Comprehensive Characterization of Armoracia rusticana Roots and Leaves: Physicochemical Properties, Functional Potential, and Nutritional Composition
Abstract
1. Introduction
2. Results
2.1. Determination of Powder Color
2.2. Moisture Analysis of Powders
2.3. Water Activity of Powders
2.4. Titratable Acidity of Powders
2.5. pH of Powders
2.6. Ash Content of the Powders
2.7. Protein Content of the Powders
2.8. Fat Content of the Powders
2.9. Fiber Content of the Powders
2.10. Carbohydrate Content and Energy Value of the Powders
2.11. Mineral Elements in Powders and Soil
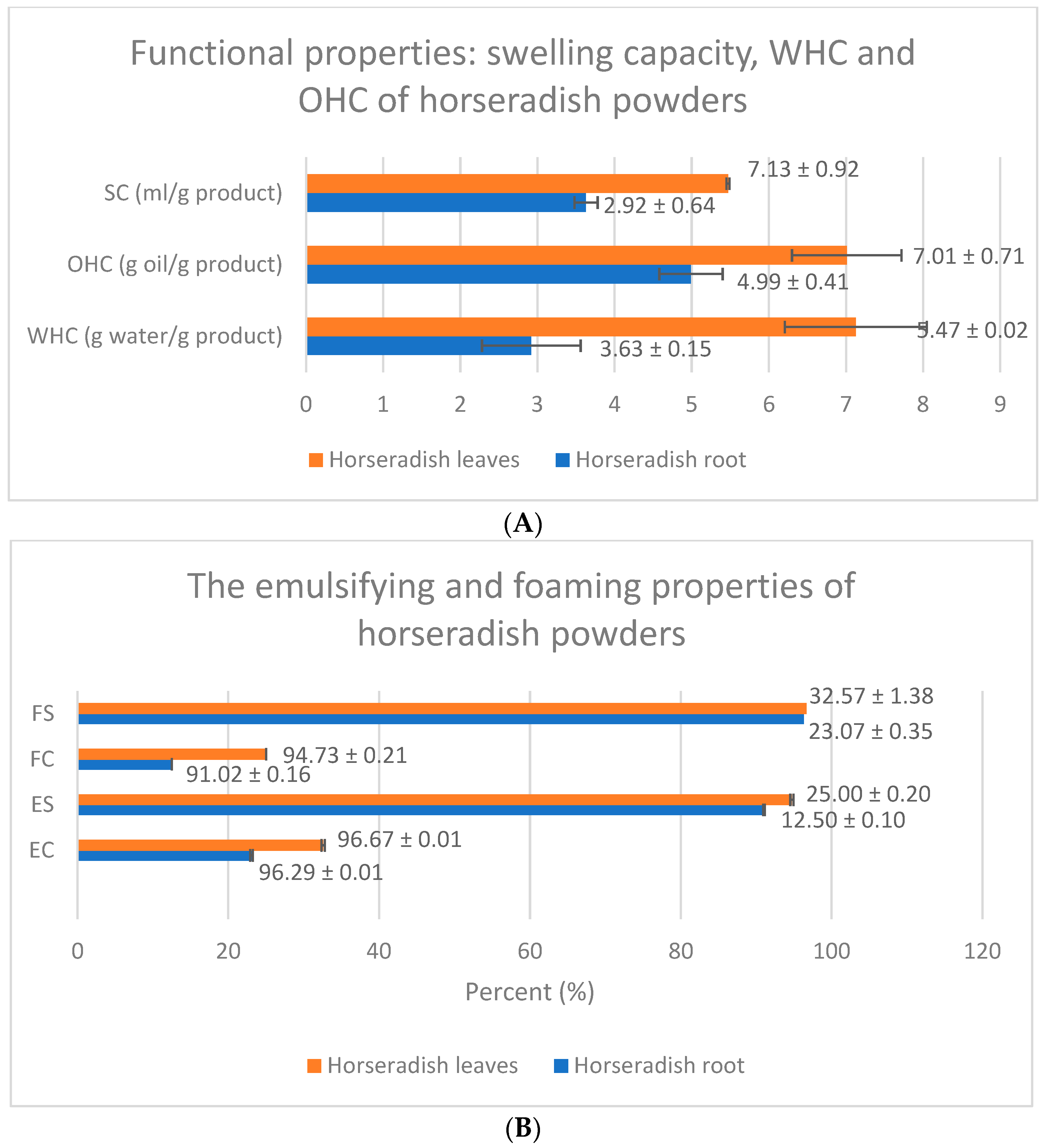
2.12. Functional Properties of the Powders
2.12.1. Water Holding Capacity
2.12.2. Oil Holding Capacity
2.12.3. Density
2.12.4. Swelling Capacity
2.12.5. Emulsifying Capacity and Emulsion Stability
2.12.6. Foaming Properties and Foam Stability
2.12.7. Gelling Properties
2.13. Fourier Transform Infrared (FTIR) Spectra
2.14. Antioxidant Activity by DPPH Assay
2.15. Chlorophyll and Carotenoid Content
2.16. Vitamin C Content
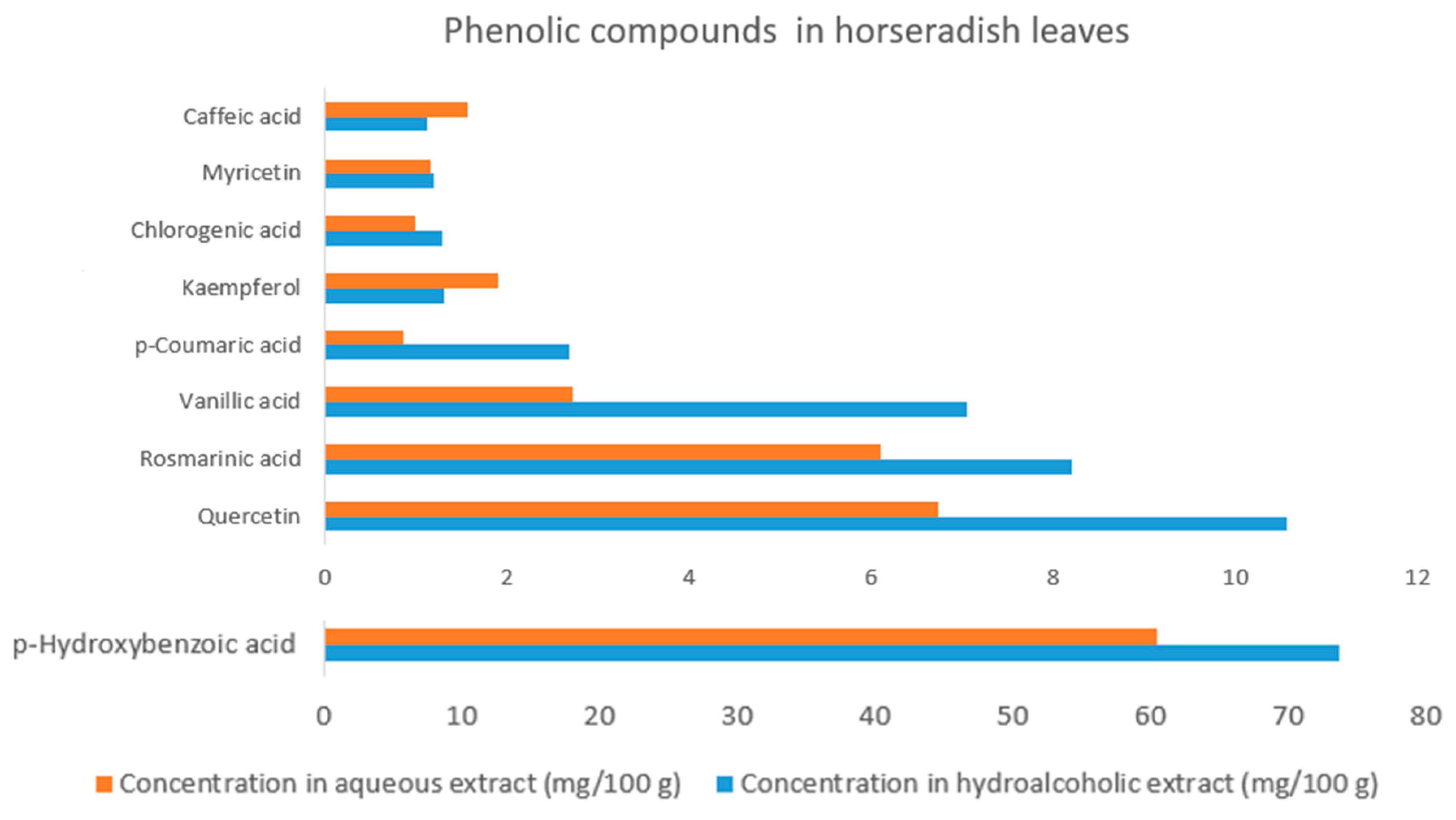
2.17. Phenolic Compound Profile
3. Discussions
3.1. Physicochemical Parameters
3.2. Functional Properties
3.3. Nutritional Composition
3.4. Antioxidant Activity and Bioactive Content
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Measurement of Color
- L, a, b = CIELAB color coordinates of the sample (measured values);L0, a0, b0 = CIELAB color coordinates of the reference (standard) sample
4.3. Determination of Moisture Content
- = initial mass before drying;= final mass after drying.
4.4. Measurement of Water Activity
4.5. Determination of Titratable Acidity
- V = volume of NaOH required for titration (mL);N = normality of the NaOH solution (eq/L);m = mass of the powder sample used in the analysis (g).
4.6. Determination of pH
4.7. Determination of Ash Content
4.8. Determination of Protein Content
- Vsample = volume of HCl used for titration of the sample (mL);Vcontrol = volume of HCl used for titration of the control (mL);z= stoichiometric factor (1 for HCl);C = concentration of HCl (mol/L);f = correction factor for the HCl solution (1);MN = mass of nitrogen (14.007 g/mol);m = mass of the sample (g);1000 = conversion from mL to L.
4.9. Determination of Fat Content
- m1 = mass of the empty container (g);m2 = mass of the container with extracted fat (g);ms = mass of the dried sample used for analysis (g).
4.10. Determination of Fiber Content
- m1 = mass of the initial sample;m2 = mass of the bag with the residue remaining after digestion;m3 = mass of the empty bag;m4 = mass of the bag with the residue remaining after calcination.
4.11. Carbohydrate Content and Energy Value Determination
4.12. Determination of Mineral Content
4.13. Determination of Functional Properties
4.13.1. Water and Oil Holding Capacity
- = mass of dry residue (g);= mass of hydrated residue (g).
4.13.2. Density
4.13.3. Swelling Capacity
4.13.4. Emulsifying Capacity and Emulsion Stability
- hemusion phase = height of the emulsified oil layer;hsuspension = total height of the suspension;hfinal suspension = total height of the suspension after heating.
4.13.5. Foaming Properties and Foam Stability
- = volume of solution after shaking (mL);= initial volume of solution (mL).
4.13.6. Gelling Properties
4.14. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
4.15. DPPH Radical Scavenging Assay Analysis
- Acontrol = absorbance of the blank sample;Asample = absorbance of the sample with extract.
4.16. Determination of Chlorophyll Content (a, b, c, and Total) and Carotenoids
4.17. Determination of Vitamin C content
4.18. Phenolic Compound Profile Determination
4.19. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tomsone, L.; Kruma, Z. Influence of freezing and drying on the phenol content and antioxidant activity of horseradish and lovage. In Proceedings of the Baltic Conference on Food Science and Technology “FOODBALT”, Jelgava, Latvia, 8–9 May 2014; pp. 192–197. [Google Scholar]
- Tomsone, L.; Galoburda, R.; Krūma, Z.; Cinkmanis, I. Changes in phenolic profile and antioxidant activity of horseradish roots during freezing and frozen storage. Proc. Latv. Acad. Sci. 2022, 76, 103–109. [Google Scholar] [CrossRef]
- Knez, E.; Kadac-Czapska, K.; Dmochowska-Ślęzak, K.; Grembecka, M. Root vegetables—Composition, health effects, and contaminants. Int. J. Environ. Res. Public Health 2022, 19, 15531. [Google Scholar] [CrossRef]
- Nguyen, N.M.; Gonda, S.; Vasas, G. A review on the phytochemical composition and potential medicinal uses of horseradish (Armoracia rusticana) root. Food Rev. Int. 2013, 29, 261–275.5. [Google Scholar] [CrossRef]
- Tomsone, L.; Kruma, Z.; Galoburda, R. Comparison of different solvents and extraction methods for isolation of phenolic compounds from horseradish roots. World Acad. Sci. Eng. Technol. 2012, 64, 903–908. [Google Scholar]
- Roșu, M.; Bordean, D.-M.; Berbecea, A.; Radulov, I.; Hotea, I.; Preda, G.; Dragomirescu, M. Horseradish, a reservoir of useful bioactive compounds. Sci. Bull. Ser. F Biotechnol. 2024, 28, 156–160. [Google Scholar]
- Tomsone, L.; Kruma, Z. Comparison of different solvents for isolation of phenolic compounds from horseradish (Armoracia rusticana L.) leaves. Res. Rural Dev. 2013, 1, 104–110. [Google Scholar]
- Tomsone, L.; Galoburda, L.; Kruma, R.; Durrieu, Z.; Cinkmanis, I. Microencapsulation of horseradish (Armoracia rusticana L.) juice using spray-drying. Foods 2020, 9, 1332. [Google Scholar] [CrossRef]
- Kim, H.Y.; Gornsawun, G.; Shin, I.S. Antibacterial activities of isothiocyanates (ITCs) extracted from horseradish (Armoracia rusticana) root in liquid and vapor phases against 5 dominant bacteria isolated from low-salt Jeotgal, a Korean salted and fermented seafood. Food Sci. Biotechnol. 2015, 24, 1405–1412. [Google Scholar] [CrossRef]
- Bladh, K.W.; Olsson, K.M.; Yndgaard, F. Evaluation of glucosinolates in Nordic horseradish (Armoracia rusticana). Bot. Lith. 2013, 19, 43–48. [Google Scholar] [CrossRef]
- Fusari, C.M.; Nazareno, M.A.; Locatelli, D.A.; Fontana, A.; Beretta, V.; Camargo, A.B. Phytochemical profile and functionality of Brassicaceae species. Food Biosci. 2020, 36, 100606. [Google Scholar] [CrossRef]
- Fernandes, F.; Valentão, P.; Sousa, C.; Pereira, J.A.; Seabra, R.M.; Andrade, P.B. Chemical and antioxidative assessment of dietary turnip (Brassica rapa var. rapa L.). Food Chem. 2007, 105, 1003–1010. [Google Scholar] [CrossRef]
- Guragain, M. Hot Air Drying of Horseradish: Empirical Drying Kinetic Modeling and Physical Quality Characteristics of Dried Horseradish. Ph.D. Thesis, University of Wisconsin–Stout, Menomonie, WI, USA, 2018. [Google Scholar]
- Marić, L.; Malešić, E.; Jurinjak Tušek, A.; Benković, M.; Valinger, D.; Jurina, T.; Kljusurić, J.G. Effects of drying on physical and chemical properties of root vegetables: Artificial neural network modelling. Food Bioprod. Process. 2020, 119, 148–160. [Google Scholar] [CrossRef]
- Kyung, K.H. Antimicrobial activity of volatile sulfur compounds in foods. In Volatile Sulfur Compounds in Food Flavors; American Chemical Society: Washington, DC, USA, 2011; Volume 1068, pp. 323–338. [Google Scholar]
- Popović, M.; Maravić, A.; Čulić, V.Č.; Đulović, A.; Burčul, F.; Blažević, I. Biological effects of glucosinolate degradation products from horseradish: A horse that wins the race. Biomolecules 2020, 10, 343. [Google Scholar] [CrossRef] [PubMed]
- Nor, N.D.M.; Lignou, S.; Bell, L.; Houston-Price, C.; Harvey, K.; Methven, L. The relationship between glucosinolates and the sensory characteristics of steamed-pureed turnip (Brassica rapa subsp. rapa L.). Foods 2020, 9, 1601. [Google Scholar] [CrossRef]
- Dekić, M.S.; Radulović, N.S.; Stojanović, N.M.; Randjelović, P.J.; Stojanović-Radić, Z.Z.; Najman, S.; Stojanović, S. Spasmolytic, antimicrobial and cytotoxic activities of 5-phenylpentyl isothiocyanate, a new glucosinolate autolysis product from horseradish (Armoracia rusticana P. Gaertn., B. Mey. & Scherb., Brassicaceae). Food Chem. 2017, 232, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Park, H.W.; Choi, K.D.; Shin, I.S. Antimicrobial activity of isothiocyanates (ITCs) extracted from horseradish (Armoracia rusticana) root against oral microorganisms. Biocontrol Sci. 2013, 18, 163–168. [Google Scholar] [CrossRef]
- Shin, I.S.; Han, J.S.; Choi, K.D.; Chung, D.H.; Choi, G.P.; Ahn, J. Effect of isothiocyanates from horseradish (Armoracia rusticana) on the quality and shelf life of tofu. Food Control 2010, 21, 1081–1086. [Google Scholar] [CrossRef]
- György, É.; Laslo, É.; Csató, E. Antibacterial activity of plant extracts against Listeria monocytogenes isolated from ready-to-eat salads. Acta Univ. Sapientiae Aliment. 2020, 13, 131–143. [Google Scholar] [CrossRef]
- Șuian, B.; Amariei, S. Extraction and Characterization of Bioactive Compounds in Horseradish (Armoracia rusticana): Focus on Polyphenols, Vitamin C, and Fatty Acids. Appl. Sci. 2025, 15, 6534. [Google Scholar] [CrossRef]
- Kaur, G.; Bhatia, S. Radish leaf protein concentrates: Optimization of alkaline extraction for production and characterization of an alternative plant protein concentrate. J. Food Meas. Charact. 2022, 16, 3166–3181. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Devahastin, S.; Liu, Y. Influence of low-temperature ball milling time on physicochemical properties, flavor, bioactive compounds contents and antioxidant activity of horseradish powder. Adv. Powder Technol. 2020, 31, 914–921. [Google Scholar] [CrossRef]
- Kotecha, J.L.; Ram, V.R. Extraction and spectrophotometric determination of chlorophyll content and carotenoids from *Cocos nucifera* L. leaf using various solvents in Saurashtra region. Int. J. Plant Environ. 2023, 9, 364–368. [Google Scholar] [CrossRef]
- Goyeneche, R.; Roura, S.; Ponce, A.; Vega-Gálvez, A.; Quispe-Fuentes, I.; Uribe, E.; Di Scala, K. Chemical characterization and antioxidant capacity of red radish (Raphanus sativus L.) leaves and roots. J. Funct. Foods 2015, 16, 256–264. [Google Scholar] [CrossRef]
- Pereyra Gonzales, A.S.; Naranjo, G.B.; Leiva, G.E.; Malec, L.S. Maillard reaction kinetics in milk powder: Effect of water activity at mild temperatures. Int. Dairy J. 2010, 20, 40–45. [Google Scholar] [CrossRef]
- Chegini, G.; HamidiSepehr, A.; Dizaji, M.F.; Mirnezami, S.V. Study of physical and chemical properties of spray drying whey powder. Int. J. Recycl. Org. Waste Agric. 2014, 3, 62. [Google Scholar] [CrossRef]
- Jiang, P.; Liu, H.F.; Zhao, X.H.; Ding, Q. Physicochemical properties of soybean protein isolate affected by the cross-linking with horseradish peroxidase, glucose oxidase and glucose. J. Food Meas. Charact. 2017, 11, 1196–1202. [Google Scholar] [CrossRef]
- Piotrowska-Cyplik, A.; Staninska-Pięta, J.; Drożdżyńska, A.; Cyplik, P. Grapevine and horseradish leaves as natural, sustainable additives for improvement of the microbial, sensory, and antioxidant properties of traditionally fermented low-salt cucumbers. Sustainability 2024, 16, 2431. [Google Scholar] [CrossRef]
- Sengul, M.; Yildiz, H.; Ercisli, S.; Yildirim, E.; Turan, M.; Ozdemir, O.; Sener, D. Some phytochemical characteristics of turnip (Brassica rapa var. rapa L.) roots. Ital. J. Food Sci. 2011, 23, 338. [Google Scholar]
- Ladjal Ettoumi, Y.; Chibane, M. Some physicochemical and functional properties of pea, chickpea and lentil whole flours. Int. Food Res. J. 2015, 22, 987–996. [Google Scholar]
- Ma, Z.; Boye, J.I.; Simpson, B.K.; Prasher, S.O.; Monpetit, D.; Malcolmson, L. Thermal processing effects on the functional properties and microstructure of lentil, chickpea, and pea flours. Food Res. Int. 2011, 44, 2534–2544. [Google Scholar] [CrossRef]
- Di Lena, G.; Del Pulgar, J.S.; Lucarini, M.; Durazzo, A.; Ondrejíčková, P.; Oancea, F.; Frincu, R.M.; Aguzzi, A.; Ferrari Nicoli, S.; Casini, I.; et al. Valorization potentials of rapeseed meal in a biorefinery perspective: Focus on nutritional and bioactive components. Molecules 2021, 26, 6980. [Google Scholar] [CrossRef] [PubMed]
- Socaciu, C.; Fetea, F.; Ranga, F.; Bunea, A.; Dulf, F.; Socaci, S.; Pintea, A. Attenuated total reflectance–fourier transform infrared spectroscopy (ATR-FTIR) coupled with chemometrics to control the botanical authenticity and quality of cold-pressed functional oils commercialized in Romania. Appl. Sci. 2020, 10, 8453. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to read and interpret FTIR spectroscope of organic material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Polanco, V.; Cerdá-Bernad, D.; Quispe-Fuentes, I.; Bernal, C.; López, J. Bioactive content and antioxidant properties of spray-dried microencapsulates of Peumus boldus M. leaf extracts. Antioxidants 2024, 13, 12. [Google Scholar] [CrossRef]
- Han, H.; Sha, R.; Dai, J.; Wang, Z.; Mao, J.; Cai, M. Garlic origin traceability and identification based on fusion of multi-source heterogeneous spectral information. Foods 2024, 13, 7. [Google Scholar] [CrossRef]
- Shi, M.; Hlaing, M.M.; Ying, D.Y.; Ye, J.H.; Sanguansri, L.; Augustin, M.A. New food ingredients from broccoli by-products: Physical, chemical and technological properties. Int. J. Food Sci. Technol. 2019, 54, 1423–1432. [Google Scholar] [CrossRef]
- Losacco, D.; Campanale, C.; Tumolo, M.; Ancona, V.; Massarelli, C.; Uricchio, V.F. Evaluating the influence of nitrogen fertilizers and biochar on Brassica oleracea L. var. botrytis by the use of Fourier transform infrared (FTIR) spectroscopy. Sustainability 2022, 14, 12345. [Google Scholar]
- Ali Redha, A.; Langston, F.; Nash, G.R.; Bows, J.R.; Torquati, L.; Gidley, M.J.; Cozzolino, D. Determination of glucosinolates in broccoli (Brassica oleracea var. italica) by combining mid-infrared (MIR) spectroscopy with chemometrics. Int. J. Food Sci. Technol. 2023, 58, 5679–5688. [Google Scholar] [CrossRef]
- Dima, Ș.-O.; Constantinescu-Aruxandei, D.; Tritean, N.; Ghiurea, M.; Capră, L.; Nicolae, C.-A.; Faraon, V.; Neamțu, C.; Oancea, F. Spectroscopic analyses highlight plant biostimulant effects foliar fertilization. Plants 2023, 12, 3016. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Mendanha, D.; Gomes, J.M.; Oliveira, J.A.S.A.; Ribeiro, C.; Miranda, A.F.; Barbosa, J.R.; Soares, O.S.; Pereira, M.F.; Santos, J.; et al. A Comprehensive Study on the Physicochemical Characterisation of Plant-Based By-Products. Materials 2025, 18, 2054. [Google Scholar] [CrossRef]
- Segneanu, A.-E.; Vlase, G.; Chirigiu, L.; Herea, D.D.; Pricop, M.-A.; Saracin, P.-A.; Tanasie, Ș.E. Romanian wild-growing Armoracia rusticana L.—Untargeted low-molecular metabolomic approach to a potential antitumoral phyto-carrier system based on kaolinite. Antioxidants 2023, 12, 6. [Google Scholar] [CrossRef]
- Tomsone, L.; Kruma, Z. Influence of harvest time on the phenolic content of horseradish leaves. In Proceedings of the Baltic Conference on Food Science and Technology—FOODBALT, Jelgava, Latvia, 27–28 April 2017; pp. 45–50. [Google Scholar]
- Vica, S.I.; Laslo, V.; Pantea, S.; Bandici, G.E. Chlorophyll and carotenoids pigments from mistletoe (Viscum album). Fasc. Biol. 2010, 20, 213–218. [Google Scholar]
- Yu, X.; Wang, H.; Xiang, X.; Fu, J.; Wang, X.; Zhou, Y.; Xing, W. Biosynthesis and extraction of chlorophyll, carotenoids, anthocyanins, and betalain in vivo and in vitro. Curr. Issues Mol. Biol. 2024, 46, 10662–10676. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and UV-VIS characterization. Curr. Protoc. Food Anal. Chem. 2001, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Seifu, M.; Tola, Y.B.; Mohammed, A.; Astatkie, T. Effect of variety and drying temperature on physicochemical quality, functional property, and sensory acceptability of dried onion powder. Food Sci. Nutr. 2018, 6, 1641–1649. [Google Scholar] [CrossRef]
- Colunga Biancatelli, R.M.L.; Berrill, M.; Catravas, J.D.; Marik, P.E. Quercetin and vitamin C: An experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19). Front. Immunol. 2020, 11, 550247. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Kim, J.E.; Song, Y.J. Antiviral activities of quercetin and isoquercitrin against human herpesviruses. Molecules 2020, 25, 10. [Google Scholar] [CrossRef]
- Wu, W.; Li, R.; Li, X.; He, J.; Jiang, S.; Liu, S.; Yang, J. Quercetin as an antiviral agent inhibits influenza A virus (IAV) entry. Viruses 2015, 8, 1. [Google Scholar] [CrossRef]
- Noor, N.; Gani, A.; Gani, A.; Shah, A.; Ashraf, Z.U. Exploitation of polyphenols and proteins using nanoencapsulation for anti-viral and brain boosting properties–Evoking a synergistic strategy to combat COVID-19 pandemic. Int. J. Biol. Macromol. 2021, 180, 375–384. [Google Scholar] [CrossRef]
- Tomsone, L.; Galoburda, R.; Kruma, Z.; Cinkmanis, I. Characterization of dried horseradish leaves pomace: Phenolic compounds profile and antioxidant capacity, content of organic acids, pigments and volatile compounds. Eur. Food Res. Technol. 2020, 246, 1647–1660. [Google Scholar] [CrossRef]
- Costache, M.A.; Campeanu, G.; Neata, G. Studies concerning the extraction of chlorophyll and total carotenoids from vegetables. Rom. Biotechnol. Lett. 2012, 17, 7702–7708. [Google Scholar]
- Matłok, N.; Piechowiak, T.; Kapusta, I. From by-product to bioactive molecular ingredient: The impact of cranberry pomace on antioxidant properties and enzyme modulation in functional biscuits. Int. J. Mol. Sci. 2025, 26, 9002. [Google Scholar] [CrossRef]
- Da Costa, J.N.; De Figueiredo, R.W.; De Sousa, P.H.M.; Da Costa Gonzaga, M.L.; Constant, P.B.L.; Soares, D.J. Study of the stability of passion fruit (Passiflora edullis f. flavicarpa) powder from organic farming. Semin. Agrar. 2013, 34, 705–716. [Google Scholar] [CrossRef]
- AOAC 935.29; Moisture in Malt. Association of Official Analytical Chemists: Arlington, VA, USA, 2003.
- Petraru, A.; Amariei, S. Rapeseed—An Important Oleaginous Plant in the Oil Industry and the Resulting Meal a Valuable Source of Bioactive Compounds. Plants 2024, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Mata, M.C.; Loera, R.D.C.; Morales, P.; Fernández-Ruiz, V.; Cámara, M.; Marqués, C.D.; Pardo-de-Santayana, M.; Tardío, J. Wild vegetables of the Mediterranean area as valuable sources of bioactive compounds. Genet. Resour. Crop Evol. 2012, 59, 431–443. [Google Scholar] [CrossRef]
- ISO 2171:2007; Cereals, Pulses and by-Products—Determination of Ash Yield by Incineration. ISO: Geneva, Switzerland, 2007.
- AOAC 985.29; Fat (Crude) or Ether Extract in Animal Feed. Association of Official Analytical Chemists: Arlington, VA, USA, 2003.
- Scripcă, L.A.; Amariei, S. The influence of chemical contaminants on the physicochemical properties of unifloral and multifloral honey. Foods 2021, 10, 1039. [Google Scholar] [CrossRef]
- Sava, C. Chimie Analitică, Metode Optice; Ovidius University Press: Constanța, Romania, 2009. [Google Scholar]


| Sample | L* | a* | b* | ||
|---|---|---|---|---|---|
| Root powder | 85.87 ± 0.06 | 0.13 ± 0.06 | 13.97 ± 0.04 | 12.89 | 24.67 |
| Leaf powder | 55.23 ± 0.15 | −14.90 ± 0.09 | 24.67 ± 0.08 | 46.29 | 57.48 |
| Evaluated Parameter | Root | Leaves |
|---|---|---|
| Moisture (%) | 7.13 ± 0.10 | 7.19 ± 0.12 |
| Water activity (aw) | 0.21 ± 0 | 0.47 ± 0 |
| Titratable acidity (meq/100 g) | 7.20 ± 0.10 | 7.22 ± 0.01 |
| pH | 5.74 ± 0.01 | 5.98 ± 0.01 |
| Ash content (%) | 5.07 ± 0.01 | 9.94 ± 0.74 |
| Protein content (%) | 12.35 ± 0.43 | 27.22 ± 0.59 |
| Fat content (%) | 1.07 ± 0.06 | 3.37 ± 0.14 |
| Fiber content (%) | 9.42 ± 0.01 | 9.72 ± 0.02 |
| Carbohydrate content (%) | 65.05 ± 0.02 | 42.83 ± 1.35 |
| Energy value (kcal/100 g) | 319.22 ± 2.26 | 310.56 ± 5.92 |
| No. | Mineral Element (mg/100 g) | Soil | Horseradish—Armoracia rusticana | p-Value | |
|---|---|---|---|---|---|
| Root | Leaves | ||||
| 1. | Sodium (Na) | 15.18 ± 0.08 | 8.91 ± 0.28 | 6.53 ± 0.48 | 0.0264 |
| 2. | Magnesium (Mg) | 30.33 ± 3.53 | 18.11 ± 0.1 | 21.71 ± 0.23 | 0.0001 |
| 3. | Calcium (Ca) | 406.07 ± 0.98 | 530.41 ± 7.75 | nd | - |
| 4. | Chromium (Cr) | 0.66 ± 0.01 | 0.95 ± 0.03 | 0.67 ± 0.06 | 0.0286 |
| 5. | Manganese (Mn) | nd | 0.23 ± 0.01 | nd | - |
| 6. | Cobalt (Co) | 10.65 ± 0.01 | nd | nd | - |
| 7. | Zinc (Zn) | 781.95 ± 0.28 | 45.59 ± 0.77 | 1.17 ± 0 | 0.0002 |
| 8. | Gallium (Ga) | 2.02 ± 0.13 | nd | nd | - |
| 9. | Silver (Ag) | 109.75 ± 0.16 | 79.96 ± 0.42 | 51.43 ± 0.07 | 0.0001 |
| 10 | Iodine (I) | 90.82 ± 0.89 | 71.69 ± 0.14 | nd | - |
| TOTAL | 1447.43 | 755.85 | 61.51 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Șuian, B.; Amariei, S.; Petraru, A. Comprehensive Characterization of Armoracia rusticana Roots and Leaves: Physicochemical Properties, Functional Potential, and Nutritional Composition. Int. J. Mol. Sci. 2025, 26, 9462. https://doi.org/10.3390/ijms26199462
Șuian B, Amariei S, Petraru A. Comprehensive Characterization of Armoracia rusticana Roots and Leaves: Physicochemical Properties, Functional Potential, and Nutritional Composition. International Journal of Molecular Sciences. 2025; 26(19):9462. https://doi.org/10.3390/ijms26199462
Chicago/Turabian StyleȘuian, Bianca, Sonia Amariei, and Ancuța Petraru. 2025. "Comprehensive Characterization of Armoracia rusticana Roots and Leaves: Physicochemical Properties, Functional Potential, and Nutritional Composition" International Journal of Molecular Sciences 26, no. 19: 9462. https://doi.org/10.3390/ijms26199462
APA StyleȘuian, B., Amariei, S., & Petraru, A. (2025). Comprehensive Characterization of Armoracia rusticana Roots and Leaves: Physicochemical Properties, Functional Potential, and Nutritional Composition. International Journal of Molecular Sciences, 26(19), 9462. https://doi.org/10.3390/ijms26199462






