholar] [CrossRef]
- Song, Y.; Kim, J.H.; Shin, E.-C.; Yoon, J.C. CD49a/b-based human natural killer cell subsets in the blood and the liver: Phenotypic and functional characteristics (INC6P.312). J. Immunol. 2015, 194 (Suppl. S1), 192.14. [Google Scholar] [CrossRef]
- Morcos, M.N.F.; Schoedel, K.B.; Hoppe, A.; Behrendt, R.; Basak, O.; Clevers, H.C.; Roers, A.; Gerbaulet, A. SCA-1 Expression Level Identifies Quiescent Hematopoietic Stem and Progenitor Cells. Stem Cell Rep. 2017, 8, 1472–1478. [Google Scholar] [CrossRef]
- Fogel, L.A.; Sun, M.M.; Geurs, T.L.; Carayannopoulos, L.N.; French, A.R. Markers of nonselective and specific NK cell activation. J. Immunol. 2013, 190, 6269–6276. [Google Scholar] [CrossRef]
- Shmerling, M.; Chalik, M.; Smorodinsky, N.I.; Meeker, A.; Roy, S.; Sagi-Assif, O.; Meshel, T.; Danilevsky, A.; Shomron, N.; Levinger, S.; et al. LY6S, a New IFN-Inducible Human Member of the Ly6a Subfamily Expressed by Spleen Cells and Associated with Inflammation and Viral Resistance. Immunohorizons 2022, 6, 253–272. [Google Scholar] [CrossRef]
- Llera, A.S.; Viedma, F.; Sanchez-Madrid, F.; Tormo, J. Crystal structure of the C-type lectin-like domain from the human hematopoietic cell receptor CD69. J. Biol. Chem. 2001, 276, 7312–7319. [Google Scholar] [CrossRef]
- Sattler, S.; Ghadially, H.; Hofer, E. Evolution of the C-type lectin-like receptor genes of the DECTIN-1 cluster in the NK gene complex. Sci. World J. 2012, 2012, 931386. [Google Scholar] [CrossRef]
- Cibrian, D.; Sanchez-Madrid, F. CD69: From activation marker to metabolic gatekeeper. Eur. J. Immunol. 2017, 47, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Cabrera, M.; Santis, A.G.; Fernandez-Ruiz, E.; Blacher, R.; Esch, F.; Sanchez-Mateos, P.; Sanchez-Madrid, F. Molecular cloning, expression, and chromosomal localization of the human earliest lymphocyte activation antigen AIM/CD69, a new member of the C-type animal lectin superfamily of signal-transmitting receptors. J. Exp. Med. 1993, 178, 537–547. [Google Scholar] [CrossRef]
- Lorenzo-Anota, H.Y.; Martinez-Loria, A.B.; Tamez-Guerra, R.S.; Scott-Algara, D.; Martinez-Torres, A.C.; Rodriguez-Padilla, C. Changes in the natural killer cell repertoire and function induced by the cancer immune adjuvant candidate IMMUNEPOTENT-CRP. Cell Immunol. 2022, 374, 104511. [Google Scholar] [CrossRef]
- Borrego, F.; Robertson, M.J.; Ritz, J.; Pena, J.; Solana, R. CD69 is a stimulatory receptor for natural killer cell and its cytotoxic effect is blocked by CD94 inhibitory receptor. Immunology 1999, 97, 159–165. [Google Scholar] [CrossRef]
- Dons’koi, B.V.; Chernyshov, V.P.; Osypchuk, D.V. Measurement of NK activity in whole blood by the CD69 up-regulation after co-incubation with K562, comparison with NK cytotoxicity assays and CD107a degranulation assay. J. Immunol. Methods 2011, 372, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Notario, L.; Alari-Pahissa, E.; de Molina, A.; Lauzurica, P. CD69 Deficiency Enhances the Host Response to Vaccinia Virus Infection through Altered NK Cell Homeostasis. J. Virol. 2016, 90, 6464–6474. [Google Scholar] [CrossRef]
- Gonzalez-Amaro, R.; Cortes, J.R.; Sanchez-Madrid, F.; Martin, P. Is CD69 an effective brake to control inflammatory diseases? Trends Mol. Med. 2013, 19, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, E.; Reynolds, G.; Botting, R.A.; Calero-Nieto, F.J.; Morgan, M.D.; Tuong, Z.K.; Bach, K.; Sungnak, W.; Worlock, K.B.; Yoshida, M.; et al. Single-cell multi-omics analysis of the immune response in COVID-19. Nat. Med. 2021, 27, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Cortada, E.; Yao, J.; Xia, Y.; Dundar, F.; Zumbo, P.; Yang, B.; Rubio-Navarro, A.; Perder, B.; Qiu, M.; Pettinato, A.M.; et al. Cross-species single-cell RNA-seq analysis reveals disparate and conserved cardiac and extracardiac inflammatory responses upon heart injury. Commun. Biol. 2024, 7, 1611. [Google Scholar] [CrossRef]
- Program, C.Z.I.C.S.; Abdulla, S.; Aevermann, B.; Assis, P.; Badajoz, S.; Bell, S.M.; Bezzi, E.; Cakir, B.; Chaffer, J.; Chambers, S.; et al. CZ CELLxGENE Discover: A single-cell data platform for scalable exploration, analysis and modeling of aggregated data. Nucleic Acids Res. 2025, 53, D886–D900. [Google Scholar] [CrossRef]
- Holl, E.; Kapinsky, M.; Larbi, A. An Update on Flow Cytometry Analysis of Hematological Malignancies: Focus on Standardization. Cancers 2025, 17, 2045. [Google Scholar] [CrossRef]
- Kondratyeva, L.G.; Rakitina, O.A.; Pleshkan, V.V.; Kuzmich, A.I.; Linge, I.A.; Kondratieva, S.A.; Snezhkov, E.V.; Alekseenko, I.V.; Sverdlov, E.D. The Cellular and Transcriptomic Early Innate Immune Response to BCG Vaccination in Mice. Cells 2024, 13, 2043. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsyplenkov, K.K.; Belousova, A.A.; Zinovyeva, M.V.; Alekseenko, I.V.; Pleshkan, V.V. Translational Insights into NK Immunophenotyping: Comparative Surface Marker Analysis and Circulating Immune Cell Profiling in Cancer Immunotherapy. Int. J. Mol. Sci. 2025, 26, 9547. https://doi.org/10.3390/ijms26199547
Tsyplenkov KK, Belousova AA, Zinovyeva MV, Alekseenko IV, Pleshkan VV. Translational Insights into NK Immunophenotyping: Comparative Surface Marker Analysis and Circulating Immune Cell Profiling in Cancer Immunotherapy. International Journal of Molecular Sciences. 2025; 26(19):9547. https://doi.org/10.3390/ijms26199547
Chicago/Turabian StyleTsyplenkov, Kirill K., Arina A. Belousova, Marina V. Zinovyeva, Irina V. Alekseenko, and Victor V. Pleshkan. 2025. "Translational Insights into NK Immunophenotyping: Comparative Surface Marker Analysis and Circulating Immune Cell Profiling in Cancer Immunotherapy" International Journal of Molecular Sciences 26, no. 19: 9547. https://doi.org/10.3390/ijms26199547
APA StyleTsyplenkov, K. K., Belousova, A. A., Zinovyeva, M. V., Alekseenko, I. V., & Pleshkan, V. V. (2025). Translational Insights into NK Immunophenotyping: Comparative Surface Marker Analysis and Circulating Immune Cell Profiling in Cancer Immunotherapy. International Journal of Molecular Sciences, 26(19), 9547. https://doi.org/10.3390/ijms26199547





