Multifaceted Biological Activity of Selected Flavone C-Monoglucosides
Abstract
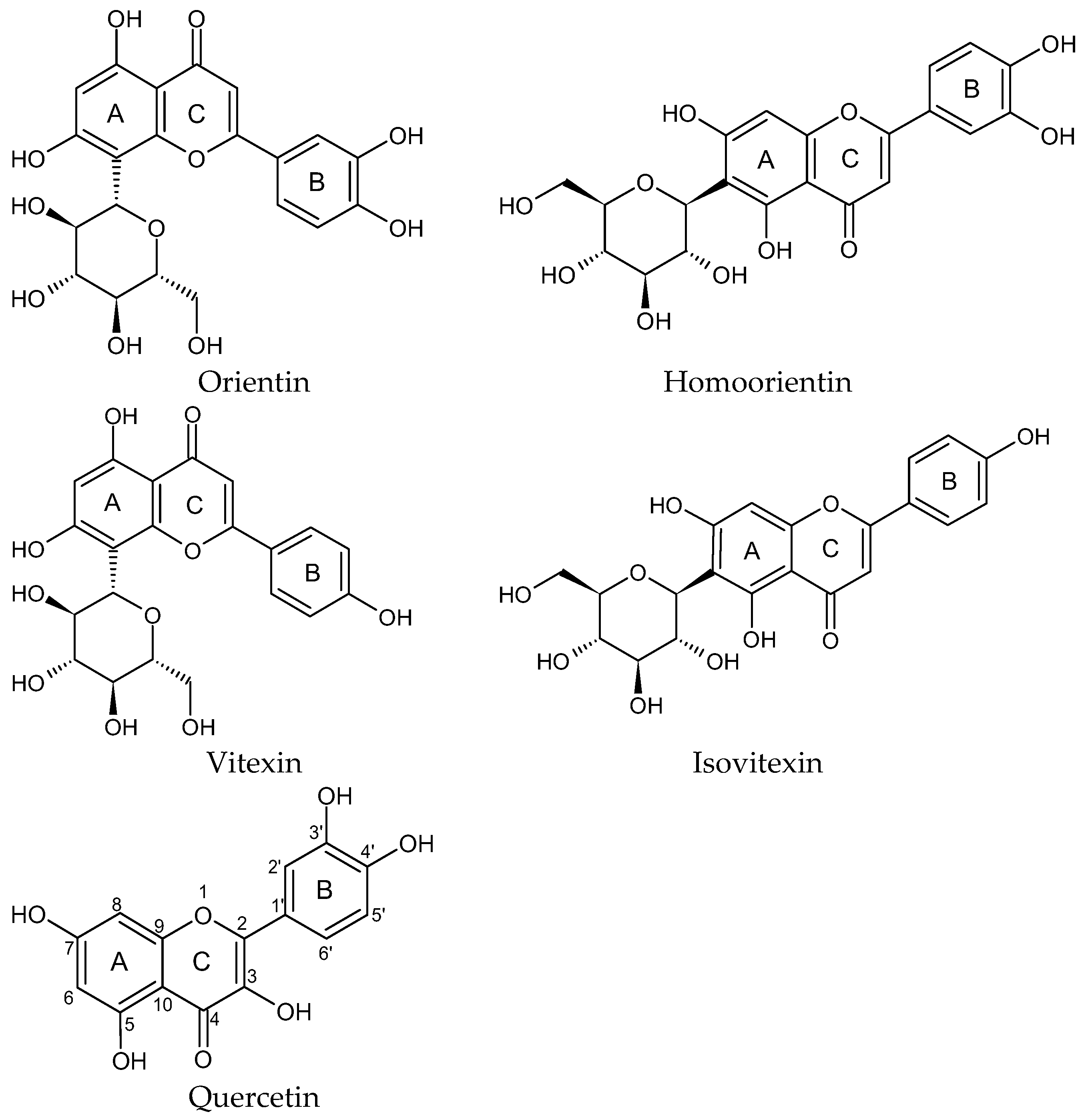
1. Introduction
2. Results
2.1. The Anodic Oxidation Potentials of Selected Flavone C-Monoglucosides in Comparison to Quercetin Provided by the Differential Pulse Voltammetry
2.2. Angiotensin-I-Converting Enzyme Inhibitory Activity of Selected Flavone C-Monoglucosides in Comparison to Quercetin
2.3. Acetylcholinesterase Inhibitory Activity of Selected Flavone C-Monoglucosides in Comparison to Quercetin
2.4. The Inhibitory Activity of Selected Flavone C-Monoglucosides in Comparison to Quercetin Against the Advanced Glycation End Products (AGEs) Formation
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Measurement of the Anodic Oxidation Potentials of Flavone C-Monoglucosides in Comparison to Quercetin with the Differential Pulse Voltammetry
4.3. Determination of the Angiotensin-I-Converting Enzyme Inhibitory Activity of Flavone C-Monoglucosides
4.4. Determination of the Acetylcholinesterase Inhibitory Activity of Flavone C-Monoglucosides Assay
4.5. Measurement of the Inhibitory Activity of Flavone C-Monoglucosides Towards Advanced Glycation End Products (AGEs) Formation
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jadreško, D.; Miličević, A.; Novak Jovanović, I. Reactivity of flavonoids toward superoxide radical: An electrochemical approach. Electrochim. Acta 2022, 421, 140501. [Google Scholar] [CrossRef]
- Speisky, H.; Shahidi, F.; Costa de Camargo, A.; Fuentes, J. Revisiting the Oxidation of Flavonoids: Loss, Conservation or Enhancement of Their Antioxidant Properties. Antioxidants 2022, 11, 133. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Oualid, T.; Artur, M.S.S. Advances in C-glycosylflavonoid research. Curr. Org. Chem. 2012, 6, 859–896. [Google Scholar] [CrossRef]
- Veitch, N.C.; Grayer, R.J. Flavonoids and their glycosides, including anthocyanins. Nat. Prod. Rep. 2011, 28, 1626–1695. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.B.; Muzashvili, T.S.; Georgiev, M.I. Advance on biotechnology for glycosylation of high-value flavonoids. Biotechnol. Adv. 2014, 32, 1145–1156. [Google Scholar] [CrossRef]
- Xiao, J.; Capanoglu, E.; Jassbi, A.R.; Miron, A. Advance on the Flavonoid C-glycosides and Health Benefits. Crit. Rev. Food Sci. Nutr. 2016, 56 (Suppl. S1), S29–S45. [Google Scholar] [CrossRef]
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Bellocco, E.; Leuzzi, U.; Gattuso, G. First evidence of C and O-glycosyl flavone in blood orange (Citrus sinensis (L.) Osbeck) juice and their influence on antioxidant properties. Food Chem. 2014, 149, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Abou-Zaid, M.M.; Lombardo, D.A.; Kite, G.C.; Grayer, R.J.; Veitch, N.C. Acylated flavone C-glycosides from Cucumis sativus. Phytochemistry 2001, 58, 167–172. [Google Scholar] [CrossRef]
- Yadaw, R.N.; Singh, R.K.R. 6-Hydroxy-3,5,7,4′-tetramethoxyflavone 6-rhamnoside from roots of Pterocarpus marsupim. Phytochemistry 1998, 48, 1259–1261. [Google Scholar]
- Zieliński, H.; Michalska, A.; Piskuła, M.K.; Kozłowska, H. Antioxidants in thermally treated buckwheat groats. Mol. Nutr. Food Res. 2006, 50, 824–832. [Google Scholar] [CrossRef]
- Kim, A.J.; Zaidul, I.S.M.; Maeda, T.; Suzuki, T.; Hashimoto, N.; Takigawa, S.; Noda, T.; Matsuura-Endo, C.; Yamauchi, H. A time-course of flavonoids in the sprouts of tartary (Fagopyrum tataricum Gaertn.) buckwheats. Sci. Hortic. 2007, 115, 13–18. [Google Scholar] [CrossRef]
- Kim, Y.C.; Jun, M.; Leong, W.S.; Chung, S.K. Antioxidant properties of flavone C-glycosides from Atractylodes japonica leaves in human low-density lipoprotein oxidation. J. Food Sci. 2005, 70, S575–S580. [Google Scholar] [CrossRef]
- Peng, X.; Zheng, Z.; Cheng, K.-W.; Shan, F.; Ren, G.-X.; Chen, F.; Wang, M. Inhibitory Effect of Mung Bean Extract and its Constituents Vitexin and Isovitexin on the Formation of Advanced Glycation End products. Food Chem. 2008, 106, 475–481. [Google Scholar] [CrossRef]
- Zhang, Y.; Bao, B.; Lu, B.; Ren, Y.; Tie, X.; Zhang, Y. Determination of flavone C-glucosides in antioxidant of bamboo leaves (AOB) fortified foods by reversed-phase high-performance liquid chromatography with ultraviolet diode array detection. J. Chromatogr. A 2005, 1065, 177–185. [Google Scholar] [CrossRef]
- Fu, Y.; Zu, Y.; Liu, W.; Hou, C.; Chen, L.; Li, S.; Shi, X.; Tong, M. Preparative separation of vitexin and isovitexin from pigeonpea extracts with macroporous resins. J. Chromatogr. A 2007, 1139, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, D.; Zieliński, H. Antioxidant activity of flavone C-glucosides determined by updated analytical strategies. Food Chem. 2011, 124, 672–678. [Google Scholar] [CrossRef]
- Magalhaes, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Chiorcea-Paquim, A.M.; Enache, T.A.; De Souza Gil, E.; Oliveira-Brett, A.M. Natural phenolic antioxidants electrochemistry: Towards a new food science methodology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1680–1726. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Arbeláez, J.; Vázquez, M.; Contreras-Calderón, J. Electrochemical methods as a tool for determining the antioxidant capacity of food and beverages: A review. Food Chem. 2017, 221, 1371–1381. [Google Scholar] [CrossRef]
- Sochor, J.; Dobes, J.; Krystofova, O.; Ruttkay-Nedecky, B.; Babula, P.; Pohanka, M.; Jurikova, T.; Zitka, O.; Adam, V.; Klejdus, B.; et al. Electrochemistry as a Tool for Studying Antioxidant Properties. Inter. J. Electroch. Sci. 2013, 8, 8464–8489. [Google Scholar] [CrossRef]
- Zielińska, D.; Zieliński, H.; Piskuła, M.K. An Electrochemical Determination of the Total Reducing Capacity of Wheat, Spelt, and Rye Breads. Antioxidants 2022, 11, 1438. [Google Scholar] [CrossRef]
- Guang, C.; Philips, R.D. Plant Food-Derived Angiotensin I Converting Enzyme Inhibitory Peptides. J. Agric. Food Chem. 2009, 57, 5113–5120. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, S. Strategies for initial management of hypertension. Indian J. Med. Res. 2010, 132, 531–542. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, L.; Han, X.; Meng, Z.; Zhang, J.; Wu, Y.; Cheng, D. Quantitative structure-activity relationship modeling coupled with molecular docking analysis in screening of angiotensin I-converting enzyme inhibitory peptides from qula casein hydrolysates obtained by two-enzyme combination hydrolysis. J. Agric. Food Chem. 2018, 66, 3221–3228. [Google Scholar] [CrossRef]
- Fujita, H.; Yokoyama, K.; Yoshikawa, M. Classification and antihypertensive activity of angiotensin I-converting enzyme inhibitory peptides derived from food proteins. J. Food Sci. 2000, 65, 564–569. [Google Scholar]
- Kim, S.K.; Byun, H.G.; Park, P.J.; Shahidi, F. Angiotensin I converting enzyme inhibitory peptides purified from bovine skin gelatin hydrolysate. J. Agric. Food Chem. 2001, 49, 2992–2997. [Google Scholar] [CrossRef]
- Wimo, A.; Winblad, B.; Aguero-Torres, H.; von Strauss, E. The magnitude of dementia occurrence in the world. Alzheimer Dis. Assoc. Disord. 2003, 17, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Pathways towards and away from Alzheimer’s disease. Nature 2004, 430, 631–639, Addendum in Nature 2005, 431, 107. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.J.; Howes, M.J. Natural products and derivatives affecting neurotransmission relevant to Alzheimer’s and Parkinson’s disease. Neurosignals 2005, 14, 6–22. [Google Scholar] [CrossRef]
- Conforti, L.; Adalbert, R.; Coleman, M.P. Neuronal death: Where does the end begin? Trends Neurosci. 2007, 30, 159–166. [Google Scholar] [CrossRef]
- Adewusi, E.A.; Steenkamp, V. In vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from southern Africa. Asian Pac. J. Trop. Med. 2011, 4, 829–835. [Google Scholar] [CrossRef]
- Williams, P.; Sorribas, A.; Howes, M.J.R. Natural products as a source of Alzheimer’s drug leads. Nat. Prod. Rep. 2011, 28, 48–77. [Google Scholar] [CrossRef] [PubMed]
- Oei, S.; Millar, C.L.; Lily, T.N.N.; Mukamal, K.J.; Kiel, D.P.; Lipsitz, L.A.; Hannan, M.T.; Sahni, S. Higher intake of dietary flavonols, specifically dietary quercetin, is associated with lower odds of frailty onset over 12 years of follow-up among adults in the Framingham Heart Study. Am. J. Clin. Nutr. 2023, 118, 27–33. [Google Scholar] [CrossRef]
- Safarzadeh, E.; Ataei, S.; Akbari, M.; Abolhasani, R.; Baziar, M.; Asghariazar, V.; Dadkhah, M. Quercetin ameliorates cognitive deficit, expression of amyloid precursor gene, and pro-inflammatory cytokines in an experimental models of Alzheimer’s disease in Wistar rats. Exp. Gerontol. 2024, 193, 112466. [Google Scholar] [CrossRef]
- Ahmed, N. Advanced glycation endproducts--role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005, 67, 3–21. [Google Scholar] [CrossRef]
- Buetler, T. The Health Risks of Dietary Advanced Glycation end-products. Mol. Nutr. Food Res. 2007, 51, 1071–1073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Y.; Fu, L. Dietary advanced glycation end-products: Perspectives linking food processing with health implications. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2559–2587. [Google Scholar] [CrossRef] [PubMed]
- Lunceford, N.; Gugliucci, A. Ilex paraguariensis extracts inhibit AGE formation more efficiently than green tea. Fitoterapia 2005, 76, 419–427. [Google Scholar] [CrossRef]
- Pashikanti, S.; de Alba, D.R.; Boissonneault, G.A.; Cervantes-Laurean, D. Rutin metabolites: Novel inhibitors of nonoxidative advanced glycation end products. Free Radic. Biol. Med. 2010, 48, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Islam, M.N.; Ali, M.Y.; Kim, E.J.; Kim, Y.M.; Jung, H.A. Effects of C-glycosylation on anti-diabetic, anti-Alzheimer’s disease and anti-inflammatory potential of apigenin. Food Chem. Toxicol. 2014, 64, 27–33. [Google Scholar] [CrossRef]
- Brett, A.M.O.; Ghica, M.E. Electrochemical oxidation of quercetin. Electroanalysis 2003, 15, 1745–1750. [Google Scholar] [CrossRef]
- Timbola, A.K.; De Souza, C.D.; Giacomelli, C.; Spineli, A. Electrochemical oxidation of quercetin in hydro-alcoholic solution. J. Braz. Chem. Soc. 2006, 17, 139–148. [Google Scholar] [CrossRef]
- Chevion, S.; Roberts, M.A.; Chevion, M. The use of cyclic voltammetry for the evaluation of antioxidant capacity. Free Radic. Biol. Med. 2000, 6, 860–870. [Google Scholar] [CrossRef]
- Zielińska, D.; Zieliński, H.; Laparra-Llopis, J.M.; Honke, J.; Giménez-Bastida, J.A. Caffeic acid modulates processes associated with intestinal inflammation. Nutrients 2021, 13, 554. [Google Scholar] [CrossRef]
- Shetti, N.P.; Malode, S.J.; Nandibewoor, S.T. Electro-oxidation of captopril at a gold electrode and its determination in pharmaceuticals and human fluids. Anal. Methods 2015, 7, 8673–8682. [Google Scholar] [CrossRef]
- Lopez, S.; Bastida, J.; Viladomat, F.; Codina, C. Acetylcholinesterase inhibitory activity of some Amaryllidaceae alkaloids and Narcissus extracts. Life Sci. 2002, 71, 2521–2529. [Google Scholar] [CrossRef]
- Hoyos-Arbeláez, J.; Blandón-Naranjo, L.; Vázquez, M.; Contreras Calderón, J. Antioxidant capacity of mango fruit (Mangifera indica). An electrochemical study as an approach to the spectrophotometric methods. Food Chem. 2018, 266, 435–440. [Google Scholar] [CrossRef]
- Besco, E.; Braccioli, E.; Vertuani, S.; Ziosi, P.; Brazzo, F.; Bruni, R.; Sacchetti, G.; Manfredini, S. The use of photochemiluminescence for the measurement of the integral antioxidant capacity of baobab products. Food Chem. 2007, 102, 1352–1356. [Google Scholar] [CrossRef]
- Martinez, S.; Valek, L.; Resetic, J.; Rusic, D.F. Cyclic voltammetry study of plasma antioxidant capacity–comparison with the DPPH and TAS spectrophotometric methods. J. Electrochem. Chem. 2006, 588, 68–73. [Google Scholar] [CrossRef]
- Alam, M.W.; Najeeb, J.; Naeem, S.; Usman, S.M.; Nahvi, I.; Alismail, F.; Abuzir, A.; Farhan, M.; Allah Nawaz, A. Electrochemical methodologies for investigating the antioxidant potential of plant and fruit extracts: A review. Antioxidants 2022, 11, 1205. [Google Scholar] [CrossRef]
- Zlatić, G.; Arapović, A.; Martinović, I.; Martinović Bevanda, A.; Bošković, P.; Prkić, A.; Paut, A.; Vukušić, T. Antioxidant Capacity of Herzegovinian Wildflowers Evaluated by UV–VIS and Cyclic Voltammetry Analysis. Molecules 2022, 27, 5466. [Google Scholar] [CrossRef]
- Vilas-Boas, Â.; Valderrama, P.; Fontes, N.; Geraldo, D.; Bento, F. Evaluation of total polyphenol content of wines by means of voltammetric techniques: Cyclic voltammetry vs. differential pulse voltammetry. Food Chem. 2019, 276, 719–725. [Google Scholar] [CrossRef]
- Osorio-Valencia, A.I.; Franco-Mejía, J.J.; Hoyos-Arbeláez, J.A.; Blandón-Naranjo, L.; Vega-Castro, O.A.; Contreras-Calderón, J.C. Evaluation of antioxidant capacity in different food matrices through differential pulse voltammetry and its correlation with spectrophotometric methods. J. Appl. Electrochem. 2023, 53, 2495–2505. [Google Scholar] [CrossRef]
- Terao, J. Potential Role of Quercetin Glycosides as Anti-Atherosclerotic Food-Derived Factors for Human Health. Antioxidants 2023, 12, 258. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.M.; Paganda, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, Y. Reducing power: The measure of antioxidant activities or reductant compounds. Redox Rep. 2004, 9, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Sarian, M.N.; Ahmed, Q.U.; Mat So’ad, S.Z.; Alhassan, A.M.; Murugesu, S.; Perumal, V.; Mohamad, S.N.A.S.; Khatib, A.; Latip, J. Antioxidant and Antidiabetic Effects of Flavonoids: A Structure-Activity Relationship Based Study. Biomed. Res. Int. 2017, 8386065. [Google Scholar] [CrossRef] [PubMed]
- Blasco, A.J.; Rogerio, M.C.; Gonzalez, M.C.; Escarpa, A. ‘‘Electrochemical Index” as a screening method to determine ‘‘total polyphenolics” in foods: A proposal. Anal. Chim. Acta 2005, 539, 237–244. [Google Scholar] [CrossRef]
- Miguel, M.; Contreras, M.M.; Recio, I.; Aleixandre, A. ACE inhibitory and antihypertensive properties of a bovine casein hydrolysate. Food Chem. 2009, 12, 211–214. [Google Scholar] [CrossRef]
- Je, J.Y.; Qian, Z.J.; Byun, H.G.; Kim, S.K. Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Proc. Biochem. 2007, 42, 840–846. [Google Scholar] [CrossRef]
- Kuba, M.; Tana, C.; Tawata, S.; Yasuda, M. Production of angiotensin I-converting enzyme inhibitory peptides from soybean protein with Monascus purpureus acid proteinase. Proc. Biochem. 2005, 40, 2191–2196. [Google Scholar] [CrossRef]
- Torino, M.I.; Limón, R.I.; Martínez-Villaluenga, C.; Mäkinen, S.; Pihlanto, A.; Vidal-Valverde, C.; Frias, J. Antioxidant and antihypertensive properties of liquid and solid state fermented lentils. Food Chem. 2013, 136, 1030–1037. [Google Scholar] [CrossRef]
- Wang, C.; Tian, J.; Wang, Q. ACE inhibitory and antihypertensive properties of apricot almond meal hydrolysate. Eur. Food Res. Technol. 2011, 232, 549–556. [Google Scholar] [CrossRef]
- Ren, X.; Ma, H.; Mao, S.; Zhou, H. Effects of sweeping frequency ultrasound treatment on enzymatic preparations of ACE-inhibitory peptides from zein. Eur. Food Res. Technol. 2014, 238, 435–442. [Google Scholar] [CrossRef]
- Iwaniak, A.; Minkiewicz, P.; Darewicz, M. Food-originating ACE inhibitors, including antihypertensive peptides, as preventive food components in blood pressure reduction. Compr. Rev. Food Sci. Food Saf. 2014, 13, 114–134. [Google Scholar] [CrossRef] [PubMed]
- Lacaille-Dubois, M.A.; Franck, U.; Wagner, H. Search for potential Angiotensin Converting Enzyme (ACE)-inhibitors from plants. Phytomedicine 2001, 8, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, H.; Honke, J.; Topolska, J.; Bączek, N.; Piskuła, M.K.; Wiczkowski, W.; Wronkowska, M. ACE inhibitory properties and phenolics profile of fermented flours and of baked and digested biscuits from buckwheat. Foods 2020, 9, 847. [Google Scholar] [CrossRef]
- Galleano, M.; Pechanova, O.; Fraga, C.G. Hypertension, nitric oxide, oxidants, and dietary polyphenols. Curr. Pharm. Biotechnol. 2010, 11, 837–848. [Google Scholar] [CrossRef]
- Cordova, A.C.; Sumpio, B.J.; Sumpio, B.E. Perfecting the plate: Adding cardioprotective compounds to the diet. J. Am. Coll. Surg. 2012, 214, 97–114. [Google Scholar] [CrossRef]
- Pan, M.-H.; Lai, C.-S.; Dushenkov, S.; Ho, C.-T. Modulation of inflammatory genes by natural dietary bioactive compounds. J. Agric. Food Chem. 2009, 57, 4467–4477. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, L.; Castillo, J.; Quinones, M.; Garcia-Vallve, S.; Arola, L.; Pujadas, G.; Muguerza, B. Inhibition of angiotensin-converting enzyme activity by flavonoids: Structure-activity relationship studies. PLoS ONE. 2012, 7, e49493. [Google Scholar] [CrossRef]
- Zielińska, D.; Starowicz, M.; Wronkowska, M.; Zieliński, H. Angiotensin-converting enzyme inhibitory activity of selected phenolic acids, flavonoids, their O-glucosides, and low-molecular-weight phenolic metabolites in relation to their oxidation potentials. Metabolites 2025, 15, 443. [Google Scholar] [CrossRef]
- Tsai, H.; Deng, H.; Tsai, S.; Hsu, Y. Bioactivity comparison of extracts from various parts of common and tartary buckwheats: Evaluation of the antioxidant- and angiotensin converting enzyme inhibitory activities. Chem. Cent. J. 2012, 6, 78–82. [Google Scholar] [CrossRef]
- Shukor, N.A.; Camp, J.V.; Gonzales, G.B.; Staljanssens, D.; Struijs, K.; Zotti, M.J.; Raes, K.; Smagghe, G. Angiotensin-Converting Enzyme Inhibitory Effects by Plant Phenolic Compounds: A Study of Structure Activity Relationships. J. Agric. Food Chem. 2013, 61, 11832–11839. [Google Scholar] [CrossRef] [PubMed]
- Persson, I.-A.-L.; Josefsson, M.; Persson, K.; Andersson, R.G.G. Tea flavanols inhibit angiotensin-converting enzyme activity and increase nitric oxide production in human endothelial cells. J. Pharm. Pharmacol. 2006, 58, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Paiva, L.; Lima, E.; Marcone, M.; Baptista, J. Angiotensin I-converting enzyme (ACE) inhibition and biological activities of green and black tea samples from Azorean Camellia sinensis. J. Funct. Foods. 2023, 107, 105701. [Google Scholar] [CrossRef]
- Şenol, F.S.; Orhan, İ.; Yilmaz, G.; Çiçek, M.; Şener, B. Acetylcholinesterase, butyrylcholinesterase, and tyrosinase inhibition studies and antioxidant activities of 33 Scutellaria L. taxa from Turkey. Food Chem. Toxicol. 2010, 48, 781–788. [Google Scholar] [CrossRef]
- Eckert, G.P. Traditional used plants against cognitive decline and Alzheimer’s disease. Front. Pharmacol. 2010, 1, 138. [Google Scholar] [CrossRef]
- Lopez-Sendon, J.; Swedberg, K.; McMurray, J.; Tamargo, J.; Maggioni, A.P.; Dargie, H.; Tendera, M.; Waagstein, F.; Kjekshus, J.; Lechat, P.; et al. Expert consensus document on angiotensin-converting enzyme inhibitors in cardiovascular disease. The Task Force on ACE-inhibitors of the European Society of Cardiology. Eur. Heart J. 2004, 25, 454–470. [Google Scholar]
- Wu, C.H.; Yen, G.C. Inhibitory effect of naturally occuring flavonoids on the formation of advanced glycation endproducts. J. Agric. Food Chem. 2005, 53, 3167–3173. [Google Scholar] [CrossRef]
- Cosio, M.S.; Buratti, S.; Mannino, S.; Benedetti, S. Use of an electrochemical method to evaluate the antioxidant activity of herb extracts from the Labiatae family. Food Chem. 2006, 97, 725–731. [Google Scholar] [CrossRef]
- Sentandreu, M.Á.; Toldrá, F. A rapid, simple and sensitive fluorescence method for the assay of angiotensin-I converting enzyme. Food Chem. 2006, 97, 546–554. [Google Scholar] [CrossRef]
- Eldeen, I.M.S.; Elgorashi, E.E.; Staden, J. Antibacterial, anti-inflammatory, anti-cholinesterase and mutagenic effects of extracts obtained from some trees used in South African traditional medicine. J. Ethnopharmacol. 2005, 102, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, D.; Starowicz, M.; Wronkowska, M.; Zieliński, H. Multifaceted Biological Activity of Rutin, Quercetin, and Quercetin’s Glucosides. Molecules 2025, 30, 2555. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Zieliński, H.; Piskula, M.; Zielińska, D.; Szawara-Nowak, D. Buckwheat bioactive compounds, their derived phenolic metabolites and their health benefits. Mol. Nutr. Food Res. 2017, 61, 1600475. [Google Scholar] [CrossRef] [PubMed]


| Compound | (mV) | Antioxidant Activity (mM Trolox) | ||
|---|---|---|---|---|
| Orientin (OR) | 345 ± 6 b | 1020 ± 9 a | - | 3.00 ± 0.14 b |
| Homoorientin (hOR) | 336 ± 5 b | 960 ± 9 b | - | 3.24 ± 0.15 b |
| Vitexin (VT) | 925 ± 8 a | - | - | 0.93 ± 0.07 c |
| Isovitexin (iVT) | 926 ± 8 a | - | - | 1.06 ± 0.07 c |
| Quercetin (Q) | 255 ± 7 c | 510 ± 8 c | 1060 ± 10 | 3.81 ± 0.19 a |
| Compound | Equation of the Linear Regression | IC50 (µM) |
|---|---|---|
| Orientin (OR) | y = 0.1807x + 27.908 R2 = 0.96 | 122.26 ± 2.66 c |
| Homoorientin (hOR) | y = 0.2431x + 22.193 R2 = 0.98 | 114.39 ± 1.23 d |
| Vitexin (VT) | y = 0.1701x + 26.982 R2 = 0.94 | 135.32 ± 2.45 b |
| Isovitexin (iVT) | y = 0.1333x + 28.498 R2 = 0.96 | 161.31 ± 3.27 a |
| Quercetin (Q) | y = 0.3831x + 27.285 R2 = 0.96 | 59.29 ± 0.48 e |
| Captopril | y = 5008.1x + 20.632 R2 = 0.98 | 0.00586 ± 0.00004 g |
| Glutathione (GSH) | y = 0.1337x + 44.429 R2 = 0.98 | 41.68 ± 3.45 f |
| Compound | Equation of the Linear Regression | IC50 (µM) |
|---|---|---|
| Orientin (OR) | y = 56.347x + 1.074 R2 = 0.99 | 0.868 ± 0.088 c |
| Homoorientin (hOR) | y = 23.817x + 36.167 R2 = 0.81 | 0.581 ± 0.096 d |
| Vitexin (VT) | y = 42.046x − 3.739 R2 = 0.99 | 1.278 ± 0.089 a |
| Isovitexin (iVT) | y = 48.34x − 2.033 R2 = 0.99 | 1.076 ± 0.011 b |
| Quercetin (Q) | y = 32.48x + 5.370 R2 = 0.99 | 1.374 ± 0.077 a |
| Galanthamine | y = 834.84x + 14.195 R2 = 0.99 | 0.043 ± 0.008 e |
| Compound | BSA-Glucose System | BSA-MGO System | ||
|---|---|---|---|---|
| Equation of the Linear Regression | IC50 (mM) | Equation of the Linear Regression | IC50 (mM) | |
| Orientin (OR) | y = 0.0205x − 1.1218 R2 = 0.83 | 0.073 ± 0.005 c | y = 0.0147x − 0.4095 R2 = 0.94 | 0.326 ± 0.019 e |
| Homoorientin (hOR) | y = 0.017x − 0.8359 R2 = 0.70 | 0.014 ± 0.008 d | y = 0.0149x − 0.3539 R2 = 0.986 | 0.391 ± 0.022 cd |
| Vitexin (VT) | y = 0.0135x − 0.4199 R2 = 0.81 | 0.255 ± 0.016 b | y = 0.0128x − 0.203 R2 = 0.98 | 0.437 ± 0.017 bc |
| Isovitexin (iVT) | y = 0.0141x − 0.491 R2 = 0.82 | 0.214 ± 0.011 b | y = 0.0139x − 0.3543 R2 = 0.94 | 0.341 ± 0.020 de |
| Quercetin (Q) | y = 0.0164x − 0.5768 R2 = 0.86 | 0.243 ± 0.012 b | y = 0.0141x − 0.2341 R2 = 0.99 | 0.466 ± 0.017 b |
| Aminoquanidine (AG) | y = 0.0167x − 0.4031 R2 = 0.94 | 0.432 ± 0.036 a | y = 0.0161x − 0.274 R2 = 0.99 | 0.531 ± 0.028 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielińska, D.; Zieliński, H. Multifaceted Biological Activity of Selected Flavone C-Monoglucosides. Int. J. Mol. Sci. 2025, 26, 10124. https://doi.org/10.3390/ijms262010124
Zielińska D, Zieliński H. Multifaceted Biological Activity of Selected Flavone C-Monoglucosides. International Journal of Molecular Sciences. 2025; 26(20):10124. https://doi.org/10.3390/ijms262010124
Chicago/Turabian StyleZielińska, Danuta, and Henryk Zieliński. 2025. "Multifaceted Biological Activity of Selected Flavone C-Monoglucosides" International Journal of Molecular Sciences 26, no. 20: 10124. https://doi.org/10.3390/ijms262010124
APA StyleZielińska, D., & Zieliński, H. (2025). Multifaceted Biological Activity of Selected Flavone C-Monoglucosides. International Journal of Molecular Sciences, 26(20), 10124. https://doi.org/10.3390/ijms262010124






