Biochemical Modification of Poly-Vinyl-Alcohol-Based Bioplastics with Citrus By-Product to Increase Its Food Packaging Application
Abstract
1. Introduction
2. Results and Discussions
2.1. Extraction and Characterization of Polyphenols Matrix from Citrus bergamia “Pastazzo”
2.2. Bioplastics Characterizations
2.2.1. ATR-FTIR Analysis
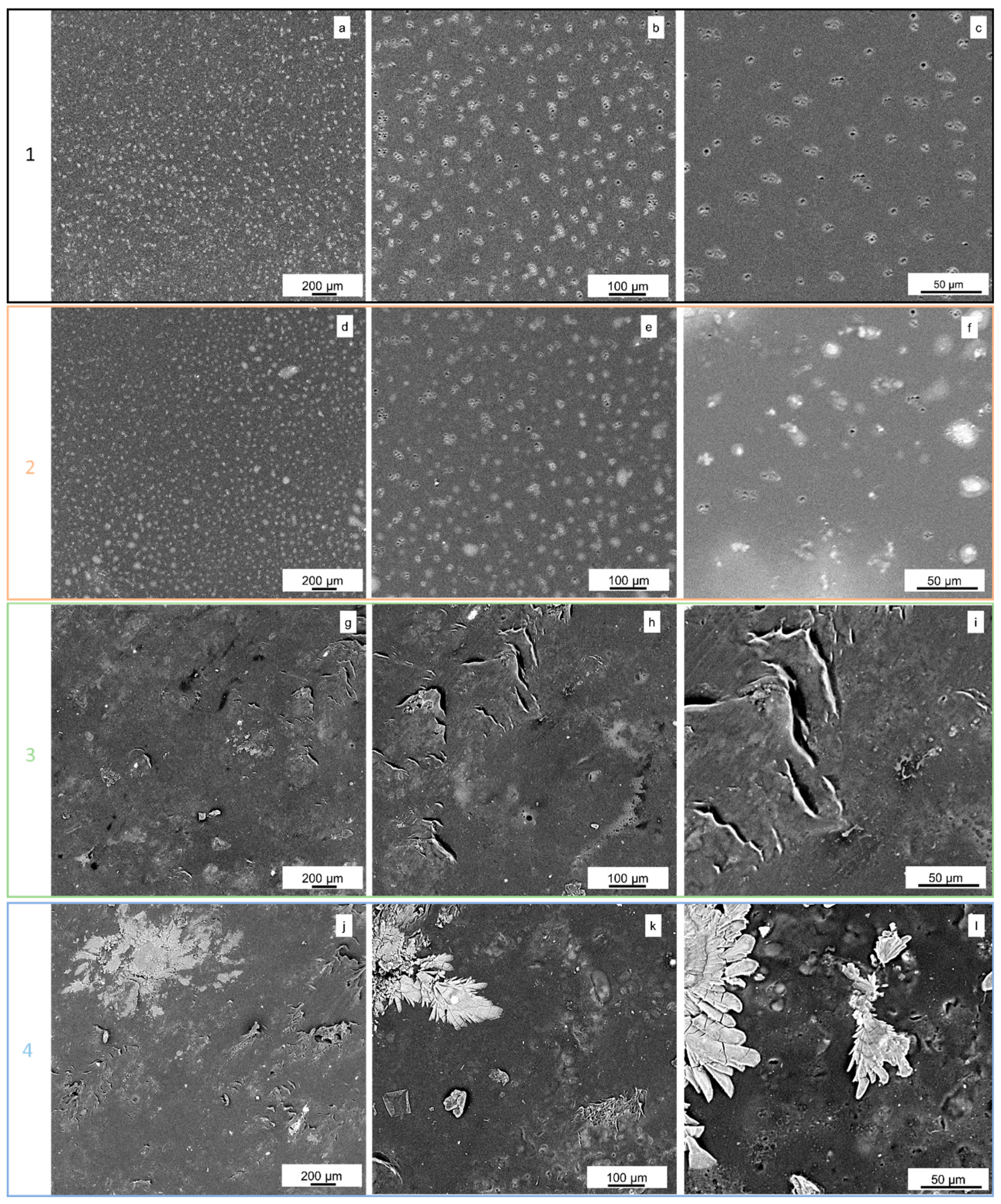
2.2.2. SEM Analysis
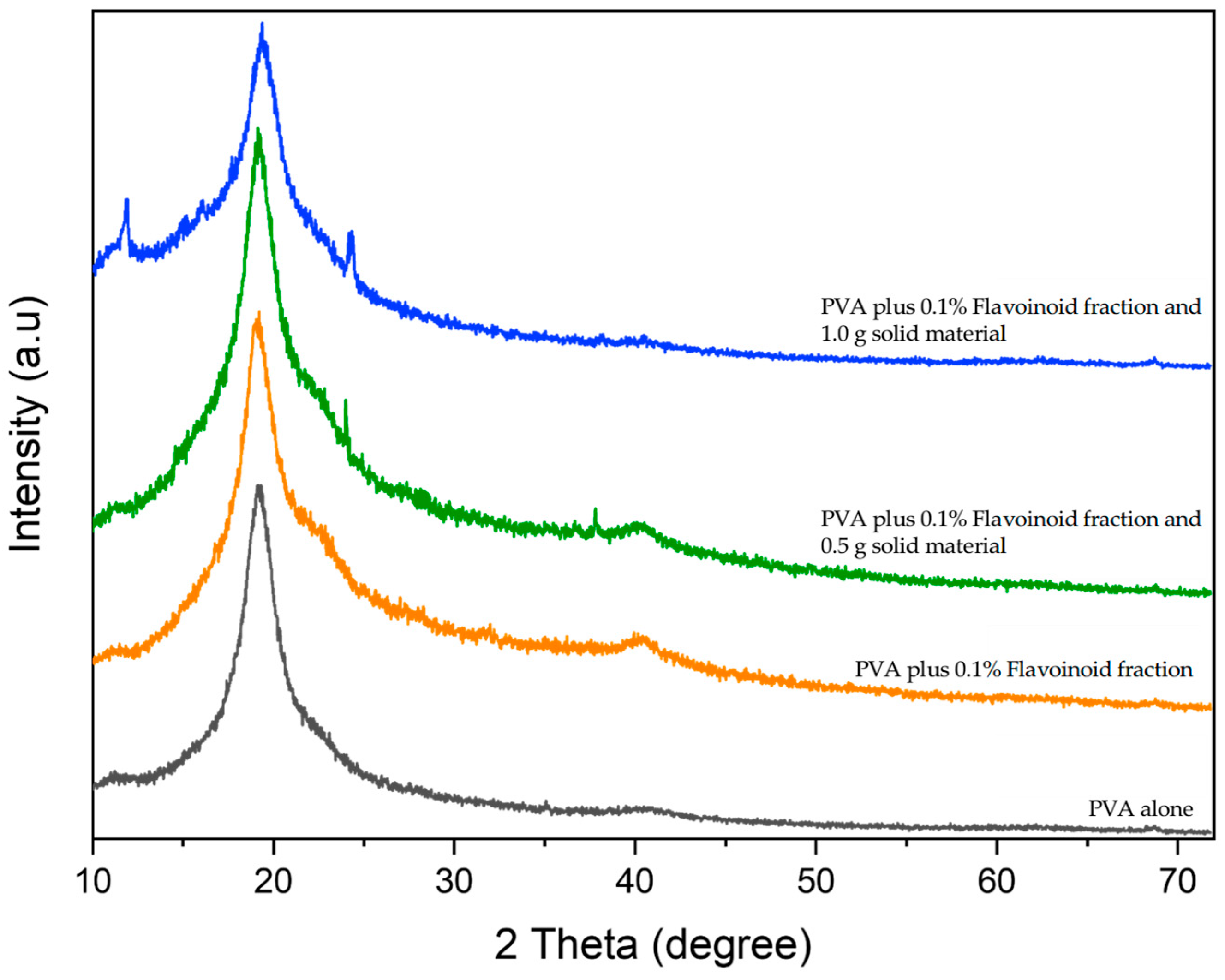
2.2.3. XRD Analysis
2.3. Thickness and Mechanical Properties of Functionalized Bioplastics
2.4. Optical Properties and Appearance
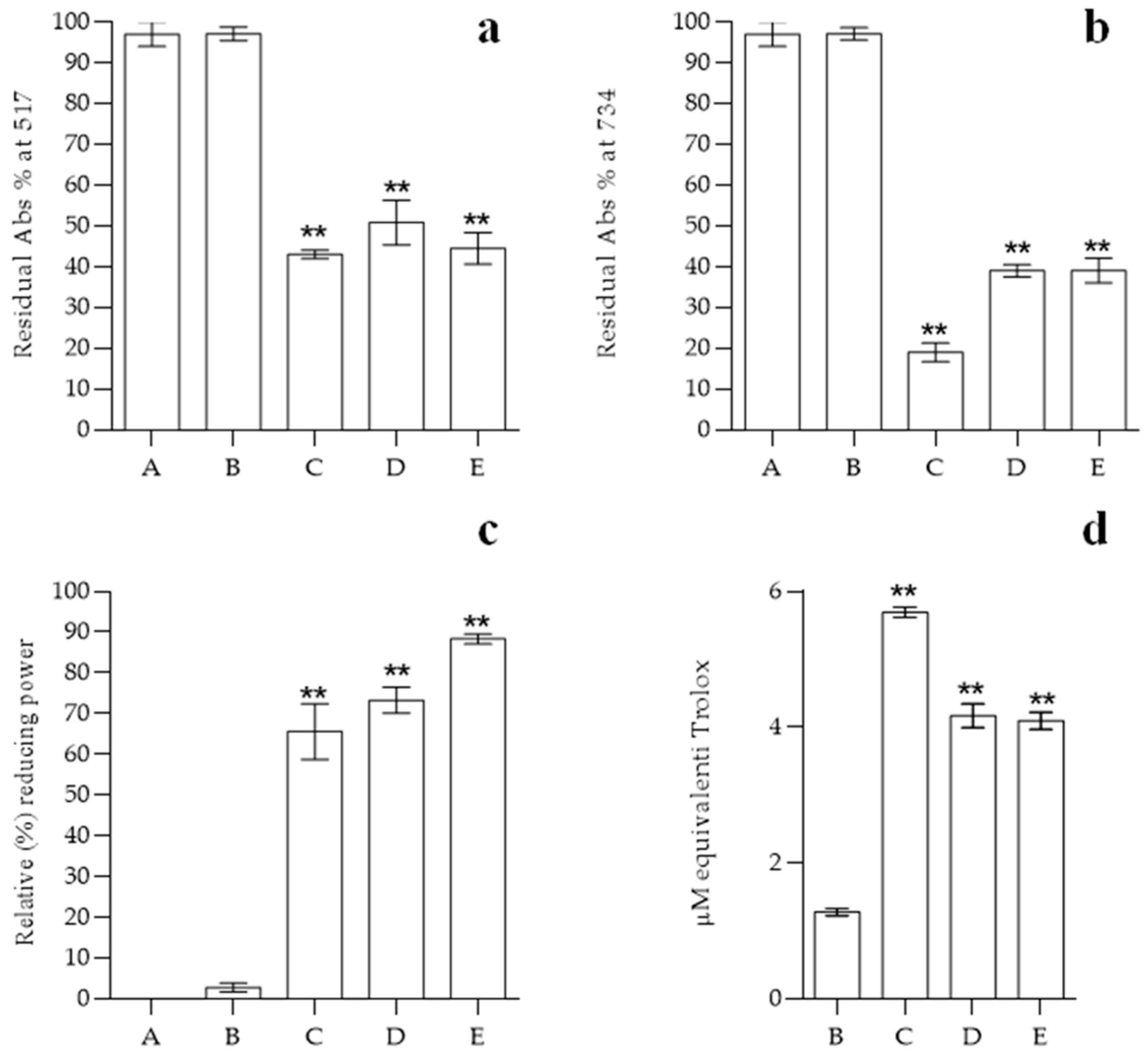
2.5. Antioxidant Power of Functionalized Bioplastics
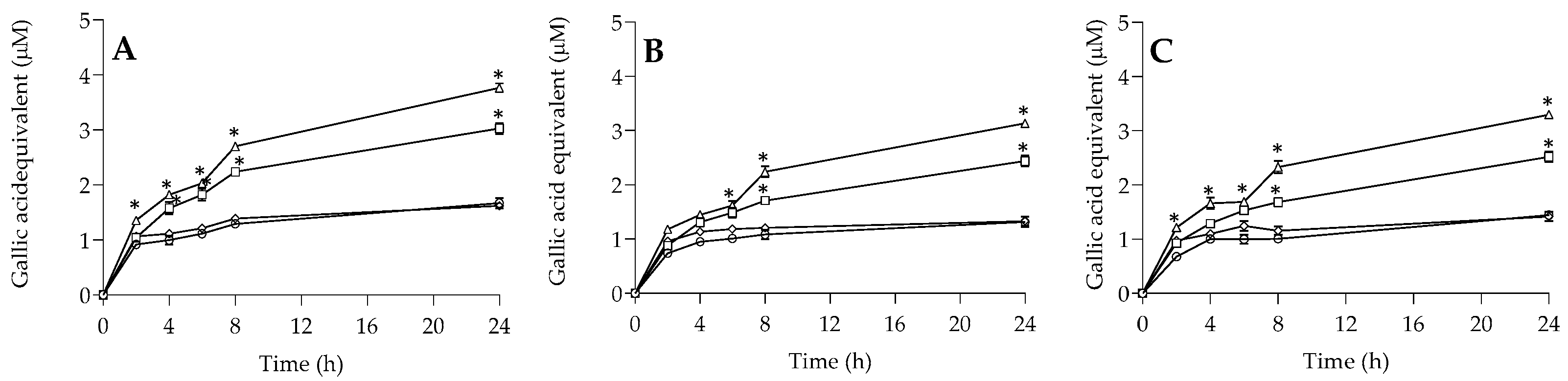
2.6. Migration Test of Polyphenols in Food Simulants
2.7. Food Fresh-Keeping Test
3. Materials and Methods
3.1. Reagents and Standard Solutions
3.2. Collection of Pastazzo and Extraction Optimization of Polyphenols
3.3. Polyphenol Extraction and Characterization by Reverse Phase High-Performance Liquid Chromatography Coupled with Diode Array (RP-HPLC-DAD)
3.4. Enzymatic Digestion of Pastazzo
3.5. PVA Based Bioplastic Production
3.6. Film Characterization
3.6.1. Attenuated Total Reflection-Infrared Spectroscopy
3.6.2. Scanning Electron Microscopy
3.6.3. X-Ray Diffractometer
3.6.4. Optical Properties
3.7. Mechanical Properties
3.8. Antioxidant Capacity of Bioplastics
3.8.1. Inhibition of the Free Radical 2,2-Diphenylpyrylhydrazyl Radical (DPPH) Assay
3.8.2. Ferric-Reducing Antioxidant Power (FRAP) Assay
3.8.3. Cupric Reducing Antioxidant Capacity (Cuprac)
3.8.4. ABTS
3.9. Release and Quantification of Polyphenols in Different Food Simulants
3.10. Packaging and Food Fresh-Keeping Test
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bucknall, D.G. Plastics as a materials system in a circular economy. Philos. Trans. A Math Phys. Eng. Sci. 2020, 378, 20190268. [Google Scholar] [CrossRef]
- Symeonides, D.; Loizia, P.; Zorpas, A.A. Tire waste management system in Cyprus in the framework of circular economy strategy. Environ. Sci. Pollut. Res. Int. 2019, 26, 35445–35460. [Google Scholar] [CrossRef]
- Visco, A.; Scolaro, C.; Facchin, M.; Brahimi, S.; Belhamdi, H.; Gatto, V.; Beghetto, V. Agri-Food Wastes for Bioplastics: European Prospective on Possible Applications in Their Second Life for a Circular Economy. Polymers 2022, 14, 2752. [Google Scholar] [CrossRef] [PubMed]
- Unni, A.B.; Muringayil Joseph, T. Enhancing Polymer Sustainability: Eco-Conscious Strategies. Polymers 2024, 16, 1769. [Google Scholar] [CrossRef]
- Beghetto, V.; Gatto, V.; Samiolo, R.; Scolaro, C.; Brahimi, S.; Facchin, M.; Visco, A. Plastics today: Key challenges and EU strategies towards carbon neutrality: A review. Environ. Pollut. 2023, 334, 122102. [Google Scholar] [CrossRef]
- Visco, A.; Scolaro, C.; Oliveri, F.; Ruta, A.J. Mathematical Modelling of Tensile Mechanical Behavior of a Bio-Composite Based on Polybutylene-Succinate and Brewer Spent Grains. Polymers 2024, 16, 2966. [Google Scholar] [CrossRef] [PubMed]
- Sari, N.; Mairisya, M.; Kurniasari, R.; Purnavita, S. Bioplastik Berbasis Galaktomanan Hasil Ekstraski Ampas Kelapa Dengan Campuran Polyvinyl Alkohol. METANA 2019, 15, 8. [Google Scholar] [CrossRef]
- Abdullah, Z.W.; Dong, Y. Biodegradable and Water Resistant Poly(vinyl) Alcohol (PVA)/Starch (ST)/Glycerol (GL)/Halloysite Nanotube (HNT) Nanocomposite Films for Sustainable Food Packaging. Front. Mater. 2019, 6, 2019. [Google Scholar] [CrossRef]
- Patane, G.T.; Calderaro, A.; Putaggio, S.; Ginestra, G.; Mandalari, G.; Cirmi, S.; Barreca, D.; Russo, A.; Gervasi, T.; Neri, G.; et al. Novel Bioplastic Based on PVA Functionalized with Anthocyanins: Synthesis, Biochemical Properties and Food Applications. Int. J. Mol. Sci. 2024, 25, 9929. [Google Scholar] [CrossRef]
- Belay, M. Review on Physicochemical Modification of Biodegradable Plastic: Focus on Agar and Polyvinyl Alcohol (PVA). Adv. Mater. Sci. Eng. 2023, 2023, 4056020. [Google Scholar] [CrossRef]
- Ismayati, M.; Fatah, N.A.N.; Ernawati, E.E.; Juliandri; Kusumaningrum, W.B.; Lubis, M.A.R.; Fatriasari, W.; Solihat, N.N.; Sari, F.P.; Halim, A.; et al. Antioxidant and UV-blocking activity of PVA/tannin-based bioplastics in food packaging application. Int. J. Biol. Macromol. 2024, 257, 128332. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Bellocco, E.; Caristi, C.; Leuzzi, U.; Gattuso, G. Flavonoids and Furocoumarins in Bergamot, Myrtle-Leaved Orange, and Sour Orange Juices: Distribution and Properties. Acs. Sym. Ser. 2012, 1093, 17–35. [Google Scholar]
- Salerno, R.; Casale, F.; Calandruccio, C.; Procopio, A. Characterization of flavonoids in Citrus bergamia (Bergamot) polyphenolic fraction by liquid chromatography–high resolution mass spectrometry (LC/HRMS). PharmaNutrition 2016, 4, S1–S7. [Google Scholar] [CrossRef]
- Adorisio, S.; Muscari, I.; Fierabracci, A.; Thuy, T.T.; Marchetti, M.C.; Ayroldi, E.; Delfino, D.V. Biological effects of bergamot and its potential therapeutic use as an anti-inflammatory, antioxidant, and anticancer agent. Pharm. Biol. 2023, 61, 639–646. [Google Scholar] [CrossRef]
- Cirmi, S.; Bisignano, C.; Mandalari, G.; Navarra, M. Anti-infective potential of Citrus bergamia Risso et Poiteau (bergamot) derivatives: A systematic review. Phytother. Res. 2016, 30, 1404–1411. [Google Scholar] [CrossRef]
- Mandalari, G.; Bennett, R.N.; Bisignano, G.; Saija, A.; Dugo, G.; Curto, R.B.L.; Faulds, C.B.; Waldron, K.W. Characterization of flavonoids and pectins from bergamot (Citrus bergamia Risso) peel, a major byproduct of essential oil extraction. J. Agric. Food Chem. 2006, 54, 197–203. [Google Scholar] [CrossRef]
- Zhang, S.; Cheng, X.; Yang, W.; Fu, Q.; Su, F.; Wu, P.; Li, Y.; Wang, F.; Li, H.; Ai, S. Converting fruit peels into biodegradable, recyclable and antimicrobial eco-friendly bioplastics for perishable fruit preservation. Bioresour. Technol. 2024, 406, 131074. [Google Scholar] [CrossRef]
- Yaradoddi, J.S.; Banapurmath, N.R.; Ganachari, S.V.; Soudagar, M.E.M.; Sajjan, A.M.; Kamat, S.; Mujtaba, M.A.; Shettar, A.S.; Anqi, A.E.; Safaei, M.R.; et al. Bio-based material from fruit waste of orange peel for industrial applications. J. Mater. Res. Technol. 2022, 17, 3186–3197. [Google Scholar] [CrossRef]
- Fiorentini, C.; Duserm Garrido, G.; Bassani, A.; Cortimiglia, C.; Zaccone, M.; Montalbano, L.; Martinez-Nogues, V.; Cocconcelli, P.S.; Spigno, G. Citrus Peel Extracts for Industrial-Scale Production of Bio-Based Active Food Packaging. Foods 2021, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, M.; Caracciolo, F.; Cembalo, L.; Chinnici, G.; Pecorino, B.; D’Amico, M. Making Virtue Out of Necessity: Managing the Citrus Waste Supply Chain for Bioeconomy Applications. Sustainability 2018, 10, 4821. [Google Scholar] [CrossRef]
- Lanfranchi, M. Economic analysis on the enhancement of citrus waste for energy production. J. Essent. Oil Res. 2012, 24, 583–591. [Google Scholar] [CrossRef]
- Chavan, P.; Singh, A.K.; Kaur, G. Recent progress in the utilization of industrial waste and by-products of citrus fruits: A review. J. Food Process Eng. 2018, 41, e12895. [Google Scholar] [CrossRef]
- Ledesma-Escobar, C.A.; de Castro, M.D.L. Towards a comprehensive exploitation of citrus. Trends Food Sci. Tech. 2014, 39, 63–75. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Hwan, M.; Lee, Y.R. Converting citrus wastes into value-added products: Economic and environmently friendly approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef]
- Mamma, D.; Christakopoulos, P. Biotransformation of Citrus By-Products into Value Added Products. Waste Biomass Valori. 2014, 5, 529–549. [Google Scholar] [CrossRef]
- Taghizadeh-Alisaraei, A.; Hosseini, S.H.; Ghobadian, B.; Motevali, A. Biofuel production from citrus wastes: A feasibility study in Iran. Renew Sust. Energ. Rev. 2017, 69, 1100–1112. [Google Scholar] [CrossRef]
- Khandan, N.; Abdollahi, H.; Gharabaghi, M.; Khoshdast, H. Valorization of biofuel produced from citrus waste as potential collector in coal flotation: Production, characterization, and flotation performance. Int. J. Coal. Prep. Util. 2025, 1–12. [Google Scholar] [CrossRef]
- Pourbafrani, M.; Forgács, G.; Horváth, I.S.; Niklasson, C.; Taherzadeh, M.J. Production of biofuels, limonene and pectin from citrus wastes. Bioresour. Technol. 2010, 101, 4246–4250. [Google Scholar] [CrossRef]
- Bampidis, V.A.; Robinson, P.H. Citrus by-products as ruminant feeds: A review. Anim. Feed Sci. Tech. 2006, 128, 175–217. [Google Scholar] [CrossRef]
- Testa, R.; Foderà, M.; Di Trapani, A.M.; Tudisca, S.; Sgroi, F. Giant reed as energy crop for Southern Italy: An economic feasibility study. Renew Sust. Energ. Rev. 2016, 58, 558–564. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE-Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, J.; Gong, J.; Li, J.; Mo, L. Preparation, characterization and acetylation of cellulose nanocrystal allomorphs. Cellulose 2018, 25, 4905–4918. [Google Scholar] [CrossRef]
- do Nascimento, J.V.; Silva, K.A.; Giuliangeli, V.C.; Mendes, A.L.D.; Piai, L.P.; Michels, R.N.; Dal Bosco, T.C.; Stroher, G.R.; Shirai, M.A. Starch-PVA based films with Clitoria ternatea flower extract: Characterization, phenolic compounds release and compostability. Int. J. Biol. Macromol. 2024, 255, 128232. [Google Scholar] [CrossRef]
- Visco, A.; Bardella, N.; Scolaro, C.; Belhamdi, H.; Brahimi, S.; Gatto, V.; Samiolo, R.; Beghetto, V. Reuse of beer spent grain for the industrial production of biodegradable bio-composites. Ind. Crops Prod. 2025, 235, 121684. [Google Scholar] [CrossRef]
- Guzman-Puyol, S.; Benitez, J.J.; Heredia-Guerrero, J.A. Transparency of polymeric food packaging materials. Food Res. Int. 2022, 161, 111792. [Google Scholar] [CrossRef]
- Wang, L.; Yang, C.; Deng, X.; Peng, J.; Zhou, J.; Xia, G.; Zhou, C.; Shen, Y.; Yang, H. A pH-sensitive intelligent packaging film harnessing Dioscorea zingiberensis starch and anthocyanin for meat freshness monitoring. Int. J. Biol. Macromol. 2023, 245, 125485. [Google Scholar] [CrossRef] [PubMed]
- Mirpoor, S.F.; Patane, G.T.; Corrado, I.; Giosafatto, C.V.L.; Ginestra, G.; Nostro, A.; Foti, A.; Gucciardi, P.G.; Mandalari, G.; Barreca, D.; et al. Functionalization of Polyhydroxyalkanoates (PHA)-Based Bioplastic with Phloretin for Active Food Packaging: Characterization of Its Mechanical, Antioxidant, and Antimicrobial Activities. Int. J. Mol. Sci. 2023, 24, 11628. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Estaca, J.; López-de-Dicastillo, C.; Hernández-Muñoz, P.; Catalá, R.; Gavara, R. Advances in antioxidant active food packaging. Trends Food Sci. Tech. 2014, 35, 42–51. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Cruz, J.M.; Sendón, R.; de Quirós, A.R.B.; Ares, A.; Castro-López, M.; Abad, M.J.; Maroto, J.; Paseiro-Losada, P. Development of antioxidant active films containing tocopherols to extend the shelf life of fish. Food Control 2013, 31, 236–243. [Google Scholar] [CrossRef]
- Gemili, S.; Yemenicioglu, A.; Altinkaya, S.A. Development of antioxidant food packaging materials with controlled release properties. J. Food Eng. 2010, 96, 325–332. [Google Scholar] [CrossRef]
- Quintero-Borregales, L.M.; Vergara-Rubio, A.; Santos, A.; Famá, L.; Goyanes, S. Black Tea Extracts/Polyvinyl Alcohol Active Nanofibers Electrospun Mats with Sustained Release of Polyphenols for Food Packaging Applications. Polymers 2023, 15, 1311. [Google Scholar] [CrossRef]
- Ambrogi, V.; Cerruti, P.; Carfagna, C.; Malinconico, M.; Marturano, V.; Perrotti, M.; Persico, P. Natural antioxidants for polypropylene stabilization. Polym. Degrad. Stab. 2011, 96, 2152–2158. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; Gil, N.J.B.; González-Martínez, C.; Chiralt, A. Antioxidant poly (lactic acid) films with rice straw extract for food packaging applications. Food Packag. Shelf Life 2022, 34, 101003. [Google Scholar] [CrossRef]
- Gattuso, A.; Piscopo, A.; Romeo, R.; De Bruno, A.; Poiana, M. Recovery of Bioactive Compounds from Calabrian Bergamot Citrus Waste: Selection of Best Green Extraction. Agriculture 2023, 13, 1095. [Google Scholar] [CrossRef]
- Fratianni, F.; Cozzolino, A.; De Feo, V.; Coppola, R.; Ombra, M.N.; Nazzaro, F. Polyphenols, Antioxidant, Antibacterial, and Biofilm Inhibitory Activities of Peel and Pulp of Citrus medica L., Citrus bergamia, and Citrus medica cv. Salo Cultivated in Southern Italy. Molecules 2019, 24, 4577. [Google Scholar] [CrossRef] [PubMed]
- Baron, G.; Altomare, A.; Mol, M.; Garcia, J.L.; Correa, C.; Raucci, A.; Mancinelli, L.; Mazzotta, S.; Fumagalli, L.; Trunfio, G.; et al. Analytical Profile and Antioxidant and Anti-Inflammatory Activities of the Enriched Polyphenol Fractions Isolated from Bergamot Fruit and Leave. Antioxidants 2021, 10, 141. [Google Scholar] [CrossRef]
- Osorio, J.; Aznar, M.; Nerin, C.; Elliott, C.; Chevallier, O. Comparison of LC-ESI, DART, and ASAP for the analysis of oligomers migration from biopolymer food packaging materials in food (simulants). Anal. Bioanal. Chem. 2022, 414, 1335–1345. [Google Scholar] [CrossRef]
- European Commission. No. 10/2011 of 14 january 2011 on plastic materials and articles intended to come into contact with food. Off. J. Eur. Union 2011, 89, 42–130. [Google Scholar]
- Song, D.; Ma, L.W.; Pang, B.; An, R.; Nie, J.H.; Guo, Y.R.; Li, S. An Active Bio-Based Food Packaging Material of ZnO@Plant Polyphenols/Cellulose/Polyvinyl Alcohol: DESIGN, Characterization and Application. Int. J. Mol. Sci. 2023, 24, 1577. [Google Scholar] [CrossRef]
- Mittal, K.L. Contact Angle, Wettability and Adhesion, 1st ed.; CRC Press: London, UK, 2003; Volume 3, p. 520. [Google Scholar]
- Özyürek, M.; Güçlü, K.; Apak, R. The main and modified CUPRAC methods of antioxidant measurement. Trac. Trend Anal. Chem. 2011, 30, 652–664. [Google Scholar] [CrossRef]
- Barreca, D.; Lagana, G.; Leuzzi, U.; Smeriglio, A.; Trombetta, D.; Bellocco, E. Evaluation of the nutraceutical, antioxidant and cytoprotective properties of ripe pistachio (Pistacia vera L., variety Bronte) hulls. Food Chem. 2016, 196, 493–502. [Google Scholar] [CrossRef]
- Barreca, D.; Laganà, G.; Ficarra, S.; Tellone, E.; Leuzzi, U.; Galtieri, A.; Bellocco, E. Evaluation of the antioxidant and cytoprotective properties of the exotic fruit Mill. (Annonaceae). Food Res. Int. 2011, 44, 2302–2310. [Google Scholar] [CrossRef]
- Apak, R.; Guclu, K.; Ozyurek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Papalia, T.; Barreca, D.; Panuccio, M.R. Assessment of Antioxidant and Cytoprotective Potential of Jatropha (Jatropha curcas) Grown in Southern Italy. Int. J. Mol. Sci. 2017, 18, 660. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; Diez Garcia, A.; Lopez, D.; Fiori, S.; Peponi, L. Antioxidant Bilayers Based on PHBV and Plasticized Electrospun PLA-PHB Fibers Encapsulating Catechin. Nanomaterials 2019, 9, 346. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.; Dominguez-López, I.; Lamuela-Raventós, R.M. The Chemistry Behind the Folin-Ciocalteu Method for the Estimation of (Poly)phenol Content in Food: Total Phenolic Intake in a Mediterranean Dietary Pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef]
- Jatav, J.; Chinchkar, A.V.; Bhattacharya, B. Chitosan film with pineapple peel extract in the extension of shelf life of Indian cottage cheese: Release kinetics and bio-accessibility studies. Food Res. Int. 2023, 174, 113580. [Google Scholar] [CrossRef]

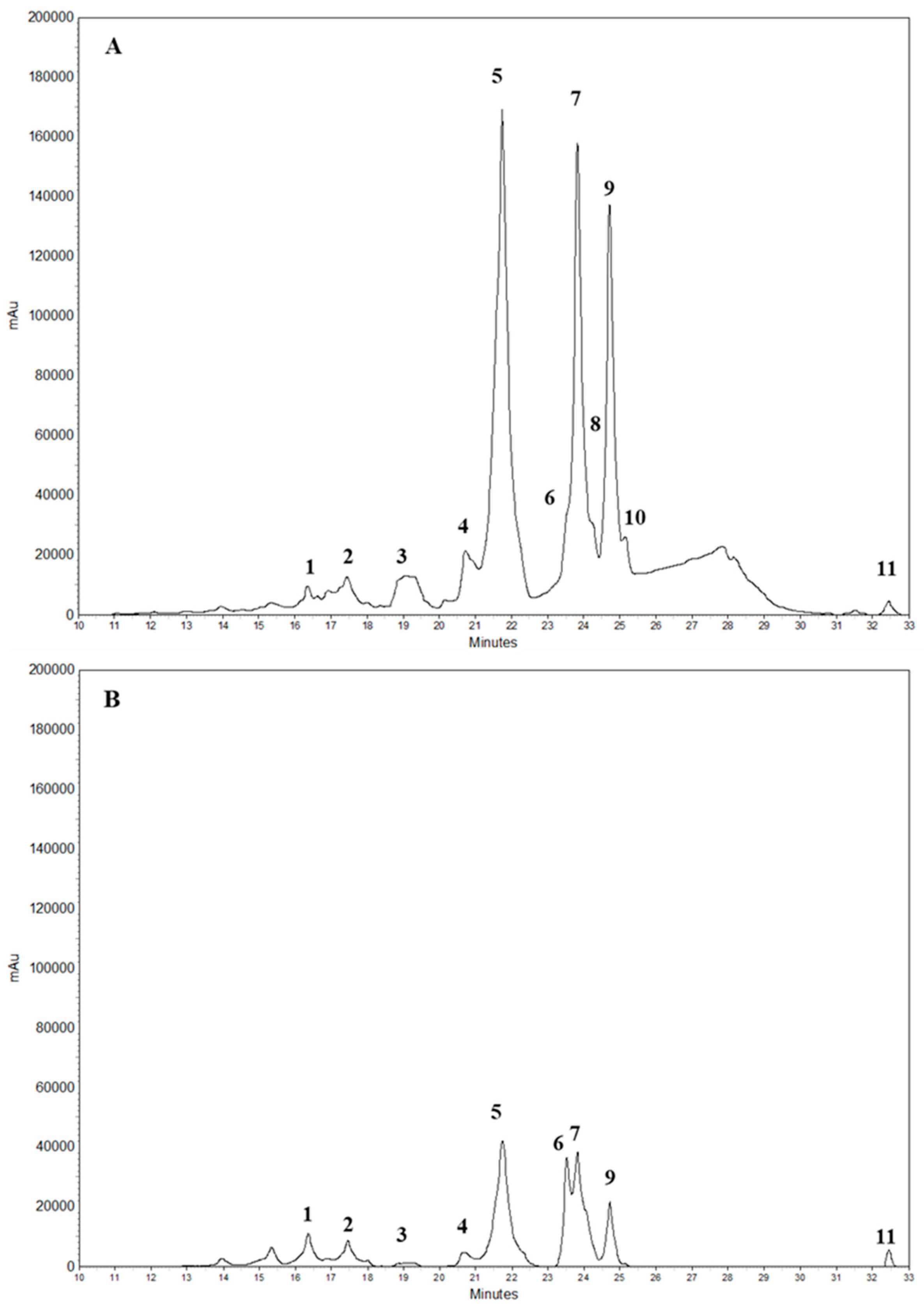
 ) PVA alone; (
) PVA alone; ( ) PVA plus 0.1% flavonoid fraction and liquid fraction obtained after digestion with cellulase and pectinase; (
) PVA plus 0.1% flavonoid fraction and liquid fraction obtained after digestion with cellulase and pectinase; ( ) PVA plus 0.1% flavonoid fraction, liquid fraction obtained after digestion with cellulase and pectinase and 0.5 g of the solid material remaining after enzyme treatment; (
) PVA plus 0.1% flavonoid fraction, liquid fraction obtained after digestion with cellulase and pectinase and 0.5 g of the solid material remaining after enzyme treatment; ( ) PVA plus 0.1% flavonoid fraction, liquid fraction obtained after digestion with cellulase and pectinase and 1.0 g of the solid material remaining after enzyme treatment. Band assignment: 3249 cm−1 (O–H stretching); 2936 cm−1 (asymmetric stretching of CH2); 2906 cm−1 (symmetric stretching of CH2); 1643 cm−1 (water absorption); 1416 cm−1 (CH2 bending); 1325 cm−1 (δ (OH); rocking with CH wagging); 1141 cm−1 (shoulder stretching of C–O) (crystalline sequence of PVA); 1083 cm−1 (stretching of C=O and bending of OH) (amorphous sequence of PVA); 1020 cm−1 (C–O symmetric stretching of the primary alcohol); 915 cm−1 (CH2 rocking); 822 cm−1 (C–C stretching).
) PVA plus 0.1% flavonoid fraction, liquid fraction obtained after digestion with cellulase and pectinase and 1.0 g of the solid material remaining after enzyme treatment. Band assignment: 3249 cm−1 (O–H stretching); 2936 cm−1 (asymmetric stretching of CH2); 2906 cm−1 (symmetric stretching of CH2); 1643 cm−1 (water absorption); 1416 cm−1 (CH2 bending); 1325 cm−1 (δ (OH); rocking with CH wagging); 1141 cm−1 (shoulder stretching of C–O) (crystalline sequence of PVA); 1083 cm−1 (stretching of C=O and bending of OH) (amorphous sequence of PVA); 1020 cm−1 (C–O symmetric stretching of the primary alcohol); 915 cm−1 (CH2 rocking); 822 cm−1 (C–C stretching).
 ) PVA alone; (
) PVA alone; ( ) PVA plus 0.1% flavonoid fraction and liquid fraction obtained after digestion with cellulase and pectinase; (
) PVA plus 0.1% flavonoid fraction and liquid fraction obtained after digestion with cellulase and pectinase; ( ) PVA plus 0.1% flavonoid fraction, liquid fraction obtained after digestion with cellulase and pectinase and 0.5 g of the solid material remaining after enzyme treatment; (
) PVA plus 0.1% flavonoid fraction, liquid fraction obtained after digestion with cellulase and pectinase and 0.5 g of the solid material remaining after enzyme treatment; (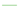 ) PVA plus 0.1% flavonoid fraction, liquid fraction obtained after digestion with cellulase and pectinase and 1.0 g of the solid material remaining after enzyme treatment. Band assignment: 3249 cm−1 (O–H stretching); 2936 cm−1 (asymmetric stretching of CH2); 2906 cm−1 (symmetric stretching of CH2); 1643 cm−1 (water absorption); 1416 cm−1 (CH2 bending); 1325 cm−1 (δ (OH); rocking with CH wagging); 1141 cm−1 (shoulder stretching of C–O) (crystalline sequence of PVA); 1083 cm−1 (stretching of C=O and bending of OH) (amorphous sequence of PVA); 1020 cm−1 (C–O symmetric stretching of the primary alcohol); 915 cm−1 (CH2 rocking); 822 cm−1 (C–C stretching).
) PVA plus 0.1% flavonoid fraction, liquid fraction obtained after digestion with cellulase and pectinase and 1.0 g of the solid material remaining after enzyme treatment. Band assignment: 3249 cm−1 (O–H stretching); 2936 cm−1 (asymmetric stretching of CH2); 2906 cm−1 (symmetric stretching of CH2); 1643 cm−1 (water absorption); 1416 cm−1 (CH2 bending); 1325 cm−1 (δ (OH); rocking with CH wagging); 1141 cm−1 (shoulder stretching of C–O) (crystalline sequence of PVA); 1083 cm−1 (stretching of C=O and bending of OH) (amorphous sequence of PVA); 1020 cm−1 (C–O symmetric stretching of the primary alcohol); 915 cm−1 (CH2 rocking); 822 cm−1 (C–C stretching).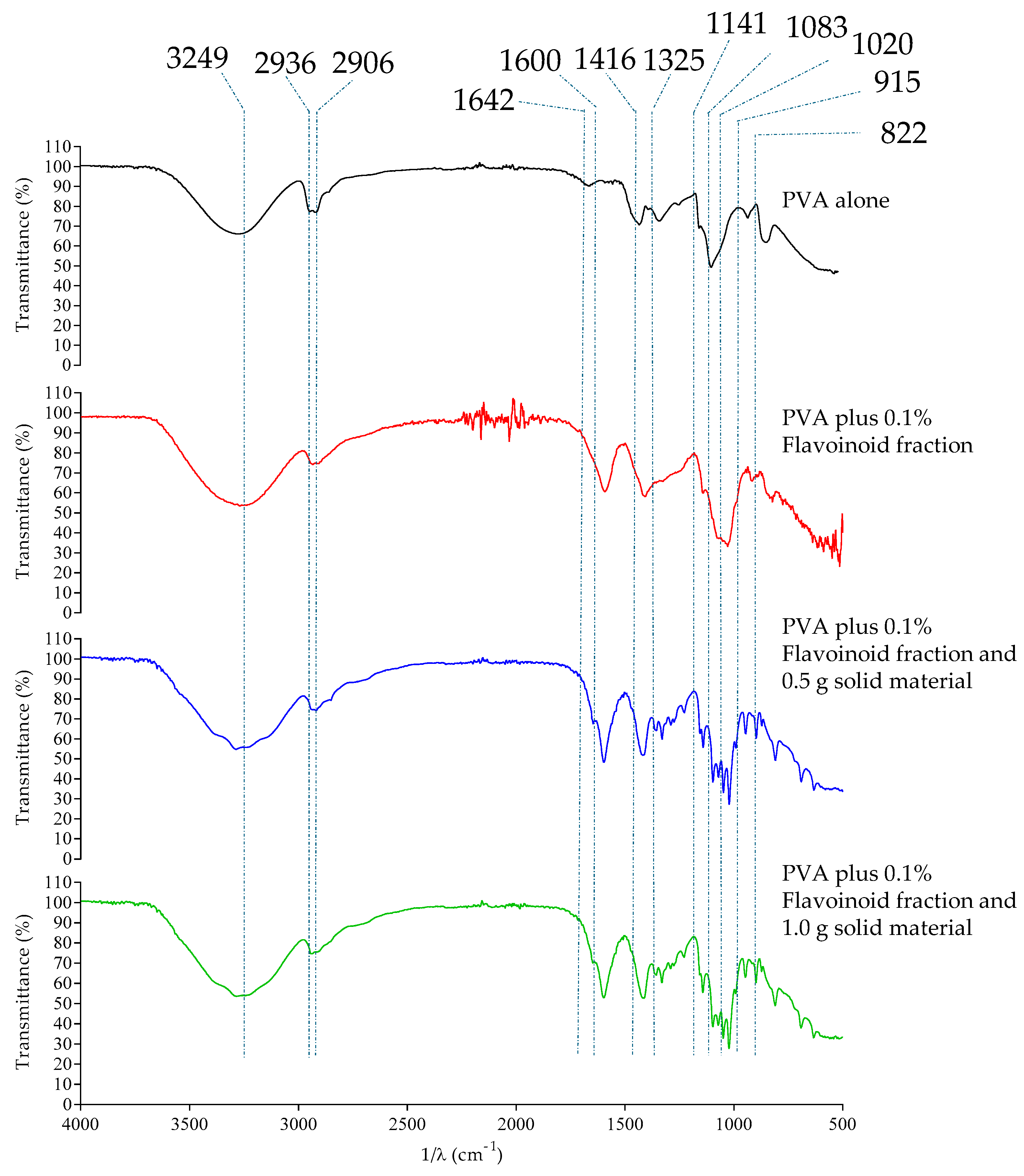


| Biofilms | Thickness [μm] | E [MPa] | σb [MPa] | εβ [%] | θw [°] |
|---|---|---|---|---|---|
| A | 32.01 ± 10.07 | 1221.69 ± 5.34 | 35.77 ± 1.40 | 118.89 ± 6.29 | 14.91 ± 1.80 |
| B | 79.86 ± 10.50 | 526.26 ± 9.66 | 79.56 ± 2.21 | 384.16 ± 12.18 | 16.87 ± 2.83 |
| C | 78.05 ± 9.29 | 623.74 ± 8.04 | 20.14 ± 2.24 | 45.77 ± 10.02 | 21.70 ± 2.11 |
| D | 67.16 ± 10.80 | 594.04 ± 7.33 | 25.76 ± 1.11 | 50.91 ± 10.49 | 18.46 ± 2.07 |
| Functionalized Bioplastics | L* | a* | b* | ΔE* | |
|---|---|---|---|---|---|
| A |  | 90.44 ± 0.16 | −1.06 ± 0.02 | 2.77 ± 0.01 | 90.49 ± 0.16 |
| B |  | 75.54 ± 1.24 | 3.85 ± 1.12 | 47.46 ± 3.24 | 89.35 ± 0.73 |
| C |  | 77.05 ± 1.59 | 2.70 ± 0.92 | 34.80 ± 2.80 | 84.63 ± 0.51 |
| D |  | 78.27 ± 2.57 | 2.31 ± 1.19 | 35.84 ± 3.04 | 86.93 ± 0.73 |
| Functionalized Bioplastics | T% | Opacity (Abs/mm−1) |
|---|---|---|
| A | 89.75667 ± 0.499433 | 1.564 |
| B | 60.1075 ± 2.753983 | 3.158 |
| C | 32.974 ± 4.532878 | 6.883 |
| D | 38.292 ± 3.265803 | 6.222 |
| Hours | L* | a* | b* | ΔE* | |
|---|---|---|---|---|---|
| Apple samples not packed | 0 | 71.38 ± 1.31 a | −1.61 ± 0.31 a | 19.24 ± 1.21 a | 73.94 ± 1.48 a |
| Apple samples not packed | 42.77 ± 1.08 b,1 | 10.61 ± 0.02 b,1 | 31.65 ± 3.96 b,1 | 50.73 ± 1.01 b,1 | |
| A | 24 | 62.15 ± 4.23 2 | 2.72 ± 0.88 2 | 26.92 ± 2.91 2 | 67.80 ± 4.92 2 |
| B | 64.58 ± 2.82 2 | 3.35 ± 2.12 2 | 26.31 ± 2.45 2 | 69.85 ± 3.11 2 | |
| C | 61.94 ± 4.79 2 | 3.97 ± 1.00 2 | 29.29 ± 0.88 2 | 68.64 ± 4.76 2 | |
| D | 61.74 ± 4.25 2 | 3.57 ± 1.20 2 | 29.02 ± 0.79 2 | 67.94 ± 4.37 2 | |
| Apple samples not packed | 42.60 ± 0.66 b,1 | 15.12 ± 1.10 c,1 | 34.82 ± 0.91 b,1 | 57.07 ± 0.64 c,1 | |
| A | 48 | 46.89 ± 0.61 1 | 14.67 ± 0.77 1 | 38.99 ± 3.15 2 | 62.75 ± 2.17 2 |
| B | 61.76 ± 0.20 2 | 11.64 ± 1.57 2 | 39.04 ± 5.86 2 | 74.11 ± 2.87 2 | |
| C | 55.62 ± 0.98 2 | 13.79 ± 0.33 2 | 42.63 ± 1.55 2 | 71.42 ± 1.75 2 | |
| D | 55.13 ± 0.87 2 | 13.57 ± 0.28 2 | 42.22 ± 1.37 2 | 71.32 ± 1.62 2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patanè, G.T.; Putaggio, S.; Barreca, D.; Russo, A.; Visco, A.; Scolaro, C.; Cigala, R.M.; Crea, F.; Abate, S.; De Luca, F.; et al. Biochemical Modification of Poly-Vinyl-Alcohol-Based Bioplastics with Citrus By-Product to Increase Its Food Packaging Application. Int. J. Mol. Sci. 2025, 26, 9470. https://doi.org/10.3390/ijms26199470
Patanè GT, Putaggio S, Barreca D, Russo A, Visco A, Scolaro C, Cigala RM, Crea F, Abate S, De Luca F, et al. Biochemical Modification of Poly-Vinyl-Alcohol-Based Bioplastics with Citrus By-Product to Increase Its Food Packaging Application. International Journal of Molecular Sciences. 2025; 26(19):9470. https://doi.org/10.3390/ijms26199470
Chicago/Turabian StylePatanè, Giuseppe Tancredi, Stefano Putaggio, Davide Barreca, Annamaria Russo, Annamaria Visco, Cristina Scolaro, Rosalia Maria Cigala, Francesco Crea, Salvatore Abate, Federica De Luca, and et al. 2025. "Biochemical Modification of Poly-Vinyl-Alcohol-Based Bioplastics with Citrus By-Product to Increase Its Food Packaging Application" International Journal of Molecular Sciences 26, no. 19: 9470. https://doi.org/10.3390/ijms26199470
APA StylePatanè, G. T., Putaggio, S., Barreca, D., Russo, A., Visco, A., Scolaro, C., Cigala, R. M., Crea, F., Abate, S., De Luca, F., Ficarra, S., Tellone, E., Laganà, G., & Calderaro, A. (2025). Biochemical Modification of Poly-Vinyl-Alcohol-Based Bioplastics with Citrus By-Product to Increase Its Food Packaging Application. International Journal of Molecular Sciences, 26(19), 9470. https://doi.org/10.3390/ijms26199470











