Abstract
(1) Chronic heart failure (CHF) is a typical component of the polymorbid profile of an elderly patient. The aim of this systematic review was to search for data from pharmacokinetic (PK) studies of any drugs in patients with CHF to systematize information on changes in PK parameters depending on the physicochemical properties (PCPs) of the drug and route of its administration. (2) A systematic review of PK studies in patients with CHF was performed using Elibrary.ru, United States National Library of Medicine (PubMed), China National Knowledge Infrastructure (CNKI), and Directory of Open Access Journals (DOAJ). The final number of included articles was 106. A descriptive and correlation analysis of PK data and PCPs of drugs included in the study was carried out. Inclusion criteria: PK study, available PK parameters, demographic data, and diagnosed CHF. Risk of bias was assessed using ROBINS-I. (3) Evaluation of correlations between PCPs of drugs and their PK revealed a link between (i) plasma protein binding (PPB) and volume of distribution for lipophilic drugs; (ii) PCPs, half-life, and clearance for drugs with high PPB; and (iii) PPB and clearance for hydrophilic and amphiphilic drugs. (4) Hypoalbuminemia associated with CHF may lead to an increased volume of distribution of lipophilic drugs; lipophilic drugs used in CHF patients may be associated with prolongation of the half-life period and reduction in clearance; highly protein-bound drugs may manifest with reduced clearance. PK characteristics identified in this review should guide modifications to dosing regimens in CHF patients receiving medications from different groups.
1. Introduction
CHF is among the most common chronic diseases worldwide. According to data from S. Jimenez et al. (2020), CHF prevalence in the European population is 2–4% [1]. The highest prevalence is typically detected in the elderly, from 7.7 to 9% [2]. According to the NHANES study (2017–2020), there is a trend toward increasing CHF prevalence in the American population aged over 20 years, reaching up to 8.7 million by 2030 [3]. The combination of pathological changes specific to CHF patients can affect drug efficacy and safety due to alterations of all PK processes: absorption, distribution, metabolism, and excretion.
In a study by Osadchiy V.A. et al. (2015), it was found that patients with CHF (NYHA functional classes I, II, and III) exhibited reduced gastric hydrochloric acid secretion due to impaired blood flow [4]. Decreased acid production by gastric parietal cells with subsequent alkalization of the gastric environment may lead to reduced solubility and absorption of drugs. A representative example is itraconazole, absorption of which decreases by 65% under achlorhydric conditions [5]. Intestinal venous congestion causes alterations in the gut microbiome [6], which can subsequently affect the absorption and metabolism of some drugs [7].
Fluid redistribution typical for CHF patients results in increased extracellular fluid compartment [8]. Consequently, the number of fluid compartments available for drug distribution expands, potentially altering drugs volume of distribution. CHF-induced hemodynamic changes may lead to congestive hepatopathy, liver fibrosis, and non-alcoholic fatty liver disease, impairing hepatic protein synthesis and consequently reducing albumin levels [9,10,11,12]. Hypoalbuminemia decreases protein-bound drug fractions, increasing pharmacologically active (unbound) drug concentrations. Elevated unbound fraction may mediate increased risks of toxicity and adverse drug reactions (ADRs). Another reason for increased risks of toxic drug effects in CHF patients may be related to the known down-regulation of hepatic CYP enzymes specific to the given population. CHF mediates hepatocyte damage, hypoxemia, increased pro-inflammatory cytokine levels, and heightened production of heme oxygenase-1 [13].
CHF-associated renal parenchymal hypoperfusion decreases drug clearance and prolongs elimination half-life, potentially leading to drug accumulation and subsequent ADRs [14].
The sum of PK changes unique to CHF patients may lead to significant variability in drug plasma levels, which can result in diminished efficacy of pharmacotherapy [15]. Existing PK studies in CHF patients are primarily focused on cardiovascular drugs, with limited data on other pharmacologic classes. The aim of this systematic review was to identify PK studies of various drugs in patients with CHF and to systematize information on PK parameter alterations based on drugs’ PCPs and administration routes.
2. Materials and Methods
Object of the study—Elibrary.ru, United States National Library of Medicine (PubMed), China National Knowledge Infrastructure (CNKI), and Directory of Open Access Journals (DOAJ) databases.
Search period: lower date limit—not applied, upper date limit—1 March 2025.
Search language: Russian and English.
Keywords for Elibrary.ru database search: “chronic heart failure”, “congestive heart failure”, “pharmacokinetics”, “clearance”, “half-life”, “volume of distribution”, “pharmacokinetic parameters”.
Keywords for PubMed, CNKI, DOAJ database search: (congestive heart failure OR chronic heart failure OR CHF) AND pharmacokinetics AND (clearance OR half-life OR volume of distribution) AND pharmacokinetic trial AND pharmacokinetic parameters.
Inclusion criteria:
- Full article text access;
- Study type: PK study;
- Study population: CHF patients;
- Detailed reporting of PK parameters for the studied drug;
- Age ≥18 years;
- Language of the article: Russian or English.
Exclusion criteria:
- Studies lacking reported PK parameters of drugs;
- Publication types: narrative reviews, case–control studies, meta-analyses, and systematic reviews.
Our systematic review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [16].
Following the application of inclusion/exclusion criteria, 106 publications were selected for inclusion in this systematic review. The PRISMA flow chart is presented in Figure 1.
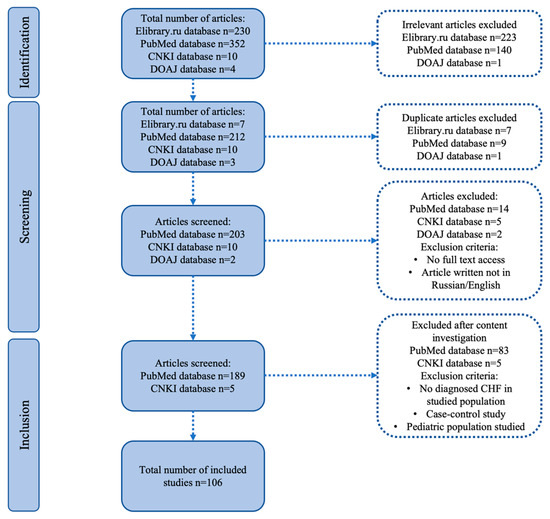
Figure 1.
PRISMA flowchart.
Data identification:
Three independent authors extracted the following data from eligible studies:
- 1.
- Demographic data: patients’ age, number of study participants, control group size (where applicable), NYHA functional class, and left ventricular ejection fraction (LVEF).
- 2.
- PK parameters: clearance, volume of distribution, half-life, and their alterations in CHF.
Risk of bias assessment:
Two authors evaluated the risk of bias using the Cochrane tool for assessing risk of bias (ROBINS-I).
Statistical analysis:
To assess correlations between physicochemical and pharmacokinetic drug properties, Spearman’s rank correlation coefficient was employed. A p-value of <0.05 was considered statistically significant.
3. Results
3.1. Study Identification
The initial number of publications was 596: 230 from Elibrary.ru, 352 from PubMed, 10 from CNKI, and 4 from DOAJ. After excluding invalid studies, duplicates, articles with no full-text access, and non-Russian/English publications, 189 articles from PubMed and 5 from CNKI remained. Further exclusion of studies without a confirmed CHF diagnosis in the study group, case–control studies, and those with pediatric populations resulted in 106 articles.
3.2. Quality Assessment
Two authors evaluated the risk of bias using the Cochrane tool for assessing risk of bias (ROBINS-I). The majority of the studies (n = 71; 67.0%) revealed a moderate risk of bias, 13 (12.3%) revealed a low risk, and 21 (20.8%) revealed a serious risk. Detailed results can be seen in Figure S1.
3.3. Study Characteristics
The search encompassed the entire period of database existence up to 1 March 2025.
The earliest identified publication date: 28 March 1974.
The most recent publication date: 25 March 2022.
The total number of included studies was 106, of which 13 (12.26%) contained data on more than one drug.
3.4. Drug Group Categorization
The first step in analysis was to classify all the drugs identified in PK studies into different groups using the Anatomical Therapeutic Chemical (ATC) classification system. Within ATC classification, substances are divided into groups according to the affected organ or system, therapeutic, pharmacological, and chemical properties (Table 1 and Figure 2). Detailed data on the structure of subgroups within ATC group C is presented in Figure 2B.

Table 1.
Drug structure based on ATC classification.
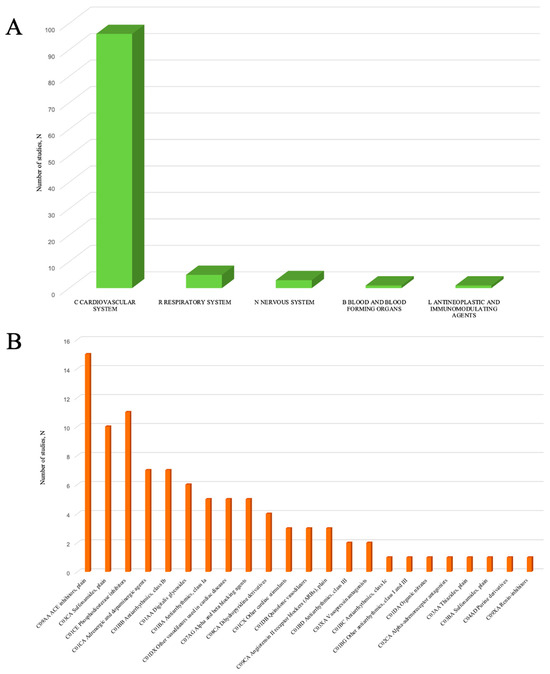
Figure 2.
ATC groups revealed in PK studies in patients with CHF. (A)—structure of ATC Drug Groups (C—cardiovascular system, R—respiratory system, L—antineoplastic and immunomodulating agents, N—nervous system, and B—blood and blood-forming organs); (B)—structure of subgroups in ATC group C.
The total number of drugs identified in PK studies included in our systematic review was 71 (full description is given in Table S1). Evaluating the number of publications dedicated to each drug, we found that the largest number of publications was reported for furosemide (n = 9, 2 of which also contained data on bumetanide), digoxin (n = 6), theophylline (n = 5), and milrinone (n = 4).
The leading group was represented by cardiovascular drugs (Group C), accounting for up to 96 studies (90.6%). Within this group, the subgroups with the largest number of studies were angiotensin-converting enzyme (ACE) inhibitors (C09AA) (n = 15; 15.6%), loop diuretics (C03CA) (n = 10; 10.4%), phosphodiesterase (PDE) inhibitors (C01CE) (n = 11; 11.5%), adrenergic agents (C01CA) (n = 7; 7.3%), and class Ib antiarrhythmics (C01BB) (n = 7; 7.3%). The structure of drug subgroups in Group C is presented in Figure 2B and Table S2.
Among ACE inhibitors (C09AA), lisinopril (n = 3) and captopril (n = 2) were leaders based on the number of studies. Considering loop diuretics (C03CA), the largest number of studies was identified for furosemide (n = 9) and torasemide (n = 3). Among PDE inhibitors (C01CE), milrinone (n = 4) and enoximone (n = 3) were leaders. Studying adrenergic drugs, the largest number of studies was found for ibopamine (n = 3) and prenalterol (n = 2). The detailed structure of drugs in Group C is presented in Table S3.
Group R was presented only with theophylline (n = 5).
Group N drugs included acetaminophen (n = 1), midazolam (n = 1), and fluvoxamine (n = 1).
Group L contained only one study with etanercept.
3.5. PCPs Evaluation
The second phase of our study was focused on evaluating PCPs of the identified drugs. The detailed structure of lipophilic, hydrophilic, and amphiphilic drugs is given in Table S4. We revealed an equal proportion of lipophilic (n = 32; 45.1%) and hydrophilic (n = 32; 45.1%) agents in PK studies in CHF patients, and a minority (n = 7; 9.9%) were amphiphilic (Table 2).

Table 2.
Drug structure according to PCPs.
Since the structural properties of a drug govern the plasma protein binding rate, we considered PPB in this section dedicated to PCPs of drugs. Most drugs included in our analysis revealed low values of PPB (LPB; clinically non-significant values) (n = 39; 54.9%). A high rate of PPB (HPB; clinically significant values) was observed in 39.4% (n = 28). For four drugs (5.6%), relevant data on PPB could not be identified in the literature. Detailed data on the PPB of drugs included in the analysis is presented in Table 3 and Table S4.

Table 3.
Drug structure according to PPB.
3.6. Evaluation of PK Parameters in CHF Patients
As the third phase of the study, we evaluated alterations of PK parameters detected in CHF patients. Given that the route of drug administration affects the PK of a drug, subdivision of all the revealed drugs into parenterally administered and orally administered was made.
The number of parenterally administered drugs was 42: 40.5% were lipophilic (n = 17), 52.4% were hydrophilic (n = 22), and 7.1% were amphiphilic (n = 3). Demographic data derived from publications involving PK studies with parenterally administered drugs is presented in Table S5.
Analysis of PK studies, including patients receiving parenteral drugs, revealed a relatively small number of participants (mean 16.28 ± 26.16; min = 5; max = 193). The mean patient age was 59.7 ± 8.7 years (min = 18.0, max = 81.8) years. NYHA class II–IV was most frequently identified (73.9%). Left ventricle ejection fraction (LVEF) was not reported in 53.9% of cases; in 46.1%, it was ≤45%.
The number of orally administered drugs was 50: 48% were lipophilic (n = 4), 38% were hydrophilic compounds (n = 19), and 14% were amphiphilic (n = 7). Demographic characteristics from PK studies, including oral drug administration, are presented in Tables S6 and S7. The mean number of CHF patients in PK studies was 20.8 ± 32.2 (min = 3, max = 116). The mean patient age was 61.8 ± 9.9 (min = 18, max = 84) years. NYHA class II–III was the most prevalent (66.3%). LVEF data was absent in 70.7%, and in 28.3% of patients, it was ≤45%.
3.6.1. Evaluation of PK Changes for Parenterally Administered Drugs
Analysis of hydrophilic drugs revealed that 62.5% exhibited LPB (a clinically non-significant rate), while 37.5% demonstrated HPB (a clinically significant rate). For further PK evaluation, we excluded studies missing the following PK parameters: clearance, volume of distribution, and half-life period. Among the total of 32 studies examining hydrophilic drugs, 2 were excluded from clearance analysis. Of the remaining 30 studies, clearance was reduced in 76.7% and increased in 23.3%. While assessing the volume of distribution, 9 of the 32 studies were excluded. Among the included 23 studies, the volume of distribution increased in 52.2% and decreased in 47.8%. The half-life period analysis excluded 8 studies, with the remaining 24 indicating a prolonged half-life in 70.8% and a reduced half-life in 29.2%.
The sum of PK changes revealed in the studies, including parenterally administered hydrophilic drugs, is shown in Table 4.

Table 4.
PK changes revealed in the studies, including parenterally administered hydrophilic drugs.
Among lipophilic drugs, LPB was predominantly observed (56.7%). Analyzing clearance changes among lipophilic agents, 4 out of 30 studies were excluded (no data on clearance). Among the remaining 26 studies, clearance decreased in 92.3% of cases and increased in only 7.7%. Assessing the volume of distribution, we excluded 11 studies (no data available). Analysis of the finally included 19 PK studies revealed a decreased volume of distribution in 52.6% and an increase in 47.4%. Half-life period evaluation resulted in the exclusion of 10 studies. Among the 20 included studies, it was prolonged in 90% and shortened in 10%.
PK changes revealed in the studies, including parenterally administered lipophilic drugs, are shown in Table 5.

Table 5.
PK changes revealed in the studies, including parenterally administered lipophilic drugs.
Among amphiphilic drugs, LPB predominated (66.7%), clearance was reduced in 66.7%, and the volume of distribution was increased in 66.7%. In the half-life analysis, one study was excluded due to the absence of data, and 100% of the included studies demonstrated prolongation of this parameter. Detailed data is presented in Table 6.

Table 6.
PK changes revealed in the studies, including parenterally administered amphiphilic drugs.
Subsequently, we performed a correlation analysis between the PCPs of the studied drugs (hydrophilicity/lipophilicity/amphiphilicity, and PPB) and their PK (clearance, volume of distribution, and half-life).
The analysis revealed a strong positive correlation (r = 0.725; p = 0.00045) between the degree of PPB of lipophilic drugs and their volume of distribution. Increased PPB among lipophilic drugs correlated with an increased volume of distribution in CHF patients. A moderate positive correlation (r = 0.433; p = 0.0346) between PCPs (hydro-/lipophilic/amphiphilic nature) of HPB drugs and their half-life period was detected. We revealed that the lower lipophilicity of HPB drugs correlates with a reduction in the half-life period. Detailed data is presented in Table 7.

Table 7.
Correlations between PCP and PK parameters for parenterally administered drugs.
3.6.2. Evaluation of PK Changes for Orally Administered Drugs
First, we studied hydrophilic drugs. Analysis of PPB rates resulted in the exclusion of eight studies. The remaining 38 studies demonstrated LPB in 71.1% of hydrophilic drugs and HPB in 29.0%. Clearance evaluation led to the exclusion of 7 out of 46 studies. Analysis disclosed decreased clearance in 76.9% and increased clearance in 23.1%. Assessing the volume of distribution, we excluded 41 studies, and only 5 were included. Analysis revealed an increased volume of distribution in 80% and a decrease in 20%. Half-life period analysis excluded 14 studies, and among the 32 remaining ones, 81.2% demonstrated prolongation, and 18.8% demonstrated reduction. A summary of PK changes of orally administered hydrophilic drugs is given in Table 8.

Table 8.
PK changes revealed in the studies, including orally administered hydrophilic drugs.
The analysis of lipophilic drugs predominantly revealed HPB (71.11%). Evaluating clearance, 1 study out of 45 was excluded, and the remaining 44 studies demonstrated decreased clearance in 88.6% and increased clearance in 11.4%. The volume of distribution assessment led to the exclusion of 37 studies. Among the eight included studies, decreased volume of distribution was detected in 37.5%, and an increase was detected in 62.5%. Analyzing the half-life period, 19 studies were excluded, with 26 included studies showing prolonged half-life in 80.8% and reduced half-life in 19.2%. Detailed data on PK changes revealed in the studies, including orally administered lipophilic drugs, is shown in Table 9.

Table 9.
PK changes revealed in the studies, including orally administered lipophilic drugs.
Assessing the PPB of amphiphilic drugs, we excluded two studies. Among the six included studies, half of the drugs were HPB, and half were LPB. PK analysis of amphiphilic drugs demonstrated decreased clearance in 62.5% of studies and an increase in 37.5%. Assessing the volume of distribution, we excluded seven studies, with only one study included. This result demonstrated a reduction in this PK parameter. Analyzing the half-life period, three studies were excluded, and all five included studies (100%) revealed prolongation. Full data highlighting PK changes revealed in the studies, including orally administered amphiphilic drugs, is given in Table 10.

Table 10.
PK changes revealed in the studies, including orally administered amphiphilic drugs.
Subsequent correlation analysis between PCPs (hydrophilicity/lipophilicity/amphiphilicity and PPB) and PK parameters (clearance, volume of distribution, and half-life) revealed several significant relationships: hydrophilic drugs demonstrated a moderate positive correlation between PPB and clearance (r = 0.5044, p = 0.0038), where increased PPB was associated with enhanced clearance; amphiphilic compounds showed a strong inverse correlation (r = −1, p < 0.05) with reduced PPB resulting in increased clearance; while HPB drugs exhibited a moderate negative correlation between hydrophilicity and clearance (r = −0.3956, p = 0.0087). Hydrophilicity was associated with increased clearance. Detailed data is presented in Table 11.

Table 11.
Correlations between PCPs and PK parameters for orally administered drugs.
4. Discussion
Our study revealed some correlations between PCPs and PK parameters of drugs in elderly patients with CHF. This study demonstrated a strong positive correlation (r = 0.725, p = 0.00045) between the PPB of lipophilic drugs administered parenterally and their volume of distribution. These results indicate that higher PPB is associated with increased volume of distribution for lipophilic compounds in CHF patients. This relationship can be attributed to CHF-related hypoproteinemia, which leads to elevated levels of unbound fraction with greater capacity for tissue penetration, ultimately resulting in larger distribution volumes [124]. A strong correlation between the unbound drug fraction and volume of distribution is supported by the work of K. Korzekwa et al. (2017), which examined the relationship between volume of distribution and PPB in both plasma and microsomes [125]. However, there was no link to be found for the PPB of parenteral hydrophilic and amphiphilic drugs. Considering the strong correlations revealed, we should also mention the small sample size of studies included in the final analysis. This may limit the significance of this phenomenon and require additional studies to verify its clinical value.
Furthermore, our results indicated a moderate positive correlation (r = 0.433; p = 0.0346) between the PCPs of HPB parenteral drugs and their half-lives. This finding suggests that decreased albumin level in CHF results in elevated unbound fraction, and lipophilic compounds exhibit prolonged half-life. This happens due to increased tissue penetration and subsequent binding with tissue proteins. This is supported by data derived from the study by H. Gunaydin et al. (2018), which concluded that reduced lipophilicity correlates with shorter half-life [126]. A high prevalence of adiposity (defined as >25% body fat in males and >35% in females) is observed in over 60% of the CHF population [127]. Patients with obesity demonstrated a direct correlation between drug lipophilicity and elimination half-life according to a study by Bruno C.D. et al. (2021) [128]. We revealed the absence of such a correlation for LPB drugs. Our study revealed a moderate negative correlation (r = −0.3956; p = 0.0087) between the PCPs of HPB orally administered drugs and their clearance.
Transferring our findings on other groups of drugs, we can suppose that there is a need to modify the dosing regimen in CHF patients. This may apply to such a group of antibiotics as beta-lactams, particularly cephalosporins. This class includes drugs with both HPB (e.g., cefoperazone) and LPB (e.g., ceftazidime), which may significantly affect clearance rates and consequently affect steady-state plasma drug concentrations [129].
For hydrophilic drugs, a moderate positive correlation (r = 0.504; p = 0.0038) was identified between PPB and clearance. In CHF patients, HPB hydrophilic drugs may demonstrate increased clearance, which stems from elevated unbound drug fractions. This happens due to hypoalbuminemia as the unbound fraction undergoes glomerular filtration [124].
A strong negative correlation (r = −1; p < 0.05) was observed between PPB and clearance for amphiphilic drugs, confirming that increased PPB reduces clearance in this drug category for CHF patients. This relationship may be particularly relevant for amphiphilic drugs among aminoglycosides described by Dezanet C. et al. (2020), suggesting the importance of monitoring PPB status to control the potential toxicity of these agents [130].
The limitations of our study are listed below:
- The relatively small sample size in the majority of included studies.
- The predominance of the moderate risk of bias among most of the included studies.
- Most of the included studies included data on cardiovascular drugs. Thus, extrapolation of the PK alterations to non-cardiovascular drugs remains speculative.
- Most studies were published prior to the year 2000.
Therefore, additional studies for non-cardiovascular drugs are required to improve our knowledge on PK alterations specific to CHF patients. Nevertheless, considering the pathophysiological CHF triad of reduced cardiac output, hepatic congestion, and hypoperfusion, we can propose practical recommendations. First, identification of high-risk drugs should be provided while planning treatment strategies in CHF patients (highly lipophilic, HPB). Administration of most highly lipophilic drugs should be started with a reduced dose and followed by a slow titration, allowing for sufficient time to assess both efficacy and toxicity. Some drugs, including those with a narrow therapeutic index and some antibiotics, require therapeutic drug monitoring (TDM) in CHF patients to guide therapy and avoid subtherapeutic or toxic plasma concentrations.
5. Conclusions
Our study revealed several consistent patterns of PK parameter changes among cardiovascular drugs with different PCPs. Based on the obtained data, the following conclusions can be drawn:
- Hypoalbuminemia associated with CHF may lead to an increased volume of distribution of lipophilic drugs.
- For lipophilic drugs used in CHF patients, potential prolongation of half-life and reduced clearance should be considered. This applies to both oral and parenteral formulations as important factors for dosing regimen modification.
- For HPB drugs used in CHF patients, reduced clearance must be considered as an important factor for dosing regimen modification.
Evaluation of correlations between drugs’ PK parameters and PCPs may provide additional insight into drug behavior in CHF patients. Some drug classes exhibit high concentration-dependent efficacy; thus, even small PK alterations may provide a pronounced effect on clinical outcomes, and antibiotics may represent an example of such drugs [131,132]. Since the data on PK changes in CHF is mainly limited to cardiovascular agents, further development of prognostic tools based on PK-PCP correlations may be helpful. Thus, additional PK studies including various pharmacological groups are required to improve our knowledge and construct proper approaches to correct dosing regimens in CHF patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26199495/s1.
Author Contributions
Conceptualization, O.B. and Y.K.; methodology, O.B. and Y.K.; software, Y.K.; validation, O.B., S.Z., and Y.K.; formal analysis, O.B., S.Z., and Y.K.; investigation, O.B. and Y.K.; resources, O.B. and Y.K.; data curation, O.B., S.Z., and Y.K.; writing—original draft preparation, O.B. and Y.K.; writing—review and editing, O.B. and Y.K.; visualization, Y.K.; supervision, S.Z.; project administration, S.Z. and O.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CHF | Chronic heart failure. |
| PCP | Physicochemical property. |
| PK | Pharmacokinetic. |
| PubMed | United States National Library of Medicine. |
| CNKI | China National Knowledge Infrastructure. |
| DOAJ | Directory of Open Access Journals. |
| PPB | Plasma protein binding. |
| ACE | Angiotensin-converting enzyme. |
| PDE | Phosphodiesterase. |
| NHANES | National Health and Nutrition Examination Survey. |
| NYHA | New York Heart Association. |
| ADR | Adverse drug reaction. |
| ATC classification | Anatomical Therapeutical Chemical classification. |
| NA | Not applicable. |
| LVEF | Left ventricle ejection fraction. |
| LPB | Low protein binding. |
| HPB | High protein binding. |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses. |
References
- Jimenez, S.; Cainzos-Achirica, M.; Monterde, D.; Garcia-Eroles, L.; Enjuanes, C.; Garay, A.; Yun, S.; Alcoberro, L.; Moliner, P.; Hidalgo, E.; et al. A population-based analysis in 375,233 cases of heart failure stages A, B and C. Real world epidemiology of prevalence and temporal trends in South-European populations. Eur. Hear. J. 2020, 41, ehaa946.0960. [Google Scholar] [CrossRef]
- Tiller, D.; Russ, M.; Greiser, K.H.; Nuding, S.; Ebelt, H.; Kluttig, A.; Kors, J.; Thiery, J.; Bruegel, M.; Haerting, J.; et al. Prevalence of symptomatic heart failure with reduced and with normal ejection fraction in an elderly general population—The CARLA study. PLoS ONE 2013, 8, e59225. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Ahmad, T.; Alexander, K.M.; Baker, W.L.; Bosak, K.; Breathett, K.; Fonarow, G.C.; Heidenreich, P.; Ho, J.E.; Hsich, E.; et al. Heart Failure Epidemiology and Outcomes Statistics: A Report of the Heart Failure Society of America. J. Card. Fail. 2023, 29, 1412–1451. [Google Scholar] [CrossRef]
- Osadchiy, V.A.; Bukanova, T.Y. Clinical and pathogenetic features of inflammatory and atrophic changes in the gastroduodenal zone in patients with varying severity of chronic heart failure associated with coronary artery disease. Ter. Arkhiv 2015, 87, 15–19. [Google Scholar] [CrossRef]
- Yang, E.; Yu, K.S.; Lee, S. Prediction of gastric pH-mediated drug exposure using physiologically-based pharmacokinetic modeling: A case study of itraconazole. CPT Pharmacomet. Syst. Pharmacol. 2023, 12, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Lupu, V.V.; Adam Raileanu, A.; Mihai, C.M.; Morariu, I.D.; Lupu, A.; Starcea, I.M.; Frasinariu, O.E.; Mocanu, A.; Dragan, F.; Fotea, S.; et al. The Implication of the Gut Microbiome in Heart Failure. Cells 2023, 12, 1158. [Google Scholar] [CrossRef]
- Cicchinelli, S.; Gemma, S.; Pignataro, G.; Piccioni, A.; Ojetti, V.; Gasbarrini, A.; Franceschi, F.; Candelli, M. Intestinal Fibrogenesis in Inflammatory Bowel Diseases: Exploring the Potential Role of Gut Microbiota Metabolites as Modulators. Pharmaceuticals 2024, 17, 490. [Google Scholar] [CrossRef]
- Hanna, D.; Baig, I.; Subbiondo, R.; Iqbal, U. The Usefulness of Bioelectrical Impedance as a Marker of Fluid Status in Patients With Congestive Heart Failure: A Systematic Review and Meta-Analysis. Cureus 2023, 15, e37377. [Google Scholar] [CrossRef]
- Haddadin, R.; Aboujaoude, C.; Trad, G. Congestive Hepatopathy: A Review of the Literature. Cureus 2024, 16, e58766. [Google Scholar] [CrossRef]
- Berezin, A.A.; Obradovic, Z.; Berezina, T.A.; Boxhammer, E.; Lichtenauer, M.; Berezin, A.E. Cardiac Hepatopathy: New Perspectives on Old Problems through a Prism of Endogenous Metabolic Regulations by Hepatokines. Antioxidants 2023, 12, 516. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Nakamura, K.; Nishihara, T.; Ichikawa, K.; Nakayama, R.; Takaya, Y.; Toh, N.; Akagi, S.; Miyoshi, T.; Akagi, T.; et al. Association between Cardiovascular Disease and Liver Disease, from a Clinically Pragmatic Perspective as a Cardiologist. Nutrients 2023, 15, 748. [Google Scholar] [CrossRef] [PubMed]
- Goliopoulou, A.; Theofilis, P.; Oikonomou, E.; Anastasiou, A.; Pantelidis, P.; Gounaridi, M.I.; Zakynthinos, G.; Katsarou, O.; Kassi, E.; Lambadiari, V.; et al. Non-Alcoholic Fatty Liver Disease and Echocardiographic Parameters of Left Ventricular Diastolic Function: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 14292. [Google Scholar] [CrossRef] [PubMed]
- Zordoky, B.N.; El-Kadi, A.O. Modulation of cardiac and hepatic cytochrome P450 enzymes during heart failure. Curr Drug Metab. 2008, 9, 122–128. [Google Scholar] [CrossRef]
- Abe, M.; Hemmi, S.; Kobayashi, H. How should we treat acute kidney injury caused by renal congestion? Kidney Res. Clin. Pract. 2023, 42, 415–430. [Google Scholar] [CrossRef]
- Lainscak, M.; Vitale, C.; Seferovic, P.; Spoletini, I.; Cvan Trobec, K.; Rosano, G.M. Pharmacokinetics and pharmacodynamics of cardiovascular drugs in chronic heart failure. Int. J. Cardiol. 2016, 224, 191–198. [Google Scholar] [CrossRef]
- Checklist Based on the Recommendations of PRISMA 2020. Available online: http://vestnik.mednet.ru/content/view/1580/1/lang,ru/ (accessed on 24 August 2023). (In Russian).
- Kostis, J.B.; Garland, W.T.; Delaney, C.; Northon, J.; Liao, W.C. Fosinopril: Pharmacokinetics and pharmacodynamics in congestive heart failure. Clin. Pharmacol. Ther. 1995, 58, 660–665. [Google Scholar] [CrossRef]
- Till, A.E.; Dickstein, K.; Aarsland, T.; Gomez, H.J.; Gregg, H.; Hichens, M. The pharmacokinetics of lisinopril in hospitalized patients with congestive heart failure. Br. J. Clin. Pharmacol. 1989, 27, 199–204. [Google Scholar] [CrossRef]
- Gautam, P.C.; Vargas, E.; Lye, M. Pharmacokinetics of lisinopril (MK521) in healthy young and elderly subjects and in elderly patients with cardiac failure. J. Pharm. Pharmacol. 1987, 39, 929–931. [Google Scholar] [CrossRef]
- Johnston, D.; Duffin, D. Pharmacokinetic profiles of single and repeat doses of lisinopril and enalapril in congestive heart failure. Am. J. Cardiol. 1992, 70, 151C–153C. [Google Scholar] [CrossRef]
- Nishida, M.; Matsuo, H.; Sano, H.; Obata, H.; Yasuda, H. Effect of captopril on congestive heart failure. Jpn. Circ. J. 1990, 54, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, T.; Shiyonoiri, H.; Takasaki, I.; Kobayashi, K.; Ishii, M. The effect of captopril on pharmacokinetics of digoxin in patients with mild congestive heart failure. Rinsho Iyaku 1990, 6, 2001–2011. [Google Scholar] [CrossRef]
- Schwartz, J.B.; Taylor, A.; Abernethy, D.; O’Meara, M.; Farmer, J.; Young, J.; Nelson, E.; Pool, J.; Mitchell, J.R. Pharmacokinetics and pharmacodynamics of enalapril in patients with congestive heart failure and patients with hypertension. J. Cardiovasc. Pharmacol. 1985, 7, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Bellissant, E.; Giudicelli, J.F. Pharmacokinetic-pharmacodynamic model for perindoprilat regional haemodynamic effects in healthy volunteers and in congestive heart failure patients. Br. J. Clin. Pharmacol. 2001, 52, 25–33. [Google Scholar] [CrossRef]
- Thuillez, C.; Richard, C.; Loueslati, H.; Auzepy, P.; Giudicelli, J.F. Systemic and regional hemodynamic effects of perindopril in congestive heart failure. J. Cardiovasc. Pharmacol. 1990, 15, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Kimata, S. Acute hemodynamic effect of quinapril on chronic heart failure. Rinsho Iyaku 1995, 11, 299–313. [Google Scholar]
- Squire, I.B.; Macfadyen, R.J.; Lees, K.R.; Hillis, W.S.; Reid, J.L. Haemodynamic response and pharmacokinetics after the first dose of quinapril in patients with congestive heart failure. Br. J. Clin. Pharmacol. 1994, 38, 117–123. [Google Scholar] [CrossRef]
- Begg, E.J.; Robson, R.A.; Ikram, H.; Richards, A.M.; Bammert-Adams, J.A.; Olson, S.C.; Posvar, E.L.; Reece, P.A.; Sedman, A.J. The pharmacokinetics of quinapril and quinaprilat in patients with congestive heart failure. Br. J. Clin. Pharmacol. 1994, 37, 302–304. [Google Scholar] [CrossRef][Green Version]
- Heintz, B.; Verho, M.; Brockmeier, D.; Luckel, G.; Maigatter, S.; Sieberth, H.G.; Rangoonwala, B.; Bender, N. Multiple-dose pharmacokinetics of ramipril in patients with chronic congestive heart failure. J. Cardiovasc. Pharmacol. 1993, 22 (Suppl. 9), S36–S42. [Google Scholar] [CrossRef]
- Wiseman, M.N.; Elstob, J.E.; Francis, R.J.; Brown, A.N.; Rajaguru, S.; Steiner, J.; Dymond, D.S. Initial and steady state pharmacokinetics of cilazapril in congestive cardiac failure. J. Pharm. Pharmacol. 1991, 43, 406–410. [Google Scholar] [CrossRef]
- Kostis, J.B.; Klapholz, M.; Delaney, C.; Vesterqvist, O.; Cohen, M.; Manning, J.A., Jr.; Jemal, M.; Kollia, G.D.; Liao, W.C. Pharmacodynamics and pharmacokinetics of omapatrilat in heart failure. J. Clin. Pharmacol. 2001, 41, 1280–1290. [Google Scholar] [CrossRef]
- Kessler, K.M.; Kayden, D.S.; Estes, D.M.; Koslovskis, P.L.; Sequeira, R.; Trohman, R.G.; Palomo, A.R.; Myerburg, R.J. Procainamide pharmacokinetics in patients with acute myocardial infarction or congestive heart failure. J. Am. Coll. Cardiol. 1986, 7, 1131–1139. [Google Scholar] [CrossRef]
- Tisdale, J.E.; Rudis, M.I.; Padhi, I.D.; Borzak, S.; Svensson, C.K.; Webb, C.R.; Acciaioli, J.; Ware, J.A.; Krepostman, A.; Zarowitz, B.J. Disposition of procainamide in patients with chronic congestive heart failure receiving medical therapy. J. Clin. Pharmacol. 1996, 36, 35–41. [Google Scholar] [CrossRef]
- Graffner, C.; Conradson, T.B.; Hofvendahl, S.; Ryden, L. Tocainide kinetics after intravenous and oral administration in healthy subjects and in patients with acute myocardial infarction. Clin. Pharmacol. Ther. 1980, 27, 64–71. [Google Scholar] [CrossRef] [PubMed]
- MacMahon, B.; Bakshi, M.; Branagan, P.; Kelly, G.J.; Walsh, M.J. Pharmacokinetics and haemodynamic effects of tocainide in patients with acute myocardial infarction complicated by left ventricular failure. Br. J. Clin. Pharmacol. 1985, 19, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, S.M.; Esterbrooks, D.; Hilleman, D.E.; Aronow, W.S.; Patterson, A.J.; Sketch, M.H.; Mooss, A.N.; Hee, T.T.; Reich, J.W. Tocainide kinetics in congestive heart failure. Clin. Pharmacol. Ther. 1983, 34, 596–603. [Google Scholar] [CrossRef]
- Yokota, M.; Miyahara, T.; Enomoto, N.; Inagaki, H.; Goto, J.; Hayashi, H. Pharmacokinetics and pharmacodynamics of SUN 1165, a novel antiarrhythmic agent, after administration of a single oral dose. Ther. Res. 1989, 10, 2135–2147. [Google Scholar]
- Rehnqvist, N.; Billing, E.; Moberg, L.; Lundman, T.; Olsson, G. Pharmacokinetics of felodipine and effect on digoxin plasma levels in patients with heart failure. Drugs 1987, 34 (Suppl. 3), 33–42. [Google Scholar] [CrossRef]
- Brater, D.C.; Day, B.; Burdette, A.; Anderson, S. Bumetanide and furosemide in heart failure. Kidney Int. 1984, 26, 183–189. [Google Scholar] [CrossRef]
- Chaturvedi, P.R.; O’Donnell, J.P.; Nicholas, J.M.; Shoenthal, D.R.; Waters, D.H.; Gwilt, P.R. Steady state absorption kinetics and pharmacodynamics of furosemide in congestive heart failure. Int. J. Clin. Pharmacol. Ther. Toxicol. 1987, 25, 123–128. [Google Scholar]
- Vasko, M.R.; Cartwright, D.B.; Knochel, J.P.; Nixon, J.V.; Brater, D.C. Furosemide absorption altered in decompensated congestive heart failure. Ann. Intern. Med. 1985, 102, 314–318. [Google Scholar] [CrossRef]
- Carlton, L.D.; Patterson, J.H.; Mattson, C.N.; Schmith, V.D. The effects of epoprostenol on drug disposition. II: A pilot study of the pharmacokinetics of furosemide with and without epoprostenol in patients with congestive heart failure. J. Clin. Pharmacol. 1996, 36, 257–264. [Google Scholar] [CrossRef]
- Gottlieb, S.S.; Khatta, M.; Wentworth, D.; Roffman, D.; Fisher, M.L.; Kramer, W.G. The effects of diuresis on the pharmacokinetics of the loop diuretics furosemide and torsemide in patients with heart failure. Am. J. Med. 1998, 104, 533–538. [Google Scholar] [CrossRef]
- Vargo, D.L.; Kramer, W.G.; Black, P.K.; Smith, W.B.; Serpas, T.; Brater, D.C. Bioavailability, pharmacokinetics, and pharmacodynamics of torsemide and furosemide in patients with congestive heart failure. Clin. Pharmacol. Ther. 1995, 57, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, F.; Mikkelsen, E. Distribution, elimination and effect of furosemide in normal subjects and in patients with heart failure. Eur. J. Clin. Pharmacol. 1977, 12, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Granneman, G.R.; Packer, M.; Boger, R.S. Simultaneous modeling of the pharmacokinetic and pharmacodynamic properties of enalkiren (Abbott-64662, a renin inhibitor). II: A dose-ranging study in patients with congestive heart failure. J. Cardiovasc. Pharmacol. 1993, 21, 834–840. [Google Scholar] [CrossRef]
- Gupta, S.K.; Granneman, G.R.; Boger, R.S.; Hollenberg, N.K.; Luther, R.R. Simultaneous modeling of the pharmacokinetic and pharmacodynamic properties of enalkiren (Abbott-64662, a renin inhibitor). I: Single dose stusy. Drug Metab Dispos. 1992, 20, 821–825. [Google Scholar] [CrossRef]
- Tilstone, W.J.; Dargie, H.; Dargie, E.N.; Morgan, H.G.; Kennedy, A.C. Pharmacokinetics of metolazone in normal subjects and in patients with cardiac or renal failure. Clin. Pharmacol. Ther. 1974, 16, 322–329. [Google Scholar] [CrossRef]
- Naafs, M.A.; van der Hoek, C.; van Duin, S.; Koorevaar, G.; Schopman, W.; Silberbusch, J. Decreased renal clearance of digoxin in chronic congestive heart failure. Eur. J. Clin. Pharmacol. 1985, 28, 249–252. [Google Scholar] [CrossRef]
- Applefeld, M.M.; Adir, J.; Crouthamel, W.G.; Roffman, D.S. Digoxin pharmacokinetics in congestive heart failure. J. Clin. Pharmacol. 1981, 21, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Carlton, L.D.; Patterson, J.H.; Mattson, C.N.; Schmith, V.D. The effects of epoprostenol on drug disposition. I: A pilot study of the pharmacokinetics of digoxin with and without epoprostenol in patients with congestive heart failure. J. Clin. Pharmacol. 1996, 36, 247–256. [Google Scholar] [CrossRef]
- Machida, M.; Komatsu, T.; Fujimoto, T.; Takechi, S.; Nomura, A. The effect of carvedilol on plasma digoxin concentration in patients with chronic heart failure. Jpn. J. Ther. Drug Monit. 2007, 24, 155–161. [Google Scholar]
- Yukawa, E.; Suematu, F.; Yukawa, M.; Minemoto, M.; Ohdo, S.; Higuchi, S.; Goto, Y.; Aoyama, T. Population pharmacokinetics of digoxin in Japanese patients: A 2-compartment pharmacokinetic model. Clin. Pharmacokinet. 2001, 40, 773–781. [Google Scholar] [CrossRef]
- Yukawa, E.; Honda, T.; Ohdo, S.; Higuchi, S.; Aoyama, T. Population-based investigation of relative clearance of digoxin in Japanese patients by multiple trough screen analysis: An update. J. Clin. Pharmacol. 1997, 37, 92–100. [Google Scholar] [CrossRef]
- Edelson, J.; Stroshane, R.; Benziger, D.P.; Cody, R.; Benotti, J.; Hood, W.B., Jr.; Chatterjee, K.; Luczkowec, C.; Krebs, C.; Schwartz, R. Pharmacokinetics of the bipyridines amrinone and milrinone. Circulation 1986, 73 Pt 2, III145–III152. [Google Scholar]
- Benotti, J.R.; Lesko, L.J.; McCue, J.E.; Alpert, J.S. Pharmacokinetics and pharmacodynamics of milrinone in chronic congestive heart failure. Am. J. Cardiol. 1985, 56, 685–689. [Google Scholar] [CrossRef]
- Taniguchi, T.; Shibata, K.; Saito, S.; Okeike, K. Pharmacokinetics of milrinone in patients with congestive heart failure during continuous venovenous hemofiltration. Intensive Care Med. 2000, 26, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Seino, Y.; Takano, T.; Hayakawa, H.; Kanmatsuse, K.; Saitoh, S.; Saitoh, T.; Kamishima, G.; Watanabe, K.; Motomiya, T.; Murata, M. Hemodynamic effects and pharmacokinetics of oral milrinone for short-term support in acute heart failure. Cardiology 1995, 86, 34–40. [Google Scholar] [CrossRef]
- Tammara, B.; Trang, J.M.; Kitani, M.; Miyamoto, G.; Bramer, S.L. The pharmacokinetics of toborinone in subjects with congestive heart failure and concomitant renal impairment and/or concomitant hepatic impairment. J. Clin. Pharmacol. 2002, 42, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Obata, K.; Yasue, H.; Yoshimura, M. Clinical effects and pharmacokinetics of alpha-human atrial natriuretic peptide (SUN 4936; carperitide) in patients with acute heart failure. Jpn. Pharmacol. Ther. 1993, 21, 1103–1114. [Google Scholar]
- Huber, T.; Grosse-Heitmeyer, W.; Rietbrock, S. Pharmacokinetics and pharmacodynamics of molsidomine in patients with liver dysfunction due to congestive heart failure. Int. J. Clin. Pharmacol. Ther. Toxicol. 1992, 30, 491–492. [Google Scholar]
- Mao, Z.L.; Stalker, D.; Keirns, J. Pharmacokinetics of conivaptan hydrochloride, a vasopressin V(1A)/V(2)-receptor antagonist, in patients with euvolemic or hypervolemic hyponatremia and with or without congestive heart failure from a prospective, 4-day open-label study. Clin. Ther. 2009, 31, 1542–1550. [Google Scholar] [CrossRef]
- Ochs, H.R.; Schuppan, U.; Greenblatt, D.J.; Abernethy, D.R. Reduced distribution and clearance of acetaminophen in patients with congestive heart failure. J. Cardiovasc. Pharmacol. 1983, 5, 697–699. [Google Scholar] [CrossRef] [PubMed]
- Soran, O.; Feldman, A.M.; Schneider, V.M.; Mann, D.L.; Korth-Bradley, J.M. The pharmacokinetics of etanercept in patients with heart failure. Br. J. Clin. Pharmacol. 2001, 51, 191–192. [Google Scholar] [CrossRef] [PubMed]
- Leuenberger, U.; Kenney, G.; Davis, D.; Clemson, B.; Zelis, R. Comparison of norepinephrine and isoproterenol clearance in congestive heart failure. Am. J. Physiol. 1992, 263 Pt 2, H56–H60. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Uno, S.; Yamasaki, Y.; Hirano, T.; Kim, S. OPC-61815 Investigators. Pharmacokinetics, Pharmacodynamics, Efficacy, and Safety of OPC-61815, a Prodrug of Tolvaptan for Intravenous Administration, in Patients With Congestive Heart Failure—A Phase II, Multicenter, Double-Blind, Randomized, Active-Controlled Trial. Circ. J. 2022, 86, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.; Rocci MLJr Weber, K.T.; Andrews, V.; Likoff, M.J. Pharmacokinetics and hemodynamics of amrinone in patients with chronic cardiac failure of diverse etiology. Res. Commun. Chem. Pathol. Pharmacol. 1987, 56, 3–19. [Google Scholar]
- Azzollini, F.; Catto, G.; Iacuitti, G.; Pelosi, G.; Picca, M.; Pocchiari, F. Ibopamine kinetics after a single oral dose in patients with congestive heart failure. Int. J. Clin. Pharmacol. Ther. Toxicol. 1988, 26, 105–112. [Google Scholar]
- Itoh, H.; Taniguchi, K.; Tsujibayashi, T.; Koike, A.; Sato, Y.; Nakamura, S. Hemodynamic effects and pharmacokinetics of longterm therapy with ibopamine in patients with chronic heart failure. Cardiology 1992, 80, 356–366. [Google Scholar] [CrossRef]
- Azzollini, F.; De Caro, L.; Longo, A.; Pelosi, G.; Rolandi, E.; Ventresca, G.P. Ibopamine kinetics after single and multiple dosing in patients with congestive heart failure. Int. J. Clin. Pharmacol. Ther. Toxicol. 1988, 26, 544–551. [Google Scholar]
- Nisi, A.; Panfili, M.; De Rosa, G.; Boffa, G.; Groppa, F.; Gusella, M.; Padrini, R. Pharmacokinetics of pentoxifylline and its main metabolites in patients with different degrees of heart failure following a single dose of a modified-release formulation. J. Clin. Pharmacol. 2013, 53, 51–57. [Google Scholar] [CrossRef]
- Tenero, D.; Boike, S.; Boyle, D.; Ilson, B.; Fesniak, H.F.; Brozena, S.; Jorkasky, D. Steady-state pharmacokinetics of carvedilol and its enantiomers in patients with congestive heart failure. J. Clin. Pharmacol. 2000, 40, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Lukas, M.A.; Tenero, D.M.; Baidoo, C.A.; Greenberg, B.H. Pharmacokinetic profile of controlled-release carvedilol in patients with left ventricular dysfunction associated with chronic heart failure or after myocardial infarction. Am. J. Cardiol. 2006, 98, 39L–45L. [Google Scholar] [CrossRef]
- Horiuchi, I.; Nozawa, T.; Fujii, N.; Inoue, H.; Honda, M.; Shimizu, T.; Taguchi, M.; Hashimoto, Y. Pharmacokinetics of R- and S-Carvedilol in routinely treated Japanese patients with heart failure. Biol. Pharm. Bull. 2008, 31, 976–980. [Google Scholar] [CrossRef][Green Version]
- Saito, M.; Kawana, J.; Ohno, T.; Hanada, K.; Kaneko, M.; Mihara, K.; Shiomi, M.; Nagayama, M.; Sumiyoshi, T.; Ogata, H. Population pharmacokinetics of R- and S-carvedilol in Japanese patients with chronic heart failure. Biol. Pharm. Bull. 2010, 33, 1378–1384. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abo, Y.; Mori, S.; Yokoi, H.; Takeda, H.; Nakano, H.; Watanabe, Y. Pharmacokinetics of candesartan cilexetil (TCV-116) in patients with chronic heart failure. J. N. Remedies Clin. 1996, 45, 1662–1668. [Google Scholar][Green Version]
- Kostis, J.B.; Vachharajani, N.N.; Hadjilambris, O.W.; Kollia, G.D.; Palmisano, M.; Marino, M.R. The pharmacokinetics and pharmacodynamics of irbesartan in heart failure. J. Clin. Pharmacol. 2001, 41, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Lo, M.W.; Toh, J.; Emmert, S.E.; Ritter, M.A.; Furtek, C.I.; Lu, H.; Colucci, W.S.; Uretsky, B.F.; Rucinska, E. Pharmacokinetics of intravenous and oral losartan in patients with heart failure. J. Clin. Pharmacol. 1998, 38, 525–532. [Google Scholar] [CrossRef]
- Lima, J.J.; Haughey, D.B.; Leier, C.V. Disopyramide pharmacokinetics and bioavailability following the simultaneous administration of disopyramide and 14C-disopyramide. J. Pharmacokinet. Biopharm. 1984, 12, 289–313. [Google Scholar] [CrossRef]
- Bonde, J.; Angelo, H.R.; Bodtker, S.; Svendsen, T.L.; Kampmann, J.P. Kinetics of disopyramide after intravenous infusion to patients with myocardial infarction and heart failure. Acta Pharmacol. Toxicol. 1985, 56, 278–282. [Google Scholar] [CrossRef]
- Kessler, K.M.; Lowenthal, D.T.; Warner, H.; Gibson, T.; Briggs, W.; Reidenberg, M.M. Quinidine elimination in patients with congestive heart failure or poor renal function. N. Engl. J. Med. 1974, 290, 706–709. [Google Scholar] [CrossRef]
- Prescott, L.F.; Adjepon-Yamoah, K.K.; Talbot, R.G. Impaired lignocaine metabolism in patients with myocardial infarction and cardiac failure. Br. Med. J. 1976, 1, 939–941. [Google Scholar] [CrossRef][Green Version]
- Thomson, P.D.; Melmon, K.L.; Richardson, J.A.; Cohn, K.; Steinbrunn, W.; Cudihee, R.; Rowland, M. Lidocaine pharmacokinetics in advanced heart failure, liver disease, and renal failure in humans. Ann. Intern. Med. 1973, 78, 499–508. [Google Scholar] [CrossRef]
- Sawyer, D.R.; Ludden, T.M.; Crawford, M.H. Continuous infusion of lidocaine in patients with cardiac arrhythmias. Unpredictability of plasma concentrations. Arch. Intern. Med. 1981, 141, 43–45. [Google Scholar] [CrossRef]
- Kobayashi, M.; Fukumoto, K.; Ueno, K. Effect of congestive heart failure on mexiletine pharmacokinetics in a Japanese population. Biol. Pharm. Bull. 2006, 29, 2267–2269. [Google Scholar] [CrossRef] [PubMed]
- Vadiei, K.; O’Rangers, E.A.; Klamerus, K.J.; Kluger, J.; Kazierad, D.J.; Leese, P.T.; Chow, M.S.; Zimmerman, J.J. Pharmacokinetics of intravenous amiodarone in patients with impaired left ventricular function. J. Clin. Pharmacol. 1996, 36, 720–727. [Google Scholar] [CrossRef]
- Tisdale, J.E.; Overholser, B.R.; Sowinski, K.M.; Wroblewski, H.A.; Amankwa, K.; Borzak, S.; Kingery, J.R.; Coram, R.; Zipes, D.P.; Flockhart, D.A.; et al. Pharmacokinetics of ibutilide in patients with heart failure due to left ventricular systolic dysfunction. Pharmacotherapy 2008, 28, 1461–1470. [Google Scholar] [CrossRef]
- Dunselman, P.H.; Edgar, B.; Scaf, A.H.; Kuntze, C.E.; Wesseling, H. Pharmacokinetics of felodipine after intravenous and chronic oral administration in patients with congestive heart failure. Br. J. Clin. Pharmacol. 1989, 28, 45–52. [Google Scholar] [CrossRef]
- Chen, D.G.; Feng, Q.P.; Wang, Z.Q.; Chen, K. Nifedipine pharmacodynamics and pharmacokinetics in treatment of congestive heart failure. Chin. Med. J. 1990, 103, 1008–1014. [Google Scholar] [PubMed]
- Muller, F.O.; Middle, M.V.; Schall, R.; Terblanche, J.; Hundt, H.K.; Groenewoud, G. An evaluation of the interaction of meloxicam with frusemide in patients with compensated chronic cardiac failure. Br. J. Clin. Pharmacol. 1997, 44, 393–398. [Google Scholar] [CrossRef]
- Hirasawa, K.; Tateda, K.; Kato, J. Clinicopharmacological study of nicardipine hydrochloride: Pharmacokinetics and hemodynamics after intravenous infusion in patients with acute heart failure. Jpn. Pharmacol. Ther. 1995, 23, 901–911. [Google Scholar]
- Marone, C.; Rivera, B.; Zwahlen, H.; Lahn, W.; Frey, F. Efficacy and pharmacokinetics of piretanide in patients with congestive heart failure. Eur. J. Clin. Investig. 1989, 19, 378–383. [Google Scholar] [CrossRef]
- Smith, N.A.; Kates, R.E.; Lebsack, C.; Ruder, M.A.; Mead, R.H.; Bekele, T.; Okerholm, R.A.; Rubin, G.M.; Winkle, R.A. Clinical pharmacology of intravenous enoximone: Pharmacodynamics and pharmacokinetics in patients with heart failure. Am. Heart J. 1991, 122 Pt 1, 755–763. [Google Scholar] [CrossRef]
- Ruder, M.A.; Lebsack, C.; Winkle, R.A.; Mead, R.H.; Smith, N.; Kates, R.E. Disposition kinetics of orally administered enoximone in patients with moderate to severe heart failure. J. Clin. Pharmacol. 1991, 31, 702–708. [Google Scholar] [CrossRef]
- Lima, J.J.; Leier, C.V.; Holtz, L.; Sterechele, J.; Shields, B.J.; MacKichan, J.J. Oral enoximone pharmacokinetics in patients with congestive heart failure. J. Clin. Pharmacol. 1987, 27, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Sandell, E.P.; Hayha, M.; Antila, S.; Heikkinen, P.; Ottoila, P.; Lehtonen, L.A.; Pentikainen, P.J. Pharmacokinetics of levosimendan in healthy volunteers and patients with congestive heart failure. J. Cardiovasc. Pharmacol. 1995, 26 (Suppl. 1), S57–S62. [Google Scholar] [CrossRef] [PubMed]
- Poder, P.; Eha, J.; Sundberg, S.; Antila, S.; Heinpalu, M.; Loogna, I.; Planken, U.; Rantanen, S.; Lehtonen, L. Pharmacokinetic-pharmacodynamic interrelationships of intravenous and oral levosimendan in patients with severe congestive heart failure. Int. J. Clin. Pharmacol. Ther. 2003, 41, 365–373. [Google Scholar] [CrossRef]
- Antila, S.; Kivikko, M.; Lehtonen, L.; Eha, J.; Heikkilä, A.; Pohjanjousi, P.; Pentikäinen, P.J. Pharmacokinetics of levosimendan and its circulating metabolites in patients with heart failure after an extended continuous infusion of levosimendan. Br. J. Clin. Pharmacol. 2004, 57, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Kieback, A.G.; Iven, H.; Stolzenburg, K.; Eichner, E.; Ruckdeschel, W.; Baumann, G. Pharmacokinetics and hemodynamic effects of the phosphodiesterase III inhibitor saterinone in patients with chronic heart failure. Int. J. Cardiol. 2003, 91, 201–208. [Google Scholar] [CrossRef]
- Mueck, W.; Frey, R. Population pharmacokinetics and pharmacodynamics of cinaciguat, a soluble guanylate cyclase activator, in patients with acute decompensated heart failure. Clin. Pharmacokinet. 2010, 49, 119–129. [Google Scholar] [CrossRef]
- Tice, F.D.; Jungbluth, G.L.; Binkley, P.F.; MacKichan, J.J.; Mohrland, J.S.; Wolf, D.L.; Leier, C.V. Clinical pharmacology of nicorandil in patients with congestive heart failure. Clin. Pharmacol. Ther. 1992, 52, 496–503. [Google Scholar] [CrossRef]
- Kates, R.E.; Leier, C.V. Dobutamine pharmacokinetics in severe heart failure. Clin. Pharmacol. Ther. 1978, 24, 537–541. [Google Scholar] [CrossRef]
- Patel, I.H.; Soni, P.P.; Fukuda, E.K.; Smith, D.F.; Leier, C.V.; Boudoulas, H. The pharmacokinetics of midazolam in patients with congestive heart failure. Br. J. Clin. Pharmacol. 1990, 29, 565–569. [Google Scholar] [CrossRef]
- Kuntz, H.D.; Straub, H.; May, B. Theophylline elimination in congestive heart failure. Klin. Wochenschr. 1983, 61, 1105–1106. [Google Scholar] [CrossRef]
- Slaughter, R.L.; Lanc, R.A. Theophylline clearance in obese patients in relation to smoking and congestive heart failure. Drug Intell. Clin. Pharm. 1983, 17, 274–276. [Google Scholar]
- Jeong, C.S.; Hwang, S.C.; Jones, D.W.; Ryu, H.S.; Sohn, K.; Sands, C.D. Theophylline disposition in Korean patients with congestive heart failure. Ann. Pharmacother. 1994, 28, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Cuzzolin, L.; Schinella, M.; Tellini, U.; Pezzoli, L.; Lippi, U.; Benoni, G. The effect of sex and cardiac failure on the pharmacokinetics of a slow-release theophylline formulation in the elderly. Pharmacol. Res. 1990, 22 (Suppl. 1), 137–138. [Google Scholar] [CrossRef] [PubMed]
- Ueno, K.; Miyai, K.; Koyama, M.; Seki, T.; Kawaguchi, Y.; Horiuchi, Y. Effect of congestive heart failure on theophylline disposition. Clin. Pharm. 1990, 9, 936–937. [Google Scholar]
- Gheorghiade, M.; Thyssen, A.; Zolynas, R.; Nadar, V.K.; Greenberg, B.H.; Mehra, M. Pharmacokinetics and pharmacodynamics of rivaroxaban and its effect on biomarkers of hypercoagulability in patients with chronic heart failure. J. Heart Lung Transplant. 2011, 30, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.M.; Shieh, S.M.; Hu, O.Y. Pharmacokinetics and pharmacodynamics of enantiomers of pimobendan in patients with dilated cardiomyopathy and congestive heart failure after single and repeated oral dosing. Clin. Pharmacol. Ther. 1995, 57, 610–621. [Google Scholar] [CrossRef]
- Sakai, M.; Ohkawa, S.; Kaku, T.; Kuboki, K.; Chida, K.; Imai, T. Pharmacokinetics of flosequinan in elderly patients with chronic congestive heart failure. Eur. J. Clin. Pharmacol. 1993, 44, 387–389. [Google Scholar] [CrossRef]
- Nicholls, D.P.; Droogan, A.; Carson, C.A.; Taylor, I.C.; Passmore, A.P.; Johnston, G.D.; Kendall, M.; Dutka, D.; Morris, G.K.; Underwood, L.M.; et al. Pharmacokinetics of flosequinan in patients with heart failure. Eur. J. Clin. Pharmacol. 1996, 50, 289–291. [Google Scholar] [CrossRef]
- Orlando, R.; De Martin, S.; Andrighetto, L.; Floreani, M.; Palatini, P. Fluvoxamine pharmacokinetics in healthy elderly subjects and elderly patients with chronic heart failure. Br. J. Clin. Pharmacol. 2010, 69, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Silke, B.; Lakhani, Z.M.; Taylor, S.H. Pharmacokinetic and pharmacodynamic studies with prazosin in chronic heart failure. J. Cardiovasc. Pharmacol. 1981, 3, 329–335. [Google Scholar] [CrossRef]
- Cook, J.A.; Smith, D.E.; Cornish, L.A.; Tankanow, R.M.; Nicklas, J.M.; Hyneck, M.L. Kinetics, dynamics, and bioavailability of bumetanide in healthy subjects and patients with congestive heart failure. Clin. Pharmacol. Ther. 1988, 44, 487–500. [Google Scholar] [CrossRef]
- Rosenthal, E.; Francis, R.J.; Brown, A.N.; Rajaguru, S.; Williams, P.E.; Steiner, J.; Curry, P.V. A pharmacokinetic study of cilazapril in patients with congestive heart failure. Br. J. Clin. Pharmacol. 1989, 27 (Suppl. 2), 267S–273S. [Google Scholar] [CrossRef]
- Lima, J.J.; Binkley, P.F.; Johnson, J.; Leier, C.V. Dose- and timedependent binding and kinetics of pindolol in patients with congestive heart failure. J. Clin. Pharmacol. 1986, 26, 253–257. [Google Scholar] [CrossRef]
- Massarella, J.W.; Silvestri, T.; DeGrazia, F.; Miwa, B.; Keefe, D. Effect of congestive heart failure on the pharmacokinetics of cibenzoline. J. Clin. Pharmacol. 1987, 27, 187–192. [Google Scholar] [CrossRef]
- Crawford, M.H.; Ludden, T.M.; Kennedy, G.T. Determinants of systemic availability of oral hydralazine in heart failure. Clin. Pharmacol. Ther. 1985, 38, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Iida, S.; Kinoshita, H.; Holford, N.H. Population pharmacokinetic and pharmacodynamic modelling of the effects of nicorandil in the treatment of acute heart failure. Br. J. Clin. Pharmacol. 2008, 66, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.W.; Armstrong, J.A.; Marks, G.S. Pharmacokinetic hemodynamic studies of intravenous nitroglycerin in congestive cardiac failure. Circulation 1980, 62, 160–166. [Google Scholar] [CrossRef]
- Sainsbury, E.J.; Fitzpatrick, D.; Ikram, H. Pharmacokinetic and plasma-concentration-effect relationships of prenalterol in cardiac failure. Eur. J. Clin. Pharmacol. 1985, 28, 397–403. [Google Scholar] [CrossRef]
- Dahlstrom, U.; Graffner, C.; Jonsson, U.; Hoffmann, K.J.; Karlsson, E.; Lagerstrom, P.O. Pharmacokinetics of prenalterol after single and multiple administration of controlled release tablets to patients with congestive heart failure. Eur. J. Clin. Pharmacol. 1983, 24, 495–502. [Google Scholar] [CrossRef]
- Pasini, E.; Comini, L.; Dioguardi, F.S.; Grossetti, F.; Olivares, A.; Zanelli, E.; Aquilani, R.; Scalvini, S. Hypoalbuminemia as a marker of protein metabolism disarrangement in patients with stable chronic heart failure. Minerva Med. 2020, 111, 226–238. [Google Scholar] [CrossRef]
- Korzekwa, K.; Nagar, S. Drug Distribution Part 2. Predicting Volume of Distribution from Plasma Protein Binding and Membrane Partitioning. Pharm. Res. 2017, 34, 544–551. [Google Scholar] [CrossRef]
- Gunaydin, H.; Altman, M.D.; Ellis, J.M.; Fuller, P.; Johnson, S.A.; Lahue, B.; Lapointe, B. Strategy for Extending Half-life in Drug Design and Its Significance. ACS Med. Chem. Lett. 2018, 9, 528–533. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bandeira, F.; Oliveira, L.B.; Siqueira, M.A.F.M.; Gadelha, R.B.M.; Correia, A.R.P.; Garcia, J.M.A.; Bandeira, F.V.; Bandeira, M.P. FRI113 Increased Body Fat Is Associated With Heart Failure Of Ischemic Cause. J. Endocr. Soc 2023, 7, bvad114.626. [Google Scholar] [CrossRef]
- Bruno, C.D.; Harmatz, J.S.; Duan, S.X.; Zhang, Q.; Chow, C.R.; Greenblatt, D.J. Effect of lipophilicity on drug distribution and elimination: Influence of obesity. Br. J. Clin. Pharmacol. 2021, 87, 3197–3205. [Google Scholar] [CrossRef]
- Jongmans, C.; Muller, A.E.; Van Den Broek, P.; Cruz De Almeida, B.D.M.; Van Den Berg, C.; Van Oldenrijk, J.; Bos, P.K.; Koch, B.C.P. An Overview of the Protein Binding of Cephalosporins in Human Body Fluids: A Systematic Review. Front. Pharmacol. 2022, 13, 900551. [Google Scholar] [CrossRef]
- Dezanet, C.; Kempf, J.; Mingeot-Leclercq, M.-P.; Décout, J.-L. Amphiphilic Aminoglycosides as Medicinal Agents. Int. J. Mol. Sci. 2020, 21, 7411. [Google Scholar] [CrossRef] [PubMed]
- Butranova, O.I.; Ushkalova, E.A.; Zyryanov, S.K.; Chenkurov, M.S.; Baybulatova, E.A. Pharmacokinetics of Antibacterial Agents in the Elderly: The Body of Evidence. Biomedicines 2023, 11, 1633. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Butranova, O.I.; Ushkalova, E.A.; Zyryanov, S.K.; Chenkurov, M.S. Developmental Pharmacokinetics of Antibiotics Used in Neonatal ICU: Focus on Preterm Infants. Biomedicines 2023, 11, 940. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).