Recent Advances in the Role of Osteocytes in Orthodontic Tooth Movement
Abstract
1. Introduction
2. Osteocyte Signaling Pathways Governing Osteoclastogenesis in OTM
3. Osteocyte Mechanotransduction and Mechanical Modulation of Bone Resorption in OTM
4. The Impact of Aging Osteocytes on OTM
5. Osteocyte Morphology and Mechano-Adaptation During OTM
6. Conclusions, Limitations, and Future Directions
- Should osteocyte-targeted interventions in aging models, particularly the use of senolytics, be pursued despite significant safety concerns such as off-target toxicity and the heterogeneity of osteocyte populations?
- Rigorous translational and clinical studies are needed to evaluate the efficacy and safety of adjunctive mechanical stimulation strategies, such as low-magnitude high-frequency vibration (LMHFV), in orthodontic patients.
- At a fundamental level, the field lacks advanced single-cell and spatial omics approaches capable of mapping osteocyte heterogeneity within bone. Applying these tools to human alveolar bone under varying mechanical strain, and in aged or diseased contexts, would provide a nuanced understanding of osteocyte biology. Such insights are critical to develop personalized, mechanism-based orthodontic therapies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reitan, K. Clinical and histologic observations on tooth movement during and after orthodontic treatment. Am. J. Orthod. 1967, 53, 721–745. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Davidovitch, Z.e. Cellular, molecular, and tissue-level reactions to orthodontic force. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 469.E1–469.E32. [Google Scholar] [CrossRef]
- Masella, R.S.; Meister, M. Current concepts in the biology of orthodontic tooth movement. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 458–468. [Google Scholar] [CrossRef]
- Wise, G.E.; King, G.J. Mechanisms of Tooth Eruption and Orthodontic Tooth Movement. J. Dent. Res. 2008, 87, 414–434. [Google Scholar] [CrossRef]
- Li, Y.; Zhan, Q.; Bao, M.; Yi, J.; Li, Y. Biomechanical and biological responses of periodontium in orthodontic tooth movement: Up-date in a new decade. Int. J. Oral. Sci. 2021, 13, 20. [Google Scholar] [CrossRef]
- Kitaura, H.; Ohori, F.; Marahleh, A.; Ma, J.; Lin, A.; Fan, Z.; Narita, K.; Murakami, K.; Kanetaka, H. The Role of Cytokines in Orthodontic Tooth Movement. Int. J. Mol. Sci. 2025, 26, 6688. [Google Scholar] [CrossRef]
- Klein-Nulend, J.; Bakker, A.D.; Bacabac, R.G.; Vatsa, A.; Weinbaum, S. Mechanosensation and transduction in osteocytes. Bone 2013, 54, 182–190. [Google Scholar] [CrossRef]
- Seddiqi, H.; Klein-Nulend, J.; Jin, J. Osteocyte Mechanotransduction in Orthodontic Tooth Movement. Curr. Osteoporos. Rep. 2023, 21, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Robling, A.G.; Bonewald, L.F. The Osteocyte: New Insights. Annu. Rev. Physiol. 2020, 82, 485–506. [Google Scholar] [CrossRef]
- Marotti, G. The structure of bone tissues and the cellular control of their deposition. Ital. J. Anat. Embryol. 1996, 101, 25–79. [Google Scholar] [PubMed]
- Buenzli, P.R.; Sims, N.A. Quantifying the osteocyte network in the human skeleton. Bone 2015, 75, 144–150. [Google Scholar] [CrossRef]
- Li, X.; Kordsmeier, J.; Xiong, J. New Advances in Osteocyte Mechanotransduction. Curr. Osteoporos. Rep. 2021, 19, 101–106. [Google Scholar] [CrossRef]
- Bonewald, L.F. The amazing osteocyte. J. Bone Min. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef]
- Franz-Odendaal, T.A.; Hall, B.K.; Witten, P.E. Buried alive: How osteoblasts become osteocytes. Dev. Dyn. 2006, 235, 176–190. [Google Scholar] [CrossRef]
- Nefussi, J.R.; Sautier, J.M.; Nicolas, V.; Forest, N. How osteoblasts become osteocytes: A decreasing matrix forming process. J. Biol. Buccale 1991, 19, 75–82. [Google Scholar] [PubMed]
- Palumbo, C.; Ferretti, M.; Marotti, G. Osteocyte dendrogenesis in static and dynamic bone formation: An ultrastructural study. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2004, 278, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, C. A three-dimensional ultrastructural study of osteoid-osteocytes in the tibia of chick embryos. Cell Tissue Res. 1986, 246, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Onal, M.; Jilka, R.L.; Weinstein, R.S.; Manolagas, S.C.; O’Brien, C.A. Matrix-embedded cells control osteoclast formation. Nat. Med. 2011, 17, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Poole, K.E.S.; Van Bezooijen, R.L.; Loveridge, N.; Hamersma, H.; Papapoulos, S.E.; Löwik, C.W.; Reeve, J. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005, 19, 1842–1844. [Google Scholar] [CrossRef]
- Robling, A.G.; Niziolek, P.J.; Baldridge, L.A.; Condon, K.W.; Allen, M.R.; Alam, I.; Mantila, S.M.; Gluhak-Heinrich, J.; Bellido, T.M.; Harris, S.E.; et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J. Biol. Chem. 2008, 283, 5866–5875. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Kang, H.; Liu, W.; Liu, P.; Zhang, J.; Harris, S.E.; Wu, D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J. Biol. Chem. 2005, 280, 19883–19887. [Google Scholar] [CrossRef]
- Marahleh, A.; Kitaura, H.; Ohori, F.; Noguchi, T.; Mizoguchi, I. The osteocyte and its osteoclastogenic potential. Front. Endocrinol. 2023, 14, 1121727. [Google Scholar] [CrossRef]
- Xiong, J.; Piemontese, M.; Onal, M.; Campbell, J.; Goellner, J.J.; Dusevich, V.; Bonewald, L.; Manolagas, S.C.; O’Brien, C.A. Osteocytes, not Osteoblasts or Lining Cells, are the Main Source of the RANKL Required for Osteoclast Formation in Remodeling Bone. PLoS ONE 2015, 10, e0138189. [Google Scholar] [CrossRef]
- Nakashima, T.; Hayashi, M.; Fukunaga, T.; Kurata, K.; Oh-hora, M.; Feng, J.Q.; Bonewald, L.F.; Kodama, T.; Wutz, A.; Wagner, E.F.; et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011, 17, 1231–1234. [Google Scholar] [CrossRef]
- Nakai, Y.; Praneetpong, N.; Ono, W.; Ono, N. Mechanisms of Osteoclastogenesis in Orthodontic Tooth Movement and Orthodontically Induced Tooth Root Resorption. J. Bone Metab. 2023, 30, 297–310. [Google Scholar] [CrossRef]
- Hamilton, J.A. CSF-1 signal transduction. J. Leukoc. Biol. 1997, 62, 145–155. [Google Scholar] [CrossRef]
- Hsu, H.; Lacey, D.L.; Dunstan, C.R.; Solovyev, I.; Colombero, A.; Timms, E.; Tan, H.L.; Elliott, G.; Kelley, M.J.; Sarosi, I.; et al. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc. Natl. Acad. Sci. USA 1999, 96, 3540–3545. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.M.; Ryan, G.R.; Hapel, A.J.; Dominguez, M.G.; Russell, R.G.; Kapp, S.; Sylvestre, V.; Stanley, E.R. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood 2002, 99, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Yashima, Y.; Kaku, M.; Yamamoto, T.; Izumino, J.; Kagawa, H.; Ikeda, K.; Shimoe, S.; Tanimoto, K. Effect of continuous compressive force on the expression of RANKL, OPG, and VEGF in osteocytes. Biomed. Res. 2020, 41, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Shoji-Matsunaga, A.; Ono, T.; Hayashi, M.; Takayanagi, H.; Moriyama, K.; Nakashima, T. Osteocyte regulation of orthodontic force-mediated tooth movement via RANKL expression. Sci. Rep. 2017, 7, 8753. [Google Scholar] [CrossRef]
- Yoshimatsu, M.; Shibata, Y.; Kitaura, H.; Chang, X.; Moriishi, T.; Hashimoto, F.; Yoshida, N.; Yamaguchi, A. Experimental model of tooth movement by orthodontic force in mice and its application to tumor necrosis factor receptor-deficient mice. J. Bone Miner. Metab. 2006, 24, 20–27. [Google Scholar] [CrossRef]
- Kitaura, H.; Yoshimatsu, M.; Fujimura, Y.; Eguchi, T.; Kohara, H.; Yamaguchi, A.; Yoshida, N. An Anti-c-Fms Antibody Inhibits Orthodontic Tooth Movement. J. Dent. Res. 2008, 87, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Marahleh, A.; Kitaura, H.; Ohori, F.; Kishikawa, A.; Ogawa, S.; Shen, W.R.; Qi, J.; Noguchi, T.; Nara, Y.; Mizoguchi, I. TNF-α Directly Enhances Osteocyte RANKL Expression and Promotes Osteoclast Formation. Front. Immunol. 2019, 10, 2925. [Google Scholar] [CrossRef] [PubMed]
- Marahleh, A.; Kitaura, H.; Ohori, F.; Noguchi, T.; Nara, Y.; Pramusita, A.; Kinjo, R.; Ma, J.; Kanou, K.; Mizoguchi, I. Effect of TNF-α on osteocyte RANKL expression during orthodontic tooth movement. J. Dent. Sci. 2021, 16, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Kitaura, H.; Marahleh, A.; Ohori, F.; Noguchi, T.; Shen, W.R.; Qi, J.; Nara, Y.; Pramusita, A.; Kinjo, R.; Mizoguchi, I. Osteocyte-Related Cytokines Regulate Osteoclast Formation and Bone Resorption. Int. J. Mol. Sci. 2020, 21, 5169. [Google Scholar] [CrossRef]
- Ohori, F.; Kitaura, H.; Marahleh, A.; Kishikawa, A.; Ogawa, S.; Qi, J.; Shen, W.R.; Noguchi, T.; Nara, Y.; Mizoguchi, I. Effect of TNF-α-Induced Sclerostin on Osteocytes during Orthodontic Tooth Movement. J. Immunol. Res. 2019, 2019, 9716758. [Google Scholar] [CrossRef]
- Kitaura, H.; Marahleh, A.; Ohori, F.; Noguchi, T.; Nara, Y.; Pramusita, A.; Kinjo, R.; Ma, J.; Kanou, K.; Mizoguchi, I. Role of the Interaction of Tumor Necrosis Factor-α and Tumor Necrosis Factor Receptors 1 and 2 in Bone-Related Cells. Int. J. Mol. Sci. 2022, 23, 1481. [Google Scholar] [CrossRef]
- Lam, J.; Takeshita, S.; Barker, J.E.; Kanagawa, O.; Ross, F.P.; Teitelbaum, S.L. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J. Clin. Investig. 2000, 106, 1481–1488. [Google Scholar] [CrossRef]
- Kobayashi, K.; Takahashi, N.; Jimi, E.; Udagawa, N.; Takami, M.; Kotake, S.; Nakagawa, N.; Kinosaki, M.; Yamaguchi, K.; Shima, N.; et al. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J. Exp. Med. 2000, 191, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ling, J.; Jiang, Q. Inflammasomes in Alveolar Bone Loss. Front. Immunol. 2021, 12, 691013. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.H.; Huang, X.; Rojas Cortez, L.; Sripinun, P.; Lee, J.-m.; Hong, J.J.; Graves, D.T. Inflammation and mechanical force-induced bone remodeling. Periodontology 2000 2024, 1–26. [Google Scholar] [CrossRef]
- Noguchi, T.; Kitaura, H.; Ogawa, S.; Qi, J.; Shen, W.R.; Ohori, F.; Marahleh, A.; Nara, Y.; Pramusita, A.; Mizoguchi, I. TNF-α stimulates the expression of RANK during orthodontic tooth movement. Arch. Oral. Biol. 2020, 117, 104796. [Google Scholar] [CrossRef]
- Odagaki, N.; Ishihara, Y.; Wang, Z.; Ei Hsu Hlaing, E.; Nakamura, M.; Hoshijima, M.; Hayano, S.; Kawanabe, N.; Kamioka, H. Role of Osteocyte-PDL Crosstalk in Tooth Movement via SOST/Sclerostin. J. Dent. Res. 2018, 97, 1374–1382. [Google Scholar] [CrossRef]
- Miura, M.; Kitaura, H.; Ohori, F.; Narita, K.; Ren, J.; Noguchi, T.; Marahleh, A.; Ma, J.; Lin, A.; Fan, Z.; et al. Role of CXCL10 released from osteocytes in response to TNF-α stimulation on osteoclasts. Sci. Rep. 2025, 15, 3040. [Google Scholar] [CrossRef] [PubMed]
- Sucur, A.; Jajic, Z.; Artukovic, M.; Matijasevic, M.I.; Anic, B.; Flegar, D.; Markotic, A.; Kelava, T.; Ivcevic, S.; Kovacic, N.; et al. Chemokine signals are crucial for enhanced homing and differentiation of circulating osteoclast progenitor cells. Arthritis Res. Ther. 2017, 19, 142. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.; Kalajzic, Z.; Choi, T.; Maleeh, I.; Ricupero, C.L.; Skelton, M.N.; Daily, M.L.; Chen, J.; Wadhwa, S. The role of inhibition of osteocyte apoptosis in mediating orthodontic tooth movement and periodontal remodeling: A pilot study. Prog. Orthod. 2021, 22, 21. [Google Scholar] [CrossRef]
- Yan, M.; Zhang, Y.; Niu, W.; Liu, K.; Xue, L.; Zhou, K. Reactive oxygen species-mediated endoplasmic reticulum stress contributes to osteocyte death induced by orthodontic compressive force. Microsc. Res. Tech. 2023, 86, 1529–1541. [Google Scholar] [CrossRef]
- Ohori, F.; Kitaura, H.; Marahleh, A.; Ma, J.; Miura, M.; Ren, J.; Narita, K.; Fan, Z.; Lin, A.; Mizoguchi, I. Osteocyte necroptosis drives osteoclastogenesis and alveolar bone resorption during orthodontic tooth movement. Sci. Rep. 2025, 15, 19413. [Google Scholar] [CrossRef]
- Zhao, W.; Qian, J.; Li, J.; Su, T.; Deng, X.; Fu, Y.; Liang, X.; Cui, H. From death to birth: How osteocyte death promotes osteoclast formation. Front. Immunol. 2025, 16, 1551542. [Google Scholar] [CrossRef]
- Uchibori, S.; Sekiya, T.; Sato, T.; Hayashi, K.; Takeguchi, A.; Muramatsu, R.; Ishizuka, K.; Kondo, H.; Miyazawa, K.; Togari, A.; et al. Suppression of tooth movement-induced sclerostin expression using β-adrenergic receptor blockers. Oral. Dis. 2020, 26, 621–629. [Google Scholar] [CrossRef]
- Xu, H.; Xia, M.; Sun, L.; Wang, H.; Zhang, W.B. Osteocytes Enhance Osteogenesis by Autophagy-Mediated FGF23 Secretion Under Mechanical Tension. Front. Cell Dev. Biol. 2021, 9, 782736. [Google Scholar] [CrossRef]
- Li, W.; Zhao, J.; Sun, W.; Wang, H.; Pan, Y.; Wang, L.; Zhang, W.B. Osteocytes promote osteoclastogenesis via autophagy-mediated RANKL secretion under mechanical compressive force. Arch. Biochem. Biophys. 2020, 694, 108594. [Google Scholar] [CrossRef]
- Jacobs, C.R.; Temiyasathit, S.; Castillo, A.B. Osteocyte mechanobiology and pericellular mechanics. Annu. Rev. Biomed. Eng. 2010, 12, 369–400. [Google Scholar] [CrossRef]
- Qin, L.; Liu, W.; Cao, H.; Xiao, G. Molecular mechanosensors in osteocytes. Bone Res. 2020, 8, 23. [Google Scholar] [CrossRef]
- Qin, L.; He, T.; Chen, S.; Yang, D.; Yi, W.; Cao, H.; Xiao, G. Roles of mechanosensitive channel Piezo1/2 proteins in skeleton and other tissues. Bone Res. 2021, 9, 44. [Google Scholar] [CrossRef]
- Nottmeier, C.; Lavicky, J.; Gonzalez Lopez, M.; Knauth, S.; Kahl-Nieke, B.; Amling, M.; Schinke, T.; Helms, J.; Krivanek, J.; Koehne, T.; et al. Mechanical-induced bone remodeling does not depend on Piezo1 in dentoalveolar hard tissue. Sci. Rep. 2023, 13, 9563. [Google Scholar] [CrossRef]
- Sakamoto, M.; Fukunaga, T.; Sasaki, K.; Seiryu, M.; Yoshizawa, M.; Takeshita, N.; Takano-Yamamoto, T. Vibration enhances osteoclastogenesis by inducing RANKL expression via NF-κB signaling in osteocytes. Bone 2019, 123, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Takeshita, N.; Fukunaga, T.; Seiryu, M.; Sakamoto, M.; Oyanagi, T.; Maeda, T.; Takano-Yamamoto, T. Vibration accelerates orthodontic tooth movement by inducing osteoclastogenesis via transforming growth factor-β signalling in osteocytes. Eur. J. Orthod. 2022, 44, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, M.; Derynck, R.; Miyazono, K. TGF-β and the TGF-β Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb. Perspect. Biol. 2016, 8, a021873. [Google Scholar] [CrossRef] [PubMed]
- Karst, M.; Gorny, G.; Galvin, R.J.; Oursler, M.J. Roles of stromal cell RANKL, OPG, and M-CSF expression in biphasic TGF-beta regulation of osteoclast differentiation. J. Cell Physiol. 2004, 200, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wu, X.; Lei, W.; Pang, L.; Wan, C.; Shi, Z.; Zhao, L.; Nagy, T.R.; Peng, X.; Hu, J.; et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat. Med. 2009, 15, 757–765. [Google Scholar] [CrossRef]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 Are Essential Components of Distinct Mechanically Activated Cation Channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef]
- Li, X.; Han, L.; Nookaew, I.; Mannen, E.; Silva, M.J.; Almeida, M.; Xiong, J. Stimulation of Piezo1 by mechanical signals promotes bone anabolism. eLife 2019, 8, e49631. [Google Scholar] [CrossRef]
- Sun, W.; Chi, S.; Li, Y.; Ling, S.; Tan, Y.; Xu, Y.; Jiang, F.; Li, J.; Liu, C.; Zhong, G.; et al. The mechanosensitive Piezo1 channel is required for bone formation. eLife 2019, 8, e47454. [Google Scholar] [CrossRef]
- Temiyasathit, S.; Jacobs, C.R. Osteocyte primary cilium and its role in bone mechanotransduction. Ann. N.Y. Acad. Sci. 2010, 1192, 422–428. [Google Scholar] [CrossRef]
- Boskey, A.L.; Coleman, R. Aging and bone. J. Dent. Res. 2010, 89, 1333–1348. [Google Scholar] [CrossRef] [PubMed]
- Mi, B.; Xiong, Y.; Knoedler, S.; Alfertshofer, M.; Panayi, A.C.; Wang, H.; Lin, S.; Li, G.; Liu, G. Ageing-related bone and immunity changes: Insights into the complex interplay between the skeleton and the immune system. Bone Res. 2024, 12, 42. [Google Scholar] [CrossRef]
- Kanou, K.; Kitaura, H.; Noguchi, T.; Ohori, F.; Marahleh, A.; Kinjo, R.; Ma, J.; Ren, J.; Ogasawara, K.; Mizoguchi, I. Effect of age on orthodontic tooth movement in mice. J. Dent. Sci. 2024, 19, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, J.; Zhang, Y.; Liu, H.; Han, B.; Li, W. Age-related alveolar bone maladaptation in adult orthodontics: Finding new ways out. Int. J. Oral. Sci. 2024, 16, 52. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, Y.; Chen, F.; Li, W. The age-related effects on orthodontic tooth movement and the surrounding periodontal environment. Front. Physiol. 2024, 15, 1460168. [Google Scholar] [CrossRef]
- Huang, W.; Hickson, L.J.; Eirin, A.; Kirkland, J.L.; Lerman, L.O. Cellular senescence: The good, the bad and the unknown. Nat. Rev. Nephrol. 2022, 18, 611–627. [Google Scholar] [CrossRef]
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef]
- Eckhardt, B.A.; Rowsey, J.L.; Thicke, B.S.; Fraser, D.G.; O’Grady, K.L.; Bondar, O.P.; Hines, J.M.; Singh, R.J.; Thoreson, A.R.; Rakshit, K.; et al. Accelerated osteocyte senescence and skeletal fragility in mice with type 2 diabetes. JCI Insight 2020, 5, e135236. [Google Scholar] [CrossRef]
- Farr, J.N.; Fraser, D.G.; Wang, H.; Jaehn, K.; Ogrodnik, M.B.; Weivoda, M.M.; Drake, M.T.; Tchkonia, T.; LeBrasseur, N.K.; Kirkland, J.L.; et al. Identification of Senescent Cells in the Bone Microenvironment. J. Bone Min. Res. 2016, 31, 1920–1929. [Google Scholar] [CrossRef] [PubMed]
- Farr, J.N.; Kaur, J.; Doolittle, M.L.; Khosla, S. Osteocyte Cellular Senescence. Curr. Osteoporos. Rep. 2020, 18, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Tilton, M.; Liao, J.; Kim, C.; Shaygani, H.; Potes, M.A.; Cordova, D.; Kirkland, J.L.; Miller, K.M. Tracing Cellular Senescence in Bone: Time-Dependent Changes in Osteocyte Cytoskeleton Mechanics and Morphology. bioRxiv 2025. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X.; Cheng, X.; Ren, T.; Xu, W.; Li, J.; Wang, H.; Zhang, J. Inflammation produced by senescent osteocytes mediates age-related bone loss. Front. Immunol. 2023, 14, 1114006. [Google Scholar] [CrossRef]
- Song, I.; Kim, P.J.; Choi, Y.J.; Chung, Y.S.; Lee, S.; Baek, J.H.; Woo, K.M. Exploring the Interplay Between Senescent Osteocytes and Bone Remodeling in Young Rodents. J. Aging Res. 2024, 2024, 4213141. [Google Scholar] [CrossRef]
- Tiede-Lewis, L.M.; Xie, Y.; Hulbert, M.A.; Campos, R.; Dallas, M.R.; Dusevich, V.; Bonewald, L.F.; Dallas, S.L. Degeneration of the osteocyte network in the C57BL/6 mouse model of aging. Aging 2017, 9, 2190. [Google Scholar] [CrossRef]
- Milovanovic, P.; Zimmermann, E.A.; Hahn, M.; Djonic, D.; Püschel, K.; Djuric, M.; Amling, M.; Busse, B. Osteocytic Canalicular Networks: Morphological Implications for Altered Mechanosensitivity. ACS Nano 2013, 7, 7542–7551. [Google Scholar] [CrossRef] [PubMed]
- Carter, Y.; Thomas, C.D.L.; Clement, J.G.; Cooper, D.M.L. Femoral osteocyte lacunar density, volume and morphology in women across the lifespan. J. Struct. Biol. 2013, 183, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Shibata, Y.; Zhu, T.; Zhou, J.; Zhang, J. Osteocytes in bone aging: Advances, challenges, and future perspectives. Ageing Res. Rev. 2022, 77, 101608. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, S.; Zhang, S.; Liu, M.; Du, H.; Sun, R.; Jing, B.; Sun, Y. Ageing characteristics of bone indicated by transcriptomic and exosomal proteomic analysis of cortical bone cells. J. Orthop. Surg. Res. 2019, 14, 129. [Google Scholar] [CrossRef]
- Busse, B.; Djonic, D.; Milovanovic, P.; Hahn, M.; Püschel, K.; Ritchie, R.O.; Djuric, M.; Amling, M. Decrease in the osteocyte lacunar density accompanied by hypermineralized lacunar occlusion reveals failure and delay of remodeling in aged human bone. Aging Cell 2010, 9, 1065–1075. [Google Scholar] [CrossRef]
- Alikhani, M.; Chou, M.Y.; Khoo, E.; Alansari, S.; Kwal, R.; Elfersi, T.; Almansour, A.; Sangsuwon, C.; Al Jearah, M.; Nervina, J.M.; et al. Age-dependent biologic response to orthodontic forces. Am. J. Orthod. Dentofac. Orthop. 2018, 153, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Peng, C.; Du, Y.; Li, Q.; Yang, K. Effect of autophagy on aging-related changes in orthodontic tooth movement in rats. BMC Oral Health 2024, 24, 785. [Google Scholar] [CrossRef]
- Youlten, S.E.; Kemp, J.P.; Logan, J.G.; Ghirardello, E.J.; Sergio, C.M.; Dack, M.R.G.; Guilfoyle, S.E.; Leitch, V.D.; Butterfield, N.C.; Komla-Ebri, D.; et al. Osteocyte transcriptome mapping identifies a molecular landscape controlling skeletal homeostasis and susceptibility to skeletal disease. Nat. Commun. 2021, 12, 2444. [Google Scholar] [CrossRef]
- Varga, P.; Hesse, B.; Langer, M.; Schrof, S.; Männicke, N.; Suhonen, H.; Pacureanu, A.; Pahr, D.; Peyrin, F.; Raum, K. Synchrotron X-ray phase nano-tomography-based analysis of the lacunar-canalicular network morphology and its relation to the strains experienced by osteocytes in situ as predicted by case-specific finite element analysis. Biomech. Model. Mechanobiol. 2015, 14, 267–282. [Google Scholar] [CrossRef]
- Okada, S.; Yoshida, S.; Ashrafi, S.H.; Schraufnagel, D.E. The canalicular structure of compact bone in the rat at different ages. Microsc. Microanal. 2002, 8, 104–115. [Google Scholar] [CrossRef]
- Mullender, M.G.; van der Meer, D.D.; Huiskes, R.; Lips, P. Osteocyte density changes in aging and osteoporosis. Bone 1996, 18, 109–113. [Google Scholar] [CrossRef]
- Currey, J.D. The many adaptations of bone. J. Biomech. 2003, 36, 1487–1495. [Google Scholar] [CrossRef]
- Qiu, S.; Rao, D.S.; Palnitkar, S.; Parfitt, A.M. Age and distance from the surface but not menopause reduce osteocyte density in human cancellous bone. Bone 2002, 31, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Kamath, T.; Mazur, C.M.; Mirzamohammadi, F.; Rotter, D.; Hojo, H.; Castro, C.D.; Tokavanich, N.; Patel, R.; Govea, N.; et al. Control of osteocyte dendrite formation by Sp7 and its target gene osteocrin. Nat. Commun. 2021, 12, 6271. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.Q.; Ward, L.M.; Liu, S.; Lu, Y.; Xie, Y.; Yuan, B.; Yu, X.; Rauch, F.; Davis, S.I.; Zhang, S.; et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat. Genet. 2006, 38, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Rangiani, A.; Cao, Z.; Sun, Y.; Lu, Y.; Gao, T.; Yuan, B.; Rodgers, A.; Qin, C.; Kuro-o, M.; Feng, J.Q. Protective Roles of DMP1 in High Phosphate Homeostasis. PLoS ONE 2012, 7, e42329. [Google Scholar] [CrossRef] [PubMed]
- Zuo, G.-l.; Zhang, L.-f.; Qi, J.; Kang, H.; Jia, P.; Chen, H.; Shen, X.; Guo, L.; Zhou, H.-b.; Wang, J.-s.; et al. Activation of HIFa Pathway in Mature Osteoblasts Disrupts the Integrity of the Osteocyte/Canalicular Network. PLoS ONE 2015, 10, e0121266. [Google Scholar] [CrossRef]
- Li, Q.; Wang, R.; Zhang, Z.; Wang, H.; Lu, X.; Zhang, J.; Kong, A.P.; Tian, X.Y.; Chan, H.F.; Chung, A.C.; et al. Sirt3 mediates the benefits of exercise on bone in aged mice. Cell Death Differ. 2023, 30, 152–167. [Google Scholar] [CrossRef]
- Rolvien, T.; Schmidt, F.N.; Milovanovic, P.; Jähn, K.; Riedel, C.; Butscheidt, S.; Püschel, K.; Jeschke, A.; Amling, M.; Busse, B. Early bone tissue aging in human auditory ossicles is accompanied by excessive hypermineralization, osteocyte death and micropetrosis. Sci. Rep. 2018, 8, 1920. [Google Scholar] [CrossRef]
- van Hove, R.P.; Nolte, P.A.; Vatsa, A.; Semeins, C.M.; Salmon, P.L.; Smit, T.H.; Klein-Nulend, J. Osteocyte morphology in human tibiae of different bone pathologies with different bone mineral density--is there a role for mechanosensing? Bone 2009, 45, 321–329. [Google Scholar] [CrossRef]
- Wu, V.; van Oers, R.F.M.; Schulten, E.; Helder, M.N.; Bacabac, R.G.; Klein-Nulend, J. Osteocyte morphology and orientation in relation to strain in the jaw bone. Int. J. Oral. Sci. 2018, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Ndemuweda, T.; Kitaura, H.; Ohori, F.; Marahleh, A.; Ma, J.; Fan, Z.; Lin, A.; Narita, K.; Itou, A.; Mizoguchi, I. Evaluation of the effects for root resorption in orthodontic tooth movement with micro-osteoperforations in mice. J. Dent. Sci. 2025, 20, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Adhikari, M.; Sabol, H.M.; Anloague, A.; Khan, S.; Kurihara, N.; Diaz-delCastillo, M.; Andreasen, C.M.; Barnes, C.L.; Stambough, J.B.; et al. Single-Cell Transcriptomic Analysis Identifies Senescent Osteocytes That Trigger Bone Destruction in Breast Cancer Metastasis. Cancer Res. 2024, 84, 3936–3952. [Google Scholar] [CrossRef]
- Wissler Gerdes, E.O.; Zhu, Y.; Tchkonia, T.; Kirkland, J.L. Discovery, development, and future application of senolytics: Theories and predictions. FEBS J. 2020, 287, 2418–2427. [Google Scholar] [CrossRef] [PubMed]
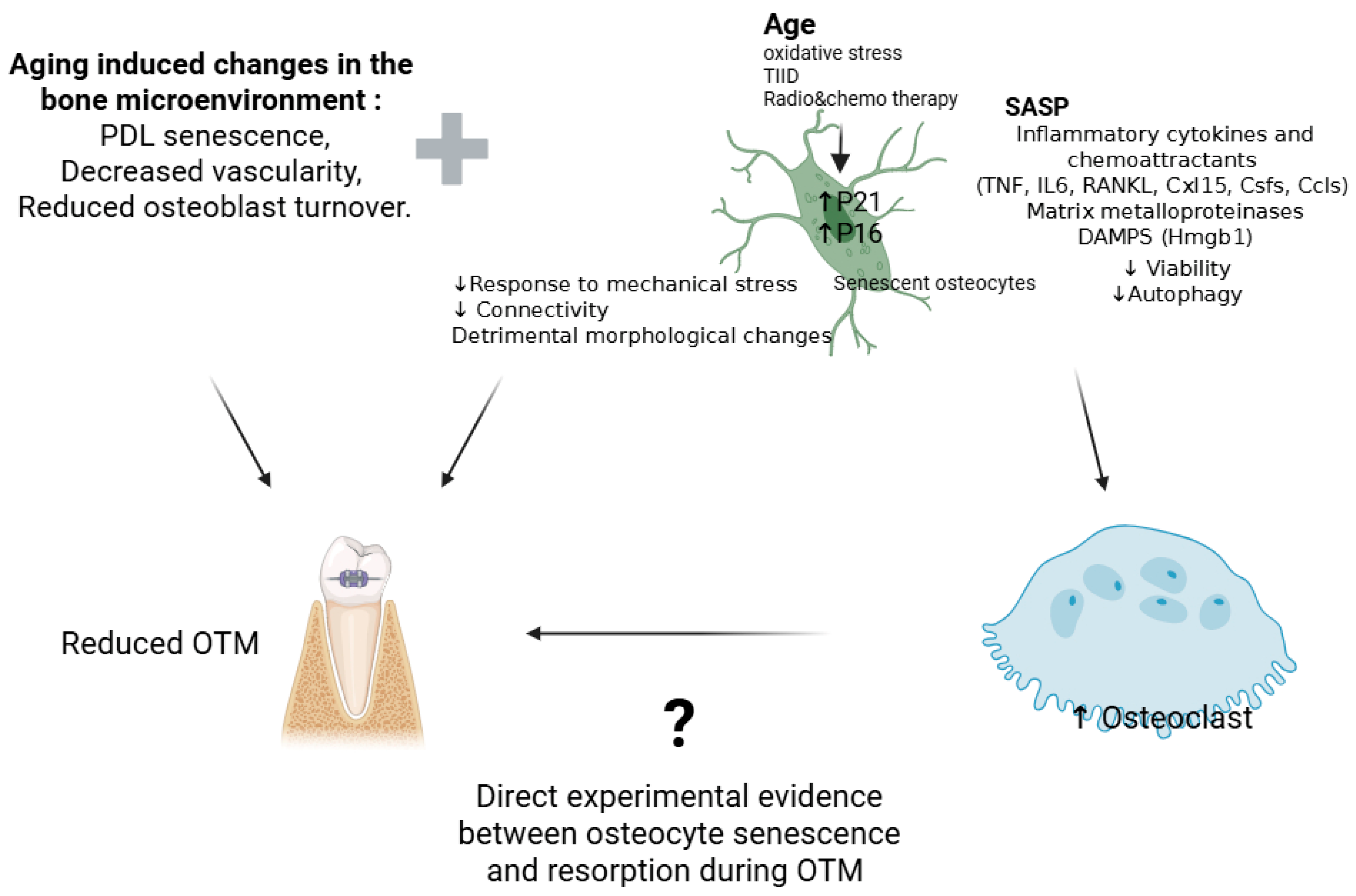
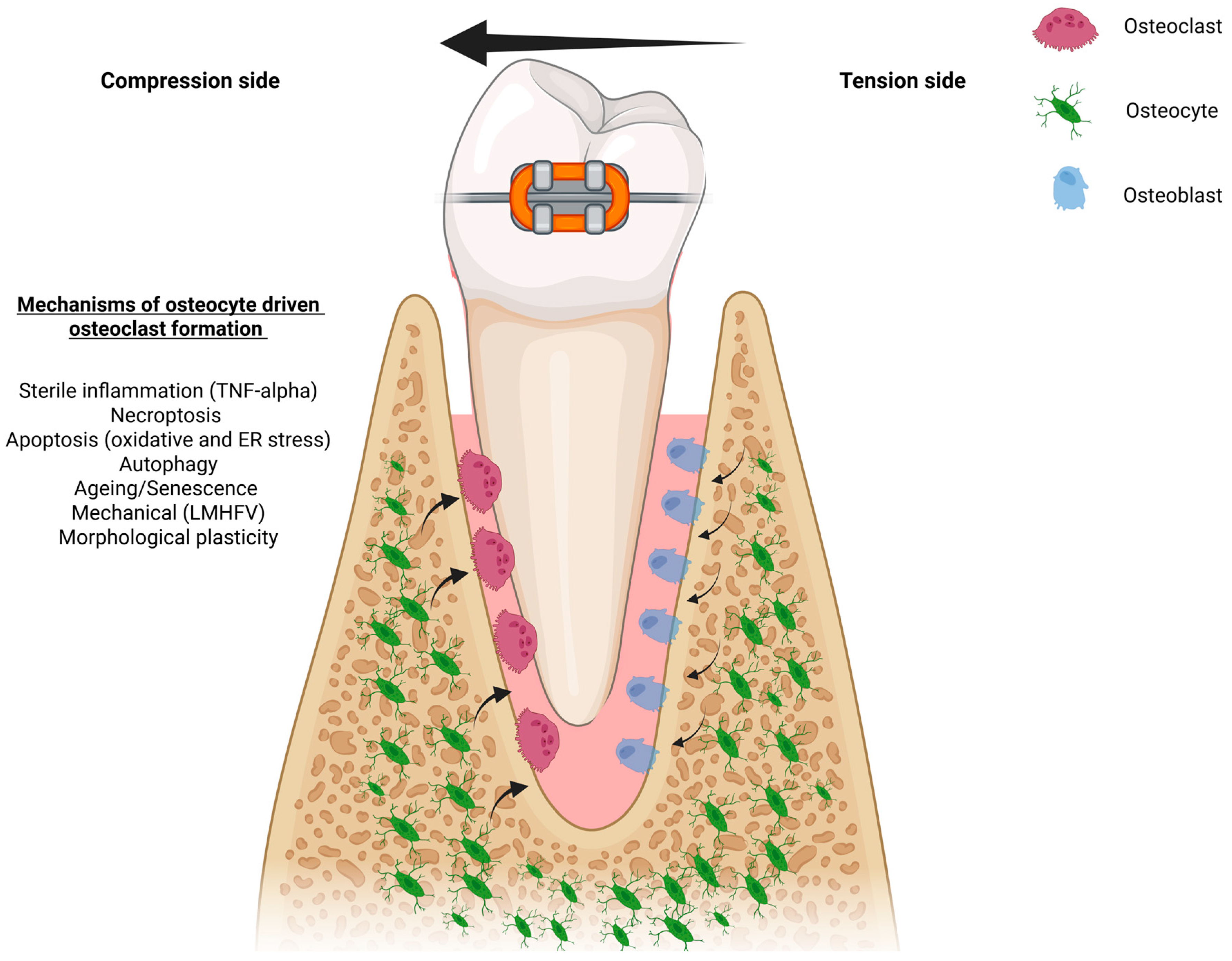
| Pathway/ Factor | Evidence in In Vivo OTM Models | Evidence from Broader In Vivo/In Vitro Studies |
|---|---|---|
| RANK–RANKL–OPG | Osteocyte-specific RANKL deletion reduces OTM distance [31]. RANKL expression upregulated in osteocytes at compression sites during OTM [35]. β-adrenergic blockade reduced osteocyte RANKL expression and osteoclastogenesis on the compression side [50]. | Osteocyte RANKL is essential for bone remodeling [18] Osteocyte-specific RANKL knockout mice develop osteopetrosis at 12 weeks of age [24]. |
| TNF-α | TNFR1/2 KO have reduced osteocytic RANKL and sclerostin expression on the compression side [35,37]. | TNF-α directly enhances osteocyte RANKL and sclerostin expression via MAPKs and NFκB pathways [34,37]. |
| Sclerostin (SOST) | OTM induces sclerostin expression in osteocytes on the compression side [37,44]. β-adrenergic blockade reduced sclerostin expression on the compression side and increased bone volume [50]. | Sclerostin increases RANKL expression [37] and neutralizing sclerostin increases OPG expression and decreases RANKL/OPG ratio [44]. |
| Stimulus | Intracellular Pathway/Process | Outcome | Source |
|---|---|---|---|
| Mechanical compressive force and Inflammation | Necroptosis via RIP3/MLKL activation | DAMPs release → RANKL-independent osteoclast differentiation | [49] |
| Mechanical compressive force | Autophagy induction (ROCK↓ → AKT↓ → TFE3 → ATG7 & LC3B upregulation) | ↑ RANKL secretion → enhanced osteoclastogenesis & localized bone resorption | [52] |
| Inflammation (TNF-α, IL1β) | MAPK (ERK1/2, JNK, p38) & NF-κB activation | ↑ RANKL & ↑ sclerostin → synergistic boost in osteoclastogenesis | [35,37] |
| Exogenous mechanical stimuli (LMHFV) | TGF-β1 release & NF-κB signaling | ↑ RANKL expression → accelerated osteoclast differentiation during vibration-assisted OTM | [58,59] |
| Sympathetic (β-adrenergic) stimulation | β-adrenergic signaling → upregulation of sclerostin and RANKL | ↑ Sclerostin & RANKL → shifts remodeling toward resorption over formation | [51] |
| Aging (osteocyte senescence) | SASP (p16INK4a/p21CIP1-mediated growth arrest) producing IL-6, IL-8, RANKL, etc. | SASP cytokines + RANKL → recruit & promote osteoclast precursors → age-related bone loss | [75,76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marahleh, A.; Ohori, F.; Ma, J.; Fan, Z.; Lin, A.; Narita, K.; Murakami, K.; Kitaura, H. Recent Advances in the Role of Osteocytes in Orthodontic Tooth Movement. Int. J. Mol. Sci. 2025, 26, 9396. https://doi.org/10.3390/ijms26199396
Marahleh A, Ohori F, Ma J, Fan Z, Lin A, Narita K, Murakami K, Kitaura H. Recent Advances in the Role of Osteocytes in Orthodontic Tooth Movement. International Journal of Molecular Sciences. 2025; 26(19):9396. https://doi.org/10.3390/ijms26199396
Chicago/Turabian StyleMarahleh, Aseel, Fumitoshi Ohori, Jinghan Ma, Ziqiu Fan, Angyi Lin, Kohei Narita, Kou Murakami, and Hideki Kitaura. 2025. "Recent Advances in the Role of Osteocytes in Orthodontic Tooth Movement" International Journal of Molecular Sciences 26, no. 19: 9396. https://doi.org/10.3390/ijms26199396
APA StyleMarahleh, A., Ohori, F., Ma, J., Fan, Z., Lin, A., Narita, K., Murakami, K., & Kitaura, H. (2025). Recent Advances in the Role of Osteocytes in Orthodontic Tooth Movement. International Journal of Molecular Sciences, 26(19), 9396. https://doi.org/10.3390/ijms26199396






